Abstract
Prior published reports have demonstrated that glucose-oxidized low-density lipoproteins (g-OxLDL) enhance the proliferative response of vascular smooth muscle cells (SMC) to IGF-I. Our previous studies have determined that the regulation of cleavage of integrin-associated protein (IAP) by matrix-metalloprotease-2 (MMP-2) in diabetic mice in response to hyperglycemia is a key regulator of the response of SMC to IGF-I. Because chronic hyperglycemia enhances glucose-induced LDL oxidation, these studies were conducted to determine whether g-OxLDL modulates the response of SMC to IGF-I by regulating MMP-2-mediated cleavage of IAP. We determined that exposure of SMC to g-OxLDL, but not native LDL, was sufficient to facilitate an increase in cell proliferation in response to IGF-I. Exposure to an anti-CD36 antibody, which has been shown to inhibit g-OxLDL-mediated signaling, inhibited the effects of g-OxLDL on IGF-I-stimulated SMC proliferation. The effect of g-OxLDL could be attributed, in part, to an associated decrease in proteolytic cleavage of IAP leading to increase in the basal association between IAP and Src homology 2 domain–containing protein tyrosine phosphatase substrate-1, which is required for IGF-I-stimulated proliferation. The inhibitory effect of g-OxLDL on IAP cleavage appeared to be due to its ability to decrease the amount of activated MMP-2, the protease responsible for IAP cleavage. In conclusion, these data provide a molecular mechanism to explain previous studies that have reported an enhancing effect of g-OxLDL on IGF-I-stimulated SMC proliferation.
The glucose-oxidized low-density lipoprotein stimulated decrease in integrin-associated protein (IAP) cleavage, leading to an increase in IAP association with SHPS-1, mediates its enhancing effects on IGF-I stimulated proliferation.
Diabetic patients have a significantly increased risk of developing atherosclerosis (1, 2, 3). The migration and proliferation of smooth muscle cells (SMC) are important components of lesion formation (4). IGF-I has been implicated in the development of atherosclerotic lesions by virtue of its ability to stimulate both SMC migration and proliferation (5, 6, 7). SMC maintained in medium containing 5 mm glucose do not significantly increase their migration or proliferation in response to IGF-I, whereas SMC, maintained in high glucose (>12.5 mm), respond to IGF-I with increases in both migration and proliferation (8). Although activation of the IGF-I receptor (IGF-IR) is required to trigger downstream signaling events that lead to SMC proliferation and migration, there is no difference in IGF-IR activation between SMC grown in normal or high glucose (8). To respond to IGF-I, these cells require the association between two proteins, Src homology 2 domain–containing protein tyrosine phosphatase substrate-1 (SHPS-1) and integrin-associated protein (IAP) (9). We have determined that this difference in IGF-I responsiveness is due, in part, to the glucose-mediated regulation of IAP proteolysis. When SMC cultures are maintained in 5 mm glucose, IAP is constitutively cleaved, and the residual fragment of IAP that remains associated with the cell is unable to bind to SHPS-1 (10). Furthermore, we have demonstrated recently that this observation is not an artifact of growing SMC in culture because a similar difference in the amount of intact IAP and IAP association with SHPS-1 was observed in normoglycemic mice compared with hyperglycemic animals (11). Although our previous studies demonstrated that IAP was cleaved by matrix metalloprotease-2 (MMP-2) and that MMP-2 cleavage of IAP was in inhibited by high glucose concentrations (>12 mm), the changes that were induced by exposure to high glucose that altered MMP-2 activity were not determined (10, 11).
The oxidation of low-density lipoproteins (LDL) and the subsequent effects of oxidized LDL (OxLDL) on SMC have been implicated as important contributors to the development of atherosclerosis (12, 13, 14, 15, 16). It has been postulated that one mechanism by which hyperglycemia may contribute to an increased rate of lesion formation in diabetic patients is as a result of increased oxidation of LDL (17). Therefore, the aim of this study was to determine whether exposure of SMC to glucose-oxidized LDL (g-OxLDL) inhibits MMP-2 cleavage of IAP and thereby enhances the response of SMC to IGF-I.
Materials and Methods
Human IGF-I was a gift from Genentech (South San Francisco, CA). Polyvinyl difluoride membranes (Immobilon P) were purchased from Millipore Corp. (Billerica, MA). Autoradiographic film was obtained from Pierce (Rockford, IL). Fetal bovine serum (FBS), DMEM, penicillin, and streptomycin were purchased from Life Technologies (Grand Island, NY). The anti-IGF-IR antibody, the monoclonal anti-phosphotyrosine antibody (PY99), the phospho c-Jun N-terminal kinase (JNK; that recognizes the phosphorylation of Thr183 and Tyr185), and total JNK antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-Shc antibody was purchased from BD Transduction Laboratories (Lexington, KY). The anti-SHPS-1 antibody was purchased from Upstate Cell Signaling Solutions (Charlottesville, VA). The anti-β3 antibody was prepared in house as we have described previously (18). All other reagents were purchased from Sigma Chemical Co. (St. Louis, MO) unless stated.
Anti-IAP and anti-CD36 antibodies
The anti-IAP monoclonal antibody, B6H12, was purified from a specific cell line derived from a B-cell hybridoma (19). This antibody recognizes both intact and the residual membrane-associated fragment that results after cleavage of IAP by MMP-2 (10). The anti-IAP antibody, R569, was prepared using a peptide, 41–61 of the extracellular domain of IAP (KGRDIYTFDGALNKSTVPTC) that was coupled to keyhole limpet hemagglutinin and then used for immunization (10). This antibody specifically recognizes intact IAP.
The anti-CD36 antibody was prepared using a peptide, homologous to amino acids 93-100 of human CD36 that was coupled to keyhole limpet hemagglutinin and then used to immunize one rabbit (20).
Characterization of the anti-CD36 antibody
OxLDL have been shown to bind CD36 (21). The specificity of this antibody for CD36 was determined by comparing it with a commercially available anti-CD36 antibody, MCA722GA (supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org), which is a mouse antihuman CD36 antibody purchased from Ab-D Serotec (Raleigh, NC). To control for nonspecific effects of adding the anti-CD36 antiserum, nonimmune rabbit serum was used at the same dilution. Western immunoblotting of lysate from SMC with the commercial anti-CD36 antibody recognized a doublet of approximately 86-88 kDa (supplemental Fig. 1, lane 1). A similar doublet was detected when samples of the same lysate were immunoblotted with our anti-CD36 antiserum (supplemental Fig. 1, lane 6). Treatment of SMC with either 5 or 10 μg/ml g-OxLDL (concentrations shown previously to stimulate SMC proliferation) (22, 23) or LDL in the absence of glucose [native LDL (n-LDL)] for 24 h did not significantly change the amount of CD36 that could be detected (lanes 2–5).
Porcine SMC
SMC were isolated from the porcine aortic explants (8) based upon a modification of the protocol described by Ross (24). Male and female pigs, spotted Poland/China cross, age 12 months, were treated according to the Guide for Care and Use of Laboratory Animals (National Institutes of Health Publication 85-23) using an approved protocol. SMC were fed every 3 d with DMEM containing 900 g/liter glucose (5 mm) plus 1.0 mm pyruvate, 10% FBS, penicillin, and streptomycin and were passaged every 6 d. All experiments were performed with SMC between passages 4 and 10. Our previous studies have determined that the level of glucose in the medium did not decline significantly if the SMC were fed every 2–3 d, and overnight incubation in serum-free medium (SFM) did not result in significant depletion of glucose from the medium (8, 10).
Preparation of g-OxLDL
Human LDL was purchased from Biodesign International (Saco, ME), and g-OxLDL was generated as described by Lamharzi et al. (16). Briefly, 1 mg/ml LDL was incubated in the presence of 25 mm glucose in PBS at 37 C for 10 d. EDTA (0.1 mmol/liter) was included to inhibit metal ion-catalyzed oxidation. Control LDL was prepared by incubation in the absence of glucose (n-LDL). Both g-OxLDL and n-LDL were stored (under nitrogen) in the dark at 4 C and used within 2 wk.
Measurement of oxidation
The extent of LDL oxidation was determined by measuring thiobarbituric acid-reactive substance (PeroXOQuant; Pierce, Rockford, IL). Quantification of thiobarbituric acid-reactive substance was performed by comparison with a standard curve of malondialdehyde (MDA) equivalents. The mean value of six separate preparations was 3 ± 0.3 nmol MDA/mg LDL protein [compared with 0.85 ± 0.06 nmol MDA/mg LDL protein of nontreated LDL and 18 ± 2.3 nmol MDA/mg LDL protein for CuSO4 (5 μm) treated LDL].
Synthetic vitronectin (Vn) heparin-binding domain (HBD) peptide synthesis
A synthetic peptide corresponding to the HBD of human Vn (25) (amino acids 365-381; LAKKQRFRHRNRKGYRS) was synthesized by the peptide synthesis facility at University of North Carolina School of Medicine. The peptide was purified by HPLC, and the sequences were verified by mass spectrometry.
JNK inhibitory peptide
The JNK inhibitory peptide is derived from the JNK-binding domain of JNK-interacting protein 1 and has been shown to act as a dominant inhibitor of the JNK pathway (26). The peptide (HKRPTTLRLTTLGAQDS) also contained the TAT sequence that confers cell permeability (YARAAARQARA) (27). The peptides were synthesized by the Protein Chemistry Core Facility, University of North Carolina, Chapel Hill. Purity and sequence confirmation were determined by mass spectrometry. The peptide was added to SMC, at a final concentration of 10 μg/ml, at the same time as g-OxLDL.
Cell proliferation
SMC were plated at 2 × 104 cells per well of 24-well plates in SFM containing 2% FBS. After overnight attachment the medium was replaced with SFM + 0.2% FBS for a further 24 h. Cells were then incubated with various treatments prepared in SFM + 0. 2% platelet poor plasma (PPP) for 48 h. G-OxLDL and n-LDL were used at a final concentration of 5 μg/ml. The Vn HBD peptide was used at 10 μg/ml. IGF-I was added at a final concentration of 50 ng/ml. The anti-CD36 and control antisera were used at a dilution of 1:1000. Cell number was determined after trypsinization using trypan blue staining and counting (28).
Cell lysis, immunoprecipitation, and Western immunoblotting
SMC were plated in 10-cm dishes and grown to confluency over 6 d with the medium being refreshed after 2–3 d. On d 6, the growth medium was removed, and the confluent monolayers were rinsed three times with SFM (5 mm glucose plus 20 mm mannitol, and incubated overnight for 16–17 h). Our previous studies did not determine any significant difference in cellular responses in the presence or absence of mannitol. To examine the effects of g-OxLDL and n-LDL on IGF-I-mediated signaling events, the LDL preparations (5 μg/ml) were added for 4 h before the addition of IGF-I (100 ng/ml), which was added for the times shown. Where indicated, the anti-CD36 antiserum or control nonimmune serum (1:1000) was added for 1 h before the LDL preparations. For immunoprecipitation studies, cell monolayers were lysed and clarified by centrifugation, and then equal amounts of lysate protein were incubated overnight at 4 C in the presence of the appropriate antibody. Immune complexes were then precipitated with protein A before separation by SDS-PAGE (8%) under reducing or nonreducing (for detection of IAP and IAP-SHPS-1 coprecipitation) conditions. After SDS-PAGE, proteins were visualized by immunoblotting (19). For immunoblotting, the antibodies were used at concentrations between 1:500 and 1:1000.
Determination of MMP-2 protease activity
SMC grown to confluency were incubated overnight in SFM. Fresh SFM was added with or without the addition of g-OxLDL or n-LDL. Four hours later, the conditioned media were collected and concentrated 10-fold using 10K NMWL membrane centrifugal filters (Millipore). The concentrated conditioned media samples were then diluted 1:1 with nonreducing Laemmli buffer and MMP-2 gelatinase activity measured described previously (10).
Statistical analysis
Chemiluminescent images obtained were scanned using a DuoScan T1200 (AGFA, Brussels, Belgium), and band intensities of the scanned images were analyzed using NIH Image, version 1.61 to obtain arbitrary scan units for each specific immunoreactive band. The differences in the scan units between treatments were determined, and the mean values (±sem) of the differences from at least three independent experiments were calculated. The Student’s t test was used to determine whether the differences were significant. The results that are shown in all experiments are representative of at least three separate experiments.
Results
Exposure of SMC to g-OxLDL enhances the proliferative response of SMC to IGF-I in a CD36-dependent manner
Because culturing SMC in medium containing more than 12 mm glucose induces multiple biochemical changes that alter the response of SMC to IGF-I, in this study we cultured the SMC in 5 mm glucose, a condition in which they do not proliferate in response to IGF-I (8). This allowed us to directly determine the ability of OxLDL to alter the cellular responses. Furthermore, because glucose oxidation of LDL generates LDL that is more mildly oxidized than that oxidized by metal ions (16) and highly OxLDL can damage SMC and induce apoptosis (29, 30), in this study we focused on specifically determining the effect of low concentrations of g-OxLDL.
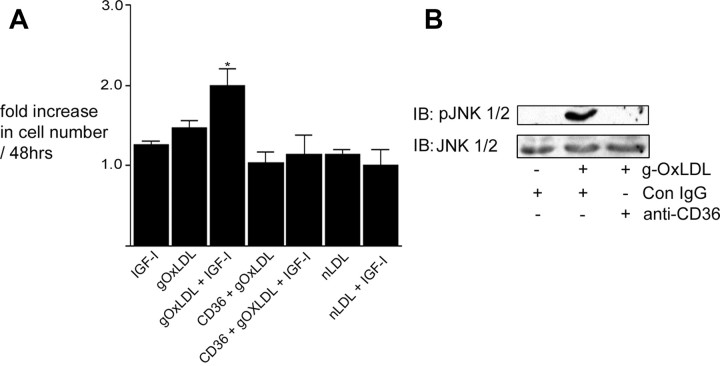
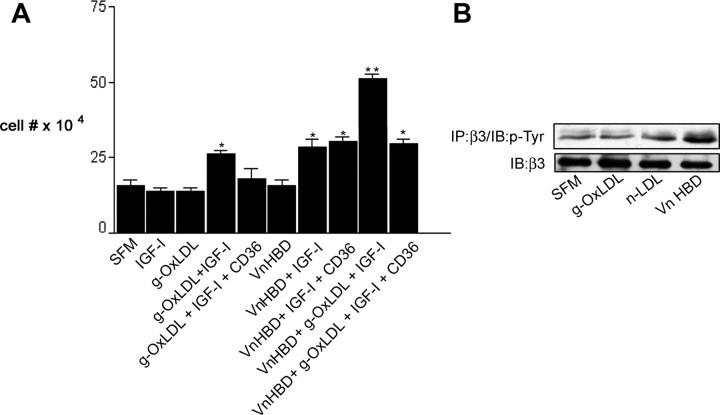
Previous studies have shown that at specific concentrations (5–10 μg/ml) of metal ion-OxLDL enhance IGF-I-stimulated SMC proliferation (22, 23). This is approximately 10-fold lower than the concentrations of OxLDL that have been shown to induce SMC apoptosis (29, 30). Because we specifically wished to examine the effects of g-OxLDL on SMC proliferation, not apoptosis, we defined the effects of 5 μg/ml g-OxLDL on SMC proliferation (Fig. 1A). Consistent with our previous results (8), IGF-I did not stimulate a significant increase in cell number in SMC grown in 5 mm glucose [a 1.3 ± 0.1-fold increase (mean ± sem, n = 3)]. The addition of 5 μg/ml g-OxLDL alone had no significant effect on SMC proliferation, but when added together with IGF-I, there was a significant increase [a 2.1 ± 0.3-fold increase (mean ± sem, n = 3; P < 0.05)] in cell number (Fig. 1A). In contrast to the effects of g-OxLDL, the addition of n-LDL had no significant effect on cell proliferation in the presence of IGF-I. Previous studies have shown that OxLDL binds to the scavenger receptor CD36 (31), and at least some of its effects have been shown to be mediated through this receptor (32). When the anti-CD36 antiserum was added with g-OxLDL, there was no enhancement of IGF-I-stimulated cell proliferation [a 1.5 ± 0.1-fold increase (mean ± sem, n = 3, P = not significant compared with IGF-I alone)], suggesting that g-OxLDL exerts its effects in a CD36-dependent manner similar to the previously reported proliferative effects of metal ion-OxLDL on SMC.
Fig. 1.
g-OxLDL increases IGF-I-stimulated SMC proliferation in a CD36-dependent manner. A, Cells (2 × 104) were plated in each well of a 24-well plate before exposure to IGF-I (50 ng/ml), g-OxLDL in the presence of control antiserum (5 μg/ml), n-LDL (5 μg/ml), or g-OxLDL in the presence of the anti-CD36 (CD36) antiserum (all prepared in DMEM plus 0.2% platelet poor plasma). At 48 h after the addition of IGF-I (50 ng/ml), cell number was determined by trypan blue staining and counting. *, P < 0.05 when cell number in response to IGF-I in the presence of g-OxLDL is compared with the number of cells in the presence of IGF-I alone. B, SMC were grown to confluency in medium containing 5 mm glucose before overnight incubation in SFM. g-OxLDL (5 μg/ml) was added for 4 h. Where indicated, either control IgG or anti-CD36 antiserum was added for 30 min before the addition of the LDL preparation. Phosphorylation of JNK was determined by immunoblotting (IB) equal amounts of cell lysates with an antibody specific for the phosphorylated form (pJNK). To demonstrate that there as no significant difference in the amount of total protein, blots were reprobed with an antibody that recognizes total JNK protein.
Regulation of the effects of g-OxLDL by CD36
Phosphorylation of JNK has been shown in a number of different cell types including SMC to be increased after OxLDL binding to CD36 (33, 34). Therefore, to determine whether the anti-CD36 antibody was effective in blocking stimulation of g-OxLDL-dependent effects, we measured the phosphorylation of JNK in response to g-OxLDL in the presence or absence of the antibody. Using an antibody that recognizes the dual phosphorylated form of JNK1/2 (Thr183/Tyr185), we determined that when SMC were treated with g-OxLDL, there was a significant increase in JNK phosphorylation, and this increase was blocked in the presence of the anti-CD36 antibody (Fig. 1B).
Exposure of SMC to g-OxLDL protects IAP from cleavage
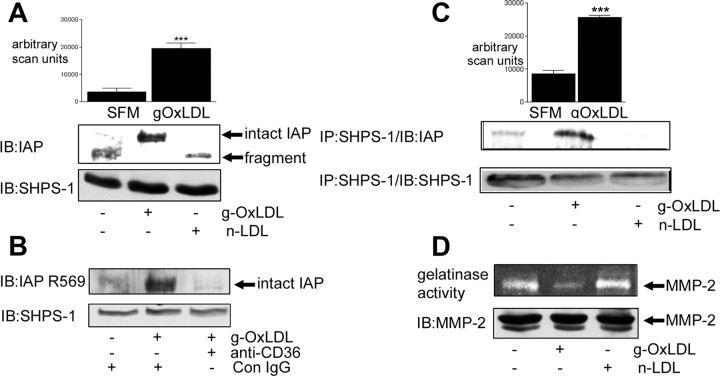
We have determined that IGF-I-stimulated SMC proliferation requires the association of the extracellular domains of two transmembrane proteins, IAP and SHPS-1 (9). This in turn permits the phosphorylation of SHPS-1 in response to IGF-I, allowing Shc recruitment to SHPS-1 and c-Src-dependent phosphorylation of Shc (35, 36). Shc phosphorylation is required for the proliferative and migratory responses of SMC to IGF-I (35). We have determined that when SMC are grown in 5 mm glucose, IAP is cleaved constitutively, which results in shedding of its extracellular domain (10). This in turn prevents the association of IAP with SHPS-1, thus accounting for the absence of a response to IGF-I under normal glucose conditions. When SMC are grown in 25 mm glucose, IAP is protected from cleavage (10). We therefore determined whether g-OxLDL increased the amount of intact IAP that could be detected in SMC grown in 5 mm glucose. Consistent with our previous observations, almost all of the IAP that could be detected in the lysate from SMC grown in 5 mm glucose was fragmented (based upon its electrophoretic mobility compared with intact IAP). Therefore, we examined the effect of incubating SMC, maintained in 5 mm glucose, with g-OxLDL. g-OxLDL resulted in a significant 82 ± 7% (mean ± sem, n = 3; P < 0.005) increase in the amount of intact IAP that could be detected in SMC grown in 5 mm glucose (Fig. 2A). The increase in intact IAP appeared to be due to its protection from cleavage because there was a corresponding 68 ± 11% (mean ± sem, n = 3; P < 0.005) decrease in the detection of the residual fragment of IAP (Fig. 2A). Similar to its effects on cell proliferation, the ability of g-OxLDL to protect IAP from cleavage was dependent upon g-OxLDL binding to CD36 because the increase in intact IAP was blocked when cells were preincubated with the anti-CD36 antibody (Fig. 2B).
Fig. 2.
g-OxLDL protects IAP from cleavage. SMC were grown to confluency in medium containing 5 mm glucose before overnight incubation in SFM. g-OxLDL (5 μg/ml) or n-LDL (5 μg/ml) was added for 4 h before collecting conditioned medium and lysing the cells. Where indicated, either control IgG or anti-CD36 antiserum was added for 30 min before the addition of the LDL preparations. A, Intact and fragmented IAP were visualized by immunoblotting (IB) cell lysates with the anti-IAP monoclonal antibody B6H12. Lysates were also immunoblotted with an anti-SHPS-1 antibody as a control for total protein. The graph shows the intensity of the intact IAP immunoreactive band expressed as arbitrary scanning units (mean ± sem, n = 3). ***, P < 0.005 when the values for IAP in the presence of g-OxLDL is compared with SFM alone. B, Intact IAP was visualized by immunoblotting (IB) cell lysates with the anti-IAP antibody that specifically recognizes intact IAP (R569). Lysates were also immunoblotted with an anti-SHPS-1 antibody as a control for total protein. C, IAP association with SHPS-1 was determined by immunoblotting cell lysates with an anti-SHPS-1 antibody and then immunoblotting with the anti-IAP monoclonal antibody B6H12. Lysates were also immunoprecipitated (IP) with an anti-SHPS-1 antibody and immunoblotted with the same antibody to demonstrate that the difference in the amount of IAP was not due to a significant change in SHPS-1 protein. The graph shows the intensity of the IAP immunoreactive band expressed as arbitrary scanning units (mean ± sem, n = 3). ***, P < 0.005 when the values for SHPS-1 association with IAP in the presence of g-OxLDL is compared with SFM alone. D, Conditioned medium was collected, concentrated, and then applied to a gelatin zymogram. After overnight incubation at 37 C (as described in Materials and Methods), the gel was stained with Coomassie blue, and cleared areas were visualized as an indicator of MMP-2 gelatinase activity (upper panel). Cell lysates were immunoblotted (IB) with an anti-MMP-2 antibody (lower panel). The results shown are representative of three independent experiments with similar results.
The addition of the JNK inhibitory peptide that blocks JNK activity did not inhibit the protective effect of g-OxLDL on IAP cleavage (data not shown).
Exposure of SMC to g-OxLDL enhances IAP association with SHPS-1
When SMC are maintained in medium containing 25 mm glucose, robust basal association between IAP and SHPS-1 is detectable, and this basal association is required for the response of SMC to IGF-I (9). In contrast, when SMC are maintained in 5 mm glucose, there is no IAP association with SHPS-1 due to the MMP-2-mediated cleavage of the extracellular domain of IAP (10). Because g-OxLDL treatment was associated with an increase in the amount of intact IAP, we next examined whether this was associated with an increase in IAP association with SHPS-1. Consistent with our previous studies (10), almost no IAP was coprecipitated with SHPS-1 in SMC grown in 5 mm glucose (Fig. 2C). After the addition of g-OxLDL, there was a significant 2.5 ± 0.3-fold increase (mean ± sem, n = 3; P < 0.005) in the association of IAP with SHPS-1 (Fig. 2C). n-LDL had no significant effect on IAP-SHPS-1 association.
g-OxLDL protects IAP from cleavage by regulating MMP-2 activity
We have determined that the cleavage of IAP is mediated by the matrix metalloprotease MMP-2 (10). OxLDL has been shown to regulate MMP-2 and -9 protein and activity levels in SMC (37, 38). We hypothesized that the effect of g-OxLDL on IAP stability could be due to its ability to decrease MMP-2 activity. We compared MMP-2 protease activity in the conditioned media from SMC, maintained in medium containing 5 mm glucose, and then incubated with g-OxLDL or n-LDL. In the presence of g-OxLDL, there was a significant 59 ± 7.5% decrease (mean ± sem, n = 3; P < 0.05) in the amount of MMP-2 protease activity that could be detected compared with the amount of MMP-2 activity from the conditioned media of untreated cells (Fig. 2D). However, there was no significant difference in the amount of MMP-2 protein after treatment with either g-OxLDL or n-LDL (Fig. 2D).
g-OxLDL enhances SHPS-1 phosphorylation in response to IGF-I
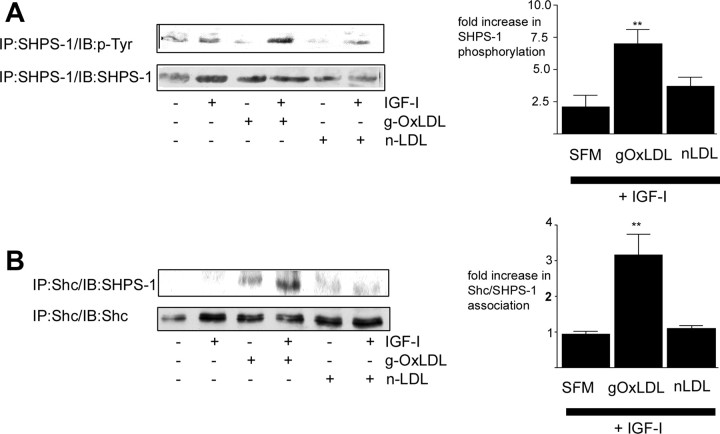
The association between IAP and SHPS-1 is required for SHPS-1 to be phosphorylated in response to IGF-I (9). Due to the cleavage of IAP and therefore lack of IAP association with SHPS-1 when SMC are maintained in medium containing 5 mm glucose, SHPS-1 is not phosphorylated after IGF-I stimulation. Given that g-OxLDL can stimulate an increase in IAP association with SHPS-1, we next examined SHPS-1 phosphorylation. IGF-I alone stimulated a 2.1 ± 1-fold (mean ± sem, n = 3) increase in SHPS-1 phosphorylation (Fig. 3A). In contrast, after incubation of SMC for 4 h with g-OxLDL, IGF-I stimulated a significant 7 ± 1.2-fold (mean ± sem, n = 3; P < 0.01) increase in SHPS-1 phosphorylation (Fig. 3A). n-LDL had no significant effect on IGF-I-stimulated SHPS-1 phosphorylation [a 3.7 ± 0.7-fold increase (mean ± sem, n = 3), P = not significant compared with IGF-I alone].
Fig. 3.
g-OxLDL enhances IGF-I-stimulated SHPS-1 phosphorylation and Shc recruitment to SHPS-1. SMC were grown to confluency in medium containing 5 mm glucose before overnight incubation in SFM. g-OxLDL (5 μg/ml) or n-LDL (5 μg/ml) was added for 4 h. IGF-I was added (100 ng/ml) for 5 min at the end of the 4-h incubation before lysing of the cells. A, SHPS-1 phosphorylation was determined by immunoprecipitating (IP) with an anti-SHPS-1 antibody and immunoblotting with an anti-phosphotyrosine antibody (p-Tyr). Equal amounts of cell lysates were also immunoprecipitated with the anti-SHPS-1 antibody and immunoblotted with the same antibody to demonstrate that the difference in response was not due to different amounts of SHPS-1 protein. The graph shows the increase in SHPS-1 phosphorylation in response to IGF-I (mean ± sem, n = 3). **, P < 0.01 when SHPS-1 phosphorylation in response to IGF-I in the presence of g-OxLDL is compared with the response to IGF-I alone. B, The extent of Shc association with SHPS-1 was determined by immunoprecipitating (IP) cell lysates with an anti-Shc antibody and then immunoblotting with an anti-SHPS-1 antibody. Equal amounts of cell lysates were also immunoprecipitated with the anti-Shc antibody and immunoblotted with the same antibody. The graph shows the increase in Shc association with SHPS-1 in response to IGF-I (mean ± sem, n = 3). **, P < 0.01 when Shc recruitment to SHPS-1 in response to IGF-I in the presence of g-OxLDL is compared with the response to IGF-I alone. The results shown are representative of three similar experiments performed independently.
Exposure of SMC to g-OxLDL enhances Shc recruitment to SHPS-1 in response to IGF-I
Recruitment of the adaptor protein Shc to the phosphorylated tyrosine residues of the cytoplasmic tail of SHPS-1 in response to IGF-I is necessary for recruitment of Shc and subsequently Shc phosphorylation (35). Because incubation of SMC maintained in 5 mm glucose with g-OxLDL increases basal IAP association with SHPS-1 and SHPS-1 phosphorylation in response to IGF-I, we next examined Shc recruitment to SHPS-1. There was no change in Shc recruitment to SHPS-1 [1 ± 0.1-fold (mean ± sem, n = 3)] in SMC grown in 5 mm glucose in response to IGF-I, consistent with the lack of SHPS-1 phosphorylation (Fig. 3B). In contrast, after a 4-h incubation with g-OxLDL, IGF-I stimulated a 3.2 ± 0.6-fold (mean ± sem, n = 3; P < 0.01) increase in Shc recruitment to SHPS-1.
Exposure of SMC to g-OxLDL enhances Shc phosphorylation in response to IGF-I in a CD36-dependent manner
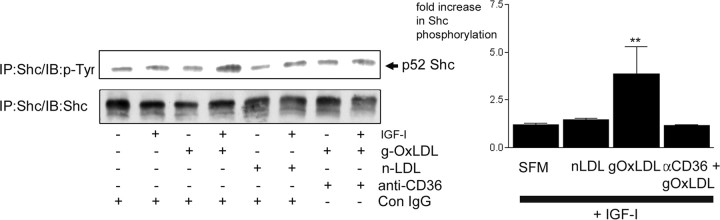
We have determined previously that the proliferative response of SMC to IGF-I requires phosphorylation of the adaptor protein Shc, which is required for activation of the MAPK pathway (35). The lack of proliferative response to IGF-I, when SMC grown are maintained 5 mm glucose, is attributable to a lack of Shc phosphorylation due to the absence of formation of the IAP-SHPS-1-Shc signaling complex (8). We next determined whether the ability of g-OxLDL to enhance cell proliferation in response to IGF-I was associated with enhanced Shc phosphorylation (8). After treatment with IGF-I alone, there was no significant increase in Shc phosphorylation [a 1.2 ± 0.1-fold increase (mean ± sem, n = 3)]. However, in the presence of g-OxLDL, IGF-I stimulated a significant 3.9 ± 1-fold (mean ± sem, n = 3; P < 0.01) increase in Shc phosphorylation (Fig. 4). Because the enhancing effect of g-OxLDL on IGF-I-stimulated proliferation was mediated in a CD36-dependent manner, we determined whether its enhancing effect on Shc phosphorylation was also dependent upon CD36. In the presence of the CD36 antiserum, the ability of g-OxLDL to enhance Shc phosphorylation in response to IGF-I was completely blocked [a 1.2 ± 0.3-fold increase (mean ± sem, n = 3)].
Fig. 4.
g-OxLDL enhances IGF-I-stimulated Shc phosphorylation in a CD36-dependent manner. SMC were grown to confluency in medium containing 5 mm glucose before overnight incubation in SFM. g-OxLDL (5 μg/ml) or n-LDL (5 μg/ml) was added for 4 h. Where indicated, either control IgG or anti-CD36 antiserum (anti-CD36) was added for 30 min before the addition of the LDL preparations. IGF-I (100 ng/ml) was added for 5 min at the end of the 4-h incubation before lysing the cells. Shc phosphorylation was determined by immunoprecipitating (IP) with an anti-Shc antibody and immunoblotting with an anti-phosphotyrosine antibody (p-Tyr). Equal amounts of cell lysates were also immunoprecipitated with the anti-Shc antibody and immunoblotted with the same antibody. The graph shows the fold increase in Shc phosphorylation in response to IGF-I (mean ± sem, n = 3). **, P < 0.01 when Shc phosphorylation in the presence of g-OxLDL and IGF-I is compared with Shc phosphorylation in the presence of IGF-I alone. The results shown are representative of the results of three similar experiments performed independently.
g-OxLDL enhances IGF-I signaling in a manner distinct from changes in αVβ3 ligand occupancy
We have determined previously that the response of SMC maintained in high glucose is dependent upon ligand occupancy of the αVβ3 integrin (8). In our previous studies, we demonstrated that there was a significant increase in the abundance of αVβ3 ligands including osteopontin, Vn, and thrombospondin in SMC grown in 25 compared with 5 mm glucose (8). Moreover, the addition of Vn to SMC maintained in 5 mm glucose is sufficient to permit a proliferative response to IGF-I (8). We next tested whether there was any cross-talk between the effects of g-OxLDL and αVβ3 ligand occupancy on IGF-I signaling responses. We have determined that binding of the Vn HBD to a specific region of the β3 subunit, referred to as the cysteine loop, is sufficient to mediate the positive effects of Vn on the response of SMC to IGF-I (20, 39). The addition of a peptide homologous to the Vn HBD was sufficient to permit a 1.8 ± 0.08-fold increase in cell number in response to IGF-I. The addition of g-OxLDL was accompanied by a 2.0 ± 0.12-fold increase in cell number in response to IGF-I, in contrast to the lack of cellular response when IGF-I was added alone (mean ± sem, n = 2; P < 0.01; Fig. 5A). However, when the Vn HBD and g-OxLDL were added at the same time, the increase in response to IGF-I was greater than with either alone (a further 1.8 ± 0.2-fold increase in response to IGF-I compared with IGF-I and g-OxLDL alone, mean ± sem; n = 2; P < 0.01). As shown in Fig. 1, addition of the anti-CD36 antibody effectively blocked the enhancing effect of g-OxLDL; however, it had no effect on the ability of the Vn HBD to enhance IGF-I-stimulated proliferation (Fig. 5A). When the anti-CD36 antibody was added at the same time as both Vn HBD and g-OxLDL, the stimulatory effect was reduced to a level similar to that seen the Vn HBD was added with IGF-I. Because the anti-CD36 antibody inhibits the effects of g-OxLDL but not the Vn HBD, this suggests that stimulation of αVβ3 by the Vn HBD is enhancing cell proliferation independently of CD36 in response to IGF-I.
Fig. 5.
CD36 blocks g-OxLDL but not Vn enhancement of IGF-I-stimulated SMC proliferation. A, Cells 2 × 104) were plated in each well of a 24-well plate before exposure to g-OxLDL (5 μg/ml) with or without the anti-CD36 antiserum or control antiserum (Con IgG), a peptide homologous to the Vn HBD (10 μg/ml) or g-OxLDL and Vn HBD with or without the anti-CD36 antiserum (CD36). All treatments were prepared in DMEM plus 0.2% platelet-poor plasma. At 48 h after the addition of IGF-I (50 ng/ml), cell number was determined by trypan blue staining and counting. *, P < 0.05 when cell number in response to IGF-I is compared with SFM alone. **, P < 0.01 when cell number in response to IGF-I in the presence of g-OxLDL/Vn HBD is compared with the number of cells in the presence of IGF-I alone. B, SMC were grown to confluency in medium containing 5 mm glucose before overnight incubation in SFM. g-OxLDL (5 μg/ml), n-LDL (5 μg/ml), or the Vn HBD (10 μg/ml) was added for 4 h. After lysis, β3 phosphorylation was detected by immunoprecipitation (IP) with an anti-β3 antibody and immunoblotting with an anti-phosphotyrosine antibody (p-Tyr). To demonstrate that an equivalent amount of protein was loaded in each lane, samples were also immunoblotted with an anti-β3 antibody.
To further exclude the possibility that g-OxLDL was signaling through αVβ3, we examined the effect of g-OxLDL on tyrosine phosphorylation of residues within the cytoplasmic domain of β3 that are critical for the enhancing effects of ligands that bind to αVβ3 on the response of SMC to IGF-I (18). Addition of either g-OxLDL or n-LDL had no effect on the level of β3 phosphorylation, whereas the Vn HBD peptide stimulated an increase in β3 phosphorylation (Fig. 5B).
Discussion
Previous studies have shown that low concentrations of OxLDL can directly stimulate or enhance IGF-I-stimulated SMC proliferation (22, 23). Our results showed that the addition of g-OxLDL can enhance the response of SMC to IGF-I when SMC are cultured in 5 mm glucose. The mechanism by which g-OxLDL enhances the response to IGF-I is due, in part, to its ability to protect IAP from cleavage. When SMC are grown in medium containing physiologically normal glucose concentrations, IAP is constitutively cleaved (10). The addition of g-OxLDL protects IAP from cleavage, leading to an increase in the formation of the IAP-SHPS-1-Shc signaling complex, thereby allowing Shc phosphorylation, which is required for the positive effect of IGF-I on SMC proliferation. The enhancing effects of g-OxLDL appear to be dependent on CD36 because the presence of the anti-CD36 antibody blocked the enhancing effects of g-OxLDL on both IGF-I-stimulated Shc phosphorylation and cell proliferation.
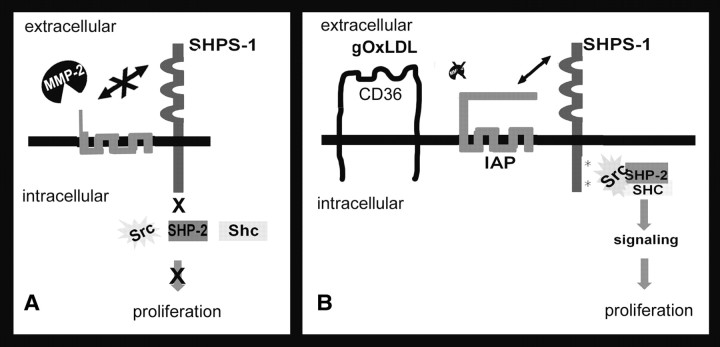
Previous studies have shown that LDL can interact with the IGF-I signaling pathway at multiple levels including enhancement of IGF-IR phosphorylation, up-regulation of the phosphatidylinositol-3 kinase and MAPK pathway activation as well as increases in IGF-IR and IGF-I (22, 23). However, in these studies, addition of g-OxLDL was not associated with an increase in intrinsic kinase activity of the IGF-IR, and consistent with our previous studies (35), g-OxLDL enhanced the ability of IGF-I to stimulate Shc phosphorylation. That Shc phosphorylation is needed to stimulate SMC growth was shown by expressing a dominant-negative form of Shc, in which the tyrosines have been substituted for phenylalanines and showing that this resulted in failure of IGF-I to stimulate a proliferative response (35). Phosphorylation of Shc requires the formation of a multiprotein complex on phosphorylated SHPS-1. Association of IAP and SHPS-1 is required to stimulate SHPS-1 phosphorylation. Culturing cells in 5 mm glucose results in a failure to form this signaling complex due to IAP cleavage. In contrast, exposure to g-OxLDL protects IAP from cleavage and is associated with an increase in IAP association with SHPS-1, leading to SHPS-1 phosphorylation and recruitment of Shc. Taken together, these observations suggest that the protection of IAP from cleavage is a major determinant of the changes in signaling in response to IGF-I that occurs in the presence of g-OxLDL (Fig. 6).
Fig. 6.
Diagrammatic representation of the regulation of IGF-I signaling by OxLDL increase in IAP association with SHPS-1. When SMC are grown in normal glucose-containing medium, the cleavage of IAP by MMP-2 prevents its interaction with SHPS-1. The consequence of this cleavage is an inability of IGF-I to stimulate SHPS-1 phosphorylation and therefore failure to form the SHPS-1-SHP-2-Src-Shc signaling complex that is required for signaling (A). In contrast, when SMC are exposed to g-OxLDL, the amount of MMP-2 activity in the conditioned medium is reduced, thus limiting the cleavage of IAP. Consequently, the association of IAP with SHPS-1 is enhanced, resulting in increased phosphorylation of SHPS-1 in response to IGF-I. The formation of this signaling complex then facilitates the proliferative response of SMC to IGF-I (B).
Our recent studies have determined that when SMC are maintained in 5 mm glucose, IAP is constitutively cleaved (10). Similarly, the IAP detected in aortic homogenates prepared from normoglycemic mice was almost completely in the fragmented state (11). Because the addition of MMP-2 inhibitors or the reduction in MMP-2 using RNA interference protects IAP from cleavage, it seems likely that MMP-2 is the protease responsible for the cleavage of IAP (10). Because incubation of SMC with g-OxLDL increased the amount of MMP-2 protease activity in the conditioned medium, it is reasonable to conclude that this protease contributes to the increase in intact IAP. OxLDL has been reported to modulate MMP-2 levels and activity (37, 38). Treatment of SMC with 5 μg/ml OxLDL, a concentration that has been shown to enhance SMC proliferation and equivalent to the concentration used in this study, was sufficient to significantly reduce the level of MMP-2 and MMP-2 protease activity released into the conditioned medium (37). However, in contrast to this previous study, we did not detect any change in MMP-2 protein. Therefore, the mechanism by which g-OxLDL decreases MMP-2 protease activity remains to be determined.
Exposure to higher concentrations of OxLDL, equivalent to those that have been shown to stimulate SMC apoptosis, were shown to increase MMP-2 concentrations and enzymatic activity (37, 38). Taken together, these results suggest that there is an association between OxLDL concentration and MMP-2 activity. The biphasic effects of OxLDL on SMC responses and MMP-2 correlate well with the changes that occur during the development of atherosclerotic lesions. Early lesions are characterized by initial accumulation of mildly OxLDL, SMC proliferation, and a decrease in MMP activity as well as an associated increase in extracellular matrix accumulation. However, in advanced lesions, there is increased accumulation of more highly OxLDL, SMC apoptosis, and an increase in MMP activity associated with degradation of the extracellular matrix.
The results from these studies suggest that g-OxLDL mediates its enhancing effects through its interaction with the scavenger receptor CD36 because protection of IAP from cleavage, Shc phosphorylation, and the increase in cell proliferation were inhibited in the presence of the anti-CD36 antibody. However, in contrast to other reports that suggested g-OxLDL exerted its effects through JNK activation (33, 34), we determined that the effect of g-OxLDL on IAP was not dependent upon JNK because a cell-permeable peptide that has been shown to block JNK activity did not prevent the protective effect of g-OxLDL.
In addition to CD36, other scavenger receptors, for example the lectin-like OxLDL receptor-1 (LOX-1) have been implicated in mediating the effects of modified LDL on SMC. LOX-1 was first identified on endothelial cells (40) but later also shown to be expressed on SMC, and its expression is increased in atherosclerotic lesions (41). We did not analyze LOX-1 and therefore cannot exclude the possibility that some of the effects that we noted after g-OxLDL addition could be mediated through this receptor. However, the fact that an antibody that is specific for the CD36 receptor inhibited these actions makes this possibility less likely.
The mechanism by which g-OxLDL stimulates the decrease in MMP-2 activity and consequently the increase in intact IAP remain to be determined. The CD36 receptor has been shown to mediate internalization of OxLDL by macrophages leading to foam cell formation (34). Although CD36 mediates OxLDL uptake in SMC (31), the roles of internalized vs. cell-membrane-associated OxLDL remain to be fully defined. Chatterjee and Ghosh (42) have shown that the growth-stimulatory effects of OxLDL do not occur at 4 C, suggesting it requires receptor-mediated uptake. They determined that OxLDL exerted its stimulatory effects on SMC growth by increasing lactosyl ceramide synthase leading to an increase in NAD(P)H oxidase, an increase in reactive oxygen species, and subsequent activation of the RAS/RAF/MAPK pathway (43). Whether OxLDL binding to CD36 induces a similar change was not investigated. Additionally, the role of the CD36 receptor and/or OxLDL uptake in modulating MMP-2 activity was not investigated (37, 38). Further studies will therefore be required to determine the molecular events that mediate the CD36-dependent effects on MMP-2 activity levels in SMC.
Accurately replicating the concentration, duration of exposure, and extent of oxidation of g-OxLDL that occurs in vivo is beyond the limitations of short-term cell culture experiments. However, it can be argued that the enhancing effect of low concentrations of g-OxLDL on SMC proliferation and the apoptotic effects of higher concentrations are good mimics of the events that occur in vivo during the development and progression of atherosclerosis, thus providing a reasonable model for understanding the contribution of OxLDL to the response of SMC to changes in the extracellular environment in patients with diabetes.
In vivo hyperglycemia is associated with a number of changes in the extracellular matrix surrounding the SMC. Our previous studies have shown that the hyperglycemia-stimulated increase in αVβ3 ligands may contribute to the enhanced response of SMC to IGF-I under these conditions (8). In this study, we demonstrate that glucose oxidation of LDL may also enhance the proliferative response of SMC. Because the anti-CD36 antibody effectively inhibited the increase in response to IGF-I in the presence of g-OxLDL, but not the Vn HBD, and only partially inhibited the response when both agents were added together, the results suggest that these two ligands enhance IGF-I-stimulated proliferation of SMC through distinct molecular pathways. Because we have shown that g-OxLDL protects IAP from cleavage and the presence of intact IAP is critical for the enhanced response of SMC in 25 mm glucose to IGF-I, these findings suggest that these two occupancy pathways converge at the point of protecting IAP from cleavage (Fig. 6). Further studies will be necessary to fully elucidate the relative contribution of g-OxLDL and αVβ3 ligand occupancy in determining the vascular SMC response to IGF-I in the presence of sustained hyperglycemia.
Footnotes
This work was funded by an American Heart Association Mid Atlantic Affiliate Beginning Grant in Aid (0465462U) and Atorvastatin Research Award to L.A.M.
Disclosure Statement: The authors have nothing to disclose.
First Published Online October 30, 2008
Abbreviations: FBS, Fetal bovine serum; g-OxLDL, glucose-oxidized LDL; HBD, heparin-binding domain; IAP, integrin-associated protein; IGF-IR, IGF-I receptor; JNK, c-Jun N-terminal kinase; LDL, low-density lipoprotein; LOX-1, lectin-like OxLDL receptor-1; MDA, malondialdehyde; MMP-2, matrix metalloprotease-2; n-LDL, native LDL; OxLDL, oxidized LDL; SFM, serum-free medium; SHPS-1, Src homology 2 domain-containing protein tyrosine phosphatase substrate-1; SMC, smooth muscle cells; Vn, vitronectin.
References
- 1.Kannel WB, McGee DL 1979. Diabetes and cardiovascular risk factors: the Framingham study. Circulation 59:8–13 [DOI] [PubMed] [Google Scholar]
- 2.Laing SP, Swerdlow AJ, Slater SD, Burden AC, Morris A, Waugh NR, Gatling W, Bingley PJ, Patterson CC 2003. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 46:760–765 [DOI] [PubMed] [Google Scholar]
- 3.Moss SE, Klein R, Klein BE 1991. Cause-specific mortality in a population-based study of diabetes. Am J Public Health 81:1158–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross R 1993. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362:801–809 [DOI] [PubMed] [Google Scholar]
- 5.Jones JI, Prevette T, Gockerman A, Clemmons DR 1996. Ligand occupancy of the α-V-β3 integrin is necessary for smooth muscle cells to migrate in response to insulin-like growth factor. Proc Natl Acad Sci USA 93:2482–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cercek B, Fishbein MC, Forrester JS, Helfant RH, Fagin JA 1990. Induction of insulin-like growth factor I messenger RNA in rat aorta after balloon denudation. Circ Res 66:1755–1760 [DOI] [PubMed] [Google Scholar]
- 7.Hayry P, Myllarniemi M, Aavik E, Alatalo S, Aho P, Yilmaz S, Raisanen-Sokolowski A, Cozzone G, Jameson BA, Baserga R 1995. Stabile d-peptide analog of insulin-like growth factor-1 inhibits smooth muscle cell proliferation after carotid ballooning injury in the rat. FASEB J 9:1336–1344 [DOI] [PubMed] [Google Scholar]
- 8.Maile LA, Capps BE, Ling Y, Xi G, Clemmons DR 2007. Hyperglycemia alters the responsiveness of smooth muscle cells to insulin-like growth factor-I. Endocrinology 148:2435–2443 [DOI] [PubMed] [Google Scholar]
- 9.Maile LA, Badley-Clarke J, Clemmons DR 2003. The association between integrin-associated protein and SHPS-1 regulates insulin-like growth factor-I receptor signaling in vascular smooth muscle cells. Mol Biol Cell 14:3519–3528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maile LA, Capps BE, Miller EC, Allen LB, Veluvolu U, Aday AW, Clemmons DR 2008. Glucose regulation of integrin-associated protein cleavage controls the response of vascular smooth muscle cells to insulin-like growth factor-I. Mol Endocrinol 22:1226–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maile LA, Capps BE, Miller EC, Aday AW, Clemmons DR 2008. Integrin-associated protein association with Src homology 2 domain containing tyrosine phosphatase substrate 1 regulates IGF-I signaling in vivo. Diabetes 57:2637–2643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yla-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S, Carew TE, Butler S, Witztum JL, Steinberg D 1989. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest 84:1086–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Illingworth DR, Durrington PN 1999. Dyslipidemia and atherosclerosis: how much more evidence do we need? Curr Opin Lipidol 10:383–386 [DOI] [PubMed] [Google Scholar]
- 14.Lusis AJ 2000. Atherosclerosis. Nature 407:233–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witztum JL, Steinberg D 2001. The oxidative modification hypothesis of atherosclerosis: does it hold for humans? Trends Cardiovasc Med 11:93–102 [DOI] [PubMed] [Google Scholar]
- 16.Lamharzi N, Renard CB, Kramer F, Pennathur S, Heinecke JW, Chait A, Bornfeldt KE 2004. Hyperlipidemia in concert with hyperglycemia stimulates the proliferation of macrophages in atherosclerotic lesions: potential role of glucose-oxidized LDL. Diabetes 53:3217–3225 [DOI] [PubMed] [Google Scholar]
- 17.Liguori A, Abete P, Hayden JM, Cacciatore F, Rengo F, Ambrosio G, Bonaduce D, Condorelli M, Reaven PD, Napoli C 2001. Effect of glycaemic control and age on low-density lipoprotein susceptibility to oxidation in diabetes mellitus type 1. Eur Heart J 22:2075–2084 [DOI] [PubMed] [Google Scholar]
- 18.Ling Y, Maile LA, Clemmons DR 2003. Tyrosine phosphorylation of the β3-subunit of the αVβ3 integrin is required for membrane association of the tyrosine phosphatase SHP-2 and its further recruitment to the insulin-like growth factor I receptor. Mol Endocrinol 17:1824–1833 [DOI] [PubMed] [Google Scholar]
- 19.Maile LA, Clemmons DR 2003. Integrin-associated protein binding domain of thrombospondin-1 enhances insulin-like growth factor-I receptor signaling in vascular smooth muscle cells. Circ Res 93:925–931 [DOI] [PubMed] [Google Scholar]
- 20.Maile LA, Busby WH, Sitko K, Capps BE, Sergent T, Badley-Clarke J, Clemmons DR 2006. IGF-I signaling in smooth muscle cells is regulated by ligand binding to the 177CYDMKTTC184 sequence of the β3 subunit of αVβ3. Mol Endocrinol 20:881–892 [DOI] [PubMed] [Google Scholar]
- 21.Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA 1993. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem 268:11811–11816 [PubMed] [Google Scholar]
- 22.Gonzalez B, Lamas S, Melian EM 2001. Cooperation between low density lipoproteins and IGF-I in the promotion of mitogenesis in vascular smooth muscle cells. Endocrinology 142:4852–4860 [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Timon B, Gonzalez-Munoz M, Zaragoza C, Lamas S, Melian EM 2004. Native and oxidized low density lipoproteins oppositely modulate the effects of insulin-like growth factor I on VSMC. Cardiovasc Res 61:247–255 [DOI] [PubMed] [Google Scholar]
- 24.Ross R 1971. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol 50:172–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki S, Pierschbacher MD, Hayman EG, Nguyen K, Ohgren Y, Ruoslahti E 1984. Domain structure of vitronectin. Alignment of active sites. J Biol Chem 259:15307–15314 [PubMed] [Google Scholar]
- 26.Kaneto H, Nakatani Y, Miyatsuka T, Kawamori D, Matsuoka TA, Matsuhisa M, Kajimoto Y, Ichijo H, Yamasaki Y, Hori M 2004. Possible novel therapy for diabetes with cell-permeable JNK-inhibitory peptide. Nat Med 10:1128–1132 [DOI] [PubMed] [Google Scholar]
- 27.Ho A, Schwarze SR, Mermelstein SJ, Waksman G, Dowdy SF 2001. Synthetic protein transduction domains: enhanced transduction potential in vitro and in vivo. Cancer Res 61:474–477 [PubMed] [Google Scholar]
- 28.Clemmons DR, Maile LA 2005. Interaction between insulin-like growth factor-I receptor and αVβ3 integrin linked signaling pathways: cellular responses to changes in multiple signaling inputs. Mol Endocrinol 19:1–11 [DOI] [PubMed] [Google Scholar]
- 29.Scheidegger KJ, James RW, Delafontaine P 2000. Differential effects of low density lipoproteins on insulin-like growth factor-1 (IGF-1) and IGF-1 receptor expression in vascular smooth muscle cells. J Biol Chem 275:26864–26869 [DOI] [PubMed] [Google Scholar]
- 30.Okura Y, Brink M, Zahid AA, Anwar A, Delafontaine P 2001. Decreased expression of insulin-like growth factor-1 and apoptosis of vascular smooth muscle cells in human atherosclerotic plaque. J Mol Cell Cardiol 33:1777–1789 [DOI] [PubMed] [Google Scholar]
- 31.Ricciarelli R, Zingg JM, Azzi A 2000. Vitamin E reduces the uptake of oxidized LDL by inhibiting CD36 scavenger receptor expression in cultured aortic smooth muscle cells. Circulation 102:82–87 [DOI] [PubMed] [Google Scholar]
- 32.Higashi Y, Peng T, Du J, Sukhanov S, Li Y, Itabe H, Parthasarathy S, Delafontaine P 2005. A redox-sensitive pathway mediates oxidized LDL-induced downregulation of insulin-like growth factor-1 receptor. J Lipid Res 46:1266–1277 [DOI] [PubMed] [Google Scholar]
- 33.Metzler B, Hu Y, Dietrich H, Xu Q 2000. Increased expression and activation of stress-activated protein kinases/c-Jun NH2-terminal protein kinases in atherosclerotic lesions coincide with p53. Am J Pathol 156:1875–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL 2006. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab 4:211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ling Y, Maile LA, Lieskovska J, Badley-Clarke J, Clemmons DR 2005. Role of SHPS-1 in the regulation of insulin-like growth factor I-stimulated Shc and mitogen-activated protein kinase activation in vascular smooth muscle cells. Mol Biol Cell 16:3353–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lieskovska J, Ling Y, Badley-Clarke J, Clemmons DR 2006. The role of Src kinase in insulin-like growth factor-dependent mitogenic signaling in vascular smooth muscle cells. J Biol Chem 281:25041–25053 [DOI] [PubMed] [Google Scholar]
- 37.Wilson D, Massaeli H, Pierce GN, Zahradka P 2003. Native and minimally oxidized low density lipoprotein depress smooth muscle matrix metalloproteinase levels. Mol Cell Biochem 249:141–149 [PubMed] [Google Scholar]
- 38.Auge N, Maupas-Schwalm F, Elbaz M, Thiers JC, Waysbort A, Itohara S, Krell HW, Salvayre R, Negre-Salvayre A 2004. Role for matrix metalloproteinase-2 in oxidized low-density lipoprotein-induced activation of the sphingomyelin/ceramide pathway and smooth muscle cell proliferation. Circulation 110:571–578 [DOI] [PubMed] [Google Scholar]
- 39.Maile LA, Busby WH, Sitko K, Capps BE, Sergent T, Badley-Clarke J, Ling Y, Clemmons DR 2006. The heparin-binding domain of vitronectin is the region that is required to enhance insulin-like growth factor-I signaling. Mol Endocrinol 20:405–411 [DOI] [PubMed] [Google Scholar]
- 40.Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, Masaki T 1997. An endothelial receptor for oxidized low-density lipoprotein. Nature 386:73–77 [DOI] [PubMed] [Google Scholar]
- 41.Draude G, Hrboticky N, Lorenz RL 1999. The expression of the lectin-like oxidized low-density lipoprotein receptor (LOX-1) on human vascular smooth muscle cells and monocytes and its down-regulation by lovastatin. Biochem Pharmacol 57:383–386 [DOI] [PubMed] [Google Scholar]
- 42.Chatterjee S, Ghosh N 1996. Oxidized low density lipoprotein stimulates aortic smooth muscle cell proliferation. Glycobiology 6:303–311 [DOI] [PubMed] [Google Scholar]
- 43.Chatterjee S, Bhunia AK, Snowden A, Han H 1997. Oxidized low density lipoproteins stimulate galactosyltransferase activity, ras activation, p44 mitogen activated protein kinase and c-fos expression in aortic smooth muscle cells. Glycobiology 7:703–710 [DOI] [PubMed] [Google Scholar]