Abstract
Portulacaria afra L. is a dominant facultative CAM species growing in the Southeastern Cape of South Africa. P. afra is well adapted to regions of the Spekboom thicket in areas of limited and sporadic rainfall. P. afra populations occur in isolated drainages. We hypothesized the utilization of CAM would vary in the different populations in response to rainfall and temperature gradients. Carbon isotope composition can be used to determine the contribution of CAM in leaf tissue. P. afra leaves of populations were analyzed in transects running south to north and east to west in locations from the coast to elevations of 1400 m. Carbon isotope values ranged from −16.1‰ in Plutosvale to −21.0‰ to −22.7‰ in Port Alfred and Grahamstown populations respectively with some values reaching −25.2‰. These values indicated an estimated variable contribution of the CAM pathway ranging from 23% to almost 60%. The results indicate a much greater range of variability than previously reported. The carbon isotope values showed no direct correlation with rainfall or maximum or minimum day/night temperatures in the summer or winter for the different locations. The results indicated the microclimate may play a more significant role in determining CAM utilization. We present evidence that CAM is a continuous trait in P. afra and CAM is operating continuously at low levels during C3 photosynthesis which may explain the high variability in its carbon isotope composition. P. afra populations illustrate a large phenotypic plasticity and further studies may indicate genotypic differences between populations. This may be valuable in ascertaining the genetic contribution to its water use efficiency and possible use in engineering higher water use efficiency in C3 plants. The results revealed here may explain P. afra’s ability to sequester carbon at high rates compared to more mesic species.
Keywords: Plant biology, Evolution, Ecology
1. Introduction
Crassulacean acid metabolism (CAM) is a metabolic and anatomical adaptation that is characterized by net nocturnal carbon dioxide uptake with a temporal separation of the C4 and C3 pathway (Osmond, 1978; Ting, 1985). The CO2 is fixed by Phosphoenolpyruvate carboxylase (PEPCase), converted to malate and stored as malic acid in the vacuole during the night. In the subsequent light period, the malate is decarboxylated to release CO2 for utilization by Rubisco in the C3 cycle. CAM plants typically have a leaf mesophyll anatomy with primarily spongy parenchyma cells with a large central vacuole (Gibson, 1982). The uptake of CO2 at night with the stomata closed during the day results in an increased water use efficiency when compared to C3 and C4 plants (Black, 1973). CAM has evolved in at least 35 different plant families including six aquatic families and over 343 genera (Borland et al., 2011).
The δ 13C values can be used as an investigative tool to determine if plants are utilizing C3, C4, or CAM photosynthesis. The δ 13C values are indicative of whether CO2 is fixed by Rubisco or are indicative of the utilization PEPCase as the main carboxylating enzyme during growth. C3 plants have carbon isotope composition values closer to −27‰ while C4 plants have values which are more positive at −11‰ (Black, 1973). CAM plants can have values ranging from −14‰ to −25‰ depending on the overall contribution of nocturnal CO2 uptake (Winter and Holtum, 2002). Obligate CAM species will have values much closer to the values of C4 plants due to primarily fixing CO2 at night with a small contribution of exogenous daytime CO2 uptake in the early morning and late afternoon. Facultative CAM species vary along the C3 to C4 continuum depending on the overall utilization and contributions of CAM to the carbon balance of the plants. Thus, plants which predominantly use CAM will have values closer to C4 species while plants which have predominantly daytime CO2 uptake will have an isotope signal in the range of C3 species.
Portulacaria afra L., a succulent member of the Didereaceae (formerly of the Portulacaceae), is native to South Africa and commonly found in semi-arid areas (Cowling and Proche Vlok, 2005). Portulacaria afra, called elephant’s food locally in South Africa has small succulent leaves. The plant can grow from 2–5 m in height as a large woody shrub or small tree (Oakes, 1973; Baran, 1999). The branches show dichotomous branching and the leaves are opposite one another. The leaves of the plants last at least one growing season or more (Guralnick et al., 1984b; Baran, 1999). P. afra currently occupies approximately 1.7 million hectares (Mills et al., 2005) in the eastern and southeastern Cape. The rainfall can occur throughout the year but spring and fall have heavier rainfall maxima with some summer rainfall in the Eastern Cape. Precipitation can range from 250 to 750 mm per year. P. afra is the dominant vegetation in parts of the Spekboom veld and in some areas can compose up to 90% of the stand. In these areas P. afra can form a closed canopy shrubland (Mills et al., 2005). P. afra grows primarily in regions that are frost free but it is found in regions where the temperature has reached −6 °C (Oakes, 1973). This ability to withstand frost is aided by the density of the P. afra stands (Palmer and Pitman, 1961).
Portulacaria afra has been shown to be a facultative Crassulacean acid metabolism species (Guralnick and Jackson, 2001). A facultative CAM species can perform daytime CO2 uptake, traditional C3 photosynthesis, but utilize the CAM pathway during times of water stress. Portulacaria afra was first shown to have nocturnal CO2 uptake and a large acid fluctuation when water stressed (Ting and Hanscom, 1977; Hanscom and Ting, 1978). Later studies of P. afra have indicated the CAM response is seasonal and related to long day photoperiods, showing more CAM activity during the summer months when temperatures are higher (Guralnick et al., 1984a, b). However, P. afra is also able to utilize the C3 pathway with predominantly daytime photosynthesis in the cooler months and thus shows a faster growth rate than obligate CAM plants (i.e., Jade plant, Crassula argenta) which can only take in exogenous CO2 during the night period and in the early morning or late afternoon of the daytime period.
P. afra is a facultative CAM species and utilizes CAM under a variety of environmental conditions. It has been shown that Portulacaria afra primarily utilizes the CAM pathway during its growth in South Africa as noted by its reported measurements of the δ13C composition (13C/12C ratios) of −17.1‰ (Mooney et al., 1977). The δ13C composition measured by Mooney et al. (1977) was taken from one population in the Cape and this measurement was taken over 35 years ago. Mills et al. (2005) found a δ13C range of −17.4 to −20.5‰ for plants in the Fish River region in the Eastern Cape at an elevation of 300–500 m.
Portulacaria afra has a widespread distribution and many of the populations are isolated in different drainages from other P. afra populations. The phenotypic variation in growth habitat may be an indicator of genotypic variation in the different populations. We hypothesized the contribution of CAM to the overall growth could vary among different populations depending on the particular environmental conditions to which the populations are acclimated. Those populations which have the least amount of rainfall would be expected to have a greater overall contribution of CAM to the δ13C composition. Our goal was to measure the δ13C composition of different P. afra populations in the Eastern Cape. These results will help to determine which populations have greater water use efficiencies and could be used for restoration. We report our findings for the δ13C composition of ten populations of P. afra.
2. Materials and methods
2.1. Climate and plant material
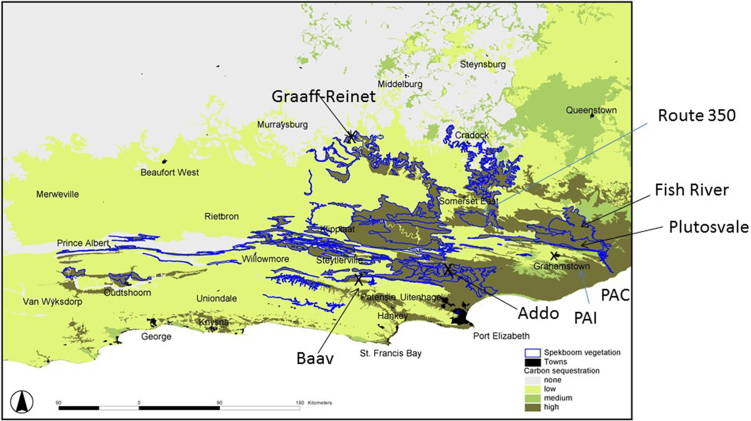
Temperature and rainfall characteristics of various locations near the sample sites in the Eastern Cape are shown in Table 1. Average daily maximum summer temperatures are similar for the region as a whole while winter temperatures show greater variation within the Eastern Cape. Populations were sampled in east to west transect and a south to north transect (Fig. 1). Rainfall varies from 214 mm in Baviaanskloof (westernmost sample; 24°61′W) to 837 mm in the Coastal region of Port Alfred (southernmost sample; 33°41′S). The elevation varied from sea level in Port Alfred to 500–600 m in Plutosvale and Grahamstown to 1400 m in Graff-Reinet (northernmost sample; 32°14′N). The easternmost sample near the Fish River Reserve was taken at 26°42′E. The summer maximum temperature in the different areas ranges from 32 °C in Addo Elephant National Park, 30 °C in Graaff-Reinet to 27 °C in Port Alfred. The winter maximum temperature varies from 16 °C in Baviaanskloof to 23 °C in Port Alfred and 25 °C in Graaff-Reinet. Baviaanskloof has the coolest average winter temperature at 3 °C. Leaf samples of different populations of Portulacaria afra (L.) Jacq. were collected in the summer during the day (March 10-March 15, 2013 and January 3-January 18, 2014) and frozen at −80 °C until processed and assayed. Mature leaf samples (second and third leaves from the apical position) were randomly collected from different plants at approximately noon from sun and shade position. New leaf growth was collected from apical positions on the branches. Reproductive tissue was also collected if the plants in the population were flowering.
Table 1.
Annual rainfall and average maximum and minimum temperatures in regions of the Eastern Cape.
Fig. 1.
Map of location of collecting sites of various populations of Portulacaria afra. The Blue represents the distribution of the Spekboom Thicket. Baav (Baviaanskloof); Addo Elephant National Park; Grahamstown; Route 350; Fish River; Plutosvale; Port Alfred; Graaff −Reinett. Map Courtesy of Mike Powell.
2.2. Carbon isotope analysis
The δ 13C was determined from frozen leaf and flower specimens, dried in an oven at 65 °C and sent to Washington State University (College of Sciences Stable Isotope Core; http://www.isotopes.wsu.edu) for analysis (Guralnick et al., 2008). Sample sized ranged from 5–36 for populations. Estimates of Nighttime CO2 uptake were calculated using equations from Winter and Holtum (2002). Data were analyzed using a one-way ANOVA; a Tukey post-hoc comparison was utilized to measure any significant differences (p < 0.05) between populations.
3. Results
3.1. Carbon isotope composition
The δ 13C varied among the populations with average values ranging from −17.7 to −22.7‰ (Table 2). The most positive values were found in Plutosvale and Route 350 which were intermediate distances along both the south/north and east/west transect. The Plutosvale population was significantly less negative than the Addo, Baviaanskloof, and the Port Alfred coastal populations. The most negative isotope values were found in Grahamstown and the Graaff-Reinet populations. The populations near Addo Elephant National park, Baviaanskloof, the Port Alfred inland population, and the population north of Grahamstown were intermediate between the Grahamstown and Plutosvale populations showing δ13C values ranging from −19.0 to −19.6‰ (Table 2). The population near Addo Elephant National park was −19.8‰ which was intermediate between the Port Alfred coastal and Plutosvale. The high Altitude population of Graaff-Reinet (1400 m) was similar in values to the Port Alfred Coastal population. The average δ 13C value of all populations was −19.6‰. An analysis of the carbon isotope values showed no correlation with summer or winter maximum temperatures, summer day/night differentials, or average rainfall.
Table 2.
Carbon Isotope values of populations of Portulacaria afra in the Eastern Cape.
| Location | δ13C Isotope Value Averagex (‰) | Range |
|---|---|---|
| Addo Elephant National Park (n = 13) | -19.0(0.3)a | -16.5/20.0 |
| Baviaanskloof (n = 16) | -19.1(0.1)ab | -18.6/-19.9 |
| Fish River (Route 67) (n = 36) | -18.1(0.1)abc | -16.2/-19.9 |
| Graaff-Reinet (n = 10) | -21.3(0.6)deg | -16.8/-23.0 |
| Grahamstown (n = 31) | -22.7(0.2)d | -20.0/-25.2 |
| Grahamstown (n = 6)y(Hellspoort Valley) | -19.6(0.8)abcef | -17.0/-21.6 |
| Plutosvale (n = 19) | -17.7(0.4)cf | -16.0/-22.0 |
| Port Alfred coast (n = 6) | -21.0(0.2)de | -20.4/-21.5 |
| Port Alfred Inland (n = 7)z | -19.5(0.9)abceg | -16.4/-23.1 |
| Route 350 (n = 5) | -17.5(0.2)abcf | -16.9/-17.9 |
Populations followed by the same letter are not significantly different from each other (p < 0.05 level). Numbers in parentheses are 1 SEM.
Grahamstown population 25 km north of city center near Hellspoort Valley turnoff.
Port Aflred Inland population (35 km from Grahamstown).
The carbon isotope composition values for new growth were not significantly different from that of the mature leaves for all but one population (Table 3). In the Fish River population, new growth was significantly more negative than the mature leaves of the population. Only the Addo population had flowers and the samples were significantly less negative than the mature leaves of the population (Table 3).
Table 3.
Carbon Isotope values of new growth in populations of Portulacaria afra in the Eastern Cape.
| Location | δ13C New Growth (‰) | δ13C Mature Leavesa (‰) |
|---|---|---|
| Addo Elephant National Park (n = 5) Addo (Reproductive tissue (n = 3) |
-17.9(0.4) -16.1(0.1)* |
-19.0 |
| Fish River (Route 67) (n = 4) | -20.5(0.3)* | -18.1 |
| Graaff-Reinet (n = 5) | -21.7(0.4) | -21.3 |
| Grahamstown (n = 5) | -23.0(0.7) | -22.7 |
| Plutosvale (n = 4) | -17.4(0.1) | -17.7 |
| Port Alfred coast (n = 4) | -21.0(0.5) | -21.0 |
| Port Alfred Inland (n = 5)b | -17.7(0.3) | -19.5 |
Samples followed by the asterisk indicate that the tissue is significantly different from mature tissue (p < 0.05 level). Numbers in parentheses are 1 SEM.
Port Alfred Inland population (35 km from Grahamstown).
Carbon isotope samples were analyzed from species growing together in the same area for comparison. P. afra showed an isotope value of −19.6‰ (Table 4). The C3 species of Plumabago auricalata had a δ13C of −28.7‰ and Pappea capensis showed a δ13C of −26.4‰. The succulent species of Crassula cultrata showed a δ13C of −17.1‰ (Table 4). Given that the C3 species showed a range of δ13C values of −26.0 to −30.2‰, we then estimated nocturnal contribution of overall CO2 uptake. The populations varied in their nighttime contribution from ∼22.75% in the Grahamstown population to over ∼50% in the Plutosvale and Route 350 population (Table 5, Winter and Holtum, 2002). The other populations ranged from 30.2 to 47.4% for an estimated overall contribution nocturnal CO2 uptake. Individual plant samples showed a much higher contribution of nocturnal CO2 uptake, in some cases up to 60% of the overall carbon balance. On the other end of the spectrum, the contribution of nighttime carbon uptake was reduced to ∼10% of the overall balance.
Table 4.
Carbon Isotope Composition of C3 species associated with Portulacaria afra.
| Species | δ13C Isotope Value Averagex(‰) | Range |
|---|---|---|
| Portulacaria afra (N = 6) | -19.6(0.8)a | -17.0–21.6 |
| Plumabago auricalata (N = 3) | -28.7(1.0)b | -26.9–30.2 |
| Pappea capensis (N = 3) | -26.4(0.5)b | -25.7–27.4 |
| Crassula cultrata (N = 3) | -17.7(0.3)a | -17.2–18.2 |
Populations followed by the same letter are not significantly different from each other (p < 0.05 level). Numbers in parentheses are 1 SEM.
Table 5.
Proportion of Nighttime CO2 Uptake of Portulacaria afra populations in the Eastern Cape.
| Location | δ13C Isotope Value Averagew,x(‰) | Nighttime CO2 Uptake (%) |
|---|---|---|
| Addo Elephant National Park (n = 13) | -19.0(0.34)a | 42.6 |
| Baviaanskloof (n = 16) | -19.1(0.09)ab | 42.1 |
| Fish River (Route 67) (n = 36) | -18.1(0.14)abc | 47.4 |
| Graaff-Reinet (n = 10) | -21.3(0.57)deg | 30.2 |
| Grahamstown (n = 31) | -22.7(0.24)d | 22.7 |
| Grahamstown (n = 6)y (Hellspoort Valley) | -19.6(0.80)abcef | 39.5 |
| Plutosvale (n = 19) | -17.7(0.39)cf | 50.1 |
| Port Alfred coast (n = 6) | -21.0(0.18)de | 31.9 |
| Port Alfred Inland (n = 7)z | -19.5(0.93)abceg | 39.9 |
| Route 350 (n = 5) | -17.5(0.18)abcf | 50.7 |
Estimates of Nighttime CO2 uptake calculated using equations from Winter and Holtum (2002).
Populations followed by the same letter are not significantly different from each other (p < 0.05 level). Numbers in parentheses are 1 SEM.
Grahamstown population 25 km north of city center near Hellspoort Valley turnoff.
Port Alfred Inland population (35 km from Grahamstown).
4. Discussion
The expected results for plants in the Eastern Cape would be that contribution of CAM would vary in accordance with either rainfall abundance, increased day/night temperature differentials or day/night maxima temperatures. The results found in this study did not support this hypothesis. The mature leaf samples from populations collected in the various locales of the Eastern Cape showed variation from −17.7‰ to −23.1‰ with individual values ranging from −16.4‰ to −25.2‰ for P. afra. Our results indicate a larger range of values than reported by Mills et al. (2005) for P. afra. Mooney et al. (1977) previously measured P. afra and reported a value of −17.5‰ from plants measured at Plutosvale (near Grahamstown). The results reported here from Plutosvale illustrated that the ∂13C values are still in a similar range after 37 years. This is under conditions of increasing atmospheric CO2 concentrations from 330 ppm to ∼400 ppm. The results reported for populations of P. afra are similar to the range of values detected in three species of Clusia from Panama (Holtum et al., 2004). New leaf tissue showed similar values to the mature leaf tissue except for one population. This result is an indicator that most carbon for the growing leaves is imported from the mature leaf tissue as reported by Winter and Holtum (2002). The reproductive tissue was more CAM like and may have been produced during a period of reduced soil water.
There appears to be a linear relationship between the carbon isotope values and the proportion of daytime and nighttime CO2 fixation (Winter and Holtum, 2002; Winter et al., 2015). Our results confirmed the C3 growing with P. afra fell in the C3 range enabling estimation using ∂13C of nighttime contribution of CO2 uptake, We estimated the nighttime contribution of CO2 uptake of P. afra populations using equations from Winter and Holtum (2002). We hypothesized the nighttime contribution of CO2 uptake would follow trends in the rainfall patterns. The Port Alfred population had a more C3 like value with an estimated contribution of ∼30% nighttime CO2 uptake (Table 5). The Addo population which still has somewhat of a coastal influence, but with less rainfall than Port Alfred, showed more of intermediate C3 and CAM value with an estimated 42% contribution of nighttime CO2 uptake. The more CAM like values were found in Route 350 and Plutosvale populations which grow further inland and show approximately a 50% contribution on nocturnal CO2 uptake. These regions have higher summer maxima day and night temperatures which are more conducive to CAM photosynthesis. Their values fell in the range in which 50–60% of the carbon uptake and indicates nighttime CO2 fixation by PEPCase (Winter and Holtum, 2002). Based on the range of δ 13C values, it appears the contribution of the CAM pathway can vary from 20% to 60% with an average for some populations between 50 and 60 percent. This is large range for the contribution of nocturnal CO2 uptake and indicates a large variation of CAM photosynthesis within the P. afra species.
The range of δ 13C values measured in this study did not show a significant correlation between the rainfall and temperature conditions that the various populations were growing in. There were slight trends, such as the Port Alfred coastal population which grows under milder conditions during both winter and summer and receives the most precipitation of all of the populations (Table 1). The results indicate the populations may have distinct microclimates which caused the contribution of CAM to vary widely even though populations are not too distant from one another. The Grahamstown population with a δ 13C of −22.7‰ is 20–25 km from the Plutosvale population (which is east of Grahamstown) which had a δ 13C of −17.7‰ and receives more rainfall. A population 20 km north of Grahamstown (Hellspoort Valley) had a δ 13C of −19.6‰ and was similar to the Port Alfred inland population which was south of Grahamstown. The Baviaanskloof population which is the furthest west had a δ 13C of −19.1‰ and has less rainfall than Plutosvale. This region may have a slight influence of the Western Cape climate as it is close to the transition zone of the Mediterranean climate and winter rainfall which is more conducive to C3 photosynthesis. Those populations further east have more of a summer rainfall pattern and experience a winter drought. Plants with summer rainfall still experience high evapotranspiration and midday loss of turgor pressure resulting in the utilization of CAM (Guralnick and Ting, 1988). This may explain the more CAM like signal in plants in summer rainfall areas.
Most CAM and C3-CAM species fall into bimodal distribution of carbon isotope values ranging from −27‰ to −14‰ Winter and Holtum (2002). The results of Winter and Holtum (2002) showed there are relatively few species found in the range from −19‰ to −22‰. The range of δ 13C values observed in the different populations of P. afra are interesting in that they fall in within the range where the majority of most facultative CAM species are conspicuously absent. The overall δ 13C average of the ten populations was −19.6‰ and falls clearly in the range where few species are found. These are values are estimated to have a contribution of nighttime CO2 uptake in the range of 40–50% of the overall carbon balance. It is believed this range is not phenotypically adaptable to most species and which is why there is a bimodal distribution of CAM species (Winter et al., 2015). Most species Winter and Holtum (2002) observed were either predominantly C3 or showed more than a 50% contribution of CAM to the carbon balance of the species.
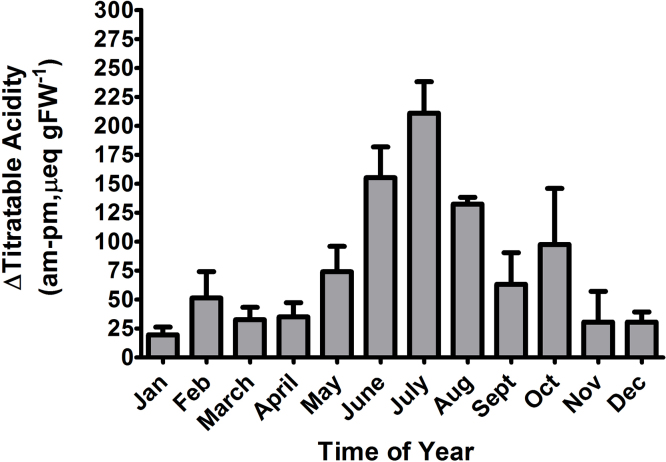
P. afra is well adapted to maximize its carbon uptake with its ability to shift between C3 and CAM on a daily basis. In P. afra CAM is operating continuously ranging from C3 to CAM-Cycling (refixation of respiratory CO2) to CAM and CAM-idling depending on environmental conditions (Guralnick et al., 1984b). This may account for its carbon isotope signal to fall in the range from −19‰ to −22‰. The utilization of CAM varies during the course of the year (Guralnick et al., 1984b). P. afra has previously reported values ranging from −27.5‰ (Sternberg et al., 1984), −25.2‰ (Neales, 1975) and −23.4‰ (Guralnick and Ting, 1986) dependent on growth conditions. The value of −23.4‰ reported by (Guralnick and Ting, 1986) were from well-watered plants growing outdoors in a Mediterranean climate of Southern California and CAM was utilized extensively for 3–4 months during the summer (Guralnick and Ting, 1988). In addition, P. afra exhibits high levels of housekeeping PEPCase activity enabling the plant to utilize CAM during the winter months and then shows an increased induction of PEPCase activity during the summer months (Guralnick and Ting, 1988). During the winter months, P. afra exhibits an average acid fluctuation in the range from 20 to 34 μeq gFW−1 which is typical of plants that are recycling respiratory CO2 at night (Fig. 2). Thus, P. afra exhibits what is termed low-level CAM (Winter et al., 2015) during the season when predominantly C3 photosynthesis is observed. During the summer months, P. afra exhibits an average acid fluctuation in the range from 125 to 210 μeq gFW−1. Thus, P. afra exhibits a strong CAM signal with substantial amounts of nocturnal CO2 uptake and carbon gain. In every month P. afra exhibits some measurable acid fluctuation which indicates within this species, CAM is a continuous trait.
Fig. 2.
The monthly average change of titratable acidity (AM- PM) of well-watered Portulacaria afra plants grown in Southern California in the Northern Hemisphere. Summer months are June, July, and August. (n = 4-9, error bars indicate 1 SEM). Data is taken from Guralnick (1983) and Guralnick (1987).
The population results of this study are similar to results of population studies of Sedum wrightii reported by Kalisz and Teeri (1986). They found δ13C values ranging from −13.8 to −22.9‰. Further research indicated genetic differences of populations where plants from the more arid environments appeared to have a higher degree of tolerance to drought and more significant utilization of CAM than the other populations (Gurevitch et al., 1986). Population level studies of P. afra will be critical to the understanding of its role in the Spekboom thicket because it is a dominant species and can account for over 90% of the plant cover. In Graaff-Reinet, P. afra occurs at a plant density of 440 plants/Ha (Guralnick, unpublished data) and other areas can even have higher plant densities. In comparison, the woody species of Clusia has densities ranging from 40–100 plants/HA in their community but do not dominate the landscape like P. afra (Quevedo et al., 2013).
The research presented here illustrates different populations show a marked phenotypic plasticity in their utilization of CAM and growth patterns, which is presumed to be environmentally related. We have started initial genetic screening of the populations and there are indications there may be genetic differences in the populations (Guralnick and Gladsky unpublished data). More information will be needed to determine if these differences may have some photosynthetic components. Plants from different populations have also been transplanted to a common garden for more detailed physiological comparisons. We have initiated microclimatic studies looking at soil variables and water relations in the different populations. This in turn may lead to populations that may be better adapted for restoration due to increased water use efficiency. The water use efficiency (WUE) of C3 P. afra appears to be higher than a typical C3 plant and is in the range of 1.2–8.3 mmol CO2/mol H2O and may be related to its leaf anatomy and reduced mesophyll airspace of 5% (Borland et al., 2009; Guralnick unpublished data). This may be important as researchers are working to genetically engineer higher water use efficiencies in C3 plants that would be more typical of CAM species (Borland et al., 2009). The results here suggest that P. afra may be an ideal candidate to study the molecular aspects of facultative CAM metabolism (Winter and Holtum, 2014).
5. Conclusions
We provide evidence which indicated a much greater range of variability in the contribution of CAM than has been previously identified in areas where P. afra is found. We also extend the altitudinal range to 1400 m in which P. afra is found and performing CAM. We are continuing our studies on the contribution of the CAM pathway to the overall carbon balance and water use efficiency in Portulacaria afra and its possible role in carbon sequestration in South Africa. The overall evidence supports that CAM is a continuous trait in P. afra.
Declarations
Author contribution statement
Lonnie J. Guralnick: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Kate Gladsky: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
Research was supported in part from a Rhode Island-INBRE fellowship awarded to Kate Gladsky. Support was also provided by an award from NSF-EPSCoR.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to thank Dr. Gerald Edwards and Dr. Ray Lee for their generous support and help with the Carbon Isotope Analysis. We thank Roger Williams University for travel support for LJG and the Provost’s Travel fund for KG. We thank Mike Powell for his time, knowledge of different populations, and use of his vehicle to collect samples. Professor Brad Ripley was available for much discussion. We thank Rhodes University for the use of their facilities.
References
- Baran R.J. 1999. Portulacaria afra the Elephant’s food or spekboom.www.users.qwest.net/∼rjbphx/Portulacaria.html [Google Scholar]
- Black C.C. Photosynthetic carbon fixation in relation to net CO2 uptake. Ann. Rev. Plant Physiol. 1973;24:253–286. [Google Scholar]
- Borland A.M., Griffiths H., Harwell J., Smith J.A.C. Exploiting the potential of plants with Crassulacean acid metabolism for bioenergy production on marginal lands. J. Exp. Bot. 2009;60:2879–2896. doi: 10.1093/jxb/erp118. [DOI] [PubMed] [Google Scholar]
- Borland A.M., Zambrano V.A.B., Ceusters J., Shorrock K. The photosynthetic plasticity of crassulacean acid metabolism: an evolutionary innovation for sustainable productivity in a changing world. New Phytol. 2011;191:619–633. doi: 10.1111/j.1469-8137.2011.03781.x. [DOI] [PubMed] [Google Scholar]
- Cowling R.M., Proche Vlok J.H.J. On the origin of southern African subtropical thicket vegetation. S. Af. J. Bot. 2005;71:1–23. [Google Scholar]
- Gibson A. Anatomy of Succulence. In: Ting I.P., Gibbs M., editors. Crassulacean acid metabolism. Proceedings of the Fifth Symposium in Botany. Waverly Press; Baltimore, MD: 1982. [Google Scholar]
- Guralnick L.J. San Diego State University; 1983. Photoperiodic control of the induction of Crassulacean acid metabolism in Portulacaria afra. MS Thesis. [Google Scholar]
- Guralnick L.J. University of California; Riverside: 1987. The effect of drought on the seasonal shift from C3 to CAM photosynthesis in Portulacaria afra (L.) Jacq. Ph.D. dissertation. [Google Scholar]
- Guralnick L.J., Jackson M.D. The occurrence and phylogenetics of Crassulacean acid metabolism activity in the Portulacaceae. Int. J. Plant Sci. 2001;162:257–262. [Google Scholar]
- Guralnick L.J., Ting I.P. Seasonal response to drought and rewatering in Portulacaria afra (L.) Jacq. Oecologia. 1986;70:85–91. doi: 10.1007/BF00377114. [DOI] [PubMed] [Google Scholar]
- Guralnick L.J., Ting I.P. Seasonal patterns of water relations and enzyme activity of the facultative CAM plant Portulacaria afra (L.) Jacq. Plant Cell Environ. 1988;11:811–818. [Google Scholar]
- Guralnick L.J., Rorabaugh P.A., Hanscom Z. Influence of photoperiod and leaf age on Crassulacean acid metabolism in Portulacaria afra (L.) Jacq. Plant Physiol. 1984;75:454–457. doi: 10.1104/pp.75.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnick L.J., Rorabaugh P.A., Hanscom Z. Seasonal shifts of photosynthesis in Portulacaria afra (L.) Jacq. Plant Physiol. 1984;76:643–646. doi: 10.1104/pp.76.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnick L.J., Cline A., Smith M., Sage R. Evolutionary Physiology: The extent of C4 and CAM photosynthesis in the Genera Anacampseros and Grahamia of the Portulacaceae. J. Exp. Bot. 2008;59:1735–1742. doi: 10.1093/jxb/ern081. [DOI] [PubMed] [Google Scholar]
- Gurevitch J., Teeri J.A., Wood A.M. Differentiation among populations of Sedum wrightii (Crassulaceae) in response to limited water availability: Water Relations, CO2 assimilation, growth and survivorship. Oecologia. 1986;70:198–204. doi: 10.1007/BF00379240. [DOI] [PubMed] [Google Scholar]
- Hanscom Z., Ting I.P. Response of succulents to plant water stress. Plant Physiol. 1978;61:327–330. doi: 10.1104/pp.61.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtum J.A.M., Aranda J., Virgo A., Gehrig H.H., Winter K. δ13C values and Crassulacean acid metabolism in Clusia species from Panama. Trees. 2004;18:658–668. [Google Scholar]
- Kalisz S., Teeri J.A. Population-level variation in Photosynthetic metabolism and Growth in Sedum wrightii. Ecology. 1986;67:20–26. [Google Scholar]
- Mills A.J., Cowling R.M., Fey M.V., Kerley G.I.H., Donaldson J.S., Lechmere-Oertel R.G., Sigwela A.M., Skowno A.L., Rundel P. Effects of goat pastoralism on ecosystem carbon storage in semiarid thicket, Eastern Cape, South Africa. Austral Ecol. 2005;30:797–804. [Google Scholar]
- Mooney H.A., Troughton J.H., Berry J.A. Carbon isotope ratio measurements of succulent plants in Southern Africa. Oecologia. 1977;30:295–305. doi: 10.1007/BF00399762. [DOI] [PubMed] [Google Scholar]
- Neales T.F. The gas exchange patterns of CAM plants. In: Marcelle R., editor. Environmental and biological control of photosynthesis. Dr. W. Junk; The Hague: 1975. pp. 299–310. [Google Scholar]
- Oakes A.J. Portulacaria afra Jacq. −A potential browse plant. Econ. Bot. 1973;27:413–416. [Google Scholar]
- Osmond C.B. Crassulacean acid metabolism: A curiosity in context. Ann. Rev. Plant Physiol. 1978;29:379–414. [Google Scholar]
- Quevedo A.A., Schleuning M., Hensen I., Saavedra F., Durka W. Forest Fragmentation and edge effects of the genetic structure of Clusia sphaerocarpa and C. lechleri (Clusiaceae) in tropical montane forests. J. Trop. Ecol. 2013;29:321–329. [Google Scholar]
- Palmer E., Pitman N. AA Balkema; Cape Town: 1961. Trees of South Africa; p. 352. [Google Scholar]
- Sternberg L.R., Deniro M.J., Ting I.P. Carbon, Hydrogen, and Oxygen Isotope Ratios of Cellulose from Plants Having Intermediary Photosynthetic Modes. Plant Physiol. 1984;74:104–107. doi: 10.1104/pp.74.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting I.P. Crassulacean acid metabolism. Ann. Rev. Plant Physiol. 1985;36:595–622. doi: 10.1104/pp.77.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting I.P., Hanscom Z. Induction of acid metabolism in Portulacaria afra. Plant Physiol. 1977;59:511–514. doi: 10.1104/pp.59.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K., Holtum J.A.M. How closely do the δ13C values of Crassulacean acid metabolism plants reflect the proportion of CO2 fixed during the day and night. Plant Physiol. 2002;129:1843–1851. doi: 10.1104/pp.002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K., Holtum J.A.M. Facultative crassulacean acid metabolism (CAM) plants: powerful tools for unraveling the functional elements of CAM photosynthesis. J. Exp. Bot. 2014;65:3425–3441. doi: 10.1093/jxb/eru063. [DOI] [PubMed] [Google Scholar]
- Winter K., Holtum J.A.M., Smith J.A.C. Crassulacean acid metabolism: a continuous or discrete trait? New Phytol. 2015;208:73–78. doi: 10.1111/nph.13446. [DOI] [PubMed] [Google Scholar]