Abstract
Type 1 diabetes is associated with subfertility in humans. The current treatment for type 1 diabetes, insulin monotherapy, is suboptimal to fully stabilize glycemia, potentially leading to this subfertility. Recent work has demonstrated that treatment with the energy-regulating hormone leptin, alone or in combination with insulin, can more effectively control glycemia in mouse models of type 1 diabetes. Here, we sought to determine whether the fertility defects in a type 1 diabetic mouse model, the Akita mouse, can be rescued with leptin monotherapy in the absence of any exogenous insulin. Akita homozygous mice treated with leptin alone had a larger total body size, testes, and seminal vesicles than their untreated siblings. Leptin treatment prevented testicular degeneration and rescued sperm motility to wild-type levels. Furthermore, sperm obtained from leptin-treated mice could successfully fertilize ooctyes in vitro. Despite completely rescuing spermatogenesis, the critical reproductive hormones LH and testosterone were only modestly higher than in untreated mice, indicating that a minimum threshold of these hormones must be met to maintain spermatogenesis. Cumulatively, these findings implicate the importance of leptin in maintaining fertility and support the use of leptin therapy in the treatment of type 1 diabetes.
Untreated type 1 diabetes causes disruptions in the hypothalamic pituitary gonadal (HPG) axis, which integrates signals from the central nervous system and the reproductive organs. As a result of endocrine disorders and damage to sperm nuclear and mitochondrial DNA from oxidative stress, men with type 1 diabetes are subfertile (1–4). Similarly, mouse models of untreated type 1 diabetes exhibit decreased levels of reproductive hormones, including LH, FSH, testosterone, insulin, and leptin, all of which affect proper function of the HPG axis (5, 6).
The Akita mouse is a useful model for studying the mechanism of type 1 diabetes-related male infertility. Akita heterozygous mice are diabetic (blood glucose levels of 600 mg/dL by 7 wk of age) (6) owing to a mutation in the insulin (ins2) gene, which produces a misfolded protein that causes endoplasmic reticulum stress and subsequent pancreatic β-cell death (7). The homozygous Akita mouse is severely diabetic, with blood glucose levels surpassing 600 mg/dL by 3 weeks of age, and has a lifespan of 8–12 weeks. The Akita homozygote is infertile and undergoes testicular atrophy beginning at 5 weeks of age and by 7–8 weeks has a reduced testis size, arrested spermatogenesis, and an absence of mature spermatozoa (6). Serum levels of critical reproductive hormones, including LH, testosterone, and leptin, are significantly reduced, indicating that the infertility is due to disruptions in the HPG axis.
Leptin, an important signaling hormone involved in energy expenditure, is decreased in newly diagnosed type 1 diabetic human patients before receiving insulin treatment and in mouse models of type 1 diabetes (8, 9). Leptin is secreted by fat cells, and its concentration in serum is directly proportional to the amount of adipose tissue present (10, 11). Type 1 diabetic animals lacking insulin have depleted leptin levels corresponding with diminished fat stores (8). Leptin signaling has a wide range of activities, including regulation of appetite, maintenance of body weight and normoglycemia, and neuroendocrine effects and low levels of leptin have detrimental effects on fertility (12). Leptin stimulates secretion of GnRH from the hypothalamus, which causes the pituitary to release LH, stimulating testosterone synthesis in the testes (13). Without LH or testosterone, spermatogenesis is impaired, resulting in infertility (14, 15). Leptin levels are also markedly decreased in cases of nutritional deprivation in humans. Leptin can restore fertility in anorexic patients with hypothalamic amenorrhea, suggesting that the maintenance of fertility is leptin dependent (16). Additionally, mice lacking either leptin or its receptor (ob/ob or db/db mice, respectively) are infertile due to a decrease in gonadotropins (17). Cumulatively, these data demonstrate the importance of leptin for maintaining fertility.
Type 1 diabetes in humans is currently treated with insulin, but a number of comorbidities, such as atherosclerosis, hypertension, nephropathy, and neuropathy, can persist (3, 18, 19). Recently, leptin monotherapy has been shown to reverse hyperglycemia in type 1 diabetic mice as well as ameliorate many other symptoms of diabetes, including hyperglucagonemia, hyperketonemia, and polyuria (20–22). Also, leptin inhibits lipogenesis (23), whereas insulin promotes lipogenesis (24). Thus, leptin therapy may prevent metabolic risks associated with the increased fat deposition associated with insulin monotherapy. Current murine data suggest that leptin therapy may result in better glycemic control than insulin monotherapy (21). Furthermore, such treatments may prevent many of the type 1 diabetes complications resulting from blood glucose fluctuations. Our previous work demonstrated that exogenous insulin treatment restored the HPG axis in Akita mice, rescuing testicular atrophy and germ cell loss (6). However, we hypothesized that the ultimate cause of infertility was not lack of insulin but rather the lack of leptin due to the decreased adipose tissue in these mice. Here, we tested the hypothesis that leptin monotherapy can restore male fertility in Akita mice.
Materials and Methods
Mice
All studies were approved by the animal studies committee at Washington University School of Medicine and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. FVB.B6-Ins2Akita/MlnJ mice were obtained from The Jackson Laboratory. Heterozygous males and females were mated to obtain homozygous, heterozygous, and wild-type males.
Leptin treatment
Akita homozygous sibling pairs were used for leptin treatment. At 5 weeks of age, 100-μg leptin was administered sc (chosen on the basis of preliminary studies in our lab) twice daily for 2 weeks; the sibling Akita homozygote received PBS. The treatment starting time point was chosen to be 5 weeks, before Akita homozygous mice lose significant body weight due to worsening diabetic state, to determine whether leptin could prevent the testicular atrophy observed in Akita mice between 5 and 7 weeks of age (6).
Insulin treatment
Akita homozygous mice were treated with insulin pellets as described previously (6).
Histology
Testes were fixed overnight in Bouin's solution, dehydrated in ethanol, and paraffin embedded. Tissue sections (5 μm) were cut, dried overnight at 60°C, rehydrated, and then stained with hemotoxylin and eosin.
Transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL)
Caudal sperm was collected into Embryomax Human Tubal Fluid media (Millipore). Cells were centrifuged at 800g, washed with PBS, and TUNEL (Roche) reactions were performed as described previously (6).
Sperm motility analysis
Cauda epididymal sperm were collected in Embryomax Human Tubal Fluid (Millipore) and allowed to disperse for 10 minutes at 37°C in 5% CO2. Computer Assisted Sperm Analysis software (Hamilton-Thorne) was used to analyze sperm motility.
In vitro fertilization
Wild-type FVB females were injected with single injections of 10-IU pregnant mare serum gonadotropin (Sigma) followed by 10-IU human chorionic gonadotropin 48 hours later. Twelve hours after human chorionic gonadotropin injection, ovulated cumulus oocyte complexes (COCs) were collected in Research Vitro Fert media (containing 5-mg/mL BSA) (Cook Medical). After capacitation for 90 minutes, 1–5 million sperm were added to COCs in 500-μL drops of media under oil. Sperm and COCs were incubated at 37°C and 5% CO2 for 6 hours, after which oocytes were washed free of sperm and incubated overnight. Fertilization rate was calculated as the number of 2-cell embryos 24 hours after fertilization. Blastocyst rate was calculated as the number of 2-cell embryos reaching blastocyst stage after 4 days.
Serum hormone measurements
Mice were housed individually for 1 week before serum collection. Serum testosterone (radioimmunoassay) and LH (Mouse LH Sandwich assay) were measured by the University of Virginia Center for Research in Reproduction Ligand assay and Analysis Core. Leptin was measured by ELISA (Millipore) and analyzed on a Molecular Devices VersaMax Microplate reader at 450 and 590 nm. Glucose was measured as described previously (13).
Intratesticular testosterone
Testes were homogenized in 500 μL of PBS with proteinase inhibitors, incubated at room temperature overnight, and extracted with 500 μL of dichloromethane. Dichloromethane was evaporated under a fume hood, and the pellet was reconstituted with 100 μL of ELISA dilution buffer (Endocrine Technologies).
Quantitative RT-PCR (qRT-PCR)
qRT-PCR was performed as described previously (6) using validated gene-specific TaqMan primers and probes.
Body fat measurement and tissue weights
Body fat was measured by EchoMRI at the Diabetes Phenotyping Core at Washington University in St Louis. Testes and seminal vesicles were removed, and testis weights were recorded as wet weight for single testis.
Statistics
Data were analyzed by using one-way ANOVA followed by a Tukey post hoc test.
Results and Discussion
Spermatogenesis is restored in leptin-treated Akita homozygotes
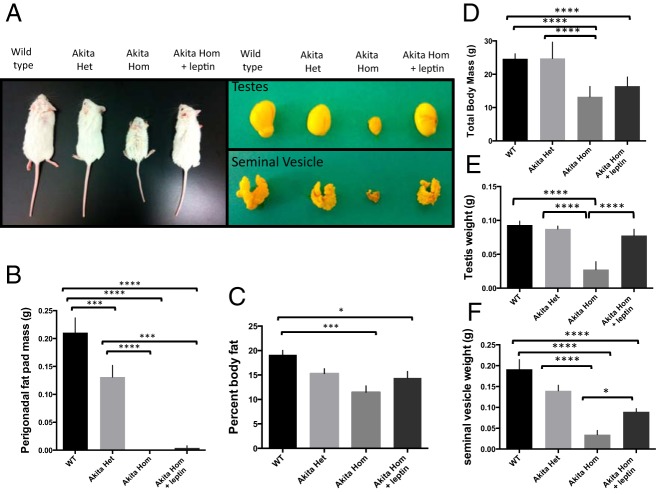
Akita homozygous mice are deficient in spermatocytes and spermatids, resulting in infertility (6). These mice also have dramatically decreased levels of leptin resulting from decreased body fat. We hypothesized that leptin deficiency may be the cause of Akita infertility and that leptin monotherapy could rescue the infertility in Akita mice. Akita homozygotes treated with leptin had a larger overall body size (average, 16.4 vs 13.2 g), testes (0.075 vs 0.032 g), and seminal vesicles (0.091 vs 0.046 g) than untreated Akita homozygote siblings (Figure 1). The rescue of the seminal vesicle size suggests a greater production of testosterone, and the larger overall body size suggests that leptin treatment may regulate growth hormones. Indeed, a recent study by Yu et al (25) showed that leptin-treated type 1 diabetic mice had higher levels of insulin growth factor 1 than untreated controls. Additionally, leptin- and insulin-signaling pathways are known to converge at the level of phosphoinositide 3-kinase, so leptin may be able to induce many of the same biochemical reactions as insulin (26).
Figure 1.
Leptin treatment rescues body size (A and D) (n = 9 wild type [WT], heterozygous [het], and homozygous [hom]+leptin; n = 16 hom), testis size (A and E), and seminal vesicle size (A and F) of Akita homozygous mice. Perigonadal fat mass and body fat percentage are decreased in both untreated and leptin-treated Akita homozygotes (B and C). Error bars, SEM. *, P < .05; ***, P < .001; ****, P < .0001.
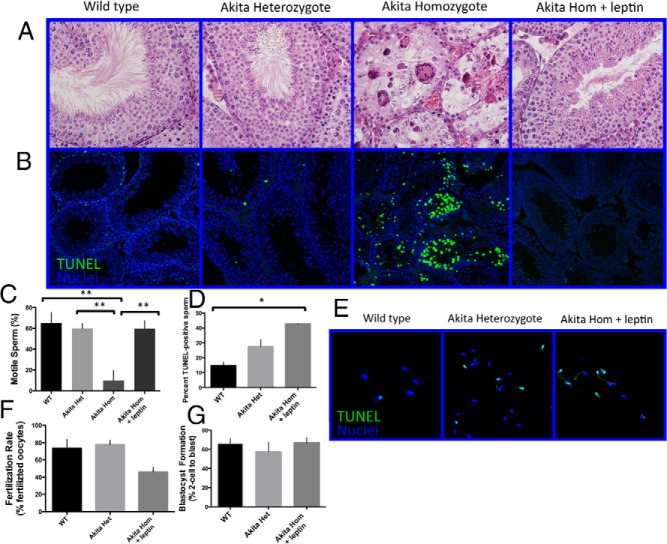
The testes of PBS-treated Akita homozygotes contained no mature spermatozoa, a high degree of vacuolization, and large, multinucleated cells. By contrast, leptin-treated Akita homozygous mice possessed all of the cell types within the testes, including mature spermatozoa (Figure 2A). The seminiferous tubule images shown are representative of full testicular cross-sections. Additionally, sperm from leptin-treated mice were significantly more motile than those from untreated mice (computer assisted sperm analysis values for Akita homozygotes without caudal sperm were reported as 0) (Figure 2C). Because DNA damage in sperm often occurs in type 1 diabetes (4), we performed a TUNEL assay to assess DNA integrity of the sperm cells. The number of DNA-damaged epididymal sperm cells was higher in sperm from both Akita heterozygous and leptin-treated homozygous mice than in sperm from wild-type mice (Figure 2, D and E). However, TUNEL analysis of testicular sections revels a low level of apoptosis in leptin-treated Akita homozygotes and Akita heterozygotes compared with untreated homozygotes (Figure 2B). This indicates that DNA damage occurs in the epididymis of leptin-treated homozygotes and heterozygous Akita mice, perhaps due to the persistent hyperglycemia in these mice. Although leptin-treated Akita homozygous mice could not sire pups, sperm obtained from these mice could fertilize wild-type oocytes, although at a lower rate than wild-type (46 ± 6% vs 74 ± 10%) (Figure 2F). Of oocytes that were fertilized, 67 ± 5% of those fertilized with sperm from leptin-treated Akita homozygotes developed to the blastocyst stage, whereas 66 ± 5% of those from wild-type sperm developed to the blastocyst stage (Figure 2G). This suggests that, despite the large percentage of sperm with DNA damage, those sperm that are able to fertilize can successfully contribute to development of the blastocyst. Nonetheless, it will be interesting in future studies to examine any potential effects of this DNA damage on offspring. Together, these data demonstrate that leptin treatment prevents the testicular atrophy observed in Akita homozygotes but that fertility is not fully rescued at the dosage and treatment duration used for these experiments.
Figure 2.
Leptin treatment of Akita homozygous mice prevents loss of germ cells, increases sperm motility, and results in production of fertilization-competent sperm. Hematoxylin and eosin sections of the testes (A), computer-assisted sperm motility analysis (B) (n = 5), DNA damage in caudal sperm cells (C and D). TUNEL-positive cells (green), nuclei (blue) (E and F) (n = 3 mice), fertilization rate (E) (n = 5), and 2-cell-to-blastocyst development rate (F) (n = 5), and arrows in A indicate mature spermatozoa. Error bars, SEM. *, P < .05; **, P < .01. WT, wild type; het, heterozygote; hom, homozygote.
Serum leptin and fat mass in leptin-treated Akitas
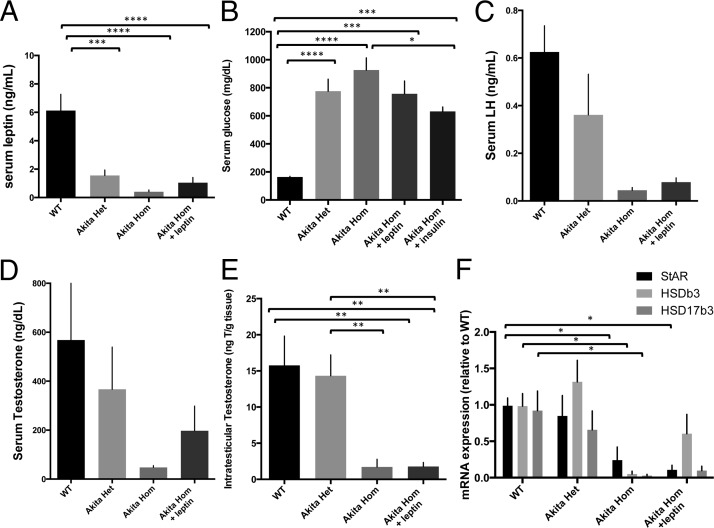
Despite injecting high concentrations of leptin (10 times that used to rescue leptin-deficient ob/ob males) (22) into Akita homozygous mice, serum leptin levels of these mice remained low. Serum leptin levels were 6.13 ± 1.14 ng/mL in wild-type mice, 1.55 ± 0.39 ng/mL in Akita heterozygotes, 0.409 ± 0.13 ng/mL (and often below the 0.2-ng/mL detection threshold) in untreated Akita homozygotes, and 1.04 ± 0.37 ng/mL in Akita homozygotes after 2 weeks of leptin treatment (Figure 3A). Thus, a low threshold of leptin is required to prevent the arrest of spermatogenesis. These data are in accordance with previous literature showing that high serum leptin levels are not required for the reversal of hyperglycemia in a streptozocin-induced mouse model of type 1 diabetes (20). Similarly, Akita heterozygotes have low serum leptin levels but maintain spermatogenesis. Blood glucose levels decreased moderately with leptin treatment, similar to insulin-treated Akita mice (Figure 3B). The hyperglycemia in Akita heterozygotes and leptin-treated homozygotes may result in oxidative stress and, thus, explain the high level of DNA damage observed in sperm. Peripheral fat storage also did not increase significantly, and perigonadal fat pads remained small or absent in leptin-treated Akita homozygotes (Figure 1B), indicating that fat storage was not drastically increased in these mice. Analysis by EchoMRI indicated that leptin-treated Akita homozygotes had a slightly higher percentage of body fat than untreated siblings (13.3 ± 1.2% vs 11.96 ± 1.2%), but this was still much lower than that of wild-type mice (19.1 ± 0.7%) (Figure 1C). Thus, it is not the presence of adipose tissue that is critical for the maintenance of fertility but the presence of sufficient leptin concentrations.
Figure 3.
Serum leptin levels (A) (n = 9), serum glucose (B) (n = 4 hom+leptin; n = 5 WT and het; n = 6 hom and hom+ins), serum LH (C) (n = 5 WT and het; n = 12 hom; n = 7 hom+lep), serum testosterone (D) (n = 7 hom and hom+lep; n = 10 WT and het), (E) intratesticular testosterone (n = 6 WT and het; n = 5 hom+lep), and (F) testicular steroidogenic mRNA expression (n = 6 WT, het and hom, n = 4 hom+ lep). Error bars, SEM. *, P < .05; **, P < .01; ***, P < .001; ****, P < .0001. WT, wild type; het, heterozygote; hom, homozygote; ins, insulin.
Gonadotropins in leptin-treated Akita homozygous mice
To test whether HPG axis activity was restored in the presence of leptin, we measured the gonadotropins LH and testosterone. Although these values were higher in leptin-treated Akita homozygotes than in the untreated mice, the differences did not reach significance. Untreated homozygous Akita mice had 0.045 ± 0.01-ng/mL LH and 47.7 ± 7.4-ng/dL testosterone, whereas leptin-treated Akita homozygotes had 0.079 ± 0.02-ng/mL LH and 197.4 ± 101-ng/dL testosterone (Figure 3, C and D). Likewise, intratesticular testosterone was low in both leptin-treated (1.1 ng/g) and untreated Akita homozygotes (0.26 ng/g), and both were much lower than wild-type levels (11.83 ng/g) (Figure 3E). Genes involved in testosterone steroidogenesis, steroidogenic acute regulatory protein, 3-β-hydroxysteroid dehydrogenase/Δ-5–4 isomerase, and hydroxysteroid (17-β) dehydrogenase 3 were all significantly decreased in Akita homozygotes, and leptin treatment did not significantly increase their expression (Figure 3F). The fact that leptin treatment rescued spermatogenesis and sizes of the testes and seminal vesicles despite minimal rescue of testosterone concentration suggests that a low threshold of gonadotropins is necessary to maintain spermatogenesis. This is in agreement with previous literature demonstrating that although initiation of spermatogenesis may require large amounts of testosterone, maintenance of spermatogenesis requires only low levels of gonadotropins (27). However, the low level of testosterone may explain why the leptin-treated Akita homozygotes did not mount female mice, because the testosterone levels may be too low to support mating behavior. In accordance with this, previous research demonstrates that sufficient testosterone levels are critical for mounting, intromission, and ejaculation in a number of mouse models (28, 29).
As with leptin, measurements of LH and testosterone in Akita homozygotes were frequently below the detectability threshold. Notably, Akita heterozygotes have decreased levels of gonadotropins, leptin, and insulin and have extreme hyperglycemia, yet maintain fertility. Although we used a large dose of leptin in these studies, it was still insufficient to completely rescue the Akita homozygote gonadotropin levels wild-type levels. The requirement for a large dose of leptin to rescue fertility is not surprising, considering that the Akita homozygous mice also remain hyperglycemic when treated with doses of insulin that rescue spermatogenesis (Figure 3B), and high doses of insulin are required to fully rescue Akita homozygote fertility (6); this is most likely due to the severity of diabetes in the Akita mouse. It is possible that starting leptin treatment earlier or providing larger doses of leptin could further rescue the Akita homozygous diabetic mouse.
Conclusions
The subfertility associated with type 1 diabetes may result from a combination of hormonal imbalances and DNA damage from hyperglycemia-induced oxidative stress. We have previously shown that high doses of insulin can rescue the fertility of Akita homozygous mice, and here, we show that high doses of leptin can prevent their testicular atrophy. Leptin treatment was able to completely prevent degeneration of the testes and maintain a viable sperm cell population. It appears that the causative factor of Akita spermatogenesis defects is not a lack of insulin but a lack of leptin. This is in agreement with data reporting subfertility in leptin-deficient and lipoatrophic mice (21). Uncontrolled diabetes in Akita homozygous mice leads to a severe depletion of adipose tissue, which in turn depletes leptin levels. The lack of leptin signals a lack of energy availability to the central nervous system, leading to hypogonadism and infertility (27, 30). It is likely that supplementing Akita mice with leptin at least partially restores the HPG axis, thereby increasing LH and testosterone above the thresholds necessary to maintain spermatogenesis.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- COC
- cumulus oocyte complex
- HPG
- hypothalamic pituitary gonadal
- TUNEL
- transferase-mediated deoxyuridine triphosphate nick-end labeling.
References
- 1. La Vignera S, Condorelli R, Vicari E, D'Agata R, Calogero AE. Diabetes mellitus and sperm parameters. J Androl. 2012;33(2):145–153. [DOI] [PubMed] [Google Scholar]
- 2. Mallidis C, Agbaje I, O'Neill J, McClure N. The influence of type 1 diabetes mellitus on spermatogenic gene expression. Fertil Steril. 2009;92(6):2085–2087. [DOI] [PubMed] [Google Scholar]
- 3. Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. 2012;6:CD007543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Agbaje IM, Rogers DA, McVicar CM, et al. . Insulin dependant diabetes mellitus: implications for male reproductive function. Hum Reprod. 2007;22(7):1871–1877. [DOI] [PubMed] [Google Scholar]
- 5. Ballester J, Muñoz MC, Domínguez J, Rigau T, Guinovart JJ, Rodríguez-Gil JE. Insulin-dependent diabetes affects testicular function by FSH- and LH-linked mechanisms. J Androl. 2004;25(5):706–719. [DOI] [PubMed] [Google Scholar]
- 6. Schoeller EL, Albanna G, Frolova AI, Moley KH. Insulin rescues impaired spermatogenesis via the hypothalamic-pituitary-gonadal axis in Akita diabetic mice and restores male fertility. Diabetes. 2012;61(7):1869–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oyadomari S, Koizumi A, Takeda K, et al. . Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109(4):525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Havel PJ, Uriu-Hare JY, Liu T, et al. . Marked and rapid decreases of circulating leptin in streptozotocin diabetic rats: reversal by insulin. Am J Physiol. 1998;274(5 pt 2):R1482–R1491. [DOI] [PubMed] [Google Scholar]
- 9. Hanaki K, Becker DJ, Arslanian SA. Leptin before and after insulin therapy in children with new-onset type 1 diabetes. J Clin Endocrinol Metab. 1999;84(5):1524–1526. [DOI] [PubMed] [Google Scholar]
- 10. Banerji MA, Faridi N, Atluri R, Chaiken RL, Lebovitz HE. Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men. J Clin Endocrinol Metab. 1999;84(1):137–144. [DOI] [PubMed] [Google Scholar]
- 11. Dua A, Hennes MI, Hoffmann RG, et al. . Leptin: a significant indicator of total body fat but not of visceral fat and insulin insensitivity in African-American women. Diabetes. 1996;45(11):1635–1637. [DOI] [PubMed] [Google Scholar]
- 12. Goumenou AG, Matalliotakis IM, Koumantakis GE, Panidis DK. The role of leptin in fertility. Eur J Obstet Gynecol Reprod Biol. 2003;106(2):118–124. [DOI] [PubMed] [Google Scholar]
- 13. Lowry O, Passonneau JV. Enzymatic Analysis: A Practical Guide. 1 ed New York, NY: Springer-Verlag, LLC; 1993. [Google Scholar]
- 14. Ma X, Dong Y, Matzuk MM, Kumar TR. Targeted disruption of luteinizing hormone β-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci USA. 2004;101(49):17294–17299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang RS, Yeh S, Tzeng CR, Chang C. Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocr Rev. 2009;30(2):119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chou SH, Chamberland JP, Liu X, et al. . Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci USA. 2011;108(16):6585–6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Swerdloff RS, Batt RA, Bray GA. Reproductive hormonal function in the genetically obese (ob/ob) mouse. Endocrinology. 1976;98(6):1359–1364. [DOI] [PubMed] [Google Scholar]
- 18. Krolewski AS, Bonventre JV. High risk of ESRD in type 1 diabetes: new strategies are needed to retard progressive renal function decline. Semin Nephrol. 2012;32(5):407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rathsman B, Rosfors S, Sjoholm A, Nystrom T. Early signs of atherosclerosis are associated with insulin resistance in non-obese adolescent and young adults with type 1 diabetes. Cardiovasc Diabetol. 2012;11:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fujikawa T, Chuang JC, Sakata I, Ramadori G, Coppari R. Leptin therapy improves insulin-deficient type 1 diabetes by CNS-dependent mechanisms in mice. Proc Natl Acad Sci USA. 2010;107(40):17391–17396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang MY, Chen L, Clark GO, et al. . Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci USA. 2010;107(11):4813–4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Denroche HC, Levi J, Wideman RD, et al. . Leptin therapy reverses hyperglycemia in mice with streptozotocin-induced diabetes, independent of hepatic leptin signaling. Diabetes. 2011;60(5):1414–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buettner C, Muse ED, Cheng A, et al. . Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med. 2008;14(6):667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kersten S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2001;2(4):282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu X, Park BH, Wang MY, Wang ZV, Unger RH. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc Natl Acad Sci USA. 2008;105(37):14070–14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest. 2005;115(4):951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Handelsman DJ, Spaliviero JA, Simpson JM, Allan CM, Singh J. Spermatogenesis without gonadotropins: maintenance has a lower testosterone threshold than initiation. Endocrinology. 1999;140(9):3938–3946. [DOI] [PubMed] [Google Scholar]
- 28. Park JH. Assessment of male sexual behavior in mice. In: Mood and Anxiety Related Phenotypes in Mice. New York: Humana Press; Vol 63 2011:357–373. [Google Scholar]
- 29. Arteaga-Silva M, Rodríguez-Dorantes M, Baig S, Morales-Montor J. Effects of castration and hormone replacement on male sexual behavior and pattern of expression in the brain of sex-steroid receptors in BALB/c AnN mice. Comp Biochem Physiol A Mol Integr Physiol. 2007;147(3):607–615. [DOI] [PubMed] [Google Scholar]
- 30. Jun JY, Ma Z, Pyla R, Segar L. Leptin treatment inhibits the progression of atherosclerosis by attenuating hypercholesterolemia in type 1 diabetic Ins2(+/Akita):apoE(−/−) mice. Atherosclerosis. 2012;225(2):341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]