Significance
The whole-cell file lineages of plant roots are derived from a small group of initials that surround a functional and structural center, called the quiescent center (QC). It’s known that the QC maintains the directly abutting stem cells undifferentiated, but the mechanism for the maintenance is not entirely clear. Using a system that effectively blocks plasmodesmata in the QC, we show that QC-derived signals symplastically instruct the status of surrounding stem cells. Via directing local auxin biosynthesis, cell-to-cell communication between the QC and neighboring cells provides positional information for stem cell maintenance. Our results reveal a tight link between symplastic communication and auxin-dependent stem cell maintenance.
Keywords: symplastic signaling, auxin, stem cell niche, PLETHORA, Arabidopsis
Abstract
Stem cells serve as the source of new cells for plant development. A group of stem cells form a stem cell niche (SCN) at the root tip and in the center of the SCN are slowly dividing cells called the quiescent center (QC). QC is thought to function as a signaling hub that inhibits differentiation of surrounding stem cells. Although it has been generally assumed that cell-to-cell communication provides positional information for QC and SCN maintenance, the tools for testing this hypothesis have long been lacking. Here we exploit a system that effectively blocks plasmodesmata (PD)-mediated signaling to explore how cell-to-cell communication functions in the SCN. We showed that the symplastic signaling between the QC and adjacent cells directs the formation of local auxin maxima and establishment of AP2-domain transcription factors, PLETHORA gradients. Interestingly we found symplastic signaling is essential for local auxin biosynthesis, which acts together with auxin polar transport to provide the guidance for local auxin enrichment. Therefore, we demonstrate the crucial role of cell-to-cell communication in the SCN maintenance and further uncover a mechanism by which symplastic signaling initiates and reinforces the positional information during stem cell maintenance via auxin regulation.
A major feature of plants is the occurrence of organogenesis throughout the entire life cycle. This is achieved by the continual maintenance of the quiescent center (QC) and stem cell niche (SCN) (1). Teasing out the factors determining the “stemness” has proved difficult. The early studies back in 1990s provided evidence for the key role of the QC in producing essential repressive signals to prevent the differentiation of surrounding stem cells (2). However, it has long been an enigma how the communication between the QC and surrounding cells leads to the quiescence in the QC and stemness in the SCN.
Plant hormones have been reported to regulate the QC and SCN (3–7). Auxin appears to be the central regulator, acting to integrate the activities of other hormones and regulators (8). Around the the QC, auxin forms a maximum, which was proposed to determine the position of the SCN within the developing root (3, 9). Consistent with the auxin enrichment, AP2-domain transcription factors, PLETHORA (PLTs), display a gradient of expression with the peak in the SCN (10, 11). The expression of PLTs is under the control of auxin, and PLT function is essential for SCN maintenance (12). A large body of evidence demonstrated that various signals are transmitted to the auxin/PLT regulatory loop to influence the SCN in Arabidopsis roots (13–15). Interestingly, the expression of auxin efflux PINs appeared to be maintained by PLTs, thus forming a feed-forward loop to reinforce the position of the SCN (1).
Residing in the center of the SCN, the QC plays a pivotal role in SCN regulation. Therefore, maintaining QC quiescence and mitotic inactivity seems to be vital for the stem cell status. A QC-specific gene, WUSCHEL-RELATED HOMEOBOX 5 (WOX5), was found indispensable for the suppression of QC division and the differentiation of columella stem cells (CSCs) (16). Recent studies have elucidated a regulatory pathway of WOX5, in which signaling peptide CLAVATA3/EMBRYOSURROUNDING REGION 40 (CLE40) and the receptor-like kinases ARABIDOPSIS CRINKLY4 (ACR4)/CLAVATA1 (CLV1) maintain the CSCs through negative regulation of WOX5 (17, 18). In the QC, WOX5 inhibits mitotic activity by repressing CYCD3;3 expression (19). Interestingly, WOX5 protein also moves into CSCs to repress a group II D of transcription factor CDF4, resulting in a noncell-autonomous inhibition of CSC differentiation (20). WOX5 also appeared to act as the integrator of activity of transcription factors, such as SCR and ROW1, as well as complex hormone regulatory networks (16, 21–23).
Despite the importance of the QC in SCN regulation, direct evidence for cell-to-cell communication between the QC and SCN is lacking, and the mechanism behind this communication is unknown. In addition, it is unclear why the direct cellular contact within the SCN is indispensable, and how cell-to-cell communication functions in SCN maintenance. The lack of tools for assessing intercellular signaling between the QC and adjacent stem cell initials had made it difficult to address these questions. Recently, a mutated CALLOSE SYNTHASE 3 (cals3m) was demonstrated to promote callose deposition around the plasmodesmata (PD), preventing transport of mobile transcription factors between cells (24). Here we developed a system in which the symplastic communication between the QC and adjacent cells is inducibly and specifically blocked by expressing icals3m (inducible form of cals3m) in the QC. Our results suggest that symplastic communication between the QC and abutting cells directs the formation of local auxin maxima and establishment of PLT gradients. Although long-distance auxin polar transport provides the source of auxin for development, local auxin biosynthesis appears to contribute to the establishment of local auxin enrichment. Through feed-forward loops, symplastic communication creates a reinforcing specification of the SCN via auxin and PLT pathways. Therefore, we provide direct evidence for the crucial role of cell-to-cell communication in SCN maintenance, and further uncover a mechanism by which symplastic signaling initiates and reinforces the position information during stem cell maintenance via auxin regulation.
Results and Discussion
Transient Callose Deposition to PD Efficiently Blocks Symplastic Communication in the QC.
Because the QC is indispensable for SCN maintenance, communication between the QC and adjacent stem cells must exist. It is thought that the conveying intercellular signals can be symplastic, juxtacrine, or mechanical, but direct evidence for any of these is lacking. To address the role of PD-mediated symplastic communication in SCN maintenance, we modified the previously reported icals3m system (24, 25) to inducibly and transiently block the PD in the QC. Compared with a previously reported laser ablation method, icals3m has an advantage of noninvasiveness. By specifically building up callose around the PD to disrupt symplastic movement, the icals3m system retains the mechanical intactness and juxtacrine regulation. To test the ability of icals3m to block movement into the QC, we expressed the icals3m transgene driven by the WOX5 promoter.
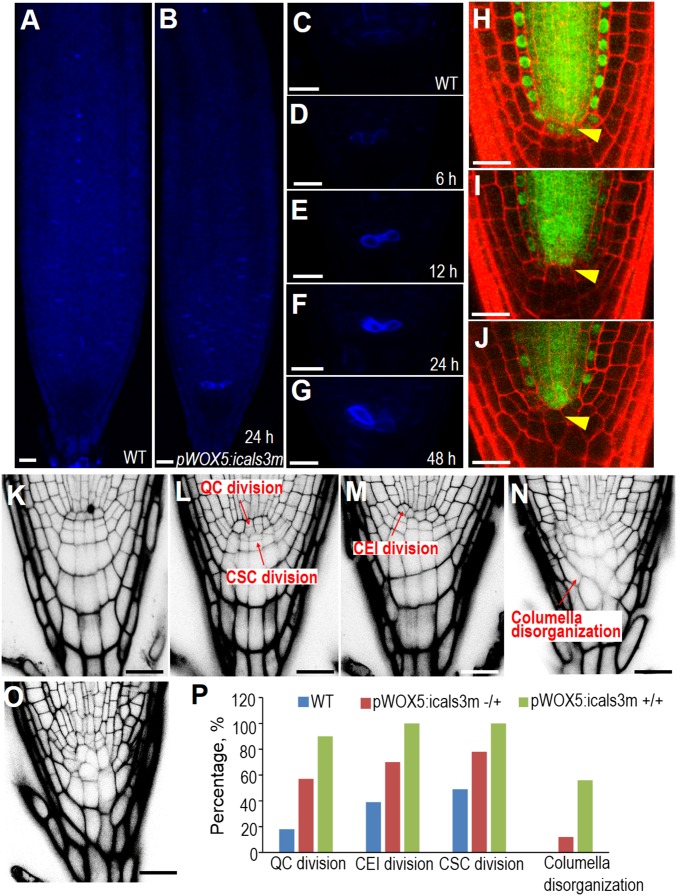
At 24-h postinduction with estradiol, we observed a clear accumulation of callose in the apical region of roots expressing the pWOX5:icals3m transgene (Fig. 1 A and B). To determine how early the icals3m can promote callose deposition, we performed time-course callose staining (Fig. 1 C–G). As early as 6 h, callose was clearly visible in two to three cells residing at the QC position, whereas the same position in controls appeared to be the background level (Fig. 1D). The callose induction in pWOX5:icals3m roots was maintained at a high level after 2-d estradiol treatment (Fig. 1G). We usually performed our observation and analysis within this time frame.
Fig. 1.
Symplastic communication is essential for SCN maintenance. (A–G) Callose staining of WT (A and C) and pWOX5:icals3m (B, D–G) after 10-μM estradiol induction for 6 h (D), 12 h (E), 24 h (B and F), and 48 h (G). Note the specific deposition of callose in the QC position in B, D, and E. (H–J) Confocal images of roots expressing the pSHR:SHR-GFP transgene before (H) and after (I and J) induction of pWOX5:icals3m for 24 h (I) and 48 h (J), respectively. Arrowheads indicate the QC position. (K–O) Phenotypes of pWOX5:icals3m-expressing roots (L–O) compared with WT (K) after 48-h induction in 10 μM estradiol medium. (L–N) the classification of observed phenotypes. (P) The quantification of the percentage of each phenotype among WT (n = 28), heterozygous pWOX5:icals3m (+/−) (n = 56) (represented by L and M), and homozygous pWOX5:icals3m (+/+) (n = 48) roots (represented by N and O). “n” represents the total number of roots used for quantification. (Scale bars, 20 μm.)
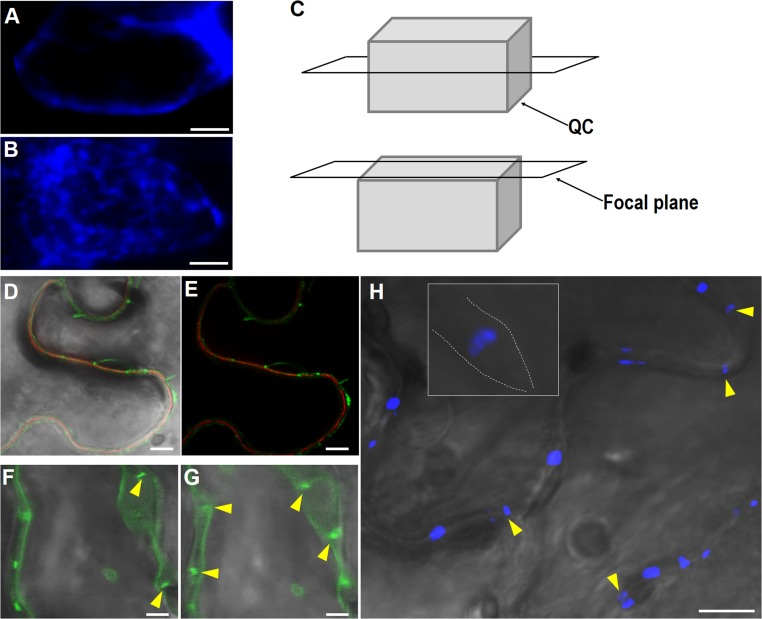
To verify the PD-localization of callose induced by icals3m, we observed the subcellular distribution of callose under Zeiss 880 using an airy scan function. The median confocal section showed cell membrane accumulation and the confocal section on the surface displayed punctae distribution of callose (Fig. S1 A–C). To further confirm that the discrete callose signal was a PD-associated structure, we imaged 35S:GFP-icals3m and its induced callose in tobacco leaves. The dot-like enrichments along the cell wall were observed, which are typical PD-localization in leaf pavement cells (Fig. S1 D and E). In addition, plasmolysis of those cells led to the strand of GFP-icals3m and callose spanning the cell wall, indicating the accumulated signals localized to PD areas (Fig. S1 F–H).
Fig. S1.
PD localization of icals3m and induced callose. (A–C) Aniline blue staining showing the accumulation of callose in the QC of pWOX5:icals3m roots after 48-h estradiol induction. The median confocal section (A) showing cell membrane localization of callose and the surface confocal section (B) showing the punctate distribution of callose on the cell surface. Cartoon showing different confocal sections (C). (D and E) Expression of 35S:GFP-icals3m in tobacco leaves. Punctate accumulation of GFP-icals3m along PI-stained cell wall. Stretched GFP-icals3m (yellow arrow heads) between membrane structure of two plasmolytic cells (F and G). (H) Aniline blue staining showing the stretched callose structures (yellow arrow heads) between two plasmolytic cells (the separated cell membranes were drawn in dotted line in the Inset). (Scale bars, 2 μm in A and B, 50 μm in D–G, and 100 μm in H.)
To determine whether this increase in callose effectively blocked transport through the PD of the QC, we introduced pSHR:SHR-GFP into the pWOX5:icals3m-expressing plants. Synthesized in the stele, SHR-GFP was demonstrated to move cell-to-cell via the PD (26). In the WT, stele-derived SHR-GFP was present in the endodermis, cortex/endodermis initials (CEI), and QC cells, in addition to stele (Fig. 1H). After 24 h in estradiol medium, SHR-GFP in the QC was reduced to the background level (Fig. 1I). At 48 h, SHR-GFP became invisible in the QC but was still present in the endodermis and CEI, suggesting a specific disruption of symplastic transport in QC cells (Fig. 1J).
Symplastic Blockage in QC Leads to Disruption of SCN Maintenance.
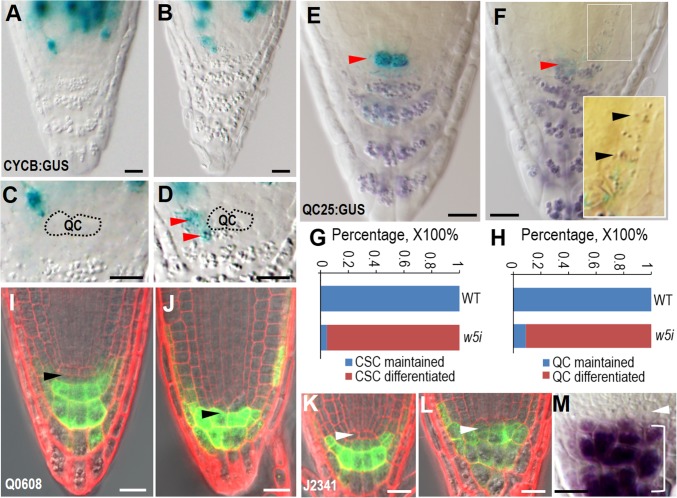
After 48-h induction with estradiol, we found profound SCN defects, including QC division, CSC division, CEI division, and disorganized columella in pWOX5:icals3m roots (Fig. 1 K–O). Compared with heterozygous pWOX5:icals3m, the homozygous lines exhibited stronger phenotypes, particularly in the organization and patterning of the columella (Fig. 1 N–P). Consistent with a defective cell division pattern in the SCN, we often detected pCYCB1;1:CYCB1;1-GUS expression in CSCs even after the granule structures appeared in these cells (Fig. 2 A–D). To determine whether the emergence of granule structures reflects the differentiated CSCs, we performed Lugol’s staining in pWOX5:icals3m roots, with the QC marked by QC25. The clear invasion of starch staining in CSCs and even in the proximal meristem indicates the repression of differentiation in those cells was abolished after symplastic communication was blocked in the QC (Fig. 2 E and F). Compared with the WT, in which none of the QC and CSCs exhibited starch-staining, 95.5% (21 of 22 roots) of CSCs and 90.9% (20 of 22 roots) of the QC in pWOX5:icals3m roots became differentiated. Consistently, the expression of the marker QC25 was reduced after symplastic signaling was disrupted, suggesting that the identity and function of QC were likely affected (Fig. 2F).
Fig. 2.
Disruption of symplastic signaling in QC causes cell differentiation in the SCN. (A–D) The expression of pCYCB1;1:CYCB1;1-GUS in WT (A and C) and pWOX5:icals3m-expressing roots after 48-h estradiol induction. The QC position is labeled and red arrowheads point to the ectopic cell division marked by the expression of CYCB1;1-GUS in SCN. (E and F) Starch granule accumulation as visualized by Lugol’s staining in the WT (E) and WOX5:icals3m (F) roots after 48-h estradiol induction. Red arrowheads point to the QC position with expression of QC25 marker. Note the starch granules in proximal meristem in zoom-in image in F. (G and H) Quantification of CSC (G) and QC (H) differentiation rate in WT and pWOX5:icals3m (w5i) roots. (I and J) Q0608 expression in WT (I) and pWOX5:icals3m (J) roots after 48-h estradiol induction. Black arrowheads point to the CSC position. (K and L) J2341 expression in WT (K) and pWOX5:icals3m (L) roots after 48-h estradiol induction. White arrowheads point to the QC position. (M) QC (pointed to by the white arrow head) was not differentiated but CSC became differentiated (included in the bracket) in the wox5-1 root. (Scale bars, 20 μm.)
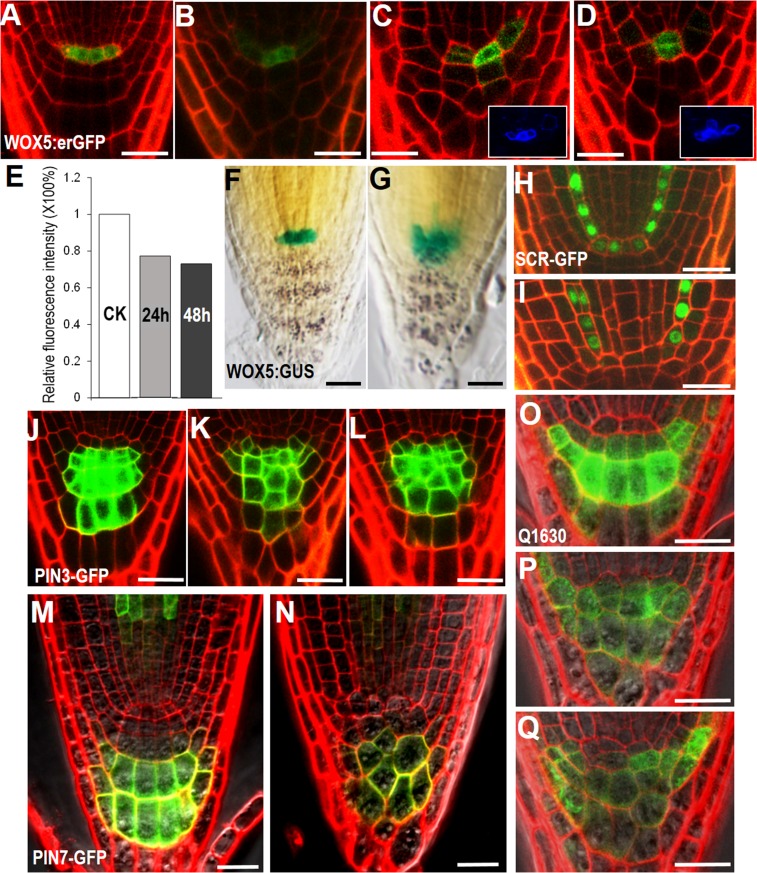
To verify the differentiation status in the SCN, we introduced several cell type-specific markers into pWOX5:icals3m roots. Q0608 was only expressed in matured columella cells of WT, but its expression expanded into CSCs in pWOX5:icals3m roots (Fig. 2 I and J). J2341 is often expressed in most cells in the SCN, except for the QC. After PD was obstructed, the QC appeared to take on the expression of J2341, suggesting the functional alteration of the QC (Fig. 2 K and L). However, QC properties were partially retained, as evidenced by the absent expression of Q0608 in the QC (Fig. 2J). Consistent with this finding, WOX5 maintained a high expression after 48-h induction with estradiol (Fig. S2 A–D). The enlarged expression zone of WOX5 was likely because of QC divisions (Fig. S2 B–D and G). However, quantification of pWOX5:erGFP showed only a negligible reduction (23% after 24 h and 27% after 48 h) of GFP fluorescence intensity (Fig. S2E).
Fig. S2.
WOX5 expression and columella patterning are well maintained in pWOX5:icals3m roots. (A–D) The expression of pWOX5:erGFP in WT (A) and pWOX5:icals3m (B–D) after 48-h estradiol induction. The Insets in C and D are representative images of callose staining after 48-h estradiol induction, indicating the pWOX5:icals3m was active at this time point. (E) Quantification of pWOX5:erGFP fluorescent intensity relative to the controls (CK); 24 h and 48 h represent pWOX5:icals3m roots treated by estradiol for 24 h and 48 h, respectively. (F and G) The expression of pWOX5:NLS-GUS in WT (F) and pWOX5:icals3m (G) after 48-h estradiol induction. (H and I) The expression of pSCR:SCR-GFP in WT (H) and pWOX5:icals3m (I) after 48-h estradiol induction. Note the absence of SCR-GFP in the QC in I. (J–L) The expression of pPIN3:PIN3-GFP in WT (J) and pWOX5:icals3m (K and L) after 48-h estradiol induction. (M and N) The expression of pPIN7:PIN7-GFP in WT (M) and pWOX5:icals3m (N) after 48-h estradiol induction. (O–Q) The expression of Q1630 in WT (O) and pWOX5:icals3m (P and Q) after 48-h estradiol induction. (Scale bars, 20 μm.)
A previously described SCN regulator, SCR was not visible in the QC of pWOX5:icals3m roots after 48-h estradiol induction, which is likely because of blocked SHR movement into the QC (Fig. S2 H and I). Thus, the WOX5 activation in the QC is not solely or directly dependent on SCR activity. Interestingly, WOX5 protein was recently shown to move into the CSC to repress its differentiation (20). However, in the wox5-1 mutant we never saw the QC differentiation that was observed in pWOX5:icals3m, suggesting the existence of additional mobile factors that regulate the QC and SCN (Fig. 2M).
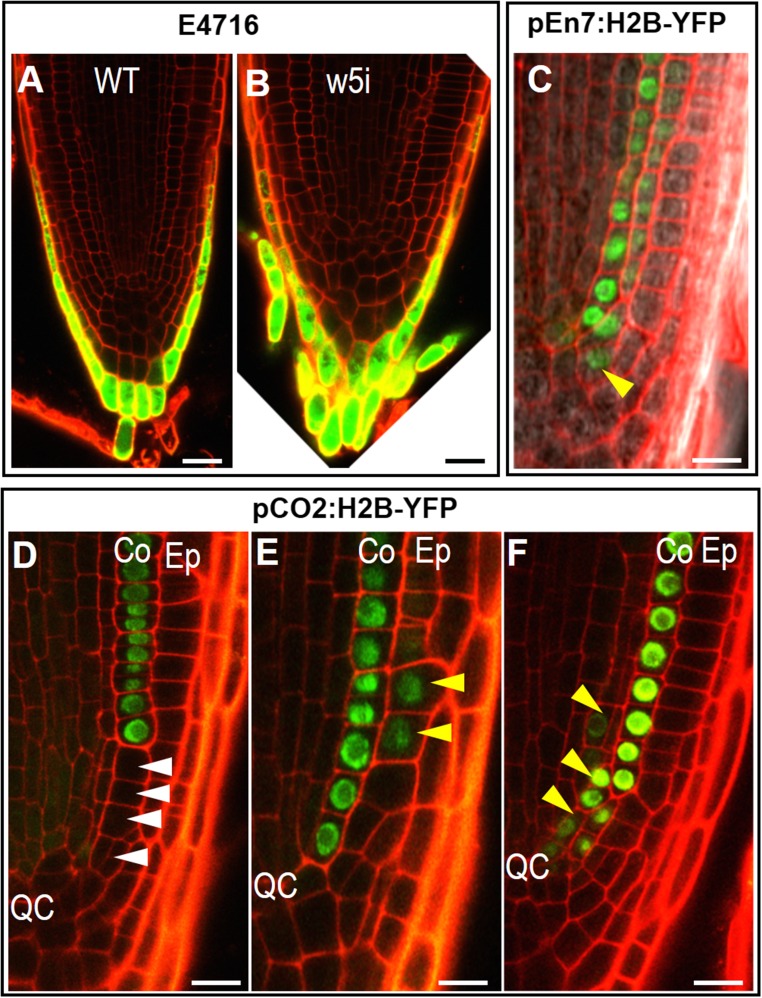
We next examined whether defects in SCN affect the patterning of the tissue nearby. In the distal meristem, specific markers, including pPIN3:PIN3-GFP, pPIN7:PIN7-GFP, Q1630, and E4716 in pWOX5:icals3m-expressing roots retained the similar expression pattern as in WT (Figs. S2 J–Q and S3 A and B). This finding indicates that the columella cell fate is not subject to the symplastic regulation from the QC. In the proximal meristem, ground tissue patterning seemed to be moderately affected in pWOX5:icals3m roots, with loss-of-expression or mis-expression of pEn7:H2B-YFP and pCO2:H2B-YFP in cells adjacent to the SCN (Fig. S3 C–F). However, these defects seemed to be corrected when cells move away from the SCN, indicating that QC-derived signaling only affects the adjacent region (Fig. S3 C–F).
Fig. S3.
Root patterning and cell fate determination outside of the SCN are not affected in pWOX5:icals3m roots. (A and B) The expression of enhancer trap line E4716 in WT and pWOX5:icals3m (w5i) roots after 48-h estradiol induction. (C) The expression of pEn7:Histone2-YFP (pEn7:H2B-YFP) in pWOX5:icals3m roots after 48-h estradiol induction. Yellow arrowheads point to mis-expression of pEn7:H2B-YFP. (D–F) The expression of pCO2:Histone2-YFP (pCO2:H2B-YFP) in pWOX5:icals3m roots after 48-h estradiol induction. Co, cortex; Ep, epidermis. White arrowheads point to loss of pCO2:H2B-YFP expression; yellow arrowheads point to mis-expression of pCO2:H2B-YFP. (Scale bars, 20 μm in A and B and 10 μm in C–F.)
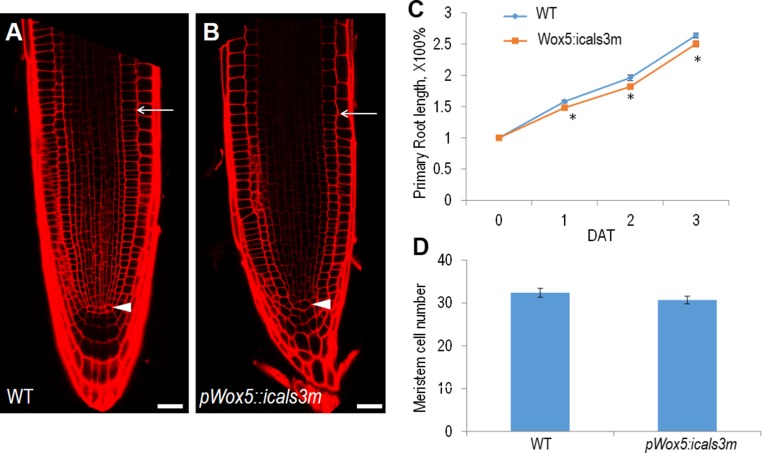
Because the SCN serves as the source of new cells for root growth, we analyzed how SCN defects affect root growth. Unexpectedly, we found the SCN defects were not translated to the root growth immediately. The meristem size of pWOX5:icals3m roots was comparable to the WT after 48 h on estradiol medium (Fig. S4 A and B). The primary root length had only a marginal decrease in pWOX5:icals3m roots but the meristemetic cell number remained at the same level as WT (Fig. S4 C and D). This result suggests that the SCN, although serving as the source for cell proliferation, its activity, and division, likely acts only as a long-run reservoir of new cells for root growth.
Fig. S4.
Root growth analysis of pWOX5:icals3m. (A and B) The root meristem size of WT (A) and pWOX5:icals3m (B) after 48-h estradiol induction. Arrowheads point to the QC and arrows point to the junction between meristem and rapid elongation zone. (C) Time-course analysis of root growth rate. DAT, days after treatment. Data are average values ± SE of >15 roots per treatment. Asterisks indicate Student’s t test significant difference between WT and pWOX5:icals3m (*P < 0.05). (D) Root meristem cell number counted in cortex cell file in WT and pWOX5:icals3m after 48-h estradiol induction. (Scale bars, 20 μm.)
Sympastic Communication Between QC and Stem Cells Instructs the Auxin Maxima and Gradients in SCN and Columella.
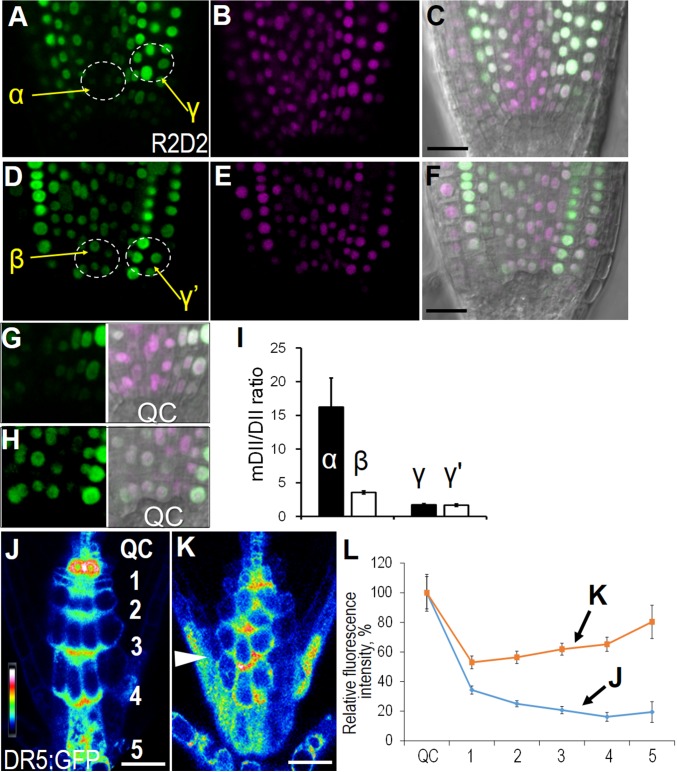
An auxin maxima is associated with SCN maintenance. In central columella cells, auxin gradients are essential for correct differentiation in CSCs (27). To investigate how sympastic signaling interplays with auxin in SCN maintenance, we examined auxin distribution using two different markers. The R2D2 marker is a recently developed tool for semiquantitative measurement of auxin concentration (28). The measurement of “auxin input” using this reporter can be represented as a reduction of green (DII-Venus) relative to magenta signal (ntdTomato-fused mDII), thus providing a more quantitative and precise way to assay auxin enrichment. In the control, auxin peaks in the SCN, evidenced by the low DII-Venus fluorescence in this area, whereas the mDII-ntdTomato signal from the same cells exhibited the normal level (Fig. 3 A–C and G). Forty-eight hours after estradiol treatment, pWOX5:icals3m roots had a significant increase in DII-Venus signal in the SCN, suggesting a reduction of auxin in this region (Fig. 3 D–F and H). Interestingly, the fluorescence change was confined to the region just above the QC, indicating a regional alteration of auxin concentration. To quantitatively compare the distribution of auxin, we analyzed the mDII/DII ratio in different areas. In the proximal SCN of WT roots, mDII/DII ratio stayed at a high level (α in Fig. 3I), but this value significantly dropped in pWOX5:icals3m roots (β in Fig. 3I). The reduction of auxin concentration was restricted in proximal SCN as the mDII/DII ratio in flanking cell layer of proximal SCN was not affected (γ and γ′ in Fig. 3I).
Fig. 3.
Auxin maximum and gradient are disturbed in pWOX5:icals3m roots. (A–H) R2D2 expression in WT (A–C, G) and pWOX5:icals3m (D–F, H). (A and D) DII-Venus; (B and E) mDII-ntdTomato; (C and F) overlay with DIC. (G and H) Zoom-in view of the SCN in A and D, respectively. α, β, γ, and γ′ represent the region that is quantified in I. (I) Quantified mDII/DII ratio in regions specified in A and D. (J and K) Auxin reporter DR5:erGFP in WT (J) and heterozygous pWOX5:icals3m (+/−) after 2-d treatment with 10 μM estradiol (K). Different cell layers in columella are labeled in J. Arrowhead in K points to the auxin distribution in the lateral root cap. (L) Quantification of the DR5:erGFP intensity in different cell tiers of columella relative to QC. The x axis represents the serial number of the cell layer from QC. Blue line represents the measurement on WT and orange line represents the measurement on pWOX5:icals3m after 48-h estradiol induction. The relative fluorescence intensity of each cell layer was normalized to the QC (100%). Data are average values ± SE of three independent experiments with >15 roots per treatment. (Scale bars, 20 μm.)
In R2D2, both DII and mDII were driven by the RPS5A promoter, which is preferentially expressed in dividing cells. Thus, to detect the auxin in the distal region of root tips, we made use of the frequently used DR5:erGFP. From this reporter, auxin forms a peak in the QC and gradually falls down along the columella (Fig. 3J). This gradient is thought to be essential for maintaining the distal stem cells (CSCs) and sustaining the precise balance of differentiation (29). Consistent with the differentiation phenotypes in columella cells, we saw a disruption of auxin gradient with the communication blocked in the QC (Fig. 3K). The quantification of erGFP in each cell layers of columella relative to that in the QC showed an elevated ratiometric value in columella cells and no gradient was detected (Fig. 3L).
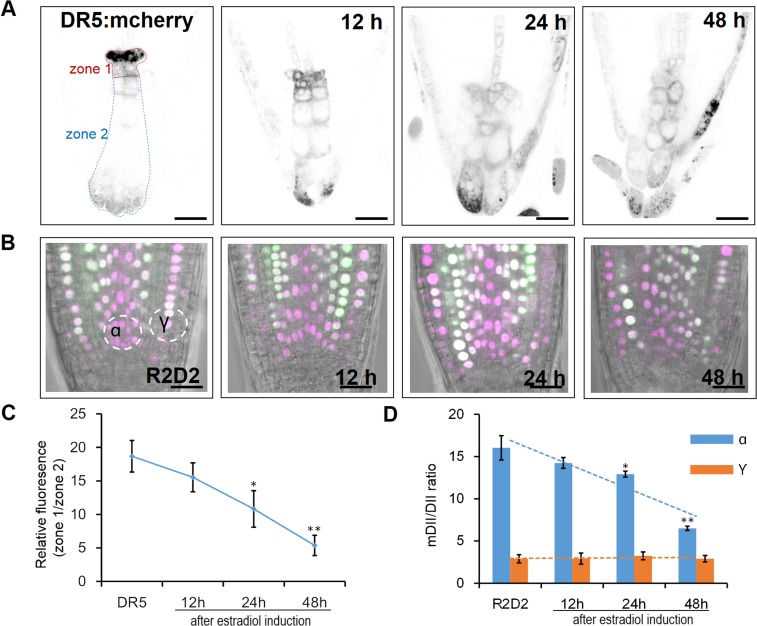
To assess how early these defects occur, we performed time-course analysis of auxin gradient in columella by DR5:mCherry and in proximal meristem using R2D2. Defective gradients in both markers were detected as early as 12 h after estradiol induction (Fig. S5).
Fig. S5.
Time-course analysis of auxin markers in pWOX5:icals3m roots. (A) DR5:mCherry expression pattern in the control (Left) and in pWOX5:icals3m roots. Times after induction are marked. (B) R2D2 expression pattern in the control (Left) and in pWOX5:icals3m roots. Times after induction are marked. (C) Quantitative measurements of auxin markers shown in A. The expression DR5:mCherry in the root tip was divided into zone 1 (including the QC and CSC) and zone 2 (columella cells). The relative fluorescence of each section was measured and calculated as zone1/zone2 (shown in A). (D) Quantitative measurements of auxin markers shown in B. The ntdTomato/Venus relative fluorescence of two circle sections (α and γ, shown in B) was measured and calculated. Data are average values ± SE of >15 roots per treatment. Asterisks indicate Student’s t test significant difference between WT and pWOX5:icals3m (**P < 0.01; *P < 0.05). (Scale bars, 20 μm.)
PLT Peak in SCN Depends on Symplastic Communication Between QC and Stem Cells.
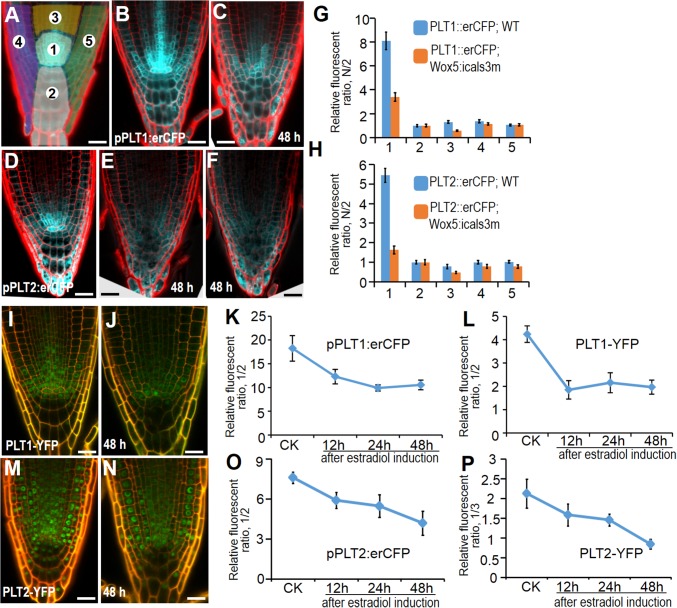
The AP2-domain PLT genes regulate SCN specification by acting downstream of auxin. Accordingly, the expression of PLTs exhibits a maximum in the SCN and tapers off along the longitudinal axis of the root, forming a gradient (Fig. 4 B and D). Evidence indicates that PLT proteins play dose-dependent roles in root development. Interestingly, the phenotypes found in pWOX5:icals3m roots, including QC division and CSC differentiation, were all observed in the plt1-4 plt2-2 double mutant roots (10). Thus, we hypothesize that PLTs participate in the symplastic regulation of the SCN by responding to the auxin maximum. We tested this hypothesis by analyzing transcriptional and translational reporter lines of PLTs.
Fig. 4.
PLT gradient is disrupted in pWOX5:icals3m expressing roots. (A) The scheme depicting sections for the fluorescence ratio measurement. Whole root tip was divided into five sections as marked. (B and C) The expression of pPLT1:erCFP in WT (B) and pWOX5:icals3m (C). (D–F) The expression of pPLT2:erCFP in WT (D) and pWOX5:icals3m (E and F). (G and H) Quantitative comparison of PLT1:erCFP (G) and PLT2:erCFP (H) in different sections between WT (blue) and pWOX5:icals3m (orange). The numbers along the horizontal axis represent each section, and the value of the vertical axis represents the relative fluorescence ratio of each section that was normalized to section 2 (labeled as N/2). (I and J) The expression of pPLT1:PLT1-YFP in WT (I) and pWOX5:icals3m (J). (M and N) The expression of pPLT2:PLT2-YFP in WT (M) and pWOX5:icals3m (N). (K–P) Time-course analysis of pPLT1:erCFP (K), pPLT1:PLT1-YFP (L), pPLT2:erCFP (O), and pPLT2:PLT2-YFP (P) in section 1 (normalized to section 2 or 3). CK represents the control root. Different induction times of pWOX5:icals3m roots are marked. Data are average values ± SE of three independent experiments with >15 roots per treatment. (Scale bars, 20 μm.)
In pWOX5:icals3m roots, the expression level of both pPLT1:erCFP and pPLT2:erCFP in pWOX5:icals3m roots was greatly reduced around the SCN compared with controls (Fig. 4 A–F). However, the effect appeared to be restricted in the proximal meristem, because the PLT expression out of this region was normal. To better quantify relative expression level in different regions, we divided the root tip into five sections (Fig. 4A). We used section 2 as the reference region and the fluorescence intensity of all other sections were normalized to section 2. As shown in Fig. 4 G and H, section 1, which is localized in the proximal meristem, exhibited a remarkable drop of fluorescent level, whereas most other sections, except section 3, had the comparable level of PLT expression to the control. Section 3, representing the region just above the proximal meristem, also showed a slight reduction of the PLT transcription. Consistent with this finding, we found a decrease in the pPLT1:PLT1-YFP translational fusion proteins in section 1 of pWOX5:icals3m roots (Fig. 4 I–N). To understand how down-regulation of PLTs in proximal meristem responds to symplastic regulation, we analyzed the transcriptional and translational level of PLTs at different induction times in pWOX5:icals3m roots. We used the same ratiometric value (section 1/section 2) to determine the level of PLTs in the SCN, except for the pPLT2:PLT2-YFP line, in which section 2 seemed also to be down-regulated. Our time-course analysis showed that both PLT1 and PLT2 exhibited gradual decline in both transcript and protein levels once the symplastic communication in the QC was turned off, which is consistent with auxin gradient disruption (Fig. 4 K–P). Taken together, our results reveal that PD-mediated symplastic signaling is essential for maintaining auxin gradient and PLT maximum in the SCN. A number of regulators were previously reported to control overall PLT expression in the root apical meristem (13–15). In contrast to those regulators, symplastic communication between the QC and abutting cells controls auxin and PLTs in a restricted area.
Symplastic Communication Modulates Auxin Maxima in SCN.
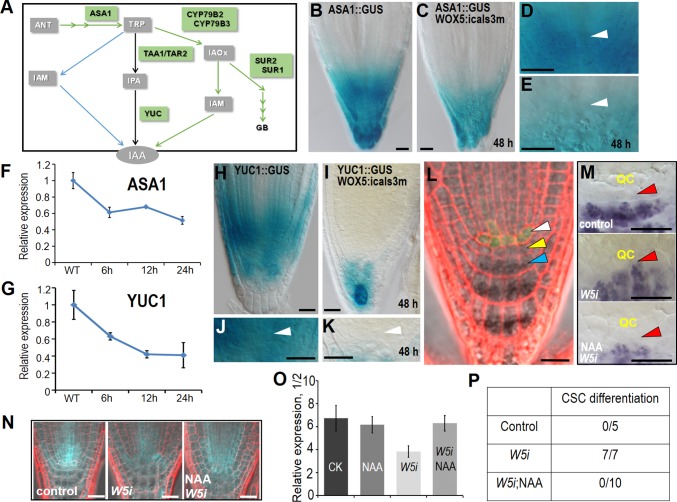
A large body of evidence has demonstrated the crucial role of polar auxin transport in establishing auxin maximum at the root tip (30–32). In addition, localized auxin production contributes to auxin local enrichment in the root tip (33, 34). To understand how auxin concentration changes in pWOX5:icals3m roots, we examined both auxin local biosynthesis and auxin polar transport in the root tip.
Recent evidence suggests that auxin is synthesized predominantly through two consecutive reactions, starting at tryptophan (Trp) (35). TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS 1 (TAA1) and its close homologs TAR1 and TAR2 convert Trp to indole-3-pyruvate (IPA), which serves as the substrate for YUCCA (YUC) to produce indole-3-acetic acid (IAA) (36). Although the reaction catalyzed by YUCs is considered as a rate-limiting step in auxin biosynthesis, other pathways, such as CYP79B2/B3 catalyzing Trp to IAOx conversion may also be involved (37, 38). Immediately upstream of Trp is a committed step catalyzed by ASA1 and ASB1 (39, 40) (Fig. 5A). A few of those enzymes, including ASA1, TAA1, and a number of YUCs, have been shown to express in the root tip. In estradiol-treated pWOX5:icals3m roots, pASA1:GUS staining became dramatically reduced compared with WT (Fig. 5 B–E). However, qRT-PCR showed that the expression of both TAA1 genes appeared to be unaffected, and TAR1 even had a slightly elevated transcript level (Fig. S6). This could be a feedback response to the reduction of ASA1. The IPA to IAA conversion was catalyzed by the YUC family encoded by 11 YUC genes in Arabidopsis, which show overlapping function and expression domains (33, 41). To understand how YUCs respond to disrupted symplastic signaling in the QC, we extracted RNA from the tip of roots (∼4–5 mm) for qRT-PCR analysis of YUC genes that were previously reported to express in roots. Compared with WT, several YUCs showed markedly repressed expression in pWOX5:icals3m roots (Fig. S6A). We further observed the expression of pYUC1:GUS that had strong expression in the root apical meristem. After 48-h estradiol induction, pYUC1:GUS expression in the SCN was markedly repressed (Fig. 5 H–K). Time-course analysis of ASA1 and YUC1 transcription showed that transcriptional reduction of these two genes occurred as early as 6 h after estradiol induction (Fig. 5 F and G). Thus, the change in auxin biosynthesis seemed to be earlier than the auxin concentration change detected by the R2D2 reporter. In accordance with this result, we also observed a moderate decline of the transcript level for STY1, which was reported to activate transcription of YUC4. In addition to the predominant Trp-IPA-IAA pathway, we also detected a considerable decline in the transcription of CYP79B2 and CYP79B3, suggesting that other metabolic pathways contributing to IAA biogenesis were also inhibited in the pWOX5:icals3m roots (Fig. S6B).
Fig. 5.
Symplastic communication is indispensable for local auxin biosynthesis in the SCN. (A) Current model of auxin biogenesis. (B–E) The expression of pASA1:GUS in WT (B and D) and pWOX5:icals3m after 48-h estradiol induction (C and E). (D and E) Zoom-in view of proximal meristem in B and C, respectively. Arrowheads indicate QC position. (F and G) qRT-PCR analysis of ASA1 (F) and YUC1 (G) in WT and pWOX5:icals3m roots with estradiol induction. (H–K) The expression of pYUC1:GUS in WT (H and J) and pWOX5:icals3m after 48-h estradiol induction (I and K). (J and K) Zoom-in view of proximal meristem in H and I, respectively. Arrowheads indicate QC position. (L) Rescued SCN in pWOX5:icals3m roots after 48-h estradiol induction by expressing pWOX5:IAAm-YFP (shown in green fluorescence). White arrowhead indicates QC position; yellow arrowhead points to CSC; blue arrowhead points to differentiated columella cells. (M) Lugol’s staining showing the recovery of CSC differentiation phenotype in pWOX5:icals3m roots by NAA treatment. QC is labeled in yellow and red arrowheads point to the position of CSC. (N and O) Defective SCN and disturbed pPLT1:erCFP gradient of pWOX5:icals3m roots can be partially rescued by NAA treatment. Representative images of pPLT1:erCFP (N) and the quantification of pPLT1:erCFP fluorescence ratio in section 1 (normalized to section 2) (O). (P) Comparison of CSC differentiation ratio among imaged roots between different treatments. W5i represents pWOX5:icals3m. (Scale bars, 20 μm.)
Fig. S6.
(A and B) qRT-PCR showing the expression change of auxin biosynthesis genes, including YUCs (A) and other auxin biosynthesis genes (B). Each data represents the mean of three biological replicates.
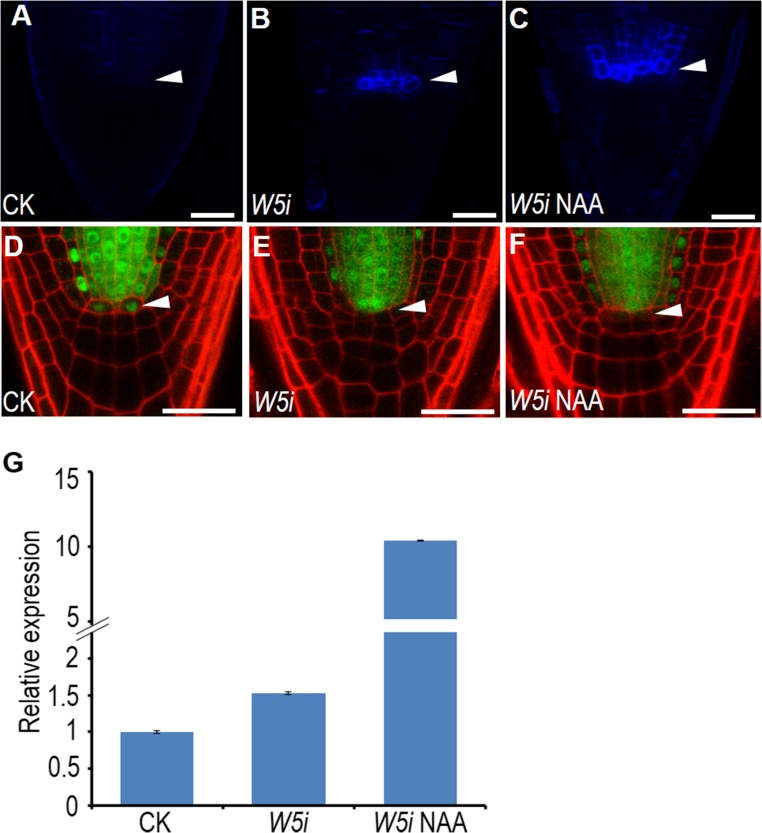
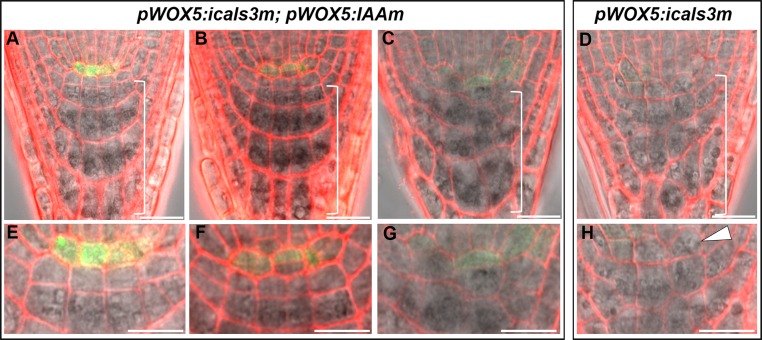
To functionally determine the association of pWOX5:icals3m phenotypes to auxin local deficiency, we added naphthalene acetic acid (NAA) to the treatments. Compared with the induction of estradiol alone, NAA plus estradiol greatly enhanced PLT1 expression in the SCN of pWOX5:icals3m roots (Fig. 5 N and O). In agreement with this, adding back NAA also rescued the CSC differentiation phenotype in pWOX5:icals3m roots (Fig. 5 M and P). To rule out the possibility that auxin treatment could reduce the callose deposition in the QC, we imaged the callose staining and SHR-GFP movement. In the QC of pWOX5:icals3m roots treated with NAA, we saw strong callose deposition and the resultant absence of SHR-GFP, suggesting NAA did not impair callose deposition (Fig. S7 A–F). Interestingly, NAA treatment appeared to boost icals3m expression as well as callose deposition in cells out of the QC (Fig. S7 C and G). This occurance is presumably because of the expanded WOX5 expression domain, which has been reported previously (27). In addition to exogenous auxin treatment, we expressed iaaM, a bacterial auxin biosynthesis enzyme, specifically in the QC under the WOX5 promoter. In 66 roots that expressed both pWOX5:icals3m and pWOX5:iaaM-YFP, 56 roots appeared to be WT-like (Fig. 5L) and 10 roots had weak phenotypes. In the 10 roots with weak phenotypes, we did not detect QC differentiation that we saw in pWOX5:icals3m roots (Fig. S8).
Fig. S7.
icals3m system functions normally with NAA treatment. (A–C) Callose staining of WT (A), pWOX5:icals3m after 48-h estradiol induction (B), and pWOX5:icals3m after 48-h estradiol induction and 24-h NAA treatment (C). (D–F) pSHR:SHR-GFP in WT (D), pWOX5:icals3m after 48-h estradiol induction (E), and pWOX5:icals3m after 48-h estradiol induction and 24-h NAA treatment (F). White arrowheads indicate the QC position. (G) qRT-PCR analysis of icals3m expression in WT, pWOX5:icals3m after 48-h estradiol induction, and pWOX5:icals3m after 48-h estradiol induction and 24-h NAA treatment. (Scale bars, 20 μm.)
Fig. S8.
Rescued SCN in pWOX5:icals3m by expressing pWOX5:IAAm-YFP. (A–G) Fully rescued SCN (A) and partially rescued SCN (B and C) in pWOX5:icals3m by expressing pWOX5:IAAm-YFP. (E–G) Zoomed images showing SCN in A, B, and C, respectively. Note the QC division in F and differentiated CSC in G. (D and H) SCN phenotypes in the root of pWOX5:icals3m. White arrowhead in H indicates the differentiated QC, which was not observed in A–G. All roots were induced in estradiol for 48 h. Brackets in A–D include the differentiated cells. (Scale bars, 20 μm.)
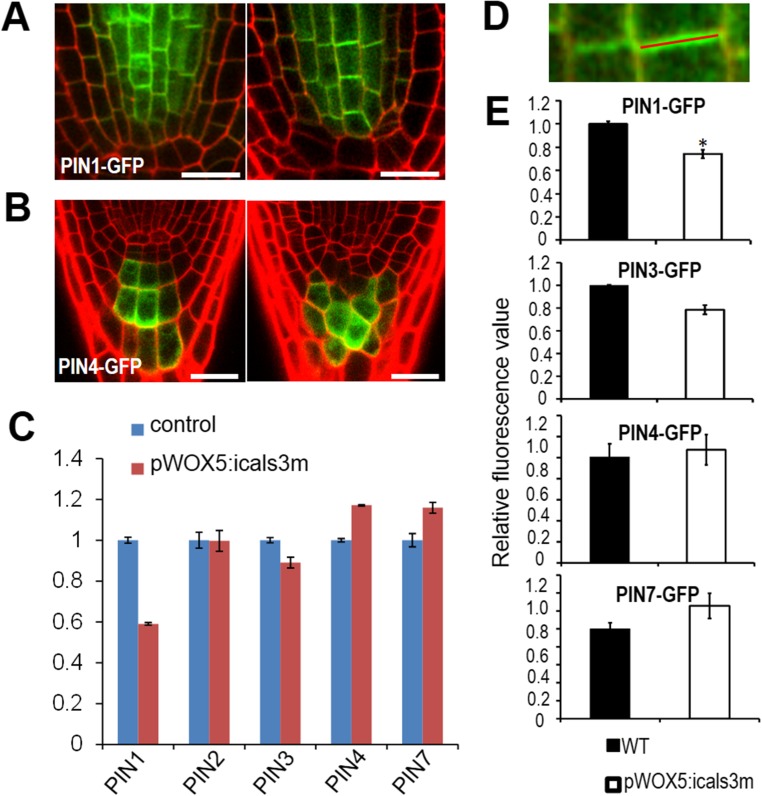
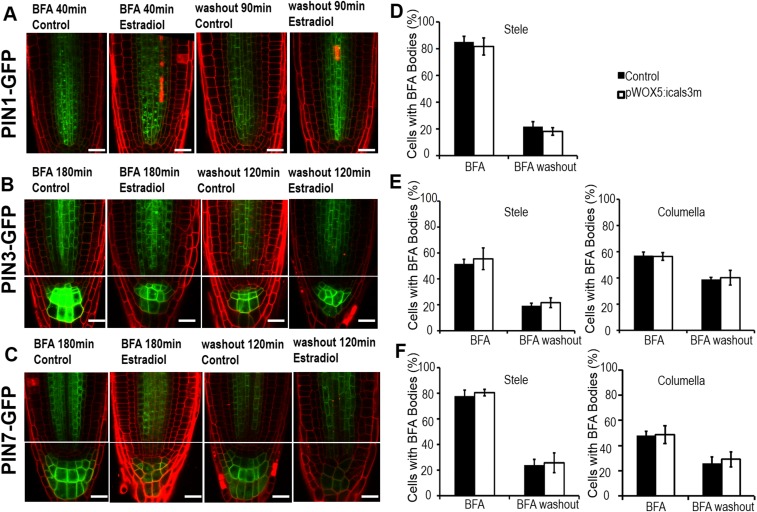
We next ask whether auxin polar transport also accounts for the defective auxin distribution in the SCN. The directional flow and accumulation in the SCN of auxin are coordinately modulated by a group of PINs (30–32). In pWOX5:icals3m-expressing roots, cell-specific expression of PIN1, PIN3, PIN4, and PIN7 were not affected (Figs. S2 K, L, and N and S9 A and B). qRT-PCR also showed that the expression level of PINs in pWOX5:icals3m roots is comparable to that in WT (with moderate reduction of PIN1 expression and marginal increase of PIN4 and -7 expression) (Fig. S9C). We further analyzed the protein level of PINs by quantifying their fluorescence intensity on the cell membrane (Fig. S9D). Similar to the transcriptional level, translational fusion of PINs exhibited the comparable level in pWOX5:icals3m roots to that in WT (Fig. S9E). Auxin directional flow relies on the polar localization of efflux carriers, which is mainly determined by dynamic cycling of PINs. The constitutive intracellular cycling between plasma membrane and endosomal compartments of PINs can be inhibited by BFA treatment (42). Treatment by BFA (40 min for pPIN1:PIN1-GFP and 180 min for pPIN3:PIN3-GFP and pPIN7:PIN7-GFP) led to visible BFA bodies in the cytoplasm of both WT and pWOX5:icals3m roots (Fig. S10 A–C). Quantification of BFA bodies showed no detectible difference between WT and pWOX5:icals3m roots (Fig. S10 D–F). To further investigate the dynamics of PINs, we examined the process of cycling back to cell membrane of PINs. BFA-pretreated PINs-GFP seedlings were washed with Murashige and Skoog (MS) liquid medium for recovery. After washout 90 min for pPIN1:PIN1-GFP and 180 min for pPIN3:PIN3-GFP and pPIN7:PIN7-GFP, accumulated PIN1-GFP (∼74%), PIN3-GFP (64%), and PIN7-GFP (69%) disappeared from the stele cells (Fig. S10 A–C). In columella cells, accumulated PIN3-GFP (32%) and PIN7-GFP (46%) were removed from the cytosol (Fig. S10 A–C). Compared with the WT, pWOX5:icals3m exhibited the negligible effect on PINs cycling after washout (Fig. S10 D–F). Therefore, the abundance and dynamics of PINs in pWOX5:icals3m are akin to those in WT, except that PIN1 had a moderately reduced expression in pWOX5:icals3m roots. Taken together, our results reveal that disruption of auxin gradient in pWOX5:icals3m-expressing root tips likely arise from impaired local auxin biosynthesis.
Fig. S9.
Transcription and protein level on membrane of PINs are not significantly influenced in pWOX5:icals3m. (A and B) pPIN1:PIN1-GFP (A) and pPIN4:PIN4-GFP (B) in WT (Left) and pWOX5:icals3m (Right) after 48-h estradiol induction. (C) qRT-PCR analysis of PINs in WT and pWOX5:icals3m roots after 48-h estradiol induction. (D) The scheme depicting the measurement of fluorescence intensity of PINs on cell membrane. (E) Comparison of PINs fluorescence intensity on cell membrane between WT (black) and pWOX5:icals3m roots (white) after 48-h estradiol induction. Data are average values ± SE of three independent experiments (>50 cells from >10 roots per treatment), and asterisks indicate significant differences by Student’s t test (*P < 0.05). (Scale bars, 20 μm.)
Fig. S10.
PINs cycling stays unaltered in pWOX5:icals3m. (A–C) The accumulation of pPIN1:PIN1-GFP (A), pPIN3:PIN3-GFP (B), and pPIN7:PIN7-GFP (C) in pWOX5:icals3m roots with BFA treatments and after washout. (D–F) Quantification of cells with BFA Bodies in pWOX5:icals3m roots shown in A–C, respectively. Data are means ± SE of >15 roots for each treatment. (Scale bars, 20 μm.)
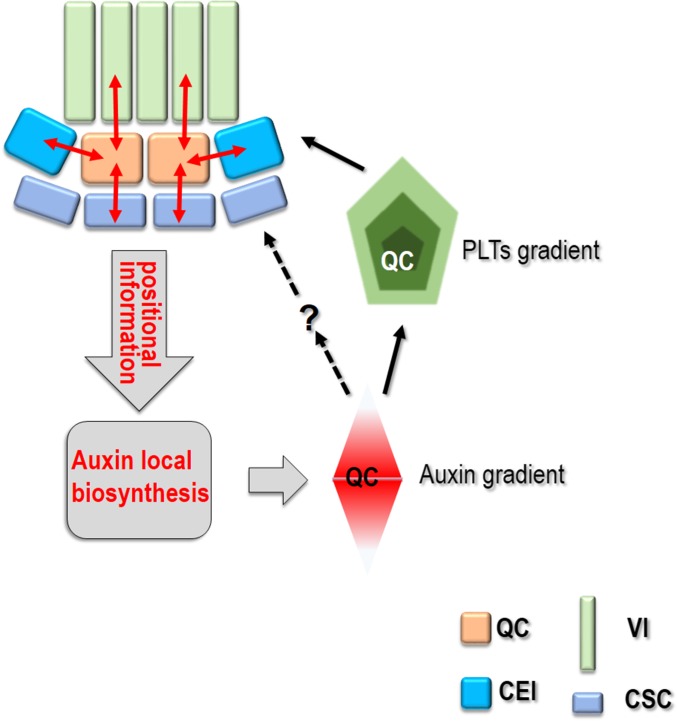
Based on these findings, we propose that communication between the QC and surrounding cells via a symplastic conduit provides positional information for local auxin biosynthesis. The locally produced auxin contributes the establishment of the auxin gradient, which instructs PLTs maximum formation in SCN. Patterned auxin and PLTs in turn reinforce the SCN recognition (Fig. S11).
Fig. S11.
Schematics of symplastic regulation of SCN maintenance. Black arrows indicate positive regulation and dashed arrows depict possible regulation; the question mark represents potential regulators.
Materials and Methods
All seedlings were grown on 0.5× MS medium with 1% agar and 1% sucrose under a 16-h light/8-h dark cycle at 23 °C. Roots were counterstained in 0.01 μg/mL propidium iodide (PI) in water. Confocal images were obtained using a 40× water-immersion lens on a Zeiss LSM 880 laser-scanning confocal microscope. All transgenic lines, plasmid construction, chemical treatments, qRT-PCR, and all other details on materials and methods are provided in SI Materials and Methods.
SI Materials and Methods
Plant Materials and Growth Condition.
The Arabidopsis thaliana Columbia ecotype (Col-0) was used as the WT throughout the experiments. The following marker lines PLT1:erCFP, PLT2:erCFP, PLT1:PLT1-YFP, PLT2:PLT2-YFP, SHR:SHR-GFP, SCR:SCR-GFP, PIN1:PIN1-GFP, PIN3:PIN3-GFP, PIN7:PIN7-GFP, CYCB:CYCB-GUS, QC25:GUS, DR5:erGFP, DII-VENUS, ASA1:GUS, WOX5:erGFP, WOX5:GUS, CO2:H2B-YFP, En7:H2B-YFP, E4716, R2D2, Q1630, Q0608, and J2341 were crossed into plants expressing the pWOX5:icals3m transgene. Homozygous lines expressing both the markers and pWOX5:icals3m were screened based on fluorescence, PCR genotyping, and the root phenotype after induction. All plants were germinated and grown vertically on 1/2 MS medium containing 0.05% (wt/vol) morpholinoethansulfonic acid monohydrate (pH 5.7), 1.0% (wt/vol) sucrose, and 1.0% agar in a growth chamber at 23 °C under a 16/8-h light/dark cycle. Plants were imaged 6–7 d after plating, unless otherwise stated. After sterilization, the seeds were germinated after incubated for 2 d at 4 °C in the dark.
Plasmid Construction and Plant Transformation.
The 4666bp WOX5 promoter fragment was amplified by PCR, then digested by KpnI/XhoI and inserted into previously published plasmid p1R4-ML:XVE (43). The resulting plasmid was recombined with pDONR221 carrying a cals3m fragment into a R4pGWB601 destination vector (44) using standard gateway protocols (Invitrogen/Life Technologies, www.thermofisher.com/us/en/home/life-science/cloning/gateway-cloning.html). The resulting binary vectors were introduced into Agrobacterium tumefaciens strain GV3101-pMP and transformed A. thaliana ecotype Col-0 using a standard floral-dip method. Transgenic plants were screened for resistance to glufosinate ammonium (Basta) in soil. Six independently transformed lines were analyzed and one of them was chosen for further analysis.
Staining, Chemical Treatments, and Confocal Microscopy Imaging.
β-Glucoronidase (GUS) staining was performed as previously described (45). Seedlings were incubated in the GUS (0.5 mg/mL) staining solution from 0.5 h to overnight at 37 °C and then cleared in 70% ethanol. For starch staining, root tips were incubated in a 1:1 dilution of Lugol’s solution (Sigma-Aldrich) for 1 min, then briefly washed with water and mounted in the HCG solution for visualization and microscopy analysis. Samples were viewed using Nikon ECLIPSE Ni-U microscope connected to a Nikon DS-Ri 2 digital camera.
Callose staining of seedling root followed the previously described protocol (46). Briefly, roots were stained in 67 mM K3PO4, 1% Aniline blue, and 1‰ Silwet in the dark for 30–60 min and then imaged under a confocal microscope. Stock solutions of 1 mM NAA, 100 μM β-estradiol (Sigma-Aldrich) were made and stored at −20 °C. For NAA treatment, seeds were germinated for 4 d on 1/2 MS medium followed by 10-μM β-estradiol treatment for 1 d, and then transferred to 1/2 MS medium supplemented with 1 μM NAA and/or 10 μM β-estradiol for another day. Roots were mounted in 0.01 μg/mL PI in water. Roots tips were then examined for the QC or stem cell differentiation. Confocal images were obtained using a 40× water-immersion lens on a Zeiss LSM 880 laser scanning confocal microscope.
Image quantification was performed using ImageJ 1.4.3 software. For PINs protein level analysis, we used the rectangular selection function to create a narrow region along the cell membrane where PINs reside. For quantification of maker expression, including pPLTs:erCFP, pPLTs:PLTs-YFP, pWOX5:erGFP, R2D2, and DR5:mCherry, we divided the root tip into several separated regions based the root anatomy, and used the polygon selection function to create the region-of-interest. The average intensity of fluorescence was calculated by the region-of-interest manager and then used to calculate the ratio of relative fluorescence intensity. Representative images were collected from 10 to 15 roots with three biological replicates.
BFA Treatment and BFA Washout Experiments.
The 6-d-old pWOX5:icals3m seedlings (including 2 d with estradiol incubation) expressing PIN1/PIN3/PIN7-GFP were immersed in 1/2 MS medium containing 100 mM BFA (Sigma-Aldrich) for various lengths (PIN1 for 40 min, PIN3 for 180 min, PIN7 for 150 min), and then observed or observed after being washed with 1/2 MS medium at various time periods (PIN1-GFP for 90 min, PIN3-GFP and PIN7-GFP for 120 min). The same growth-phase PIN1/PIN3/PIN7-GFP seedlings were used as controls. For quantitative analysis of BFA bodies, at least 60 cells from 5 to 10 roots were used for each assay.
Quantitative Real-Time RT-PCR Analysis.
cDNA was prepared from the total RNA extracted from the root tip (∼1 cm) of 6-d-old pWOX5:icals3m seedlings after 2-d estradiol incubation. qRT-PCR was performed on a Stratagen Mx3005P (Agilent Technologies) with the TransStart Top Green qPCR SuperMix (Transgen), according to the manufacturer’s instructions. The primers of interested genes were listed as follows: YUCCA1 (5′-CCTCACAACAAAACTGACCAGACC-3′ and 5′-AAGGGACTCCACGGCTCG-3′), YUCCA2 (5′-TATTTCATCATTAGATTTACCCTGG-3′ and 5′-ATACTCTCTTTTGTGACTCGTGGA-3′), YUCCA3 (5′-ATGGTCGTTCGTAGCGCTGTTC-3′ and 5′-GCGAGCCAAACGGGCATATACTTC-3′), YUCCA4 (5′-TGGGCACTTGTAGAGAATCAGA-3′ and 5′-GGATGGGTATTTGGGGAAG-3′); YUCCA5 (5′-AACGCGTGGAAAGGGAAATCGG-3′ and 5′-TCTGCTGATGCTCCAGCCAATC-3′), YUCCA7 (5′-ACTCAGGCATGGAAGTCTCTCTTG-3′ and 5′-AACGGAGCTTCGAACGACCATTG-3′), YUCCA8 (5′-GCGGTTGGGTTTACGAGGAAAG-3′ and 5′-TGCGATCTTAACCGCGTCCATTG-3′), YUCCA9 (5′-AGTCCGGCGAGAAATTCAGAGG-3′ and 5′-AACATGAACCGAGCTTCTAACGAC-3′), YUCCA10 (5′-TCCGTTTGCAATTGGTTAGAGGAC-3′ and 5′-TTTGGCATCGGTGCTTTGGG-3′), TAA1 (5′-ATACAAACGACCAAACCAAGAAGA-3′ and 5′-CCATCTTCCTCCAGTATTCTTCGTA-3′), TAR2 (5′-GCTCTTCACTGCTTCAAAGAGCAC-3′ and 5′-TCTGTCTTTCACCAAAGCCCATCC-3′), STY1 (5′-ACCGGCAACTTCATCGTCTCTTG-3′ and 5′-TGCCAACTTCTAGCCCTGAATGAG-3′), CYP7B2 (5′-TCACTACCACTGCAACCGAA-3′ and 5′-GAGTGTTTCCTAACTTCACGCAT-3′), CYP7B3 (5′-TTGCGTCAAGACCACTCACT-3′ and 5′-AAGCCTCTTGATTGCATTTCC-3′), ASA1 (5′-GTAGAGAAGCTTATGAACATCGA-3′ and 5′-GGTGCACCACTAACTGTTCCCAC-3′), PIN1 (5′-GGCGAACAAAAGATGATTACGG-3′ and 5′-AGATTTTCCACCATTTGACAGAGC-3′), PIN2 (5′-CCTCGCCGCACTCTTTCTTT-3′ and 5′-CGTACATCGCCCTAAGCAAT-3′), PIN3 (5′-TGTTGTCACTTTTAGTCCTATGGGC-3′ and 5′-AGCAGCCGTCTCAGGGAACT-3′), PIN4 (5′-TTGCTTGTGGGAACTCTGTCG-3′ and 5′-AAGGTCGCCGTGTAAGCCAAT-3′), and PIN7 (5′-GAGGAAACTCATAAGAAACCCAA-3′ and5′-CCAAGACCAGCATCAGAAAGA-3′). ACTIN2 (5′-TTGACTACGAGCAGGAGATGG-3′ and 5′-ACAAACGAGGGCTGGAACAAG-3′) was used as reference gene.
Acknowledgments
We thank Ykä Helariutta, Ari Pekka Mähönen, Ben Scheres, Philip N. Benfey, Jiri Friml, Lin Xu, Xu Chen, and Scott Poethig for materials; Zhenbiao Yang for comments on the manuscript; and Lei Shi for technical assistance.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1616387114/-/DCSupplemental.
References
- 1.Bennett T, Scheres B. Root development-two meristems for the price of one? Curr Top Dev Biol. 2010;91:67–102. doi: 10.1016/S0070-2153(10)91003-X. [DOI] [PubMed] [Google Scholar]
- 2.van den Berg C, Willemsen V, Hendriks G, Weisbeek P, Scheres B. Short-range control of cell differentiation in the Arabidopsis root meristem. Nature. 1997;390:287–289. doi: 10.1038/36856. [DOI] [PubMed] [Google Scholar]
- 3.Sabatini S, et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell. 1999;99:463–472. doi: 10.1016/s0092-8674(00)81535-4. [DOI] [PubMed] [Google Scholar]
- 4.Ortega-Martínez O, Pernas M, Carol RJ, Dolan L. Ethylene modulates stem cell division in the Arabidopsis thaliana root. Science. 2007;317:507–510. doi: 10.1126/science.1143409. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Swarup R, Bennett M, Schaller GE, Kieber JJ. Cytokinin induces cell division in the quiescent center of the Arabidopsis root apical meristem. Curr Biol. 2013;23:1979–1989. doi: 10.1016/j.cub.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Vilarrasa-Blasi J, et al. Regulation of plant stem cell quiescence by a brassinosteroid signaling module. Dev Cell. 2014;30:36–47. doi: 10.1016/j.devcel.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Heyman J, et al. ERF115 controls root quiescent center cell division and stem cell replenishment. Science. 2013;342:860–863. doi: 10.1126/science.1240667. [DOI] [PubMed] [Google Scholar]
- 8.Jiang K, Feldman LJ. Regulation of root apical meristem development. Annu Rev Cell Dev Biol. 2005;21:485–509. doi: 10.1146/annurev.cellbio.21.122303.114753. [DOI] [PubMed] [Google Scholar]
- 9.Vanneste S, Friml J. Auxin: A trigger for change in plant development. Cell. 2009;136:1005–1016. doi: 10.1016/j.cell.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Aida M, et al. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell. 2004;119:109–120. doi: 10.1016/j.cell.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Galinha C, et al. PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature. 2007;449:1053–1057. doi: 10.1038/nature06206. [DOI] [PubMed] [Google Scholar]
- 12.Mähönen AP, et al. PLETHORA gradient formation mechanism separates auxin responses. Nature. 2014;515:125–129. doi: 10.1038/nature13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Q, et al. The basic helix-loop-helix transcription factor MYC2 directly represses PLETHORA expression during jasmonate-mediated modulation of the root stem cell niche in Arabidopsis. Plant Cell. 2011;23:3335–3352. doi: 10.1105/tpc.111.089870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou W, et al. Arabidopsis tyrosylprotein sulfotransferase acts in the auxin/PLETHORA pathway in regulating postembryonic maintenance of the root stem cell niche. Plant Cell. 2010;22:3692–3709. doi: 10.1105/tpc.110.075721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen M, et al. RopGEF7 regulates PLETHORA-dependent maintenance of the root stem cell niche in Arabidopsis. Plant Cell. 2011;23:2880–2894. doi: 10.1105/tpc.111.085514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarkar AK, et al. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature. 2007;446:811–814. doi: 10.1038/nature05703. [DOI] [PubMed] [Google Scholar]
- 17.Stahl Y, Wink RH, Ingram GC, Simon R. A signaling module controlling the stem cell niche in Arabidopsis root meristems. Curr Biol. 2009;19:909–914. doi: 10.1016/j.cub.2009.03.060. [DOI] [PubMed] [Google Scholar]
- 18.Stahl Y, et al. Moderation of Arabidopsis root stemness by CLAVATA1 and ARABIDOPSIS CRINKLY4 receptor kinase complexes. Curr Biol. 2013;23:362–371. doi: 10.1016/j.cub.2013.01.045. [DOI] [PubMed] [Google Scholar]
- 19.Forzani C, et al. WOX5 suppresses CYCLIN D activity to establish quiescence at the center of the root stem cell niche. Curr Biol. 2014;24:1939–1944. doi: 10.1016/j.cub.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pi L, et al. Organizer-derived WOX5 signal maintains root columella stem cells through chromatin-mediated repression of CDF4 expression. Dev Cell. 2015;33:576–588. doi: 10.1016/j.devcel.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 21.Sabatini S, Heidstra R, Wildwater M, Scheres B. SCARECROW is involved in positioning the stem cell niche in the Arabidopsis root meristem. Genes Dev. 2003;17:354–358. doi: 10.1101/gad.252503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Jiao Y, Liu Z, Zhu YX. ROW1 maintains quiescent centre identity by confining WOX5 expression to specific cells. Nat Commun. 2015;6:6003. doi: 10.1038/ncomms7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee Y, Lee WS, Kim SH. Hormonal regulation of stem cell maintenance in roots. J Exp Bot. 2013;64:1153–1165. doi: 10.1093/jxb/ers331. [DOI] [PubMed] [Google Scholar]
- 24.Vatén A, et al. Callose biosynthesis regulates symplastic trafficking during root development. Dev Cell. 2011;21:1144–1155. doi: 10.1016/j.devcel.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Wu S, et al. Symplastic signaling instructs cell division, cell expansion, and cell polarity in the ground tissue of Arabidopsis thaliana roots. Proc Natl Acad Sci USA. 2016;113:11621–11626. doi: 10.1073/pnas.1610358113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakajima K, Sena G, Nawy T, Benfey PN. Intercellular movement of the putative transcription factor SHR in root patterning. Nature. 2001;413:307–311. doi: 10.1038/35095061. [DOI] [PubMed] [Google Scholar]
- 27.Ding Z, Friml J. Auxin regulates distal stem cell differentiation in Arabidopsis roots. Proc Natl Acad Sci USA. 2010;107:12046–12051. doi: 10.1073/pnas.1000672107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao CY, et al. Reporters for sensitive and quantitative measurement of auxin response. Nat Methods. 2015;12:207–210, 2 pages following 210. doi: 10.1038/nmeth.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian H, et al. WOX5-IAA17 feedback circuit-mediated cellular auxin response is crucial for the patterning of root stem cell niches in Arabidopsis. Mol Plant. 2014;7(2):277–289. doi: 10.1093/mp/sst118. [DOI] [PubMed] [Google Scholar]
- 30.Friml J, et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell. 2002;108:661–673. doi: 10.1016/s0092-8674(02)00656-6. [DOI] [PubMed] [Google Scholar]
- 31.Blilou I, et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- 32.Grieneisen VA, Xu J, Marée AF, Hogeweg P, Scheres B. Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature. 2007;449:1008–1013. doi: 10.1038/nature06215. [DOI] [PubMed] [Google Scholar]
- 33.Cheng Y, Dai X, Zhao Y. Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev. 2006;20:1790–1799. doi: 10.1101/gad.1415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinon V, Prasad K, Grigg SP, Sanchez-Perez GF, Scheres B. Local auxin biosynthesis regulation by PLETHORA transcription factors controls phyllotaxis in Arabidopsis. Proc Natl Acad Sci USA. 2013;110:1107–1112. doi: 10.1073/pnas.1213497110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Won C, et al. Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proc Natl Acad Sci USA. 2011;108:18518–18523. doi: 10.1073/pnas.1108436108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stepanova AN, et al. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell. 2008;133:177–191. doi: 10.1016/j.cell.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y, et al. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science. 2001;291:306–309. doi: 10.1126/science.291.5502.306. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y, et al. Trp-dependent auxin biosynthesis in Arabidopsis: Involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes Dev. 2002;16:3100–3112. doi: 10.1101/gad.1035402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ljung K, et al. Sites and regulation of auxin biosynthesis in Arabidopsis roots. Plant Cell. 2005;17:1090–1104. doi: 10.1105/tpc.104.029272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM. A Link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell. 2005;17:2230–2242. doi: 10.1105/tpc.105.033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mashiguchi K, et al. The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci USA. 2011;108:18512–18517. doi: 10.1073/pnas.1108434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geldner N, Friml J, Stierhof YD, Jürgens G, Palme K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature. 2001;413:425–428. doi: 10.1038/35096571. [DOI] [PubMed] [Google Scholar]
- 43.Siligato R, et al. MultiSite gateway-compatible cell type-specific gene-inducible system for plants. Plant Physiol. 2016;170:627–641. doi: 10.1104/pp.15.01246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka Y, Nakamura S, Kawamukai M, Koizumi N, Nakagawa T. Development of a series of gateway binary vectors possessing a tunicamycin resistance gene as a marker for the transformation of Arabidopsis thaliana. Biosci Biotechnol Biochem. 2011;75:804–807. doi: 10.1271/bbb.110063. [DOI] [PubMed] [Google Scholar]
- 45.Weijers D, et al. An Arabidopsis Minute-like phenotype caused by a semi-dominant mutation in a RIBOSOMAL PROTEIN S5 gene. Development. 2001;128:4289–4299. doi: 10.1242/dev.128.21.4289. [DOI] [PubMed] [Google Scholar]
- 46.Ingram P, Dettmer J, Helariutta Y, Malamy JE. Arabidopsis Lateral Root Development 3 is essential for early phloem development and function, and hence for normal root system development. Plant J. 2011;68:455–467. doi: 10.1111/j.1365-313X.2011.04700.x. [DOI] [PubMed] [Google Scholar]