Abstract
Objectives
This UK-wide study defines the natural history of argininosuccinic aciduria and compares long-term neurological outcomes in patients presenting clinically or treated prospectively from birth with ammonia-lowering drugs.
Methods
Retrospective analysis of medical records prior to March 2013, then prospective analysis until December 2015. Blinded review of brain MRIs. ASL genotyping.
Results
Fifty-six patients were defined as early-onset (n = 23) if symptomatic < 28 days of age, late-onset (n = 23) if symptomatic later, or selectively screened perinatally due to a familial proband (n = 10). The median follow-up was 12.4 years (range 0–53). Long-term outcomes in all groups showed a similar neurological phenotype including developmental delay (48/52), epilepsy (24/52), ataxia (9/52), myopathy-like symptoms (6/52) and abnormal neuroimaging (12/21). Neuroimaging findings included parenchymal infarcts (4/21), focal white matter hyperintensity (4/21), cortical or cerebral atrophy (4/21), nodular heterotopia (2/21) and reduced creatine levels in white matter (4/4). 4/21 adult patients went to mainstream school without the need of additional educational support and 1/21 lives independently. Early-onset patients had more severe involvement of visceral organs including liver, kidney and gut. All early-onset and half of late-onset patients presented with hyperammonaemia. Screened patients had normal ammonia at birth and received treatment preventing severe hyperammonaemia. ASL was sequenced (n = 19) and 20 mutations were found. Plasma argininosuccinate was higher in early-onset compared to late-onset patients.
Conclusions
Our study further defines the natural history of argininosuccinic aciduria and genotype–phenotype correlations. The neurological phenotype does not correlate with the severity of hyperammonaemia and plasma argininosuccinic acid levels. The disturbance in nitric oxide synthesis may be a contributor to the neurological disease. Clinical trials providing nitric oxide to the brain merit consideration.
Electronic supplementary material
The online version of this article (doi:10.1007/s10545-017-0022-x) contains supplementary material, which is available to authorized users.
Introduction
In the central nervous system, nitric oxide (NO) is involved in crucial processes including neurotransmission (Garthwaite 2008), neuronal differentiation (Peunova and Enikolopov 1995) and migration (Nott et al. 2013). Argininosuccinate lyase (ASL) cleaves argininosuccinate into arginine and fumarate as part of the NO-citrulline cycle that regulates NO production in multiple tissues (e-Figure 1) (Nagamani et al. 2012a). ASL deficiency causes argininosuccinic aciduria (ASA; OMIM 207900), the only inherited condition proven to cause systemic NO deficiency (Erez et al. 2011). ASL is also required for the liver-based urea cycle, which detoxifies ammonia produced by amino acid catabolism. ASA is the second most common urea cycle defect (UCD) with an incidence of 1:70,000 live-births (Nagamani et al. 2012a) and presents clinically either as an early neonatal-onset (<28 days of age) hyperammonaemic coma, or a later-onset hyperammonaemic crisis (Nagamani et al. 2012a). A chronic phenotype with neurocognitive, gastrointestinal and liver symptoms without severe hyperammonaemia is also recognised (Nagamani et al. 2012a). Conventional treatment aims to decrease ammonia by use of a protein-restricted diet and ammonia scavenger drugs (sodium benzoate and phenylbutyrate) and to correct arginine deficiency by L-arginine supplementation (Haberle et al. 2012).
The phenotype in ASA differs from other UCD by the higher incidence of neurocognitive symptoms, liver fibrosis, renal impairment and systemic hypertension (Nagamani et al. 2012a; Kolker et al. 2015). These symptoms are observed in patients with early- or late-onset forms and in those without documented episodes of hyperammonaemia (Marble et al. 2008). Among UCD, ASA patients have the lowest frequency of hyperammonaemic crises (23%) but the second highest frequency of cognitive impairment (65–74%) after arginase deficiency (Ruegger et al. 2014; Waisbren et al. 2016). This paradox raises questions about the role of hyperammonaemia in causing the neurological problems. Newborns screened and treated prospectively from birth have been reported to have a better neurological outcome (Widhalm et al. 1992; Ficicioglu et al. 2009; Mercimek-Mahmutoglu et al. 2010). As conventional treatment decreases ammonia levels, it was suggested that neurological complications were caused by unrecognised hyperammonaemic episodes (Widhalm et al. 1992). However, newborn screening programmes can capture a wide phenotypic spectrum, including patients who would remain asymptomatic without treatment. Some of these screened patients had high residual ASL activity (Ficicioglu et al. 2009), suggesting that the prospectively treated cohort might have had an increased number of mild cases, introducing a bias into the comparison.
We describe a United Kingom (UK) wide cohort of ASA patients expanding the disease natural history, reporting long-term neurological outcomes with a focus on neuroimaging and genotype–phenotype correlations. The outcomes in patients treated prospectively (10/56) were compared with those who presented with symptoms before diagnosis (46/56).
Material and methods
Patients
Anonymised data were collected prospectively from March 2013 and retrospectively before, from five tertiary metabolic centres in the UK: Birmingham Children’s Hospital, Birmingham; Guy’s and St Thomas’ Hospital, London; Great Ormond Street Hospital for Children, London; the National Hospital for Neurology and Neurosurgery, London; the Royal Manchester Children Hospital, Manchester. Molecular analysis of patients was approved by the National Research Ethics Service Committee London-Bloomsbury (13/LO/0168). Patients included had plasma argininosuccinic acid levels > 5 μmol/L, and/or pathogenic mutations in ASL. Patients were considered lost to follow-up if no clinical assessment was performed during the last 3 years at the relevant metabolic centre. The database was closed on 31st December 2015.
Neurological outcome was assessed with physical neurological examination regarding developmental impairment, epilepsy, ataxia, myopathy-like symptoms and brain MRI features and was performed as follows: if neuropsychological assessment was unavailable, cognitive impairment was determined by clinical judgement of the metabolic specialist or neuropaediatrician or by the need for additional support in school or subsequently at the workplace. Epilepsy was defined as the occurrence of two or more seizures without accompanying hyperammonaemia. MR spectroscopy was performed as described previously (Davison et al. 2011). Indication for neuroimaging was an unexplained and/or severe neurological disease. Brain MRIs were analysed by two neuroradiologists blinded to the report of each other. Magnetic resonance spectroscopy (MRS) studies were performed concurrently with clinically indicated MRI scans at 1.5 T. Comparison was made with MRS metabolite data from a standard cohort of children with normal appearing MRI as described previously. Liver involvement was considered using the following parameters: hepatomegaly, increased levels of transaminases (alanine aminotransferase ALT > 50 IU/L). Nephromegaly was defined as renal length on ultrasound imaging above the 95th centile for the age and sex. Biochemical data were assessed using the mean of at least the last ten results available during compensated metabolic state. Plasma ammonia levels were considered elevated if >100 μmol/L before 28 days of life or >45 μmol/L subsequently. Hypokalaemia was defined as a plasma potassium level lower than 3.5 mmol/L and judged as “transient” if observed in a single sample, or “persistent” if measured in ≥ 2 samples separated by ≥ 1 month. Plasma arginine and argininosuccinic acid reflect the last ten measurements performed during follow-up in a compensated metabolic state on the patient’s standard treatment. For analysis, patients were divided into three groups: (i) early-onset form (hyperammonaemic symptoms started on/before 28 days of life), (ii) late-onset form (presentation after 28 days of life), (iii) perinatally screened patients diagnosed after a family proband and treated prospectively from birth. For the last group, the status (early- or late-onset) of the familial proband was investigated but missing data prevented inclusion of these index cases into the study.
ASL sequencing
The 16 coding exons of ASL (NM_001024943.1; Ensembl ENST00000395332) and the intron-exon boundaries were PCR-amplified. Sequencing performed with the Big Dye Terminator Cycle Sequencing System version 1.1 (Applied Biosystems/ThermoFisher Scientific) was run on an ABI PRISM 3730 DNA Analyzer (Applied Biosystems/ThermoFisher Scientific) (e-Methods).
Statistical analysis
Statistical analyses used Fisher’s exact test for investigating the association between categorical data and the patient groups (www.vassarstats.net). Continuous variables between groups were compared using the Student’s t test or one-way ANOVA with Bonferroni correction for pairwise comparison (p values detailed in e-Table 1) (GraphPad Prism 5.0, San Diego, CA, USA). p values ≤ 0.05 were considered statistically significant. Kaplan-Meier survival curves were compared with the log-rank test. Patients 10, 18 and 52, who died during the first month of life, and patient 53, for whom very limited clinical information was available, were excluded from long-term analysis. For patients lost during follow-up (n = 6), the assessments at their last follow-up visits were used for analysis.
Results
Patients
Fifty-six patients were classified as early-onset (n = 23/56), late-onset (n = 23/56) or screened patients (n = 10/56). Ethnic origins were White British (n = 24; 44%), Pakistani (n = 16; 29%), Chinese (n = 6; 11%), Indian (n = 5; 9%), Bangladeshi (n = 3; 5%), other White European (n = 1; 2%) and missing data (n = 1; 2%) (e-Table 3). Screened patients, diagnosed either antenatally or neonatally, had an affected familial proband with early-onset (n = 5), late-onset (n = 3) or unknown (n = 2) phenotype (e-Table 3). One pair of siblings with early-onset form (patients 15 and 16) was included in the study. Mean follow-up was not significantly different between the early-onset (EO) or late-onset (LO) groups compared to the screened group (SCR) (p = 0.19 and p = 0.19 respectively) (Table 1).
Table 1.
Epidemiological and clinical data for the three analysed cohorts: early-onset, late-onset and screened patients
| Early onset | Late onset | Screened | Total | |||
|---|---|---|---|---|---|---|
| Epidemiology | Number (adult) | 23 (3 = 10%) | 23 (16 = 70%) | 10 (1 = 10%) | 56 (20 = 36%) | |
| Sex (M/F) | 12/11 | 11/12 | 8/2 | 31/25 | ||
| Consanguinity | 12/23 (52%) | 2/23 (9%) | 5/8 (62%) | 21/52 (40%) | ||
| Patients still living | 16/23 (73%) | 21/23 (91%) | 9/10 (90%) | 46/56 (82%) | ||
| At diagnosis | Age | 4 days (2–8) | 2.75 years (0.25–12) | 2 antenatally 8 neonatally |
2 years (0–12) | |
| Ammonia (RI < 100 μmol/L if <28 days of life; <50 μmol/L if >28 days of life) | 861 ± 120 | 212 ± 67 | 84 ± 18 | 530 ± 85 | ||
| Follow up | Mean follow-up (years) | 11 (1.9–25.7) | 15.1 (1–53) | 15.6 (8–18.2) | 12.4 (0–53) | |
| Patients lost | 2/23 (9%) | 4/23 (18%) | 2/10 (20%) | 8/56 (14%) | ||
| Age (years) | 11 (1.9–25.7) | 23 (16.7–57) | 15.6 (8–18.2) | 15.6 (1.9–57) | ||
| Phenotype | Neurology | Developmental delay | 18/21 (86%) | 23/23 (100%) | 7/8 (88%) | 48/52 (92%) |
| Age when first reported (years) | 2 (0.1–4) | 2.5 (1–6) | 3.1 (2–4) | 2 (0.1–6) | ||
| Epilepsy | 8/21 (38%) | 11/23 (48%) | 3/8 (38%) | 22/52 (42%) | ||
| Age when first reported (years) | 9 (1.5–13) | 2 (0.7–11) | 8.5 (8–9) | 5.5 (0.7–13) | ||
| Ataxia | 3/21 (14%) | 6/23 (26%) | 0/8 (0%) | 9/52 (17%) | ||
| Myopathic features | 4/21 (19%) | 3/23 (13%) | 0/8 (0%) | 7/52 (13%) | ||
| Abnormal brain MRI | 5/9 (56%) | 5/10 (50%) | 2/4 (50%) | 12/23 (52%) | ||
| Liver | Hepatomegaly | 17/21 (81%) | 3/23 (13%) | 5/8 (62%) | 25/51 (49%) | |
| Age when first reported (years) | 2.5 (0–12) | 13.0 | 07.5 (0.9–11) | 2.5 (0–12) | ||
| Raised ALT | 18/21 (86%) | 4/23 (17%) | 6/8 (75%) | 28/51 (55%) | ||
| Age when first reported (years) | 0.15 (0–6) | 23 (1–53) | 7 (3–7) | 1 (0–53) | ||
| ALT (RI 20–50 IU/L) | 238 ± 77 | 81 ± 24 | 181 ± 50 | 169 ± 37 | ||
| Kidney appearance (ultrasound) | Enlargement (>95th centile) | 8/18 (44%) | 2/9 (22%) | 2/5 (40%) | 12/32 (38%) | |
| Poor corticomedullar differentiation | 2/7 (29%) | 0/3 (0%) | 1/2 (50%) | 3/12 (25%) | ||
| Miscellaneous | Hypokalaemia: total | 16/23 (70%) | 4/23 (17%) | 6/10 (60%) | 26/56 (46%) | |
| Persistent | 3/23 (13%) | 3/23 (13%) | 1/10 (10%) | 7/56 (12%) | ||
| Intermittent | 13/23 (56%) | 1/23 (4%) | 5/10 (50%) | 19/56 (34%) | ||
| Arterial hypertension | 1 | 0 | 0 | 1 | ||
| Age when first reported (years) | 11 | / | / | 11 | ||
| Trichorrhexis nodosa | 0/23 (0%) | 5/23 (22%) | 0/10 (0%) | 5/56 (9%) | ||
| Age when first reported (years) | / | 7.3 ± 2.2 | / | |||
| Severe diarrhoea | 10/21 (48%) | 3/20 (15%) | 4/10 (40%) | 17/51 (33%) | ||
| Biology | Plasma arginine (RI 30-126 μmol/L) | 126 ± 19 | 102 ± 12 | 134 ± 15 | 116 ± 9 | |
| Plasma argininosuccinic acid (RI <5 μmol/L) | 512 ± 92 | 234 ± 64 | 238 ± 206 | 356 ± 62 | ||
| Therapeutics | Protein restricted diet | Frequency | 20/20 (100%) | 16/20 (80%) | 6/7 (86%) | 42/47 (89%) |
| Daily protein allowance (g/kg/day) | 1.2 ± 0.14 | 1.2 ± 0.1 | 1.4 ± 0.08 | 1.2 ± 0.07 | ||
| L-arginine supplementation | Frequency | 20/20 (100%) | 20/20 (100%) | 7/7 (100%) | 47/47 (100%) | |
| L-arginine (mg/kg/day) | 239 ± 28 | 155 ± 25 | 251 ± 45 | 201 ± 20 | ||
| Na benzoate supplementation | Frequency | 17/19 (89%) | 2/22 (9%) | 6/7 (72%) | 25/48 (52%) | |
| Na benzoate (mg/kg/day) | 215 ± 18 | 134 ± 33 | 167 ± 17 | 191 ± 15 | ||
| Na phenylbutyrate supplementation | Frequency | 7/19 (37%) | 2/22 (9%) | 1/7 (14%) | 10/48 (21%) | |
| Na phenylbutyrate (mg/kg/day) | 200 ± 50 | 57 ± 12 | NA | 143 ± 31 | ||
Age at diagnosis, currently, at first occurrence of symptom and duration of follow-up are presented as median ± range. Other figures show mean ± standard error. Hypokalaemia-total includes patients with intermittent and persistent hypokalaemia. Follow-up is considered until December 2015. Plasma arginine and argininosuccinic acid concentrations reflect the last ten measurements performed during follow-up when patients were in a compensated metabolic state on their standard treatment
ALT alanine aminotransferase, NA not available, RI range interval
Neurological phenotype
The frequency and median age of onset of the neurological features were not significantly different between the groups (e-Table 1).
Developmental impairment was reported in 48/52 patients (92%) and was the most common symptom. The median age at diagnosis was 2 years (range 0.1–6 years) (Table 1) and when observed, developmental impairment was present before the age of 6 years in all but two patients. Only four patients were reported with normal neurocognitive function: three early-onset patients aged <6 months (patient 22), 23 months (patient 15) and 11 years old at last assessment (patient 11) and one patient screened at birth (patient 47; sibling to a late-onset proband, aged 8 years old at last assessment) (e-Table 2). Developmental impairment was mild or moderate affecting predominantly speech and learning ability. Detailed information about schooling was available in 35 patients (e-Table 2). Only 6/35 patients (17%) attended mainstream school without the need for additional educational support (patients 9, 11, 35, 36, 47 and 48 with last assessment at 25, 11, 22, 20, 8 and 16 years old respectively). Most patients (20/35; 57%) required speech and language therapy. The neuropsychological assessments identified behavioural difficulties with auto- or hetero-aggression (n = 3) and learning disabilities in logic and reasoning. Twenty-one adults (EO n = 3; LO n = 17; SCR n = 1) with a median age of 22.3 years (range 18–57 years) were assessed for socioeconomic status. Five (25%) had semi-skilled employment. Independent living was reported in 1/12 (8%), and long-term relationships in 3/12 (25%). Patients not living independently were accommodated in the parental (9/11; 82%) or care (2/11; 18%) homes.
Epilepsy was observed in 22/52 patients (42%) with no significant difference of the median age of onset between groups (Table 1). Various seizure types were reported including generalised, partial and complex, febrile and afebrile seizures. Tonic-clonic seizures were most frequent (n = 16), followed by absence seizures (n = 5), myoclonic jerks (n = 4), atonic seizures (n = 2) and occasionally status epilepticus (n = 1) (e-Table 2). Electroencephalogram was performed in three non-epileptic patients and showed an abnormal pattern in two patients. 17/22 patients (77%) were treated with an average of 1.5 antiepileptic drugs (range 0–4).
Cerebellar dysfunction was detected in early-onset and late-onset patients (n = 3 and n = 6 respectively) with the incidence of 9/52 (17%) (Table 1). Ataxia was first noticed at a median age of 8.5 years (range 1–12) with two main age groups at first observation, early around the age of 1 year (n = 2) or later as teenagers (n = 7). Three patients had dyskinesia and tremor and one had nystagmus (e-Table 2). The mild inconvenience caused did not require any specific medical or surgical treatment.
Episodes of myopathy-like symptoms, reported in 7/52 patients (13%) (Table 1), included global hypotonia with a hypomimic facial expression, and unexplained recurrent episodes of general weakness persisting several days before spontaneous recovery. One patient (patient 28, currently aged 15.9 years) was reported with fatigable ptosis from 12 years old onwards. Four patients were investigated with electromyogram, which were always normal, including a Tensilon test in one patient. One patient (patient 33 currently aged 26 years) presented with an electrophysiologically confirmed episode of Guillain-Barré syndrome at 8 years old (e-Table 2).
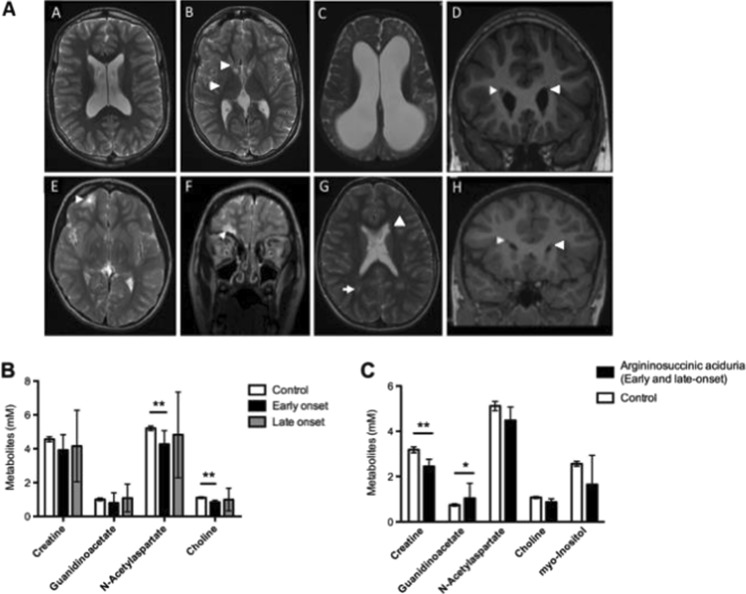
MRI brain was performed as part of the clinical work-up in 21 patients with unexplained or severe neurological features. The average age at the time of MRI was 12 years (range 0–23). Twelve scans were reported as abnormal (52%) (Table 1). Neuroimaging performed during follow-up showed small parenchymal infarcts (n = 4), foci of white matter hyperintensity on T2-weighted sequences (n = 4), nodular heterotopia (n = 2), cortical atrophy (n = 2), cerebellar atrophy (n = 2), perirolandic gliosis (n = 1), thalamic atrophy (n = 1), hyperintensity of caudate head and posterior putamen (n = 1) or isosignal between pallidi and putamen (n = 1) (Fig. 1A and e-Table 2). Spectroscopy of basal ganglia (n = 8; 3 early-onset, 5 late-onset) indicated a significant decrease of N-acetylaspartate and choline in early-onset patients compared to controls (p = 0.001 and p = 0.008, respectively). Creatine and guanidinoacetate levels in the basal ganglia did not differ significantly between controls, early- or late-onset groups (Fig. 1B). Spectroscopy of the white matter (n = 4; 3 early-onset, 1 late-onset) showed a significant decrease in creatine levels (p = 0.003) and an increase of guanidinoacetate (p = 0.01) (Fig. 1C) in patients compared to controls.
Fig. 1.
Neuroimaging. A: Morphological brain MRI features. A, B: T2-weighted axial images showing brain matter volume loss and mild ex vacuo dilatation of ventricles (A) and high signal in bilateral caudate heads and posterior putamina (arrows). C: T2-weighted axial images with severe diffuse cerebral atrophy and ventricular dilatation. D, H: T1-weighted coronal image with right periventricular heterotopia (arrowheads). E, F: T2-weighted axial (E) and coronal (F) images with evidence of right inferior frontal lobe infarct (arrow). G: T2-weighted axial image with bilateral high signal of the peritrigonal white matter (arrow). B 1 H MR spectroscopy features in basal ganglia. Assessment in early-onset (n = 5), late-onset (n = 3) and control (n = 63) patients analysed using a paired t test. c 1 H MR spectroscopy features in white matter. Patients affected by argininosuccinic aciduria (n = 4) and controls (n = 53) analysed with one way ANOVA. Graphs represent mean ± 95% confidence interval. * p < 0.05; ** p < 0.01
Systemic phenotype
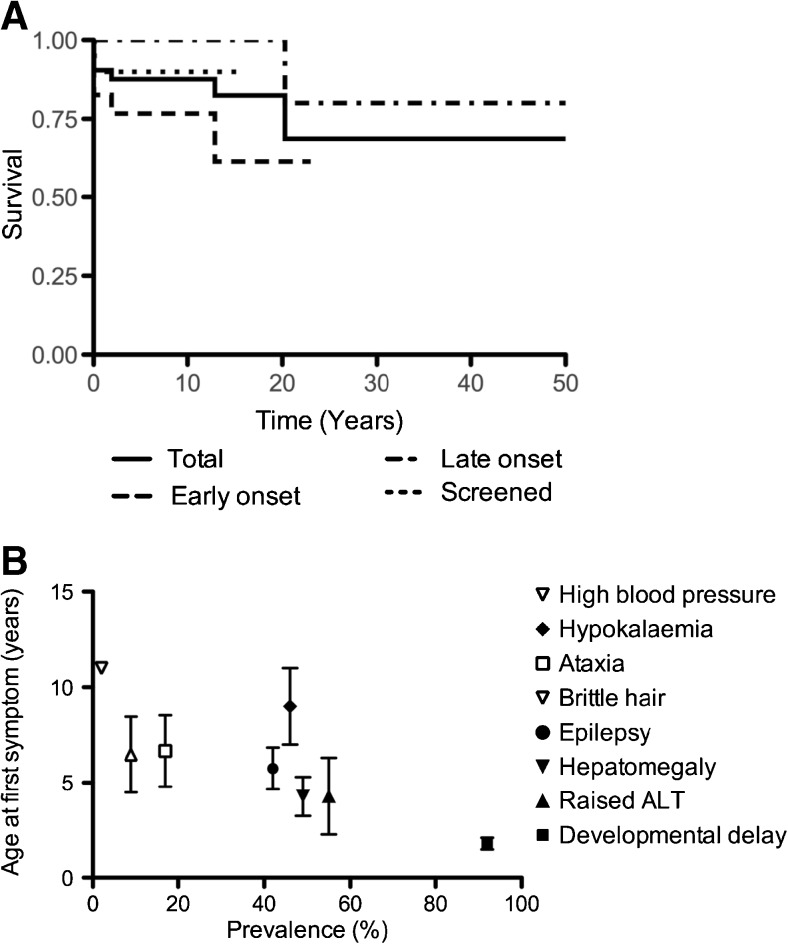
47/56 patients (84%) were alive at the time of assessment with no significant difference between groups, with a median follow-up of 12.4 years (range 0–53) (Fig. 2A). Cause and age of death were hyperammonaemic decompensation at presentation (n = 2; patients aged day 3 and 4 of life), sepsis (n = 3; patients aged 7 days, 11 years and 20 years), extradural hematoma (n = 1; patient aged 2 years), hepatocellular carcinoma (n = 1; patient aged 4.5 years), acute pancreatitis (n = 1; patient aged 12 years) and a possible arrhythmia (n = 1; patient aged 52 years).
Fig. 2.
Natural history of argininosuccinic aciduria. A Kaplan-Meier survival curves for all (solid line), early-onset (dashed line), late-onset (dashed dotted line) and screened (dotted line) patients. B Natural history of the systemic phenotype of argininosuccinic aciduria. Mean ± standard error of age of onset of each symptom from data of the whole cohort when information available: developmental delay (n = 7), abnormal LFTs (n = 8), hepatomegaly (n = 18), epilepsy (n = 15), brittle hair (n = 4), ataxia (n = 6), hypokalaemia (n = 2), high blood pressure (n = 1). Symptom frequency in the total population of patients studied is presented in brackets. ALT: plasma alanine aminotransferase activity. It was assumed that patients had normal blood pressure if hypertension was not specifically mentioned in medical records
Natural history data included the age of onset of organ involvement or symptoms (Fig. 2B). Among neurological symptoms, developmental delay was the first observed usually during the second or third year of life followed by epilepsy and ataxia.
The commonest hepatic involvement was a persistent rise in plasma alanine transaminase activity, usually accompanied by hepatomegaly. These were significantly more frequent in early-onset and screened patients than in late-onset patients (p < 0.00001 and p < 0.005, respectively; e-Table 1). In screened patients, the likelihood of hepatic abnormalities depended on the age of onset of the disease in the familial proband: 4/4 of screened patients with an early-onset familial history had hepatic abnormalities compared to 1/3 with a familial late-onset phenotype (Table 1).
There was no evidence of differences between groups for nephromegaly and poor corticomedullary differentiation assessed by ultrasound (Table 1). Transient or persistent hypokalaemia occurred more frequently in early-onset versus late-onset patients (p < 0.03) (Table 1). Acute metabolic decompensation, gastroenteritis and acute diarrhoea were significantly associated with transient hypokalaemia (p < 0.005).
Trichorrhexis nodosa was observed only in late-onset patients before diagnosis and normalised with treatment (Table 1).
Chronic profuse diarrhoea was observed in 17 patients (33%; including early-onset n = 10, late-onset n = 3, screened patients n = 4; Table 1). This symptom was refractory to symptomatic and immunosuppressive treatments and caused nutritional difficulties in several early-onset patients. Two patients had colonoscopies performed at 5 years of age and repeated at ages 7 and 10. Intestinal biopsies showed non-specific mild inflammation. Chronic pancreatitis was observed in one early-onset patient.
Refractory arterial hypertension was diagnosed in one early-onset patient (patient 6) at the age of 9 years and was sub-optimally controlled despite three antihypertensive medications. This patient died at 12 years old from acute pancreatitis. One late-onset patient developed atrial flutter at 60 years.
Ammonaemia, ASA levels and therapies
None of the patients in the screened group suffered severe or prolonged hyperammonaemia. Three of these patients had an initial ammonia level >100 μmol/L (133, 134 and 190 μmol/L), which normalised in less than 24 hours. All early-onset patients had hyperammonaemia at diagnosis with values significantly higher than in the late-onset and screened groups (p < 0.001; e-Table 1). Only 50% of the late-onset patients were hyperammonaemic at diagnosis.
A protein-restricted diet was used in 89% of patients (100% of the early-onset group and 80% of the late-onset group; Table 1). All patients were treated with L-arginine with no significant difference in the dose between groups (Table 1). Ammonia scavenger drugs (sodium benzoate and phenylbutyrate) were prescribed significantly more often in the early-onset and screened groups than in the late-onset group (p < 0.001 and p < 0.01 respectively) and at higher doses in the early-onset group (p = 0.03) (e-Table 1).
Plasma argininosuccinate levels were higher in early-onset (512 ± 92 μmol/L) compared to late-onset (234 ± 64 μmol/L) (p = 0.03) (e-Table 1).
Genotype–phenotype correlation
The genotype was available for 19 patients (Table 2). Twenty mutations (including eight novel) were identified: 11 were missense, five splice site, two nonsense mutations and two deletions. A sequence alignment of ASL showed that all missense mutations affect amino acid residues that are highly conserved across species. The deletions included a 13 base pair deletion (c.1045_1057del, p.(Val349Cysfs*72)) and one large deletion of approximately 2 kb which included exons 15 and 16 (c.1143+117_*1353del). Homozygous mutations observed with early onset disease included c.437G>A p.(Arg146Gln), c.749T>A p.(Met250Lys), c.1045_1057del p.(Val349Cysfs*3), c.1143+117_*1353del and c.1153C>T p.(Arg385Cys). Homozygous mutations observed with late-onset disease included c.35G>A p.(Arg12Gln), c.377G>A p.(Arg126Gln) and c.1138A>G p.(Lys380Glu). The c.1045_1057del deletion is predicted to cause a frameshift and introduction of a premature stop codon and the c.1143+117_*1353del deletion is predicted to cause the loss of exons 15 and 16, and both were associated with early-onset phenotype. Patients homozygous for c.1143+117_*1353del were younger brothers of a proband who was not genotyped but presented with the early onset phenotype (Table 2).
Table 2.
Genotype–phenotype correlation of hASL
| Patient number | Allele 1 | Allele 2 | Presumed effect on protein | Severity in this study | Reported severity in the literature | |
|---|---|---|---|---|---|---|
| 1 | c.35G>A | c.35G>A | p.(Arg12Gln) | p.(Arg12Gln) | Late onset | Unknown (n = 1) (Balmer et al. 2014) |
| 2 | c.348+1G>A | c.532G>A | Splicing effect | p.(Val178Met) | Late onset | New genotype |
| 3 | c.349-1G>A | c.532G>A | Splicing effect | p.(Val178Met) | Early onset | New genotype |
| 4, 5 | c.377G>A | c.377G>A | p.(Arg126G1n) | p.(Arg126G1n) | Late onset | New genotype |
| 6 | c.437G>A | c.437G>A | p.(Arg146G1n) | p.(Arg146G1n) | Early onset | New genotype |
| 7 | c.437G>A | c.446+1G>A | p.(Arg146G1n) | Splicing effect | Late onset | New genotype |
| 8 | c.544C>T | c.772G>A | p.(Arg182*) | p.(Glu258Lys) | Late onset | New genotype |
| 9 | c.719-2A>G | c.857A>G | Splicing effect | p.(Gln286Arg) | Early onset | New genotype |
| 10 | c.721G>A | c.918+5G>A | p.(Glu241Lys) | Splicing effect | Early onset | New genotype |
| 11, 12 | c.749T>A | c.749T>A | p.(Met250Lys) | p.(Met250Lys) | Early onset | New genotype |
| 13 | c.1045_1057del | c.1045_1057del | p.(Val349Cysfs*72) | p.(Val349Cysfs*72) | Early onset | New genotype |
| 14 | c.1138A>G | c.1138A>G | p.(Lys380Glu) | p.(Lys380Glu) | Late onset | Unknown (n = 1) (Balmer et al. 2014) |
| 15, 16a | c.1143+117_*1353del | c.1143+117_*1353del | Loss of exons 15 and 16 | Loss of exons 15 and 16 | Early onset | New genotype |
| 17, 18 | c.1153C>T | c.1153C>T | p.(Arg385Cys) | p.(Arg385Cys) | Early onset | Prenatal diagnosis (n = 3, Keskinen et al. 2008; Balmer et al. 2014), early onset (n = 4, this study, Kleijer et al. 2002; Keskinen et al. 2008), late onset (n = 7, Kleijer et al. 2002; Keskinen et al. 2008), unknown (n = 3, Balmer et al. 2014) |
| 19 | c.1284G>A | c.1366C>T | p.(Trp428*) | p.(Arg456Trp) | Late onset | New genotype |
New genotype refers to combination of mutations not described before. Presumed protein effect is mentioned within brackets for novel mutations. For screened patients, the severity of the phenotype is deducted from the symptomatic familial proband
aPatients 15 and 16 are siblings
Discussion
This study describes three groups of ASA patients (early-onset, late-onset and perinatally screened after a familial proband) with prolonged follow-up periods and compares the long-term outcome with regard to the time at initiation of treatment. In contrast to previously reported patients diagnosed by newborn screening, this study describes screened patients, who had a familial index case with a known phenotype.
Neurological outcome
The most common complications of ASA were neurological. Comparison between groups demonstrates a homogeneous long-term neurological outcome, with no significant difference in frequency, severity and age of onset for all neurological features assessed. Previously unreported neuroimaging findings such as focal infarcts or heterotopia might be related to impaired NO-dependent neuronal migration or microcirculation.
The early-onset group had higher ammonia levels compared to the late-onset group, as evidenced by differences in the ammonia levels at diagnosis, and need for ammonia scavenger medications and protein restriction. The screened patients also needed more treatment to control their ammonia levels than patients in the late-onset group because most of them (5/8) had siblings with early-onset disease. Plasma ASA levels were higher in early-onset compared to late-onset and screened patients. However, these differences did not affect the neurological phenotype. These observations show that hyperammonaemia and ASA levels are not the dominant factors causing the long-term neurological phenotype in ASA. Previous publications have suggested that neonatal screening and early treatment may prevent or ameliorate the neurological disease (Widhalm et al. 1992; Ficicioglu et al. 2009). However, an extended Austrian cohort of neonatally screened patients, initially reported with normal neurocognitive outcome at a mean age of 6 years (Widhalm et al. 1992), showed that 35% of patients (6/17; median age 13 years) had an IQ of less than 80 (Mercimek-Mahmutoglu et al. 2010). As the neurological disease is progressive, duration of the follow-up is essential to determine the outcome objectively. At the end of the first year, the frequency of developmental impairment is similar to other UCD (64%) attributable to sequelae of neonatal hyperammonaemia in early-onset patients (Burgard et al. 2016). This frequency increases to 65–100% with time (this study; Keskinen et al. 2008; Tuchman et al. 2008; Ah Mew et al. 2013; Ruegger et al. 2014).
Aggressive behaviour and psychiatric problems such as psychosis and paranoid ideation were previously reported in ASA (von Wendt et al. 1982; Odent et al. 1989; Lagas and Ruokonen 1991; Sijens et al. 2006), although these features were not observed in this cohort.
Systemic phenotype
Our study found a wide range of systemic complications. All of them were more frequent in the early-onset group, apart from trichorrhexis nodosa, although blood pressure was not systematically investigated. Chronic diarrhoea, not reported previously, was a major problem in many patients with endoscopy showing mild inflammation. This has been observed in an enterocyte-specific conditional knockout mouse model, in which a loss of ASL was associated with necrotizing enterocolitis (Premkumar et al. 2014). A similar pattern of systemic complications was found in the screened group, suggesting that prospective treatment has no preventative effect.
Genotype–phenotype correlation
Mutation analysis of our cohort identified genotype/phenotype correlation for some of the mutations in agreement with the literature.
The frequently occurring mutation c.35G>A p.(Arg12Gln) was associated with the late-onset phenotype in one patient as previously suggested for homozygous and compound heterozygous patients (Sampaleanu et al. 2002; Mercimek-Mahmutoglu et al. 2010; Balmer et al. 2014). It has been reported that the arginine 12 residue on the N-terminal loop close to the catalytic site of ASL might influence the binding/exit of the substrate without affecting the catalytic site explaining the milder phenotype (Sampaleanu et al. 2002).
The novel homozygous mutation c.749T>A p.(Met250Lys) was observed in two unrelated patients with early-onset phenotype. This mutation involves changes in the protein sequence close to two other amino acid modifications also associated with early-onset phenotype p.(Glu241Lys) and p.(Trp245fs) (Balmer et al. 2014).
The homozygous mutation c.1153C>T p.(Arg385Cys) has been associated previously with both early-onset (n = 4) (Kleijer et al. 2002; Keskinen et al. 2008) or late-onset phenotypes (n = 7) (Kleijer et al. 2002; Keskinen et al. 2008). However, all patients with late-onset phenotype were diagnosed before 20 months of life. p.(Arg385Cys) has been reported as a founder mutation in the Finnish population (Keskinen et al. 2008) and is associated with very low ASL activity affecting an amino acid near the catalytic site (Hu et al. 2015).
Pathophysiology of ASA
Various pathophysiological mechanisms have been proposed to account for the long-term complications of ASA. Argininosuccinic acid may be toxic to the brain, either directly or via the formation of guanidino compounds. Raised guanidinoacetate was reported on brain spectroscopy of ASA patients in the grey (3.63 ± 0.6 mmol/L) and the white matter (3.52 ± 0.09 mmol/L) (Sijens et al. 2006; van Spronsen et al. 2006) and may be explained by L-arginine supplementation (Sijens et al. 2006). In our study, levels of guanidinoacetate were similar to controls in basal ganglia but slightly elevated in white matter (1.05 ± 0.41 mmol/L). Patients with guanidinoacetate methyltransferase (GAMT) deficiency have much higher guanidinoacetate concentrations in brain (3.4-3.6 mmol/L) (Stockler et al. 1994) and CSF (11–12 μmol/L) (Stockler-Ipsiroglu et al. 2014). There is also some evidence of raised guanidinoacetate in patients with hyperargininaemia due to Arginase deficiency, with variable CSF guanidinoacetate concentrations (up to 0.127 μmol/L versus controls 0.049 μmol/L) (Deignan et al. 2010), although the spectral peak of guanidinoacetate at 3.8 ppm was not seen in a cohort of adult patients with hyperargininaemia (Carvalho et al. 2014), while in a 3 year old patient a prominent peak at 3.8 ppm was ascribed to arginine, which in vitro has resonances at 3.75 and 3.23 ppm (Wishart et al. 2007) and may have masked any detectable guanidinoacetate. Guanidinosuccinic acid can be neurotoxic (D’Hooge et al. 1992) and activates N-methyl-D-aspartate receptors (Aoyagi et al. 2001). However, this hypothesis is not supported by the observation of the early-onset group, which has higher levels of argininosuccinic acid but neurological outcomes similar to the other groups.
In humans, ASL is crucial for the synthesis of L-arginine, which becomes an essential amino acid in ASA. Arginine deprivation, associated with altered NO-mediated immune responses, can lead to site-specific neuronal loss in animal models of neurodegenerative diseases (Kan et al. 2015). Arginine is a precursor for the synthesis of creatine and agmatine (e-Figure 1). Brain spectroscopy showed creatine deficiency (this study; Sijens et al. 2006; van Spronsen et al. 2006; Boenzi et al. 2012). However, the role of secondary creatine deficiency in cerebral dysfunction has not been convincingly demonstrated (Boenzi et al. 2012). Agmatine is involved in learning (Leitch et al. 2011), neuroprotection (Molderings and Haenisch 2012) and anticonvulsant effect (Demehri et al. 2003). Thus, secondary agmatine deficiency could explain some of the neurological symptoms.
Finally, several symptoms may be caused by impaired NO synthesis. Using an Asl Neo/Neo mouse model, Erez et al. (2011) showed that defective ASL is responsible for the loss of the catalytic function of the enzyme and affects the structure of a multi-protein complex incorporating NOS. This disrupts the NOS-dependent NO synthesis and leads to systemic NO deficiency. Hypoargininaemia can lead to uncoupling of NOS, decreased NO production and increased generation of free radicals that damage tissues (e-Figure 1) (Nagamani et al. 2012b). Reactive oxygen species interfere with NO production and regulation of the microcirculation (Shu et al. 2015). In addition decreased NO levels might affect protein S-nitrosylation (Jaffrey et al. 2001), which in turn regulates histone methylation and gene expression (Nott and Riccio 2009). In the brain, NO plays a key-role as a signalling molecule (Riccio 2010) involved in neurotransmission (Garthwaite 2008), regulation of neuronal differentiation (Peunova and Enikolopov 1995; Lameu et al. 2012) and migration (Bredt and Snyder 1994; Nott et al. 2013). In human, NO therapy was reported to have mild neurocognitive benefit (Nagamani et al. 2012b). In this study, NO deficiency might account for the neuropathology underlying the neuroimaging findings such as local parenchymal infarcts or nodular heterotopia due to impaired microcirculation and abnormal neuronal migration during development respectively. Besides neurological implications, NO is involved in various physiological processes such as vasodilatation (Cosby et al. 2003), liver fibrosis (Diesen and Kuo 2011), muscle strength and performance (De Palma et al. 2014), kidney filtration rate (Satriano et al. 2008) and gut physiology (Vallance and Charles 1998; Premkumar et al. 2014; Bogdan 2015). Therefore, NO deficiency might be involved at least partially in various symptoms highlighted in this study including chronic hepatitis, myopathy-like phenotype, chronic diarrhoea and systemic hypertension.
Optimising therapeutics in ASA
This study demonstrates persisting neurological and systemic disease not obviously related to hyperammonaemia in ASA patients on conventional treatment. Although some organs (liver, kidney, gut) are more frequently affected in early-onset patients, who have higher ammonia and ASA levels, this is strikingly not the case for the brain. Our observation of parenchymal infarcts, nodular heterotopia and the report of mild neurological improvement after NO therapy (Nagamani et al. 2012b) support the role of NO deficiency in the pathophysiology of the brain disease in ASA. Currently correction of NO deficiency is not considered in the conventional treatment of ASA. Liver transplantation (Marble et al. 2008; Newnham et al. 2008) cures the urea cycle but would not be expected to correct the systemic NO-arginine cycle defect. Similarly, successful liver-targeted gene therapy in Asl Neo/Neo mouse did not correct extra-hepatic features such as defective NO-mediated vascular relaxation (Nagamani et al. 2012b). Future therapeutic approaches in ASA might consider targeting the NO deficiency, which could include the use of an enriched nitrate diet, nitrate therapy (Nagamani et al. 2012b; Erez 2013) or multiorgan-targeted gene replacement.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOCX 194 kb)
(DOCX 145 kb)
(DOCX 291 kb)
(DOCX 269 kb)
(DOCX 50 kb)
Acknowledgements
Study funded by Action Medical Research for Children Charity (grant GN2137).
PBM is in receipt of a Great Ormond Street Hospital (GOSH) Children’s Charity Leadership award (V2516) and is supported by the National Institute for Health Research Biomedical Research Centre at GOSH for Children NHS Foundation Trust and University College London. Miss Emma Reid and Mr Matthew Wilson provided technical assistance in ASL sequencing. The authors are indebted to the patients and families for their participation in this study. The authors thank the metabolic teams involved in the care of the patients, especially dieticians.
Abbreviations
- ALT
Alanine aminotransferase
- ASA
Argininosuccinic aciduria
- ASL
Argininosuccinate lyase
- CSF
Cerebro spinal fluid
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- UCD
Urea cycle defects
Compliance with ethical standards
Conflict of interest
None.
Contributor Information
Julien Baruteau, Phone: 0044 2078138331, Email: julien.baruteau@gosh.nhs.uk.
Elisabeth Jameson, Email: Elisabeth.jameson@cfmt.nhs.uk.
Andrew A. Morris, Email: Andrew.morris@cfmt.nhs.uk
Anupam Chakrapani, Email: anupam.chakrapani@gosh.nhs.uk.
Saikat Santra, Email: saikat.santra@bch.nhs.uk.
Suresh Vijay, Email: suresh.vijay@bch.nhs.uk.
Huriye Kocadag, Email: huriyegkocadag@gmail.co.
Clare E. Beesley, Email: clare.beesley@gosh.nhs.uk
Stephanie Grunewald, Email: Stephanie.grunewald@gosh.nhs.uk.
Elaine Murphy, Email: Elaine.murphy@uclh.nhs.uk.
Maureen Cleary, Email: Maureen.cleary@gosh.nhs.uk.
Helen Mundy, Email: Helen.mundy@gstt.nhs.uk.
Lara Abulhoul, Email: lara.abulhoul@gosh.nhs.uk.
Alexander Broomfield, Email: alexander.broomfield@cfmt.nhs.uk.
Robin Lachmann, Email: robin.lachmann@uclh.nhs.uk.
Yusof Rahman, Email: yusof.rahman@gstt.nhs.uk.
Peter H. Robinson, Email: peter.robinson3@nhs.net
Lesley MacPherson, Email: Lesley.macpherson@bch.nhs.uk.
Katharine Foster, Email: Katharine.foster@bch.nhs.uk.
W. Kling Chong, Email: Kling.chong@gosh.nhs.uk.
Deborah A. Ridout, Email: d.ridout@ucl.ac.uk
Kirsten McKay Bounford, Email: Kirsten.mckaybounford@bwnft.nhs.uk.
Simon N. Waddington, Email: S.waddington@ucl.ac.uk
Philippa B. Mills, Email: p.mills@ucl.ac.uk
Paul Gissen, Email: p.gissen@ucl.ac.uk.
James E. Davison, Email: James.davison@gosh.nhs.uk
References
- Ah Mew N, Krivitzky L, McCarter R, Batshaw M, Tuchman M, Urea Cycle Disorders Consortium of the Rare Diseases Clinical Research Network (2013) Clinical outcomes of neonatal onset proximal versus distal urea cycle disorders do not differ. J Pediatr 162:324–329 e321 [DOI] [PMC free article] [PubMed]
- Aoyagi K, Shahrzad S, Iida S, et al. Role of nitric oxide in the synthesis of guanidinosuccinic acid, an activator of the N-methyl-D-aspartate receptor. Kidney Int Suppl. 2001;78:S93–S96. doi: 10.1046/j.1523-1755.2001.59780093.x. [DOI] [PubMed] [Google Scholar]
- Balmer C, Pandey AV, Rufenacht V, et al. Mutations and polymorphisms in the human argininosuccinate lyase (ASL) gene. Hum Mutat. 2014;35:27–35. doi: 10.1002/humu.22469. [DOI] [PubMed] [Google Scholar]
- Boenzi S, Pastore A, Martinelli D, et al. Creatine metabolism in urea cycle defects. J Inherit Metab Dis. 2012;35:647–653. doi: 10.1007/s10545-012-9494-x. [DOI] [PubMed] [Google Scholar]
- Bogdan C. Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol. 2015;36:161–178. doi: 10.1016/j.it.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Transient nitric oxide synthase neurons in embryonic cerebral cortical plate, sensory ganglia, and olfactory epithelium. Neuron. 1994;13:301–313. doi: 10.1016/0896-6273(94)90348-4. [DOI] [PubMed] [Google Scholar]
- Burgard P, Kolker S, Haege G, Lindner M, Hoffmann GF. Neonatal mortality and outcome at the end of the first year of life in early onset urea cycle disorders—review and meta-analysis of observational studies published over more than 35 years. J Inherit Metab Dis. 2016;39:219–229. doi: 10.1007/s10545-015-9901-1. [DOI] [PubMed] [Google Scholar]
- Carvalho DR, Farage L, Martins BJ, Brum JM, Speck-Martins CE, Pratesi R. Brain MRI and magnetic resonance spectroscopy findings in patients with hyperargininemia. J Neuroimaging. 2014;24:155–160. doi: 10.1111/j.1552-6569.2012.00739.x. [DOI] [PubMed] [Google Scholar]
- Cosby K, Partovi KS, Crawford JH, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- Davison JE, Davies NP, Wilson M, et al. MR spectroscopy-based brain metabolite profiling in propionic acidaemia: metabolic changes in the basal ganglia during acute decompensation and effect of liver transplantation. Orphanet J Rare Dis. 2011;6:19. doi: 10.1186/1750-1172-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma C, Morisi F, Pambianco S, et al. Deficient nitric oxide signalling impairs skeletal muscle growth and performance: involvement of mitochondrial dysregulation. Skelet Muscle. 2014;4:22. doi: 10.1186/s13395-014-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deignan JL, De Deyn PP, Cederbaum SD, et al. Guanidino compound levels in blood, cerebrospinal fluid, and post-mortem brain material of patients with argininemia. Mol Genet Metab. 2010;100(Suppl 1):S31–S36. doi: 10.1016/j.ymgme.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Demehri S, Homayoun H, Honar H, et al. Agmatine exerts anticonvulsant effect in mice: modulation by alpha 2-adrenoceptors and nitric oxide. Neuropharmacology. 2003;45:534–542. doi: 10.1016/S0028-3908(03)00199-0. [DOI] [PubMed] [Google Scholar]
- D’Hooge R, Pei YQ, Marescau B, De Deyn PP. Convulsive action and toxicity of uremic guanidino compounds: behavioral assessment and relation to brain concentration in adult mice. J Neurol Sci. 1992;112:96–105. doi: 10.1016/0022-510X(92)90138-B. [DOI] [PubMed] [Google Scholar]
- Diesen DL, Kuo PC. Nitric oxide and redox regulation in the liver: part II. Redox biology in pathologic hepatocytes and implications for intervention. J Surg Res. 2011;167:96–112. doi: 10.1016/j.jss.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez A. Argininosuccinic aciduria: from a monogenic to a complex disorder. Genet Med. 2013;15:251–257. doi: 10.1038/gim.2012.166. [DOI] [PubMed] [Google Scholar]
- Erez A, Nagamani SC, Shchelochkov OA, et al. Requirement of argininosuccinate lyase for systemic nitric oxide production. Nat Med. 2011;17:1619–1626. doi: 10.1038/nm.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficicioglu C, Mandell R, Shih VE. Argininosuccinate lyase deficiency: longterm outcome of 13 patients detected by newborn screening. Mol Genet Metab. 2009;98:273–277. doi: 10.1016/j.ymgme.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci. 2008;27:2783–2802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberle J, Boddaert N, Burlina A, et al. Suggested guidelines for the diagnosis and management of urea cycle disorders. Orphanet J Rare Dis. 2012;7:32. doi: 10.1186/1750-1172-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Pandey AV, Balmer C, et al. Unstable argininosuccinate lyase in variant forms of the urea cycle disorder argininosuccinic aciduria. J Inherit Metab Dis. 2015;38:815–827. doi: 10.1007/s10545-014-9807-3. [DOI] [PubMed] [Google Scholar]
- Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- Kan MJ, Lee JE, Wilson JG, et al. Arginine deprivation and immune suppression in a mouse model of Alzheimer’s disease. J Neurosci. 2015;35:5969–5982. doi: 10.1523/JNEUROSCI.4668-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskinen P, Siitonen A, Salo M. Hereditary urea cycle diseases in Finland. Acta Paediatr. 2008;97:1412–1419. doi: 10.1111/j.1651-2227.2008.00923.x. [DOI] [PubMed] [Google Scholar]
- Kleijer WJ, Garritsen VH, Linnebank M, et al. Clinical, enzymatic, and molecular genetic characterization of a biochemical variant type of argininosuccinic aciduria: prenatal and postnatal diagnosis in five unrelated families. J Inherit Metab Dis. 2002;25:399–410. doi: 10.1023/A:1020108002877. [DOI] [PubMed] [Google Scholar]
- Kolker S, Valayannopoulos V, Burlina AB, et al. The phenotypic spectrum of organic acidurias and urea cycle disorders. Part 2: the evolving clinical phenotype. J Inherit Metab Dis. 2015;38:1059–1074. doi: 10.1007/s10545-015-9840-x. [DOI] [PubMed] [Google Scholar]
- Lagas PA, Ruokonen A. Late onset argininosuccinic aciduria in a paranoid retardate. Biol Psychiatry. 1991;30:1229–1232. doi: 10.1016/0006-3223(91)90159-J. [DOI] [PubMed] [Google Scholar]
- Lameu C, Trujillo CA, Schwindt TT, et al. Interactions between the NO-citrulline cycle and brain-derived neurotrophic factor in differentiation of neural stem cells. J Biol Chem. 2012;287:29690–29701. doi: 10.1074/jbc.M111.338095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch B, Shevtsova O, Reusch K, Bergin DH, Liu P. Spatial learning-induced increase in agmatine levels at hippocampal CA1 synapses. Synapse. 2011;65:146–153. doi: 10.1002/syn.20828. [DOI] [PubMed] [Google Scholar]
- Marble M, McGoey RR, Mannick E, et al. Living related liver transplant in a patient with argininosuccinic aciduria and cirrhosis: metabolic follow-up. J Pediatr Gastroenterol Nutr. 2008;46:453–456. doi: 10.1097/MPG.0b013e3180ca8720. [DOI] [PubMed] [Google Scholar]
- Mercimek-Mahmutoglu S, Moeslinger D, Haberle J, et al. Long-term outcome of patients with argininosuccinate lyase deficiency diagnosed by newborn screening in Austria. Mol Genet Metab. 2010;100:24–28. doi: 10.1016/j.ymgme.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Molderings GJ, Haenisch B. Agmatine (decarboxylated L-arginine): physiological role and therapeutic potential. Pharmacol Ther. 2012;133:351–365. doi: 10.1016/j.pharmthera.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Nagamani SC, Erez A, Lee B. Argininosuccinate lyase deficiency. Genet Med. 2012;14:501–507. doi: 10.1038/gim.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamani SC, Campeau PM, Shchelochkov OA, et al. Nitric-oxide supplementation for treatment of long-term complications in argininosuccinic aciduria. Am J Hum Genet. 2012;90:836–846. doi: 10.1016/j.ajhg.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newnham T, Hardikar W, Allen K, et al. Liver transplantation for argininosuccinic aciduria: clinical, biochemical, and metabolic outcome. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2008;14:41–45. doi: 10.1002/lt.21297. [DOI] [PubMed] [Google Scholar]
- Nott A, Riccio A. Nitric oxide-mediated epigenetic mechanisms in developing neurons. Cell Cycle. 2009;8:725–730. doi: 10.4161/cc.8.5.7805. [DOI] [PubMed] [Google Scholar]
- Nott A, Nitarska J, Veenvliet JV, et al. S-nitrosylation of HDAC2 regulates the expression of the chromatin-remodeling factor Brm during radial neuron migration. Proc Natl Acad Sci U S A. 2013;110:3113–3118. doi: 10.1073/pnas.1218126110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odent S, Roussey M, Journel H, Betremieux P, David V, Le Marec B. Argininosuccinic aciduria. A new case revealed by psychiatric disorders. J Hum Genet. 1989;37:39–42. [PubMed] [Google Scholar]
- Peunova N, Enikolopov G. Nitric oxide triggers a switch to growth arrest during differentiation of neuronal cells. Nature. 1995;375:68–73. doi: 10.1038/375068a0. [DOI] [PubMed] [Google Scholar]
- Premkumar MH, Sule G, Nagamani SC, et al. Argininosuccinate lyase in enterocytes protects from development of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2014;307:G347–G354. doi: 10.1152/ajpgi.00403.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A. Dynamic epigenetic regulation in neurons: enzymes, stimuli and signaling pathways. Nat Neurosci. 2010;13:1330–1337. doi: 10.1038/nn.2671. [DOI] [PubMed] [Google Scholar]
- Ruegger CM, Lindner M, Ballhausen D, et al. Cross-sectional observational study of 208 patients with non-classical urea cycle disorders. J Inherit Metab Dis. 2014;37:21–30. doi: 10.1007/s10545-013-9624-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaleanu LM, Yu B, Howell PL. Mutational analysis of duck delta 2 crystallin and the structure of an inactive mutant with bound substrate provide insight into the enzymatic mechanism of argininosuccinate lyase. J Biol Chem. 2002;277:4166–4175. doi: 10.1074/jbc.M107465200. [DOI] [PubMed] [Google Scholar]
- Satriano J, Cunard R, Peterson OW, Dousa T, Gabbai FB, Blantz RC. Effects on kidney filtration rate by agmatine requires activation of ryanodine channels for nitric oxide generation. Am J Physiol Renal Physiol. 2008;294:F795–F800. doi: 10.1152/ajprenal.00392.2007. [DOI] [PubMed] [Google Scholar]
- Shu X, Keller TC, Begandt D, et al. Endothelial nitric oxide synthase in the microcirculation. Cell Mol Life Sci. 2015;72:4561–4575. doi: 10.1007/s00018-015-2021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijens PE, Reijngoud DJ, Soorani-Lunsing RJ, Oudkerk M, van Spronsen FJ. Cerebral 1H MR spectroscopy showing elevation of brain guanidinoacetate in argininosuccinate lyase deficiency. Mol Genet Metab. 2006;88:100–102. doi: 10.1016/j.ymgme.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Stockler S, Holzbach U, Hanefeld F, et al. Creatine deficiency in the brain: a new, treatable inborn error of metabolism. Pediatr Res. 1994;36:409–413. doi: 10.1203/00006450-199409000-00023. [DOI] [PubMed] [Google Scholar]
- Stockler-Ipsiroglu S, van Karnebeek C, Longo N, et al. Guanidinoacetate methyltransferase (GAMT) deficiency: outcomes in 48 individuals and recommendations for diagnosis, treatment and monitoring. Mol Genet Metab. 2014;111:16–25. doi: 10.1016/j.ymgme.2013.10.018. [DOI] [PubMed] [Google Scholar]
- Tuchman M, Lee B, Lichter-Konecki U, et al. Cross-sectional multicenter study of patients with urea cycle disorders in the United States. Mol Genet Metab. 2008;94:397–402. doi: 10.1016/j.ymgme.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallance P, Charles I (1998) Nitric oxide as an antimicrobial agent: does NO always mean NO? Gut 42:313–314 [DOI] [PMC free article] [PubMed]
- van Spronsen FJ, Reijngoud DJ, Verhoeven NM, Soorani-Lunsing RJ, Jakobs C, Sijens PE. High cerebral guanidinoacetate and variable creatine concentrations in argininosuccinate synthetase and lyase deficiency: implications for treatment? Mol Genet Metab. 2006;89:274–276. doi: 10.1016/j.ymgme.2006.02.005. [DOI] [PubMed] [Google Scholar]
- von Wendt L, Simila S, Ruokonen A, Puukka M. Argininosuccinic aciduria in a Finnish woman presenting with psychosis and mental retardation. Ann Clin Res. 1982;14:145–147. [PubMed] [Google Scholar]
- Waisbren SE, Gropman AL, Members of the Urea Cycle Disorders Cosortium. Batshaw ML. Improving long term outcomes in urea cycle disorders—report from the Urea Cycle Disorders Consortium. J Inherit Metab Dis. 2016;39:573–584. doi: 10.1007/s10545-016-9942-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widhalm K, Koch S, Scheibenreiter S, et al. Long-term follow-up of 12 patients with the late-onset variant of argininosuccinic acid lyase deficiency: no impairment of intellectual and psychomotor development during therapy. Pediatrics. 1992;89:1182–1184. [PubMed] [Google Scholar]
- Wishart DS, Tzur D, Knox C, et al. HMDB: the Human Metabolome Database. Nucleic Acids Res. 2007;35:D521–D526. doi: 10.1093/nar/gkl923. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 194 kb)
(DOCX 145 kb)
(DOCX 291 kb)
(DOCX 269 kb)
(DOCX 50 kb)