Abstract
Insulin resistance and type 2 diabetes mellitus are associated with impaired postprandial secretion of glucagon-like peptide-1 (GLP-1), a potent insulinotropic hormone. The direct effects of insulin and insulin resistance on the L cell are unknown. We therefore hypothesized that the L cell is responsive to insulin and that insulin resistance impairs GLP-1 secretion. The effects of insulin and insulin resistance were examined in well-characterized L cell models: murine GLUTag, human NCI-H716, and fetal rat intestinal cells. MKR mice, a model of chronic hyperinsulinemia, were used to assess the function of the L cell in vivo. In all cells, insulin activated the phosphatidylinositol 3 kinase-Akt and MAPK kinase (MEK)-ERK1/2 pathways and stimulated GLP-1 secretion by up to 275 ± 58%. Insulin resistance was induced by 24 h pretreatment with 10−7 m insulin, causing a marked reduction in activation of Akt and ERK1/2. Furthermore, both insulin-induced GLP-1 release and secretion in response to glucose-dependent insulinotropic peptide and phorbol-12-myristate-13-acetate were significantly attenuated. Whereas inhibition of phosphatidylinositol 3 kinase with LY294002 potentiated insulin-induced GLP-1 release, secretion was abrogated by inhibiting the MEK-ERK1/2 pathway with PD98059 or by overexpression of a kinase-dead MEK1-ERK2 fusion protein. Compared with controls, MKR mice were insulin resistant and displayed significantly higher fasting plasma insulin levels. Furthermore, they had significantly higher basal GLP-1 levels but displayed impaired GLP-1 secretion after an oral glucose challenge. These findings indicate that the intestinal L cell is responsive to insulin and that insulin resistance in vitro and in vivo is associated with impaired GLP-1 secretion.
Insulin is a novel secretagogue of the incretin hormone, glucagon-like peptide-1 (GLP-1), and L cell insulin resistance impairs heterologous secretagogue-induced GLP-1 secretion in vitro and in vivo.
Glucagon-like peptide (GLP)-1 is a 30-amino acid peptide secreted from intestinal epithelial L cells after a meal (1, 2). The main physiological role of this endocrine hormone is as an incretin, enhancing glucose-stimulated insulin secretion from the β-cell. GLP-1 demonstrates a number of additional antidiabetic effects including suppression of glucagon release, inhibition of food intake, and enhancement of growth and survival of β-cells (2, 3, 4). In light of these antidiabetic functions, GLP-1 mimetics and pharmacological approaches to increase circulating levels of endogenous GLP-1 via decreased degradation are currently being used in the treatment of type 2 diabetes. Moreover, it has also been suggested that stimulation of endogenous GLP-1 release, possibly in association with reduced GLP-1 degradation, may be feasible as an alternative approach to enhance GLP-1 action (1, 5).
In response to ingestion of a meal, nutrients, particularly glucose and monounsaturated fatty acids, are the primary stimulus of GLP-1 secretion from the intestinal L cell (1, 6, 7, 8, 9). L cells are mainly located in the distal ileum and colon (10); however, GLP-1 release occurs rapidly after a meal, with a first peak of secretion occurring at 15–30 min, followed by a later peak at 90–120 min (11). In rodents, the first phase of secretion is initiated by nutrients in the proximal intestine through a vagal-mediated pathway, whereas the second phase of secretion is believed to occur through direct interaction of the ingested nutrients with L cells in the distal gut (1, 12, 13, 14). Several circulating hormones and neuropeptides, including leptin, glucose-dependent insulinotropic peptide (GIP), and gastrin-releasing peptide, are also able to stimulate GLP-1 secretion in vitro and in vivo, thereby providing another level of control over the release of this intestinal hormone (8, 15, 16, 17).
Given the importance of GLP-1 actions to normal glucose homeostasis, reports of decreased GLP-1 levels in subjects with type 2 diabetes, independent of obesity, suggest a role for this peptide in the etiology and/or pathophysiology of this disease (18, 19, 20, 21, 22). As the rate of clearance of bioactive GLP-1 between normal subjects and those with type 2 diabetes is not different (20), these findings suggest a secretory defect in the L cell. Furthermore, Rask et al. (23, 24) demonstrated in a cohort of normal male subjects with varying degrees of insulin sensitivity that insulin resistance is negatively correlated with GLP-1 secretion. The notion that impaired insulin action reduces secretion by the endocrine intestine is not without precedence because release of the orexigenic hormone, ghrelin, is also decreased in nonobese subjects with insulin resistance (25). We therefore hypothesized that the intestinal L cell is sensitive to the effects of insulin, and that insulin resistance in the L cell directly impairs the secretion of GLP-1. These hypotheses were tested with the use of several well-characterized models of GLP-1 secretion; murine GLUTag cells, human NCI-H716 cells, and fetal rat intestinal cultures (1, 6, 9, 13, 14, 15). Additionally, nonobese, insulin-resistant MKR mice were used to examine the effects of chronic hyperinsulinemia on GLP-1 secretion from the enteroendocrine L cell in vivo (26, 27).
Materials and Methods
In vitro models
The mouse L cell model, GLUTag, was generated from a large bowel tumor in mice carrying a proglucagon/simian virus 40 large T-antigen transgene, whereas human NCI-H716 L cells were derived from a poorly differentiated adenocarcinoma of the cecum (9, 14, 15). Both cell models were grown in high glucose media, unless otherwise indicated. Fetal rat intestinal cell (FRIC) cultures are a heterogeneous primary L cell model, cultured from fetal intestines collected from term pregnant Wistar rats, as previously described in detail (6, 9, 13, 15). All cell models release GLP-1 appropriately in response to a variety of known secretagogues, making them ideal models to study GLP-1 secretion from the L cell (1). Furthermore, GLUTag cells also release cholecystokinin, whereas FRIC cultures have been shown to secrete peptide YY and somatostatin, albeit at levels insufficient to alter L cell secretion (28, 29, 30). For all in vitro studies, experiments were performed on different batches of cultures to ensure reproducibility of results. Insulin resistance was induced by a 24-h pretreatment with media containing 10−7 m insulin (Eli Lilly, Toronto, Ontario, Canada). After pretreatment, cells were washed for 3 × 40 min with media containing 1% BSA before addition of test agents for 2 h. Previous studies demonstrated that similar conditions are sufficient to decrease insulin action in adipocytes and myotubes (31, 32, 33, 34). This protocol did not alter total GLP-1 content in any cell model (data not shown).
To study GLP-1 secretion, cells were washed with Hanks’ balanced salt solution and treated with insulin, IGF-I (Long R3 IGF-1; Novozymes GroPep, Adelaide, Australia), GIP (10−6 m; Bachem Inc., Torrance, CA-positive control), or phorbol 12-myristate 13-acetate (PMA; 10−6 m, positive control; Sigma-Aldrich, St. Louis, MO), and treatments were prepared as previously described (9, 14, 15). Some cells were also pretreated with the pharmacological inhibitors, LY294002 (Sigma-Aldrich) or PD98059 (Calbiochem, La Jolla, CA; each at 50 μm) for 15 min. After the 2-h treatment, peptides in supernatants or cell extracts were collected by reversed-phase extraction, as previously described (9, 12, 13, 14, 15).
Total GLP-1 was assayed in cell and medium samples by RIA with a GLP-1 antiserum (Affinity Research Products, Nottingham, UK) that targets the carboxy terminus of GLP-17–36NH2, as previously described (9, 12, 13, 14, 15). Secretion was expressed as the total amount of GLP-1 in the medium, normalized to the total cell content of GLP-1 (media plus cells) and expressed as a percent of control. Basal media content and cell content in GLUTag cells were 28.3 ± 2.8 and 273.1 ± 76.9 pg/ml (n = 17), respectively. In NCI-H716 cells, basal media content and cell content were 40.5 ± 8.1 and 5201.7 ± 1274.3 pg/ml (n = 18), respectively, whereas in FRIC cultures, basal media content and cell content were 50.6 ± 9.8 and 394.3 ± 56.1 pg/ml (n = 8), respectively.
In vivo studies
MKR mice were generated by the expression of a dominant-negative IGF-I receptor in skeletal muscle, resulting in insulin resistance in not only skeletal muscle but also fat and liver (26, 27). In all experiments, age- and sex-matched FVB mice (Charles River, St. Laurent, Québec, Canada), were used as wild-type controls. All mice were housed four per cage with ad libitum access to food and water under a 12-h light, 12-h dark cycle. At 11 wk of age, insulin sensitivity was assessed by ip insulin tolerance test (1 U/kg) after a 3-h fasting period, and blood glucose concentrations were determined with a glucometer (OneTouch Ultra; LifeScan Canada, Burnaby, British Columbia, Canada). After 2 d of recovery, mice were fasted for 24 h and divided into two groups for the measurement of basal, fasting glucose, insulin, and GLP-1 levels at t = 0 and then 10 min after an oral glucose load. Pilot studies with 1.5 and 3.0 g/kg glucose loads did not elicit detectable responses in GLP-1 secretion; as such, 6 g/kg was used for all experiments. Mice from each group were anesthetized with isofluorane and immediately exsanguinated by cardiac puncture. Blood was collected into Trasylol, EDTA, and Diprotin-A, and plasma was collected and stored at −80 C (15). Plasma levels of insulin were measured by ELISA (Crystal Chem, Downers Grove, IL). Bioactive GLP-17-36NH2 was measured using an ELISA (Meso Scale Discovery, Gaithersburg, MD) that is specific to GLP-17-36NH2 and does not recognize the closely related GLP-17-37 or the degradation product GLP-19-36NH2. The detection limit of this assay was 2 pg/ml. Because no significant differences in any of these parameters were detected between male and female MKR or FVB mice, with the exception of slightly reduced basal blood glucose levels in FVB females, all data were combined for analysis. All animal procedures were approved by the Animal Care Committee of the University of Toronto.
Immunohistochemistry
Sections of mouse ileum or jejunum were fixed in 10% neutral buffered formalin, embedded in paraffin, and cut to 5 μm thickness. Mouse and 5-μm human jejunal sections (a gift from Dr. S. L. Asa, University of Toronto, Toronto, Ontario, Canada), and all cell models were subjected to immunohistochemistry or -cytochemistry, as previously described (9, 14, 15, 35). In brief, tissues and cells were incubated overnight at 4 C with primary antibodies against GLP-1 (mouse antihuman, a gift from Dr. D. A. D’Alessio, University of Cincinnati, Cincinnati, OH), the α- or β-subunits of the insulin receptor (rabbit antimouse; Santa Cruz Biotechnology, Santa Cruz, CA), or phosphatidylinositol-(3,4,5)-phosphate (PIP3; mouse anti-PIP3; Echelon Biosciences, Salt Lake City, UT). The absence of primary antisera was used as a negative control. Furthermore, mouse and human intestinal sections were examined in the presence of only the secondary fluorescent-labeled secondary antibodies for the insulin receptor and GLP-1 to examine the contributions of each secondary to nonspecific staining. To further test the specificity of the insulin receptor antibodies, pancreatic sections from wild-type and β-cell-specific insulin receptor knockout mice (a gift from Dr. R. N. Kulkarni, Joslin Diabetes Center, Boston, MA) were also stained for the insulin receptor using the insulin receptor α-subunit antibody. Images were visualized with a Carl Zeiss Axioplan deconvolution microscope (Carl Zeiss Canada, Ltd., Don Mills, Ontario, Canada).
RNA isolation and semiquantitative RT-PCR
Total RNA extracted from white adipose tissue of CD1 mice and GLUTag and NCI-H716 cells (RNeasy kit; QIAGEN Inc., Mississauga, Ontario, Canada) along with human jejunal (Ambion, Austin, TX), and human placental RNA (a gift from Dr. J. R. G. Challis, University of Toronto, Ontario, Canada) were reverse transcribed and amplified with a OneStep RT-PCR kit (QIAGEN). As a negative control, template RNA was replaced with ribonuclease-free water. Identification of mouse and human insulin receptor transcripts was based on primers and conditions reported to generate PCR products of 500 or 600 (isoform A)/636 (isoform B) bp, respectively (36, 37). Primers used to amplify IGF-I receptor mRNA transcripts in mouse and human RNA samples generated PCR products of 71 and 300 bp, respectively (38, 39).
Construction of adenoviruses and adenoviral infection
Recombinant adenoviruses expressing green fluorescent protein (GFP) or a dominant-negative form of ERK-2 (Ad-MEK1-ERK2 KR) (40) were generated and purified as previously described (41, 42). Adenoviral titers were determined by infecting GLUTag cells (∼70% confluency in six-well plates) with Adv-GFP or Adv-MEK1-ERK2 KR (0.5–5.0 × 109 PFU/ml) for 2 h. After viral infection, media were replaced with complete growth medium, and cells were incubated for a further 48 h before extraction for Western blot of phospho- and total ERK1/2. For secretion experiments, cells were infected, as above, with 5.0 × 109 PFU/ml of the constructs, followed by treatment with GLP-1 secretagogues, as above.
Cell lysis, immunoprecipitation, and immunoblotting
After treatment with insulin, IGF-I, pharmacological inhibitors, or adenovirus constructs, cell lysates were subjected to insulin receptor immunoprecipitation and/or Western blot analysis, as previously described (9, 14, 15, 36). Proteins of interest were detected with antibodies targeted against phosphotyrosine (clone 4G10, 1:1000; Upstate, Lake Placid, NY); insulin receptor (β-subunit, 1:400; Calbiochem); phospho-Akt (Ser473), Akt, phospho-ERK1/2 (Thr202/Tyr204), ERK1/2 (1:1000; Cell Signaling, Danvers, MA); and actin (1:5000; Sigma-Aldrich).
Statistical analysis
All data are expressed as mean ± sem. In some experiments, data were log10 transformed to normalize variance for statistical analysis. Data were analyzed by Student’s t test or one- or two-factor ANOVA, followed by appropriate post hoc testing (SAS version 9.1.3, Cary, NC). Factors for ANOVA analysis consisted of the effects of doses of insulin or IGF-I for one-way analyses and the effects of glucose, insulin, 24 h insulin pretreatment, LY294002, PD98059, adenovirus infections, or genotype for two-way analyses. Statistical significance was assumed at P < 0.05.
Results
Expression of the insulin receptor in L cells
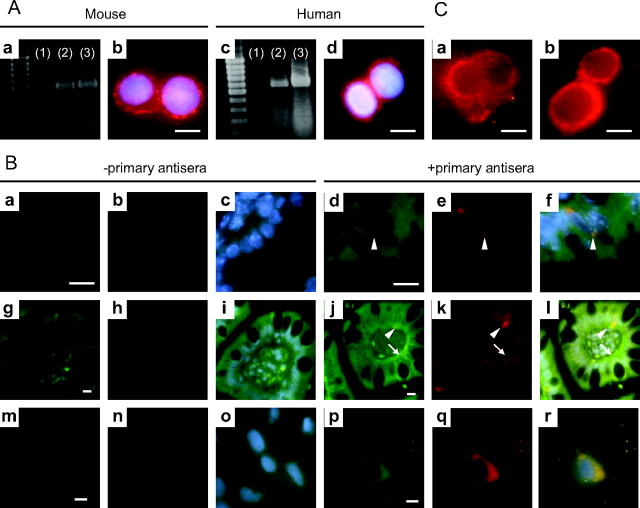
Both mRNA transcripts and protein for the insulin receptor were detected in GLUTag and NCI-H716 cells, as determined by semiquantitative RT-PCR and immunocytochemistry (Fig. 1A). To establish whether primary L cells also express the insulin receptor, double-immunofluorescence staining for the insulin receptor and GLP-1 was performed on sections of mouse ileum and human jejunum and on FRIC cultures. Positive immunoreactivity for the insulin receptor α-subunit was observed on all cells along the cypt-villus axis in the mouse and human intestinal epithelium (Fig. 1B, d and j); moreover, positive staining for the insulin receptor was also observed along the basolateral membrane in human jejunal sections (Fig. 1Bj and Supplemental Fig. 1A, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org) but not in β-cells lacking the insulin receptor (β-cell specific insulin receptor knockout mice, negative control, Supplemental Fig. 2). The expression of the insulin receptor throughout the intestine is not surprising because insulin has been found to regulate lipoprotein synthesis from enterocytes (43). Furthermore, L cells that stained positive for GLP-1 were also found to express insulin receptor α-subunit protein (Fig. 1B). When an antiserum against the insulin receptor β-subunit was used, a similar colocalization of the insulin receptor protein and GLP-1 was found (Supplemental Fig. 1B).
Fig. 1.
Expression of the insulin receptor and activation of PI3 kinase in L cells. A, Semiquantitative RT-PCR analysis for insulin receptor mRNA transcripts in GLUTag (a: lane 2) and NCI-H716 cells (c: lane 2). Mouse fat (a: lane 3) and human placenta (c: lane 3) were used as positive controls, respectively, whereas the omission of template RNA was used as negative controls (a: lane 1, c: lane 1). Murine GLUTag (b) and human NCI-H716 (d) cells were subjected to immunofluorescent staining for the insulin receptor β-subunit (red); 4′,6′-diamino-2-phenylindole (DAPI; blue) was used to visualize nuclei. B, Mouse (a–f) and human (g–l) intestinal sections and FRIC cultures (m–r) were subjected to double-immunofluorescent staining for the insulin receptor α-subunit (green) and GLP-1 (red); DAPI (blue) was used to visualize nuclei. The absence of primary antisera was used as negative controls (mouse: a–c; human: g–i; FRIC: m–o). Arrowheads indicate L cells, and arrows indicate basolateral staining for the insulin receptor α-subunit. Colocalization of the insulin receptor and GLP-1is indicated by yellow in the overlay (f, l, and r). C, Immunofluorescent detection of PIP3 production (red) in response to treatment of GLUTag cells with media alone (a) or 10−7 m insulin (b) for 5 min. Identical camera exposure times were used. Scale bars, 10 μm.
Insulin signaling and actions on the mouse L cell
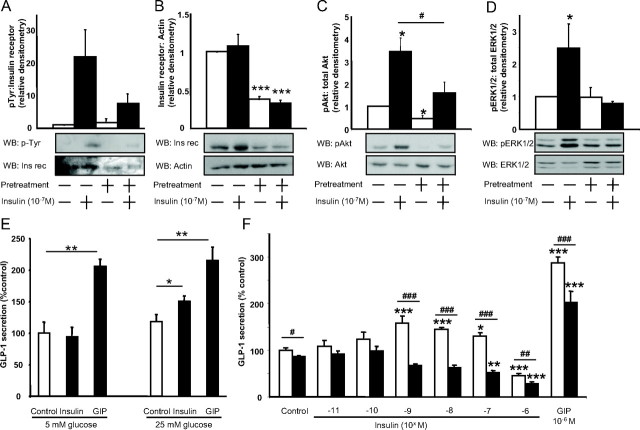
Treatment of GLUTag cells with 10−7 m insulin for 5 min induced a 21.9-fold increase in insulin receptor phosphorylation (Fig. 2A) in addition to a robust increase in cytosolic PIP3, demonstrating phosphatidylinositol 3 (PI3) kinase activation (Fig. 1C). No change in total insulin receptor protein was observed (Fig. 2B); however, Akt and ERK1/2 phosphorylation were increased by 3.4- and 2.5-fold (P < 0.05), as determined by Western blot analysis (Fig. 2, C and D). In preliminary studies (Fig. 2E), it was observed that GLUTag cells required high-glucose (25 mm) conditions to demonstrate insulin-induced GLP-1 secretion (overall two factor ANOVA: P < 0.001; F value: 13.01), and all subsequent experiments with these cells were therefore performed under these conditions. In response to an acute treatment of GLUTag cells with 10−11 to 10−6 m insulin, a hyperbolic pattern of GLP-1 secretion was observed, such that 10−9 to 10−7 m insulin significantly stimulated GLP-1 release, to 144.5 ± 9.1% of control (P < 0.05), whereas10−6 m insulin significantly inhibited GLP-1 secretion, to 45.6 ± 3.1% of control (P < 0.001) (overall one factor ANOVA: P < 0.0001; F value: 19.24) (Fig. 2F). GIP, used as a positive control for the rodent L cell (1), increased GLP-1 secretion to 286.5 ± 13.8% (P < 0.001). However, no additive effects of insulin and GIP on GLP-1 release were detected (data not shown). Because these findings suggested that desensitization of the insulin receptor could modulate GLP-1 release, we next determined the effects of insulin resistance on the L cell.
Fig. 2.
Effects of insulin and insulin resistance on murine GLUTag cells. Cells were exposed for 24 h to either media alone or high insulin to induce insulin resistance and, after three 40-min washes with serum-free media containing 1% BSA, were treated acutely with 10−7 m insulin for 5 min for Western blot analysis (WB) or with the indicated concentrations of insulin or GIP for 2 h to measure GLP-1 secretion by RIA. A–D, Crude protein lysates or immunoprecipitated protein extracts were subjected to immunoblot analysis for insulin receptor (Ins rec) phosphorylation (n = 3/group) (A), total insulin receptor expression (n = 4/group) (B), Akt phosphorylation (n = 4/group) (C), or ERK1/2 phosphorylation (n = 7/group) (D). Representative blots are shown, and all values were expressed relative to the untreated control. *, P < 0.05, **, P < 0.01, ***, P < 0.001 when compared with untreated control; #, P < 0.05 as indicated. E, GLP-1 secretion in response to medium alone (control), insulin (10−8 m), and GIP (10−6 m) in the presence of 5 or 25 mm glucose. GLP-1 secretion was determined by RIA, and all data were expressed as a percent of the untreated control (n = 3–4/group. *, P < 0.05; **, P < 0.01. F, GLP-1 secretion in response to medium alone (control), insulin, or GIP from normal (open bars) or insulin-resistant (closed bars) GLUTag cells. GLP-1 secretion was determined by RIA, and all data were expressed as a percent of the untreated control (n = 5–6/group). *, P < 0.05; **, P < 0.01; ***, P < 0.001 when compared with untreated control; #, P < 0.05; ##, P < 0.01; ###, P < 0.001 as indicated. p, Phospho.
Insulin resistance was induced in GLUTag (IR-GLUTag) cells by 24 h pretreatment with media containing 10−7 m insulin. Insulin-induced insulin receptor phosphorylation in IR-GLUTag cells was decreased from 21.9- to 7.5-fold as a result of the pretreatment, and total insulin receptor protein levels were decreased by 64.5 ± 4.0% (P < 0.001) (Fig. 2, A and B). Moreover, in IR-GLUTag cells, insulin-induced Akt phosphorylation was decreased to 1.6-fold (P < 0.05) and insulin-induced ERK1/2 phosphorylation was completely abolished (Fig. 2, C and D). A small but significant 13.4 ± 2.6% decrease in basal GLP-1 release was observed in IR-GLUTag cells (P < 0.05), whereas the stimulatory effect of insulin on GLP-1 secretion was completely abrogated (overall two factor ANOVA: P < 0.0001; F value: 21.34). Unexpectedly, insulin resistance also caused a significant decrease in GIP-mediated GLP-1 release compared with normal GLUTag cells (from 286.9 ± 13.8 to 202.5 ± 24.3%, P < 0.001) (Fig. 2F).
Insulin and the human L cell
To confirm the findings in the murine GLUTag L cell model, human NCI-H716 cells were similarly treated with 10−7 m insulin, resulting in 7.6- and 3.0-fold increases in Akt and ERK1/2 phosphorylation, respectively (P < 0.05–0.01) (Fig. 3, A and B). Increased GLP-1 secretion was also observed after acute treatment with insulin, whereby concentrations greater than 10−8 m insulin significantly stimulated GLP-1 release by up to 275.2 ± 57.6% of control (P < 0.05) (overall one factor ANOVA: effect: P = 0.003; F value: 4.84) (Fig. 3C). As found in IR-GLUTag cells, IR-NCI-H716 cells displayed a marked impairment in activation of downstream insulin signaling pathways due to 24 h pretreatment with 10−7 m insulin, such that insulin-induced Akt and ERK1/2 phosphorylation was reduced to 3.0- and 1.7-fold (P < 0.05), respectively. These defects were accompanied by an inability of insulin to stimulate GLP-1 secretion (overall two factor ANOVA: P < 0.0001; F value: 5.43) (Fig. 3, A–C). Although GIP does not enhance GLP-1 secretion from the human L cell (1), heterologous desensitization was observed in IR-NCI-H716 cells in response to the known secretagogue PMA (1, 9), decreasing GLP-1 secretion from 409.3 ± 68.2 to 189.4 ± 15.4% of controls (P < 0.001).
Fig. 3.
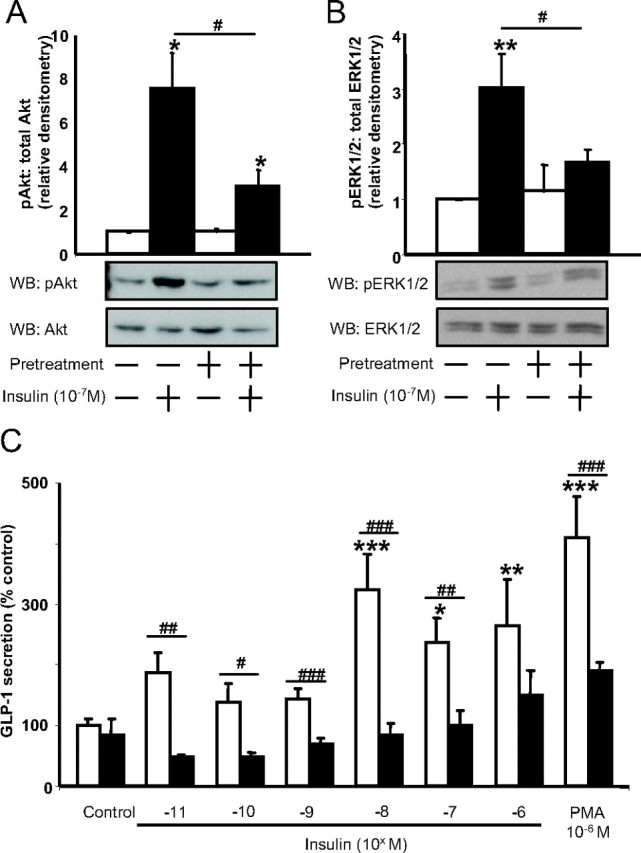
Effect of insulin and insulin resistance on human NCI-H716 cells. Cells were exposed for 24 h to either media alone or high insulin to induce insulin resistance and, after three 40-min washes with serum-free media containing 1% BSA, were treated acutely with 10−7 m insulin for 5 min for Western blot analysis (WB) or with graded concentrations of insulin or PMA for 2 h to measure GLP-1 secretion. A and B, Crude protein lysates were subjected to immunoblot analysis for Akt phosphorylation (n = 4/group) (A) or ERK1/2 phosphorylation (n = 4/group) (B). All values were expressed relative to the untreated control, and representative blots are shown. *, P < 0.05, **, P < 0.01 when compared with untreated control; #, P < 0.05 as indicated. C, GLP-1 secretion in response to medium alone (control), graded concentrations of insulin, or PMA from normal (open bars) or insulin-resistant (closed bars) NCI-H716 cells. GLP-1 secretion was determined by RIA, and all data were expressed as a percent of the untreated control (n = 5–6/group). *, P < 0.05, **, P < 0.01, ***, P < 0.001 when compared with untreated control; #, P < 0.05, ##, P < 0.01, ###, P < 0.001 as indicated. p, Phospho.
Activation of the IGF-I receptor does not stimulate GLP-1 secretion
Because it has been reported that nonphysiological concentrations of insulin may cause activation of the IGF-I receptor, and that IGF-I can exert similar effects to insulin (44, 45, 46), the contribution of IGF-I receptor activation was next examined. Expression of IGF-I receptor mRNA transcripts was detected in GLUTag and NCI-H716 cells, as determined by RT-PCR (Fig. 4A). Unexpectedly, a higher molecular weight band was also amplified from NCI-H716 mRNA, which was not seen in control human jejunal RNA. However, IGF-I had no effect on GLP-1 release from the GLUTag cells and exhibited variable but inhibitory effects on GLP-1 release at 10−10 to 10−8 m (P < 0.05) in NCI-H716 cells (overall one factor ANOVA: P = 0.028; F value: 2.750) (Fig. 4, B and C). These findings suggest that the stimulatory effects of insulin on the L cell are not mediated via the IGF-I receptor.
Fig. 4.
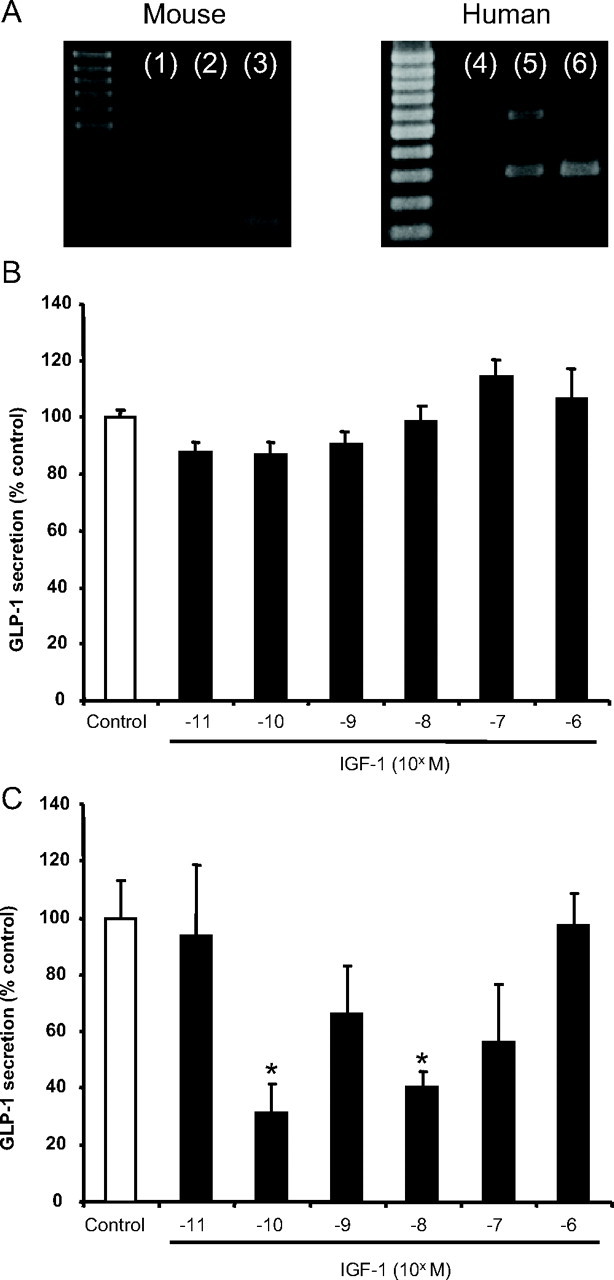
Contribution of IGF-I receptor activation to GLP-1 secretion. A, Semiquantitative RT-PCR was performed on isolated RNA from GLUTag (lane 2) and NCI-H716 (lane 5) cells for IGF-I receptor mRNA transcripts. Total RNA from mouse fat (lane 3) or human jejunal tissue (lane 6) was used as a positive control, and the omission of template RNA was used as a negative control (lanes 1 and 4). B and C, The effect of IGF-I receptor activation on GLP-1 release was analyzed by treating GLUTag (n = 6/group) (B) and NCI-H716 (n = 4–6/group) (C) cells with graded concentrations of human Long-Arg3-IGF-I for 2 h. GLP-1 secretion was determined by RIA, and all data are expressed as a percent of the untreated control. *, P < 0.05.
Mechanism of insulin-induced GLP-1 secretion
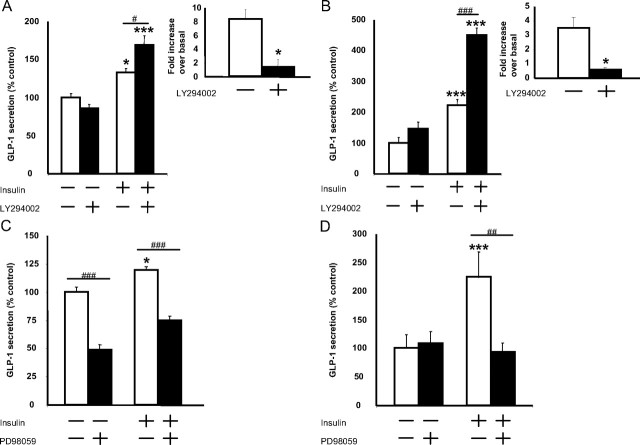
To determine the mechanism of action of insulin in stimulating GLP-1 release from the L cell, PI3 kinase-Akt and MAPK kinase (MEK)-ERK1/2 signaling were inhibited with LY294002 and PD98059, respectively. Inhibition of PI3 kinase did not prevent insulin-induced GLP-1 secretion but unexpectedly potentiated the response to insulin in both GLUTag and NCI-H716 cells (P < 0.05–0.001) (overall two factor ANOVA and F values for GLUTag and NCI-H716 cells: P < 0.0001 and 21.10; and P < 0.0001 and 56.42, respectively) (Fig. 5, A and B). The PI3 kinase-inhibitory activity of the LY294002 was confirmed in independent experiments by demonstrating its ability to decrease insulin-stimulated Akt phosphorylation in both cell lines (P < 0.05). In contrast, basal GLP-1 secretion from GLUTag cells was attenuated by 59.9 ± 1.2% in the presence of PD98059 (P < 0.001), whereas insulin-induced GLP-1 release was decreased slightly in the GLUTag cells (P < 0.001) (overall two factor ANOVA: P < 0.0001; F value: 24.27). Furthermore, insulin-stimulated GLP-1 release was completely abrogated by PD98059 pretreatment of NCI-H716 cells (P < 0.01) (overall two factor ANOVA: P = 0.0004; F value: 9.93) (Fig. 5, C and D). Together, these findings suggest a role for the MEK-ERK1/2 pathway in insulin-induced GLP-1 release from the L cell.
Fig. 5.
Effect of PI3 kinase-Akt and MEK-ERK1/2 inhibition on insulin-mediated GLP-1 secretion. GLUTag (A and C) and NCI-H716 (B and D) cells were pretreated with 50 μm LY294002 (A, n = 4–5/group; B, n = 6/group) or 50 μm PD98059 (C, n = 6–9/group; D, n = 5–6/group) for 15 min and subsequently treated with 10−8 or 10 −7 m insulin, respectively, for 2 h. GLP-1 secretion was determined by RIA, and all data were expressed as a percent of the untreated control. Insets, PI3 kinase inhibitory activity of the LY294002 was confirmed by immunoblot analysis of Akt phosphorylation in response to insulin in both cell models (n = 4/group). *, P < 0.05, ***, P < 0.001 when compared with untreated control; #, P < 0.05, ##, P < 0.01, ###, P < 0.001 as indicated.
To confirm the results of the PD98059 studies, we also examined the effect of overexpressing a kinase-dead MEK1-ERK2 fusion protein on GLP-1 release (40). GLUTag cells were infected with AdvMEK1-ERK2 KR or the control adenovirus, Adv-GFP (0.5–5.0 × 109 PFU/ml) to determine optimal viral titers, and cell lysates were immunoblotted for ERK1/2 to detect expression of the fusion protein (∼80 kDa) (Fig. 6A). As previously reported (40), expression of the MEK1-ERK2 KR fusion protein did not alter endogenous ERK1/2 phosphorylation or total ERK1/2 expression, nor did overexpression of GFP (Fig. 6A). GLUTag cells infected with Adv-GFP displayed a normal, 1.6-fold (P < 0.05) increase in insulin-stimulated GLP-1 secretion. Overexpression of MEK1-ERK2 KR resulted in a slight but nonsignificant increase in basal GLP-1 secretion (P = 0.19); however, insulin-induced GLP-1 release was completely abrogated (overall two factor ANOVA: P = 0.033; F value: 2.82) (Fig. 6B). Cells that overexpressed GFP or MEK1-ERK2 KR were also treated with 10−6 m PMA, as a positive control (9) and demonstrated no differences in their GLP-1 secretory responses (up to 1.6-fold, P < 0.05). Taken together these findings highlight the importance of the MEK-ERK1/2 pathway in insulin-stimulated GLP-1 secretion.
Fig. 6.
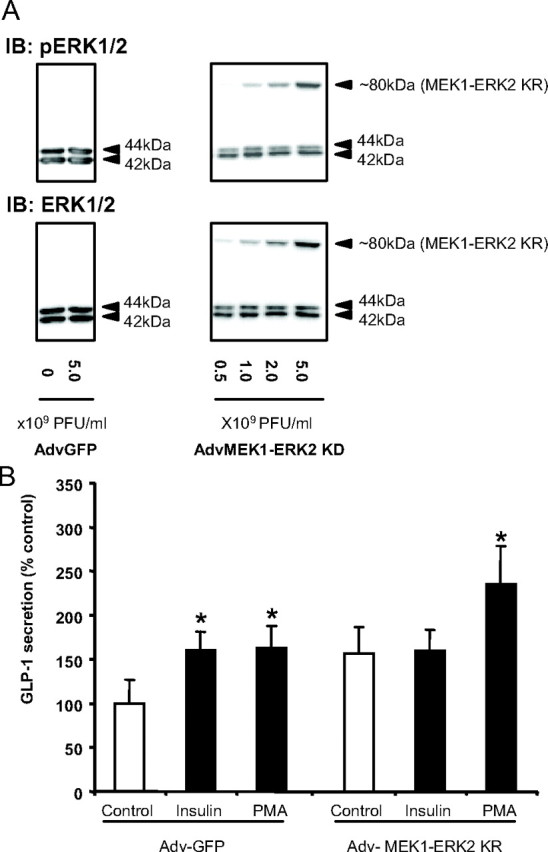
Effect of overexpression of kinase-dead MEK1-ERK2 on GLP-1 secretion. A, GLUTag cells were infected with different titers of Adv-GFP or Adv-MEK1-ERK2 KR, and expression of the fusion protein (∼80 kDa) was detected by immunoblotting (IB) for phospho- and total ERK1/2 (representative of n = 4/group). B, GLUTag cells infected with 5 × 109 plaque-forming units (PFU)/ml Adv-GFP or Adv-MEK1-ERK2 KR were treated with media alone (control), insulin (10−8 m), and PMA (10−6 m) for 2 h, and GLP-1 secretion was measured by RIA. All data were expressed as a percent of the untreated Adv-GFP control (n = 5–6/group). *, P < 0.05 when compared with the untreated control. p, Phospho.
Effect of insulin on the primary rodent L cell
To further confirm the effects of insulin and insulin resistance on the L cell, we examined insulin-induced GLP-1 secretion in primary FRIC cultures and MKR mice. Similar to the findings with both GLUTag and NCI-H716 cells, insulin treatment increased Akt phosphorylation by 2.8-fold and ERK1/2 phosphorylation by 3.3-fold (P < 0.05) in FRIC cultures (Fig. 7, A and B). However, insulin significantly increased GLP-1 secretion only at 10−6 m, by 50.6 ± 18.6% over control (P < 0.05) (overall one-factor ANOVA: P = 0.002; F value: 6.55) (Fig. 7C). Furthermore, although insulin receptors were detected on the fetal rat L cells (Fig. 1B, m–r), no staining of the insulin receptor could be detected on the adult rat L cell (data not shown), suggesting that the rat may not be a good model to study insulin-induced GLP-1 secretion.
Fig. 7.
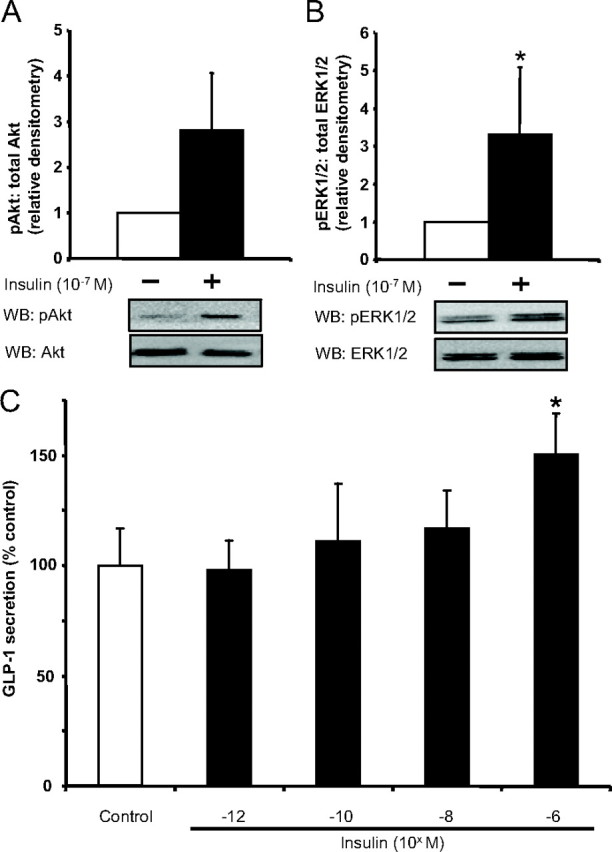
Effects of insulin on FRIC cultures. A and B, FRIC cultures were treated with 10−7 m insulin for 5 min, and cell lysates were collected for analysis of Akt (n = 4/group) (A) and ERK1/2 (n = 4/group) (B) phosphorylation by Western blot analysis (WB). *, P < 0.05. All data were expressed as a fold over control, and representative blots are shown. C, Separate FRIC cultures were treated for 2 h with different concentrations of insulin, and GLP-1 secretion was determined by RIA (n = 8/group). *, P < 0.05 when compared with the untreated control. p, Phospho.
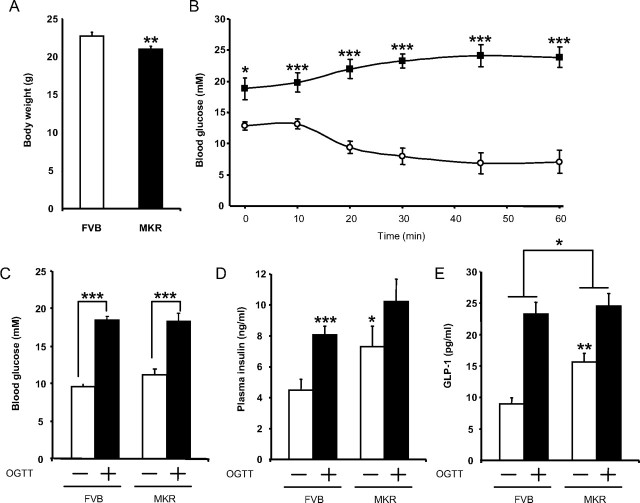
Therefore, to directly assess the effects of insulin resistance on GLP-1 secretion in vivo, MKR mice were used as a model. These mice are insulin resistant due to overexpression of a dominant-negative human IGF-I receptor in skeletal muscle and, as a consequence, develop chronic hyperinsulinemia in addition to insulin resistance in liver and fat (26, 27). MKR mice were slightly lighter than their wild-type counterparts (20.9 vs. 22.7 g, P < 0.01) (Fig. 8A). However, an insulin tolerance test revealed a profound metabolic difference between the two strains, such that after a 3-h fast, basal blood glucose levels were 1.5-fold higher than in wild-type controls (P < 0.05) and were not suppressed over the 60-min period after ip administration of insulin (P < 0.001) (Fig. 8B).
Fig. 8.
GLP-1 secretion in MKR mice, a nonobese model of chronic hyperinsulinemia. A, Age- and sex-matched MKR mice and wild-type FVB mice were analyzed for body weights at 11 wk of age (n = 31–36/group). **, P < 0.01. B, After a 3-h fast, insulin sensitivity of MKR (black squares, n = 9) and FVB (white circles, n = 14) mice were assessed by ip insulin tolerance test (n = 9–14). *, P < 0.05; ***, P < 0.001. C–E, Mice were fasted overnight and administered an oral glucose tolerance test (6 g/kg). Blood glucose (C), plasma insulin (D), and plasma bioactive GLP-1 (E) levels were determined at t = 0 min and 10 min after oral glucose administration (n = 14–17/group). *, P < 0.05; **, P < 0.01; ***, P < 0.001. OGTT, Oral glucose tolerance test.
To measure GLP-1 secretion in vivo, wild-type and MKR mice were fasted overnight and administered an oral glucose challenge (6 g/kg). Fasting blood glucose levels were similar between the two groups, and no difference in the glucose excursion was observed 10 min after the glucose load (Fig. 8C). Basal insulin levels were elevated by 1.6-fold (P < 0.05) in the MKR mice and increased normally in response to oral glucose (Fig. 8D). In contrast, basal GLP-1 levels were increased 1.7-fold (P < 0.05), but the L cell response to the glucose load was significantly impaired in MKR mice when compared with wild-type controls, with GLP-1 release reduced from 2.6- to 1.6-fold (P < 0.05) (overall two factor ANOVA: P < 0.0001; F value: 18.41) (Fig. 8E). These findings indicate that chronic hyperinsulinemia is associated with impaired GLP-1 secretion from the intestinal L cell in response to an oral nutrient load.
Discussion
Given the demonstrated importance of endogenous GLP-1 release to the maintenance of normal insulin secretion and glucose homeostasis (47, 48), it is therefore somewhat paradoxical that postprandial GLP-1 release is decreased in subjects with type 2 diabetes and insulin resistance, independent of obesity (18, 19, 20, 21, 22, 23, 24). It has been proposed that the release of GLP-1 from the intestinal L cell is impaired in such individuals, but direct evidence demonstrating the responsiveness of the L cell to insulin has yet to be described. The findings of the present study demonstrate, for the first time, that insulin exerts a direct stimulatory effect on GLP-1 secretion from the intestinal L cell and that insulin resistance in the L cell impairs both homologous and heterologous secretagogue-induced GLP-1 secretion in vitro and in vivo.
The pancreatic β-cell is a known target of GLP-1 action, releasing insulin in a glucose-dependent fashion (2, 3, 4). However, little evidence is available to show a reciprocal effect of insulin on the L cell. Nonetheless, normal murine, human, and fetal rodent intestinal L cells were found to express the insulin receptor, as were both the GLUTag and NCI-H716 L cell lines. Furthermore, treatment of the L cells in vitro with insulin resulted in the phosphorylation of both Akt and ERK1/2, two classical effectors of insulin action. Insulin has also been reported to stimulate proglucagon gene expression, as well as GLP-1 synthesis, in GLUTag cells through an Akt-glycogen synthase kinase-3 pathway that involves the bipartite transcription factor, T cell transcription factor-4 (49). Collectively, these findings indicate that the intestinal L cell should be included among the known targets of insulin action.
Slightly different patterns of insulin-induced GLP-1 release were observed for the GLUTag and NCI-H716 cells and FRIC cultures; however, similar differences have been reported for the dose-dependent responses of these in vitro L cell models to leptin (15). These findings nevertheless suggest that the L cell secretes GLP-1 in response to insulin, thereby suggesting the existence of a positive feedback loop in vivo. Such interactions are not common in physiology but are well established to occur during the potentiation of LH release during the menstrual cycle. Moreover, a positive feedback loop between the duodenal hormone, cholecystokinin, and leptin secreted from the gastric mucosa, has been reported (50). Although previous studies have demonstrated that insulin does not stimulate GLP-1 secretion in fasting human subjects undergoing hyperinsulinemic clamps or from the perfused porcine ileum (51, 52, 53, 54), our findings clearly demonstrate that primary murine and human L cells express the insulin receptor and that insulin stimulates GLP-1 release from immortalized mouse and human L cell lines. We speculate that the absence of an insulin effect in the aforementioned studies may be due to different experimental paradigms wherein either low levels of nutrients in the lumen due to fasting or perfusate conditions or low circulating glucose levels do not permit the effect of insulin on GLP-1 secretion. Therefore, just as GLP-1 requires the presence of elevated glucose levels to stimulate insulin secretion by the β-cell (2), insulin may not stimulate GLP-1 release from the enteroendocrine L cell unless high glucose levels are present, either in the intestinal lumen or circulation. Alternatively, the lack of effect of insulin in the L cell in vivo may also be due to hormonal control by somatostatin.
We and others have demonstrated that GLP-1 stimulates somatostatin secretion from intestinal cultures and perfused ileum, and somatostatin has been found to inhibit GLP-1 secretion, thereby suggesting a feedback loop between GLP-1 and local somatostatin secretion (1, 54, 55, 56). The apparent lack of effect of insulin in the previously mentioned studies could therefore have been consequent to intestinal-derived somatostatin production in these experiments, which may then be alleviated during postprandial conditions. Finally, whereas other studies demonstrated that insulin inhibits GIP secretion during conditions of euglycemia, this inhibitory effect is reversed when blood glucose levels are elevated, suggesting either a loss of the inhibitory effect of insulin on GIP release or a possible stimulatory effect of insulin on the K cells during hyperglycemic conditions (57, 58). Our finding that the adult rat L cell does not express the insulin receptor precludes the use of this animal as a model to study insulin-induced GLP-1 secretion, but this hypothesis should be directly testable in future human or mouse studies using carefully controlled fasting vs. fed hyperinsulinemic plus high glucose conditions.
In the present study, the importance of the MEK-ERK1/2 pathway in mediating insulin-induced GLP-1 secretion from the L cell was demonstrated through pharmacological and adenoviral overexpression studies. MEK-ERK1/2 signaling has also been found to play a role in the release of other hormones, including insulin (59, 60) and the catecholamines (61, 62); moreover, an essential role for this pathway in the human L cell has been demonstrated for meat hydrolysate- and amino acid-induced GLP-1 secretion (63). In contrast to these findings, inhibition of PI3 kinase did not prevent insulin-induced GLP-1 release in either of the L cell lines but instead potentiated both basal and insulin-induced GLP-1 secretion, possibly due to nonspecific effects (64). When taken together, therefore, the present findings indicate a role for the MEK-ERK1/2 pathway in mediating insulin-induced GLP-1 release from the intestinal L cell.
Surprisingly, IGF-I was found to have no effect on the murine L cell and a slight inhibitory effect on GLP-1 secretion from the L human cell, even though both insulin and IGF-I receptors share high sequence homology and activate similar downstream components. Studies performed with overexpression models of either receptor or chimeric receptors have revealed differential effects of these hormones on other cell types (65, 66, 67), which may account for the contrasting effects of IGF-I and insulin on the L cell. Nonetheless, further studies are required to examine the role of IGF-I in the L cell.
The finding that induced-cellular insulin resistance impaired the GLP-1 secretory response to insulin is consistent with the concomitant down-regulation that was observed in both insulin receptor protein expression and insulin receptor phosphorylation. Moreover, desensitization to heterologous secretagogues, most notably GIP and PMA, was also observed in the insulin-resistant L cells in vitro, suggesting that insulin resistance affected the actions of these GLP-1 secretagogues downstream of the changes in insulin receptor activation and expression. Decreased insulin-mediated GLP-1 secretion in insulin resistance may be partly explained by the down-regulation observed in MEK-ERK1/2 activation, as seen with decreased ERK1/2 phosphorylation, possibly in association with known effects of insulin to up-regulate the expression and activity of dual-specificity MAPK phosphatases (68, 69). Interestingly, although GIP and PMA stimulate GLP-1 secretion through the activation of protein kinase A and protein kinase C isoenzymes, respectively (9, 70), recent studies demonstrated that these secretagogues can also activate the MEK-ERK1/2 pathway in other cell models (71, 72). Therefore, because insulin resistance decreased the response to GIP and PMA, as well as insulin, a decrease in the activity of the MEK-ERK1/2 pathway in the L cell may account for the diminished responses to all of these secretagogues.
Previously we demonstrated that high-fat feeding-induced obesity results in altered basal GLP-1 levels in mice as well as impairments in oral glucose-induced GLP-1 release (15). Because obesity is associated with a multitude of metabolic impairments, the exact cause of the impaired GLP-1 release in that study was not known. Therefore, we used the MKR mice as a nonobese model of insulin resistance, demonstrating that chronic hyperinsulinemia is associated with increased basal levels of circulating GLP-1 in these mice but with decreased GLP-1 secretion in response to an oral glucose load. These findings are in accordance with reports that decreased insulin sensitivity is associated with impaired GLP-1 release in response to a mixed meal (23, 24). Moreover, based on the in vitro findings of the present study whereby insulin resistance in vitro impaired GLP-1 secretion in response to heterologous secretagogues, these data suggest that chronic hyperinsulinemia may decrease the responsiveness of the L cell to nutrient and hormonal secretagogues.
In summary, enteroendocrine L cells secrete GLP-1 in response to insulin in a glucose-dependent manner, and in the mouse and human L cell, this effect is mediated through a MEK-ERK1/2-dependent pathway. Insulin resistance in vitro is associated with loss of both of these actions of insulin as well as an impaired response to heterologous secretagogues, suggesting that insulin resistance induces a defect in a distal exocytotic process that governs GLP-1 release. Consistent with these findings, GLP-1 secretion is also attenuated in response to an oral glucose load in MKR mice, further demonstrating the deleterious effect of chronic hyperinsulinemia on the function of the L cell. Further studies will be necessary to determine whether treatment or prevention of insulin resistance normalizes GLP-1 release from the L cell, thereby providing a potential therapeutic approach to restore the defective GLP-1 secretion that occurs in subjects with type 2 diabetes and insulin resistance.
Acknowledgments
The authors are grateful to Drs. S. L. Asa, J. R. G. Challis, and M. B. Wheeler (University of Toronto), Dr. D. A. D’Alessio (University of Cincinnati), and Dr. R. N. Kulkarni (Joslin Diabetes Center) for the gifts of reagents.
Footnotes
This work was supported by an Operating Grant 2374 from the Canadian Diabetes Association. G.E.L. was supported by a National Science and Engineering Research Canada Graduate Scholarship, a Banting and Best Diabetes Centre (BBDC) Novo Nordisk Studentship (University of Toronto), and a Canadian Institutes of Health Research (CIHR) Frederick Banting and Charles Best Canada Graduate Scholarship. G.J.H. was supported by a partnered Crohn’s and Colitis Foundation of Canada, Canadian Association of Gastroenterology, and CIHR Summer Studentship. N.F. was supported by a BBDC Charles Hollenberg Summer Studentship and P.L.B. by the Canada Research Chairs Program.
Disclosure Statement: G.E.L., G.J.H., N.F., and D.L. have nothing to declare. C.J.R. has equity interests in EnGene, Inc. and consults for Amylin, Inc., Merck, Inc., and Metabolex, Inc. P.L.B. consults for and has received lecture fees from Johnson and Johnson, Inc., Merck, Inc., and Metabolex, Inc.
First Published Online September 25, 2008
Abbreviations: FRIC, Fetal rat intestinal cell; GFP, green fluorescent protein; GIP, glucose-dependent insulinotropic peptide; GLP, glucagon-like peptide; MEK, MAP/ERK kinase; PI3, phosphoinositide 3; PIP3, phosphatidylinositol-(3,4,5)-phosphate; PMA, phorbol 12-myristate 13-acetate.
References
- 1.Lim GE, Brubaker PL 2006. Glucagon-like peptide-1 secretion by the L-cell: the view from within. Diabetes 55:S70–S77
- 2.Drucker DJ 2006. The biology of incretin hormones. Cell Metab 3:153–165 [DOI] [PubMed] [Google Scholar]
- 3.Wong VS, Brubaker PL 2006. From cradle to grave: pancreatic β-cell mass and glucagon-like peptide-1. Minerva Endocrinol 31:107–124 [PubMed] [Google Scholar]
- 4.Gallwitz B 2005. Glucagon-like peptide-1 as a treatment option for type 2 diabetes and its role in restoring β-cell mass. Diabetes Technol Ther 7:651–657 [DOI] [PubMed] [Google Scholar]
- 5.Furman B, Pyne N 2006. Modulation of cyclic nucleotides and cyclic nucleotide phosphodiesterases in pancreatic islet β-cells and intestinal L-cells as targets for treating diabetes mellitus. Curr Opin Investig Drugs 7:898–905 [PubMed] [Google Scholar]
- 6.Rocca AS, Brubaker PL 1995. Stereospecific effects of fatty acids on proglucagon-derived peptide secretion in fetal rat intestinal cultures. Endocrinology 136:5593–5599 [DOI] [PubMed] [Google Scholar]
- 7.Gribble FM, Williams L, Simpson AK, Reimann F 2003. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes 52:1147–1154 [DOI] [PubMed] [Google Scholar]
- 8.Roberge JN, Brubaker PL 1993. Regulation of intestinal proglucagon-derived peptide secretion by glucose-dependent insulinotropic peptide in a novel enteroendocrine loop. Endocrinology 133:233–240 [DOI] [PubMed] [Google Scholar]
- 9.Iakoubov R, Izzo A, Yeung A, Whiteside CI, Brubaker PL 2007. Protein kinase Cζ is required for oleic acid-induced secretion of glucagon-like peptide-1 by intestinal endocrine L cells. Endocrinology 148:1089–1098 [DOI] [PubMed] [Google Scholar]
- 10.Eissele R, Goke R, Willemer S, Harthus HP, Vermeer H, Arnold R, Goke B 1992. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest 22:283–291 [DOI] [PubMed] [Google Scholar]
- 11.Vilsboll T, Krarup T, Sonne J, Madsbad S, Volund A, Juul AG, Holst JJ 2003. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab 88:2706–2713 [DOI] [PubMed] [Google Scholar]
- 12.Rocca AS, Brubaker PL 1999. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology 140:1687–1694 [DOI] [PubMed] [Google Scholar]
- 13.Anini Y, Hansotia T, Brubaker PL 2002. Muscarinic receptors control postprandial release of glucagon-like peptide-1: in vivo and in vitro studies in rats. Endocrinology 143:2420–2426 [DOI] [PubMed] [Google Scholar]
- 14.Anini Y, Brubaker PL 2003. Muscarinic receptors control glucagon-like peptide 1 secretion by human endocrine L cells. Endocrinology 144:3244–3250 [DOI] [PubMed] [Google Scholar]
- 15.Anini Y, Brubaker PL 2003. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes 52:252–259 [DOI] [PubMed] [Google Scholar]
- 16.Persson K, Gingerich RL, Nayak S, Wada K, Wada E, Ahren B 2000. Reduced GLP-1 and insulin responses and glucose intolerance after gastric glucose in GRP receptor-deleted mice. Am J Physiol Endocrinol Metab 279:E956–E962 [DOI] [PubMed]
- 17.Roberge JN, Gronau KA, Brubaker PL 1996. Gastrin-releasing peptide is a novel mediator of proximal nutrient-induced proglucagon-derived peptide secretion from the distal gut. Endocrinology 137:2383–2388 [DOI] [PubMed] [Google Scholar]
- 18.Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ 2001. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab 86:3717–3723 [DOI] [PubMed] [Google Scholar]
- 19.Mannucci E, Ognibene A, Cremasco F, Bardini G, Mencucci A, Pierazzuoli E, Ciani S, Fanelli A, Messeri G, Rotella CM 2000. Glucagon-like peptide (GLP)-1 and leptin concentrations in obese patients with type 2 diabetes mellitus. Diabetes Med 17:713–719 [DOI] [PubMed] [Google Scholar]
- 20.Vilsboll T, Agerso H, Krarup T, Holst JJ 2003. Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects. J Clin Endocrinol Metab 88:220–224 [DOI] [PubMed] [Google Scholar]
- 21.Vilsboll T, Krarup T, Deacon CF, Madsbad S, Holst JJ 2001. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes 50:609–613 [DOI] [PubMed] [Google Scholar]
- 22.Muscelli E, Mari A, Casolaro A, Camastra S, Seghieri G, Gastaldelli A, Holst JJ, Ferrannini E 2008. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes 57:1340–1348 [DOI] [PubMed] [Google Scholar]
- 23.Rask E, Olsson T, Soderberg S, Johnson O, Seckl J, Holst JJ, Ahren B 2001. Impaired incretin response after a mixed meal is associated with insulin resistance in nondiabetic men. Diabetes Care 24:1640–1645 [DOI] [PubMed] [Google Scholar]
- 24.Rask E, Olsson T, Soderberg S, Holst JJ, Tura A, Pacini G, Ahren B 2004. Insulin secretion and incretin hormones after oral glucose in non-obese subjects with impaired glucose tolerance. Metabolism 53:624–631 [DOI] [PubMed] [Google Scholar]
- 25.McLaughlin T, Abbasi F, Lamendola C, Frayo RS, Cummings DE 2004. Plasma ghrelin concentrations are decreased in insulin-resistant obese adults relative to equally obese insulin-sensitive controls. J Clin Endocrinol Metab 89:1630–1635 [DOI] [PubMed] [Google Scholar]
- 26.Asghar Z, Yau D, Chan F, Leroith D, Chan CB, Wheeler MB 2006. Insulin resistance causes increased β-cell mass but defective glucose-stimulated insulin secretion in a murine model of type 2 diabetes. Diabetologia 49:90–99 [DOI] [PubMed] [Google Scholar]
- 27.Fernandez AM, Kim JK, Yakar S, Dupont J, Hernandez-Sanchez C, Castle AL, Filmore J, Shulman GI, Leroith D 2001. Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes Dev 15:1926–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sidhu SS, Thompson DG, Warhurst G, Case RM, Benson RS 2000. Fatty acid-induced cholecystokinin secretion and changes in intracellular Ca2+ in two enteroendocrine cell lines, STC-1 and GLUTag. J Physiol 528(Pt 1):165–176 [DOI] [PMC free article] [PubMed]
- 29.Brubaker PL, Drucker DJ, Greenberg GR 1990. Synthesis and secretion of somatostatin-28 and -14 by fetal rat intestinal cells in culture. Am J Physiol 258:G974–G981 [DOI] [PubMed]
- 30.Brubaker PL, Drucker DJ, Asa SL, Greenberg GR 1991. Regulation of peptide-YY synthesis and secretion in fetal rat intestinal cultures. Endocrinology 129:3351–3358 [DOI] [PubMed] [Google Scholar]
- 31.Huang C, Somwar R, Patel N, Niu W, Torok D, Klip A 2002. Sustained exposure of L6 myotubes to high glucose and insulin decreases insulin-stimulated GLUT4 translocation but upregulates GLUT4 activity. Diabetes 51:2090–2098 [DOI] [PubMed] [Google Scholar]
- 32.Thomson MJ, Williams MG, Frost SC 1997. Development of insulin resistance in 3T3-L1 adipocytes. J Biol Chem 272:7759–7764 [DOI] [PubMed] [Google Scholar]
- 33.Pirola L, Bonnafous S, Johnston AM, Chaussade C, Portis F, Van OE 2003. Phosphoinositide 3-kinase-mediated reduction of insulin receptor substrate-1/2 protein expression via different mechanisms contributes to the insulin-induced desensitization of its signaling pathways in L6 muscle cells. J Biol Chem 278:15641–15651 [DOI] [PubMed] [Google Scholar]
- 34.Tang S, Le-Tien H, Goldstein BJ, Shin P, Lai R, Fantus IG 2001. Decreased in situ insulin receptor dephosphorylation in hyperglycemia-induced insulin resistance in rat adipocytes. Diabetes 50:83–90 [DOI] [PubMed] [Google Scholar]
- 35.Niswender KD, Gallis B, Blevins JE, Corson MA, Schwartz MW, Baskin DG 2003. Immunocytochemical detection of phosphatidylinositol 3-kinase activation by insulin and leptin. J Histochem Cytochem 51:275–283 [DOI] [PubMed] [Google Scholar]
- 36.Entingh AJ, Taniguchi CM, Kahn CR 2003. Bi-directional regulation of brown fat adipogenesis by the insulin receptor. J Biol Chem 278:33377–33383 [DOI] [PubMed] [Google Scholar]
- 37.Pandini G, Frasca F, Mineo R, Sciacca L, Vigneri R, Belfiore A 2002. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J Biol Chem 277:39684–39695 [DOI] [PubMed] [Google Scholar]
- 38.Zhao FQ, Sheng ZM, Tsai MM, Hubbs AE, Wang R, O'Leary TJ, Izon DJ, Taubenberger JK 2002. Serial analysis of gene expression in murine fetal thymocyte cell lines. Int Immunol 14:1383–1395 [DOI] [PubMed] [Google Scholar]
- 39.Monti S, Di SF, Iraci R, Martini C, Lanzara S, Falasca P, Poggi M, Stigliano A, Sciarra F, Toscano V 2001. Regional variations of insulin-like growth factor I (IGF-I), IGF-II, and receptor type I in benign prostatic hyperplasia tissue and their correlation with intraprostatic androgens. J Clin Endocrinol Metab 86:1700–1706 [DOI] [PubMed] [Google Scholar]
- 40.Robinson MJ, Stippec SA, Goldsmith E, White MA, Cobb MH 1998. A constitutively active and nuclear form of the MAP kinase ERK2 is sufficient for neurite outgrowth and cell transformation. Curr Biol 8:1141–1150 [DOI] [PubMed] [Google Scholar]
- 41.Dickson LM, Lingohr MK, McCuaig J, Hugl SR, Snow L, Kahn BB, Myers Jr MG, Rhodes CJ 2001. Differential activation of protein kinase B and p70(S6)K by glucose and insulin-like growth factor 1 in pancreatic β-cells (INS-1). J Biol Chem 276:21110–21120 [DOI] [PubMed] [Google Scholar]
- 42.Lingohr MK, Dickson LM, McCuaig JF, Hugl SR, Twardzik DR, Rhodes CJ 2002. Activation of IRS-2-mediated signal transduction by IGF-1, but not TGF-α or EGF, augments pancreatic β-cell proliferation. Diabetes 51:966–976 [DOI] [PubMed] [Google Scholar]
- 43.Federico LM, Naples M, Taylor D, Adeli K 2006. Intestinal insulin resistance and aberrant production of apolipoprotein B48 lipoproteins in an animal model of insulin resistance and metabolic dyslipidemia: evidence for activation of protein tyrosine phosphatase-1B, extracellular signal-related kinase, and sterol regulatory element-binding protein-1c in the fructose-fed hamster intestine. Diabetes 55:1316–1326 [DOI] [PubMed] [Google Scholar]
- 44.Li G, Barrett EJ, Wang H, Chai W, Liu Z 2005. Insulin at physiological concentrations selectively activates insulin but not insulin-like growth factor I (IGF-I) or insulin/IGF-I hybrid receptors in endothelial cells. Endocrinology 146:4690–4696 [DOI] [PubMed] [Google Scholar]
- 45.Ueki K, Okada T, Hu J, Liew CW, Assmann A, Dahlgren GM, Peters JL, Shackman JG, Zhang M, Artner I, Satin LS, Stein R, Holzenberger M, Kennedy RT, Kahn CR, Kulkarni RN 2006. Total insulin and IGF-I resistance in pancreatic β cells causes overt diabetes. Nat Genet 38:583–588 [DOI] [PubMed] [Google Scholar]
- 46.Baudry A, Lamothe B, Bucchini D, Jami J, Montarras D, Pinset C, Joshi RL 2001. IGF-1 receptor as an alternative receptor for metabolic signaling in insulin receptor-deficient muscle cells. FEBS Lett 488:174–178 [DOI] [PubMed] [Google Scholar]
- 47.Scrocchi LA, Brown TJ, MaClusky N, Brubaker PL, Auerbach AB, Joyner AL, Drucker DJ 1996. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med 2:1254–1258 [DOI] [PubMed] [Google Scholar]
- 48.Edwards CM, Todd JF, Mahmoudi M, Wang Z, Wang RM, Ghatei MA, Bloom SR 1999. Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9-39. Diabetes 48:86–93 [DOI] [PubMed] [Google Scholar]
- 49.Yi F, Sun J, Lim GE, Fantus IG, Brubaker PL, Jin T 2008. Crosstalk between the insulin and Wnt signaling pathways: Evidence from intestinal endocrine L cells. Endocrinology 149:2341–2351 [DOI] [PubMed] [Google Scholar]
- 50.Guilmeau S, Buyse M, Tsocas A, Laigneau JP, Bado A 2003. Duodenal leptin stimulates cholecystokinin secretion: evidence of a positive leptin-cholecystokinin feedback loop. Diabetes 52:1664–1672 [DOI] [PubMed] [Google Scholar]
- 51.Nauck MA, Heimesaat MM, Behle K, Holst JJ, Nauck MS, Ritzel R, Hufner M, Schmiegel WH 2002. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab 87:1239–1246 [DOI] [PubMed] [Google Scholar]
- 52.Orskov L, Holst JJ, Moller J, Orskov C, Moller N, Alberti KG, Schmitz O 1996. GLP-1 does not acutely affect insulin sensitivity in healthy man. Diabetologia 39:1227–1232 [DOI] [PubMed] [Google Scholar]
- 53.Ryan AS, Egan JM, Habener JF, Elahi D 1998. Insulinotropic hormone glucagon-like peptide-1-(7-37) appears not to augment insulin-mediated glucose uptake in young men during euglycemia. J Clin Endocrinol Metab 83:2399-2404 [DOI] [PubMed] [Google Scholar]
- 54.Hansen L, Hartmann B, Mineo H, Holst JJ 2004. Glucagon-like peptide-1 secretion is influenced by perfusate glucose concentration and by a feedback mechanism involving somatostatin in isolated perfused porcine ileum. Regul Pept 118:11–18 [DOI] [PubMed] [Google Scholar]
- 55.Brubaker PL, Efendic S, Greenberg GR 1997. Truncated and full-length glucagon-like peptide-1 (GLP-1) differentially stimulate intestinal somatostatin release. Endocrine 6:91–95 [DOI] [PubMed] [Google Scholar]
- 56.Brubaker PL 1991. Regulation of intestinal proglucagon-derived peptide secretion by intestinal regulatory peptides. Endocrinology 128:3175–3182 [DOI] [PubMed] [Google Scholar]
- 57.Bryer-Ash M, Cheung A, Pederson RA 1994. Feedback regulation of glucose-dependent insulinotropic polypeptide (GIP) secretion by insulin in conscious rats. Regul Pept 51:101–109 [DOI] [PubMed] [Google Scholar]
- 58.Creutzfeldt W, Talaulicar M, Ebert R, Willms B 1980. Inhibition of gastric inhibitory polypeptide (GIP) release by insulin and glucose in juvenile diabetes. Diabetes 29:140–145 [DOI] [PubMed] [Google Scholar]
- 59.Longuet C, Broca C, Costes S, Hani EH, Bataille D, Dalle S 2005. Extracellularly regulated kinases 1/2 (p44/42 mitogen-activated protein kinases) phosphorylate synapsin I and regulate insulin secretion in the MIN6 β-cell line and islets of Langerhans. Endocrinology 146:643–654 [DOI] [PubMed] [Google Scholar]
- 60.Tomas A, Yermen B, Min L, Pessin JE, Halban PA 2006. Regulation of pancreatic β-cell insulin secretion by actin cytoskeleton remodelling: role of gelsolin and cooperation with the MAPK signalling pathway. J Cell Sci 119:2156–2167 [DOI] [PubMed] [Google Scholar]
- 61.Ait-Ali D, Turquier V, Grumolato L, Yon L, Jourdain M, Alexandre D, Eiden LE, Vaudry H, Anouar Y 2004. The proinflammatory cytokines tumor necrosis factor-α and interleukin-1 stimulate neuropeptide gene transcription and secretion in adrenochromaffin cells via activation of extracellularly regulated kinase 1/2 and p38 protein kinases, and activator protein-1 transcription factors. Mol Endocrinol 18:1721–1739 [DOI] [PubMed] [Google Scholar]
- 62.Cox ME, Ely CM, Catling AD, Weber MJ, Parsons SJ 1996. Tyrosine kinases are required for catecholamine secretion and mitogen-activated protein kinase activation in bovine adrenal chromaffin cells. J Neurochem 66:1103–1112 [DOI] [PubMed] [Google Scholar]
- 63.Reimer RA 2006. Meat hydrolysate and essential amino acid-induced glucagon-like peptide-1 secretion, in the human NCI-H716 enteroendocrine cell line, is regulated by extracellular signal-regulated kinase1/2 and p38 mitogen-activated protein kinases. J Endocrinol 191:159–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.El-kholy W, MacDonald PE, Lin JH, Wang J, Fox JM, Light PE, Wang Q, Tsushima RG, Wheeler MB 2003. The phosphatidylinositol 3-kinase inhibitor LY294002 potently blocks K(V) currents via a direct mechanism. FASEB J 17:720–722 [DOI] [PubMed] [Google Scholar]
- 65.Amoui M, Craddock BP, Miller WT 2001. Differential phosphorylation of IRS-1 by insulin and insulin-like growth factor I receptors in Chinese hamster ovary cells. J Endocrinol 171:153–162 [DOI] [PubMed] [Google Scholar]
- 66.Kalloo-Hosein HE, Whitehead JP, Soos M, Tavare JM, Siddle K, O'Rahilly S 1997. Differential signaling to glycogen synthesis by the intracellular domain of the insulin versus the insulin-like growth factor-1 receptor. Evidence from studies of TrkC-chimeras. J Biol Chem 272:24325–24332 [DOI] [PubMed] [Google Scholar]
- 67.Mulligan C, Rochford J, Denyer G, Stephens R, Yeo G, Freeman T, Siddle K, O'Rahilly S 2002. Microarray analysis of insulin and insulin-like growth factor-1 (IGF-1) receptor signaling reveals the selective up-regulation of the mitogen heparin-binding EGF-like growth factor by IGF-1. J Biol Chem 277:42480–42487 [DOI] [PubMed] [Google Scholar]
- 68.Xu H, Yang Q, Shen M, Huang X, Dembski M, Gimeno R, Tartaglia LA, Kapeller R, Wu Z 2005. Dual specificity MAPK phosphatase 3 activates PEPCK gene transcription and increases gluconeogenesis in rat hepatoma cells. J Biol Chem 280:36013–36018 [DOI] [PubMed] [Google Scholar]
- 69.Kusari AB, Byon J, Bandyopadhyay D, Kenner KA, Kusari J 1997. Insulin-induced mitogen-activated protein (MAP) kinase phosphatase-1 (MKP-1) attenuates insulin-stimulated MAP kinase activity: a mechanism for the feedback inhibition of insulin signaling. Mol Endocrinol 11:1532–1543 [DOI] [PubMed] [Google Scholar]
- 70.Usdin TB, Mezey E, Button DC, Brownstein MJ, Bonner TI 1993. Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology 133:2861–2870 [DOI] [PubMed] [Google Scholar]
- 71.Benes C, Poitout V, Marie JC, Martin-Perez J, Roisin MP, Fagard R 1999. Mode of regulation of the extracellular signal-regulated kinases in the pancreatic β-cell line MIN6 and their implication in the regulation of insulin gene transcription. Biochem J 340(Pt 1):219–225 [PMC free article] [PubMed]
- 72.Ehses JA, Pelech SL, Pederson RA, McIntosh CH 2002. Glucose-dependent insulinotropic polypeptide activates the Raf-Mek1/2-ERK1/2 module via a cyclic AMP/cAMP-dependent protein kinase/Rap1-mediated pathway. J Biol Chem 277:37088–37097 [DOI] [PubMed] [Google Scholar]