Abstract
BACKGROUND
The aim of this study was to evaluate the prognostic performance of novel echocardiographic (transthoracic echocardiography, or TTE) parameters for grading aortic insufficiency (AI) severity in patients with continuous-flow left ventricular assist devices (CF-LVADs). The development of AI after CF-LVAD implantation is common, although the clinical significance remains unclear. We previously described novel TTE parameters that outperformed traditional TTE parameters in grading AI severity in these patients.
METHODS
CF-LVAD patients with varying degrees of AI (N = 57) underwent Doppler TTE of the LVAD outflow cannula. Patients had AI severity graded by the novel parameters (systolic/diastolic velocity ratio and the diastolic acceleration of the LVAD outflow cannula) and the traditional vena contracta. The prognostic performance of novel and traditional AI parameters was determined by comparing rates of congestive heart failure re-admission, need for aortic valve intervention, urgent transplantation and death (composite end-points) for each parameter.
RESULTS
Grading AI severity using novel AI parameters led to reclassification of 32% of patients from trace/mild AI to moderate or greater AI (N =18). Using traditional AI parameters, there was no difference in the occurrence of the composite end-point between the moderate or greater group and the trace/mild group (1.50 vs 1.18 events/person, p = 0.46). With the novel AI parameters, there were significantly more events in the patients with moderate or greater AI compared to those with trace/mild AI (1.57 vs 0.13 events/person, p = 0.002). Novel parameters also better predicted the need for aortic valve intervention, urgent transplantation or death than traditional methods (p = 0.024 vs p = 0.343).
CONCLUSIONS
In patients with CF-LVADs, traditional parameters tend to underestimate AI severity and future cardiac events. Novel AI TTE parameters are better able to discriminate AI severity and predict clinically meaningful outcomes.
Keywords: left ventricular assist device (LVAD), aortic insufficiency, diastolic acceleration of the LVAD outflow cannula, systolic to diastolic velocity (S/D) ratio of the LVAD outflow cannula, clinical outcomes
De-novo aortic insufficiency (AI) after implantation of a continuous-flow left ventricular assist device (CF-LVAD) is a common occurrence. Up to 25% to 30% of patients will develop at least mild or moderate AI within 1 year after implantation with a CF-LVAD.1–4 The reverse transaortic–valvular gradient generated by constant left ventricular (LV) unloading by CF-LVADs leads to a limited aortic valve opening, which subsequently promotes aortic valve commissural fusion and leaflet deterioration.1,5,6 AI in patients with CF-LVADs is unique, as flow tends to be pancyclical and eccentric.1,7–10 In addition, traditional transthoracic echocardiography (TTE) measurements for grading AI severity, including vena contracta, jet width/LV outflow tract (LVOT) diameter and proximal isovelocity surface area, are less accurate in a continuous-flow system and tend to be less reliable with eccentric regurgitant flow.11–13 Despite the prevalence of the condition, the clinical significance of AI in patients with CF-LVADs remains unclear. The increased regurgitant flow caused by significant AI results in elevated cardiac filling pressures and signs and symptoms of congestion.7 However, studies evaluating outcomes after the development of de-novo AI have shown mixed results with respect to subsequent morbidity and mortality.1,3
Recently, we introduced 2 novel TTE measurements for grading AI severity in patients with CF-LVADs.13 Peak systolic-to-diastolic (S/D) velocity ratio of the LVAD outflow cannula and diastolic acceleration of LVAD outflow cannula correlated better with clinical filling pressures and regurgitant fraction than traditional TTE parameters, including vena contracta.13–15 Furthermore, we found that traditional parameters tend to underestimate AI severity.13,15
Here, for the first time, we directly compare the prognostic performance of the traditional echocardiographic parameter, vena contracta, to the novel echocardiographic parameters, S/D ratio and diastolic acceleration of the LVAD outflow cannula, in an attempt to better understand the clinical significance of AI in CF-LVAD patients.
Methods
Patient population
In this prospective study, 57 sequential LVAD patients (HeartMate II, Thoratec Corp., Pleasanton, CA, or HeartWare HVAD, HeartWare International, Inc., Framingham, MA) were enrolled. All patients underwent echocardiographic image acquisition, including outflow cannula assessment, by 1 of 2 sonographers trained in imaging the LVAD outflow cannula, between September 2014 and June 2015. TTE images were obtained either during routine outpatient follow-up or after a hospital re-admission by a clinician blinded to the study. Enrollment in the study began after the first TTE was acquired during this period. Patients were excluded if they had poor echocardiographic windows, known intracardiac shunt, device malfunction or suspected pump thrombosis. Clinicians caring for the patients were blinded to study enrollment and were not provided with information regarding AI severity assessment by the novel TTE parameters. Demographic information, type and duration of LVAD implant and clinical outcomes were obtained from a chart review. The study was approved by the institutional review board at the University of Chicago.
Echocardiographic imaging
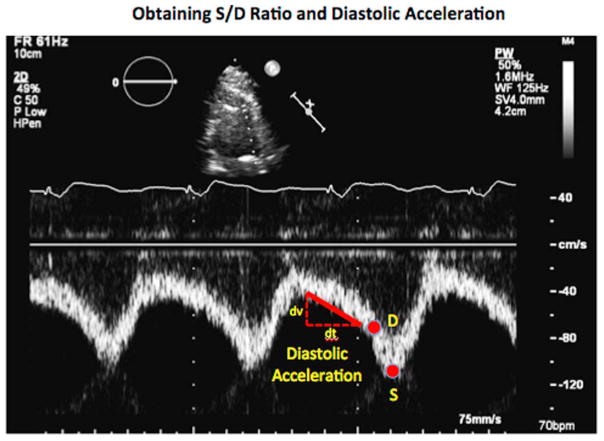
All patients underwent comprehensive 2-dimensional and Doppler evaluation of the heart and LVAD circuit by an experienced sonographer using the iE33 imaging system and S5 transducer (Philips Healthcare, Andover, MA). From the parasternal window, linear measurements were made to measure the LV chamber size, and the aortic valve opening was assessed using the M-mode technique, averaged over 10 cardiac cycles. Traditional assessments of aortic regurgitation severity were made using vena contracta width when available and qualitative assessment of AI severity when vena contracta was unavailable, as per guidelines.14,16 The S/D ratio and diastolic acceleration of the LVAD outflow cannula was assessed by pulse-wave Doppler signal of the LVAD outflow cannula from a modified, right parasternal view, as previously described.13,17 Briefly, the LVAD outflow cannula was aligned with the transducer at the point of anastomosis with the ascending aorta. All signals were acquired within 1 cm from the site of anastomosis with the ascending aorta. The diastolic acceleration of the LVAD outflow cannula was obtained by measuring the diastolic slope from the onset of diastole to end-diastole. The S/D ratio of the LVAD outflow cannula was calculated by dividing the peak systolic velocity by the end-diastolic peak velocity of the LVAD outflow cannula (Figure 1).
Figure 1.
Measurement of the LVAD outflow cannula S/D ratio and diastolic acceleration by pulsed-wave Doppler echocardiography.
As described in previous studies, diastolic acceleration is directly proportional to AI severity, whereas the S/D ratio is inversely proportional to AI severity.13–15 Moderate or greater AI, correlating with a regurgitant fraction (RF) of >30%, was defined as an S/D ratio of <5.0 or a diastolic acceleration of >49.0 cm/s2, as previously reported.13 To calculate the combined AI severity score, regurgitant fractions were extrapolated from the corresponding correlation lines for the S/D ratio vs regurgitant fraction relationship [RF = –(S/D – 10.8) / 19.3] and diastolic acceleration (DA) vs regurgitant fraction (RF) relationship [RF = (DA + 0.5)/165.4]. The regurgitant fractions obtained from the S/D ratio and diastolic acceleration were averaged for each patient to determine the combined regurgitant fraction for the patient. Moderate or greater AI was defined as a regurgitant fraction of >30%.
Hemodynamic testing
A subset of 23 patients underwent right heart catheterization (RHC) simultaneously with echocardiography in the catheterization laboratory using a 7-French Swan–Ganz catheter (Edwards Lifesciences, Irvine, CA). RHC was performed through the right internal jugular vein while on therapeutic anticoagulation. The following values were measured: right atrial pressure (RAP); systolic pulmonary artery pressure (SPAP); diastolic pulmonary artery pressure (DPAP); mean pulmonary artery pressure (MPAP); pulmonary capillary wedge pressure (PCWP); and pulmonary artery saturation (PASAT). Cardiac output (CO) and cardiac index (CI) were calculated by the indirect Fick equation with estimated oxygen consumption of 125 ml/min/m2. Hemoglobin was measured from the venous blood gas, and arterial oxygen saturation was measured by non-invasive pulse oximetry.
Statistical methods
Data was collected using EXCEL software (2011 Microsoft Corp., Redmond, WA) and analyzed using PRISM (GraphPad Software, Inc., San Diego, CA). Continuous variables were evaluated for normality using the D’Agostino–Pearson test for normal distribution, and Student’s t-test was used to determine differences in the means of normally distributed data. Categorical variables were analyzed using Fisher’s exact test. Log-rank testing was used to analyze Kaplan–Meier freedom-from-event data. For the outcome analysis, events were defined as a heart failure–related admission, urgent cardiac transplantation, surgical or percutaneous aortic valve intervention or death after LVAD implantation between October 1, 2014 and June 30, 2015. The primary end-point was a composite of the heart failure readmissions and the need for aortic valve intervention, urgent transplantation or death over this period, as documented by chart review. Outcomes were analyzed by AI severity stratified by: (1) traditional methods; (2) S/D ratio; (3) diastolic acceleration; and (4) a combined severity score that incorporates both S/D ratio and diastolic acceleration. Freedom from aortic valve intervention, death or urgent transplantation after the diagnosis of AI by TTE was compared between traditional methods of grading AI severity and the combined severity score incorporating both S/D ratio and diastolic acceleration using Kaplan–Meier analysis. To assess the reproducibility of the S/D ratio and diastolic acceleration measurements, the measurements were repeated in the first 15 patients enrolled in this study by a second observer and interobserver variability was assessed by intraclass correlation measurement. Similarly, intraclass correlation measurements were obtained to determine the variability of regurgitant fraction calculated from the S/D ratio and diastolic acceleration.
Results
Baseline characteristics
Fifty-seven patients were enrolled in the study. Four patients were excluded from the final analysis due to suboptimal echocardiographic image quality. Patient’s age ranged from 28 to 77 (mean 58) years, and 72% were male. Seventy-seven percent of patients had a HeartMate II (HMII) device and 23% of patients had an HVAD. LVAD was implanted as destination therapy in the majority of patients (72%). Patients with more severe AI trended to be on LVAD support for a longer duration of time. Baseline characteristics of the cohort are reported in Table 1.
Table 1.
Baseline Characteristics as Sorted by Traditional TTE Parametersa
| All patients (n = 57) | No AI (n = 17) | Trace or mild AI (n = 28) | Moderate or greater AI (n = 12) | p-value | |
|---|---|---|---|---|---|
| General characteristics | |||||
| Age (years) | 58.1 ± 11.5 | 54.5 ± 7.4 | 57.4 ± 12.0 | 64.7 ± 12.9 | 0.09 |
| Male | 41 (71.9) | 11 (64.7) | 21 (75.0) | 9 (75.0) | |
| LVAD characteristics | |||||
| Duration of LVAD (months) | 18.4 ± 11.5 | 11.8 ± 17.2 | 17.5 ± 15.9 | 29.8 ± 24.0 | 0.06 |
| Destination | |||||
| BTT | 16 (28.1) | 5 (29.4) | 10 (35.7) | 1 (8.3) | 0.12 |
| DT | 41 (71.9) | 12 (70.6) | 18 (64.3) | 11 (91.7) | |
| Type of LVAD | |||||
| HMII | 44 (77.2) | 15 (88.2) | 18 (64.3) | 11 (91.7) | 0.12 |
| HVAD | 13 (22.8) | 2 (11.8) | 10 (35.7) | 1 (8.3) | |
| Average speed (rpm) | |||||
| HMII | 9,158 ± 392 | 9,133 ± 335 | 9,165 ± 396 | 9,182 ± 485 | 0.92 |
| HVAD | 2,701 ± 137 | 2,640 ± 170 | 2,734 ± 124 | 2,500 ± NA | NA |
| Pulsatility index | 5.7 ± 1.1 | 6.4 ± 0.07 | 5.6 ± 1.3 | 5.6 ± 1.3 | 0.98 |
| Power | 5.7 ± 1.3 | 5.1 ± 0.07 | 6.2 ± 0.7 | 5.6 ± 1.6 | 0.55 |
| Flow | 5.2 ± 0.8 | 4.9 ± 0.8 | 5.8 ± 1.1 | 5.1 ± 0.8 | 0.27 |
| Origin of cardiomyopathy | |||||
| Ischemic | 29 (50.9) | 7 (41.1) | 15 (53.6) | 7 (58.3) | 1.0 |
| Non-ischemic | 28 (49.1) | 10 (58.9) | 13 (46.4) | 5 (41.7) | |
| Medical history | |||||
| Hypertension | 35 (61.4) | 11 (64.7) | 19 (67.9) | 5 (41.7) | 0.17 |
| Hyperlipidemia | 25 (43.9) | 6 (35.2) | 14 (50.0) | 5 (41.7) | 0.74 |
| Atrial fibrillation | 15 (26.3) | 4 (23.5) | 7 (25.0) | 4 (33.3) | 0.70 |
| DM | 22 (38.6) | 8 (47.1) | 10 (35.7) | 4 (33.3) | 1.0 |
| COPD | 8 (14.0) | 2 (11.8) | 2 (7.1) | 4 (33.3) | 0.05 |
| PAD | 6 (10.5) | 3 (17.6) | 0 (0) | 3 (25.0) | 0.02 |
| CVA | 13 (22.8) | 3 (17.6) | 8 (28.6) | 2 (16.7) | 0.69 |
| Prior sternotomy | 13 (22.8) | 2 (11.8) | 8 (28.6) | 3 (25.0) | 1.0 |
| TTE measurements | |||||
| Vena contracta (cm) | 0.17 ± 0.15 | 0 ± 0 | 0.20 ± 0.06 | 0.37 ± 0.08 | <0.0001 |
| S/D ratio | 3.0 ± 1.5 | NA | 3.4 ± 1.8 | 2.0 ± 0.5 | 0.02 |
| Diastolic acceleration (cm/s2) | 43.0 ± 34.2 | 2.5 ± 4.7 | 52.3 ± 26.0 | 79.8 ± 14.8 | 0.001 |
| Regurgitant fraction (%) | 27.6 ± 19.2 | 2.4 ± 4.8 | 35.0 ± 12.2 | 46.9 ± 4.9 | 0.001 |
| LVEDD (cm) | 6.0 ± 1.5 | 6.2 ± 1.5 | 6.1 ± 1.7 | 5.6 ± 1.6 | 0.39 |
| Aortic root (SOV) (cm) | 3.12 ± 0.45 | 3.08 ± 0.51 | 3.13 ± 0.46 | 3.14 ± 0.36 | 0.95 |
| Aortic valve opening | |||||
| No opening | 34 (59.6) | 14 (82.4) | 13 (46.6) | 7 (58.3) | 0.40 |
| Intermittent | 5 (8.8) | 0 (0) | 5 (17.9) | 0 (0) | |
| Regular | 18 (31.6) | 3 (17.6) | 10 (35.7) | 5 (41.7) | |
BTT, bridge to transplantation; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DM, diabetes mellitus; DT, destination therapy; HMII, HeartMate II; HVAD, HeartWare ventricular assist device; LVAD, left ventricular assist device; LVEDD, left ventricular end-diastolic diameter; NA, not available; PAD, peripheral arterial disease; S/D, systolic/diastolic velocity; SOV, sinus of Valsalva; TTE, transthoracic echocardiography.
Data expressed as mean ± standard deviation or as number (%).
Reclassification of AI by the novel parameters
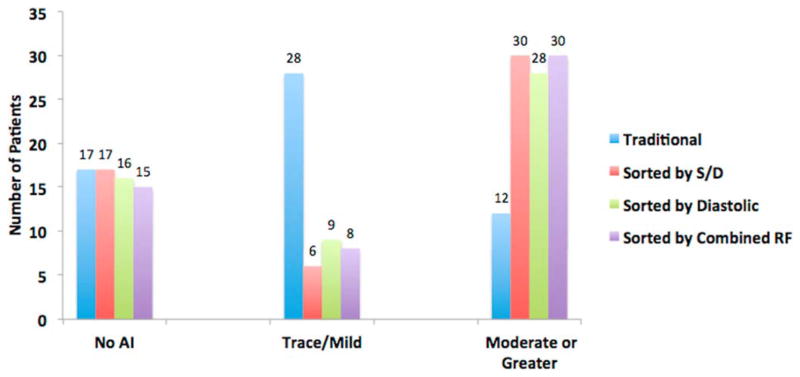
Classification of AI severity based on traditional echocardiographic parameters of vena contracta and visual estimation identified the following: 17 patients (30%) had no AI; 28 patients had trace or mild AI (49%); and 12 patients had moderate or greater AI (21%). S/D ratio and diastolic acceleration were both successfully measured in 53 of 57 patients (93%). In the 4 patients for whom the S/D ratio and diastolic acceleration were unable to be measured, the LVAD cannula was orthogonal to the transducer, and therefore reliable Doppler signals could not be obtained. Reclassification of AI severity based on S/D ratio included the following: 19 patients (33%) previously classified as trace/mild AI by the traditional methods were reclassified as moderate or greater AI by the S/D ratio. Grading with the diastolic acceleration reclassified 17 patients (30%) from trace/mild AI by traditional methods to moderate or greater AI severity. Using a combined severity score that incorporates both S/D ratio and diastolic acceleration led to the reclassification of 19 patients (33%) from trace/mild AI by traditional methods to moderate or greater AI (Table 2 and Figure 2). The interobserver variability was low for both the S/D ratio as well as the diastolic acceleration, as represented by the high intraclass correlation coefficient for both variables (r = 0.92, 95% confidence interval [CI] 0.79 to 0.97; and r = 0.97, 95% CI 0.92 to 0.99, respectively). Similarly, there was excellent agreement between the regurgitant fractions calculated from the S/D ratio and diastolic acceleration (r = 0.97, 95% CI 0.95 to 0.98).
Table 2.
Characteristics and Outcomes of Patients With Less than Moderate AI by Traditional Methods Reclassified by Novel Methodsa
| Remained trace or mild AI (n = 8) | Reclassified moderate or greater AI (n = 19) | p-value | |
|---|---|---|---|
| General characteristics | |||
| Age (years) | 54.0 ± 9.1 | 57.3 ± 12.7 | 0.51 |
| Male | 5 (62.5) | 15 (78.9) | 0.63 |
| LVAD characteristics | |||
| Duration of LVAD (months) | 12.1 ± 15.5 | 19.9 ± 16.3 | 0.26 |
| Destination | |||
| BTT | 3 (37.5) | 7 (36.8) | |
| DT | 5 (62.5) | 12 (63.2) | 1.0 |
| Type of LVAD | |||
| HMII | 7 (87.5) | 10 (57.9) | 0.19 |
| HVAD | 1 (12.5) | 9 (42.1) | |
| Average speed (rpm) | |||
| HMII | 9,200 ± 231 | 9,107 ± 501 | 0.64 |
| HVAD | 2,640 ± NA | 2,738 ± 134 | NA |
| Origin of cardiomyopathy | |||
| Ischemic | 4 (50.0) | 9 (47.4) | 1.0 |
| Non-ischemic | 4 (50.0) | 10 (52.6) | |
| Medical history | |||
| Hypertension | 6 (75.0) | 13 (68.4) | 1.0 |
| Hyperlipidemia | 3 (37.5) | 9 (47.4) | 0.70 |
| Atrial fibrillation | 1 (12.5) | 6 (31.6) | 0.37 |
| DM | 5 (62.5) | 6 (31.6) | 0.21 |
| COPD | 0 (0) | 1 (5.3) | 1.0 |
| PAD | 0 (0) | 0 (0) | 1.0 |
| CVA | 1 (12.5) | 7 (36.8) | 0.36 |
| Prior sternotomy | 1 (12.5) | 5 (26.3) | 0.63 |
| TTE measurements | |||
| Vena contracta (cm) | 0.14 ± 0.09 | 0.20 ± 0.07 | 0.14 |
| S/D ratio | 5.3 ± 1.8 | 2.6 ± 1.0 | <0.0001 |
| Diastolic acceleration (cm/s2) | 23.9 ± 8.8 | 62.4 ± 19.9 | <0.0001 |
| Regurgitant fraction (%) | 21.6 ± 5.2 | 39.9 ± 7.7 | <0.0001 |
| LVEDD (cm) | 5.8 ± 1.5 | 6.2 ± 1.3 | 0.42 |
| Aortic root (SOV) (cm) | 3.01 ± 0.45 | 3.09 ± 0.49 | 0.70 |
| Aortic valve opening | |||
| No opening | 5 (62.5) | 7 (36.8) | |
| Intermittent | 1 (12.5) | 4 (21.1) | |
| Regular | 2 (25.0) | 8 (42.1) | 0.54 |
| Outcomes | |||
| CHF re-admissions/patient | 0.13 ± 0.35 | 1.32 ± 1.20 | 0.01 |
| Aortic valve interventions | 0 (0) | 1 (5.3) | |
| Transplants | 0 (0) | 2 (10.5) | |
| Deaths | 0 (0) | 1 (5.3) | 0.29 |
BTT, bridge to transplantation; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DM, diabetes mellitus; DT, destination therapy; HMII, HeartMate II; HVAD, HeartWare ventricular assist device; LVAD, left ventricular assist device; LVEDD, left ventricular end-diastolic diameter; NA, not available; PAD, peripheral arterial disease; S/D, systolic/diastolic velocity; SOV, sinus of Valsalva; TTE, transthoracic echocardiography.
Data expressed as mean ± standard deviation or as number (%).
Figure 2.
Reclassification of AI severity using the S/D ratio, diastolic acceleration or the combination of novel parameters.
Hemodynamic implications of AI by parameter
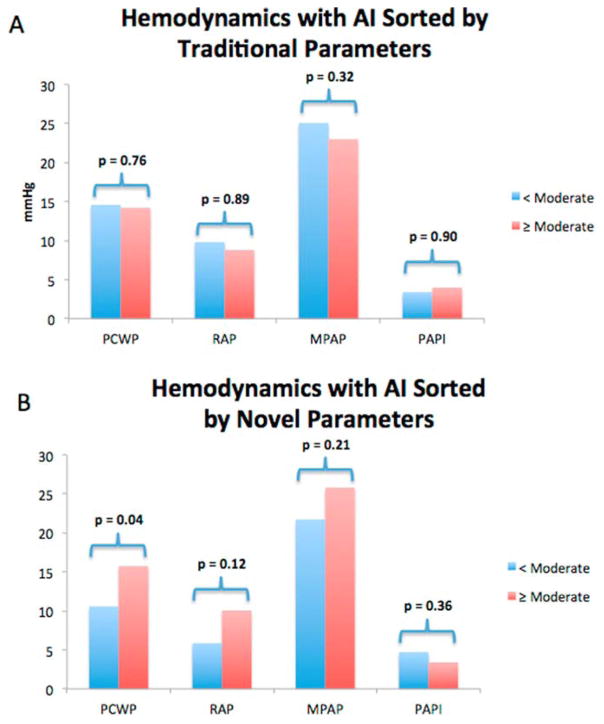
In a subset of 23 patients, simultaneous echocardiography was obtained at the time invasive right heart catheterization. Despite similar levels of LVAD support (average HMII speed 9,092 rpm vs 9,160 rpm, p = 0.82; average HVAD speed 2,700 rpm vs 2,720 rpm, p = 0.91), patients with greater than moderate AI, as classified using the novel TTE parameters, had a higher PCWP than those with less than moderate AI (15.8 mm Hg vs 10.6 mm Hg, p = 0.04). There was also a non-significant trend toward worsening markers of right ventricular (RV) function among those with moderate or greater AI assessed by novel TTE parameters (RAP: 10.1 mm Hg vs 5.6 mm Hg, p = 0.12; MPAP: 25.8 mm Hg vs 21.7 mm Hg, p = 0.21; pulmonary artery pulsatility index [PAPI]: 3.4 vs 4.7, p = 0.36) (Figure 3B). Conversely, when AI was graded by traditional TTE parameters, there was no difference in PCWP (14.2 mm Hg vs 14.6 mm Hg, p = 0.76), RAP (8.8 mm Hg vs 9.8 mm Hg, p = 0.89), MPAP (23.0 mm Hg vs 25.1 mm Hg, p = 0.32) and PAPI (4.0 vs 3.4, p = 0.90) (Figure 3A).
Figure 3.
Hemodynamic effect of AI severity classified by traditional TTE parameters (A) and novel TTE parameters (B).
Prognostic performance of traditional and novel parameters
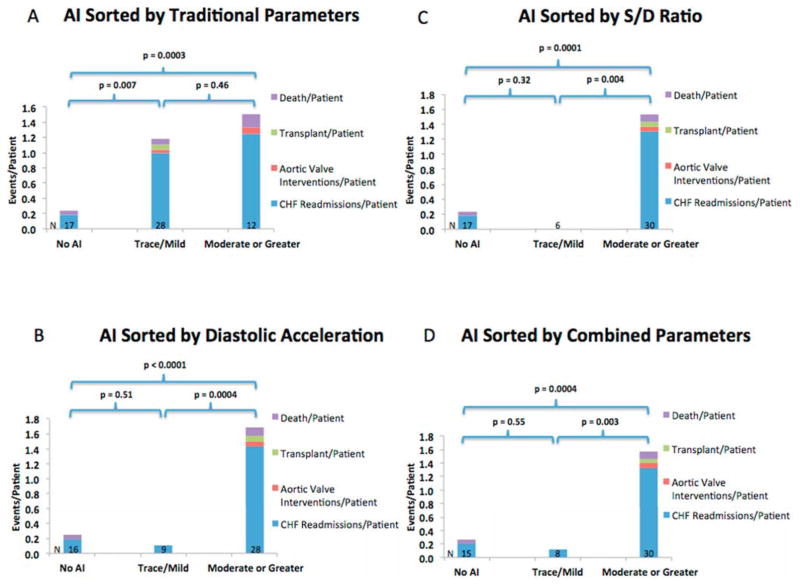
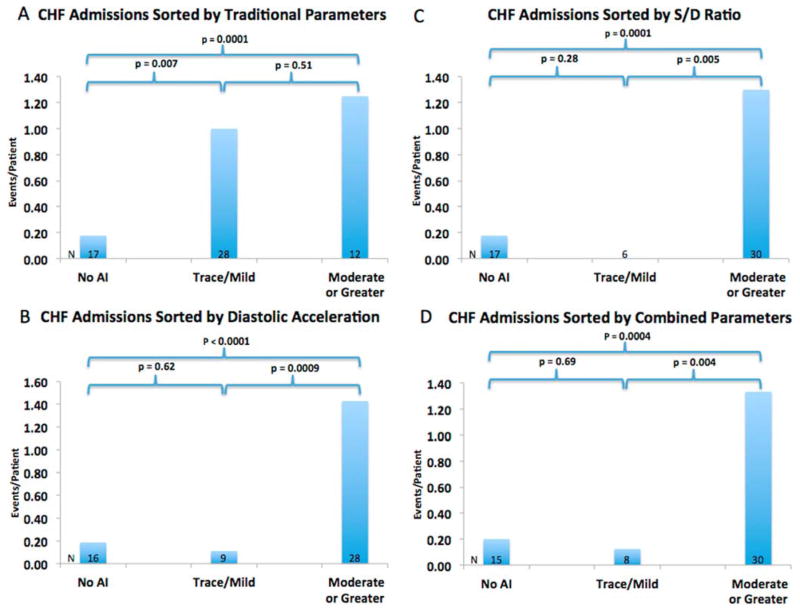
AI severity, when stratified using traditional AI parameters, including vena contracta, revealed no difference in the occurrence of the composite end-point of heart failure readmissions, aortic valve intervention, urgent transplantation or death between patients with trace/mild AI and patients with moderate or greater AI (1.50 vs 1.18 events/person, p = 0.46) (Figure 4A). Similarly, there was no difference in the rates of heart failure re-admission between the trace/mild and moderate AI groups (1.25 vs 1.00 events/person, p = 0.51) (Figure 5A). When AI severity was stratified by the S/D ratio, the occurrence of the composite end-point as well as the individual end-point of heart failure re-admission was significantly higher in the moderate or greater group than the trace/mild group (1.53 vs 0 events/person, p = 0.004; and 1.30 vs 0 event/person, p = 0.005, respectively) (Figures 4B and 5B). All patients with events who were previously classified as trace/mild AI by the traditional parameters were reclassified into the moderate or greater AI group using the S/D ratio. Grading AI severity using diastolic acceleration also led to a significant difference in the composite end-point and individual end-point of heart failure re-admission (1.68 vs 0.11 events/person, p = 0.0004; and 1.43 vs 0.11 events/person, p = 0.0009, respectively) (Figures 4C and 5C). Using a combined severity score that incorporates both S/D ratio and diastolic acceleration similarly resulted in a significant difference in the composite end-point and the individual end-point of heart failure re-admission (1.57 vs 0.13 events/person, p = 0.002; and 1.33 vs 0.13 events/person, p = 0.004, respectively) (Table 2 and Figures 4D and 5D).
Figure 4.
Prognostic performance of traditional parameters (A), S/D ratio (B), diastolic acceleration (C) or a combination of the novel parameters (D) in predicting the composite end-point of future heart failure re-admissions, aortic valve interventions, transplantation or death.
Figure 5.
Prognostic performance of traditional parameters (A), S/D ratio (B), diastolic acceleration (C) or a combination of the novel parameters (D) in predicting future heart failure re-admissions.
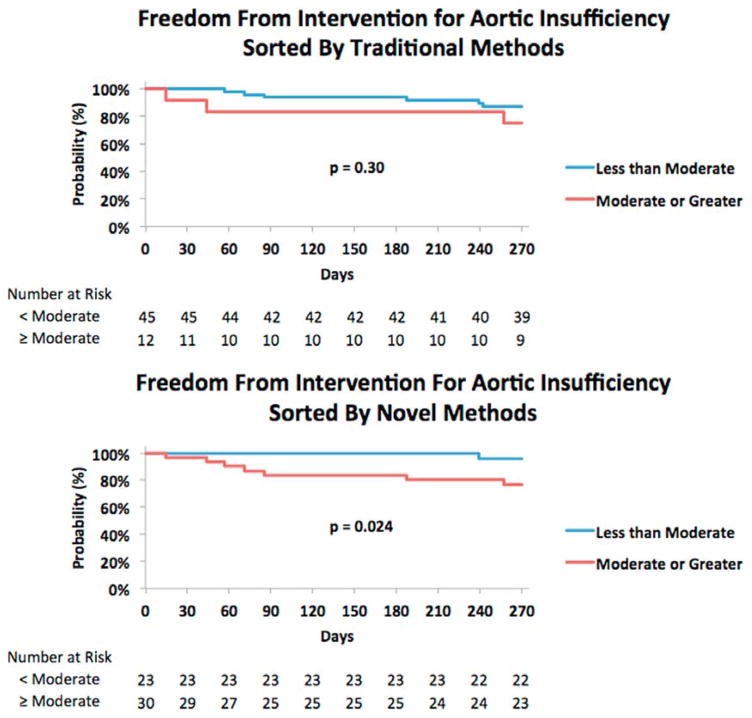
There were a total of 4 deaths, 2 urgent Status 1A heart transplantations and 2 aortic valve interventions in the cohort of 53 patients in whom the novel TTE parameters could be measured. One of the 4 deaths was in a patient without AI who died from septic shock. Two patients were transplanted urgently due to recurrent re-admissions for volume overload and failure to thrive with LVAD support. Two patients underwent an aortic valve intervention with percutaneous closure of the aortic valve with an Amplatzer device in 1 and percutaneous transcatheter aortic valve replacement in the other. The combined parameter incorporating S/D ratio and diastolic acceleration successfully identified and reclassified all patients who ultimately underwent aortic valve intervention, urgent transplantation or death from less than moderate AI to greater than moderate AI (Table 2). Kaplan–Meier analysis of freedom from an intervention or death in patients with aortic insufficiency further supports the improved prognostic performance of the novel parameters compared with the traditional parameters (Figure 6A and B). When AI severity was graded by traditional parameters, 13% of patients in the less than moderate AI group, compared with 25% in the moderate or greater group, underwent aortic valve intervention, urgent transplantation or died (p = 0.30). When AI severity was graded by the novel parameters, 4% of patients in the less than moderate AI group, compared with 23% of patients in the moderate or greater group, underwent aortic valve intervention, urgent transplantation or died (p = 0.02). No patients (0%) with trace or mild AI needed an aortic valve intervention, urgent transplantation or died when AI was graded by the novel methods, whereas 5 of 28 patients (18%) with trace or mild AI graded by the traditional parameters required an intervention or died.
Figure 6.
Kaplan–Meier analysis of freedom from intervention or death after the diagnosis of AI, stratified by traditional parameters (A) or a combination of the novel parameters (B).
Discussion
In the current study, we evaluated the clinical significance of AI in patients with CF-LVADs by comparing the prognostic performance of the traditional echocardiographic parameters of AI severity (vena contracta and visual estimation) with the novel echocardiographic parameters (S/D ratio and diastolic acceleration). The main findings of our study are as follows: (1) traditional echocardiographic parameters underestimate AI in 33% of the cases; (2) grading AI severity using traditional TTE parameters alone did not detect a difference in morbidity or mortality between patients with less than moderate AI and patients with moderate or greater AI; and (3) the novel TTE parameters predict future heart failure admissions, need for aortic valve interventions, urgent transplantation or death better than traditional parameters.
De-novo AI after implantation with a CF-LVAD is a common and progressive condition that ultimately leads to signs and symptoms of worsening heart failure.1,2,4,18,19 Regurgitant flow across the aortic valve reduces the efficiency of the LVAD circuit and has the potential to compromise systemic perfusion.6 LVAD speed optimization, either by bedside TTE or during an invasive ramp study, can help determine the optimal device setting to maximize LVAD efficiency and function in the setting of significant AI, although such measures are only temporizing.20,21 Given the progressive nature of the disease, symptomatic aortic insufficiency will ultimately require an intervention in the form of aortic valve replacement, repair, closure or cardiac transplantation.22,23
Despite the theoretical ramifications of AI after CF-LVAD implantation, outcome studies, to date, among patients who develop AI, have been mixed. Using the traditional TTE parameters of AI jet width/LVOT diameter and vena contraca, Cowger et al performed serial TTEs on 166 patients after implantation with a CF-LVAD and found no difference in survival rates or rates of urgent transplantation after the development of moderate or worse AI.3 They did find that patients with moderate AI were more likely to develop mitral regurgitation, hemolysis and worsening RV dysfunction than patients without AI. In the subgroup of patients with pre-existing RV dysfunction before device implantation, patients who developed moderate or worse AI after CF-LVAD implantation had worse survival than those without clinically relevant AI.3,7 Conversely, Jorde et al followed 232 patients with CF-LVADs and found that 7 of 21 patients (33%) with moderate or greater AI developed symptoms of heart failure requiring urgent transplantation or aortic valve closure/repair.1 Forty percent of their cohort required an intervention within 3 months of developing symptomatic AI.
There are currently no guidelines to grade AI severity in patients with CF-LVADs, and most centers use American Society of Echocardiography guidelines, which were never validated in a continuous-flow model.7 In a pulsatile model (normal heart or pulsatile pump), AI only occurs during diastole. In contrast, in a continuous-flow model, regurgitant flow often occurs throughout the entirety of the cardiac cycle. As such, the regurgitant volume for a given vena contracta or AI jet width/LVOT diameter would be expected to be greater. Accordingly, we previously showed that traditional TTE indices for grading AI severity in patients with CF-LVADs tend to underestimate severity, particularly among patients with less than moderate regurgitation.13 We also showed that the novel TTE parameters of S/D ratio and diastolic acceleration correlated better with regurgitant fraction and pulmonary capillary wedge pressure than vena contracta.13 Given that traditional TTE parameters appear sub-optimal at assessing AI severity in patients with CF-LVADs, particularly among patients with lesser degrees of AI, it is not surprising that the true clinical significance of AI has been challenging to ascertain.
This is the first report to demonstrate that the novel TTE parameters, S/D ratio and diastolic acceleration, are better able to risk-stratify and predict future disease than traditional TTE parameters in patients who develop AI after implantation with CF-LVAD. Similar to the Cowger et al group, we found that, when AI severity was graded by traditional TTE parameters, there was no difference in the rates of heart failure re-admissions, aortic valve intervention, urgent transplantation or death in patients with moderate or greater AI compared to those with less severe AI. However, when the same patients had their AI re-graded using the S/D ratio and/or diastolic acceleration, 33% were reclassified as having moderate or greater AI, resulting in a clear line of distinction with more heart failure readmissions, aortic valve interventions, urgent transplantations or deaths in the moderate or severe group. In other words, the novel TTE parameters of S/D ratio and diastolic acceleration were able to identify the high-risk patients who may have otherwise been missed using traditional TTE parameters alone.
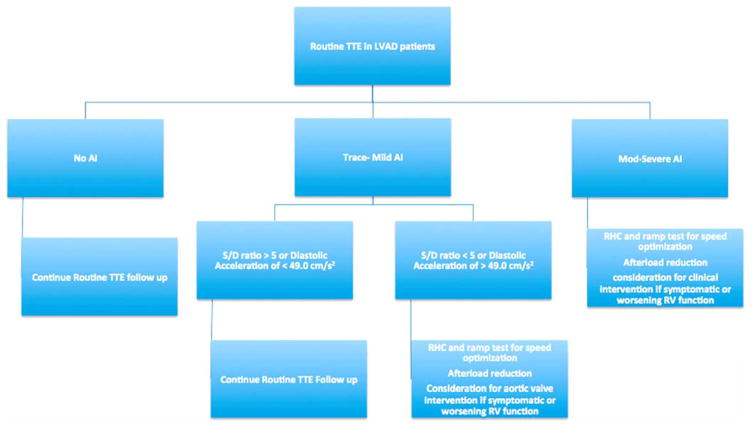
There continues to be much uncertainty about the optimal treatment strategy for managing symptomatic or hemodynamically relevant AI in patients with CF-LVAD. Surgical and percutaneous valve replacement, repair and closure have been reported with varying degrees of success.22,24 To date, surgical and percutaneous aortic valve procedures have traditionally been reserved for patients with moderate to severe AI, as graded by traditional means, who fail to symptomatically improve with increased LVAD speeds or after-load reduction. Long-term exposure to high degrees of regurgitant flow across the aortic valve ultimately increases LV pressures, leading to heightened RV after-load and strain on the RV. Accordingly, after aortic valve interventions, it is not uncommon for patients to develop worsening RV dysfunction, leading to morbidity and mortality. In light of our findings, we now know that hemodynamically and clinically relevant AI can be detected earlier using the S/D ratio and diastolic acceleration. It is hoped that more timely recognition of clinically relevant AI may allow for earlier recognition of the disease before significant RV strain occurs. In such a setting, aortic valve interventions may be associated with improved outcomes, although prospective studies are needed to better evaluate this hypothesis. Figure 7 presents a proposed clinical algorithm to evaluate and manage AI.
Figure 7.
Clinical algorithm to evaluate AI in patients with continuous-flow
Limitations
Our investigation was a single-center study and thus subject to bias related to our institution’s surgical techniques, LVADs. management style and TTE image acquisition. In addition, we only examined the performance of the traditional TTE parameters, vena contracta and visual estimation of the jet width. Thus, additional TTE parameters for grading AI severity, such as proximal isovelocity surface area and jet width/LVOT diameter, remained uncertain. Our study has strongly demonstrated the prognostic performance of the S/D ratio and diastolic acceleration in patients with AI, although it remains uncertain whether these parameters can predict events in the absence of AI.
In conclusion, traditional TTE parameters of AI severity in patients with CF-LVADs have failed to effectively risk-stratify patients. The novel TTE parameters, S/D ratio and diastolic acceleration, reclassify more than a third of patients with less than moderate AI to moderate or greater AI and are more successful at predicting future heart failure re-admissions, need for aortic valve intervention, urgent transplantation or death when compared with traditional methods. We call for adaptation of the novel echocardiographic parameters of S/D ratio and diastolic acceleration for the accurate assessment of AI severity in CF-LVAD patients.
Footnotes
Disclosure statement
N.U. is a consultant for St. Jude (Thoratec) and HeartWare. V.J. is a consultant for St. Jude (Thoratec) and HeartWare. R.L. is a consultant and is on the speaker’s bureau for Philips. The other authors have no conflicts of interest to disclose.
References
- 1.Jorde UP, Uriel N, Nahumi N, et al. Prevalence, significance, and management of aortic insufficiency in continuous flow left ventricular assist device recipients. Circ Heart Fail. 2014;7:310–9. doi: 10.1161/CIRCHEARTFAILURE.113.000878. [DOI] [PubMed] [Google Scholar]
- 2.Pak SW, Uriel N, Takayama H, et al. Prevalence of de novo aortic insufficiency during long-term support with left ventricular assist devices. J Heart Lung Transplant. 2010;29:1172–6. doi: 10.1016/j.healun.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Cowger JA, Aaronson KD, Romano MA, et al. Consequences of aortic insufficiency during long-term axial continuous-flow left ventricular assist device support. J Heart Lung Transplant. 2014;33:1233–40. doi: 10.1016/j.healun.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Soleimani B, Haouzi A, Manoskey A, et al. Development of aortic insufficiency in patients supported with continuous flow left ventricular assist devices. ASAIO J. 2012;58:326–9. doi: 10.1097/MAT.0b013e318251cfff. [DOI] [PubMed] [Google Scholar]
- 5.Mudd JO, Cuda JD, Halushka M, et al. Fusion of aortic valve commissures in patients supported by a continuous axial flow left ventricular assist device. J Heart Lung Transplant. 2008;27:1269–74. doi: 10.1016/j.healun.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 6.Holtz J, Teuteberg J. Management of aortic insufficiency in the continuous flow left ventricular assist device population. Curr Heart Fail Rep. 2014;11:103–10. doi: 10.1007/s11897-013-0172-6. [DOI] [PubMed] [Google Scholar]
- 7.Cowger J, Rao V, Massey T, et al. Comprehensive review and suggested strategies for the detection and management of aortic insufficiency in patients with a continuous-flow left ventricular assist device. J Heart Lung Transplant. 2015;34:149–57. doi: 10.1016/j.healun.2014.09.045. [DOI] [PubMed] [Google Scholar]
- 8.Martina J, de Jonge N, Sukkel E, et al. Left ventricular assist device-related systolic aortic regurgitation. Circulation. 2011;124:487–8. doi: 10.1161/CIRCULATIONAHA.111.020891. [DOI] [PubMed] [Google Scholar]
- 9.Kato TS, Maurer MS, Sera F, et al. Aortic regurgitation during systolic-phase accompanied by mitral regurgitation in patients with continuous-flow left ventricular assist device. Eur Heart J Cardiovasc Imaging. 2013;14:1022. doi: 10.1093/ehjci/jet054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siontis KC, Nkomo VT, Pislaru C, et al. Mechanism of aortic valve opening: beyond the pressure gradient. JACC Cardiovasc Imaging. 2014;7:633–4. doi: 10.1016/j.jcmg.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Estep JD, Stainback RF, Little SH, et al. The role of echocardiography and other imaging modalities in patients with left ventricular assist devices. JACC Cardiovasc Imaging. 2010;3:1049–64. doi: 10.1016/j.jcmg.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Grinstein J, Kruse E, Sayer G, et al. Accurate quantification methods for aortic insufficiency severity in patients with LVAD: role of diastolic flow acceleration and systolic-to-diastolic peak velocity ratio of the outflow cannula. JACC Cardiovascular Imaging. 2016;9:641–51. doi: 10.1016/j.jcmg.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 14.Grinstein J, Kruse E, Sayer G, et al. Quantification of aortic insufficiency in patients with left ventricular assist devices: a novel approach combining invasive hemodynamics and echocardiography. J Heart Lung Transplant. 2015;34(suppl):S154. [Google Scholar]
- 15.Grinstein J, Kruse E, Sayer G, et al. Novel parameters for grading aortic insufficiency severity in patients with left ventricular assist devices. J Am Soc Echocardiogr. 2015;28:B52–3. [Google Scholar]
- 16.Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 17.Horton SC, Khodaverdian R, Chatelain P, et al. Left ventricular assist device malfunction: an approach to diagnosis by echocardiography. J Am Coll Cardiol. 2005;45:1435–40. doi: 10.1016/j.jacc.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 18.Toda K, Fujita T, Domae K, et al. Late aortic insufficiency related to poor prognosis during left ventricular assist device support. Ann Thorac Surg. 2011;92:929–34. doi: 10.1016/j.athoracsur.2011.04.115. [DOI] [PubMed] [Google Scholar]
- 19.Aggarwal A, Raghuvir R, Eryazici P, et al. The development of aortic insufficiency in continuous-flow left ventricular assist device-supported patients. Ann Thorac Surg. 2013;95:493–8. doi: 10.1016/j.athoracsur.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Uriel N, Morrison KA, Garan AR, et al. Development of a novel echocardiography ramp test for speed optimization and diagnosis of device thrombosis in continuous-flow left ventricular assist devices: the Columbia ramp study. J Am Coll Cardiol. 2012;60:1764–75. doi: 10.1016/j.jacc.2012.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myers TJ, Frazier OH, Mesina HS, et al. Hemodynamics and patient safety during pump-off studies of an axial- flow left ventricular assist device. J Heart Lung Transplant. 2006;25:379–83. doi: 10.1016/j.healun.2005.11.459. [DOI] [PubMed] [Google Scholar]
- 22.Atkins BZ, Hashmi ZA, Ganapathi AM, et al. Surgical correction of aortic valve insufficiency after left ventricular assist device implantation. J Thorac Cardiovasc Surg. 2013;146:1247–52. doi: 10.1016/j.jtcvs.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 23.Retzer E, Tabit CE, Estrada JR, et al. TCT-78 Predictors of survival in patients undergoing trans-catheter aortic valve closure for significant left ventricular assist device associated aortic insufficiency. J Am Coll Cardiol. 2014:64. [Google Scholar]
- 24.Parikh KS, Mehrotra AK, Russo MJ, et al. Percutaneous transcatheter aortic valve closure successfully treats left ventricular assist device-associated aortic insufficiency and improves cardiac hemodynamics. JACC Cardiovasc Interv. 2013;6:84–9. doi: 10.1016/j.jcin.2012.08.021. [DOI] [PubMed] [Google Scholar]