Abstract
Summary: Nanoparticle drug carriers consist of solid biodegradable particles in size ranging from 10 to 1000 nm (50–300 nm generally). They cannot freely diffuse through the blood-brain barrier (BBB) and require receptor-mediated transport through brain capillary endothelium to deliver their content into the brain parenchyma. Polysorbate 80-coated polybutylcyanoacrylate nanoparticles can deliver drugs to the brain by a still debated mechanism. Despite interesting results these nanoparticles have limitations, discussed in this review, that may preclude, or at least limit, their potential clinical applications. Long-circulating nanoparticles made of methoxypoly(ethylene glycol)- polylactide or poly(lactide-co-glycolide) (mPEG-PLA/PLGA) have a good safety profiles and provide drug-sustained release. The availability of functionalized PEG-PLA permits to prepare target-specific nanoparticles by conjugation of cell surface ligand. Using peptidomimetic antibodies to BBB transcytosis receptor, brain-targeted pegylated immunonanoparticles can now be synthesized that should make possible the delivery of entrapped actives into the brain parenchyma without inducing BBB permeability alteration. This review presents their general properties (structure, loading capacity, pharmacokinetics) and currently available methods for immunonanoparticle preparation.
Keywords: Nanoparticle, immunonanoparticle, brain targeting, blood brain barrier, transcytosis, PEG
INTRODUCTION
Nanoparticles are solid colloidal matrix-like particles made of polymers1 or lipids.2 Generally administered by the intravenous route like liposomes, they have been developed for the targeted delivery of therapeutic or imaging agents. Their main advantages over liposomes are the low number of excipients used in their formulations, the simple procedures for preparation, a high physical stability, and the possibility of sustained drug release that may be suitable in the treatment of chronic diseases. Until the mid 1990s, their development as drug carriers was seriously limited by the lack of long-circulating properties.3 Therefore, in contrast to liposomes and despite the abundance of experimental works and achievements in the field of nanoparticle technology, no nanoparticle-based drug formulation has been marketed so far. Due to their size ranging from 10 to 1000 nm (generally 50–300 nm), and like liposomes, they are unable to diffuse through the blood-brain barrier (BBB) to reach the brain parenchyma. Based on general parenteral formulation considerations and specific BBB features, Table 1 summarizes the ideal nanoparticle properties required for drug brain delivery.4 One particularly interesting application of nanoparticule could be the drug brain delivery, accompanied with the local sustained release, of the new large molecule therapeutics now available to treat the CNS: peptides, proteins, genes, antisense drugs. Due to their poor stability in biological fluids, rapid enzymatic degradation, unfavorable pharmacokinetic properties, and lack of diffusion toward the CNS, they may be advantageously formulated in brain-targeted protective nanocontainers.5 Compared with conventional drugs, they possess a high intrinsic pharmacological activity. The small dose requested for therapeutic efficiency could easily fit the loading capacity of nanoparticles and would not require the administration of large amount of potentially toxic nanoparticle excipient. Because of the large variety of the nanoparticles developed so far, this review will focus on nanoparticles investigated for brain delivery. Nanoparticles made of polybutylcyanoacrylate (PBCA, FIG. 1) have been intensely investigated since the first papers in 1995 showing that when coated with the nonionic surfactant polysorbate 80 they permitted to deliver drugs to the brain.6,7 Despite interesting results, PBCA nanoparticles have limitations, discussed in this review, that may preclude, or at least limit, their potential clinical applications. Nanoparticles made of polylactide homopolymers (PLA) or poly(lactide-co-glycolide) heteropolymers (PLGA) may be a promising alternative. In the mid 1990s, long-circulating pegylated PLA or PLGA nanoparticles have been made available that opened great opportunities for drug targeting.3 Pegylated nanoparticles are made of methoxypoly(ethylene glycol)-PLA/PLGA (mPEG-PLA/PLGA, FIG. 1), i.e., esters of PLA or PLGA with PEG of various molecular weights. More recently, the synthesis of functionalized pegylated PLA/PLGA nanoparticles opened new perspectives for targeted drug delivery in general, and for drug brain targeting in particular. This review will present their general properties and will propose preparation methods of brain-targeted pegylated nanoparticles.
TABLE 1.
Ideal Properties of Nanoparticles for Drug Brain Delivery
|
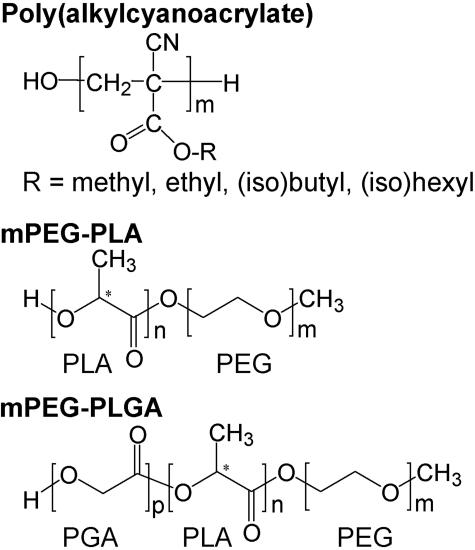
FIG. 1.
Structure of poly(alkylcyanoacrylate), methoxypoly(ethylene glycol)-polylactide [or poly(lactic acid)] (mPEG-PLA) and methoxypoly(ethylene glycol)-poly(lactide-co-glycolide) [or poly(lactic-co-glycolic acid)] (mPEG-PLGA).
PBCA NANOPARTICLES
General considerations
Nanoparticles made of poly(alkylcyanoacrylate) polymers (FIG. 1) were first described in 19778 and were recently the subject of a comprehensive review of their properties, preparation methods and potential therapeutic applications.9 They are generally prepared from (iso)butylcyanoacrylate or (iso)hexylcyanoacrylate monomers by emulsion anionic polymerization in an acidic aqueous solution of a colloidal stabilizer such as dextran 70, polysorbates, and poloxamers. Inclusion of drug can be made during the polymerization process or by adsorption onto preformed nanoparticles. Using the first method, chemical reactions may occur between drugs and monomers.10 Alternatively, the interaction between adsorbed drugs and the nanoparticle may lack stability, especially when a surfactant is subsequently added to the preparation11 or, once the nanoparticles are dispersed in blood, by a combined effect of serum protein competition and polymer degradation.12 The length of the alkyl pendant governs degradation rates13,14 and toxicity15,16 of poly(alkylcyanoacrylate) nanoparticles, which decrease in the order methyl>ethyl>butyl/isobutyl>hexyl/isohexyl. Phase I trials were therefore carried out with poly(isohexyl cyanoacrylate) nanoparticles that have the best safety profile and an appropriate degradation rate.17 Lacking stealth properties, poly(alkylcyanoacrylate) nanoparticles administered intravenously are rapidly cleared from the blood stream by the monuclear phagocyte system (MPS) and mainly accumulate in liver and spleen,18–20 together with the entrapped compounds.21–23 Only pegylated polyalkylcyanoacrylate nanoparticles have lower MPS uptake and prolonged blood circulation in vivo.24
Brain delivery with PBCA nanoparticles
Adsorbed onto polysorbate 80-coated PBCA nanoparticles administered intravenously compounds with poor brain diffusion as diverse as doxorubicin,25,26 loperamide,27 tubocurarine,28 the hexapeptide dalargin6,7 were successfully delivered to the brain, where they induced a pharmacological effect (for review, see Kreuter29). The chemical nature of the overcoating surfactant is of importance, because only polysorbates, not poloxamers (184, 188, 388, or 407), poloxamine 908, Cremophors (EZ or RH40) or polyoxyethylene(23)-laurylether, led to a CNS pharmacological effect of dalargin.30 As the mechanism of action, it was hypothesized that polysorbate-coated nanoparticles were transported across the BBB via endocytosis by the brain capillary endothelial cells.29 This endocytosis would be triggered by a serum protein, apolipoprotein E, reported to adsorb on polysorbate 20, 40, 60, or 80-coated nanoparticles after a 5-min incubation in citrate-stabilized plasma at 37°C, but not on nanoparticles coated with poloxamers 338, 407, Cremophor EL, or RH 40.29 Despite numerous arguments listed by Kreuter,29 this hypothesis raises questions, based on the following observations. 1) Apolipoprotein E adsorption is not specific of polysorbate 80-coated surfaces because it was shown to adsorb onto pegylated PLA nanoparticles.31,32 2) Polysorbate 80-coated poly(methylmethacrylate) nanoparticles are not distributed into the brain after IV administration.33 3) Replacing polysorbate 80-coated PBCA nanoparticles with polysorbate 80-coated polystyrene nanoparticles completely abolished dalargin brain delivery.11 4) The pharmacokinetic profile of polysorbate 80-coated nanoparticles is not favorable to brain distribution, due to a massive uptake by the MPS resulting in liver and spleen accumulation.33 5) Polysorbate 80 and serum protein competition, as well as the rapid nanoparticle degradation in serum/plasma, were shown to induce desorption of compounds adsorbed onto PBCA nanoparticles within a few minutes.11,12 As an evidence of this desorption, blood pharmacokinetic profiles of drugs adsorbed onto polysorbate 80-coated PBCA nanoparticles administered intravenously were actually similar to free solutions,25,34,35 and not at all typical of drugs associated to nonstealth colloidal drug carriers.21,22,23,36 Therefore, as an alternative to the brain uptake of nanoparticles, we hypothesized a nanoparticle-induced nonspecific BBB permeabilization.11 It has been known for a long time that polysorbate 80 causes BBB disturbance at intravenous systemic doses as low as 3 mg/kg37 (25–100 mg/kg polysorbate 80 doses were used in brain targeting experiments7,25,27). Recently, Calvo et al.36 showed that a polysorbate 80 intravenous dose of 20 mg/kg in rats dramatically increased BBB permeability to sucrose. In rats treated with polysorbate 80-coated PBCA nanoparticles (polysorbate 80: 25 mg/kg, nanoparticles: 50 mg/kg) inulin spaces increased by 10% (not significant) after 10 min and by 99% (significant) after 45 min compared with control.38 Because apparently no brain uptake was observed with control drug-polysorbate 80 solutions, the toxicity of PBCA nanoparticles was proposed as a synergistic factor for BBB permeabilization.11 The nanoparticle doses permitting brain delivery (100–166 mg/kg generally) were close to the lethal dose 50% of PBCA nanoparticles (230 mg/kg in mice16). Polysorbate 80-coated or uncoated PBCA nanoparticles (unloaded with drug) induced a dramatic decrease in mice locomotor activity (associated with obvious signs of distress) at a nanoparticle dose of 135 mg/kg and the permeabilization of an in vitro BBB model at a concentration of 10 μg/ml (to be compared to the 1.5 mg/ml theoretical concentration reached in mice blood after dosing animals with a 135 mg/kg nanoparticle dose).11 In contrast, the nontoxic polysorbate 80-coated polystyrene nanoparticles were ineffective at delivering dalargin to the brain.11 In a context of general toxicity induced by the high dose of PBCA nanoparticles and associated to the synergistic BBB permeabilization effect of polysorbate 80, major damage to the BBB cannot be excluded. Beyond the ongoing controversy about their mechanism of action, polysorbate 80-coated PBCA nanoparticles should be evaluated in term of benefit/risk ratio and of innovative therapeutics. In addition to the toxicity issue, the short duration of the pharmacological effect observed after administration of drugs formulated with this carrier (210 min at the best39) would probably necessitate daily intravenous administrations, a perspective not suitable for the treatment of chronic brain diseases.
PEGYLATED PLA OR PLGA NANOPARTICLES
General considerations
Among the few biodegradable polymers, polymers derived from glycolic acid and from d,l-lactic acid enantiomers are presently the most attractive compounds because of their biocompatibility and their resorbability through natural pathways.40,41 They are widely used for the preparation of biodegradable medical devices and of drug-sustained release microspheres or implants marketed in Europe, Japan, and the U.S.42 Degradation of PLA or PLGA occurs by autocatalytic cleavage of the ester bonds through spontaneous hydrolysis into oligomers and d,l-lactic and glycolic acid monomers.43 Lactate converted into pyruvate and glycolate enter the Krebs' cycle to be degraded into CO2 and H2O. After intravenous administration of 14C-PLA18000 radiolabeled nanoparticles to rats, 90% of the recovered 14C was eliminated within 25 days, among which 80% was as CO2.44 Degradation rate depends on four basic parameters: hydrolysis rate constant (depending on the molecular weight, the lactic/glycolic ratio, and the morphology), amount of water absorbed, diffusion coefficient of the polymer fragments through the polymer matrix, and solubility of the degradation products in the surrounding aqueous medium.40,41 All of these parameters are influenced by temperature, additives (including drug molecules), pH, ionic strength, buffering capacity, size and processing history, steric hindrance etc. Despite a higher water uptake the PLA or PLGA blocks of mPEG-PLA/PLGA block copolymers have similar degradation behaviors.45,46 mPEG blocks are released (10–25% within 3 days and 30–50% within 20 days at pH 7.4, 37°C) after cleavage of the ester bonds,47–49 and, in the range of molecular weights of 1000–20,000, are mainly excreted via the kidney.50 Up to an extensive PLA/PLGA polymer degradation, nanoparticle morphology and size are generally preserved.48,51 Generally considered as biocompatible,41 PLA or PLGA microspheres have also a good CNS biocompatibility.52,53 No mortality was reported with albumin-coated nanoparticles in mice with up to a 2000 mg/kg dose.44 However, PLA60000 nanoparticles stabilized with sodium cholate were much more toxic with two of five deaths at a 220 mg/kg dose and five of five at a 440 mg/kg dose associated with marked clinical signs (dyspnea, reduced locomotor activity), alteration of hematological and biochemical parameters and lung hemorrhage.54 This toxicity was attributed to a disseminated intravascular coagulation and associated events related to the physical surface properties of the nanoparticles rather than to the chemical toxicity of cholate or PLA. In contrast, mPEG2000-PLA30000 nanoparticles were shown to have a good safety profile, with no apparent signs of toxicity at the highest studied dose of 440 mg/kg in mice.54
Nanoparticle preparation
Nanoparticles made of mPEG-PLA/PLGA copolymers are mainly prepared using the emulsion/solvent evaporation technique or the precipitation solvent diffusion technique.1 In the first method, copolymers are dissolved in an organic solvent immiscible to water (such as dichloromethane, chloroform, ethylacetate) and emulsified in an aqueous phase generally containing an emulsifying agent (mainly polyvinylalcohol and sodium cholate). Then the solvent is evaporated off under normal or low pressure to form nanoparticles. Hydrophobic compounds (drug or else) to be incorporated are dissolved in the organic phase. Hydrosoluble compounds are first dissolved in water and emulsified in the polymer-dissolving organic phase. The primary water-in-oil emulsion thus formed is then processed like the organic polymer phase described above. This variant of the first method is called [(water-in-oil) in water] (or multiple emulsion) solvent evaporation technique. In the second method, polymers are dissolved in an organic solvent miscible to water (such as acetone or ethanol) and dispersed in an aqueous phase generally containing a colloid stabilizer. The almost instantaneous diffusion of the organic solvent into the aqueous phase results in the precipitation of the copolymers as nanoparticles. Finally, the solvent is evaporated off as above or extracted by dialysis against water.55 In principle, only compounds soluble in the organic solvent can be incorporated using the second method. Both basic methods require formulation optimization depending on the type of polymers/copolymers used, their molecular weights, the compound to be incorporated, the nanoparticle size to be achieved, etc.56–59 Other less frequently used methods include the emulsion solvent diffusion in an oil phase60,61 and the salting out process.62,63 Because of their different water solubility, the hydrophobic PLA/PLGA and hydrophilic PEG blocks of the mPEG-PLA/PLGA copolymer tend to phase-separate in the presence of water. Therefore, during the organic solvent evaporation or diffusion, the PEG moieties migrate toward the aqueous phase, whereas the hydrophobic PLA/PLGA moieties aggregate as the nanoparticle core. mPEG-PLA copolymers with relatively high PEG to PLA weight ratio (e.g., mPEG5000-PLA2000-3000) may self-assemble as polymeric micelles.59,64–66 Depending on the copolymer solubility in water, polymeric micelles may be prepared either by self-dispersion in water (mPEG5000-PLA1500-200065,67) or by the precipitation/solvent evaporation technique using a classical solvent extraction procedure (mPEG5000-PLA3000-11090067) or by dialysis.55 Self-dispersing mPEG-PLA copolymers are also used as emulsion stabilizers in the preparation of PLA nanoparticles.57 The size of mPEG-PLA nanoparticles prepared with constant PEG5000 was found to increase with the PLA block molecular weight.59 With mPEG5000-PLA2000-30000 nanoparticle diameters (from 26 to 64 nm in diameter) were shown to be independent of the copolymer concentration in the organic phase, whereas with higher PLA block molecular mass (45,000 Da) nanoparticle size was dependent on the copolymer concentration in the organic phase.59 After preparation, nanoparticles can be freeze-dried in the presence of appropriate cryoprotector for long-term preservation.51,62,63
Pegylated nanoparticle structure
Nanoparticles prepared from mPEG-PLA/PLGA copolymers are constituted of a PLA/PLGA hydrophobic core surrounded by a hydrophilic PEG corona or outer shell. In mPEG5000-PLA2000-75000 nanoparticles, negligible penetration of the PEG into the solid-like PLA core was reported, whereas as much of 25% PEG is entrapped within the nanoparticle core in the case of mPEG5000-PLA110000.59 It is likely that the [(water-in-oil) in water] solvent evaporation technique increases PEG entrapment, compared with the precipitation/solvent evaporation technique.46 The water content of mPEG5000PLA45000 nanoparticles (200 nm diameter) is around 30% compared with around 10% for PLA nanoparticles.46 At room temperature and 37°C, a solid-like central core and more mobile interfacial region coexist within the PLA core of nanoparticles made of mPEG5000-PLA [glass transition temperature of around 333K], whereas the PEG corona layer situated on the nanoparticle surface is in the liquid phase.67 The PLA chain packing density increases with the PLA molecular weights due to an increase in the number of attractive hydrophobic interactions between lactic acid units.59 In nanoparticles made of mPEG5000-PLA2000–3000, PLA chains possess some mobility.59 Because of the relatively high critical micellar concentration, these nanoparticles may dissociate upon dilution in blood.65 PEG conformation at the PLA-PEG nanoparticle surface is of utmost importance for the opsonin-repelling function of the PEG layer and has been extensively studied.57–59,66,68 The PEG layer thickness depends on the PEG molecular weight and surface density.57 Depending on their surface density, PEG blocks have brush-like (elongated coil, high density) or mushroom-like (random coil, low density) conformations.66,68 PEG surfaces in brush-like and intermediate configurations reduced phagocytosis and complement activation, whereas PEG surfaces in mushroom-like configuration were potent complement activators and favored phagocytosis.32,47,69,70 Based on the Alexander-de Gennes model, the distance between PEG chains should be around 1 nm to repel small globular proteins (approximately 2 nm radius) and 1.5 nm to repel large ones (6-8 nm).32 Due to the large choice in the PLA or PEG molecular weights available, the conformation of PEG blocks at the PEG-PLA nanoparticle surface is a complex issue to be addressed. At nanoparticle surface, the area available per PEG chain at the outer boundary of the shell is dependent on PEG to PLA molecular weight ratio that governs the PLA packing density and the surface curvature (linked to the nanoparticle size) of the assembly.57,58,71 As an example, an increase in the diameter of nanoparticles made of mPEG5000-PLA45000 results in a lower surface curvature, thus in an apparent increase in PEG surface coverage59 and in an improved colloidal stability.58
Pharmacokinetics
Like any colloidal drug carrier not especially designed to escape from MPS uptake, PLA or PLGA nanoparticles are rapidly removed from the blood stream after vascular administration and preferentially accumulate in liver and spleen.44,72 Blood half-lives are generally around 2-3 min.44,73–75 After intravenous administration, the first step of the process that leads to the nanoparticle uptake by the MPS is the opsonization phenomenon. Opsonins, including complement proteins, apolipoproteins, fibronectin, and Igs,31 interact with specific membrane receptors of monocytes and tissue macrophages, resulting in recognition and phagocytosis. It is generally admitted that hydrophobic surfaces promote protein adsorption and that negative surfaces are activators of the complement system.76 Following the rule hydrophobic and negative PLA or PLGA nanoparticle surfaces57,58 activate the complement system32 and coagulation factors77 in vitro. In contrast, hydrophilic coating with PEG sterically stabilizes PLA or PLGA nanoparticles and reduces opsonization and phagocytosis in vitro32 or ex vivo,78 and uptake by neutrophilic granulocytes in vivo.79 Compared with nonpegylated PLA nanoparticles, pegylated nanoparticle surfaces have lower negative ζ potential values, due to the surface shielding by the PEG corona.3,57,58 mPEG2000-PLA nanoparticles did not activate the complement47 and the coagulation77 systems in vitro and did not alter coagulation parameters in vivo.54 Gref et al.32 showed a maximum antiopsonic effect with PEG molecular weights of 5000 and above. Covalent linkage of the PEG coating and sufficient PLA block molecular weight is essential to ensure a sufficient stability and to avoid loss of the coating benefit by desorption and/or displacement in vivo.57,72,73,80 In mice, blood circulation times of 111In-labeled mPEG-PLGA5000-20000 nanoparticles (140 ± 10 nm diameter) increased compared to PLGA ones with an advantage to the higher PEG molecular weight.81 Within 5 min, however, ∼50% (PEG20000) to 75% (PEG5000) of injected nanoparticles (estimated from the blood clearance curves) had been cleared from the blood compartment (compared with 95% with control PLGA nanoparticles). In another study performed in rats, the blood half-lives of [14C]PLA-labeled mPEG-PLA30,000 nanoparticles with PEG molecular weight of 200073 (205 nm diameter) or 500078 (140 ± 60 nm) were markedly higher (6 h) and independent of the PEG molecular weights. Less prolonged blood circulation times were observed with PLGA nanoparticles coated with PLA3000-PEG4000 (147 ± 3.6nm) or PLA3000-PEG5000 (161 ± 3.7nm) (T[1/2] = 15 min and T[1/2] = 1 h, respectively, estimations from the blood clearance curves).72 With nanoparticles made of mPEG5000-PLA7000-125I (150 ± 2nm diameter) or of mPEG14000-PLA6000-125I (35.8 ± 0.5nm) blood half-lives determined in rats were 29.9 ± 12.4 and 42.3 ± 16.2 min respectively (no statistical difference).82 In rats, a blood half-life of 270.9 min was determined for 125I-BSA loaded in mPEG5000-PLGA45000 nanoparticles (around 200 nm diameter), compared with 13.6 min when formulated in PLGA nanoparticles.75 The large variability in blood half-lives determined in those works, even with the same PEG block molecular weight of 5000, may be ascribed to the above discussed density-related PEG conformation in the coating layer. The polydispersity of the PLA block molecular weights should be also considered, which renders the pegylated nanoparticle system more complex than liposomes (the molecular weight of the hydrophobic moieties of the pegylated phospholipids are constant and the fluidity of the lipidic membrane permits a statistically homogeneous distribution of pegylated phospholipids) and could lead to a surface heterogeneity pointed out by Gbadamosi et al.69 Such a surface heterogeneity may explain the rapid clearance of a significant fraction of intravenously injected long-circulating nanoparticles by the MPS.72,81,83 Because of this polydispersity, space available for PEG block expansion is likely to be variable on nanoparticle surface. Mushroom-like and brush-like conformations may coexist within a single nanoparticle or among a population of polydispersed nanoparticles (the size of micellar-like mPEG-PLA nanoparticles and therefore the PEG conformation in the corona are dependent on the PLA molecular weight, see above), thus explaining variability observed in blood half-lives. Therefore, molecular weights of PEG and PLA block, as well as polydispersity of copolymers, should be carefully selected in designing long-circulating pegylated nanoparticles.
Drug loading
Conventional drugs and general principles.
Various kinds of conventional drugs were formulated as PLA, PLGA, or mPEG-PLA nanoparticles. Examples are savoxepine,84 doxorubicin,85 irinotecan,86 paclitaxel,87,88 antiestrogen RU58668,89 tyrphostin AG-1295,90 lidocaine,91 propranolol hydrochloride,92 heparin,93 and enalaprilat.94 Basically, drug entrapment efficiency depends on the solid-state drug solubility in PLA/PLGA polymer (solid dissolution or dispersion), which is related to the polymer composition (lactic/glycolic ratio), the molecular weight, the drug-polymer interaction and the presence of end-functional groups (ester or carboxyl).95–99 The PEG moiety has no or little effect on drug loading.91 Because PLA and PLGA are hydrophobic polymers, lipophilic drugs are easier to formulate (in dissolved state) in PLA/mPEG-PLA nanoparticles, than hydrosoluble ones (segregation in separate domains). Despite the [(water-in-oil) in water] solvent evaporation technique, the entrapment of hydrophilic drugs may be a challenge due to the drug diffusion from the inner to the outer aqueous phases promoted by the large surface area developed. Nanoparticle formulators have nevertheless several means to optimize drug encapsulation: the selection of the preparation procedure,61,84,87,100 the use of additives,96,97 the pH optimization of the aqueous phases,92 the use of unionized base or acid form of drugs,84,86,96,97 the PLA/PLGA block polymer molecular weight. The incorporation of carboxylic groups to mPEG-PLA55 or the drug chemical conjugation via cleavable linkage101 may be interesting alternatives to improve drug loading efficiency and adjust release rates. Early drug release during storage may be solved by freeze-drying. Drug entrapment efficiency can reach more than 80%84,92,91 and drug content up to 50%.91 In most cases, however, drug contents are 5-10% (wt/wt) of nanoparticle weights86,88,94,96,97,102 or even less.87 Therefore, when formulating drug nanoparticles, it should always be kept in mind that generally as high as 90% of the material to be administered will likely be nanoparticle excipients with their potential toxicity. Drugs with high intrinsic pharmacological activities should be preferred to avoid the administration of massive dose of nanoparticle material. Drug release from biodegradable polymeric nanoparticles depends on the Fickian diffusion through the polymer matrix and on the degradation rate of the polymer. The prediction of the release profile is complex because it results from a combined effect of various parameters: solid-state drug polymer solubility98 and drug-polymer interactions,55,91,92,100 polymer degradation rate,61 block copolymer molecular weight and polydispersity,103 PEG content and molecular weight,89,91,103 water uptake by nanoparticles48 and drug solubility in the biological medium. In most studies, in vitro release profiles are characterized by an initial fast release (burst) of drug close to or at the surface followed by a sustained release.91,92,103 Removing the low molecular weight fraction from the polymer was shown to reduce the initial burst of drug release.103 Depending on formulations in vitro, drug releases last from a few hours84,91,92 or a few days87 to several weeks.61,84,88,90 Administered locally, betamethasone sodium phosphate-loaded PLGA nanoparticles were efficient at controlling inflammation over at least 3 weeks in a rabbit model of arthritis, compared with one day for the solution.61
Peptides, polypeptides, and protein drugs.
Certainly one of the most promising, and challenging, applications of nanoparticles in brain delivery are the sustained release of therapeutic peptides and proteins. Due to their hydrosolubility the preparation method is generally based on the [(water-in-oil)-in water] solvent evaporation technique.75,104,105 Entrapment efficiencies generally range from 10% to 90%,75,104,106 and nanoparticle contents from 1% or less99,107,108 to more than 15%.106 Apart from formulation issues inherent to peptide chemical instability or chemical reaction between peptides and polymer degradation products,109 the formulation of peptide-loaded nanoparticles is similar to conventional drugs.99,107,108 Proteins, however, are highly organized, complex structures that have to be preserved to maintain biological activity (receptor binding, antigenicity, enzymatic activity, etc.). The general issues of the protein stability and assessment and stabilization methods in PLA or PLGA delivery systems have been extensively reviewed.110–112 Structural and chemical integrity are lost during nanoparticle preparation and storage by protein exposure to damaging conditions, such as interfaces (aqueous/organic in emulsions, hydrophobic surfaces of polymers), elevated temperatures (e.g., by sonication), shear force (e.g., sonication, vigorous stirring, extrusion, high pressure homogenization process), surfactants, (freeze-) drying etc.110 Moreover, upon administration, proteins are exposed to physiological temperature and acidic by-products of PLA/PLGA polymer degradation within nanoparticles for long time periods that can also affect their stability.110,112 The study of the physical and chemical structure of the entrapped protein accompanied with an appropriate evaluation of the biological activity of the released material is the only way to confirm the maintenance of the protein integrity and activity.112 Each nanoparticle formulation of protein is unique and requires specific adaptation and evaluation. Improved protein stability was achieved by altering preparation processes,113 by changing polymer/copolymer,105,114 by changing or mixing solvents,49,113 by adding protective additives110,111 such as hydrophilic polymers (PEG115,116), surfactants (poloxamer 188104,117), proteins (serum albumin,118 gelatin105), cyclodextrins118,119 to the inner aqueous phase. Such formulation optimizations permitted sustained release of active protein over several weeks in vitro.105,114
Plasmid DNA, oligonucleotides.
Plasmid DNA-loaded nanoparticles are generally prepared using the [(water-in-oil)-in water] solvent evaporation technique.120,121 The plasmid DNA loading, release rates, and transfection efficiency were shown to be dependent of the nature and the molecular weight of the polymer,122 the nanoparticle size121 and the colloid stabilizer.122 An in vitro plasmid gene sustained release over several weeks was achieved with PLGA123 or mPEG-PLA nanoparticles.120 PLGA nanoparticles were shown to be endocytosed by cells in vitro124. After endocytosis, PLGA nanoparticles escape from the endolysosomal compartment to the cytoplasm and gradually release their content, resulting in sustained gene expression.125,126 In a rat osteotomy model, PLGA nanoparticles administered in the bone-gap tissue permitted a plasmid gene expression for at least 5 weeks demonstrating their sustained release properties.123 Like PLGA nanoparticles with an important poly(vinyl alcohol) coating,127 pegylated nanoparticles may interact poorly with cells, which may result in low, or even no gene expression. Such a problem may be overcome with appropriate targeting ligands able to trigger endocytosis.128
Oligonucleotides were successfully encapsulated within PLA129–131 or mPEG-PLA132 nanoparticles. In vitro-sustained release and intracellular delivery were demonstrated.131,133
Perspectives in brain targeting.
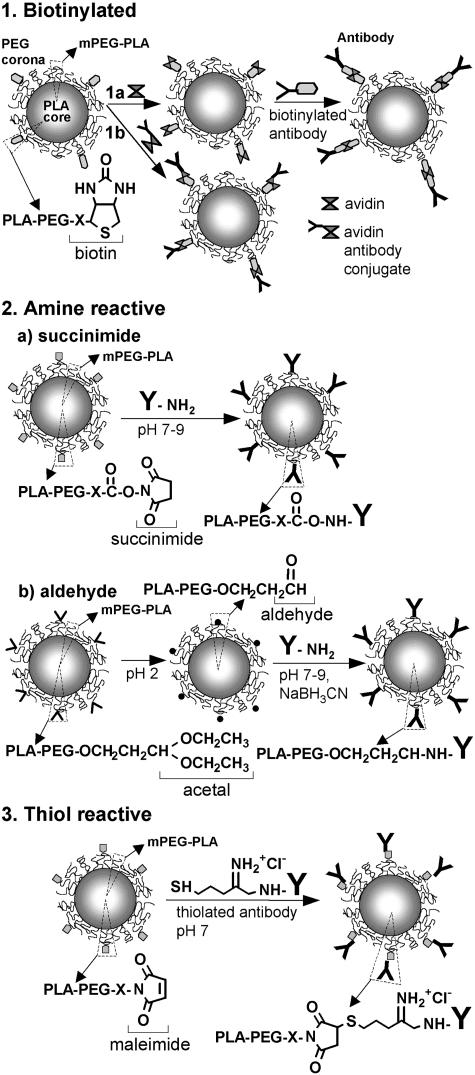
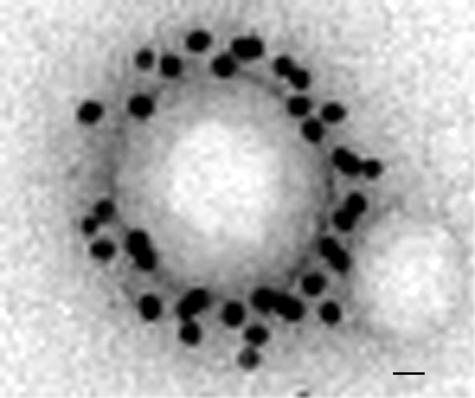
The most achieved work in the field of brain targeting with colloidal drug carriers has been carried out with pegylated immunoliposomes that access the brain from blood via receptor-mediated transcytosis and deliver their content (small drug molecules, plasmid) into the brain parenchyma, without damaging the BBB.134–137 This requires the presence of receptor-specific targeting ligands at the tip of 1-2% of the PEG2000 strands. Targeting ligands are peptidomimetic monoclonal antibodies, i.e., able to trigger the activation of receptors (transferrin or insulin receptors) that are highly expressed on the brain capillary endothelium.134,136,137 These antibodies directed against external receptor epitopes do not interfere with the natural ligand binding sites, thus avoiding competition. Colloidal carriers should have diameter less than 100 nm to fit the loading capacity of these transport systems. Because immunoliposomes are not able of sustained release of transported compounds, as shown by the relatively short-lasting plasmid expression in brain,136 they require frequent administrations to sustain a pharmacological effect.138 Pegylated PLA immunonanoparticles with sustained release properties may offer an interesting alternative. Because of the presence of unreactive methoxy terminal groups, mPEG-PLA copolymers do not permit ligands to be tethered to the PEG chain. The covalent conjugation of protein ligands to pegylated nanoparticles requires chemically reactive functions at the free end of 1-2% of the PEG strands of the PEG corona. Several functionalized copolymers have been recently synthesized: the biotinylated,139 the amine-reactive64,140 and the thiol-reactive copolymers141,142 that permit protein chemical conjugation in nondenaturing conditions143 (FIG. 2). They are generally synthesized by ring opening polymerization starting from heterobifunctional PEG and lactide and/or glycolide.64,140–142 Polymer block conjugation is an alternative method.144 Biotinylated PEG-PLA nanoparticles may link biotinylated antibodies through an avidin spacer145 (FIG. 3, panel 1a), or avidin-antibody conjugates146 (FIG. 3, panel 1b). Amine-reactive PEG-PLA (succinimide and aldehyde derivatives) can directly react with ε-amino groups of the lysine residues of antibodies in mild conditions (FIG. 3, panel 2). An α-acetal-PEG-PLA block copolymer is required to prepare aldehyde-functionalized PEG-PLA nanoparticles64,147,148 (FIG. 3, panel 2b). After nanoparticle preparation, the acetal groups are converted by mild acid treatment (pH 2) into aldehyde functions that are reactive with amine of peptidyl ligand at pH 7.147,149 Antibodies may be chemically linked through Schiff base formation and successive reductive amination using NaBH3CN.147 Due to the lack of free thiol, antibody conjugation to thiol-reactive functions (maleimide) requires the introduction of thiol residues by reacting 2-iminothiolane (Traut's reagent) with ε-amino groups of the lysine residues. The thiolation was shown not to interfere with target recognition.150 In mild conditions that preserve antibody reactivity, a stable thioether bond can be established between maleimide and thiol (FIG. 3, panel 3). Such a method was successfully applied to the preparation of brain-targeted immunoliposomes.134 In a recent work, we used the same procedure to design brain-targeted pegylated immunonanoparticles.142 Maleimide-functionalized pegylated nanoparticles were prepared with maleimide-PEG3500-PLA40000 and mPEG2600-PLA40000 (according to a 1:40 molar ratio) using the [(water-in-oil) in water] solvent evaporation technique. Thiolated mouse OX26 anti-rat transferrin receptor monoclonal antibodies were then successfully conjugated to the functionalized nanoparticles. The mean number of antibodies per nanoparticles was determined to be 67 and visualized at the nanoparticle surface by transmission electron microscopy after labeling with an anti-mouse IgG antibody gold conjugate (FIG. 4).
FIG. 2.
Structure of functionalized PEG-PLA. Biotin-PEG-PLA (a); succinimidyl tartrate PEG-PLA (b), succinimidyl succinate PEG-PLA (c), aldehyde-PEG-PLA (d), maleinimido propionate PEG-PLA (e), and maleimide-PEG-PLA (f).
FIG. 3.
Currently available conjugation techniques to prepare pegylated PLA immunonanoparticles. For comments, see text.
FIG. 4.
Transmission electron micrograph of pegylated immunonanoparticles negatively stained with phosphotungstic acid solution. Antibodies conjugated to the nanoparticle are revealed by binding with a 10-nm gold-labeled secondary antibody. The magnification bar is 15 nm. Reprinted with permission from Olivier et al. Synthesis of pegylated immunonanoparticles. Pharm Res 19:1137–1143. Copyright © 2002, Kluwer Academic Publishers, with kind permission of Springer Science and Business Media. All rights reserved.142
CONCLUSION
Even though being effective at delivering drug to the brain by a still-debated mechanism, polysorbate 80-coated PBCA nanoparticles may have limited clinical applications due to a potential toxicity, BBB permeabilization, and short lasting delivery. Technology now exists to prepare safe brain-targeted long-circulating nanoparticles, the pegylated PLA immunonanoparticles, capable of sustained drug release. Their physicochemical and biological properties and methods of preparation have been extensively described. Various drug molecules, including proteins, plasmid DNA, and oligonucleotides, were formulated and preservation of activity was demonstrated. A long way of optimization and evaluation is still, however, needed before potential clinical application. Providing PLA nanoparticles with stealth properties is a complex issue that involves the optimization of combined parameters, such as PEG molecular weight, PEG/PLA molecular weight ratio, and nanoparticle size. Stealth properties and BBB transportation of immunonanoparticles, as well as effective drug release in the brain parenchyma, remain to be investigated.
REFERENCES
- 1.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release 70: 1–20, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Wissing SA, Kayser O, Muller RH. Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev 56: 1257–1272, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Gref R, Domb A, Quellec P, Blunk T, Müller RH, Verbavatz JM, et al. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Adv Drug Deliv Rev 16: 215–233, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lockman PR, Mumper RJ, Khan MA, Allen DD. Nanoparticle technology for drug delivery across the blood-brain barrier. Drug Dev Ind Pharm 28: 1–13, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Pardridge WM. Brain drug targeting: the future of brain drug development. Cambridge, UK: Cambridge University Press, Inc., 2001.
- 6.Alyautdin R, Gothier D, Petrov V, Kharkevich D, Kreuter J Analgesic activity of the hexapeptide dalargin adsorbed on the surface of polysorbate 80-coated poly(butyl cyanoacrylate) nanoparticles. Eur J Pharm Biopharm 41: 44–48, 1995. [Google Scholar]
- 7.Kreuter J, Alyautdin RN, Kharkevich DA, Ivanov AA. Passage of peptides through the blood-brain barrier with colloidal polymer particles (nanoparticles). Brain Res 674: 171–174, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Couvreur P, Tulkens P, Roland M, Trouet A, Speiser P. Nanocapsules: a new type of lysosomotropic carrier. FEBS Lett 84: 323–326, 1977. [DOI] [PubMed] [Google Scholar]
- 9.Vauthier C, Dubernet C, Fattal E, Pinto-Alphandary H, Couvreur P. Poly(alkylcyanoacrylates) as biodegradable materials for biomedical applications. Adv Drug Deliv Rev 55: 519–548, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Guise V, Drouin JY, Benoit J, Mahuteau J, Dumont P, Couvreur P. Vidarabine-loaded nanoparticles: a physicochemical study. Pharm Res 7: 736–741, 1990. [DOI] [PubMed] [Google Scholar]
- 11.Olivier JC, Fenart L, Chauvet R, Pariat C, Cecchelli R, Couet W. Indirect evidence that drug brain targeting using polysorbate 80-coated polybutylcyanoacrylate nanoparticles is related to toxicity. Pharm Res 16: 1836–1842, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Olivier JC, Vauthier C, Taverna M, Puisieux F, Ferrier D Couvreur P. Stability of orosomucoid-coated polyisobutylcyanoacrylate nanoparticles in the presence of serum. J Control Release 40: 157–168, 1996. [Google Scholar]
- 13.Muller RH, Lherm C, Herbort J, Couvreur P. In vitro model for the degradation of alkylcyanoacrylate nanoparticles. Biomaterials 11: 590–595, 1990. [DOI] [PubMed] [Google Scholar]
- 14.Müller R H, Lherm C, Herbort J, Blunk T, Couvreur P. Alkylcyanoacrylate drug carriers: I. Physicochemical characterization of nanoparticles with different alkyl chain length. Int J Pharm 84: 1–11, 1992. [Google Scholar]
- 15.Lherm C, Müller RH, Puisieux F, Couvreur P. Alkylcyanoacrylate drug carriers: II. Cytotoxicity of cyanoacrylate nanoparticles with different alkyl chain length. Int J Pharm 84: 13–22, 1992. [Google Scholar]
- 16.Kante B, Couvreur P, Dubois-Krack G, De Meester C, Guiot P, Roland M, et al. Toxicity of polyalkylcyanoacrylate nanoparticles I: free nanoparticles. J Pharm Sci 71: 786–790, 1982. [DOI] [PubMed] [Google Scholar]
- 17.Kattan J, Droz JP, Couvreur P, Marino JP, Boutan-Laroze A, Rougier P, et al. Phase I clinical trial and pharmacokinetic evaluation of doxorubicin carried by polyisohexylcyanoacrylate nanoparticles. Invest New Drugs 10: 191–199, 1992. [DOI] [PubMed] [Google Scholar]
- 18.Grislain L, Couvreur P, Lenaerts V, Roland M, Deprez-Decampeneere D, Speiser P. Pharmacokinetics and distribution of a biodegradable drug-carrier. Int J Pharm 15: 335–345, 1983. [Google Scholar]
- 19.Douglas S J, Davis SS, Illum L. Biodistribution of poly(butyl 2-cyanoacrylate) nanoparticles in rabbits. Int J Pharm 34: 145–152, 1986. [Google Scholar]
- 20.Waser PG, Müller U, Kreuter J, Berger S, Munz K, Kaiser E, et al. Localization of colloidal particles (liposomes, hexylcyanoacrylate nanoparticles and albumin nanoparticles) by histology and autoradiography in mice. Int J Pharm 39: 213–227, 1987. [Google Scholar]
- 21.Simeonova M, Ivanova T, Raikova E, Georgieva M, Raikov Z. Tissue distribution of polybutylcyanoacrylate nanoparticles carrying spin-labelled nitrosourea. Int J Pharm 43: 267–271, 1988. [PubMed] [Google Scholar]
- 22.Verdun C, Brasseur F, Vranckx H, Couvreur P, Roland M. Tissue distribution of doxorubicin associated with polyisohexylcyanoacrylate nanoparticles. Cancer Chemother Pharmacol 26: 13–18, 1990. [DOI] [PubMed] [Google Scholar]
- 23.Lobenberg R, Araujo L, von Briesen H, Rodgers E, Kreuter J Body distribution of azidothymidine bound to hexyl-cyanoacrylate nanoparticles after i.v. injection to rats. J Control Release 50: 21–30, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Peracchia MT, Fattal E, Desmaele D, Besnard M, Noel JP, Gomis JM, et al. Stealth PEGylated polycyanoacrylate nanoparticles for intravenous administration and splenic targeting. J Control Release 60: 121–128, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Gulyaev AE, Gelperina SE, Skidan IN, Antropov AS, Kivman GY, Kreuter J Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles. Pharm Res 16: 1564–1569, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Steiniger SC, Kreuter J, Khalansky AS, Skidan IN, Bobruskin AI, Smirnova ZS, et al. Chemotherapy of glioblastoma in rats using doxorubicin-loaded nanoparticles. Int J Cancer 109: 759–767, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Alyautdin RN, Petrov VE, Langer K, Berthold A, Kharkevich DA, Kreuter J. Delivery of loperamide across the blood-brain barrier with polysorbate 80-coated polybutylcyanoacrylate nanoparticles. Pharm Res 14: 325–328, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Alyautdin RN, Tezikov EB, Ramge P, Kharkevich DA, Begley DJ, Kreuter J Significant entry of tubocurarine into the brain of rats by adsorption to polysorbate study. J Microencapsul 15: 67–74, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev 47: 65–81, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Kreuter J, Petrov VE, Kharkevich DA, Alyautdin RN. Influence of the type of surfactant on the analgesic effects induced by the peptide dalargin after its delivery across the blood-brain barrier using surfactant-coated nanoparticles. J Control Release 49: 81–87, 1997. [Google Scholar]
- 31.Allemann E, Gravel P, Leroux JC, Balant L, Gurny R. Kinetics of blood component adsorption on poly(D,L-lactic acid) nanoparticles: evidence of complement C3 component involvement. J Biomed Mater Res 37: 229–234, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Gref R, Lück M, Quellec P, Marchand M, Dellacherie E, Harnisch S. ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surfaces B: Biointerfaces 18: 301–313, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Lode J, Fichtner I, Kreuter J, Berndt A, Diederichs JE, Reszka R. Influence of surface-modifying surfactants on the pharmacokinetic behavior of 14C-poly (methylmethacrylate) nanoparticles in experimental tumor models. Pharm Res 18: 1613–1619, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Darius J, Meyer FP, Sabel BA, Schroeder U. Influence of nanoparticles on the brain-to-serum distribution and the metabolism of valproic acid in mice. J Pharm Pharmacol 52: 1043–1047, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Schroeder U, Schroeder H, Sabel BA. Body distribution of 3H-labelled dalargin bound to poly(butyl cyanoacrylate) nanoparticles after i.v. injections to mice. Life Sci 66: 495–502, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Calvo P, Gouritin B, Chacun H, Desmaele D, D'Angelo J, Noel JP, Georgin D, Fattal E, Andreux JP, Couvreur P. Long-circulating PEGylated polycyanoacrylate nanoparticles as new drug carrier for brain delivery. Pharm Res 18: 1157–1166, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Azmin MN, Stuart JF, Florence AT. The distribution and elimination of methotrexate in mouse blood and brain after concurrent administration of polysorbate 80. Cancer Chemother Pharmacol 14: 238–242, 1985. [DOI] [PubMed] [Google Scholar]
- 38.Alyaudtin RN, Reichel A, Lobenberg R, Ramge P, Kreuter J, Begley DJ. Interaction of poly(butylcyanoacrylate) nanoparticles with the blood-brain barrier in vivo and in vitro. J Drug Target 9: 209–221, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Friese A, Seiller E, Quack G, Lorenz B, Kreuter J. Increase of the duration of the anticonvulsive activity of a novel NMDA receptor antagonist using poly(butylcyanoacrylate) nanoparticles as a parenteral controlled release system. Eur J Pharm Biopharm 49: 103–109, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Vert M, Schwach G, Engel R, Coudane J. Something new in the field of PLA/GA bioresorbable polymers? J Control Release 53: 85–92, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev 28: 5–24, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Ueda H, Tabata Y. Polyhydroxyalkanonate derivatives in current clinical applications and trials. Adv Drug Deliv Rev 55: 501–518, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Li S. Hydrolytic degradation characteristics of aliphatic polyesters derived from lactic and glycolic acids. J Biomed Mater Res 48: 342–353, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Bazile DV, Ropert C, Huve P, Verrecchia T, Marlard M, Frydman A, et al. Body distribution of fully biodegradable [14 C]-poly(lactic acid) nanoparticles coated with albumin after parenteral administration to rats. Biomaterials 13: 1093–1102, 1992. [DOI] [PubMed] [Google Scholar]
- 45.von Burkersroda F, Gref R, Gopferich A. Erosion of biodegradable block copolymers made of poly(D,L-lactic acid) and poly(ethylene glycol). Biomaterials 18: 1599–1607, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Quellec P, Gref R, Perrin L, Dellacherie E, Sommer F, Verbavatz JM, Alonso MJ. Protein encapsulation within polyethylene glycol-coated nanospheres. I. Physicochemical characterization. J Biomed Mater Res 42: 45–54, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Vittaz M, Bazile D, Spenlehauer G, Verrecchia T, Veillard M, Puisieux F, et al. Effect of PEO surface density on long-circulating PLA-PEO nanoparticles which are very low complement activators. Biomaterials 17: 1575–1581, 1996. [DOI] [PubMed] [Google Scholar]
- 48.Quellec P, Gref R, Dellacherie E, Sommer F, Tran MD, Alonso MJ. Protein encapsulation within polyethylene glycol-coated nanospheres. II. Controlled release properties. J Biomed Mater Res 47: 388–395, 1999. [DOI] [PubMed] [Google Scholar]
- 49.Zambaux MF, Bonneaux F, Gref R, Dellacherie E, Vigneron C. Protein C-loaded monomethoxypoly (ethylene oxide)-poly(lactic acid) nanoparticles. Int J Pharm 212: 1–9, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Working PK, Newman MS, Johnson J, Cornacoff JB. Safety of poly(ethylene glycol) and poly(ethylene glycol) derivatives. In: Poly(ethylene glycol): chemistry and biological applications (Harris JM, Zalipsky S, eds.) ACS Symposium Series, No 680, pp 45–57. Washington, DC: American Chemical Society, 1997.
- 51.Lemoine D, Francois C, Kedzierewicz F, Preat V, Hoffman M, Maincent P. Stability study of nanoparticles of poly(epsilon-caprolactone), poly(D,L-lactide) and poly(D,L-lactide-co-glycolide). Biomaterials 17: 2191–2197, 1996. [DOI] [PubMed] [Google Scholar]
- 52.Emerich DF, Tracy MA, Ward KL, Figueiredo M, Qian R, Henschel C, et al. Biocompatibility of poly (DL-lactide-co-glycolide) microspheres implanted into the brain. Cell Transplant 8: 47–58, 1999. [DOI] [PubMed] [Google Scholar]
- 53.Menei P, Daniel V, Montero-Menei C, Brouillard M, Pouplard-Barthelaix A, Benoit JP. Biodegradation and brain tissue reaction to poly(D,L-lactide-co-glycolide) microspheres. Biomaterials 14: 470–478, 1993. [DOI] [PubMed] [Google Scholar]
- 54.Plard JP, Didier B. Comparison of the safety profiles of PLA50 and Me.PEG-PLA50 nanoparticles after single dose intravenous administration to rat. Colloids Surfaces B: Biointerfaces 16: 173–183, 1999. [Google Scholar]
- 55.Lee J, Cho EC, Cho K. Incorporation and release behavior of hydrophobic drug in functionalized poly(D,L-lactide)-block-poly(ethylene oxide) micelles. J Control Release 94: 323–335, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Zambaux MF, Bonneaux F, Gref R, Maincent P, Dellacherie E, Alonso MJ, Labrude P, Vigneron C. Influence of experimental parameters on the characteristics of poly(lactic acid) nanoparticles prepared by a double emulsion method. J Control Release 50: 31–40, 1998. [DOI] [PubMed] [Google Scholar]
- 57.Chognot D, Six JL, Leonard M, Bonneaux F, Vigneron C, Dellacherie E. Physicochemical evaluation of PLA nanoparticles stabilized by water-soluble MPEO-PLA block copolymers. J Colloid Interface Sci 268: 441–447, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Riley T, Govender T, Stolnik S, Xiong CD, Garnett MC, Illum L, et al. Colloidal stability and drug incorporation aspects of micellar-like PLA-PEG nanoparticles. Colloids Surfaces B: Biointerfaces 16: 147–159, 1999. [Google Scholar]
- 59.Riley T, Stolnik S, Heald CR, Xiong CD, Garnett MC, Illum L, et al. Physicochemical evaluation of nanoparticles assembled from poly(lactic acid)-poly(ethylene glycol) (PLA-PEG) block copolymers as drug delivery vehicles. Langmuir 17: 3168–3174, 2001. [Google Scholar]
- 60.Niwa T, Takeuchi H, Hino T, Nohara M, Kawashima Y. Biodegradable submicron carriers for peptide drugs: preparation of -lactide/glycolide copolymer (PLGA) nanospheres with nafarelin acetate by a novel emulsion-phase separation method in an oil system. Int J Pharm 121: 45–54, 1995. [Google Scholar]
- 61.Horisawa E, Hirota T, Kawazoe S, Yamada J, Yamamoto H, Takeuchi H, Kawashima Y. Prolonged anti-inflammatory action of DL-lactide/glycolide copolymer nanospheres containing betamethasone sodium phosphate for an intra-articular delivery system in antigen-induced arthritic rabbit. Pharm Res 19: 403–410, 2002. [DOI] [PubMed] [Google Scholar]
- 62.Konan YN, Gurny R, Allemann E. Preparation and characterization of sterile and freeze-dried sub-200 nm nanoparticles. Int J Pharm 233: 239–252, 2002. [DOI] [PubMed] [Google Scholar]
- 63.De Jaeghere F, Allemann E, Leroux JC, Stevels W, Feijen J, Doelker, E, et al. Formulation and lyoprotection of poly(lactic acid-co-ethylene oxide) nanoparticles: influence on physical stability and in vitro cell uptake. Pharm Res 16: 859–866, 1999. [DOI] [PubMed] [Google Scholar]
- 64.Scholz C, Iijima M, Nagasaki Y, Kataoka K. A novel reactive polymeric micelle with aldehyde groups on its surface. Macromolecules 28: 7295–7297, 1995. [Google Scholar]
- 65.Hagan SA, Coombes AGA, Garnett MC, Dunn SE, Davies MC, Illum L, et al. Polylactide-poly(ethylene glycol) copolymers as drug delivery systems. 1. Characterization of water dispersible micelle-forming systems. Langmuir 12: 2153–2161, 1996. [Google Scholar]
- 66.Stolnik S, Heald CR, Neal J, Garnett MC, Davis SS, Illum L, et al. Polylactide-poly(ethylene glycol) micellar-like particles as potential drug carriers: production, colloidal properties and biological performance. J Drug Target 9: 361–378, 2001. [DOI] [PubMed] [Google Scholar]
- 67.Heald CR, Stolnik S, Kujawinski KS, De Matteis C, Garnett MC, Illum L. Poly(lactic acid)-poly(ethylene oxide) (PLA-PEG) nanoparticles: NMR studies of the central solidlike PLA core and the liquid PEG corona. Langmuir 18: 3669–3675, 2002. [Google Scholar]
- 68.Gref R, Babak B, Bouillot P, Lukina I, Bodorev M, Dellacherie E. Interfacial and emulsion stabilising properties of amphiphilic water-soluble poly(ethylene glycol)-poly(lactic acid) copolymers for the fabrication of biocompatible nanoparticles. Colloids Surfaces A 143: 413–420, 1998. [Google Scholar]
- 69.Gbadamosi JK, Hunter AC, Moghimi SM. PEGylation of microspheres generates a heterogeneous population of particles with differential surface characteristics and biological performance. FEBS Lett 532: 338–344, 2002. [DOI] [PubMed] [Google Scholar]
- 70.Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog Lipid Res 42: 463–478, 2003. [DOI] [PubMed] [Google Scholar]
- 71.Heald CR, Stolnik S, De Matteis C, Garnett MC, Illum L, Davis SS, et al. Characterisation of poly(lactic acid):poly(ethyleneoxide) (PLA:PEG) nanoparticles using the self-consistent theory modelling approach. Colloids Surfaces A 212: 57–64, 2003. [Google Scholar]
- 72.Stolnik S, Dunn SE, Garnett MC, Davies MC, Coombes AG, Taylor DC, et al. Surface modification of poly(lactide-co-glycolide) nanospheres by biodegradable poly(lactide)-poly(ethylene glycol) copolymers. Pharm Res 11: 1800–1808, 1994. [DOI] [PubMed] [Google Scholar]
- 73.Verrecchia T, Spenlehauer G, Bazile DV, Murry-Brelier A, Archimbaud Y, Veillard M. Non-stealth (poly(lactic acid/albumin)) and stealth (poly(lactic acid-polyethylene glycol)) nanoparticles as injectable drug carriers. J Control Release 36: 49–61, 1995. [Google Scholar]
- 74.Le Ray AM, Vert M, Gautier JC, Benoît JP. Fate of [14C]poly(-lactide-co-glycolide) nanoparticles after intravenous and oral administration to mice. Int J Pharm 106: 201–211, 1994. [Google Scholar]
- 75.Li Y, Pei Y, Zhang X, Gu Z, Zhou Z, Yuan W, et al. PEGylated PLGA nanoparticles as protein carriers: synthesis, preparation and biodistribution in rats. J Control Release 71: 203–211, 2001. [DOI] [PubMed] [Google Scholar]
- 76.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev 53: 283–318, 2001. [PubMed] [Google Scholar]
- 77.Sahli H, Tapon-Bretaudiere J, Fischer AM, Sternberg C, Spenlehauer G, Verrecchia T, et al. Interactions of poly(lactic acid) and poly(lactic acid-co-ethylene oxide) nanoparticles with the plasma factors of the coagulation system. Biomaterials 18: 281–288, 1997. [DOI] [PubMed] [Google Scholar]
- 78.Bazile D, Prud'homme C, Bassoullet MT, Marlard M, Spenlehauer G, Veillard M. Stealth Me.PEG-PLA nanoparticles avoid uptake by the mononuclear phagocytes system. J Pharm Sci 84: 493–498, 1995. [DOI] [PubMed] [Google Scholar]
- 79.Zambaux MF, Faivre-Fiorina B, Bonneau F, Marchal S, Merlin JL, Dellacherie E, et al. Involvement of neutrophilic granulocytes in the uptake of biodegradable non-stealth and stealth nanoparticles in guinea pig. Biomaterials 21: 975–980, 2000. [DOI] [PubMed] [Google Scholar]
- 80.Mosqueira VC, Legrand P, Gulik A, Bourdon O, Gref R, Labarre D, et al. Relationship between complement activation, cellular uptake and surface physicochemical aspects of novel PEG-modified nanocapsules. Biomaterials 22: 2967–2979, 2001. [DOI] [PubMed] [Google Scholar]
- 81.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science 263: 1600–1603, 1994. [DOI] [PubMed] [Google Scholar]
- 82.Novakova K, Laznicek M, Rypacek F, Machova L. Pharmacokinetics and distribution 125 I-PLA-b-PEO block copolymers in rats. Pharm Dev Technol 8: 153–161, 2003. [DOI] [PubMed] [Google Scholar]
- 83.Moghimi SM. Chemical camouflage of nanospheres with a poorly reactive surface: towards development of stealth and target-specific nanocarriers. Biochim Biophys Acta 1590: 131–139, 2002. [DOI] [PubMed] [Google Scholar]
- 84.Allemann E, Leroux JC, Gurny R, Doelker E. In vitro extended-release properties of drug-loaded poly(DL-lactic acid) nanoparticles produced by a salting-out procedure. Pharm Res 10: 1732–1737, 1993. [DOI] [PubMed] [Google Scholar]
- 85.Yoo HS, Park TG. Biodegradable polymeric micelles composed of doxorubicin conjugated PLGA-PEG block copolymer. J Control Release 70: 63–70, 2001. [DOI] [PubMed] [Google Scholar]
- 86.Onishi H, Machida Y, Machida Y. Antitumor properties of irinotecan-containing nanoparticles prepared using poly(DL-lactic acid) and poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol). Biol Pharm Bull 26: 116–119, 2003. [DOI] [PubMed] [Google Scholar]
- 87.Dong Y, Feng SS. Methoxy poly(ethylene glycol)-poly(lactide) (MPEG-PLA) nanoparticles for controlled delivery of anticancer drugs. Biomaterials 25: 2843–2849, 2004. [DOI] [PubMed] [Google Scholar]
- 88.Feng SS, Mu L, Win KY, Huang G. Nanoparticles of biodegradable polymers for clinical administration of paclitaxel. Curr Med Chem 11: 413–424, 2004. [DOI] [PubMed] [Google Scholar]
- 89.Ameller T, Marsaud V, Legrand P, Gref R, Renoir JM. Pure antiestrogen RU 58668-loaded nanospheres: morphology, cell activity and toxicity studies. Eur J Pharm Sci 21: 361–370, 2004. [DOI] [PubMed] [Google Scholar]
- 90.Fishbein I, Chorny M, Rabinovich L, Banai S, Gati I, Golomb G. Nanoparticulate delivery system of a tyrphostin for the treatment of restenosis. J Control Release 65: 221–229, 2000. [DOI] [PubMed] [Google Scholar]
- 91.Peracchia MT, Gref R, Minamitake Y, Domb A, Lotan N, Langer R. PEG-coated nanospheres from amphiphilic diblock and multiblock copolymers: investigation of their drug encapsulation and release characteristics. J Control Release 46: 223–231, 1997. [Google Scholar]
- 92.Ubrich N, Bouillot P, Pellerin P, Hoffman M, Maincent P. Preparation and characterization of propranolol hydrochloride nanoparticles: a comparative study. J Control Release 97: 291–300, 2004. [DOI] [PubMed] [Google Scholar]
- 93.Hoffart V, Ubrich N, Simonin C, Babak V, Vigneron C, Hoffman M, et al. Low molecular weight heparin-loaded polymeric nanoparticles: formulation, characterization, and release characteristics. Drug Dev Ind Pharm 28: 1091–1099, 2002. [DOI] [PubMed] [Google Scholar]
- 94.Ahlin P, Kristl J, Kristl A, Vrecer F. Investigation of polymeric nanoparticles as carriers of enalaprilat for oral administration. Int J Pharm 239: 113–120, 2002. [DOI] [PubMed] [Google Scholar]
- 95.Nicoli S, Santi P, Couvreur P, Couarraze G, Colombo P, Fattal E. Design of triptorelin loaded nanospheres for transdermal iontophoretic administration. Int J Pharm 214: 31–35, 2001. [DOI] [PubMed] [Google Scholar]
- 96.Govender T, Stolnik S, Garnett MC, Illum L, Davis SS. PLGA nanoparticles prepared by nanoprecipitation: drug loading and release studies of a water soluble drug. J Control Release 57: 171–185, 1999. [DOI] [PubMed] [Google Scholar]
- 97.Govender T, Riley T, Ehtezazi T, Garnett MC, Stolnik S, Illum L, et al. Defining the drug incorporation properties of PLA-PEG nanoparticles. Int J Pharm 199: 95–110, 2000. [DOI] [PubMed] [Google Scholar]
- 98.Panyam J, Williams D, Dash A, Leslie-Pelecky D, Labhasetwar V. Solid-state solubility influences encapsulation and release of hydrophobic drugs from PLGA/PLA nanoparticles. J Pharm Sci 93: 1804–1814, 2004. [DOI] [PubMed] [Google Scholar]
- 99.Hiroaki O, Yamamoto M, Heya T, Inoue Y, Kamei S, Ogawa Y, et al. Drug delivery using biodegradable microspheres. J Control Release 28: 121–129, 1994. [Google Scholar]
- 100.Niwa T, Takeuchi H, Hino T, Kunou N, Kawashima Y. In vitro drug release behavior of D,L-lactide/glycolide copolymer (PLGA) nanospheres with nafarelin acetate prepared by a novel spontaneous emulsification solvent diffusion method. J Pharm Sci 83: 727–732, 1994. [DOI] [PubMed] [Google Scholar]
- 101.Yoo HS, Lee EA, Park TG. Doxorubicin-conjugated biodegradable polymeric micelles having acid-cleavable linkages. J Control Release 82: 17–27, 2002. [DOI] [PubMed] [Google Scholar]
- 102.Leo E, Brina B, Forni F, Vandelli MA. In vitro evaluation of PLA nanoparticles containing a lipophilic drug in water-soluble or insoluble form. Int J Pharm 278: 133–141, 2004. [DOI] [PubMed] [Google Scholar]
- 103.Matsumoto J, Nakada Y, Sakurai K, Nakamura T, Takahashi Y. Preparation of nanoparticles consisted of poly(L-lactide)-poly(ethyleneglycol)-poly(L-lactide) and their evaluation in vitro. Int J Pharm 185: 93–101, 1999. [DOI] [PubMed] [Google Scholar]
- 104.Blanco MD, Alonso MJ. Development and characterization of protein-loaded poly(lactide-co-glycolide) nanospheres. Eur J Pharm Biopharm 43: 287–294, 1997. [Google Scholar]
- 105.Tobio M, Gref R, Sanchez A, Langer R, Alonso MJ. Stealth PLA-PEG nanoparticles as protein carriers for nasal administration. Pharm Res 15: 270–275, 1998. [DOI] [PubMed] [Google Scholar]
- 106.Gref R, Quellec P, Sanchez A, Calvo P, Dellacherie E, Alonso MJ. Development and characterization of CyA-loaded poly(lactic acid)-poly(ethylene glycol)PEG micro- and nanoparticles. Comparison with conventional PLA particulate carriers. Eur J Pharm Biopharm 51: 111–118, 2001. [DOI] [PubMed] [Google Scholar]
- 107.Kawashima Y, Yamamoto H, Takeuchi H, Hino T, Niwa T. Properties of a peptide containing DL-lactide/glycolide copolymer nanospheres prepared by novel emulsion solvent diffusion methods. Eur J Pharm Biopharm 45: 41–48, 1998. [DOI] [PubMed] [Google Scholar]
- 108.Elamanchili P, Diwan M, Cao M, Samuel J. Characterization of poly(d,l-lactic-co-glycolic acid) based nanoparticulate system for enhanced delivery of antigens to dendritic cells. Vaccine 22: 2406–2412, 2004. [DOI] [PubMed] [Google Scholar]
- 109.Lucke A, Fustella E, Tessmar J, Gazzaniga A, Gopferich A. The effect of poly(ethylene glycol)-poly(D,L-lactic acid) diblock copolymers on peptide acylation. J Control Release 80: 157–168, 2002. [DOI] [PubMed] [Google Scholar]
- 110.van de Weert M, Hennink WE, Jiskoot W. Protein instability in poly(lactic-co-glycolic acid) microparticles. Pharm Res 17: 1159–1167, 2000. [DOI] [PubMed] [Google Scholar]
- 111.Schwendeman SP. Recent advances in the stabilization of proteins encapsulated in injectable PLGA delivery systems. Crit Rev Ther Drug Carrier Syst 19: 73–98, 2002. [DOI] [PubMed] [Google Scholar]
- 112.Cleland JL. Protein delivery from biodegradable microspheres. Pharm Biotechnol 10: 1–43, 1997. [DOI] [PubMed] [Google Scholar]
- 113.Zambaux MF, Bonneaux F, Gref R, Dellacherie E, Vigneron C. Preparation and characterization of protein C-loaded PLA nanoparticles. J Control Release 60: 179–188, 1999. [DOI] [PubMed] [Google Scholar]
- 114.Gaspar MM, Blanco D, Cruz MEM, Alonso MJ. Formulation of -asparaginase-loaded poly(lactide-co-glycolide) nanoparticles: influence of polymer properties on enzyme loading, activity and in vitro release. J Control Release 52: 53–62, 1998. [DOI] [PubMed] [Google Scholar]
- 115.Pean JM, Boury F, Venier-Julienne MC, Menei P, Proust JE, Benoit JP. Why does PEG 400 co-encapsulation improve NGF stability and release from PLGA biodegradable microspheres? Pharm Res 16: 1294–1299, 1999. [DOI] [PubMed] [Google Scholar]
- 116.Pean JM, Menei P, Morel O, Montero-Menei CN, Benoit JP. Intraseptal implantation of NGF-releasing microspheres promote the survival of axotomized cholinergic neurons. Biomaterials 21: 2097–2101, 2000. [DOI] [PubMed] [Google Scholar]
- 117.Sanchez A, Tobio M, Gonzalez L, Fabra A, Alonso MJ. Biodegradable micro- and nanoparticles as long-term delivery vehicles for interferon-α. Eur J Pharm Sci 18: 221–229, 2003. [DOI] [PubMed] [Google Scholar]
- 118.Sah H. Stabilization of proteins against methylene chloride/water interface-induced denaturation and aggregation. J Control Release 58: 143–151, 1999. [DOI] [PubMed] [Google Scholar]
- 119.Morlock M, Koll H, Winter G, Kissel T. Microencapsulation of rh-erythropoietin, using biodegradable poly(D,L-lactide-co-glycolide): protein stability and the effects of stabilizing excipients. Eur J Pharm Biopharm 43: 29–36, 1997. [Google Scholar]
- 120.Perez C, Sanchez A, Putnam D, Ting D, Langer R, Alonso MJ. Poly(lactic acid)-poly(ethylene glycol) nanoparticles as new carriers for the delivery of plasmid DNA. J Control Release 75: 211–224, 2001. [DOI] [PubMed] [Google Scholar]
- 121.Prabha S, Zhou WZ, Panyam J, Labhasetwar V. Size-dependency of nanoparticle-mediated gene transfection: studies with fractionated nanoparticles. Int J Pharm 244: 105–115, 2002. [DOI] [PubMed] [Google Scholar]
- 122.Prabha S, Labhasetwar V. Critical determinants in PLGA/PLA nanoparticle-mediated gene expression. Pharm Res 21: 354–364, 2004. [DOI] [PubMed] [Google Scholar]
- 123.Labhasetwar V, Bonadio J, Goldstein SA, Levy RJ. Gene transfection using biodegradable nanospheres: results in tissue culture and a rat osteotomy model. Colloids Surfaces B: Biointerfaces 16: 281–290, 1999. [Google Scholar]
- 124.Davda J, Labhasetwar V. Characterization of nanoparticle uptake by endothelial cells. Int J Pharm 233: 51–59, 2002. [DOI] [PubMed] [Google Scholar]
- 125.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev 55: 329–347, 2003. [DOI] [PubMed] [Google Scholar]
- 126.Panyam J, Zhou WZ, Prabha S, Sahoo SK, Labhasetwar V. Rapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. FASEB J 16: 1217–1226, 2002. [DOI] [PubMed] [Google Scholar]
- 127.Sahoo SK, Panyam J, Prabha S, Labhasetwar V. Residual polyvinyl alcohol associated with poly (D,L-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J Control Release 82: 105–114, 2004. [DOI] [PubMed] [Google Scholar]
- 128.Zhang Y, Jeong Lee H, Boado RJ, Pardridge WM. Receptor-mediated delivery of an antisense gene to human brain cancer cells. J Gene Med 4: 183–194, 2002. [DOI] [PubMed] [Google Scholar]
- 129.Delie F, Berton M, Allemann E, Gurny R. Comparison of two methods of encapsulation of an oligonucleotide into poly(D,L-lactic acid) particles. Int J Pharm 214: 25–30, 2001. [DOI] [PubMed] [Google Scholar]
- 130.Berton M, Allemann E, Stein CA, Gurny R. Highly loaded nanoparticulate carrier using an hydrophobic antisense oligonucleotide complex. Eur J Pharm Sci 9: 163–170, 1999. [DOI] [PubMed] [Google Scholar]
- 131.Berton M, Benimetskaya L, Allemann E, Stein CA, Gurny R. Uptake of oligonucleotide-loaded nanoparticles in prostatic cancer cells and their intracellular localization. Eur J Pharm Biopharm 47: 119–123, 1999. [DOI] [PubMed] [Google Scholar]
- 132.Emile C, Bazile D, Herman F, Helene C, Vieillard M. Encapsulation of nucleotides in stealth Me.PEG-PLA50 nanoparticles by complexation with structures oligopeptides. Drug Deliv 3: 187–195, 1996. [DOI] [PubMed] [Google Scholar]
- 133.Berton M, Benimetskaya L, Allemann E, Stein CA, Gurny R. Uptake of oligonucleotide-loaded nanoparticles in prostatic cancer cells and their intracellular localization. Eur J Pharm Biopharm 47: 119–123. [DOI] [PubMed]
- 134.Huwyler J, Wu D, Pardridge WM. Brain drug delivery of small molecules using immunoliposomes. Proc Natl Acad Sci USA 93: 14164–14169, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang Y, Schlachetzki F, Pardridge WM. Global non-viral gene transfer to the primate brain following intravenous administration. Mol Ther 7: 11–18, 2003. [DOI] [PubMed] [Google Scholar]
- 136.Shi N, Zhang Y, Zhu C, Boado RJ, Pardridge WM. Brain-specific expression of an exogenous gene after i.v. administration. Proc Natl Acad Sci USA 98: 12754–12759, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shi N, Pardridge WM. Noninvasive gene targeting to the brain. Proc Natl Acad Sci USA 97: 7567–7572, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang Y, Boado RJ, Pardridge WM. Absence of toxicity of chronic weekly intravenous gene therapy with pegylated immunoliposomes. Pharm Res 20: 1779–1785, 2003. [DOI] [PubMed] [Google Scholar]
- 139.Salem AK, Cannizzaro SM, Davies MC, Tendler SJ, Roberts CJ, Williams PM, et al. Synthesis and characterisation of a degradable poly(lactic acid)-poly(ethylene glycol) copolymer with biotinylated end groups. Biomacromolecules 2: 575–580, 2001. [DOI] [PubMed] [Google Scholar]
- 140.Tessmar J, Mikos A, Gopferich A. Amine-reactive biodegradable diblock copolymers. Biomacromolecules 3: 194–200, 2002. [DOI] [PubMed] [Google Scholar]
- 141.Tessmar J, Mikos A, Gopferich A. The use of poly(ethylene glycol)-block-poly(lactic acid) derived copolymers for the rapid creation of biomimetic surfaces. Biomaterials 24: 4475–4486, 2003. [DOI] [PubMed] [Google Scholar]
- 142.Olivier JC, Huertas R, Lee HJ, Calon F, Pardridge WM. Synthesis of pegylated immunonanoparticles. Pharm Res 19: 1137–1143, 2002. [DOI] [PubMed] [Google Scholar]
- 143.Roberts MJ, Bentley MD, Harris JM. Chemistry for peptide and protein PEGylation. Adv Drug Deliv Rev 54: 459–476, 2002. [DOI] [PubMed] [Google Scholar]
- 144.Yoo HS, Park TG. Folate receptor targeted biodegradable polymeric doxorubicin micelles. J Control Release 96: 273–283, 2004. [DOI] [PubMed] [Google Scholar]
- 145.Gref R, Couvreur P, Barratt G, Mysiakine E. Surface-engineered nanoparticles for multiple ligand coupling. Biomaterials 24: 4529–4537, 2003. [DOI] [PubMed] [Google Scholar]
- 146.Kang YS, Saito Y, Pardridge WM. Pharmacokinetics of [3H]biotin bound to different avidin analogues. J Drug Target 3: 159–165, 1995. [DOI] [PubMed] [Google Scholar]
- 147.Yamamoto Y, Nagasaki Y, Kato M, Kataoka K. Surface charge modulation of poly(ethylene glycol)poly(L-lactide) block copolymer micelles: conjugation of charged peptides. Colloids Surfaces B: Biointerfaces 16: 135–146, 1999. [Google Scholar]
- 148.Emoto K, Nagasaki Y, Iijima M, Kato M, Kataoka K. Preparation of non-fouling surface through the coating with core-polymerized block copolymer micelles having aldehyde-ended PEG shell. Colloids Surfaces B: Biointerfaces 18: 337–346, 2000. [DOI] [PubMed] [Google Scholar]
- 149.Yamamoto Y, Nagasaki Y, Kato Y, Sugiyama Y, Kataoka K. Long-circulating poly(ethylene glycol)-poly(-lactide) block copolymer micelles with modulated surface charge. J Control Release 77: 27–38, 2001. [DOI] [PubMed] [Google Scholar]
- 150.Pardridge WM, Boado RJ, Kang YS. Vector-mediated delivery of a polyamide (“peptide”) nucleic acid analogue through the blood-brain barrier in vivo. Proc Natl Acad Sci USA 92: 5592–5596, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]