Significance
Despite extraordinary advances in the treatment of HIV, the global pandemic has yet to be reversed. We developed a mathematical model for 127 countries to evaluate Joint United Nations Program on HIV/AIDS (UNAIDS) targets for expanding diagnosis and treatment of the infected, and partially efficacious HIV vaccination. Under the current levels of diagnosis and treatment, we estimated 49 million new HIV cases globally from 2015 to 2035. Achieving the ambitious UNAIDS target is predicted to avert 25 million of these new infections, with an additional 6.3 million averted by the 2020 introduction of a 50%-efficacy vaccine. Our study provides country-specific impacts of a partially effective HIV vaccine and demonstrates its importance to the elimination of HIV transmission globally.
Keywords: HIV, vaccination, mathematical model, effectiveness
Abstract
The HIV pandemic continues to impose enormous morbidity, mortality, and economic burdens across the globe. Simultaneously, innovations in antiretroviral therapy, diagnostic approaches, and vaccine development are providing novel tools for treatment-as-prevention and prophylaxis. We developed a mathematical model to evaluate the added benefit of an HIV vaccine in the context of goals to increase rates of diagnosis, treatment, and viral suppression in 127 countries. Under status quo interventions, we predict a median of 49 million [first and third quartiles 44M, 58M] incident cases globally from 2015 to 2035. Achieving the Joint United Nations Program on HIV/AIDS 95–95–95 target was estimated to avert 25 million [20M, 33M] of these new infections, and an additional 6.3 million [4.8M, 8.7M] reduction was projected with the 2020 introduction of a 50%-efficacy vaccine gradually scaled up to 70% coverage. This added benefit of prevention through vaccination motivates imminent and ongoing clinical trials of viable candidates to realize the goal of HIV control.
Despite decades of treatment innovations, HIV remains a significant cause of morbidity and mortality because of low rates of testing and variable linkage to care (1–3). In 2013, the Joint United Nations Program on HIV/AIDS (UNAIDS) established a three-part global target for 2020 (4): diagnosing 90% of all people living with HIV (PLHIV), providing antiretroviral therapy (ART) to 90% of those diagnosed, and achieving viral suppression from adherence to a responsive ART regimen in 90% of those treated (4). This 90–90–90 target corresponds to 81% of PLHIV on ART and 73% of PLHIV virally suppressed worldwide. UNAIDS then extended their goal (5) to 95–95–95 by 2030, compared with the overall global status quo of 53–75–77, amounting to viral suppression among only 31% of PLHIV (6). Underlying this global average is considerable variation among countries. For example, Botswana, currently at 22% prevalence, reports 80–97–90 (7), whereas neighboring South Africa has similar prevalence (19%) but reports 57–84–71 (8). Among developed countries, Australia achieves the greatest proportion of PLHIV virally suppressed, with rates of 90–99–93, whereas the United States ranks 50th with its attainment of only 87–52–82 (8).
The last few years have witnessed promising advances in the development of HIV vaccines and several candidates are under investigation. For example, the phase III RV144 trial in Thailand of an ALVAC-HIV/gp120 regimen demonstrated efficacies of 60.5% after 1 y and 31.2% after 3.5 y among participants aged 18–30 y, with vaccine doses administered at weeks 0, 4, 12, and 24 (9). Results of the HVTN 100 trial in South Africa, which used an updated ALVAC-HIV/gp120 regimen, showed a more robust immune response than RV144 in participants aged 18–40 (10). Given the safety and immunogenicity demonstrated by HVTN 100, the HVTN 702 study was initiated in November 2016 to determine vaccine efficacy in 14 sites across South Africa (11). The trial is enrolling 18 to 35 y olds with vaccination scheduled at months 0, 1, 3, 6, and 12 (12). Other advances in research on HIV vaccines have included candidates based on adenovirus vectors (13). Motivated by strong preclinical scientific underpinnings (14), three phase I/II trials have been initiated as part of the Ad26/MVA/trimer gp140 program and an efficacy study is planned for later in 2017 (15). In addition, investigation into the prophylactic potential of broadly acting neutralizing antibodies has provided preliminary evidence for enhanced viral clearance through immunotherapy (16). Likewise, at the frontier of vaccinology for myriad other diseases, including malaria, TB, hookworm, and dengue, is the development of partially efficacious vaccines (17–19). In cases where vaccines may not alone be sufficient for disease elimination, they may nonetheless be a component of disease control in combination with drug therapies. Here, we evaluate such vaccine-linked chemotherapy approaches (20) for HIV.
Quantifying the levels of new and existing interventions that are required to turn the tide on the HIV epidemic in each country is fundamental to national and international policies. To evaluate the impacts of achieving the UNAIDS targets and rolling out a partially efficacious vaccine, both individually and in combination, we developed a model of HIV progression, transmission, and intervention that we tailored to each of 127 countries that collectively harbor more than 99% of PLHIV worldwide (Methods and SI Appendix). Previous analyses that have considered the 90–90–90 targets in a total of 45 countries have found them likely to be effective (7, 21–23). Likewise, a hypothetical 70%-efficacy vaccine was predicted to be effective in the 24 low- and middle-income countries considered (24, 25). We further evaluated the 90–90–90 targets and HIV vaccination in 103 countries that have not been considered. Moreover, the recent addition of the 95–95–95 target for 2030 has yet to be evaluated beyond UNAIDS preliminary estimates (5). We fit our model to country-specific estimates of HIV incidence, prevalence, and progress toward the UNAIDS targets (SI Appendix), also taking into account the effects of treatment on both improving survival and reducing transmission. For each country, we then projected the epidemiological trajectories to 2035 of new infections, incidence, PLHIV, and HIV-related mortality under status quo interventions, compared with the UNAIDS targets. To quantify the potential effectiveness of HIV vaccination, we considered a base-case rollout of a 50%-efficacy vaccine initiated in 2020, with scale up of 25% coverage annually to a maximum of 70% coverage. Through scenario analysis, we also assessed alternative efficacies of 30% and 70%, maximum coverages of 50% and 90%, initiation in 2025, and more gradual scale up at 10% coverage annually. Our results demonstrate the substantial added benefit of vaccination in reversing the pandemic, irrespective of whether the highly optimistic UNAIDS targets are met.
Global Projections
Under status quo rates of diagnosis and treatment, our model projects a median of 49 million [first and third quartiles 44M, 58M] new infections globally (Figs. 1 and 2, and SI Appendix, Fig. S4). Our projections indicate that the 90–90–90 target could avert 22 million [17M, 29M] of these infections, whereas the 95–95–95 target could avert a further 3.3 million [2.8M, 3.8M] infections. By comparison, vaccination alone is predicted to avert 17 million [15M, 23M] infections beyond status quo interventions. Combining vaccination with the 95–95–95 target would avert 31 million [26M, 39M] infections.
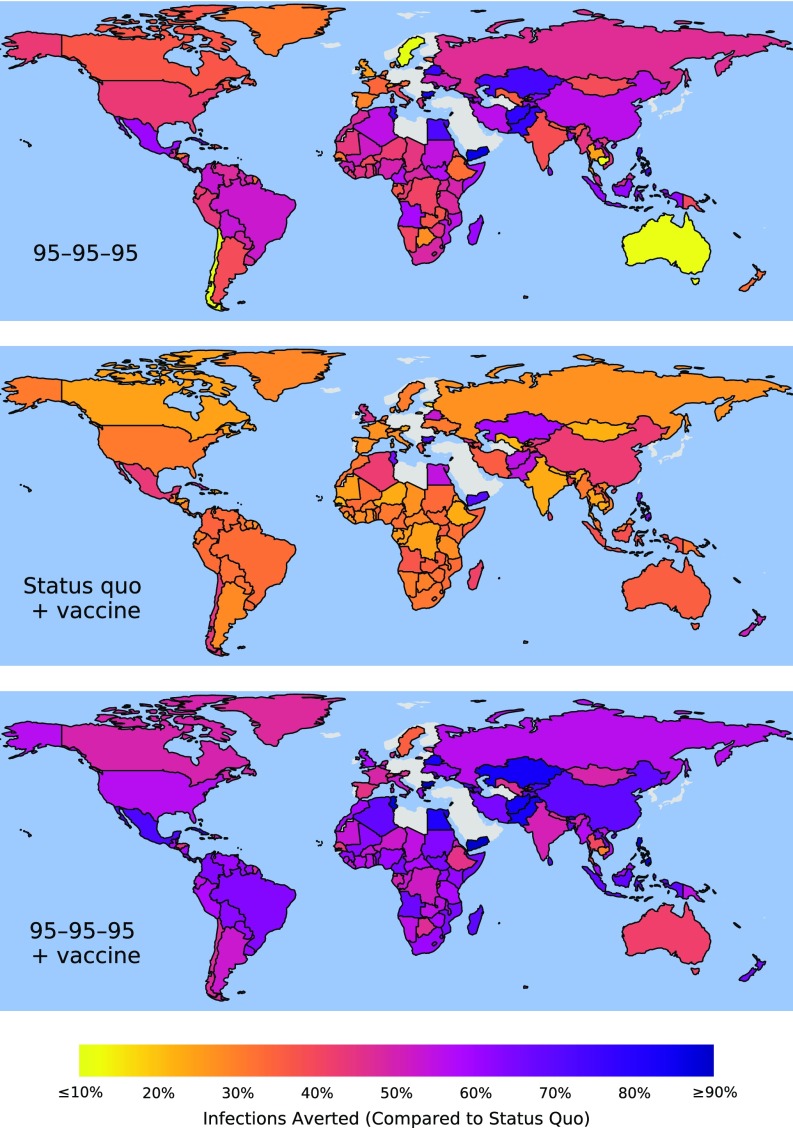
Fig. 1.
Infections averted between 2015 and 2035, compared with maintaining status quo diagnosis, treatment, and viral suppression, by the UNAIDS 95–95–95 target (Top); rollout of vaccination in 2020 while maintaining status quo levels of diagnosis, treatment, and viral suppression (Middle); and the UNAIDS 95–95–95 target combined with 2020 vaccination rollout (Bottom). Predictions are medians over 1,000 model simulations in which parameter values were sampled from data-driven distributions. See SI Appendix, Figs. S4–S140 for projections and uncertainty estimation globally, by region, and by country.
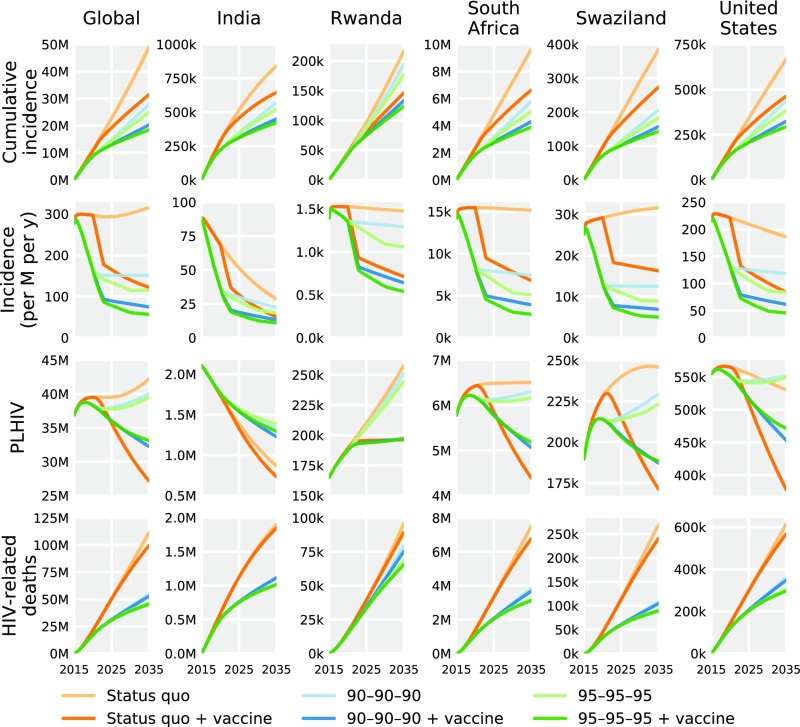
Fig. 2.
Effectiveness of HIV interventions globally (Left) and for selected countries (remaining columns) in terms of cumulative incidence (top row), annual incidence per million population (second row), PLHIV (third row), and cumulative HIV mortality (bottom row). Predictions are medians over 1,000 model simulations in which parameter values were sampled from data-driven distributions. See SI Appendix, Figs. S4–S140 for median and uncertainty estimates of effectiveness of HIV interventions for all 127 countries.
Regional Projections
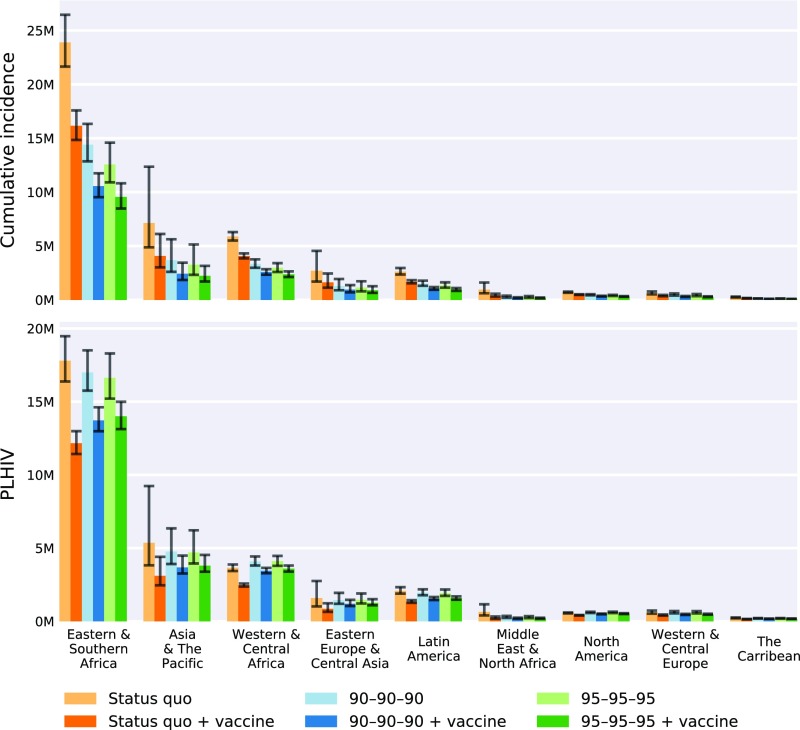
Underlying these global averages, there is regional variability that arises from country-specific transmission rates and prevalence. Both vaccination and UNAIDS targets averted the most infections in Eastern and Southern Africa (Fig. 3 and SI Appendix, Fig. S11). In this region, vaccination with status quo interventions averted 7.7 million [6.8M, 8.9M] infections, whereas vaccination combined with the 95–95–95 target averted 14.2 million [12.2M, 16.6M] infections. For the same strategy combinations in North America, 216,000 [197,000, 240,000] and 391,000 [345,000, 444,000] infections were averted (Fig. 3 and SI Appendix, Fig. S8), respectively, although this region is only 19% less populous than Eastern and Southern Africa. The UNAIDS targets based on antiviral treatment affect the number of PLHIV by prolonging survival (which increases PLHIV compared with status quo) and by reducing transmission (which decreases PLHIV). By contrast, vaccination averts new infections, reducing PLHIV without the concomitant improvement in survival. This transmission reduction achieved by vaccination is greater in African regions that have a high incidence, including Swaziland, Uganda, and South Africa, than in Asian countries with lower incidence, such as India (Fig. 2).
Fig. 3.
Effectiveness of HIV interventions for UNAIDS regions in terms of cumulative incidence from 2015 to 2035 (Upper) and number of PLHIV in 2035 (Lower). Medians (colored bars) and first and third quartiles (black error bars) were generated from 1,000 model simulations in which parameter values were sampled from data-driven distributions. See SI Appendix, Figs. S4–S140 for median and uncertainty estimates of the effectiveness of HIV interventions for UNAIDS regions.
Country-Level Projections
Viral suppression both increases the number of PLHIV by prolonging survival and reduces new PLHIV by lowering transmission. The balance between these factors, and thus the overall impact of UNAIDS targets on PLHIV varied (Fig. 2 and SI Appendix, Figs. S4–S140). In countries with increasing per-capita incidence, such as South Africa and Swaziland, the UNAIDS targets reduced PLHIV. For example, the projected number of PLHIV in Swaziland under the 90–90–90 target was 6% [-4%, 14%] lower, and under 95–95–95 was 8% [-3%, 18%] lower compared with projections under status quo interventions. Conversely, in countries where per-capita incidence is already declining, such as United States and India, the UNAIDS targets have a relatively greater benefit on improving survival than reducing transmission, thereby increasing PLHIV. In India, for instance, the predicted number of PLHIV was 54% [48%, 59%] greater under 90–90–90 and 60% [53%, 67%] greater under 95–95–95 compared with status quo projections. By contrast, vaccination universally reduces PLHIV (Fig. 2 and SI Appendix, Figs. S4–S140).
Even when status quo diagnosis, treatment, and viral suppression are not improved, vaccination was able to reverse the trend of increasing global incidence (Fig. 2 and SI Appendix, Fig. S4). Specifically, the rollout of a 50%-efficacy vaccine in 2020, with scale up at 25% coverage annually to a maximum of 70% coverage, was projected to reduce PLHIV by 36% [32%, 41%] and HIV-related mortality by 11% [9%, 13%]. Combining vaccination with the 95–95–95 target reduced PLHIV by 22% [12%, 34%] and HIV-related mortality by 59% [57%, 62%], compared with projections under status quo interventions. This synergy was predicted to be most substantial where UNAIDS targets are insufficient to reverse the epidemiological trajectory, such as in Rwanda. Nonetheless, even in the United States, where the 95–95–95 target reduced 2035 per-capita incidence by 55% [42%, 65%], the addition of a 50%-efficacy vaccine achieved a 76% [69%, 81%] reduction.
Uncertainty Analysis of Vaccine Characteristics
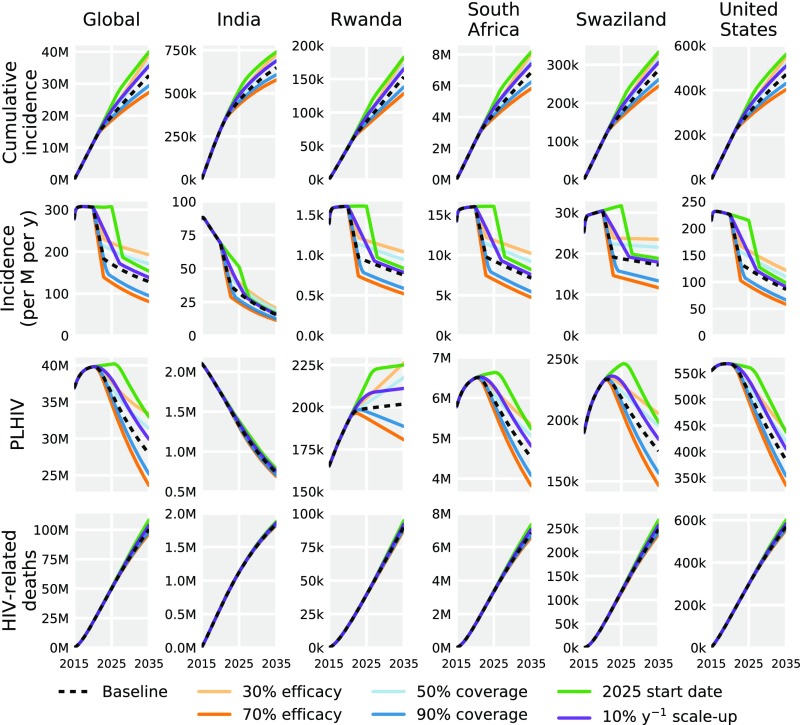
Given uncertainty of the prospective vaccine profile, we simulated model projections across a range of possible scenarios (Fig. 4). Initiating rollout in 2025 (compare 2020) reduced infections averted from 18.7 million to 11.3 million. A slower scale-up of 10% (compare 25%) annually averted a predicted 15.7 million infections, 3 million less than the base-case vaccination scenario. A lower coverage of 50% (compare 70%) averted 14.7 million infections, whereas a higher coverage of 90% averted 21.8 million infections. Considering alternative efficacies, infections averted ranged from 12.3 million with a conservative 30% efficacy to 24.0 million with an optimistic 70% efficacy, compared with 18.7 million with the base case of 50% efficacy.
Fig. 4.
Scenario analysis for vaccination under six strategies, globally (Left) and for selected countries (remaining columns), with variation in vaccine efficacy, ultimate coverage, initiation date of vaccination rollout, and rate of scale-up. Vaccine scenario projections are generated from simulations parametrized with modal values.
Sensitivity Analysis
Sensitivity analysis regarding the epidemiological, clinical, behavioral, and treatment parameters showed that predictions of vaccination effectiveness were more sensitive to the transmission rate, acute progression rate, and transmissibility within and after the acute phase than to the reduction in transmissibility during viral suppression (SI Appendix, Fig. S3). By contrast, the effectiveness of UNAIDS goals were more sensitive to transmission rate, relative transmissibility during viral suppression, and acute progression rate than to transmissibility within and after the acute phase. The projected effectiveness of both vaccination and UNAIDS goals were relatively insensitive to delay between ART initiation and viral suppression, annual number of coital acts, and improved survival due to viral suppression.
Discussion
Our results demonstrate the importance of evaluating country-level and global intervention effectiveness. For example, in Rwanda, where viral suppression is estimated at 74% of PLHIV, the 95–95–95 UNAIDS target would avert 19% [14%, 25%] of new infections, whereas vaccination alone would avert 33% [32%, 34%]. In South Africa, where rates of viral suppression are substantially lower at 34%, the UNAIDS 95–95–95 target would avert 49% [41%, 55%] of new infections, whereas vaccination alone would avert 31% [31%, 32%]. That is, vaccination was projected to have 1.7 [1.4, 2.4] times the impact of the 95–95–95 target in Rwanda and 0.6 [0.6, 0.8] times the impact in South Africa. Consequently, in Rwanda and other countries already achieving high levels of viral suppression, the benefit of further improving treatment is limited, whereas vaccination would be paramount for HIV elimination. Country-specific PLHIV trends also have implications for estimating the future morbidity and economic burdens of HIV in different regions. Projections of growing numbers of PLHIV underscore the urgency of expanding capacity to treat cardiovascular, cognitive, and liver complications associated with long-term ART use, particularly in resource-limited settings.
The feasibility of achieving these UNAIDS goals is challenged by interruptions across the HIV/AIDS treatment cascade (26, 27). For example, civil unrest in Afghanistan and Yemen, and draconian drug laws in Indonesia (28), have hampered diagnosis. Conversely, other countries, including Malaysia, the United States, and India, attain high diagnosis rates but struggle to engage people in treatment, because of inadequate infrastructure and coordinated health care or fear of stigma (29). Although the UNAIDS targets may be more aspirational than practical (30), obstacles to vaccination will likewise include its accessibility to at-risk populations, in addition to the immunological challenges of developing an efficacious and durable vaccine. Given the challenges inherent in treatment as prevention and in vaccination, a combined approach would be the most feasible and effective strategy to address the HIV pandemic in each of the 127 countries considered.
To facilitate model fitting to a variety of countries, for many of which only estimates of prevalence, incidence, as well as proportions diagnosed, treated, and with viral suppression were available, simplifying assumptions were made. Although we did not consider the vaccine waning exhibited by the RV144 candidate, the robust, but imperfect, vaccine-mediated immune protection that we modeled could correspond to a regime of boosters, as indeed has been expanded in the HVTN 100 candidate protocol (10). The vaccination and treatment-as-prevention approaches considered here would be complementary to other HIV prevention modalities, such as adult medical male circumcision (31), preexposure prophylaxis (PrEP; ref. 32) and antibody-mediated protection (33). The combination of these interventions is likely to have synergistic effects on mitigating HIV transmission. Our findings and modeling approaches may have conceptual applicability beyond HIV by showing the added benefits of partially protective vaccines for other diseases when implemented in conjunction with drug therapies. In general, rather than expecting vaccines with perfect efficacy to replace existing drug therapies, a multifaceted strategy that includes partially protective vaccines may nonetheless be necessary to achieve elimination, as we demonstrated for HIV.
While we explicitly model the clinical stages of acute, chronic without viral suppression, chronic with viral suppression, and AIDS, we did not incorporate explicit CD4 strata, both because population-level data on CD4 counts are not available for most countries and because CD4 levels are no longer pertinent as thresholds for treatment decisions (34, 35). Similarly, given the lack of estimates for most countries assessed regarding high-risk groups, such as sex workers, people who share needles, men who have sex with men, and prisoners, we did not incorporate risk-group strata. Nevertheless, our fitting of transmission rates to each country will subsume transmission within these risk groups. Targeting interventions to high-risk groups would likely achieve greater effectiveness for a lower overall population coverage. Therefore, the effectiveness of the measures considered here would be conservative, particularly for vaccination, relative to campaigns that integrated risk-group targeting of intervention. Despite the simplifications, our projections of new infections, PLHIV, and HIV-related deaths are consistent with other studies (22–24, 36, 37) of the 90–90–90 targets and vaccination based on more complex models with CD4, age, and risk strata for selected countries. Furthermore, model complexity limits the broad applicability of a model. Consequently, previous studies did not evaluate interventions in 103 of the 127 countries that we considered.
Recent results from the HTVN 100 vaccine trial have bolstered optimism for the development and deployment of an HIV vaccine in the near term. HIV vaccination would enable a strategic shift from reactive to proactive control, as suggested by our finding that an HIV vaccine with even moderate efficacy rolled out in 2020 could avert 17 million [15M, 23M] new infections by 2035 relative to expectations under status quo interventions. While overarching targets proposed by UNAIDS encourage global efforts to meet shared goals, it will become increasingly important to tailor control strategies at the country level. In many countries, including the United States, Uganda, and Nigeria, the UNAIDS goals, as ambitious as they are, would be insufficient to reverse the growth of PLHIV without at least a partially efficacious vaccine. Even in countries for which UNAIDS goals are sufficient to turn the tide on the HIV epidemic, such as India, Tanzania, and Ethiopia, vaccination would greatly accelerate elimination, saving millions of lives annually.
Methods
We developed a model of HIV transmission and progression that stratifies HIV infection into acute, chronic undiagnosed, chronic diagnosed, chronic treated, chronic virally suppressed, and AIDS (SI Appendix, Fig. S1). Viral suppression (defined as plasma HIV RNA below 1,000 copies per mL) not only extends survival but also reduces transmission, both aspects of which were incorporated into our model. Country-specific transmission rates were fitted to estimates of the historical trajectories of incidence and prevalence, spanning from as early as 1990 for some countries. Simulations of our model project the number of people in each HIV stratum from 2015 to 2035, from which statistics such as prevalence and incidence were calculated. For every scenario of intervention combinations, we conducted 1,000 model simulations, sampling values of model parameters from empirical distributions for each simulation, and summarized the results with median and percentiles. See SI Appendix, SI Methods and Datasets S1–S5 for a more detailed description of the model.
Supplementary Material
Acknowledgments
J.M. was supported by National Institutes of Health Grant U01 GM070694, A.P. was supported by a Brown Coxe Postdoctoral Fellowship in the Medical Sciences. A.S.P. and A.P.G. were supported by National Institutes of Health Grants U01 GM15627 and U01 GM087719, A.T. was supported by a Yale College Dean’s Research Fellowship, and L.A.S. was supported by National Institutes of Health Grant T32 AI007404.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 3798.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620788114/-/DCSupplemental.
References
- 1.Fauci AS, Folkers GK. Investing to meet the scientific challenges of HIV/AIDS. Health Aff (Millwood) 2009;28(6):1629–1641. doi: 10.1377/hlthaff.28.6.1629. [DOI] [PubMed] [Google Scholar]
- 2.Folkers GK, Fauci AS. Controlling and ultimately ending the HIV/AIDS pandemic: A feasible goal. JAMA. 2010;304(3):350–351. doi: 10.1001/jama.2010.957. [DOI] [PubMed] [Google Scholar]
- 3.Fauci AS, Marston HD. Focusing to achieve a world without AIDS. JAMA. 2015;313(4):357–358. doi: 10.1001/jama.2014.17454. [DOI] [PubMed] [Google Scholar]
- 4.UNAIDS 2014 90–90–90: An ambitious treatment target to help end the AIDS epidemic. Available at www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf. Accessed August 22, 2016.
- 5.UNAIDS 2014 Fast-track: Ending the AIDS epidemic by 2030. Available at www.unaids.org/sites/default/files/media_asset/JC2686_WAD2014report_en.pdf. Accessed August 16, 2016.
- 6.Levi J, et al. Can the UNAIDS 90–90–90 target be achieved? A systematic analysis of national HIV treatment cascades. BMJ Global Health. 2016;1(2):e000010. doi: 10.1136/bmjgh-2015-000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karim SA. Is the UNAIDS target sufficient for HIV control in Botswana? Lancet HIV. 2016;3(5):e195–e196. doi: 10.1016/S2352-3018(16)30008-X. [DOI] [PubMed] [Google Scholar]
- 8.UNAIDS 2014 The gap report. Available at www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf. Accessed August 22, 2016.
- 9.Rerks-Ngarm S, et al. MOPH-TAVEG Investigators Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 10.Gray GE, Laher F, Lazarus E, Ensoli B, Corey L. Approaches to preventative and therapeutic HIV vaccines. Curr Opin Virol. 2016;17:104–109. doi: 10.1016/j.coviro.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institute of Allergy and Infectious Diseases 2016 First new HIV vaccine efficacy study in seven years has begun. Available at https://www.niaid.nih.gov/news-events/first-new-hiv-vaccine-efficacy-study-seven-years-has-begun. Accessed January 19, 2017.
- 12.AVAC 2016 HVTN 702. Available at www.avac.org/trial/hvtn-702. Accessed January 31, 2017.
- 13.Baden LR, et al. B003-IPCAVD004-HVTN091 Study Group Assessment of the safety and immunogenicity of 2 novel vaccine platforms for HIV-1 prevention: A randomized trial. Ann Intern Med. 2016;164(5):313–322. doi: 10.7326/M15-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borducchi EN, et al. Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature. 2016;540(7632):284–287. doi: 10.1038/nature20583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AVAC 2016 HIV vaccine research: An update. Available at www.avac.org/sites/default/files/resource-files/HIV_VAX_research_update_sep_2016_web.pdf. Accessed January 30, 2017.
- 16.Fauci AS. An HIV vaccine: Mapping uncharted territory. JAMA. 2016;316(2):143–144. doi: 10.1001/jama.2016.7538. [DOI] [PubMed] [Google Scholar]
- 17.Evans TG, Schrager L, Thole J. Status of vaccine research and development of vaccines for tuberculosis. Vaccine. 2016;34(26):2911–2914. doi: 10.1016/j.vaccine.2016.02.079. [DOI] [PubMed] [Google Scholar]
- 18.RTS,S Clinical Trials Partnership Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386(9988):31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hotez PJ, et al. The human hookworm vaccine. Vaccine. 2013;31(Suppl 2):B227–B232. doi: 10.1016/j.vaccine.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotez P. Enlarging the “Audacious Goal”: Elimination of the world’s high prevalence neglected tropical diseases. Vaccine. 2011;29(Suppl 4):D104–D110. doi: 10.1016/j.vaccine.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 21.Stover J, et al. Fast Track modeling working group What is required to end the AIDS epidemic as a public health threat by 2030? The cost and impact of the Fast-Track approach. PLoS One. 2016;11(5):e0154893. doi: 10.1371/journal.pone.0154893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walensky RP, et al. The anticipated clinical and economic effects of 90–90–90 in South Africa. Ann Intern Med. 2016;165(5):325–333. doi: 10.7326/M16-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maddali MV, Gupta A, Shah M. Epidemiological impact of achieving UNAIDS 90-90-90 targets for HIV care in India: A modelling study. BMJ Open. 2016;6(7):e011914. doi: 10.1136/bmjopen-2016-011914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harmon TM, et al. Exploring the potential health impact and cost-effectiveness of AIDS vaccine within a comprehensive HIV/AIDS response in low- and middle-income countries. PLoS One. 2016;11(1):e0146387. doi: 10.1371/journal.pone.0146387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moodley N, Gray G, Bertram M. Projected economic evaluation of the national implementation of a hypothetical HIV vaccination program among adolescents in South Africa, 2012. BMC Public Health. 2016;16:330. doi: 10.1186/s12889-016-2959-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skarbinski J, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med. 2015;175(4):588–596. doi: 10.1001/jamainternmed.2014.8180. [DOI] [PubMed] [Google Scholar]
- 27.Cohen J. South Africa’s bid to end AIDS. Science. 2016;353(6294):18–21. doi: 10.1126/science.353.6294.18. [DOI] [PubMed] [Google Scholar]
- 28.Associated Press 2016 Indonesia’s president is defending his country’s use of the death penalty for drug offenses, arguing that drug abuse constitutes an emergency. Available at bigstory.ap.org/article/18994955e4074a3d949b7c4bf45392c7/indonesian-president-defends-death-penalty-drug-crimes. Accessed August 20, 2016.
- 29.Mahajan AP, et al. Stigma in the HIV/AIDS epidemic: A review of the literature and recommendations for the way forward. AIDS. 2008;22(Suppl 2):S67–S79. doi: 10.1097/01.aids.0000327438.13291.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson DP, Stoové MA, Hellard M. A reality check for aspirational targets to end HIV. Lancet HIV. 2015;2(1):e11. doi: 10.1016/S2352-3018(14)00038-1. [DOI] [PubMed] [Google Scholar]
- 31.Bailey RC, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: A randomised controlled trial. Lancet. 2007;369(9562):643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 32.Walensky RP, et al. The cost-effectiveness of pre-exposure prophylaxis for HIV infection in South African women. Clin Infect Dis. 2012;54(10):1504–1513. doi: 10.1093/cid/cis225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byrareddy SN, et al. Targeting α4β7 integrin reduces mucosal transmission of simian immunodeficiency virus and protects gut-associated lymphoid tissue from infection. Nat Med. 2014;20(12):1397–1400. doi: 10.1038/nm.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Senthilingam M. World Health Organization to recommend early treatment for everyone with HIV. Nature. July 20, 2015 doi: 10.1038/nature.2015.18017. [DOI] [Google Scholar]
- 35.Eholié SP, et al. Antiretroviral treatment regardless of CD4 count: The universal answer to a contextual question. AIDS Res Ther. 2016;13:27. doi: 10.1186/s12981-016-0111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korenromp EL, et al. Impact and cost of the HIV/AIDS national strategic plan for Mozambique, 2015–2019: Projections with the Spectrum/Goals model. PLoS One. 2015;10(11):e0142908. doi: 10.1371/journal.pone.0142908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stover J, et al. New Prevention Technology Study Group How can we get close to zero? The potential contribution of biomedical prevention and the investment framework towards an effective response to HIV. PLoS One. 2014;9(11):e111956. doi: 10.1371/journal.pone.0111956. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.