Significance
In Parkinson’s disease (PD), dopamine (DA)-producing neurons gradually degenerate, leading to DA deficiency and to the main symptoms of PD. Current medications do not impede neurodegeneration, but relieve symptoms by replenishing DA; however, their chronic use causes serious side effects. We targeted a protein required for the development and function of DA neurons by designing a chemical compound that, by activating this protein, increases DA and improves symptoms without current treatment side effects while simultaneously preventing neuron loss in PD mice. Our findings point to a monotherapy that can both impede PD progression and concurrently improve symptoms of PD.
Keywords: Parkinson's disease, target validation, neuroprotection
Abstract
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by the loss of dopaminergic (DAergic) neurons in the substantia nigra and the gradual depletion of dopamine (DA). Current treatments replenish the DA deficit and improve symptoms but induce dyskinesias over time, and neuroprotective therapies are nonexistent. Here we report that Nuclear receptor-related 1 (Nurr1):Retinoid X receptor α (RXRα) activation has a double therapeutic potential for PD, offering both neuroprotective and symptomatic improvement. We designed BRF110, a unique in vivo active Nurr1:RXRα-selective lead molecule, which prevents DAergic neuron demise and striatal DAergic denervation in vivo against PD-causing toxins in a Nurr1-dependent manner. BRF110 also protects against PD-related genetic mutations in patient induced pluripotent stem cell (iPSC)-derived DAergic neurons and a genetic mouse PD model. Remarkably, besides neuroprotection, BRF110 up-regulates tyrosine hydroxylase (TH), aromatic l-amino acid decarboxylase (AADC), and GTP cyclohydrolase I (GCH1) transcription; increases striatal DA in vivo; and has symptomatic efficacy in two postneurodegeneration PD models, without inducing dyskinesias on chronic daily treatment. The combined neuroprotective and symptomatic effects of BRF110 identify Nurr1:RXRα activation as a potential monotherapeutic approach for PD.
Parkinson’s disease (PD) is a common neurodegenerative disease characterized by the progressive loss of dopaminergic (DAergic) neurons in the substantia nigra (SN), leading to striatal dopamine (DA) deficiency (1). Current medications replenish DA and offer temporary symptomatic relief to patients; however, chronic treatments cause serious side effects in almost all patients, including abnormal involuntary movements (AIMs) and dyskinesias, limiting their efficacy (2). Moreover, DA replacement does not impede neurodegeneration, and PD pathology progresses (3). Despite considerable advances in our understanding of PD pathophysiology, pharmacologic strategies to prevent neurodegeneration remain elusive, and the disease remains incurable. Therefore, validation of novel targets that diminish DA replacement side effects and halt or slow disease progression is an urgent unmet clinical need (4).
Nurr1 (NR4A2), a nuclear receptor, is a promising candidate, implicated in both DA biosynthesis and DAergic neuron survival. Nurr1 is expressed in developing and mature DAergic neurons and is required for both survival and final complete DAergic differentiation (5, 6). Nurr1 enhances in vitro and in vivo transcription of tyrosine hydroxylase (TH), the rate-limiting enzyme of DA biosynthesis, and of GTP cyclohydrolase I (GCH1), the first enzyme in the biosynthesis of tetrahydrobiopterin (BH4), an essential cofactor for TH activity (7, 8). Decreased Nurr1 levels have been strongly associated with PD and reduced DAergic neuron survival. Nurr1 ablation in adult mice leads to dystrophic DAergic axons (9), loss of striatal DA (10), and behavioral features of parkinsonism during aging (11). Nurr1 mutations decreasing its mRNA have been found in patients with familial and sporadic PD (12, 13). Given the role of Nurr1 in PD, we theorized that pharmacologic activation of Nurr1 could serve as monotherapy for PD, offering both disease modification and symptomatic relief.
Nurr1 binds to DNA as a monomer, homodimer, or heterodimer with retinoid X receptor (RXR)α or RXRγ. Because Nurr1 heterodimerizes with RXRα in midbrain DAergic neurons (14), we postulated that synthetic ligands that bind to the RXRα-binding pocket would be the preferred approach. RXRα is a heterodimer partner of several nuclear receptors, and existing synthetic RXRα ligands activate several RXRα heterodimers (15). Two such ligands, XCT0135908 and bexarotene (14, 16), have been shown to activate Nurr1:RXRα as well as other RXRα heterodimers. Bexarotene, an approved antineoplastic agent activates RXRα heterodimers with liver X receptor (LXR), peroxisome proliferator-activated receptor gamma (PPARγ), and Nurr1, has shown promising results in animal models of Alzheimer’s disease and PD (16, 17), but these results have not been replicated (18, 19). In vitro, XCT0135908 (14) activates Nurr1:RXRα heterodimers and RXRα:RXRα homodimers (17), but its bioavailability is unknown.
Here we show that XCT0135908 does not reach the brain and is essentially inactive in vivo. Rational compound design and synthetic chemistry enabled us to design BRF110, a selective Nurr1:RXRα agonist with combinatorial neuroprotective and symptomatic benefits in preclinical mouse models, validating Nurr1:RXRα heterodimer activation as a promising monotherapy for PD.
Results
XCT0135908 in Vivo: Low Stability and Low Brain Penetration.
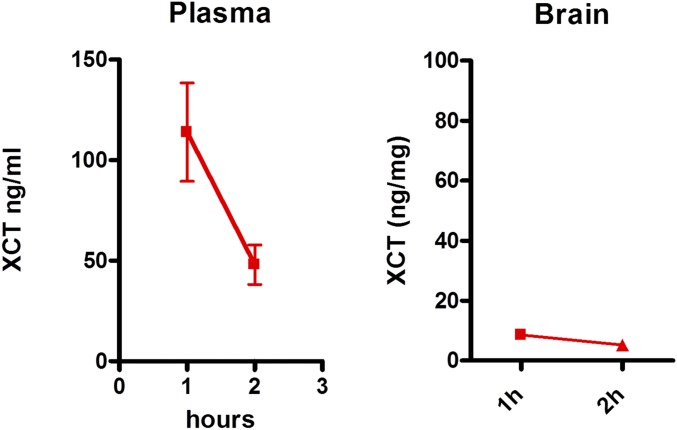
XCT0135908 was administered to mice to test its bioactivity. Intraperitoneal or intracerebroventricular injections of XCT0135908 (1 and 10 mg/kg) did not result in any changes in expression of midbrain genes, such as c-jun or TH, at various time points after administration. LC-MS/MS (liquid chromatography-tandem mass spectrometry) analysis of blood plasma or brain homogenates and targeted search of the parent compound at 1 and 2 h after i.p. administration of XCT0135908 (1 μg/kg) indicated low compound stability and minimal brain penetration (brain/blood <0.03) (Fig. S1).
Fig. S1.
Pharmacokinetics of XCT0135908. Intraperitoneal administration of XCT0135908 in mice (1 mg/kg) resulted in minimal penetration of the compound in the brain. Three serial tail bleeds were performed to detect the compound in plasma at 1 and 2 h after i.p. administration. Similarly, mice (n = 3) were killed at 1 and 2 h after i.p. administration, and brains were dissected. The concentrations of the parent compound in plasma and brain were determined by LC-MS/MS.
Discovery of the Nurr1:RXRα Activator BRF110.
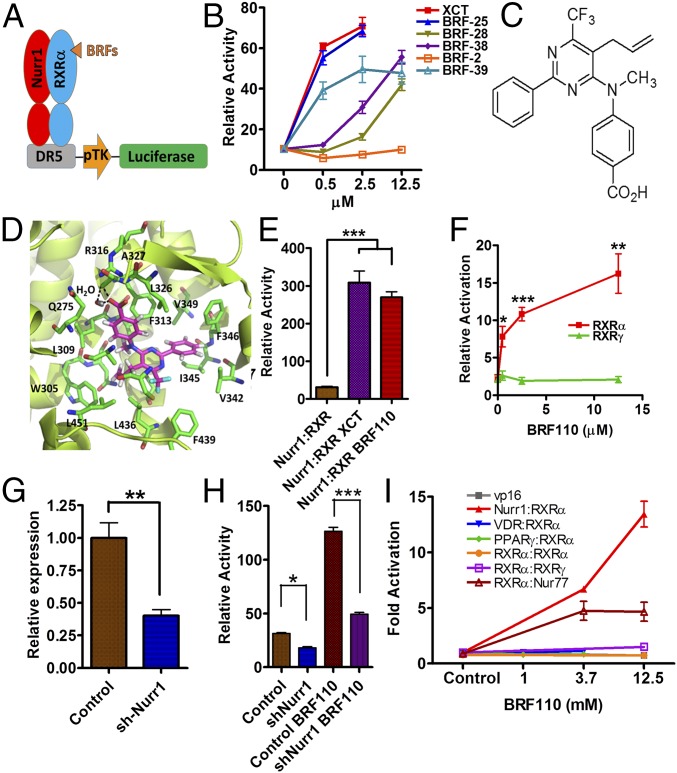
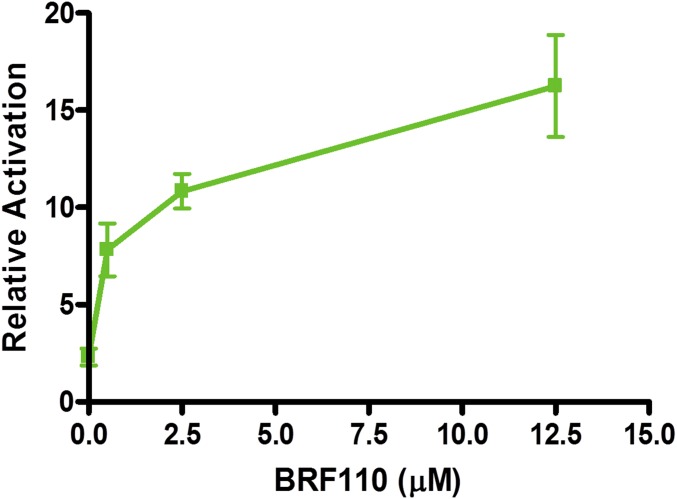
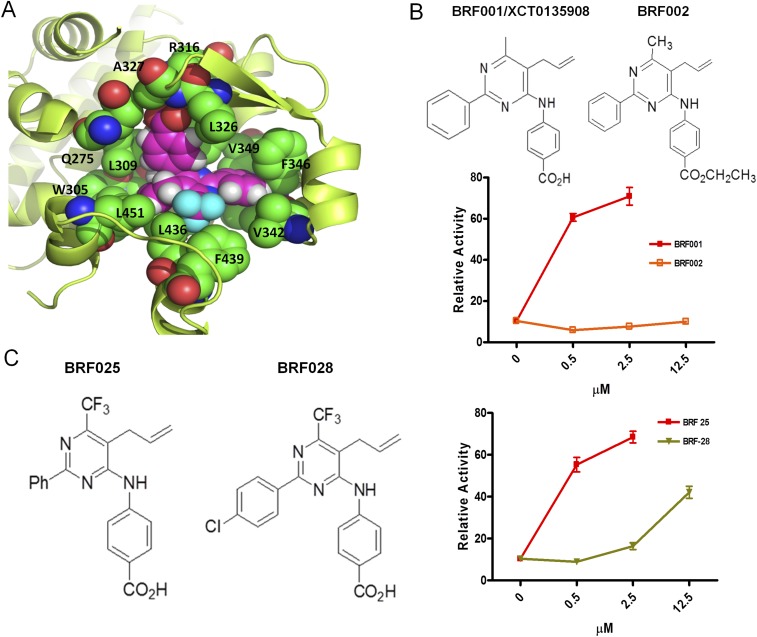
Because specific Nurr1:RXRα activators do not exist (20), we confronted the challenge to discover compounds that would be specific for Nurr1:RXRα heterodimers, stable in vivo, and brain-penetrant. Based on existing chemical structures and their function on RXRα in a variety of assays, we synthesized several series of compounds (termed BRFs). Human DAergic neuroblastoma SHSY-5Y cells cotransfected with a DR5-luciferase reporter construct, along with human Nurr1 and RXRα expression plasmids (Fig. 1 A and B), were treated with each of the experimental compounds at varying concentrations. Compounds active in vitro were routinely administered i.p. in mice (at 1 μg/kg), and brain penetration was evaluated by LC-MS/MS. This scheme and meticulous structure–activity relation analysis resulted in the identification of BRF110 (Fig. 1C and Figs. S2 and S3), which activates Nurr1:RXRα heterodimers (EC50 ∼900 nM; Fig. S4).
Fig. 1.
Discovery of BRF110, specificity for Nurr1:RXRα heterodimers, and i.p. administration in mice. (A) Schematic representation of the screening transactivation assay. (B) Typical results. (C) Chemical structure of BRF110. (D) Schematic representation of BRF110 interactions within the RXRα (PDB ID code 1MV9)-binding pocket. BRF110 (magenta) docked onto RXRα (green; PDB ID code 1MV9). Hydrogen bonds and amino acids are indicated. (E) Comparison of DR5-Luc induction by BRF110 (1 μM) and XCT (1 μM) via activation of Nurr1:RXRα heterodimers. (F) Cellular assay indicating that BRF110 activates Nurr1:RXRα heterodimers but not Nurr1:RXRγ heterodimers. (G) Retroviral-mediated knockdown of endogenous Nurr1 in SHSY5Y cells as measured by qPCR. (H) Activation by BRF110 of DR5-luciferase reporter plasmid introduced into SHSY-5Y cells infected with either a control retrovirus or the retrovirus carrying shNurr1 sequences. (I) Gal4-DNA–binding domain and Nurr1, Nur77, VDR, RXRγ, PPARγ, and RXRα ligand-binding domain fusions cotransfected with RXRα:vp16 fusions activated by BRF110 at the indicated concentrations.
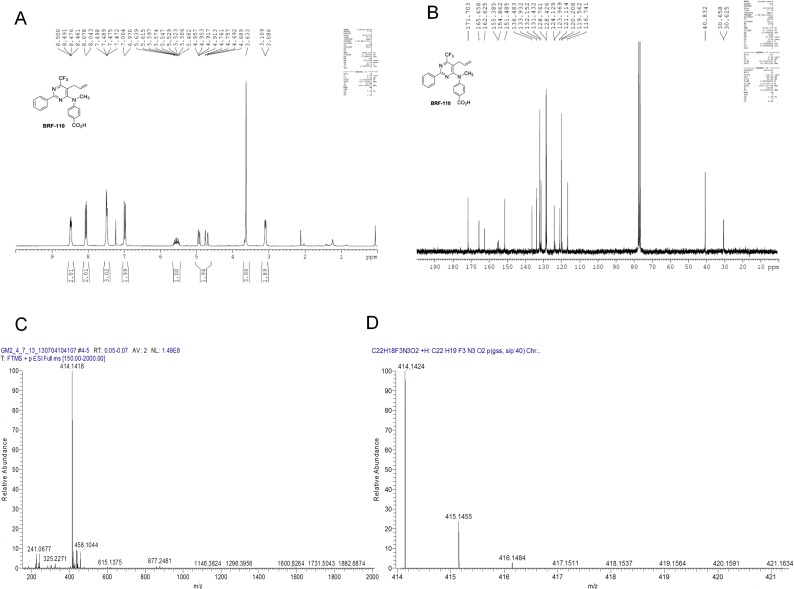
Fig. S2.
Characterization data for BRF110 4-((5-allyl-2-phenyl-6-(trifluoromethyl)pyrimidin-4-yl)(methyl)amino)benzoic acid. MW = 413.1351, cLogP = 6.14 (for neutral), cLogD = 3.33 at pH 7.4, PSA = 66.32 Å2, HBD = 1, logS = −3.60, log1/S for neutral comp = 6.41, lolubility (S) = 0.10 g/L at pH 7.4. (A) 1H NMR of BRF110. 1H NMR spectra were recorded on a 250-MHz Bruker Avance FT-NMR spectrometer. 1H NMR (250 MHz, CDCl3) δ 8.47 (m, 2 H), 8.05 (d, 2 H, J = 8.8 Hz), 7.47 (m, 3 H), 6.98 (d, 2 H, J = 8.8 Hz), 5.55 (m, 1 H), 4.93 (dd, 1 H, J = 1.0, 10.0 Hz), 4.72 (dd, J = 0.9, 17.1 Hz), 3.62 (s, 3 H), 3.08 (d, 2 H, J = 5.8 Hz). (B) 13C NMR of BRF110. 13C NMR spectra were recorded at 62.9 MHz. Distortionless enhancement by polarization transfer spectra [DEPT (135)] were recorded at 62.9 MHz. 13C NMR and [DEPT (135)] data are combined and represented as follows: chemical shift, carbon type obtained from [DEPT (135)] experiments. Chemical shifts are reported in ppm relative to solvent signal. Multiplicity is indicated as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br, broad; dd, doublet of doublets; ddd, doublet of doublets of doublets. 13C NMR (62.9 MHz, CDCl3) δ 171.7 (C), 165.6 (C), 162.6 (C), 155.1 (C, q, JC–F = 32.9 Hz), 151.5 (C), 136.5 (C), 133.9 (CH), 132.1 (CH), 131.4 (CH), 128.8 (CH), 128.4 (CH), 124.1 (C), 121.8 (C, d, JC–F = 277.2 Hz), 121.1 (C), 120.2 (CH), 116.7 (CH2), 40.8 (CH3), 30.6 (CH2, d, JC–F = 2.1 Hz). (C) HRMS of BRF110. (D) Elemental composition search on mass 414.1418 (isotopes O16, C12, H1, N14, F19), m/z 414.1418; theoretical mass, 414.1424; delta (ppm), −1.52; RDB equivalent, 13.5; composition, C22 H19 O2 N3 F3.
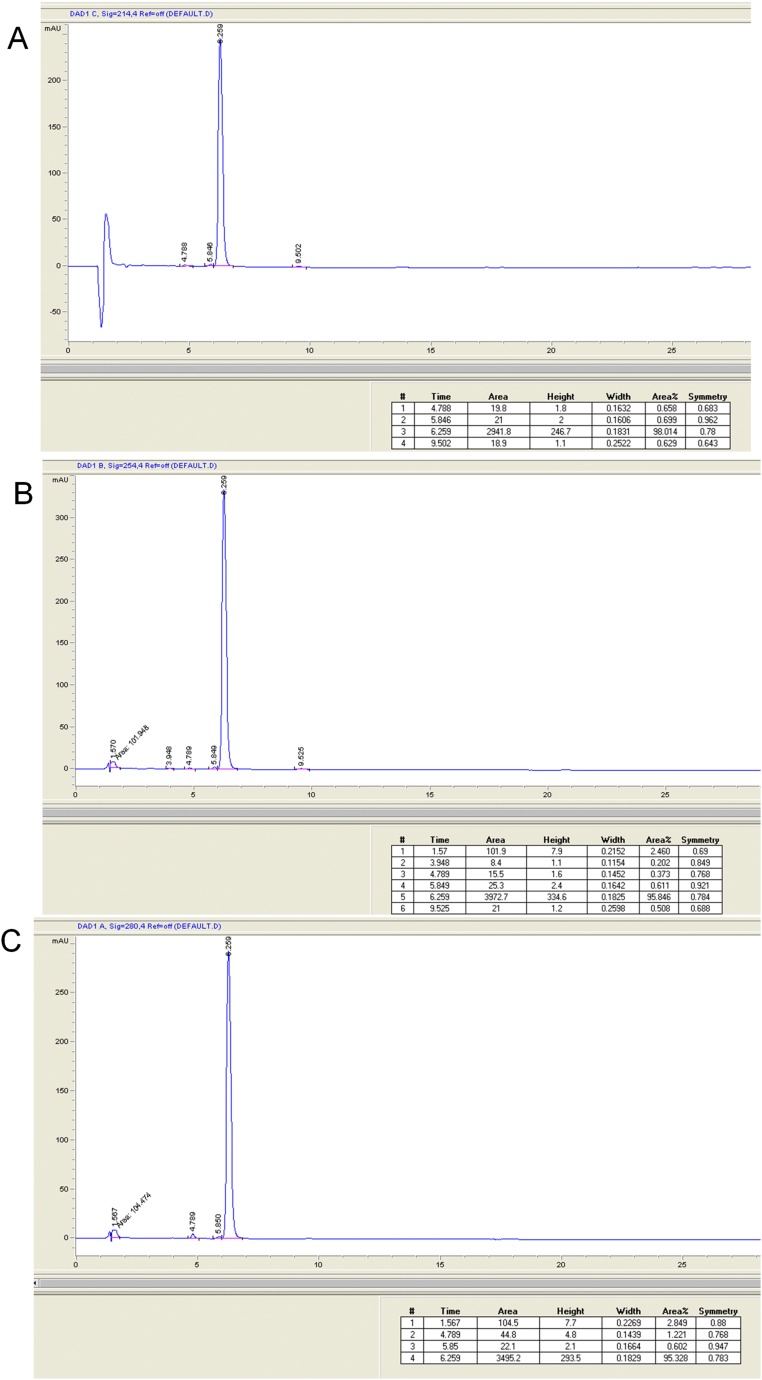
Fig. S3.
HPLC analysis of BRF110 at 214 nm (98% purity) (A), 254 nm (95.8% purity) (B), and 280 nm (95.3% purity) (C). HPLC analysis of BRF110 was performed on an Agilent Eclipse XDB-C18 column with particle size of 5 μm (15 cm × 4.6 mm i.d.), using an Agilent 1200 series liquid chromatograph equipped with a 20-μL sample loop injector. The peaks representing the target analyte(s) were recognized by both their retention time and their spectrum pattern recorded on a diode array detector running Agilent ChemStation chromatography software. Elution was accomplished isocratically with a solvent system consisting of 75% CH3CN-25% H2O (0.1% TFA). HPLC chromatograms of BRF110 were recorded in three different wavelengths to assess purity.
Fig. S4.
Dose-response of BRF110. The EC50 of BRF110 was measured in a cellular assay in SHSY5Y cells cotransfected with a DR5-driven luciferase reporter plasmid and CMV-driven Nurr1 and RXRα plasmids. A CMV β-galactosidase plasmid served as a transfection control. Varyious concentrations of BRF110 were added to the culture medium at 24 h after transfection. Cells were harvested 24 h later, and activation of the Nurr1:RXRα heterodimer was determined by luciferase assays (Promega).
In silico docking simulations show that BRF110 complements the hydrophobic L-shaped binding pocket of RXRα [Protein Data Bank (PDB) ID code 1MV9]. The carboxylic group of BRF110, with the participation of a water molecule, forms a hydrogen bond with the backbone amide of A327 and a salt bridge with the side chain of R316. The phenyl ring of BRF110 participates in π–π interactions with F346 and stabilizes the molecule in the RXRα-binding pocket, contributing to target affinity (calculated ΔG, −16.7 kcal/mol) (Fig. 1D and Fig. S5A). These simulations were validated by methyl or ethyl ester carboxylic group modifications, which abolished activation, increasing EC50 by 50- to 100-fold (Fig. S5B). In addition, halogenation of the BRF110 phenyl ring disturbs the π–π interactions with F346 and also increases the EC50 by approximately sevenfold (Fig. S5C).
Fig. S5.
Structure of BRF110 docked in the X-ray structure of RXRα (PDB ID code 1MV9). (A) In silico docking simulations using the Glide “protein and ligand preparation” module. BRF110 is represented by magenta spheres, and neighboring amino acids are shown as green spheres. The surrounding protein is shown in yellow cartoon representation. The binding pocket of RXRα is purely hydrophobic except for the side chain of R316, which forms a salt bridge with the carboxylic acid of BRF110. The carboxylic acid also forms two hydrogen bonds with A327 and a neighboring water molecule. The hydrophobic enclosure of BRF110 is exemplified by its perfect shape complementarity with the L-shaped binding pocket of RXRα through favorable van der Waals interactions. Α271, I268, C269, and Α272 seal the hydrophobic pocket from the top and thus are hidden for clarity. (B) Modification of the carboxy group of various BRF compounds blocks activation of Nurr1:RXRalpha heterodimers. Examples of BRF001 (XCT0135908) and BRF002 are shown. (C) Disturbance of the electron cloud of the phenyl ring of various BRF compounds by the substitution of a hydrogen (BRF025) atom with a halogen (BRF028) atom decreased affinity for the RXRα-binding pocket.
Specificity of BRF110 for Nurr1:RXRα Heterodimers and Brain Penetration.
BRF110 activated Nurr1:RXRα heterodimers (Fig. 1E), but did not activate Nurr1:RXRγ heterodimers (Fig. 1F), indicating that it is specific for RXRα and does not bind to Nurr1. In naïve SHSY-5Y cells, BRF110 activated endogenous Nurr1:RXRα heterodimers, as verified by loss of activity after knockdown of Nurr1 by ∼60% using a retrovirus carrying shNurr1 sequences (Fig. 1G), indicating that Nurr1 is required for BRF110 activity (Fig. 1H).
To test off-target effects of BRF110 besides Nurr1:RXRα heterodimers, we fused the ligand-binding domains of Nurr1, RXRα, and a variety of related nuclear receptors to the GAL4 DNA-binding domain to create chimeric proteins, and also fused the ligand-binding domain of RXRα with VP16. These molecular chimeras were cotransfected in pairs with RXRα:VP16 along with a GAL4-responsive luciferase reporter in SHSY-5Y cells stimulated with BRF110. BRF110 strongly activated Nurr1GAL4:RXRαVP16 heterodimer chimeras, but failed to activate other RXRαVP16 heterodimer chimeras with vitamin D receptor VDRGAL4, RXRγGAL4, PPARγGAL4, or RXRαGAL4 homodimer chimeras (Fig. 1I). Nur77GAL4:RXRαVP16 heterodimers were partially activated, but Nur77, unlike Nurr1, is not associated with PD, and Nur77 knockdown enhances cell survival (21). The foregoing experiments indicate the high degree of selectivity of BRF110.
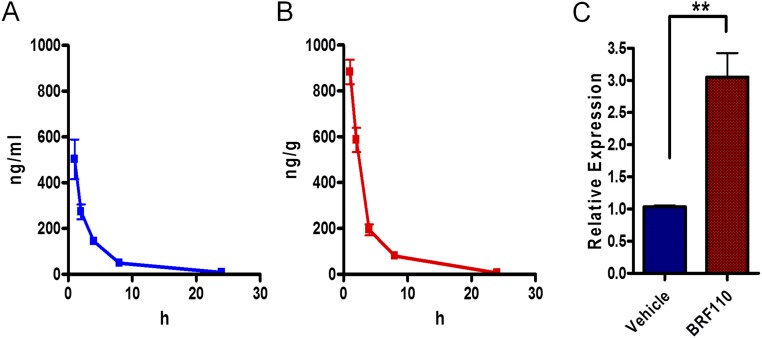
BRF110 (1 mg/kg) administered i.p. in mice reached the brain, with an approximate half-life of ∼1.5 h in both blood and brain as assessed by LC-MS/MS (brain/blood concentration area under the curve ratio, 1.7; Fig. S6) and was bioactive, increasing midbrain c-jun expression. These experiments indicate that BRF110 has the necessary properties to test our monotherapeutic PD hypothesis of Nurr1:RXRα heterodimer activation.
Fig. S6.
Brain penetration and half-life of BRF110. The concentration of the parent compound in plasma and brain were determined by LC/MSMS. (A) Detection of BRF110 in plasma at 1, 2, 4, 8, and 24 h after i.p. administration. (B) Intraperitoneal administration of BRF110 in mice (1 mg/kg) resulted in appreciable penetration of the compound in the brain. Mice (n = 5) were killed and brains dissected at 1, 2, 4, 8, and 24 h after i.p. administration. (C) c-jun transcriptional activation in the midbrain as determined by qPCR at 2 h after i.p. administration of BRF110.
BRF110 Induces Transcription of DA Biosynthesis Genes and Protects DAergic Cell Lines and Human Induced Pluripotent Stem Cell-Derived DAergic Neurons Against PD-Associated Damage.
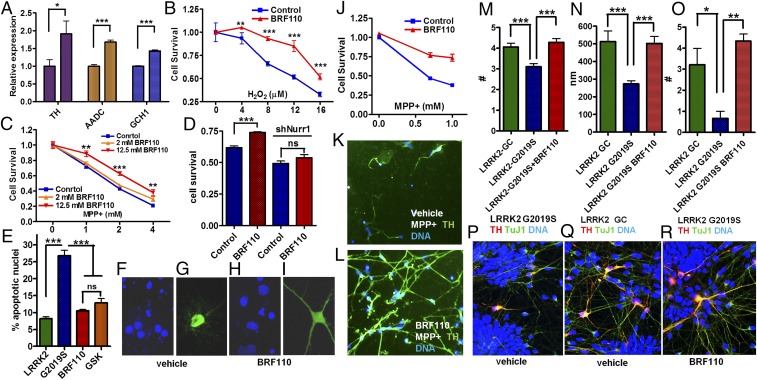
Nurr1 regulates the transcription of DA biosynthesis genes (7, 8); however, whether this is mediated by Nurr1:RXRα heterodimers is unknown. In SHSY-5Y cells, BRF110 increased the expression of the three genes required for DA biosynthesis, TH by ∼90% (n = 6; P = 0.0374, t test), aromatic l-amino acid decarboxylase (AADC) by ∼70% (n = 6; P < 0.0001, t test), and GCH1 by ∼42% (n = 6; P < 0.0001, t test) (Fig. 2A), indicating that this up-regulation depends on Nurr1:RXRα heterodimer activation.
Fig. 2.
Induction of DA biosynthesis gene expression and of neuroprotection in cell lines and in patient iPSC-derived DAergic neurons. (A) Expression levels of the three DA biosynthesis genes (TH, AADC, and GCH1) mediated by the activation of Nurr1:RXRα by BRF110 (12.5 μM), as assessed by qPCR. n = 8. (B) SHSY-5Y cell viability increases with BRF110 treatment, as measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, after exposure to varying concentrations (0–16 μM) of H2O2. (C) BRF110 concentration-dependent SHSY-5Y cell viability, as measured by the MTT assay, after exposure to varying concentrations (0–4 mM) of MPP+. (D) BRF110-mediated SHSY-5Y cell viability against MPP+ (2 mM) is dependent on Nurr1 levels, as determined by retroviral knockdown of Nurr1. (E) BRF110 treatment of primary rat cortical neurons reduces apoptotic death induced by cotransfecting LRRK2-G2019S or control WT LRRK2 cDNAs with CMV GFP, as measured by DAPI staining. (F–I) Representative DAPI (F and H) and GFP (G and I) confocal images. (J) Survival of human iPSC-derived DAergic neurons exposed to MPP+ (0.5 and 1.0 mM for 24 h) and receiving vehicle or BRF110. (K and L) BRF110 treatment preserves DAergic neuron projections, as determined by TH immunofluorescence of surviving iPSC-derived DAergic neurons after exposure to MPP+. (M–R) PD patient iPSC-derived DAergic neurons with the LRRK2-G2019S mutation or corrected mutation (GC). BRF110 treatment rescues neurite number (M), neurite length (N), and neurite branching (O) phenotypes. (P–R) Representative images stained with TuJ, TH, and DAPI.
We examined the neuroprotective capability of Nurr1:RXRα heterodimer activation in SHSY-5Y cells in which death was induced by either oxidative stress-related H2O2 or mitochondria complex I inhibitor 1-methyl-4-phenylpyridinium (MPP+) (Fig. 2B). BRF110 (12.5 μM) did not promote cell differentiation or cell proliferation, but significantly increased cell survival against varying concentrations of the toxic stimuli (P < 0.0001, two-way ANOVA) in a dose-dependent manner (Fig. 2C). The protection conferred by BRF110 was Nurr1-dependent, because it was abrogated by knockdown of endogenous Nurr1 mRNA (Figs. 1G and 2D). The neuroprotective effects of BRF110 extend to damage induced by the PD-associated mutation G2019S in the leucine‐rich repeat kinase 2 (LRRK2) gene, the most common genetic defect associated with clinical PD (22). Rat cortical neurons cotransfected with CMV-GFP (cytomegalovirus promoter-driven GFP) for identification and CMV-LRRK2-G2019S cDNAs show increased apoptosis, as assessed by DAPI staining and compared with control neurons cotransfected with a WT LRRK2 cDNA. We did not observe neuronal differentiation effects on BRF110 treatment, although it reduced apoptosis to control levels, comparable to those of the LRRK2 inhibitor GSK2578215A (P < 0.0001, one-way ANOVA) (Fig. 2 E–I).
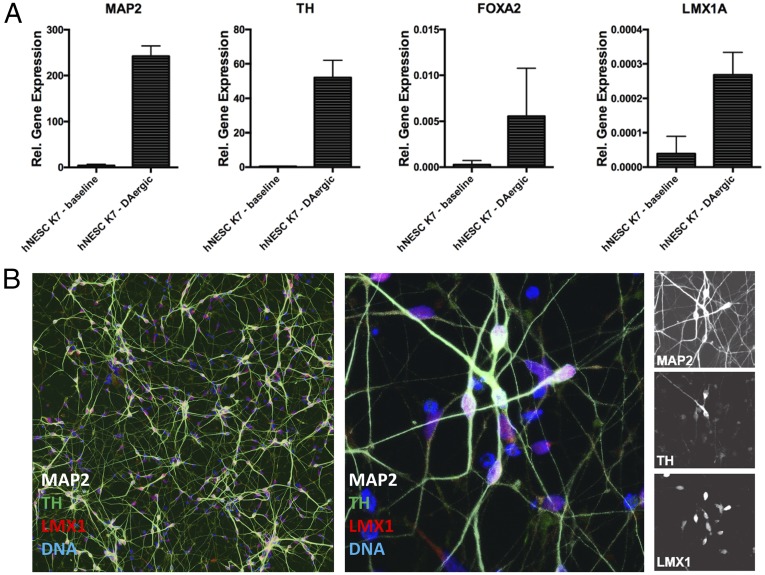
The translational therapeutic potential of BRF110 was assessed in human midbrain-specific induced pluripotent stem cell (iPSC)-derived DAergic neurons, as indicated by positive staining and quantitative PCR (qPCR) for the neuronal markers MAP2, TH, FoxA2, Lmx1, and En1 (Fig. S7). BRF110 doubled the number of surviving MPP+-intoxicated neurons compared with vehicle (P < 0.0008, two-way ANOVA) (Fig. 2 J–L). The surviving control neurons had fewer and retreating projections, indicating compromised function (Fig. 2K), whereas the neurons that received BRF110 retained a complex network of projections and contacts (Fig. 2L).
Fig. S7.
Characterization of iPSC-derived human DAergic neurons. Human DAergic neurons were differentiated from neural progenitor cells for 2 wk. (A) Expression of relevant markers detected by qPCR for MAP2, TH, FOXA2, and LMX1 (n = 3). (B) Differentiation into midbrain-specific DAergic neurons, shown by immunohistochemistry staining for MAP2, TH, and LMX1. (Scale bars: 50 µm.)
In addition, we tested BRF110 in iPSC-derived DAergic neurons from a PD patient carrying the LRRK2-G2019S mutation. These neurons showed contracted neurites with reduced branching, phenomena that can be reversed on correction (LRRK2GC) of the LRRK2-G2019S mutation (22). Treatment of the LRRK2-G2019S DAergic neurons with BRF110 (12.5 μM) for 14 d (Fig. 2 M–Q) increased neurite length by 83% (P < 0.0007, one-way ANOVA; P < 0.01, Kruskal–Wallis test), neurite number by 38% (P < 0.0001, one-way ANOVA; P < 0.001, Kruskal–Wallis test), and neurite branching by 650% (P < 0.01, one-way ANOVA; P < 0.05, Kruskal–Wallis test) (Fig. 2 M–O). During the 14-d BRF110 treatment of these cells, we did not see any effect on TuJ1(+)/TH(−) neurons or on DAergic neurons with a WT genotype.
The foregoing experiments demonstrate the in vitro potential of Nurr1:RXRα activation to shield human DAergic neurons from diverse PD-related neuronal death stimuli, such as toxins and the LRRK2-G2019S mutation.
Neuroprotection of BRF110 in Vivo in Preclinical Mouse PD Models.
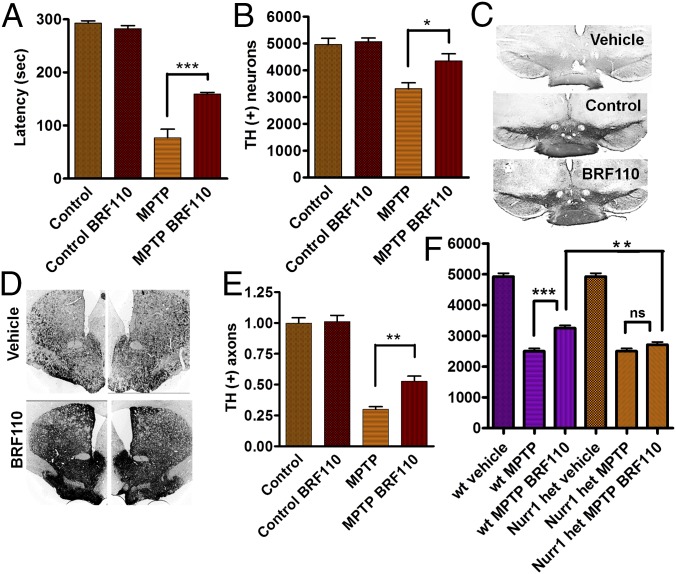
We assessed the neuroprotective effects of Nurr1:RXRα heterodimer activation in vivo in two established preclinical C57BL/6 mouse PD models: acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and unilateral 6-hydroxydopamine (6-OHDA) injection in the SN. Intraperitoneal injections of BRF110 (10 mg/kg) every 12 h, starting 12 h before toxin administration, were continued for 6 d in the MPTP model and for 14 d in the 6-OHDA model. To distinguish phenotypic effects, lasting 4–8 h (see below), from neuroprotection, BRF110 administration was discontinued 24–36 h before behavioral and histological examination of the mice. In the MPTP model, mice receiving BRF110 had considerably improved motor coordination (>100%) (n = 5; P < 0.0001, one-way ANOVA) compared with vehicle-injected mice, as assessed by the accelerating rotarod test (Fig. 3A). SN unbiased stereologic neuronal counting showed that TH(+) midbrain neuron survival per side was increased by 31% (n = 5; P = 0.0003, one-way ANOVA) in BRF110-treated mice, to a value not significantly different from that in control mice (Fig. 3 B and C). In addition, BRF110 protected SN axonal projections to the striatum, doubling the number of remaining terminals (n = 5; P < 0.0001, one-way ANOVA) (Fig. 3 D and E). This neuroprotective effect was BRF110-dependent, because a once-daily injection regimen (20 mg/kg) was ineffective. BRF110 neuroprotection against MPTP toxicity was equally effective in 129sv WT mice (vehicle 2,500 ± 91 vs. BRF110 3,250 ± 92; n = 4; P = 0.000571, t test; P < 0.001, one-way ANOVA), but was abolished in Nurr1+/− 129sv mice. The number of TH(+) neurons was significantly lower in Nurr1+/− 129sv mice compared with 129sv WT mice (2,710 ± 91, n = 4 vs. 3,250 ± 92, n = 4; P = 0.00298, t test), confirming that the in vivo neuroprotective effects of BRF110 require Nurr1:RXRα heterodimers (Fig. 3F).
Fig. 3.
BRF110 protects DAergic neurons against MPTP toxicity. (A) Improved rotarod test performance of C57BL/6 mice exposed to MPTP and receiving BRF110 treatment for 7 d. (B) Stereological counting of SN TH(+) neurons (average/side) in control C57BL/6 mice and mice exposed to MPTP and receiving either vehicle or BRF110 treatment. (C) Images of TH immunohistochemistry (IHC) in the SN of control C57BL/6 mice and mice exposed to MPTP and receiving either vehicle or BRF110 treatment. (D) Images of TH IHC of SN DAergic projections to the striatum in C57BL/6 mice exposed to MPTP and receiving either vehicle or BRF110 treatment. (E) Quantitation of TH IHC DAergic projections to the striatum of C57BL/6 mice exposed to MPTP and receiving either vehicle or BRF110 treatment. (F) Stereological counting of SN TH(+) neurons of control WT 129SV mice and mice exposed to MPTP and receiving either vehicle or BRF110 treatment (average/side) (first three bars) and of Nurr1+/− 129SV mice exposed to MPTP and receiving either vehicle or BRF110 treatment (last three bars).
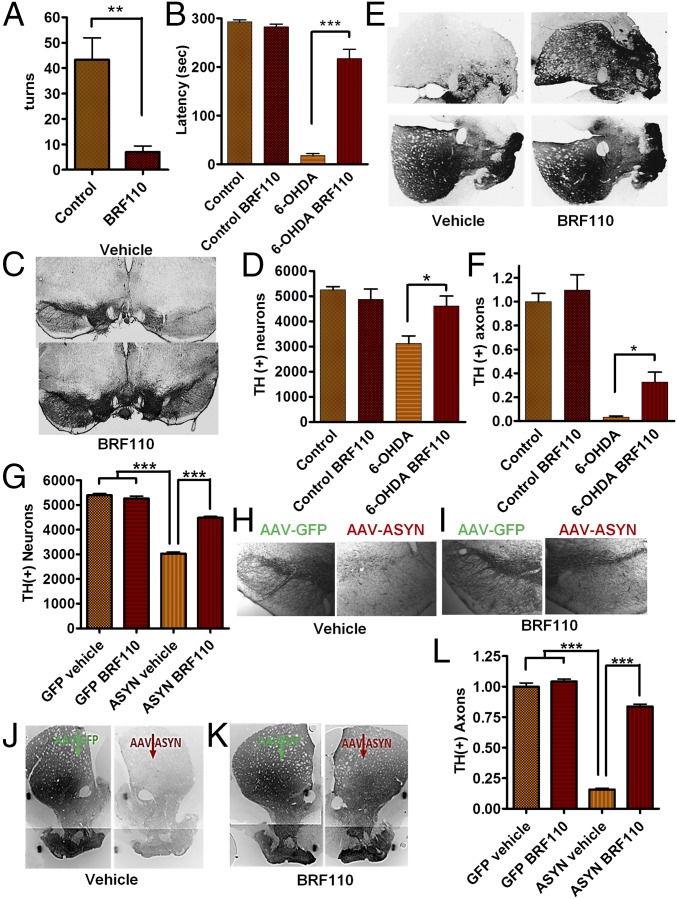
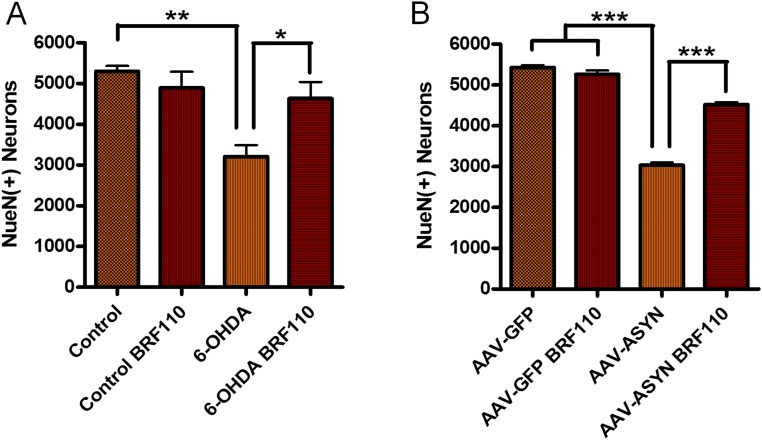
Nurr1:RXRα activation showed even more striking effects in the 6-OHDA model. Mice receiving BRF110 exhibited a dramatic reduction (by approximately eightfold) in apomorphine-induced contralateral turns (n = 5; P = 0.0159, Mann–Whitney U test) (Fig. 4A), whereas motor coordination was almost entirely restored to control levels, as evaluated by the rotarod test (P < 0.0001, one-way ANOVA; P = 0.0079, Mann–Whitney U test) (Fig. 4B). The number of surviving TH(+) neurons in the SN of BRF110-treated mice was increased by 47% (P < 0.05, one-way ANOVA; P = 0.0317, Mann–Whitney U test) to the level of complete restoration, as indicated by unbiased stereological neuronal counting (Fig. 4 C and D). Total midbrain neuronal determination by NueN staining showed similar results (Fig. S8A). Striatal innervation, which was practically obliterated in the vehicle-treated control mice, also showed a 10-fold higher preservation of TH(+) DAergic projections (P < 0.0001, one-way ANOVA; P = 0.0159, Mann–Whitney U test) (Fig. 4 E and F).
Fig. 4.
BRF110 neuroprotection against 6-OHDA and AAV-ASYN toxicity in mice. (A) Decreased number of apomorphine-induced contralateral turns in C57BL/6 mice injected unilaterally with 6-OHDA and treated daily with either vehicle or BRF110 for 13 d, showing greater than eightfold improvement. n = 5. (B) Rotarod test of mice subjected to unilateral injection of 6-OHDA and receiving either vehicle or BRF110, showing 12-fold improvement with BRF110 treatment. n = 5. (C) SN TH IHC images of mice receiving unilateral injections of 6-OHDA and treated with either vehicle or BRF110. (D) Stereological counting of SN TH(+) neurons in mice receiving unilateral injections of 6-OHDA and treated daily with either vehicle or BRF110, which increased the number of TH(+) neurons by 47%. n = 5. (E and F) Striatum TH IHC of mice receiving 6-OHDA injections and treatment with either vehicle or BRF110, showing 10-fold increased innervation. n = 5. (G) Stereological counting of SN TH(+) neurons of mice receiving unilateral injections of AAV-ASYN and treated daily with either vehicle or BRF110, which increased the number of TH(+) neurons by 48%. n = 7. (H and I) SN TH IHC images of mice receiving unilateral injections of AAV-ASYN and contralateral injections of AAV-GFP and treated with either vehicle or BRF110. (J and K) Striatum TH IHC of mice receiving injections of AAV-ASYN or AAV-GFP and treated with either vehicle or BRF110, showing 10-fold increased innervation (L). n = 7.
Fig. S8.
Total SN neuron counts. (A) Total SN neuron counts of 6-OHDA unilaterally injected mice were determined in mice receiving vehicle and those receiving BRF110 by unbiased stereological counting of NueN(+) neurons. BRF110 administration (2 × 10 mg/kg/d for 2 wk) increased the number of neurons by 44% in the mice receiving BRF110 compared with those receiving vehicle (n = 5, P < 0.0018, one-way ANOVA; n = 2, P = 0.0087, Mann–Whitney U test). (B) Total SN neuron counts of AAV-ASYN or AAV-GFP unilaterally injected mice were determined in mice receiving vehicle and those receiving BRF110 by unbiased stereologic counting of NueN(+) neurons. BRF110 administration (2 × 10 mg/kg/d for 2 wk) increased the number of neurons by 48% in the mice receiving BRF110 compared with those receiving vehicle (n = 7, P < 0.0001, one-way ANOVA; P = 0.0006, Mann–Whitney U test).
Because the predictive validity of toxin-based mouse PD models for neuroprotection in humans is questionable, we proceeded to test the effect of BRF110 in a mouse model, in which adeno-associated viruses (AAVs) overexpressing WT α-synuclein (ASYN) under the chicken β-actin promoter were injected unilaterally, along with contralateral injections of AAV-GFP. Treatment with BRF110 (10 mg/kg every 12 h for 2 wk) or vehicle was started the day after surgery. Unbiased stereology showed that the vehicle-treated AAV-ASYN–injected animals had a 44% decrease in TH(+) and NeuN(+) midbrain neurons compared with the AAV-GFP–injected side (Fig. 4 G–I and Fig. S8B). In contrast, in the BRF110-treated AAV-ASYN–injected animals, unbiased stereology showed that the number of TH(+) midbrain neurons was increased by ∼47% (P < 0.05, Wilcoxon test; P = 0.00214, Mann–Whitney U test) and NeuN(+) midbrain neurons (P < 0.05, Wilcoxon test; P = 0.00214, Mann–Whitney U test) compared with AAV-ASYN–injected animals treated with vehicle (Fig. 4G). Striatal innervation of unilaterally AAV-ASYN–injected brains resulted in a dramatic depletion of TH(+) axons by almost 85% in vehicle-treated mice, whereas in BRF110-treated mice, striatal TH(+) axons were increased by more than fivefold (P < 0.001, one-way ANOVA; P < 0.0156, Wilcoxon test) (Fig. 4 J L).
The foregoing experiments demonstrate the DAergic neuroprotective effects of Nurr1:RXRα activation in both toxin-based and ASYN genetic preclinical animal models of PD.
A Single Dose of BRF110 Increases DA Biosynthesis in Vivo and Improves Symptoms in Two Postneurodegeneration PD Models.
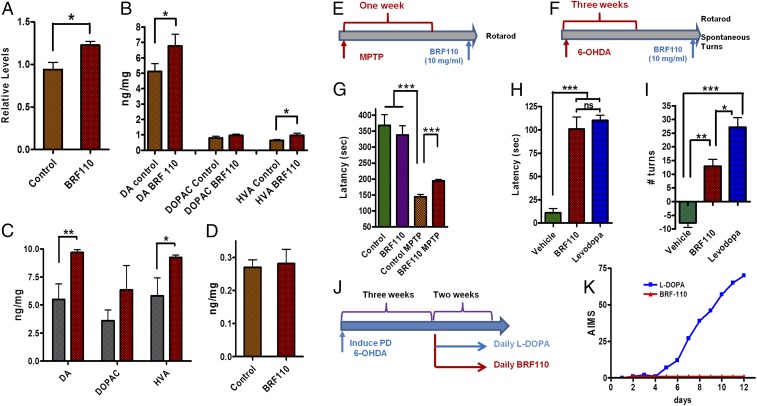
Based on the Nurr1:RXRα activation in the transcriptional regulation of DA biosynthesis genes, we tested whether BRF110 could increase DA levels in vivo. A single i.p. injection of BRF110 (10 mg/kg) in WT mice increased midbrain TH gene expression (n = 4; P = 0.0396, t test) within 4 h (Fig. 5A). Striatal DA and DA metabolite levels were increased as well, and their ratio remained constant, indicating physiological DA catabolism (Fig. 5B). Aiming to model PD based on human genetic data, we also tested whether BRF110 could increase DA levels in ASYN transgenic mice (23). A single i.p. injection of BRF110 (10 mg/kg) increased striatal DA levels (n = 4; P = 0.01069, t test) and DA metabolite levels in the ASYN transgenic mice compared with mice receiving vehicle (Fig. 5C). The effect of BRF110 was limited to DA biosynthesis, because noradrenaline levels remained unaffected (Fig. 5D).
Fig. 5.
BRF110 induces DA biosynthesis and symptomatic relief without dyskinesias in PD mouse models. (A) qPCR of TH expression levels in mouse midbrain at 4 h after i.p. administration of vehicle or BRF110 (10 mg/kg). (B) DA and DA metabolite levels at 4 h after i.p. administration of vehicle or BRF110 (10 mg/kg) in WT mice as assessed by HPLC. n = 4. (C) DA and DA metabolite levels at 4 h after i.p. administration of vehicle or BRF110 (10 mg/kg) in ASYN transgenic mice, as assessed by HPLC. n = 4. (D) Noradrenaline levels at 4 h after i.p. administration of vehicle or BRF110 (10 mg/kg), as assessed by HPLC. (E and F) Schematic representations of the treatment regimens. (G) Accelerating rotarod latency times of MPTP-treated mice at 4 h after i.p. administration of vehicle or BRF110 (10 mg/kg). (H) Accelerating rotarod latency times of 6-OHDA–treated mice at 4 h after i.p. administration of vehicle or BRF110 (10 mg/kg). (I) Spontaneous contralateral turns per minute of 6-OHDA–treated mice at 4 h after i.p. administration of vehicle, BRF110 (10 mg/kg), or L-DOPA. (J and K) Schematic of the treatment regimen (J) and AIMS (K) of mice at 5 wk after 6-OHDA treatment, after 2 wk of daily i.p. administration of BRF110 (10 mg/kg) or L-DOPA.
We subsequently tested whether the DA increase could translate to symptomatic relief in postdegeneration PD mouse models based on MPTP or 6-OHDA (Fig. 5 E and F). A single dose of BRF110 (10 mg/kg i.p.) at 8 d after acute MPTP injection or 5–6 wk after unilateral 6-OHDA injection significantly improved temporarily motor coordination in both models (P = 0.01 and 0.0001, respectively, one-way ANOVA) at 4 h after dosing (Fig. 5 G and H) and induced contralateral turns in the 6-OHDA model (P < 0.0001, one-way ANOVA) (Fig. 5I), an effect similar to that of levodopa (L-DOPA). In these models, symptom improvement from BFF110 disappeared by 8 h after dosing. These experiments indicate that Nurr1:RXRα activation can indeed offer symptomatic relief in rodents, further supporting our monotherapy hypothesis.
BRF110 Chronic Dosing Does Not Induce Dyskinesias.
We next compared BRF110 with L-DOPA in terms of causing AIMs. Mice treated daily with L-DOPA starting at 21 d after unilateral 6-OHDA injection exhibited severe hyperkinetic dyskinesias/AIMs within 7 d (Fig. 5 J and K). In contrast, similar daily BRF110 (10 mg/kg) administration for at least 2 wk resulted in consistently improved motor coordination (Fig. 5 H and I) without dyskinesias/AIMs (P = 0.0061, two-way ANOVA; P = 0.0043, Mann–Whitney U test) (Fig. 5K). These experiments exemplify the simultaneous symptomatic usefulness of Nurr1:RXRα activation devoid of undesirable complications.
Discussion
Drug discovery efforts for PD traditionally have been aimed either at symptomatic DA replacement treatments and alleviation of dyskinesias or at neuroprotection (24, 25). Neuroprotective approaches, although aimed at the core of PD pathology, so far have not delivered the expected results (26).
We selected Nurr1 as the target for disease-modifying and symptomatic relief for PD because it is strongly associated with PD. Nurr1 mutations have been identified in familial and sporadic cases of the disease (12, 13), and reduced levels of Nurr1 are associated with PD in both patients and animal models. In addition, Nurr1 is involved in DA biosynthesis (6, 7). We chose to target Nurr1 through its heterodimerization partner RXRα, limiting the activation of other Nurr1 molecules. Our data indicate that activation of Nurr1:RXRα by BRF110 can provide neuroprotection that can halt PD-associated neuronal loss in both toxin and genetic preclinical mouse models of PD. In addition, we have demonstrated that the effects of BRF110 both in vitro and in vivo depend on Nurr1 expression, validating the therapeutic target. Because of the divergent toxin and genetic insults that we used in our PD models, our data indicate that various pathways leading to DAergic neuron demise can be overcome by BRF110 activation, implying coordinated control of a complex neuroprotective network by Nurr1:RXRα.
BRF110 also increases DA levels in vivo and offers symptomatic relief in two postdegeneration PD animal models at the neuroprotective dose. This dual activity indicates that unlike most efforts aimed at finding new therapeutic approaches for the treatment of PD, Nurr1:RXRα activation offers a unique combined efficacy distinct from that of other targets. Moreover, as opposed to the excessive DA stimulation induced by DA replacement therapies, BRF110 apparently finely regulates DA production in a more physiological manner via the transcriptional activation of the DA biosynthesis genes (TH, GCH1, and AADC), without affecting DA catabolism and without eliciting dyskinesias. This effect has been corroborated by the use of a lentiviral tricistronic vector expressing TH, GCH1, and AADC that alleviates PD symptoms in primates without dyskinesias (27). A current phase 1/2 clinical trial using a virtually identical lentiviral vector offers similar symptomatic relief in PD patients (28).
Our experiments show that a single molecule activating a single target achieves a double therapeutic advantage for PD in vivo. Because BRF110 neuroprotection extends to PD patient iPSC-derived DAergic neurons, Nurr1:RXRα activation has the potential to benefit PD patients. We believe that our findings strongly support the use of Nurr1:RXRα activation as a monotherapy for PD.
Materials and Methods
The materials and methods used in these experiments are described in detail in SI Materials and Methods.
In Vitro Studies in Neuroblastoma Cell Lines.
Naive SH-SY5Y neuroblastoma cells were cultured in RPMI 1640 and 10% FBS and harvested at indicated time points. Transient transfections were performed using Lipofectamine 2000 (Invitrogen) for 4–6 h in Opti-MEM I (Invitrogen).
Mice.
Mice were housed at the Animal Care Facilities of the Academy of Athens in a pathogen-free room under a controlled 12-h light/12-h dark cycle and with free access to food and water. Animal breeding and handling were performed in accordance with the European Communities Council Directive 86/609/EEC guidelines, and all animal procedures were approved by the Academy of Athens Institutional Animal Care and Use Committee (certified with ISO 9001:2008). PD mouse models for MPTP, 0.6-OHDA, and ASYN AAV were generated following established protocols. In brief, we used an acute MPTP regimen of four 20-mg/kg MPTP injections given 2 h apart. 6-OHDA-HCl in saline containing 0.02% ascorbic acid was injected intracerebrally and unilaterally at the level of the median forebrain bundle (MFB) in anesthetized mice. Recombinant AAVs (AAV-ASYN and AAV-GFP) were injected stereotactically into the right and left SN, respectively. Experimenters were blinded to the animal groups for all animal experiments and analyses.
Statistics.
Statistical analyses were performed using GraphPad Prism 4. Significance was assessed with one-way ANOVA followed by Bonferroni's post hoc test and by the nonparametric Mann–Whitney U test or Kruskal–Wallis test unless indicated otherwise. A P value <0.05 was considered significant. In the figures, error bars represent ± SEM, and *P < 0.05, **P < 0.01, and ***P < 0.001.
SI Materials and Methods
Neuroblastoma Cell Lines.
Naive SH-SY5Y neuroblastoma cells were cultured in RPMI 1640 and 10% FBS. Transient transfections were performed using Lipofectamine 2000 (Invitrogen) for 4–6 h in Opti-MEM I Reduced Serum Medium (Invitrogen). Cells were harvested at the indicated time points.
Luciferase activity was assessed using a luciferase assay system (Promega) in accordance with the manufacturer’s instructions. As a reference plasmid to normalize transfection efficiency, a CMV-galactosidase plasmid (Promega) was cotransfected in all experiments. All reported values are mean ± SEM for at least three independent experiments.
BRF110 specifically activates Nurr1:RXRα heterodimers. Specificity assays fusing DNA sequences of the gal4 DNA-binding domain to the nuclear receptor ligand-binding domain (LBD) and to vp16 were performed as follows. Human SHSY-5Y cells were cotransfected with 20 ng of MH100-tk-luciferase, 10 ng of expression vector CMX-bgal, 20 ng of CMX-GAL4-RXRα-LBD, and 20 ng of CMX-VP16-NR-LBD, where NR (nuclear receptor) represents Nurr1, VDR, or PPARγ. CMX-VP16-VDR-LBD and CMX-VP16-PPARγ-LBD were kindly provided by M. Makishima, Nihon University School of Medicine, Tokyo, Japan. CMX-VP16-Nurr1-LBD was produced by cloning the Nurr1 LBD in the CMX-VP16 vector using KpnI. More specifically, Nurr1-LBD was isolated by PCR from a vector containing the Nurr1 cDNA using a set of primers flanking the Nurr1 LBD sequence and carrying the KpnI restriction sites, namely Nurr1 forward tgcggtaccaggagccctctcccccttc and Nurr1 reverse tgcggtaccttagaaaggtaaagtgtcca, on both sides. To exclude the possibility that BRF110 might transactivate RXRγ:Nurr1 heterodimers, CMX-GAL4-RXRγ-LBD was constructed by cloning the RXRγ LBD that was isolated by PCR from a vector containing the RXRγ cDNA (RIKEN) using the following forward and reverse primers carrying KpnI and NheI restriction sites respectively: RXRγ forward, tgcggtaccgagcgagctgagagtgag, and RXRγ LBD reverse, tgcgctagctcaggtgatctgcagc. At 17 h after transfection, the compound was added to the medium at the corresponding concentrations and left for another 12 h. Then the cells were assayed for luciferase activity normalized to galactosidase activity. Each experiment was performed at least in triplicate.
MPP+ and H2O2 Cellular Neuroprotective Assays: Neuroprotection in Vitro.
MPP+ eradicated ∼75% of the cells in 24 h. The addition of BRF110 approximately doubled the number of surviving cells. Naive Neuro2A cells or SH-SY5Y neuroblastoma cells were cultured in RPMI 1640 and 10% FBS at 37 °C. Cells were pretreated with BRF110 for 24 h. Subsequent changes of culture medium with the addition of 0, 1, 2, or 4 mM MPP+ or 4, 8, 12, or 16 mM H2O2 induced cell death. MTT assays determined the relative number of surviving cells. Each experiment was done in octuplicate.
Rat Cortical Neurons.
These neurons were prepared from E17 rat cortices as described previously (29). They were transfected with Lipofectamine (Invitrogen) in accordance with the manufacturer's instructions on day 4 after plating, and kept in Neurobasal medium (Thermo Fisher Scientific) supplemented with B27 and penicillin-streptomycin as usual. The neurons were then plated on glass coverslips coated with poly-d-lysine at a density of ∼125,000 per cm2. They were cotransfected with human LRRK2 in pcDNA3.1 and pCMS-EGFP at a ratio of 4:1. Treatments with BRF110 (12 μM, dissolved in DMSO) and GSK2578215A (1 μM, obtained from Tocris) were started on the same day as transfection, with the medium changed after 4 h and then replenished every 24 h. The cells were fixed after 72 h of expression and stained for GFP and DAPI. There were two or three replicate coverslips per condition, and the experiment was repeated in two independent cultures. From each coverslip, 100 GFP-positive neurons were counted and graded as to the presence of apoptotic nuclear features (i.e., condensed chromatin with two or more apoptotic nuclear bodies).
iPSC-Derived DAergic Neurons.
Differentiation of human DAergic neurons from neural progenitor cells, as well as their analysis via immunofluorescence and qPCR, have been described previously (23, 30). Neurite number, length, and branching assays were performed essentially as described previously (3). The iPSC DAergic neurons with the LRRK2 G2019S mutation or LRRK2 GC were cultured with either BRF110 (12 μM, dissolved in DMSO, added to the medium and replaced daily) or vehicle (DMSO) as a control. After 14 d, cells were stained with TH and β-III-tubuline (Tuj1) for visualization and neurite measurements, and then fixed with 4% paraformaldehyde. Plates were photographed with a Zeiss LSM 710 confocal microscope. For each iPSC line, approximately 20 randomly selected TH-positive neurons were analyzed with the ImageJ plug-in Neuron J to determine the length, number, and branches of neurites per cell.
Animals.
Mice were housed at the Animal Care Facilities of the Academy of Athens in a pathogen-free room under a controlled light-dark cycle (12-h light/12-h dark) and with free access to food and water. Animal breeding and handling were performed in accordance with guidelines of the European Communities Council Directive 86/609/EEC, and all animal procedures were approved by the Academy of Athens Institutional Animal Care and Use Committee. Nurr1 heterozygous mice were provided by Thomas Perlmann, Karolinska Institute, Solna, Sweden, and ASYN transgenic mice were provided by Maria Grazia Spillantini, Cambridge University, Cambridge, UK. Genotyping was performed by PCR analysis of DNA samples from mouse tails. Experimenters were blinded to the animal groups for all animal experiments.
MPTP.
We used an acute MPTP (1) regimen of four 20 mg/kg MPTP injections, given 4 h apart, on the first day of treatment. We had previously determined that at 7 d after the MPTP injections, there was an ∼80% reduction in striatal projections and 40%-50% DAergic neuron death in the SN following this protocol. Administration of BRF110 (10 mg·kg-1·d-1) to mice preceded MPTP treatment by 12 h. BRF110 administration at the same dose was continued daily, except on the day of the MPTP injections, for 7 d until completion of the experiment. Behavioral experiments were conducted 1–2 d after BRF110 treatment. Then mice were killed, and striatal projections and DAergic neuron death were assessed by TH (Calbiochem) IHC stereological neuronal counting at 24–36 h after the last BRF110 injection (at least four per condition). Independent experiments were done in adult C57BL/6 and in 129sv mice.
6-OHDA.
6-OHDA-HCl dissolved in saline containing 0.02% ascorbic acid at a concentration of 3.7 mg/mL was injected intracerebrally and unilaterally at the level of the MFB in adult C57BL/6 anesthetized mice. (The research facility is certified with ISO 9001:2008.) This regimen led to 70–80% DAergic cell death within 2 wk after surgery. BRF110 administration at 10 mg/kg/d preceded the stereotactic 6-OHDA injection by 12 h and was continued daily for 14 d until completion of the experiment. Behavioral experiments were conducted 1–2 d after BRF110 treatment. Then mice were killed, and striatal projections and DAergic neuron death were assessed by TH (Calbiochem) IHC and NeuN (MAB377 Millipore) stereological neuronal counting (n = 5 per condition).
ASYN-AAV.
Recombinant AAVs under the chicken beta actin promoter (AAV-CBA-ASYN and AAV-CPA-GFP were obtained from the University of North Carolina Vector Core (1013 vector genomes/mL) (31, 32). Adult C57BL/6 mice under anesthesia were injected stereotactically with 2 μL of AAV-SYN or AAV2-GFP into the right and left SN (33). BRF110 treatment (10 mg/kg/d every 12 h) was started 1 d after surgery and continued for 2 wk. Then mice were killed at 24–36 h after the last BRF110 injection (n = 7 per condition), and striatal projections and DAergic neuron death were assessed by TH (Calbiochem) IHC and NeuN (MAB377; EMD Millipore) stereological neuronal counting.
Behavioral Experiments.
All behavioral experiments were performed during the light phase, at the same time of the day at least 24 h after discontinuation of BRF110 treatment. Rotarod motor coordination and balance were tested on a Rota-Rod treadmill (Ugo Basile, Comerio, Italy), set for accelerating revolutions (4–40 revolutions/min) over a 5-min period. Each mouse was placed on a rotating drum and the latency to fall from the rod was measured. Mice were subjected to one training trial and two experimental trials over 2 d.
Apomorphine.
6-OHDA lesions were validated using apomorphine. At 2 wk postlesion, mice received an s.c. injection of apomorphine (0.05 mg/kg body weight; Sigma-Aldrich). Each mouse was placed in a cylindrical Plexiglas bottle and allowed to habituate for 5 min before the apomorphine injection. The number of contralateral turns to the lesion, reflecting the degree of neurodegeneration, was recorded over the next 20 min.
Immunohistochemistry.
Mice were anesthetized deeply with CO2 and perfused intracardially with ice-cold PBS (pH 7.2) and subsequently with 4% paraformaldehyde in 0.1 M PBS (PFA). Brains were quickly removed, postfixed in PFA for 1 h at 4 °C, cryoprotected in 15% sucrose in 0.1 M PBS for 24 h and in 30% sucrose in 0.1 M PBS for 24 h at 4 °C, frozen, and stored at −80 °C until sectioning. Free-floating cryostat-cut sections (35 μm) were collected using a Bright cryostat at −20 °C at the levels of striatum (AP, 0.2 mm from bregma) and the entire midbrain (AP, −5.6 mm from bregma) (33). As described previously (34), sections first were quenched for 10 min in 3% H2O2/10% methanol. Then sections were preincubated with 10% goat serum for 1 h and then incubated with a polyclonal anti-TH antibody (1:2,000; Calbiochem) or NeuN (MAB377; EMD Millipore) for 48 h at 4 °C, followed by incubation with biotinylated anti-rabbit antibody (1:1,500; Vector Laboratories) in 1% goat serum for 1 h and avidin-biotin peroxidase (ABC Elite; Vector Laboratories) for 1 h at room temperature. Staining was visualized using 3,3′-diaminobenzidene (Sigma-Aldrich) as a chromogen (34). The specificity was tested in adjacent sections with the primary or secondary antibody omitted. Total numbers of TH-positive and NeuN-positive cells were counted in both hemi-brain stems using stereological methods.
Cell Counting and TH Fiber Density.
The total numbers of TH- and NeuN-stained cells in the SN pars compacta (SNpc) were counted using an optical fractionator, which is an unbiased method of cell counting unaffected by either the volume of reference or the size of the counted neurons. The SNpc was delineated using a computer-assisted image analysis system. TH- and NeuN-stained neurons were counted in every fourth section throughout the entire the SNpc. The standard mouse atlas was used as an anatomic reference (33). For the MPTP-intoxicated mice, each side of each mouse brain was counted separately, and the average of the two sides for each mouse is reported (35). The quantification of DAergic striatal innervation was performed using ImageJ software for measuring TH fiber density in every sixth striatal section (n = 4–7).
HPLC Determination of Striatal DA and Metabolite Levels.
Animals were euthanized by cervical dislocation, brains were removed, and the striata were dissected out on ice. The samples were immediately frozen and stored at −80 °C until use. The tissues were processed for the analysis by HPLC with electrochemical detection. Dissected tissues were weighed, homogenized, and deproteinized in 0.2 N perchloric acid solution containing 7.9 mM Na2S2O5 and 1.3 mM Na2EDTA. The homogenate was centrifuged at 37,000 × g for 30 min, and the supernatant was stored at −80 °C until use. Reversed-phase ion-pair chromatography was used for all analyses. The mobile phase consisted of an acetonitrile–50 mM phosphate buffer, pH 3.0, containing 300 mg/L5-octylsulfate sodium salt as the ion-pair reagent and 20 mg/L Na2EDTA. Reference standards were prepared in 0.2 N perchloric acid solution containing 7.9 mM Na2S2O5 and 1.3 mM Na2EDTA. Samples were quantified by comparison of the areas under the peaks with those of reference standards using HPLC software (Chromatography Station for Windows; Watrex International). The ratio of DA metabolites to DA (DOPAC + HVA/DA) was calculated as an index of the DA turnover rates, which reflect the DAergic activity, including release and/or metabolism function.
In Silico Chemistry.
Docking calculations were performed with Glide 6.4 (Schrödinger). Hydrogen atoms were added to the crystal structure, and the complex was submitted to a series of restrained, partial minimizations using the OPLS-AA force field within the “protein and ligand preparation” module of Glide. Because the protein is treated as rigid by the docking software, the van der Waals radii for nonpolar ligand atoms were scaled by a factor of 0.8, thereby decreasing penalties for close contacts. Receptor atoms were not scaled. For the protein preparation, grid generation, and ligand docking procedures, the default Glide settings were used. The simple precision scoring function was used to screen the full database, and then the top 1,000 compounds were then rescreened with the extra precision scoring function (36) of Glide.
Chemistry.
BRF110 was prepared in six steps from commercially available reagents and was isolated as an off-white solid. Experimental details of the synthesis of BRF110 will be reported elsewhere and will be available on request. High-resolution mass spectra were obtained under electron spray ionization conditions on a LC-MS/LTQ-Orbitrap mass spectrometer (Thermo Fisher Scientific). HRMS (ESI-LTQ) for C22H19F3N3O2 [M+H]: calculated, 414.1424; found, 414.1418.
Pharmacokinetics.
All animal procedures were approved by the Athens Academy Bioethical Committee on the basis of the European Directive 86/609 on the protection of animals used for experimental purposes. Animals (male C57BL/6N inbred strain, obtained from Charles River Laboratories) at age 10 wk (n = 5 per group) were weighed and fasted overnight before dosing. Dosing solutions of 1 mg/kg BRF110 in ethanol:propylene glycol:saline (buffered to pH 10 using NaOH) 10:30:60 vehicle were administered i.p. Blood samples (200 μL) were collected by serial tail bleeding at selected time points (1, 2, 4, 8, and 24 h) in 1.5-mL tubes containing 800 μL of sodium citrate (0.1 M, pH 4.5). After anesthesia by isoflurane and cervical dislocation, mice were killed and brains removed and washed with PBS. Blood and brain samples were stored at −80 °C until analysis. For LC-MS/MS analysis, brains were homogenized in sodium citrate 0.1 M, pH 4.5 (1 g tissue weight:4 mL of buffer). BRF110 was quantified by LC-MS/MS.
Dyskinesias.
Intraperitoneal administration of BRF110 (10 mg/kg) repeated for 14 d did not induce dyskinesias with AIMs in the WT C57/BL6 PD mice treated with 6-OHDA. 6-OHDA-HCl dissolved in saline containing 0.02% ascorbic acid at a concentration of 3.7 mg/mL was injected intracerebrally and unilaterally at the MFB level in anesthetized mice. This regimen led to 70–80% DAergic cell death within 2 wk after surgery. The 6-OHDA mice were left to recover after the surgery for 3 wk, at which time the degree of DAergic degeneration was assessed based on the number of the apomorphine-induced turns they made contralateral to the lesion. This test was used to create a relatively homogeneous group of mice with approximately equal degrees of neurodegeneration. This group was subsequently separated into three groups that were treated daily with an i.p. injections of saline, L-DOPA, or BRF110 (10 mg/kg) for 14 d. At that time, AIMs were evaluated for each of the three groups. In brief, each mouse was observed individually for 1 min every 20 min for 3 h, starting at 20 min after L-DOPA/BRF110/saline administration. The AIMs were classified into three different subtypes based on their topographic distribution: axial AIMs, marked by twisting of the neck and upper body toward the side contralateral to the lesion; orolingual AIMs, jaw movements and contralateral tongue protrusion; and forelimb AIMs, purposeless movements of the contralateral forelimb.
The mice were evaluated using this test a total of three times during the chronic L-DOPA treatment period. It was concluded that i.p. administration of BRF110 (10 mg/kg) repeated for 12–17 d did not induce dyskinesias with AIMs. In contrast, treatment of mice with L-DOPA used as a reference induced dyskinesias with AIMs within 7 d.
Statistics.
GraphPad Prism 4 software was used for statistical analysis. Significance was assessed with the one-way or two-way ANOVA followed by Bonferroni's post hoc test and by nonparametric statistical tests (Mann–Whitney, Wilcoxon, or Kruskal–Wallis tests) unless indicated otherwise. Power analysis was performed using GPower 3.1 software. In the figures, error bars represent ± SEM, and P < 0.05 is considered to indicate statistical significance.
Acknowledgments
We thank Drs. L. Stefanis, A. S. Charonis, K. Vougas, and A. Efstratiadis for encouragement; Drs. T. Perlmann and M. Spillantini for the Nurr1+/− and ASYN transgenic mice, respectively, and Dr. M. Makishima and RIKEN for constructs. This work was partially supported by the Michael J. Fox Foundation (D.K.V.), a Cooperation grant from the General Secretariat of Research and Technology (to D.K.V.), TransMed Grant EU FP7 REGPOT CT-2010-245928 (to D.K.V.), and SEE-DRUG Grant EU FP7 REGPOT CT-2011-285950 (to S.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.K.L. is a Guest Editor invited by the Editorial Board.
See Commentary on page 3795.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1616874114/-/DCSupplemental.
References
- 1.Meissner WG. When does Parkinson’s disease begin? From prodromal disease to motor signs. Rev Neurol (Paris) 2012;168(11):809–814. doi: 10.1016/j.neurol.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Athauda D, Foltynie T. The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat Rev Neurol. 2015;11(1):25–40. doi: 10.1038/nrneurol.2014.226. [DOI] [PubMed] [Google Scholar]
- 3.Fahn S. A new look at levodopa based on the ELLDOPA study. J Neural Transm Suppl. 2006;70(Suppl):419–426. doi: 10.1007/978-3-211-45295-0_63. [DOI] [PubMed] [Google Scholar]
- 4.Meissner WG, et al. Priorities in Parkinson’s disease research. Nat Rev Drug Discov. 2011;10(5):377–393. doi: 10.1038/nrd3430. [DOI] [PubMed] [Google Scholar]
- 5.Zetterström RH, et al. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 1997;276(5310):248–250. doi: 10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
- 6.Saucedo-Cardenas O, et al. Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc Natl Acad Sci USA. 1998;95(7):4013–4018. doi: 10.1073/pnas.95.7.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim KS, et al. Orphan nuclear receptor Nurr1 directly transactivates the promoter activity of the tyrosine hydroxylase gene in a cell-specific manner. J Neurochem. 2003;85(3):622–634. doi: 10.1046/j.1471-4159.2003.01671.x. [DOI] [PubMed] [Google Scholar]
- 8.Gil M, et al. Regulation of GTP cyclohydrolase I expression by orphan receptor Nurr1 in cell culture and in vivo. J Neurochem. 2007;101(1):142–150. doi: 10.1111/j.1471-4159.2006.04356.x. [DOI] [PubMed] [Google Scholar]
- 9.Kadkhodaei B, et al. Nurr1 is required for maintenance of maturing and adult midbrain dopamine neurons. J Neurosci. 2009;29(50):15923–15932. doi: 10.1523/JNEUROSCI.3910-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadkhodaei B, et al. Transcription factor Nurr1 maintains fiber integrity and nuclear-encoded mitochondrial gene expression in dopamine neurons. Proc Natl Acad Sci USA. 2013;110(6):2360–2365. doi: 10.1073/pnas.1221077110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Le W, Xie W, Dani JA. Age-related changes in dopamine signaling in Nurr1-deficient mice as a model of Parkinson’s disease. Neurobiol Aging. 2012;33(5):1001.e7–1001.e16. doi: 10.1016/j.neurobiolaging.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le WD, et al. Mutations in NR4A2 associated with familial Parkinson disease. Nat Genet. 2003;33(1):85–89. doi: 10.1038/ng1066. [DOI] [PubMed] [Google Scholar]
- 13.Sleiman PM, et al. Characterisation of a novel NR4A2 mutation in Parkinson’s disease brain. Neurosci Lett. 2009;457(2):75–79. doi: 10.1016/j.neulet.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallen-Mackenzie A, et al. Nurr1-RXR heterodimers mediate RXR ligand-induced signaling in neuronal cells. Genes Dev. 2003;17(24):3036–3047. doi: 10.1101/gad.276003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pérez E, Bourguet W, Gronemeyer H, de Lera AR. Modulation of RXR function through ligand design. Biochim Biophys Acta. 2012;1821(1):57–69. doi: 10.1016/j.bbalip.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Cramer PE, et al. ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science. 2012;335(6075):1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McFarland K, et al. Low-dose bexarotene treatment rescues dopamine neurons and restores behavioral function in models of Parkinson’s disease. ACS Chem Neurosci. 2013;4(11):1430–1438. doi: 10.1021/cn400100f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veeraraghavalu, et al. Comment on “ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models.”. Science. 2013;340(6135):924-f. doi: 10.1126/science.1235505. [DOI] [PubMed] [Google Scholar]
- 19.Volakakis N, et al. Nurr1 and retinoid X receptor ligands stimulate Ret signaling in dopamine neurons and can alleviate α-synuclein–disrupted gene expression. J Neurosci. 2015;35(42):14370–14385. doi: 10.1523/JNEUROSCI.1155-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaz B, de Lera ÁR. Advances in drug design with RXR modulators. Expert Opin Drug Discov. 2012;7(11):1003–1016. doi: 10.1517/17460441.2012.722992. [DOI] [PubMed] [Google Scholar]
- 21.Wei X, et al. Contra-directional coupling of Nur77 and Nurr1 in neurodegeneration: A novel mechanism for memantine-induced anti-inflammation and anti-mitochondrial impairment. Mol Neurobiol. 2016;53(9):5876–5892. doi: 10.1007/s12035-015-9477-7. [DOI] [PubMed] [Google Scholar]
- 22.Reinhardt P, et al. Genetic correction of a LRRK2 mutation in human iPSCs links parkinsonian neurodegeneration to ERK-dependent changes in gene expression. Cell Stem Cell. 2013;12(3):354–367. doi: 10.1016/j.stem.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Tofaris GK, et al. Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1-120): Implications for Lewy body disorders. J Neurosci. 2006;26(15):3942–3950. doi: 10.1523/JNEUROSCI.4965-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rascol O, Perez-Lloret S, Ferreira JJ. New treatments for levodopa-induced motor complications. Mov Disord. 2015;30(11):1451–1460. doi: 10.1002/mds.26362. [DOI] [PubMed] [Google Scholar]
- 25.Schapira AH. Monoamine oxidase B inhibitors for the treatment of Parkinson’s disease: A review of symptomatic and potential disease-modifying effects. CNS Drugs. 2011;25(12):1061–1071. doi: 10.2165/11596310-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Stocchi F. Therapy for Parkinson’s disease: What is in the pipeline? Neurotherapeutics. 2014;11(1):24–33. doi: 10.1007/s13311-013-0242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarraya B, et al. Dopamine gene therapy for Parkinson’s disease in a nonhuman primate without associated dyskinesia. Sci Transl Med. 2009;1(2):2ra4. doi: 10.1126/scitranslmed.3000130. [DOI] [PubMed] [Google Scholar]
- 28.Palfi S, et al. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson’s disease: A dose-escalation, open-label, phase 1/2 trial. Lancet. 2014;383(9923):1138–1146. doi: 10.1016/S0140-6736(13)61939-X. [DOI] [PubMed] [Google Scholar]
- 29.Ho CC, Rideout HJ, Ribe E, Troy CM, Dauer WT. The Parkinson disease protein leucine-rich repeat kinase 2 transduces death signals via Fas-associated protein with death domain and caspase-8 in a cellular model of neurodegeneration. J Neurosci. 2009;29(4):1011–1016. doi: 10.1523/JNEUROSCI.5175-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reinhardt P, et al. Derivation and expansion using only small molecules of human neural progenitors for neurodegenerative disease modeling. PLoS One. 2013;8(3):e59252. doi: 10.1371/journal.pone.0059252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein RL, King MA, Hamby ME, Meyer EM. Dopaminergic cell loss induced by human A30P alpha-synuclein gene transfer to the rat substantia nigra. Hum Gene Ther. 2002;13(5):605–612. doi: 10.1089/10430340252837206. [DOI] [PubMed] [Google Scholar]
- 32.Cao S, Theodore S, Standaert DG. Fcγ receptors are required for NF-κB signaling, microglial activation, and dopaminergic neurodegeneration in an AAV-synuclein mouse model of Parkinson’s disease. Mol Neurodegener. 2010;5:42. doi: 10.1186/1750-1326-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2nd Ed Academic; San Diego: 2001. [Google Scholar]
- 34.Vila M, et al. Alpha-synuclein up-regulation in substantia nigra dopaminergic neurons following administration of the parkinsonian toxin MPTP. J Neurochem. 2000;74(2):721–729. doi: 10.1046/j.1471-4159.2000.740721.x. [DOI] [PubMed] [Google Scholar]
- 35.Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2007;2(1):141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- 36.Friesner RA, et al. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem. 2006;49(21):6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]