Abstract
Estrogenic signaling shapes and modifies daily and circadian rhythms, the disruption of which has been implicated in psychiatric, neurologic, cardiovascular, and metabolic disease, among others. However, the activational mechanisms contributing to these effects remain poorly characterized. To determine the activational impact of estrogen on daily behavior patterns and differentiate between the contributions of the estrogen receptors ESR1 and ESR2, ovariectomized adult female mice were administered estradiol, the ESR1 agonist propylpyrazole triol, the ESR2 agonist diarylpropionitrile, or cholesterol (control). Animals were singly housed with running wheels in a 12-hour light, 12-hour dark cycle or total darkness. Estradiol increased total activity and amplitude, consolidated activity to the dark phase, delayed the time of peak activity (acrophase of wheel running), advanced the time of activity onset, and shortened the free running period (τ), but did not alter the duration of activity (α). Importantly, activation of ESR1 or ESR2 differentially impacted daily and circadian rhythms. ESR1 stimulation increased total wheel running and amplitude and reduced the proportion of activity in the light vs the dark. Conversely, ESR2 activation modified the distribution of activity across the day, delayed acrophase of wheel running, and advanced the time of activity onset. Interestingly, τ was shortened by estradiol or either estrogen receptor agonist. Finally, estradiol-treated animals administered a light pulse in the early subjective night, but no other time, had an attenuated response compared with controls. This decreased phase response was mirrored by animals treated with diarylpropionitrile, but not propylpyrazole triol. To conclude, estradiol has strong activational effects on the temporal patterning and expression of daily and circadian behavior, and these effects are due to distinct mechanisms elicited by ESR1 and ESR2 activation.
Circadian timekeeping systems use approximately 24-hour endogenous rhythms to adapt and respond to cyclically changing environments. Disruptions of these rhythms are associated with increased incidence of chronic and acute disease, including cancer, obesity, metabolic disorder, compromised immunity, cardiovascular disease, sleep disorders, and a variety of psychiatric conditions (1–7). There are sex differences in the manifestation of these conditions. For example, cardiovascular disease and hypertension are more prevalent among women subjected to circadian disruptions than in men (8). This is perhaps not surprising considering that sex differences in circadian rhythms are found across a variety of species (7, 9–12). Importantly, daily and circadian rhythms are modulated in part by shifts in hormone signaling during the perinatal period, pregnancy, menopause, and throughout the reproductive cycle (13–16), suggesting ovarian hormones such as estradiol play a role in modifying circadian rhythms.
Indeed, the maximum daily activity in intact female rats and hamsters is observed during proestrus, corresponding to peak serum estradiol levels (17). Removal of circulating estrogens via ovariectomy (OVX) reduces total wheel running activity in female mice, rats, and degus (18–21), an effect reversed by exogenous estradiol replacement (22, 23). Estradiol shortens the length of the free running period (τ) (18, 24–26) and advances the onset of wheel running activity in mice, rats, and hamsters (18, 27). It also consolidates activity to the dark phase of the light/dark (LD) cycle in mice (20, 21, 28). Female OVX hamsters have greater variability in the timing of activity onset compared with intact or estradiol-treated counterparts, whereas the incidence of asynchronous or split wheel running rhythms in female OVX hamsters is reduced by estradiol replacement (13). Furthermore, intact female estrogen receptor knockout mice (αERKO) in which estrogen receptor 1 (ESR1) (formerly ERα), but not ESR2 (formerly ERβ) has been removed (29), exhibit a truncated response to a light pulse given in the early subjective night, but a more robust behavioral shift when the pulse is administered in the late subjective night compared with intact wild-type (WT) female mice (30). Taken together, these data suggest that estrogens regulate both the magnitude and timing of wheel running activity, strengthen entrainment to LD transitions (31), and modulate behavioral shifts in response to a photic cue.
The mechanisms underlying estradiol's effects on circadian and daily activity rhythms, including the roles of ESR1 and ESR2, have yet to be determined. Ogawa et al (20) used αERKO and βERKO mice to demonstrate that ESR1, but not ESR2, regulates the estradiol-induced increase in activity. The difference in total wheel running between OVX WT and αERKO, but not βERKO, females treated with estradiol (20) suggests the predominance of an activational, rather than organizational mechanism that is dependent on ESR1. However, the use of global knockout mice and the potential for developmental effects of absent estrogen signaling confound the interpretation of these previous studies. Moreover, total daily activity is the only variable that has been shown to be under the control of a specific estrogen receptor. To study the activational effects of estrogenic signaling on the expression of daily and circadian activity rhythms, we pharmacologically manipulated the activity of ESR1 and/or ESR2 in OVX WT female mice. We show here that receptor activation differentially modulates behavioral rhythmicity, suggesting unique roles for ESR1 and ESR2.
Materials and Methods
Animals and housing
Circadian activity patterns in ESR1-knockout (αERKO; −/−) and WT (+/+) littermates have been described previously (30). To ensure this work would be comparable to previous studies, αERKO heterozygotes (+/−) on the C57BL6/J background were crossed, and the resulting WT female mice were used. Mice were maintained in accordance with the University of Illinois Urbana-Champaign Institutional Animal Care and Use Committee guidelines and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animals were maintained at a constant temperature (22°C) in 12-hour light, 12-hour dark (LD) or constant darkness (DD) and were given food and water ad libitum. Animals housed in DD were exposed to a dim red light briefly each day (<5 minutes) to assess animal health but otherwise were not exposed to any light.
Animals were fed Teklad 2016 rodent diet throughout the study because it contains undetectable to 20 mg/kg levels of soy estrogen isoflavones, including daidzein and genistein. Animals were group-housed with same-sex littermates from weaning (postnatal d 21 [p21]) until after sexual maturation (p50), at which time they were singly housed in cages (28 cm long × 16 cm wide × 12 cm high) outfitted with a metal running wheel (11 cm diameter) affixed to the lid. During the light phase, the light intensity measured at the top of the cage ranged from 220 to 360 lux (average 290 lux).
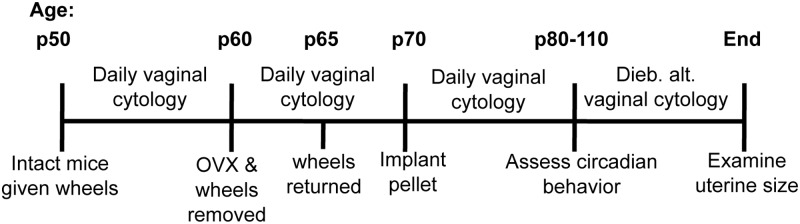
The experimental timeline is shown in Figure 1. Beginning on p50, and continuing through 2 estrous cycles, daily vaginal cytology was performed on each animal to confirm cyclicity before OVX. Throughout the study, all vaginal cytology samples were collected at varying times of day to avoid the introduction of a confounding time cue. On p60, running wheels were removed, and mice were anesthetized with ketamine/xylazine (100 and 10 mg/kg, respectively, ip) and maintained with isoflurane gas as necessary. Ovaries were removed through bilateral incisions, and the muscle/fascia and overlying skin were closed with surgical silk and staples. Vaginal cytology was examined daily for the next 8 to 10 days (Figure 1) to verify that animals were no longer exhibiting signs of cyclicity. Wheels were returned after 5 days of recovery (Figure 1).
Figure 1.
Methodological timeline. Abbreviation: Dieb. alt., every other day.
Drug pellets and administration
To manipulate estrogen receptor activity, we used specific ESR1 and ESR2 agonists. The ESR1 agonist 4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT) has a higher relative binding affinity for ESR1 over ESR2 and an estimated 1000 times higher relative potency for ESR1 (32, 33). Conversely, the relative binding affinity and relative potent selectivity for ESR2 exhibited by 2,3-bis[4-hydroxyphenyl]-propionitrile (DPN) are 70- and 170-fold greater than those observed for ESR1 (34).
DPN (rac-DPN) (1) was synthesized in the Katzenellenbogen laboratory at the University of Illinois Urbana-Champaign as described (34, 35). PPT was purchased from Orbiter Research. Administered doses were as follows: 25 μg estradiol (LowE), 50 μg estradiol (HighE), 2 mg PPT (LowPPT), 5 mg PPT (HighPPT), 2 mg DPN (LowDPN), and 5 mg DPN (HighDPN). The estradiol concentrations used were selected based on those that increased wheel-running activity, uterine weight, and the expression of corneated vaginal epithelial cells; these changes occur predominantly through the activation of ESR1 (30, 36–38). The physiologic pattern of activated ESR1 and ESR2 throughout the estrous cycle is unknown. Accordingly, doses of PPT or DPN 100-fold greater were chosen to align with previously reported efficacious doses (37, 39–42) and to account for the reduced transcriptional activity of these compounds compared with estradiol (33, 34). Specifically, our doses were selected based on studies that found PPT treatment caused estrous-like epithelial cytology (43, 44) and uterine hypertrophy (37). DPN doses were selected based on studies that found it reduced glucose-6-phosphate dehydrogenase and increased the expression of progesterone receptor (PR) and androgen receptor (AR) message and protein without altering uterine size or the proliferation of luminal epithelial cells (37, 43, 44). Each drug was compounded with ≥99% cholesterol (Sigma-Aldrich) to produce a cholesterol pellet with a total weight of 20 mg (45, 46). Control (CTL) pellets contained 20 mg cholesterol alone. Approximately 10 days after OVX (∼p70), experimental mice were anesthetized under isoflurane gas, and pellets were implanted sc (Figure 1). Mice were randomly assigned to treatment groups before pellet implantation.
To compare the efficacy of our doses with previously published work, vaginal smears were examined for 8 to 10 consecutive days after pellet implantation. Mice implanted with LowE, HighE, or HighPPT pellets exhibited vaginal cytology containing exclusively, or nearly exclusively, corneated cells, whereas cytology from CTL, LowDPN, or HighDPN-treated animals had zero to very few corneated cells. Animals treated with LowPPT pellets persistently exhibited a mixture of corneated cells, nucleated cells, and leukocytes. Daily wheel running activity was quantified from 10 days to 4 weeks after pellet implantation (Figure 1), as previously described (20). During this experimental phase, vaginal cytology was collected every other day at varying times during the day to avoid providing a nonphotic cue. Cytology for each mouse remained consistent throughout the study. Uterine size was examined at the end of the study (Figure 1) to further confirm sustained drug pellet efficacy. Consistent with previous findings (37, 43, 44), mice administered either dose of estradiol or PPT had increased uterine size compared with CTL, LowDPN, or HighDPN mice.
Assessment of daily activity patterns and circadian variables
Wheel revolutions were detected via a magnetic switch affixed to each cage. All data were recorded in 10-minute bins using VitalView and were visualized with ActiView (Mini Mitter). An individual bin was considered active if the total amount of activity during that period was equal to or exceeded at least 10% of the animal's maximum daily activity. The following parameters were assessed in LD: average daily activity, the proportion of activity in light vs dark (LD proportion), the distribution of wheel running across the total 24-hour day as well as the 12-hour dark phase, acrophase of wheel running activity, amplitude, and phase angle of activity onset. The duration of the free running period (τ) and the active phase (α) were determined in animals housed in DD. The first 3 days of DD data were ignored to account for potential acute after-effects. To evaluate the effect of estrogenic modulation on the responsiveness to light at different times of the subjective day, a modified Aschoff type II method (described below) was completed with light pulses at times corresponding to the former Zeitgeber time (ZT) 4, 16, and 22 (ZT 0 and 12 = lights on and off, respectively). Where possible, animals were used for multiple analyses and were allowed at least 1 week to re-entrain after a transition from DD to LD.
For parameters assessed in LD, animals were housed in 12:12 LD conditions for at least 1 week before data collection. Consistent phase angle during the last 4 days of this period was required to confirm entrainment (data not shown). Mean daily wheel running activity was determined by averaging the number of wheel revolutions per 10-minute bin for each animal over 3 consecutive days in LD. Data from each individual animal were normalized to prepellet baseline, which was similarly calculated by taking the mean activity for 3 days in LD after OVX and prepellet implantation. Total wheel running activity for each treatment group was then divided by the CTL group value to determine fold change.
To analyze temporal patterning of daily activity across the dark phase as well as in the light compared with dark phase, each 10-minute activity bin was averaged over 3 consecutive days, summed into 1-hour bins, and plotted over time. To determine the distribution of activity within the first and second halves of the dark phase, the average wheel revolutions per 10-minute bin over 3 days were grouped by treatment and then summed into ZT12 to ZT18 and ZT18 to ZT24 bins. The LD proportion describes the number of wheel revolutions completed during the light phase (L) compared with the total amount in the light and dark (D) and is defined as L/(L+D).
Acrophase of wheel running activity in LD corresponds to the time (10-minute bin) of maximum activity. It was determined using a cosine fitting function (Actiview) using 3 days of activity. Amplitude reflects the difference between the largest activity peak (10-minute bin) and mesor (47) observed from averaging 3 days of wheel running in LD. Subtracting the mesor from the peak enabled us to compare the magnitude of the rhythm in relation to each animal's baseline activity. Phase angle of activity onset was determined by measuring the difference in minutes between lights off and the onset of activity across 5 consecutive days in LD. Onset was defined as the first 10-minute bin of activity that equaled or exceeded 10% of the maximum activity peak that was not followed by more than 2 consecutive 10-minute bins of inactivity (28).
The length of the free running period (τ) was measured by fitting a line of best fit through activity onset for the last 5 consecutive days in DD as previously described (48, 49). Minimally, data from the first 3 days of DD were omitted from the analysis to limit the contribution of transition effects. The length of the active period (α) was determined by averaging the duration between the time of activity onset and when activity stopped over 5 days in DD. Activity cessation was defined as the last 10-minute bin with wheel revolutions ≥10% of peak activity before a break in activity lasting 2 or more hours.
We determined the effect of estrogenic signaling on the behavioral shift in response to light pulses at varying times of day (21). Briefly, animals entrained to 12:12 LD were placed in DD for at least 24 hours. A pulse of light (∼290 lux) was given for 1 hour at times corresponding to ZT4, ZT16, or ZT22 followed by 4 to 6 days in DD. Shams were treated the same but were not subjected to a light pulse. The phase shift was calculated as the difference between the pre- and post-pulse onset of wheel running activity. This experimental design allowed us to use the uniformity of the entrained animals' subjective day before their free running rhythms drifted (50).
Statistical analyses
Results are reported as mean ± SEM. For total daily activity, LD proportion, α, τ, phase angle, acrophase of wheel running activity, and amplitude, between-group differences were assessed using one-way ANOVA with treatment group (CTL, LowE, HighE, LowPPT, HighPPT, LowDPN, and HighDPN) as the independent variable, followed by Tukey's post hoc tests as appropriate. For phase response to light pulses, we used ANOVA to analyze differences across treatment groups within each time point and to identify differences between nonpulsed and pulsed animals receiving the same treatment. Distribution of activity across the 24-hour day or the 12-hour dark phase was assessed using two-way repeated-measures ANOVA with treatment and time as independent variables. All analyses were performed with SigmaPlot version 12.0 (Systat Software, Inc). Comparisons resulting in an priori P value <.05 were considered significantly different.
Results
Estradiol increases total wheel running activity through activation of ESR1, but not ESR2
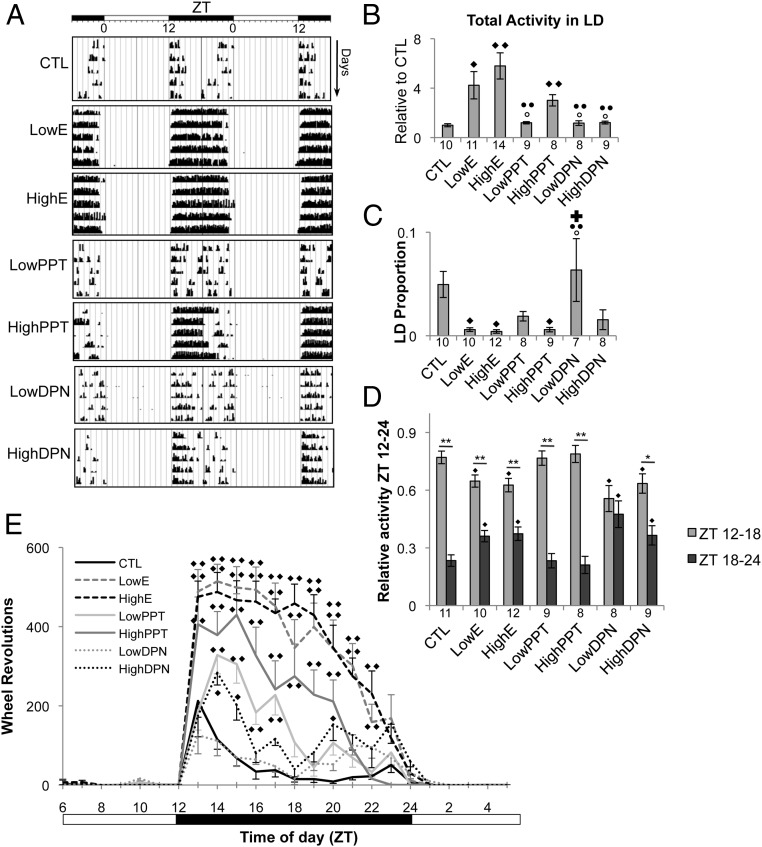
The effect of estradiol and selective estrogen receptor stimulation on wheel running activity is shown in Figure 2A, whereas total wheel running activity across all treatment groups is quantified in Figure 2B. Standardized post-pellet activity is expressed as fold change compared with pre-pellet CTL. ESR1 activation played a predominant role in the increase in daily activity that occurred after activational estradiol treatment. Both the LowE (fold change compared with CTL, 4.233 ± 1.106; P < .001) and HighE doses (fold change compared with CTL, 5.798 ± 1.061; P < .001) increased relative wheel running compared with CTL (1.00 ± 0.123). Like estradiol, PPT administration increased relative wheel running (fold change compared with CTL, 3.012 ± 0.459; P < .001). HighPPT animals did not differ from LowE or HighE animals (P > .05 for all comparisons between HighE, LowE, and HighPPT). The magnitude of wheel running observed in the LowPPT, LowDPN, and HighDPN treatment groups was not different from CTL and was significantly lower than estradiol-treated animals (P < .05).
Figure 2.
Effects of estrogenic signaling modification on circadian wheel running activity. A, Representative double-plotted actograms depicting the activational effect of differential estrogen receptor stimulation on daily wheel running activity in OVX female mice measured for 5 consecutive days in 12:12 LD (lights off at ZT12). White and black bars at the top indicate whether the lights were on or off, respectively. Consecutive days are plotted on the y-axis, whereas the ZT over 48 hours is shown on the x-axis. Each black bar represents a 10-minute bin, and the height indicates the amount of wheel running occurring during the period. B–D, Quantified treatment effects on circadian activity (mean ± SEM), including total wheel revolutions per day in LD (B), LD proportion (C), and fractional breakdown of total activity occurring during the first vs second half of the dark phase (relative activity ZT12–ZT24) (D). Sample size (n) is indicated below each bar. ♦, ○, ●, and ✚, denote significantly different comparisons made with CTL, LowE, HighE, and HighPPT respectively. In D, within-group differences in relative activity ZT12 to ZT24 are indicated by asterisks: *, P < .05; **, P < .001. E, Wheel running activity from 3 consecutive days in LD was averaged into 1-hour bins. ♦ and ♦♦, P < .05 and .001, respectively, compared with CTL (two-way repeated-measures ANOVA). Sample sizes per time points are as follows: CTL (n = 10–11), LowE (n = 9–10), HighE (n = 11–12), LowPPT (n = 8–9), HighPPT (n = 8), LowDPN (n = 7–8), and HighDPN (n = 7–9).
ESR1 stimulation mimics the estradiol-induced consolidation of activity to the dark
All animals housed in LD were more active during the dark phase than the light phase (Figure 2, A, C, and E). To determine whether activational estradiol is sufficient to consolidate activity to the dark phase as reported previously (20, 21, 28), we calculated the LD proportion. Both LowE (0.006 ± 0.002; P = .02) and HighE (0.004 ± 0.002; P = .008) significantly reduced the LD proportion compared with CTL (0.05 ± 0.01), indicating that nearly all wheel running took place during the dark phase in these mice. This effect was mirrored by administration of HighPPT (0.006 ± 0.002; P = .025). The LowDPN dose had no effect compared with CTL, and nominal reductions observed after either LowPPT or HighDPN administration were not significant (Figure 2C).
ESR2 activation results in a greater distribution of activity across the dark phase
Wheel running increased rapidly after lights out in all treatment groups (Figure 2, A and E). To further assess the effect of estrogen receptor activation throughout the dark phase, mean wheel revolutions per treatment group were binned in 1-hour increments and plotted over time (Figure 2E). Two-way repeated-measures ANOVA revealed a significant effect of time (F23 = 107.457, P < .001) and drug treatment (F6 = 19.022, P < .001) as well as a significant interaction (F138 = 8.706, P < .001). Overall, more wheel revolutions were completed in the first 6 hours of the dark phase compared with the later night. Additionally, mice receiving LowE, HighE, or HighPPT pellets were more active than CTL mice throughout most of the dark phase. In contrast, LowPPT animals were only more active than CTL animals from ZT14 to ZT17. HighDPN mice were not more active than CTL animals overall but did complete more wheel revolutions at ZT14, ZT15, and ZT20 compared with CTL mice. At no time were LowDPN mice more active than their CTL counterparts.
Animals treated with estradiol had sustained activity over a greater length of time than CTL animals, which completed their maximum amount of wheel running within the first 2 hours of darkness. Furthermore, although HighPPT treatment statistically recapitulated the estradiol-induced increase in total wheel running (Figure 2B), activity was consolidated to the early (ZT12–ZT18), rather than later (ZT18–ZT24), subjective night (Figure 2A). To further assess this interaction, we investigated whether ESR1 or ESR2 stimulation contribute to when activity occurs during the dark phase. The total number of wheel revolutions completed during the dark phase for each treatment group was summed and set at 1.0 (100%). The fraction of total activity completed during the first (ZT12–AT18) and second (ZT18–ZT24) half of the dark phase was determined and is shown in Figure 2D. In all treatment groups, except LowDPN, two-way repeated-measures ANOVA revealed that a greater amount of the total activity was completed from ZT12 to ZT18 than from ZT18 to ZT24. Interestingly, compared with the amount observed in the CTL group (ZT12–ZT18, 0.77 ± 0.03; ZT18–ZT24, 0.23 ± 0.03), both estradiol doses (LowE: ZT12–ZT18, 0.64 ± 0.03, P = .007; ZT18–ZT24, 0.36 ± 0.03, P = .007; HighE: ZT12–ZT18, 0.63 ± 0.04, P = .007; ZT18–ZT24, 0.37 ± 0.04, P = .007) and DPN-treated animals (LowDPN: ZT12–ZT18, 0.56 ± 0.07, P = .006; ZT18–ZT24, 0.44 ± 0.07, P = .006; HighDPN, ZT12–ZT18, 0.64 ± 0.05, P = .031; ZT18–ZT24, 0.37 ± 0.05, P = .031) completed a smaller fraction of their total wheel running in the first half of the night. Despite the difference in magnitude of activity between PPT and CTL (Figure 2B), there was no difference in the temporal distribution of activity across the dark phase between these 2 groups (Figure 2D).
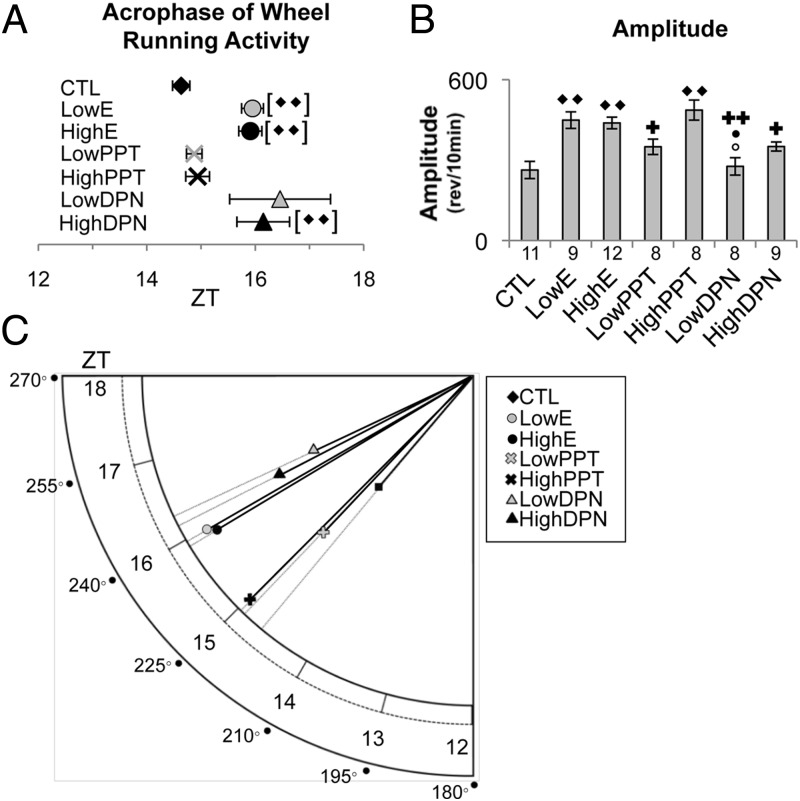
Estradiol increases amplitude and delays acrophase of wheel running activity through ESR1 and ESR2 activation, respectively
All of the mice tested demonstrated behavioral rhythmicity with clearly evident active and inactive periods (Figure 2A). To assess whether the timing and magnitude of these activity patterns were manipulated by selective estrogen receptor stimulation, we compared both the phase peak of activity (acrophase of wheel running activity) (Figure 3, A and C) and amplitude (Figure 3, B and C) across treatments. We found that ESR2 activation via DPN shifted the acrophase of wheel running activity, whereas ESR1 activation via PPT increased amplitude. There was a significant drug effect on acrophase of wheel running activity (P = .002). The acrophase for wheel running behavior (Figure 3, A and C) was delayed in animals treated with estradiol (LowE, ZT15.95 ± 0.20; HighE, ZT15.91 ± 0.21) compared with CTL (ZT14.63 ± 0.16; P < .05 for both comparisons). Post hoc analysis revealed a similar effect in animals treated with HighDPN (ZT16.15 ± 0.49; P < .05).
Figure 3.
Estradiol increases amplitude through ESR1 and delays acrophase of wheel running activity via ESR2. A, Acrophase of wheel running activity averaged over 3 days in LD across CTL, LowE, HighE, LowPPT, HighPPT, LowDPN, and HighDPN OVX female mice. Plotted between ZT12 and ZT18 (mean ± S.E.M.), the sample sizes per group are as follows: CTL (n = 10), LowE (n = 10), HighE (n = 11), LowPPT (n = 8), HighPPT (n = 8), LowDPN (n = 8), and HighDPN (n = 9). ♦♦, P < .001 compared with CTL. B, Amplitude plotted as the difference between the daily wheel running peak and mesor. Values represent the mean ± SEM. The number of mice (n) per treatment is shown below the corresponding bar. ♦, ○, ●, and ✚ denote significant comparisons made with CTL, LowE, HighE, and HighPPT, respectively; 1 symbol, P < .05; 2 symbols, P < .001. C, Polargram representation of the effects of estradiol, PPT, and DPN administration on acrophase of wheel running activity and amplitude. The radial axis value intersected by a given treatment group's vector corresponds to the acrophase of wheel running activity. Both degrees and ZT are shown. Vector length is indicative of amplitude magnitude. Variance is shown in A and B.
Amplitude (Figure 3, B and C) was higher in estradiol-treated animals compared with CTL animals (CTL, 263.14 ± 31.73 wheel revolutions; Low E, 449.06 ± 30.91 wheel revolutions; P < .001; High E, 438.57 ± 21.57 wheel revolutions; P < .001). This effect was recapitulated by treatment with HighPPT (486.52 ± 37.44 wheel revolutions; P < .001). Administration of LowPPT (349.63 ± 28.76 wheel revolutions) or either dose of DPN (LowDPN, 276.61 ± 32.34 wheel revolutions; High DPN, 350.69 ± 16.81 wheel revolutions) failed to modify amplitude compared with CTL. However, the amplitudes observed after these treatments were significantly different from those induced by HighPPT (P < .05 for all comparisons) (Figure 3B). Similarly, the amplitude observed in estradiol-treated animals was greater than that observed in LowDPN animals (P < .05). Overlapping the observed amplitude and acrophase of wheel running activity for each treatment group (Figure 3C) demonstrates the similarity between estradiol treatment and ESR2 activation with respect to acrophase of wheel running activity and, conversely, the role of ESR1 in the increased amplitude induced by LowE or HighE treatment.
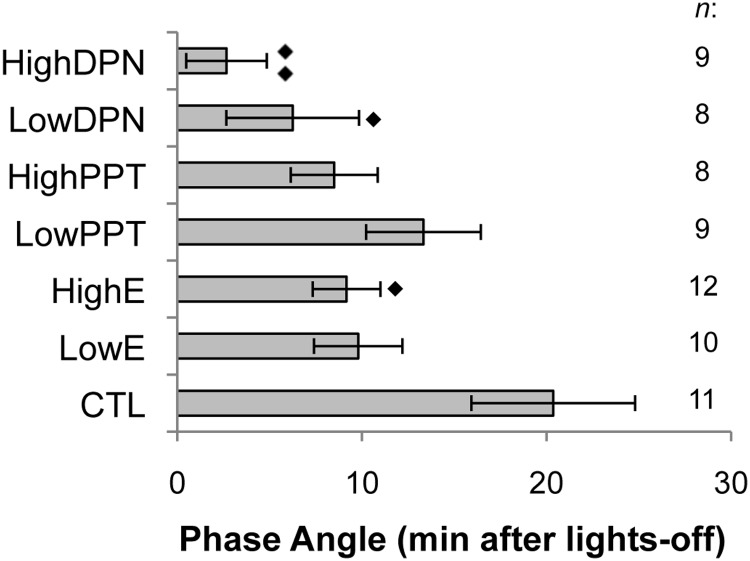
Estradiol or DPN advances activity onset
We calculated the mean phase angle of activity onset for each treatment group to determine the effects of selective estrogen receptor stimulation (Figure 4). Compared with CTL animals, in which activity began 20 ± 4 minutes after lights off, HighE mice became active significantly earlier (9 ± 1 minute after lights off; P = .0254). Similarly, both LowDPN (6 ± 3 minutes) and HighDPN pellets (2 ± 2 minutes) significantly advanced the phase of activity onset (P = .029 and .002, respectively). In contrast, selective activation of ESR1 did not alter the phase angle compared with CTL. Similarly, the low dose of estradiol had no effect on phase angle.
Figure 4.
Phase angle of activity onset (mean minutes ± SEM) for CTL, LowE, HighE, LowPPT, HighPPT, LowDPN, and HighDPN animals. Sample size per treatment group is indicated on the right. ♦ denotes comparisons made with CTL; 1 symbol, P < .05; 2 symbols, P < .001.
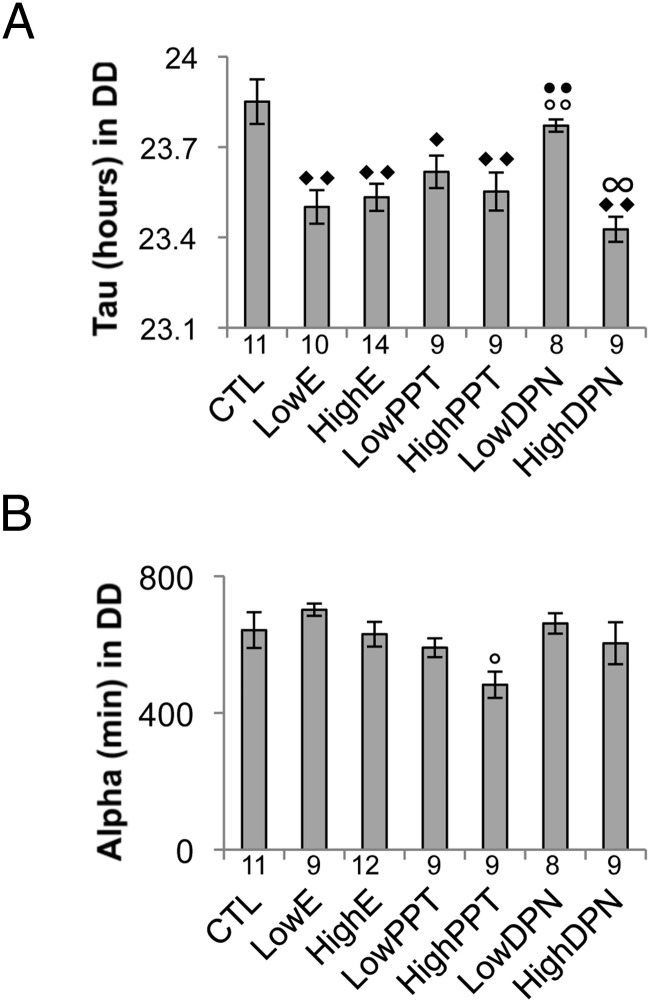
Estrogen receptor activation shortens the total period without altering the length of the active period
Under free running conditions (DD), modulation of estrogen receptor activation altered period length (Figure 5A). A one-way ANOVA revealed a significant treatment effect (P < .001). The average period length of animals treated with either dose of estradiol was shorter than that observed in CTL animals (CTL, 23.85 ± 0.07 hours; LowE, 23.50 ± 0.06 hours in LowE; HighE, 23.53 ± 0.04 hours; P < .001 for both comparisons). A similar effect was observed in animals treated with either dose of PPT (LowPPT, 23.62 ± 0.05 hours; P = .047 compared with CTL; HighPPT, 23.55 ± 0.06 hours; P = .004 compared with CTL). HighDPN (23.43 ± 0.04 hours), but not LowDPN, also decreased τ compared with CTL (P < .001). LowDPN administration had no effect on τ. However, these mice differed significantly from mice treated with LowE (P = .021), HighE (P = .04), and HighDPN (P = .002). One-way ANOVA revealed a significant drug effect on the length of the active phase (α, P = .01) (Figure 5B). However, Tukey's post hoc analysis found no difference between CTLs and any other group. The only difference observed across α was that HighPPT animals had a shorter α (482 ± 38 minutes) than animals treated with the lower dose of estrogen (702 ± 17 minutes; P < .05).
Figure 5.
Effects of estradiol, PPT, and DPN on τ and α. A, The mean free running period (τ) (± SEM). B, Average duration of the active period (α) (± SEM). Both parameters were determined from 5 days of data from animals housed in DD. Samples sizes for each group are indicated below the corresponding bar. ♦, ○, and ● and ∞ denote comparisons made with CTL, LowE, HighE, and Low DPN, respectively; 1 symbol, P < .05; 2 symbols, P < .001.
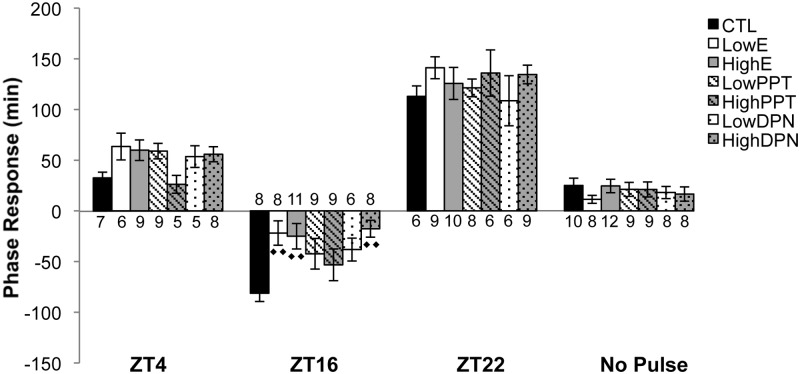
Estradiol or ESR2 activation attenuates the phase delay associated with a light pulse at ZT16
We sought to determine the impact of estradiol on behavioral phase response in response to photic stimuli. Mice housed in DD were subjected to a 1-hour pulse of light at ZT4, ZT16, or ZT22 or remained nonpulsed (Figure 6). A pulse delivered in the early subjective night at ZT16 caused a phase delay of −81 ± 8 minutes in CTL animals. Relative to CTL mice, there was a significantly dampened delay in animals administered LowE (−21 ± 12 minutes; P = .002), HighE (−25 ± 13 minutes; P = .005), or HighDPN (−17 ± 8 minutes; P < .001). Although PPT at either dose or LowDPN reduced the phase delay compared with CTL treatment, the differences were not significant. Comparisons within each treatment group revealed that CTL mice pulsed with light at ZT16 had a significantly delayed activity onset compared with nonpulsed CTL animals (P < .001). Similarly, LowPPT (P < .001), HighPPT (P < .001), LowDPN (P = .01), and HighE (P = .02) animals pulsed at ZT16 exhibited a significant phase delay compared with their nonpulsed counterparts. In contrast, the phase shift observed in LowE or HighDPN animals was not different from that observed in nonpulsed animals given the same treatment.
Figure 6.
Photic phase response of OVX female mice treated with estradiol, PPT, DPN, or CTL. Entrained animals were housed in DD for 24 hours and were either maintained in the dark (no pulse) or pulsed with light for 1 hour at a time corresponding to the previous LD schedule's ZT4, ZT16, or ZT22. Values represent the average (± SEM) relative difference between the pre- and post-pulse onset of wheel running activity. Negative values indicate a phase delay, whereas positive values signify a phase advance. Sample sizes are shown for each treatment group at each time point. ♦ denotes comparisons made with CTL; 1 symbol, P < .05; 2 symbols, P < .001.
We did not find that estradiol, PPT, or DPN resulted in a behavioral shift in response to a 1-hour light pulse administered at other times. A light pulse during the subjective day (ZT4) induced phase advances that ranged between 26 and 63 minutes, but there was no treatment effect, nor did pulsed animals differ from nonpulsed counterparts. A 1-hour pulse at ZT22, corresponding to the late subjective night, resulted in a relatively large phase advance across all groups of animals. Group means varied from 108 to 141 minutes, but there were no significant differences. Within each treatment group, the phase advance was significant relative to nonpulsed counterparts (P < .001 for all comparisons). Because the innate free running period in mice is less than the 24-hour day they are entrained to in 12:12 LD housing conditions (51), the nonpulsed sham animals were all slightly phase-advanced (range, 11–25 minutes across groups). No difference was observed between the treatment groups that did not receive a light pulse.
Discussion
We tested the hypothesis that estradiol exerts activational control over daily and circadian rhythms in female mice. Furthermore, we sought to differentiate between the roles of ESR1 and ESR2 in these behaviors. We found that ESR1 primarily regulates estradiol-induced increases in activity magnitude, whereas ESR2 controls phase and temporal distribution of activity. The total amount and amplitude of wheel running, as well as the LD proportion were modulated by estradiol through an ESR1-dependent mechanism. Conversely, ESR2 activation delayed acrophase of wheel running activity, distributed activity across the dark phase, and advanced phase angle of activity onset. Interestingly, mice exhibited a shorter τ when either ESR1 or ESR2 were stimulated. Furthermore, behavioral phase shifts in response to a light pulse in the early subjective night, but at no other time, was muted by activational estrogen signaling through a mechanism involving ESR2. These results provide novel insight into the activational effects of estradiol, increase our knowledge regarding their underlying mechanisms, and provide evidence of the differential roles played by ESR1 and ESR2.
Ogawa et al (20) demonstrated that ESR1, but not ESR2, loss of function prevents the estradiol-induced increase in wheel running activity in both males and females. Here we expand upon this work and demonstrate that ESR1 stimulation is sufficient to increase total daily wheel running. Furthermore, we observed an increased activity amplitude in mice administered estradiol or HighPPT. Because entrained mice exhibit clear peaks and troughs of activity across a single day in LD, elevations in amplitude are expected to coincide with increased total activity, assuming the animals remain rhythmic as they did here. It is possible that ESR1 stimulation increases activity levels overall, but due to masking effects, the increase is observed only in the dark phase, thus altering LD proportion. We also cannot rule out that increased wheel running itself is enough to drive activity-mediated feedback that alters outputs of the master circadian clock, located in the suprachiasmatic nucleus (SCN) (52). Regardless of the locus for controlling activity in the current study, these effects can still be considered under the control of ESR1 because the only difference across all mice tested was the drug they received.
The phase angle of activity onset relative to the time of lights-off is advanced in intact female rats on the day of estrus when serum estradiol levels peak and progesterone levels are at their minimum (17, 53). It is similarly advanced in female hamsters administered estradiol (27) but is unaffected in female Octodon degus treated with progesterone (19). Although these data support the hypothesis that increased estrogenic signaling strengthens entrainment to light (31), they do not rule out the possibility that changes in progesterone signaling may also modulate the phase angle of activity onset. Although changes in activity corresponding to the stage of the estrus cycle are well documented in rats and hamsters (17, 18, 24, 55), these rhythms have not been observed in mice. Accordingly, we did not anticipate that selective estrogen receptor stimulation would alter phase angle of activity onset in our mice. However, we found that estradiol advanced activity onset by approximately 50% in female mice. Treatment with DPN was sufficient to recapitulate this result, suggesting this is an ESR2-dependent mechanism. To our knowledge, no previous report has outlined the relationship between ESR2 and phase angle. Blattner and Mahoney (28) showed that selective removal of classical estrogen receptor signaling (mutation of the ESR1 estrogen response element-binding domain in NERKI mice) or complete loss of ESR1 failed to alter phase angle, leading to the conclusion that phase angle is not regulated by an ESR1-dependent mechanism. Our study also points to a role for ESR2-dependent distribution of activity across the dark phase, perhaps accounting for the delayed acrophase of wheel running activity observed when this receptor subtype was stimulated.
The length of the free running period under constant lighting conditions is shorter in female rats, hamsters, and humans than in males and is shorter still in females administered exogenous estradiol (10, 18, 24, 56, 57). Recent work using intact WT, αERKO, and NERKI female mice found no differences in τ, demonstrating that removal of ESR1 signaling does not alter period length (28). Interestingly, we show here that estradiol, both doses of PPT, and HighDPN all shorten τ in a similar manner. Our data suggest that stimulation of either ESR1 or ESR2 is sufficient to shorten period and that this effect is activational. Although it is possible that the shortening of τ induced by estradiol is ESR1-dependent only and that the dose of DPN administered was high enough to act on both receptors, at no time did we observe physiologic evidence of ESR1 activation in either LowDPN or HighDPN mice. For example, we did not detect corneated vaginal cells or uterine hypertrophy in mice treated with DPN. These data allude to a common regulatory mechanism for ESR1 and ESR2 control of period, but not other tested variables in which differential results were produced. Although unlikely, there also exists the possibility that a different estrogen-dependent pathway capable of using all 3 compounds as ligands, but not directly tested here, is responsible.
We also measured the length of the active period under constant darkness. Although estradiol had no effect, the higher dose of PPT shortened α compared with LowE animals. Interestingly, αERKO mice have a longer α (28), suggesting that the length of the active phase is at least partially controlled by estrogenic signaling. However, in the current study, α was not affected by circulating DPN alone, a treatment that should have been similar to αERKO animals in that only ESR2 is acted on by estradiol. Because the critical difference in these studies is the use of transgenic mice compared with acute hormone receptor activation, these data seem to support an organizational, but not activational, role for estradiol in the regulation of this circadian variable, but warrants future developmental research.
We sought to determine whether estradiol exerts an activational effect on the magnitude of light-induced behavioral phase shifting across the subjective day. We found that a light pulse in the late subjective night (ZT22) resulted in a large phase advance in all treatment groups, whereas light pulses administered in the subjective day (ZT4) had no effect (Figure 6). However, a light pulse in the early subjective evening (ZT16) resulted in a differential response dependent upon treatment. Because removal of endogenous hormone signaling truncates the behavioral phase delay in response to light in WT OVX (21) and intact αERKO females (30), we anticipated that mice treated with PPT or estradiol would have a more robust phase delay than CTL animals. Rather than a larger phase delay, we found that estradiol-treated mice had a significantly truncated response to a 1-hour light pulse at ZT16 relative to CTL mice. Animals treated with DPN also had a reduced phase delay when pulsed at ZT16 compared with CTL mice. These data suggest that estradiol exerts an activational effect on phase response to light when the pulse is administered in the early subjective night, but no other time, and that this response is likely mediated by an ESR2-dependent mechanism. Although these current findings are somewhat incongruous with previous studies, they do not take into account organizational effects that may also be impacting photic phase responsiveness. Future study is needed to determine the exclusively organizational regulation.
It remains unknown how estradiol mechanistically enacts these effects on daily and circadian activity measures, including whether estradiol acts directly or indirectly on central oscillators. Multiple types of estrogen receptors are expressed within structures that input directly or indirectly onto the SCN (7, 58–61) as well as within the SCN itself. ESR1 and ESR2 are localized to the SCN in rats and humans (62, 63), although their expression is relatively small in mice (64). There is evidence that GABAergic neurons within the SCN express estrogen receptors, especially ESR2 (62), and that estradiol administration enhances SCN neuronal excitability (66). Furthermore, pharmacologic GABAA or GABAB receptor agonists injected into the SCN of nocturnal or diurnal rodents dampens phase shifting in response to a light pulse in the early subjective night (54, 65, 67). Although not directly tested here, these data combined with our current findings support the possibility that estradiol blocks the phase delay induced by a ZT16 light pulse through an ESR2-mediated mechanism that drives excitability, resulting in greater GABA release, and subsequent GABAA and GABAB receptor binding. Further work is needed to assess the validity of this model. Importantly, the mechanism and the locus of action are likely dependent on the specific measure being examined. For example, previous work suggests that estradiol's effect on total wheel running involves the medial preoptic area (20, 63–65), but the involvement of additional structures and downstream pathways in this and the other measures reported upon here remain work for future study.
Conclusions
We demonstrate here that estradiol increases total activity and amplitude, reduces the LD proportion, delays acrophase of wheel running activity, shortens τ, and dampens the light pulse-induced phase shift in wheel running in the early subjective night. Importantly, activational stimulation of ESR1 or ESR2 differentially impacts daily and circadian activity outputs. ESR1 regulates estradiol-associated elevations in total wheel running activity and amplitude, while also consolidating activity to the dark phase. Conversely, ESR2 stimulation more significantly mediates the timing of activity, including sustaining more constant levels of activity across the active period, delaying acrophase of wheel running activity, advancing phase angle, and occluding the phase response associated with a pulse of light in the early subjective night. Together, our data contribute to a more complete understanding of the activational influence of estradiol on the expression of daily and circadian rhythms in female mice. Furthermore, we suggest, for the first time, distinct mechanisms by which ESR1 and ESR2 activity shape biorhythmic behavior.
Acknowledgments
We are grateful to members of the Mahoney laboratory for assistance with animal husbandry and experimental support as well as Dr A. Parent and Mr I. J. Bourgeois for fruitful discussion and critical reading of this manuscript.
This work was supported by the University of Illinois Urbana-Champaign Research Board (to M.M.M.), a Neuroscience Scholars Program Predoctoral Fellowship (to S.E.R.), and U.S. Public Health Service Grant 5R01DK015556 (to J.A.K.).
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the University of Illinois Urbana-Champaign Research Board (to M.M.M.), a Neuroscience Scholars Program Predoctoral Fellowship (to S.E.R.), and U.S. Public Health Service Grant 5R01DK015556 (to J.A.K.).
Footnotes
- CTL
- control
- DD
- constant darkness
- DPN
- 2,3-bis[4-hydroxyphenyl]-propionitrile
- ESR1
- estrogen receptor 1
- LD
- light/dark
- OVX
- ovariectomy
- PPT
- 4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol
- SCN
- suprachiasmatic nucleus
- WT
- wild-type
- ZT
- Zeitgeber time.
References
- 1. Turek FW, Dugovic C, Zee PC. Current understanding of the circadian clock and the clinical implications for neurological disorders. Arch Neurol. 2001;58(11):1781–1787. [DOI] [PubMed] [Google Scholar]
- 2. Wirz-Justice A. Chronobiology and mood disorders. Dialogues Clin Neurosci. 2003;5(4):315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosenwasser AM. Circadian clock genes: non-circadian roles in sleep, addiction, and psychiatric disorders? Neurosci Biobehav Rev. 2010;34(8):1249–1255. [DOI] [PubMed] [Google Scholar]
- 4. Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9(10):764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reddy AB, O'Neill JS. Healthy clocks, healthy body, healthy mind. Trends Cell Biol. 2010;20(1):36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karatsoreos IN. Effects of circadian disruption on mental and physical health. Curr Neurol Neurosci Rep. 2012;12(2):218–225. [DOI] [PubMed] [Google Scholar]
- 7. Bailey M, Silver R. Sex differences in circadian timing systems: Implications for disease. Front Neuroendocrinol. 2014;35(1):111–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kivimäki M, Virtanen M, Elovainio M, Väänänen A, Keltikangas-Järvinen L, Vahtera J. Prevalent cardiovascular disease, risk factors and selection out of shift work. Scand J Work Environ Health. 2006;32(3):204–208. [DOI] [PubMed] [Google Scholar]
- 9. Karatsoreos IN, Silver R. The neuroendocrinology of the suprachiasmatic nucleus as a conductor of body time in mammals. Endocrinology. 2007;148(12):5640–5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duffy JF, Cain SW, Chang AM, et al. . Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci U S A. 2011;108(Suppl 3):15602–15608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mong JA, Baker FC, Mahoney MM, et al. . Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J Neurosci. 2011;31(45):16107–16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuljis DA, Loh DH, Truong D, et al. . Gonadal- and sex-chromosome-dependent sex differences in the circadian system. Endocrinology. 2013;154(4):1501–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morin LP, Cummings LA. Splitting of wheelrunning rhythms by castrated or steroid treated male and female hamsters. Physiol Behav. 1982;29(4):665–675. [DOI] [PubMed] [Google Scholar]
- 14. Baker FC, Kahan TL, Trinder J, Colrain IM. Sleep quality and the sleep electroencephalogram in women with severe premenstrual syndrome. Sleep. 2007;30(10):1283–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahoney MM. Shift work, jet lag, and female reproduction. Int J Endocrinol. 2010;2010:813764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shechter A, Boivin DB. Sleep, hormones, and circadian rhythms throughout the menstrual cycle in healthy women and women with premenstrual dysphoric disorder. Int J Endocrinol. 2010;2010:259345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wollnik F, Turek FW. Estrous correlated modulations of circadian and ultradian wheel-running activity rhythms in LEW/Ztm rats. Physiol Behav. 1988;43(3):389–396. [DOI] [PubMed] [Google Scholar]
- 18. Albers HE. Gonadal hormones organize and modulate the circadian system of the rat. Am J Physiol. 1981;241(1):R62–R66. [DOI] [PubMed] [Google Scholar]
- 19. Labyak SE, Lee TM. Estrus- and steroid-induced changes in circadian rhythms in a diurnal rodent, Octodon degus. Physiol Behav. 1995;58(3):573–585. [DOI] [PubMed] [Google Scholar]
- 20. Ogawa S, Chan J, Gustafsson JA, Korach KS, Pfaff DW. Estrogen increases locomotor activity in mice through estrogen receptor α: specificity for the type of activity. Endocrinology. 2003;144(1):230–239. [DOI] [PubMed] [Google Scholar]
- 21. Brockman R, Bunick D, Mahoney MM. Estradiol deficiency during development modulates the expression of circadian and daily rhythms in male and female aromatase knockout mice. Horm Behav. 2011;60(4):439–447. [DOI] [PubMed] [Google Scholar]
- 22. Rodier WI. Progesterone-estrogen interactions in the control of activity-wheel running in the female rat. J Comp Physiol Psychol. 1971;74(3):365–373. [DOI] [PubMed] [Google Scholar]
- 23. Gentry RT, Wade GN. Sex differences in sensitivity of food intake, body weight, and running-wheel activity to ovarian steroids in rats. J Comp Physiol Psychol. 1976;90(8):747–754. [DOI] [PubMed] [Google Scholar]
- 24. Morin LP, Fitzgerald KM, Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science. 1977;196(4287):305–307. [DOI] [PubMed] [Google Scholar]
- 25. Zucker I, Fitzgerald KM, Morin LP. Sex differentiation of t-e circadian system in the golden hamster. Am J Physiol. 1980;238(1):R97–R101. [DOI] [PubMed] [Google Scholar]
- 26. Blattner MS, Mahoney MM. Estrogen receptor 1 modulates circadian rhythms in adult female mice [published online February 14, 2014]. Chronobiol Int. doi:10.3109/07420528.2014.885528. [DOI] [PubMed] [Google Scholar]
- 27. Morin LP, Fitzgerald KM, Rusak B, Zucker I. Circadian organization and neural mediation of hamster reproductive rhythms. Psychoneuroendocrinology. 1977;2(1):73–98. [DOI] [PubMed] [Google Scholar]
- 28. Blattner MS, Mahoney MM. Circadian parameters are altered in two strains of mice with transgenic modifications of estrogen receptor subtype 1. Genes Brain Behav. 2012;11(7):828–836. [DOI] [PubMed] [Google Scholar]
- 29. Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127(19):4277–4291. [DOI] [PubMed] [Google Scholar]
- 30. Blattner MS, Mahoney MM. Photic phase-response curve in 2 strains of mice with impaired responsiveness to estrogens. J Biol Rhythms. 2013;28(4):291–300. [DOI] [PubMed] [Google Scholar]
- 31. Gorman MR, Lee TM. Hormones and biological rhythms. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, eds. Behavioral Endocrinology. 2nd ed Cambridge, MA: Massachusetts Institute of Technology; 2002:451–494. [Google Scholar]
- 32. Sun J, Meyers MJ, Fink BE, Rajendran R, Katzenellenbogen JA, Katzenellenbogen BS. Novel ligands that function as selective estrogens or antiestrogens for estrogen receptor-alpha or estrogen receptor-β. Endocrinology. 1999;140(2):800–804. [DOI] [PubMed] [Google Scholar]
- 33. Stauffer SR, Coletta CJ, Tedesco R, et al. . Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-α-selective agonists. J Med Chem. 2000;43(26):4934–4947. [DOI] [PubMed] [Google Scholar]
- 34. Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-β potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44(24):4230–4251. [DOI] [PubMed] [Google Scholar]
- 35. Carroll VM, Jeyakumar M, Carlson KE, Katzenellenbogen JA. Diarylpropionitrile (DPN) enantiomers: synthesis and evaluation of estrogen receptor β-selective ligands. J Med Chem. 2012;55:528–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carroll JC, Pike CJ. Selective estrogen receptor modulators differentially regulate Alzheimer-like changes in female 3xTg-AD mice. Endocrinology. 2008;149(5):2607–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Frasor J, Barnett DH, Danes JM, Hess R, Parlow AF, Katzenellenbogen BS. Response-specific and ligand dose-dependent modulation of estrogen receptor (ER) α activity by ERβ in the uterus. Endocrinology. 2003;144(7):3159–3166. [DOI] [PubMed] [Google Scholar]
- 38. Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us [published erratum appears in Endocr Rev. 1999;20(4):459]? Endocr Rev. 1999;20(3):358–417. [DOI] [PubMed] [Google Scholar]
- 39. Harris HA, Katzenellenbogen JA, Katzenellenbogen BS. Characterization of the biological roles of the estrogen receptors, ERα and ERβ, in estrogen target tissues in vivo through the use of an ERα-selective ligand. Endocrinology. 2002;143(11):4172–4177. [DOI] [PubMed] [Google Scholar]
- 40. Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-β on anxiety-related behaviors. Endocrinology. 2005;146(2):797–807. [DOI] [PubMed] [Google Scholar]
- 41. Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor β knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol Learn Mem. 2008;89(4):513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile decrease anxiety-like behavior of wildtype, but not estrogen receptor beta knockout, mice. Behav Neurosci. 2008;122(5):974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sánchez-Criado JE, Martín De Las Mulas J, et al. . Biological role of pituitary estrogen receptors ERα and ERβ on progesterone receptor expression and action and on gonadotropin and prolactin secretion in the rat. Neuroendocrinology. 2004;79(5):247–258. [DOI] [PubMed] [Google Scholar]
- 44. Sánchez-Criado JE, Trudgen K, Millán Y, et al. . Estrogen receptor (ESR) 2 partially offsets the absence of ESR1 in gonadotropes of pituitary-specific Esr1 knockout female mice. Reproduction. 2012;143(4):549–558. [DOI] [PubMed] [Google Scholar]
- 45. Ogura Y, Ishii K, Kanda H, et al. . Bisphenol A induces permanent squamous change in mouse prostatic epithelium. Differentiation. 2007;75(8):745–756. [DOI] [PubMed] [Google Scholar]
- 46. Risbridger GP, Wang H, Frydenberg M, Cunha G. The metaplastic effects of estrogen on mouse prostate epithelium: proliferation of cells with basal cell phenotype. Endocrinology. 2001;142(6):2443–2450. [DOI] [PubMed] [Google Scholar]
- 47. Refinetti R. The circadian rhythm of body temperature. Front Biosci (Landmark Ed). 2010;15:564–594. [DOI] [PubMed] [Google Scholar]
- 48. Agostino PV, Plano SA, Golombek DA. Sildenafil accelerates reentrainment of circadian rhythms after advancing light schedules. Proc Natl Acad Sci U S A. 2007;104:9834–9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ogeil RP, Rajaratnam SM, Redman JR, Broadbear JH. Acute MDMA administration alters the distribution and circadian rhythm of wheel running activity in the rat. Brain Res. 2010;1359:128–136. [DOI] [PubMed] [Google Scholar]
- 50. Mrosovsky N. Methods of measuring phase shifts: why I continue to use an Aschoff type II procedure despite the skepticism of referees. Chronobiol Int. 1996;13(5):387–392. [DOI] [PubMed] [Google Scholar]
- 51. Refinetti R. Daily activity patterns of a nocturnal and a diurnal rodent in a seminatural environment. Physiol Behav. 2004;82(2–3):285–294. [DOI] [PubMed] [Google Scholar]
- 52. van Oosterhout F, Lucassen EA, Houben T, vanderLeest HT, Antle MC, Meijer JH. Amplitude of the SCN clock enhanced by the behavioral activity rhythm. PLoS One. 2012;7:e39693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wood GA, Fata JE, Watson KL, Khokha R. Circulating hormones and estrous stage predict cellular and stromal remodeling in murine uterus. Reproduction. 2007;133:1035–1044. [DOI] [PubMed] [Google Scholar]
- 54. Gillespie CF, Mintz EM, Marvel CL, Huhman KL, Albers HE. GABA(A) and GABA(B) agonists and antagonists alter the phase-shifting effects of light when microinjected into the suprachiasmatic region. Brain Res. 1997;759(2):181–189. [DOI] [PubMed] [Google Scholar]
- 55. Fitzgerald K, Zucker I. Circadian organization of the estrous cycle of the golden hamster. Proc Natl Acad Sci U S A. 1976;73:2923–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Davis FC, Darrow JM, Menaker M. Sex differences in the circadian control of hamster wheel-running activity. Am J Physiol. 1983;244(1):R93–R105. [DOI] [PubMed] [Google Scholar]
- 57. Schull J, Walker J, Fitzgerald K, et al. . Effects of sex, thyro-parathyroidectomy, and light regime on levels and circadian rhythms of wheel-running in rats. Physiol Behav. 1989. September;46(3):341–346. [DOI] [PubMed] [Google Scholar]
- 58. King JM. Effects of lesions of the amygdala, preoptic area, and hypothalamus on estradiol-induced activity in the female rat. J Comp Physiol Psychol. 1979;93(2):360–367. [DOI] [PubMed] [Google Scholar]
- 59. Fahrbach SE, Meisel RL, Pfaff DW. Preoptic implants of estradiol increase wheel running but not the open field activity of female rats. Physiol Behav. 1985;35(6):985–992. [DOI] [PubMed] [Google Scholar]
- 60. Takeo T, Sakuma Y. Diametrically opposite effects of estrogen on the excitability of female rat medial and lateral preoptic neurons with axons to the midbrain locomotor region. Neurosci Res. 1995;22(1):73–80. [DOI] [PubMed] [Google Scholar]
- 61. Abizaid A, Mezei G, Thanarajasingam G, Horvath TL. Estrogen enhances light-induced activation of dorsal raphe serotonergic neurons. Eur J Neurosci. 2005;21(6):1536–1546. [DOI] [PubMed] [Google Scholar]
- 62. Su JD, Qiu J, Zhong YP, Chen YZ. Expression of estrogen receptor -α and -β immunoreactivity in the cultured neonatal suprachiasmatic nucleus: with special attention to GABAergic neurons. Neuroreport. 2001;12(9):1955–1959. [DOI] [PubMed] [Google Scholar]
- 63. Kruijver FP, Swaab DF. Sex hormone receptors are present in the human suprachiasmatic nucleus. Neuroendocrinology. 2002;75(5):296–305. [DOI] [PubMed] [Google Scholar]
- 64. Vida B, Hrabovszky E, Kalamatianos T, Coen CW, Liposits Z, Kalló I. Oestrogen receptor α and β immunoreactive cells in the suprachiasmatic nucleus of mice: distribution, sex differences and regulation by gonadal hormones. J Neuroendocrinol. 2008;20(11):1270–1277. [DOI] [PubMed] [Google Scholar]
- 65. Novak CM, Albers HE. Circadian phase alteration by GABA and light differs in diurnal and nocturnal rodents during the day. Behav Neurosci. 2004;118(3):498–504. [DOI] [PubMed] [Google Scholar]
- 66. Fatehi M, Fatehi-Hassanabad Z. Effects of 17β-estradiol on neuronal cell excitability and neurotransmission in the suprachiasmatic nucleus of rat. Neuropsychopharmacology. 2008;33(6):1354–1364. [DOI] [PubMed] [Google Scholar]
- 67. Gillespie CF, Huhman KL, Babagbemi TO, Albers HE. Bicuculline increases and muscimol reduces the phase-delaying effects of light and VIP/PHI/GRP in the suprachiasmatic region. J Biol Rhythms. 1996;11(2):137–144. [DOI] [PubMed] [Google Scholar]