Abstract
Background
Echocardiographic assessment of left ventricular assist devices (LVADs) is used as a screening tool to evaluate the integrity and mechanics of the pump and circuit. We aimed to 1) establish the normal range and upper reference limit of peak velocity of the outflow cannula for the modern era of LVADs and 2) assess the clinical performance of the currently cited and newly proposed reference limits in patients with continuous-flow LVADs as a screening tool for cannula malfunction.
Methods
LVAD outflow peak CW velocities were measured with the use of Doppler transthoracic echocardiography (TTE) in 57 patients with LVADs (44 with Heartmate II (HM2), 13 with Heartware (HW)). The average velocity and the upper and lower normal reference limits (defined as ±2 standard deviations from the mean) for each LVAD type was calculated. The upper reference limit was then used as a screening threshold for cannula malfunction.
Results
The average outflow cannula peak velocity for the normal HM2 cohort was 1.86 ± 0.44 m/s with upper and lower reference limits of 2.73 m/s and 0.98 m/s, respectively. The average outflow cannula peak velocity for the normal HW cohort was 2.36 ±0.53 m/s with upper and lower reference limits of 3.42 m/s and 1.3 m/s, respectively, which was significantly higher than the HM2 cohort (P = .004).
Conclusions
In both HM2 and HW LVADs, the average peak outflow velocity and reference limit for the normal population, as measured by Doppler TTE, was markedly higher than the currently used LVAD reference limits of 2 m/s and are significantly different between devices. Patients with peak outflow velocities above our upper reference limits should be evaluated for LVAD outflow cannula malfunction.
Keywords: LVAD, outflow cannula, device malfunction
Left ventricular assist devices (LVADs) have rapidly become the mainstay of care for patients with advanced heart failure both as bridge to transplantation and as destination therapy. Given the current environment of heart allocation limitations, together with a growing list of transplant-ineligible patients, LVAD support is expected to rapidly increase over the coming years.1 Transthoracic echocardiography (TTE) has emerged as an important imaging modality for the assessment of both normal LVAD physiology and device malfunction. Much of our current knowledge about LVAD imaging, including the cited upper reference limit of the LVAD outflow cannula of 2 m/s, was derived from studies investigating currently discontinued pulsatile-flow LVADs or 1st-generation continuous-flow LVADs (CF-LVADs), namely the Jarvik 2000 and the DeBakey pump, with different outflow graft size and properties.2–4 Currently, the armamentarium of LVADs has evolved, and 2nd- and 3rd-generation LVADs with either axial or centrifugal flow, that can be placed intrathoracically or span both the thoracic and the abdominal compartments, are used.5 Imaging studies involving the newer generation of LVADs are less prevalent, and accordingly our knowledge of normal parameters assessing flow through the LVAD circuits are often extrapolated from previous-generation LVADs and may be inaccurate for the current generation of devices.2
The LVAD outflow cannula is a key component of the LVAD circuit that can readily be imaged with the use of TTE. After blood passes through the LVAD rotor, it is shuttled into the LVAD outflow cannula before rejoining the circulation at the anastomotic site of the cannula with the ascending aorta. Doppler TTE imaging of the LVAD outflow cannula can also be used to assess for cannula thrombosis, kinking, or excessive angulation at the site of anastomosis.6,7 Currently, a peak continuous-wave Doppler velocity of the LVAD outflow cannula of >2 m/s is thought to be suggestive of outflow cannula malfunction for CF-LVADs.2,4 Doppler TTE of the outflow cannula is also used to screen for device thrombosis and assess for the presence and severity of aortic insufficiency.8–11 The currently available newer CF-LVADs have different LVAD outflow cannula properties, including different size (14 mm for Heartmate 2 and 10 mm for HVAD), and therefore are not be expected to have the same flow properties as previous-generation LVADs when assessed with the use of Doppler TTE.12,13 Currently used reference limits for interpreting LVAD outflow cannula flow velocities do not take into account the known differences in both pump design and outflow cannula size of the different devices. In the present study, we provide, for the 1st time, the normal velocity profiles of the 2 most commonly used CF-LVADs and demonstrate the clinical value of these new reference values as a screening tool for outflow cannula malfunction.
Methods
Patient Population
Fifty-seven sequential LVAD patients (44 with Heartmate II [HM2; Thoratec Corp, Pleasanton, California] and 13 with Heartware HVAD [HVAD; Heartware International, Framingham, Massachusetts) were prospectively enrolled in this study. All patients underwent echocardiographic image acquisition from September 2014 to June 2015 including Doppler outflow cannula velocity assessment by 2 sonographers trained in imaging the LVAD outflow cannula. Images were acquired during either routine outpatient follow-up or a subsequent hospitalization. Images were not acquired during the index hospitalization. Patients were excluded from analysis if they had inadequate echocardiographic windows. Demographic information, type and duration of LVAD implant, and complication rates of the LVAD outflow cannula were recorded. This study was approved by the Institutional Review Board at the University of Chicago.
Echocardiographic Imaging
All patients underwent a complete 2-dimensional and Doppler evaluation of the heart and LVAD circuit with the use of the iE33 imaging system and an S5 transducer (Philips Healthcare, Andover, Massachusetts). Offline analysis of the images was performed with the use of the Xcelera Workstation (Philips Healthcare). From the parasternal window, linear measurements were obtained to measure left ventricular chamber size.14 Aortic valve opening was assessed with the use of M-mode echocardiography. Mitral and aortic regurgitation severity assessment was made using vena contracta width when available, and a qualitative assessment was performed when the vena contracta was unavailable, per guidelines.15 To allow quantitative comparison between groups, regurgitation severity was scored as follows: No mitral or aortic regurgitation was assigned a value of 0, trace regurgitation 0.5, mild regurgitation 1, mild to moderate regurgitation 1.5, moderate regurgitation 2, moderate to severe regurgitation 2.5, and severe regurgitation 3. The LVAD outflow cannula was imaged with the use of 2-dimensional imaging together with continuous- and pulse-wave Doppler signals acquired from a modified right parasternal view, as previously described.9,16 The LVAD outflow cannula was aligned with the transducer just proximal to the anastomotic site with the ascending thoracic aorta. The cannula diameter was measured at the site of Doppler acquisition. Peak velocity was defined as the end-systolic velocity of the outflow cannula on continuous-wave Doppler acquisition. For patients with irregular rhythms, the average peak velocity of no fewer than 3 measurements was used for analysis.
Statistical Methods
Data was collected with the use of Excel software (2011 Microsoft Corp, Redmond, Washington) and analyzed with the use of Graphpad Prism (Graphpad Software, San Diego, California). Normality of continuous variables was assessed with the use of the d’Agostino–Pearson test for normal distribution and the Student t test to determine differences in the means of normally distributed data. Categoric variables were analyzed with the use of the Fisher exact test. The upper and lower reference limits for the outflow cannula peak velocity was defined as the 95% prediction interval (±2 standard deviations from the mean) for the population of patients with normally functioning devices without cannula malfunction. Interobserver variability and intra-observer variability was assessed with the use of intraclass correlation measurements of the peak systolic velocity of the LVAD outflow cannula. Each patient was analyzed separately and given equal weight throughout the analysis.
Results
Baseline Characteristics
TTE was performed in 57 patients, and the baseline characteristics of the cohort are reported in Table 1. The patients’ ages ranged from 28 to 77 (mean 58) years and 72% were male; 23% (n = 13) of the patients had HVAD and 77% (n = 44) of patients had HM2. LVAD was implanted as destination therapy in the majority of patients in the HM2 group but not in the HVAD group (79.5% vs 46.2%; P = .03). Images were acquired an average of 20.4 months (range 1–65 months) after LVAD implantation in the HM2 group and 11.8 months (range 1–42 months) after implantation in the HVAD group (P = .15). Comorbid conditions were similar between both groups. There was a trend toward having more strokes in the HVAD group despite a trend toward more incidence of atrial fibrillation in the HM2 group. There was no difference in mean arterial pressure at the time of TTE acquisition between the HM2 and HVAD groups (88 mm Hg vs 83 mm Hg, P = .24) or between the normal population and the patients with confirmed outflow cannula malfunction (87 mm Hg and 82 mm Hg; P = .45). Ten patients had mean blood pressures =100 mm Hg. The mean outflow cannula velocity of these 10 patients was 1.93 ± 0.66 m/s. One HVAD patient had a mean arterial pressure of =60 mm Hg, and the outflow cannula peak velocity for this patient was 2.6 m/s.
Table 1.
Baseline Characteristics Stratified by Left Ventricular Assist Device (LVAD) Type
| Characteristics | All Patients (n = 57) | HM2 (n = 44) | HVAD (n = 13) | P Value |
|---|---|---|---|---|
| General characteristics | ||||
| Age, y, mean ± SD | 58.1 ± 11.5 | 59.4 ± 11.1 | 53.3 ± 11.8 | .09 |
| Male, % | 71.9 | 72.7 | 69.2 | 1.0 |
| Origin of cardiomyopathy, % | .53 | |||
| Ischemic | 50.9 | 47.7 | 61.5 | |
| Nonischemic | 49.1 | 52.3 | 38.5 | |
| LVAD characteristics | ||||
| Duration of LVAD, mo, mean ± SD | 18.4 ± 19.0 | 20.4 ± 20.1 | 11.8 ± 12.8 | .15 |
| Destination, %: | .03 | |||
| BTT | 28.1 | 20.5 | 53.8 | |
| DT | 71.9 | 79.5 | 46.2 | |
| Average speed, rpm, mean ± SD | NA | 9158 ± 392 | 2695 ± 134 | <.0001 |
| Medical history, % | ||||
| Hypertension | 61.4 | 65.9 | 46.1 | .21 |
| Hyperlipidemia | 43.9 | 40.9 | 53.8 | .53 |
| Atrial fibrillation | 26.3 | 31.8 | 7.7 | .15 |
| DM | 38.6 | 34.1 | 53.8 | .22 |
| COPD | 14.0 | 15.9 | 7.7 | .67 |
| PAD | 10.5 | 9.1 | 15.4 | .61 |
| CVA, % | 22.8 | 18.2 | 38.4 | .15 |
| Prior sternotomy | 22.8 | 18.2 | 38.4 | .15 |
HM2, Heartmate II; HVAD, Heartware HVAD; BTT, bridge to transplant; DT, destination therapy; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; PAD, peripheral artery disease; CVA, cardiovascular accident;
LVAD Outflow Cannula Imaging
The LVAD outflow cannula was successfully imaged in all patients. Adequate Doppler images were obtained in 54 of 57 patients (95%). In the 3 patients in whom Doppler signals were not measurable, the outflow cannula was found to be orthogonal to the transducer at the image acquisition site. The LVAD outflow cannula diameter was significantly smaller in the HVAD cohort compared with the HM2 cohort (8.4 ± 1 mm vs 12.8 ± 1 mm; P < .0001).
Screening for Outflow Cannula Malfunction
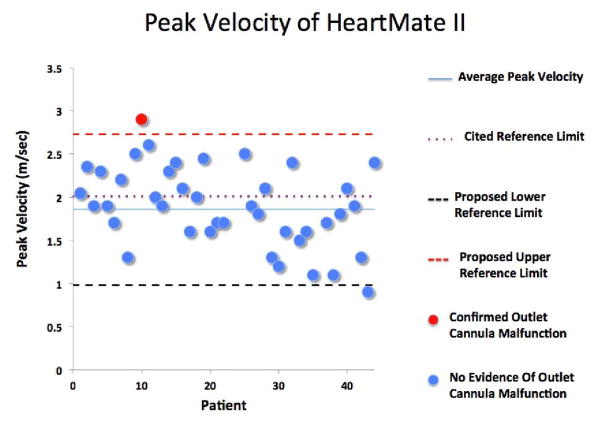
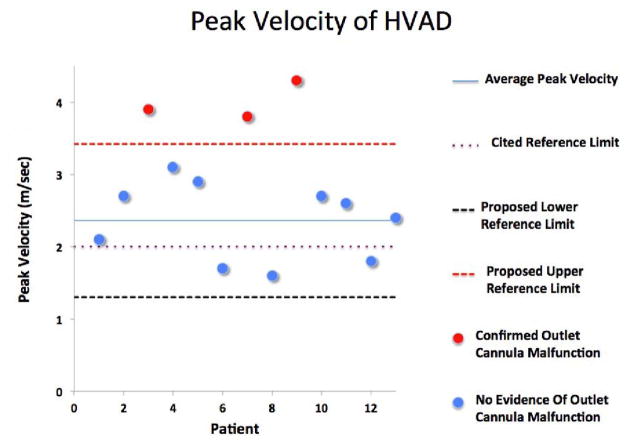
A complete chart review revealed that 4 patients (7%) developed outflow cannula obstruction (1 with HM2, 3 with HVAD). All decisions to evaluate for suspected LVAD outflow graft malfunction were made by clinicians who were blinded to the results of this analysis. Three of the patients had peak systolic velocities of the outflow cannula of =3.8 m/s as detected by echocardiography, and this together with clinical signs of deterioration prompted further investigation (Table 2). The 4th patient had evidence of outflow graft malfunction incidentally noted on computerized tomography (CT) imaging of the chest, which had been ordered for failure to thrive. Three patients had obstruction at the anastomotic site with the aorta, and 1 patient had a kink of the outflow cannula proximal to the anastomotic site. Outflow cannula malfunction was confirmed by either CT angiography and/or invasive catheterization of the outflow cannula in all patients (Fig. 1). Three of the 4 with confirmed outflow cannula obstruction had a clinical intervention. One patient had her outflow cannula stented with a covered stent; 1 patient later had LVAD pump thrombosis and catastrophic device failure and stoppage leading to percutaneous decommissioning of his device; and a 3rd patient was urgently transplanted. Fifty-three patients (93%) did not have outflow graft malfunction (normal population). The mean peak velocity of the outflow cannula for the normal population supported by the HM2 cohort was 1.86 ± 0.44 m/s with an upper reference limit for the normal HM2 cohort of 2.73 m/s (Table 3; Fig. 2). For the HVAD cohort, the mean peak velocity of the LVAD outflow cannula for the normal population was 2.36 ± 0.53 m/s with an upper reference limit for the normal HM2 cohort of 3.42 m/s (Table 3; Fig. 3). The mean peak velocity of the outflow cannula of the HVAD was significantly higher than the mean peak velocity of the HM2 (P = .004). Three patients were found to have myocardial recovery with an ejection fraction >40%. The outflow cannula velocities of these patients were 1.7 m/s, 2.6 m/s, and 1.7 m/s. Twelve patients with significant aortic insufficiency were identified. The mean outflow cannula flow velocity in those patients was 2.1 ± 0.73 m/s. The currently cited reference limit of 2 m/s poorly discriminated normal from abnormal LVAD outflow grafts (Figs. 2 and 3): 18 of 44 HM2 patients (41%) and 10 of 13 HVAD patients (77%) had peak systolic velocities of the outflow graft >2 m/s despite only 7% of the patients having confirmed malfunction of their graft. Using the new upper reference limits, as defined above, for the respective LVADs identified all 4 patients with LVAD cannula obstruction (sensitivity 100%, specificity 100%, and positive predictive value 100%; Figs. 2 and 3). In contrast, use of the commonly cited upper reference limit of 2 m/s poorly discriminated normal from abnormal outflow cannulas (sensitivity 100%, specificity 52.0%, positive predictive value 14.3%) (Table 4; Figs. 2 and 3). Interobserver and intra-observer variability for measuring the peak systolic velocity of the outflow cannula, as assessed by the intraclass correlation coefficient, were low (rI 0.99 and 0.99, respectively).
Table 2.
Transthoracic Echocardiographic (TTE) Parameters of Patients With Confirmed Outflow Cannula Malfunction
| Patients With Obstruction | |
|---|---|
| TTE Measurement | (n = 4) |
| AR grade, mean ± SD | 0.4 ± 0.3 |
| MR grade, mean ± SD | 0.6 ± 0.6 |
| Aortic root (SOV), cm, mean ± SD | 3.05 ± 0.76 |
| LVEDD, cm, mean ± SD | 6.7 ± 0.67 |
| Septum position, % | |
| Leftward | 0 |
| Midline | 75 |
| Rightward | 25 |
| Aortic valve opening, % | |
| No opening | 0 |
| Intermittent | 0 |
| Regular | 100 |
| Peak outflow cannula velocity in patients with confirmed obstruction, m/s, mean ± SD | 3.9 ± 0.8 |
AR, aortic regurgitation; MR, mitral regurgitation; SOV, sinus of Valsalva; LVEDD, left ventricular end-diastolic dimension.
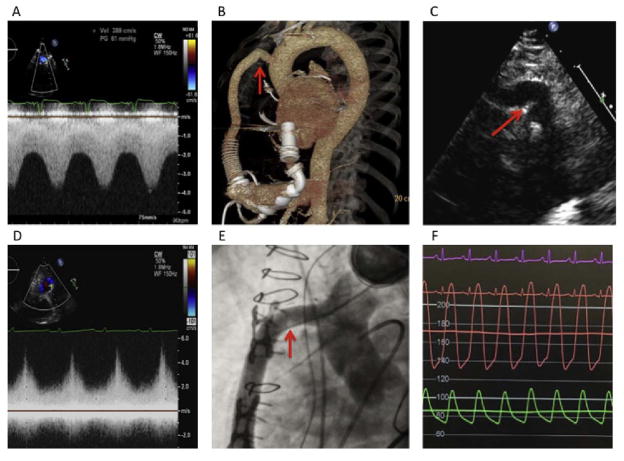
Fig. 1.
Screening for left ventricular assist device (LVAD) outflow cannula malfunction. (A) Elevated peak outflow cannula velocity in a patient with an outflow cannula kink as confirmed by (B) 3-dimensional computerized tomography and (C) 2-dimensional echocardiography. (D) Elevated peak outflow cannula velocity in a patient with and outflow cannula obstruction as confirmed by (E) fluoroscopy and (F) invasive hemodynamic assessment.
Table 3.
Transthoracic Echocardiographic Parameters Including LVAD Outflow Cannula Parameters Stratified by LVAD Type
| TTE Measurement | All Patients (n = 57) | HM2 (n = 44) | HVAD (n = 13) | P Value |
|---|---|---|---|---|
| AR grade, mean ± SD | 0.8 ± 0.8 | 0.8 ± 0.8 | 0.7 ± 0.5 | .49 |
| MR grade, mean ± SD | 0.6 ± 0.7 | 0.6 ± 0.8 | 0.7 ± 0.5 | .62 |
| Aortic root (SOV), cm, mean ± SD | 3.12 ± 0.45 | 3.12 ± 0.44 | 3.11 ± 0.52 | .93 |
| LVEDD, cm ± SD | 6.0 ± 1.5 | 5.8 ± 1.5 | 6.8 ± 0.9 | .03 |
| Septum position, % | .33 | |||
| Leftward | 10.5 | 13.6 | 0 | |
| Midline | 61.4 | 56.8 | 76.9 | |
| Rightward | 28.1 | 29.5 | 23.1 | |
| Aortic valve opening, % | .03 | |||
| No opening | 59.6 | 68.2 | 30.1 | |
| Intermittent | 8.8 | 9.1 | 7.7 | |
| Regular | 31.6 | 22.7 | 61.5 | |
| Outflow cannula size, mm, mean ± SD | 12.0 ± 2.0 | 12.8 ± 1.1 | 8.4 ± 1.1 | <.0001 |
| Peak outflow cannula velocity of normal population, m/s, mean ± SD | 1.97 ± 0.49 | 1.86 ± 0.44 | 2.36 ± 0.53 | .004 |
| Lower reference limit of outflow cannula, m/s | 0.98 | 0.98 | 1.30 | NA |
| Upper reference limit of outflow cannula, m/s | 2.96 | 2.73 | 3.42 | NA |
LVAD, left ventricular assist device; other abbreviations as in Table 2.
Fig. 2.
Average peak velocity of the outflow cannula for the Heartmate II, including the upper and lower reference limits for the normal population defined as the 95% prediction interval for the normal population.
Fig. 3.
Average peak velocity of the outflow cannula for the Heartware HVAD, including the upper and lower reference limits for the normal population defined as the 95% prediction interval for the normal population.
Table 4.
Performance Characteristics of the Traditional Upper Reference Limit for the LVAD Outflow Cannula Peak Velocity Compared With the Proposed New Reference Limits
| Characteristic | Traditional Reference Limit (2 m/s) | Proposed Reference Limit (2.7 m/s for HM2; 3.4 m/s for HVAD) |
|---|---|---|
| False positives, n | 24 | 0 |
| True positives, n | 4 | 4 |
| False negatives, n | 0 | 0 |
| True negatives, n | 26 | 50 |
| Sensitivity, % | 100 | 100 |
| Specificity, % | 52.0 | 100 |
| Negative predictive value | 100 | 100 |
| Positive predictive value | 14.3 | 100 |
Discussion
This study aimed to evaluate the outflow cannula velocity in CF-LVAD patients. First, we were able image the outflow cannula in 95% of the patients. Second, we found that the normal range for outflow cannula velocity for patients supported with the HM2 is <2.7 m/s and for HVAD patients <3.4 m/s. Those values are significantly higher than the values that were historically recorded from pulsatile LVADs.
The outflow cannula is an integral component of the LVAD circuitry that can be easily imaged with Doppler echocardiography to provide important physiologic information regarding LVAD functioning. Each type of LVAD has unique cannula properties, including distinctive graft materials and diameters as well as variable surgical techniques for graft trajectory and anastomosis. All of these variables combine to result in a unique LVAD type–dependent normal flow-velocity pattern across the outflow cannula.17,18 Outflow cannula malfunction adds yet another layer of complexity to the flow patterns of LVAD outflow cannulas. Accurate knowledge of the normal flow properties of a particular LVAD is therefore of particular importance because variations may indicate LVAD malfunction. The currently cited upper reference limits for peak velocity of LVAD outflow cannulas were established in a small population of patients using a 1st-generation CF-LVAD that is rarely currently used.4,6 The present study is the largest study to report normal flow velocities of the LVAD outflow cannula, with the use of Doppler TTE, in the contemporary generation of CF-LVADs. Furthermore, we present new upper reference limits for LVAD outflow cannulas that can be used for outflow cannula malfunction screening and that outperform the previously reported reference limits. LVAD outflow cannula obstruction is an increasingly recognized complication of LVADs that can lower the efficiency of the LVAD pump and circuit. Left untreated, cannula obstruction can lead to worsening signs and symptoms of congestion and end-organ hypoperfusion. Outflow cannula obstruction can result from intrinsic causes (ie, thrombus or infectious vegetation) or external causes (ie, kinking of graft, compression after sternal closure, excessive suturing at the anastomotic site or compression from mediastinal hemorrhage or tumor).6,19 Depending on the etiology, outflow cannula obstruction can be treated pharmacologically with anticoagulants or thrombolytic agents, percutaneously, or surgically.19–21 Timely recognition of LVAD outflow cannula malfunction is critical to enable timely appropriate treatment.
In this study, we demonstrate that Doppler TTE analysis of the LVAD outflow cannula can be readily acquired in the majority of patients. We also demonstrate that the 2 most commonly used LVADs, HM2 and HVAD, have statistically different peak LVAD outflow cannulas velocities; accordingly, individual upper reference limits should be used for specific LVAD types. We further report that the currently cited upper reference limit for LVAD outflow cannula peak velocity is significantly lower than that observed in our cohort and that the use of the current reference limits poorly discriminate between normal and abnormal outflow cannula mechanics. Using the proposed new upper reference limits of 2.7 m/s for the HM2 and 3.4 m/s for the HVAD, we were able to improve the screening for LVAD cannula malfunction compared with the currently used reference limit of 2 m/s.22
As part of our analysis of LVAD outflow cannulas, we noted that the outflow cannula diameter was highly variable among patients and that mean diameters were smaller than the manufacturer-stated diameters. The HM2 outflow cannula is manufactured as a 14 mm graft, and the HVAD outflow cannula is manufactured as a 10 mm graft.23,24 In this study, we measured mean outflow cannula diameters of 12.8 mm for the HM2 and 8.4 mm for the HVAD. To the best of our knowledge, this is the 1st report of smaller-than-expected outflow cannula graft diameters. Changes in the outflow cannula cross-sectional area would alter the resistance and flow through the LVAD circuit and alter the expected pump performance curve (H-Q) curves for the LVADs. Further studies are needed to better understand the anatomy of the outflow cannula before further conclusions are drawn.
Study Limitations
This was a single-center study with a small to moderately sized cohort and thus is prone to bias related to our own imaging and surgical techniques. However, this is the largest study to report the normal parameters of the 2 most currently used LVADs, the HM2 and HVAD. In this study, we established new reference limits for a normal population of the HM2 and HVAD by excluding patients with known outflow cannula malfunction. The analysis of the reference limits was performed retrospectively, and only patients with a clinical suspicion for outflow cannula malfunction were screened. Therefore, it is possible that a small percentage of the normal population might have subclinical malfunction of the outflow graft. However, the normal population was clinically followed for an additional 11 months (until May 2016) and there have been no signs of clinical obstruction to flow in that cohort. The clinical value of the proposed upper reference limits as a screening tool for outflow cannula malfunction was also demonstrated. Here, we define the reference limits for the outflow cannula of the HM2 and HVAD only, which currently are the 2 most commonly used LVADs. Future generations of LVADs will have unique flow patterns and will similarly require their own LVAD-specific reference values.
Conclusion
For both the HM2 and the HVAD, the average peak outflow velocity and reference limit for the normal population as measured with the use of Doppler TTE are markedly higher than the currently cited LVAD outflow cannula reference limits. New reference limits of peak outflow cannula velocity of 2.7 m/s for the HM2 and 3.4 m/s for the HVAD are proposed. These new reference limits can effectively screen for LVAD outflow cannula malfunction.
Footnotes
Disclosures
Dr Uriel is a consultant to Heartware and Thoratec; Dr Jeevanandam is a consultant to Thoratec; Dr Lang is a consultant and is on the speaker’s bureau for Philips; all other authors have no relevant disclosures.
References
- 1.Stewart GC, Stevenson LW. Keeping left ventricular assist device acceleration on track. Circulation. 2011;123:1559–68. doi: 10.1161/CIRCULATIONAHA.110.982512. discussion 1568. [DOI] [PubMed] [Google Scholar]
- 2.Estep JD, Stainback RF, Little SH, Torre G, Zoghbi WA. The role of echocardiography and other imaging modalities in patients with left ventricular assist devices. JACC Cardiovasc Imaging. 2010;3:1049–64. doi: 10.1016/j.jcmg.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Stainback RF, Croitoru M, Hernandez A, Myers TJ, Wadia Y, Frazier OH. Echocardiographic evaluation of the Jarvik 2000 axial-flow LVAD. Tex Heart Inst J. 2005;32:263–70. [PMC free article] [PubMed] [Google Scholar]
- 4.Catena E, Milazzo F, Montorsi E, Bruschi G, Cannata A, Russo C, et al. Left ventricular support by axial flow pump: the echocardiographic approach to device malfunction. J Am Soc Echocardiogr. 2005;18:1422. doi: 10.1016/j.echo.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant. 2014;33:555–64. doi: 10.1016/j.healun.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Chumnanvej S, Wood MJ, MacGillivray TE, Melo MF. Perioperative echocardiographic examination for ventricular assist device implantation. Anesth Analg. 2007;105:583–601. doi: 10.1213/01.ane.0000278088.22952.82. [DOI] [PubMed] [Google Scholar]
- 7.Catena E, Milazzo F. Echocardiography and cardiac assist devices. Minerva Cardioangiol. 2007;55:247–65. [PubMed] [Google Scholar]
- 8.Fine NM, Topilsky Y, Oh JK, Hasin T, Kushwaha SS, Daly RC, et al. Role of echocardiography in patients with intravascular hemolysis due to suspected continuous-flow LVAD thrombosis. JACC Cardiovasc Imaging. 2013;6:1129–40. doi: 10.1016/j.jcmg.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Grinstein J, Kruse E, Sayer G, Fedson S, Kim GH, Jorde UP, et al. Accurate quantification methods for aortic insufficiency severity in patients with LVAD: role of diastolic flow acceleration and systolic-to-diastolic peak velocity ratio of outflow cannula. JACC Cardiovasc Imaging. 2016;9:641–51. doi: 10.1016/j.jcmg.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Grinstein J, Kruse E, Sayer G, Fedson S, Kim GH, Jorde UP, et al. Quantification of aortic insufficiency in patients with left ventricular assist devices: a novel approach combining invasive hemodynamics and echocardiography. J Heart Lung Transplant. 2015;34:S154. [Google Scholar]
- 11.Grinstein J, Kruse E, Sayer G, Fedson S, Kim GH, Jorde UP, et al. Novel parameters for grading aortic insufficiency severity in patients with left ventricular assist devices. J Am Soc Echocardiogr. 2015;28:B52–3. [Google Scholar]
- 12.May-Newman KD, Hillen BK, Sironda CS, Dembitsky W. Effect of LVAD outflow conduit insertion angle on flow through the native aorta. J Med Eng Technol. 2004;28:105–9. doi: 10.1080/0309190042000193865. [DOI] [PubMed] [Google Scholar]
- 13.Topilsky Y, Maltais S, Oh JK, Atchison FW, Perrault LP, Carrier M, et al. Focused review on transthoracic echocardiographic assessment of patients with continuous axial left ventricular assist devices. Cardiol Res Pract. 2011;2011:187434. doi: 10.4061/2011/187434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 16.Horton SC, Khodaverdian R, Chatelain P, McIntosh ML, Horne BD, Muhlestein JB, et al. Left ventricular assist device malfunction: an approach to diagnosis by echocardiography. J Am Coll Cardiol. 2005;45:1435–40. doi: 10.1016/j.jacc.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 17.Kar B, Delgado RM, Frazier OH, Gregoric ID, Harting MT, Wadia Y, et al. The effect of LVAD aortic outflow-graft placement on hemodynamics and flow: implantation technique and computer flow modeling. Tex Heart Inst J. 2005;32:294–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Litwak KN, Koenig SC, Tsukui H, Kihara S, Wu Z, Pantalos GM. Effects of left ventricular assist device support and outflow graft location upon aortic blood flow. ASAIO J. 2004;50:432–7. doi: 10.1097/01.mat.0000136505.27884.f8. [DOI] [PubMed] [Google Scholar]
- 19.Weitzel N, Puskas F, Cleveland J, Levi ME, Seres T. Left ventricular assist device outflow cannula obstruction by the rare environmental fungus Myceliophthora thermophila. Anesth Analg. 2009;108:73–5. doi: 10.1213/ane.0b013e318187b8fc. [DOI] [PubMed] [Google Scholar]
- 20.Abraham J, Remick JD, Caulfield T, Puhlman M, Evenson K, Ott G, et al. Left ventricular assist device outflow cannula obstruction treated with percutaneous endovascular stenting. Circ Heart Fail. 2015;8:229–30. doi: 10.1161/CIRCHEARTFAILURE.114.001891. [DOI] [PubMed] [Google Scholar]
- 21.Retzer EM, Tannenbaum SA, Fedson SE, Kim GH, Sayer GT, Paul JD, et al. Successful percutaneous trans-catheter treatment of left ventricular assist device outflow graft stenosis with a covered stent. ESC Heart Fail. 2015;2:100–2. doi: 10.1002/ehf2.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stainback RF, Estep JD, Agler DA, Birks EJ, Bremer M, Hung J, et al. Echocardiography in the management of patients with left ventricular assist devices: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2015;28:853–909. doi: 10.1016/j.echo.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Franco KL, Verrier ED. Advanced therapy in cardiac surgery. 2. Hamilton, Ontario, and Lewiston, New York: BC Decker; 2003. [Google Scholar]
- 24.Kormos RL, Miller LW. Mechanical circulatory support: a companion to Braunwald’s heart disease. Philadelphia: Elsevier/Saunders; 2012. [Google Scholar]