Abstract
The mutual interplay between energy homeostasis and bone metabolism is an important emerging concept. Ghrelin and leptin antagonize each other in regulating energy balance, but the role of this interaction in bone metabolism is unknown. Using ghrelin receptor and leptin-deficient mice, we show that ghrelin has dual effects on osteoclastogenesis, inhibiting osteoclast progenitors directly and stimulating osteoclastogenesis via a more potent systemic/central pathway. Using mice with combined ghrelin receptor and leptin deficiency, we find that this systemic osteoclastogenic activity is suppressed by leptin, thus balancing the two counterregulatory ghrelin pathways and leading to an unchanged bone structure. With aging, this osteoclastogenic ghrelin pathway is lost, unmasking the direct protective effect of ghrelin on bone structure. In conclusion, we identify a novel regulatory network linking orexigenic and anorectic metabolic factors with bone metabolism that is age dependent.
There is now strong evidence that skeletal turnover is coupled with energy metabolism. The mechanism by which these processes are linked involves the adipose tissue-derived factor leptin (1, 2). Leptin acts as a signal from fat, by which bone can adapt to alterations in body weight and metabolism caused by changes in adiposity. Various routes of leptin action on bone have been described. Leptin can act directly on osteoblasts and osteoclasts to increase cortical bone mass (3), and leptin effects may also be mediated via the GH-IGF axis (4). In addition, leptin can act via neuro-endocrine circuits by both inhibiting bone resorption via stimulation of hypothalamic cocaine- and amphetamine-regulated transcript (CART) expression (5) and stimulating bone resorption and inhibiting bone formation via central circuits that stimulate sympathetic nervous system (SNS) function (5, 6). It has also been determined that there is a feed-forward regulation loop from osteoblasts involving osteocalcin that enhances pancreatic insulin production, modulates insulin sensitivity at peripheral sites, and increases energy expenditure (7). These processes enable interplay between the regulation of skeletal and energy metabolism and body weight.
An important counterregulator of leptin action is the gut-derived peptide hormone ghrelin, interacting predominantly at hypothalamic agouti-related protein/neuropeptide Y neurons of the arcuate nucleus, where both ghrelin [GH secretagogue receptor (GHS-R)] and leptin receptors are expressed (8). Ghrelin modulates long-term energy homeostasis by stimulating weight gain (9) and has direct effects on the pancreas to suppress insulin secretion, together leading to worsened glucose homeostasis (e.g. Ref. 10), although this appears to be an important beneficial function of ghrelin under conditions of starvation (11). We have exemplified some of these effects in mouse models of ghrelin (Ghrl) and ghrelin receptor (Ghsr) deficiency (12). We found that age plays an important role in the function of the ghrelin axis. Ghsr deficiency, in particular, prevents the deleterious effects of aging on glucose, lipid, and energy metabolism. Relatively little is known about the impact of ghrelin on bone metabolism. We and others have shown a direct effect of ghrelin on osteoblasts in vitro (13, 14) and that infusion of ghrelin stimulates bone growth in vivo, independently of GH (15).
Despite a well-characterized antagonistic interaction between leptin and ghrelin in the control of energy metabolism, the interplay between these factors in regulating bone metabolism is unexplored. Therefore, we focused on the role of ghrelin and its interaction with leptin in regulating bone metabolism, with the aim of describing more completely the network linking nutrition and energy homeostasis to bone metabolism. These studies provide for the first time evidence that the orexigenic factor ghrelin and the anorectic factor leptin interact in the control of bone metabolism and demonstrate the impact of age on this interaction.
Materials and Methods
Construction of the mice
Ghsr−/− mice were constructed as described by Sun et al. (16). ob/ob.Ghsr−/− mice were obtained using similar methods as described for ob/ob.Ghrl−/− by Sun et al. (17). Mice were backcrossed at least 10 times, so that they were greater than 99.9% congenic with C57BL/6J wild type (WT). Mice were housed at 22 C three to four per cage in a room with a 12-h light, 12-h dark cycle and ad libitum access to food and water. All analyses described were performed using bones from male mice at 3 and 6 months. Baylor College of Medicine Animal Care and Use Committee approved all animal protocols.
Ex vivo osteoclast and osteoblast cultures
Male mice (n = 4 per genotype) were killed with a lethal dose of isoflurane (Nicholas Piramal India Ltd., Mumbai, India). Both tibiae and one femur were cleaned of soft tissue, cut open at the distal ends, and placed in an Eppendorf tube. Bone marrow was removed by centrifugation (5 min at 1500 rpm at 4 C), and subsequently, erythrocytes were lysed with a hypoosmotic buffer (155 mm NH4Cl, 10 mm KHCO3, and 0.1 mm EDTA). Cells were washed, counted, and seeded in six-replicate cultures from each of four mice per genotype for 6 d in the presence of 30 ng/ml recombinant macrophage colony-stimulating factor (R&D Systems, Minneapolis, MI) and 20 ng/ml recombinant murine receptor activator of nuclear factor κB ligand (RANKL) (R&D Systems), and the media were refreshed at d 3 (18). At the end of the culture period, cells were washed with PBS, fixed in PBS-buffered formalin (10% vol/vol), and stored at 4 C for tartrate-resistant acid phosphatase (TRAP) staining, as described previously (18). Osteoclast numbers in the bone marrow cultures were determined using ImageJ software (version 1.41; http://rsbweb.nih.gov/ij/).
Osteoblasts were grown in four independent cultures derived from four mice per genotype for 9 d in the presence of 100 μm vitamin C and 10 mm β-glycerophosphate. At the end of the cultures, cells were washed with PBS, fixed in 70% ethanol, and stored at 4 C for alkaline phosphatase (ALP) staining as previously described (18).
Murine ghrelin and leptin were obtained from Phoenix Pharmaceuticals (Burlingame, CA). Osteoclast and osteoblast cultures were treated with murine acylated ghrelin and/or leptin for 6 or 9 d, respectively.
Bone structural analysis
Left femurs (3 months, n = 4–9 per genotype) were fixed in formalin and scanned with a voxel size of 8.88 μm using the Skyscan 1076 x-ray microtomograph (Skyscan, Aartselaar, Belgium). X-ray power and tube current were 40 kV and 0.25 mA, respectively. Left femurs (6 months, n = 4 per genotype) were scanned with a voxel size of 7.81 μm using the Skyscan 1072 x-ray microtomograph (Skyscan). X-ray power and tube current were 80 kV and 0.1 mA, respectively. In both scanners, beam hardening was reduced using a 1-mm aluminum filter. To distinguish calcified tissue from noncalcified tissue, the reconstructed greyscale images were segmented by an automated algorithm using local thresholds (19). The femoral head and diaphysis were chosen as the regions of interest to study trabecular and cortical bone, respectively. The trabeculae and cortex were separated using in-house-developed automated software. The following three-dimensional (3D) bone morphometric parameters were determined using the freely available software package 3D-Calculator (http://www.erasmusmc.nl/47460/386156/Downloads): cortical volume (Ct.V), cortical thickness (Ct.Th), endocortical volume (Ec.V), trabecular bone volume fraction [bone volume/tissue volume (BV/TV)], trabecular BV, trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular connectivity density (Conn.D). Perimeter was determined using Matlab software (MathWorks, Natick, NA). Moment of inertia (MOI) is a proxy for mechanical bone strength (resistance to rotation) and was determined using PrStackBot-New (in-house-developed software; Orthopaedic Research Laboratory, Erasmus MC).
Collection of femurs and serum
After microcomputed tomography (μCT), left femurs (3 months, n = 4 per genotype) were dehydrated and embedded in paraffin. Of these embedded bones, 6-μm sections were cut. Serum was collected from the mice (n = 4 per genotype), and serum procollagen type 1 amino-terminal propeptide (P1NP) was determined using the Rat/Mouse P1NP EIA assay (Immunodiagnostics Systems Ltd., Scottsdale, AZ).
RNA isolation, cDNA synthesis, and real-time PCR
Osteoclast and osteoblast cultures were scraped in 100-μl TRIzol (Invitrogen, Breda, The Netherlands), and total RNA was isolated according to the manufacturer's protocol. RNA isolation and cDNA synthesis were performed as described earlier (20). Expression levels of the genes listed in Table 1 were quantified by SybrGreen real-time PCR using an Applied Biosystems Prism 7700 sequence detection system. The different primers for the genes of interest were designed with Primer Express software (Applied Biosystems, Foster City, CA) and in every case spanned introns to prevent amplification of genomic DNA. Data (n = at least 4 per genotype or treatment) are presented as relative mRNA levels calculated by the formula: relative expression = 2 ^ (CtGapdh − CtGene of interest).
Table 1.
Oligonucleotide sequences
| Gene | Description | Primers (5′-3′) |
|---|---|---|
| Acp5 | Acid phosphatase 5, tartrate resistant (TRAP) | Fwd. AAGAACTTGCGACCATTGTTAGC |
| Rev. CCTGAAGATACTGCAGGTTGTGG | ||
| Ctsk | Cathepsin K | Fwd. TGATGAAAATTGTGACCGTGATAA |
| Rev. CTCTCTCCCCAGCTGTTTTTAATTA | ||
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase | Fwd. CCTGGAGAAACCTGCCAAGTAT |
| Rev. GGTCCTCAGTGTAGCCCAAGAT | ||
| Ghrl | Ghrelin | Fwd. CATGGCAGGCTCCAGCTT |
| Rev. TGACCCCCGTGCTTTCCTTCTCCT | ||
| Ghsr | GHS-R | Fwd. AAACAGACAGTGAAGATGCTTGCT |
| Rev. GGTGGTGTTTGCTTTCATCCTCTGCTG | ||
| Tcirg1 | T-cell, immune regulator 1, ATPase, H+ transporting, lysosomal V0 protein A | Fwd. TCAGATCCTAAGCCGAAGTTGAG |
| Rev. GACCAAGGCCACCTCTTCAC | ||
| Tm7sf4 | Transmembrane 7 superfamily member 4 (dendritic cell-specific transmembrane protein) | Fwd. ACTACGTGGAGAGAAGCAAGGAA |
| Rev. AGGAATGCAGCTCGGTTCAA | ||
| Tnfsf11 | RANKL | Fwd. CTGAGGCCCAGCCATTTG |
| Rev. CTTGGCCCAGCCTCGAT | ||
| Tnfrsf11b | Osteoprotegerin | Fwd. TGGAGATCGAATTCTGCTTGAA |
| Rev. ATACAGGGTGCTTTCGATGAAGTC | ||
| Runx2 | Runt-related transcription factor 2 | Fwd. GACCCAAGGCAGTGATGGA |
| Rev. TCGGACAATACTGTAGGGAATTTTC | ||
| Alpl | ALP, liver/bone/kidney | Fwd. AAGTGCGGTGCAAACTTTCT |
| Rev. TCTCGGTGGCTGGTAGTGA |
Fwd., Forward; Rev., reverse.
TRAP staining
After μCT assessment, femurs (n = 4–9) were fixed in 10% formalin, dehydrated, embedded in paraffin, and 6-mm sections were cut. After deacrylation, these were first incubated in 0.2 m Tris buffer (pH 9.0) for 90 min to reactivate TRAP in the sections and then rinsed in 0.2 m sodium acetate and 50 mm tartaric acid for 5 min as described previously (21). Naphthol AS-MX (0.5 mg/ml) and 1.1 mg/ml Fast red TR salt (both from Sigma, St. Louis, MO) were added and the tibial and femoral (3 months, n = 4 per genotype) sections incubated for 2 h at 37 C. Counterstaining was performed with hematoxylin for 5 sec, and after air-drying, the sections were embedded in Permount (Thermo Fischer Scientific, Waltham, MA). Fixed cells were stained for TRAP with an acid phosphatase leukocyte kit (Sigma) with small modifications. In particular, to visualize osteoclasts specifically, we used a 1 m tartrate solution instead of the supplied 0.33 m solution. Osteoclast numbers (TRAP positive) in the bone sections were determined using Bioquant Novoprime digital-imaging software (Bioquant, Nashville, TN). After the ex vivo cultures, osteoclasts that were TRAP positive and contained at least two nuclei were analyzed using ImageJ software.
Statistics
In all experiments values are expressed as mean ± sem. Differences between groups were tested for significance by using Student's t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001). Interaction between Ghsr and ob/ob or ghrelin and leptin effects were tested for significance using a two-way ANOVA (#, P < 0.05; ##, P < 0.01; ###, P < 0.001).
Results
Ghrelin-mediated inhibition of osteoclastogenesis and reduction in bone loss is age dependent
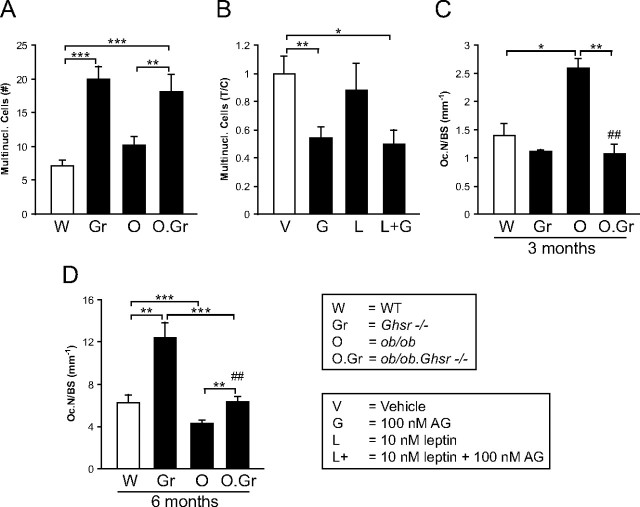
mRNA of ghrelin (encoded by Ghrl) and its receptor (encoded by Ghsr) were expressed in osteoclasts and osteoblasts and expression declined with age (data not shown). To clarify the role of ghrelin in osteoblastogenesis and osteoclastogenesis, we cultured osteoblasts and osteoclasts from bone marrow of 3-month-old WT and GHS-R-deficient (Ghsr−/−) mice. Deletion of ghrelin signaling had no impact on osteoblastogenic potential of bone marrow-derived cells, because the number and size of ALP-positive colonies, and Runx2 and Alpl expression was unaffected in Ghsr−/− cultures (Table 2 and Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). The number of multinucleated osteoclasts was increased in Ghsr−/− cultures (Fig. 1A and Table 2) as well as the expression of genes necessary for osteoclast differentiation, including dendritic cell-specific transmembrane protein (Tm7sf4), a key factor during osteoclast fusion, and Cathepsin K (Ctsk), d2 isoform of vacuolar ATPase Vo domain (Tcirg1), and TRAP (Acp5) were significantly increased (Table 2). Taken together, deletion of GHS-R increased osteoclast differentiation and fusion without affecting osteoblastogenesis. The expression of the proosteoclastogenic RANKL (Tnfrsf11) and the antiosteoclastogenic osteoprotegerin (Tnfrsf11b) was not significantly different between WT and Ghsr−/− osteoblasts (Table 2), indicating that the inhibitory effect of ghrelin on osteoclastogenesis is primarily direct and not via the osteoblast. Ghrelin treatment of WT cultures caused a 48% (P < 0.002) decrease in number of multinucleated cells, supporting our observations in Ghsr−/− cultures (Fig. 1B). Again in accord with observations on Ghsr−/− osteoblasts, ghrelin treatment did not change osteoblastogenesis (data not shown). In conclusion, ghrelin directly inhibits osteoclastogenesis at 3 months of age.
Table 2.
Osteoclastogenesis in WT and Ghsr−/− mice
| WT | Ghsr−/− | P | |
|---|---|---|---|
| Multinucleated cells (no.) | 7.17 (0.863) | 20.00 (1.806) | c |
| Mononucleated cells (no.) | 11.62 (1.73) | 6.7 (0.68) | a |
| Tm7sf4 mRNA | 0.022 (0.004) | 0.052 (0.006) | b |
| Ctsk mRNA | 0.447 (0.062) | 0.944 (0.083) | b |
| Tcirg1 mRNA | 0.008 (0.003) | 0.115 (0.063) | ns |
| Acp5 mRNA | 0.181 (0.070) | 0.469 (0.068) | a |
Data are shown as average (sem), mRNA expression is corrected for Gapdh. Differences between WT and Ghsr−/− were tested for significance by using Student's t test. ns, Not significant.
P < 0.05.
P < 0.01.
P < 0.001.
Fig. 1.
Ex vivo multinucleated osteoclast formation in bone marrow cultures of 3-month-old WT, Ghsr−/−, ob/ob, and ob/ob.Ghsr−/− mice (A) and of WT mice treated with 100 nm ghrelin (AG), 10 nm leptin, or a combination (B). Number of osteoclasts in distal metaphysis of 3-month-old (C) and 6-month-old (D) male WT, Ghsr−/−, ob/ob, and ob/ob.Ghsr−/− mice. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
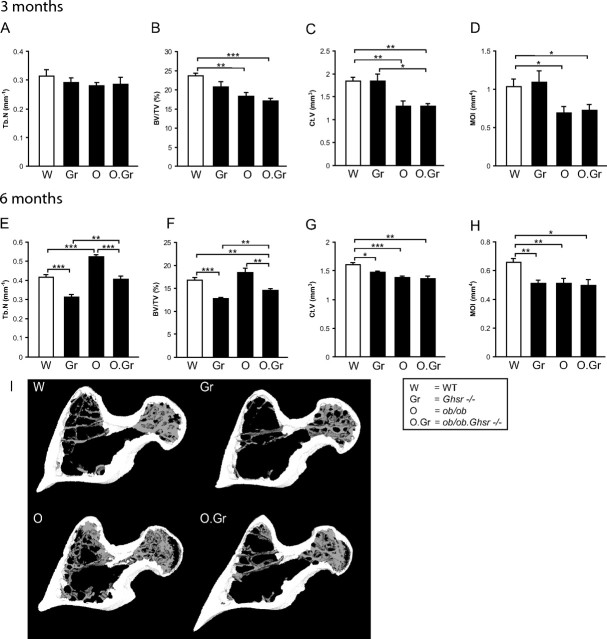
Deletion of GHS-R had no effect on cortical or trabecular bone structural parameters (Fig. 2, A–D, and Table 3). Although bone marrow cells from 3-month-old Ghsr−/− mice have increased osteoclastogenic potential ex vivo, the absence of a bone phenotype in vivo suggests that this effect is inhibited at this age. Furthermore, in contrast to the ex vivo observations, no differences in TRAP-positive osteoclast number between WT and Ghsr−/− mice were detected in femoral metaphyseal sections (Fig. 1C). Serum levels of the bone formation marker P1NP were unchanged, indicating that bone formation is unaffected by GHS-R deficiency (Table 3).
Fig. 2.
Bone morphometric parameters as determined by μCT in the femoral diaphysis and head of 3-month-old (A–D) and 6-month-old (E–H) male WT, Ghsr−/−, ob/ob, and ob/ob.Ghsr−/− mice. A and E, Tb.N. B and F, Trabecular bone volume fraction (BV/TV). C and G, Ct.V. D and H, Polar MOI. I, Representative slices through the femoral head, neck, and lesser trochanter from 3D μCT models to visualize differences in trabecular structure of 6-month-old WT, Ghsr−/− mice, ob/ob, and ob/ob.Ghsr−/− mice. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Table 3.
Bone microarchitecture and bone formation in 3-month-old WT, Ghsr−/−, ob/ob, and ob/ob.Ghsr−/− mice
| WT | Ghsr−/− | P | ob/ob | P | ob/ob.Ghsr−/− | P | |
|---|---|---|---|---|---|---|---|
| Cortical bone | |||||||
| Ct.Th (μm) | 261 (6.48) | 253 (10.5) | ns | 210 (9.56) | b | 211 (4.63) | c |
| Ec.V (mm3) | 1.52 (0.04) | 1.63 (0.07) | ns | 1.63 (0.04) | ns | 1.70 (0.07) | ns |
| Perimeter (mm) | 6.15 (0.20) | 6.14 (0.23) | ns | 5.18 (0.27) | a | 5.23 (0.19) | a |
| Trabecular bone | |||||||
| Tb.Th (μm) | 81.9 (2.01) | 80.0 (4.40) | ns | 61.8 (0.63) | c | 64.7 (1.27) | c |
| Conn.D (mm−3) | 146 (12.3) | 118 (7.52) | ns | 185 (23.9) | ns | 139 (4.8) | ns |
| Bone formation | |||||||
| P1NP (ng/ml) | 101 (18.6) | 94.6 (14.0) | ns | 60.5 (6.92) | b | 44.8 (7.38) | c |
Data are shown as average (sem). ns, Not significant.
P < 0.05 vs. WT.
P < 0.01 vs. WT.
P < 0.001 vs. WT.
Bone phenotypes in mouse models may depend on age (22), and we have shown that Ghsr deficiency has age-dependent effects on metabolic profile (12). We tested whether a bone phenotype in Ghsr−/− mice may become apparent with age by measuring cortical and trabecular bone microarchitecture in 6-month-old WT and Ghsr−/− mice. Trabecular bone volume fraction (BV/TV), Tb.N, and Conn.D were significantly decreased, whereas Tb.Th showed a trend to lower values in the GHS-R-deficient mice (Fig. 2, E, F, and I, and Table 4). Ghsr−/− mice also had decreased Ct.V, Ec.V, perimeter, and MOI but unaffected Ct.Th (Fig. 2, G and H, and Table 4). Concordant with the decrease in trabecular bone, histomorphometric analyses showed that the number of osteoclasts in the distal metaphysis was doubled (Fig. 1D). The serum bone formation marker P1NP was unaltered (Table 4).
Table 4.
Bone microarchitecture and bone formation in 6-month-old WT, Ghsr−/−, ob/ob, and ob/ob.Ghsr−/− mice
| WT | Ghsr−/− | P | ob/ob | P | ob/ob.Ghsr−/− | P | |
|---|---|---|---|---|---|---|---|
| Cortical bone | |||||||
| Ct.Th (μm) | 233 (4.49) | 243 (3.90) | ns | 232 (2.88) | ns | 232 (6.31) | ns |
| Ec.V (mm3) | 1.61 (0.06) | 1.34 (0.06) | a | 1.59 (0.06) | ns | 1.68 (0.1) | d |
| Perimeter (mm) | 6.70 (0.13) | 6.03 (0.13) | b | 6.11 (0.17) | a | 6.00 (0.22) | a |
| Trabecular bone | |||||||
| Tb.Th (μm) | 74.1 (1.67) | 68.7 (2.47) | ns | 66.6 (0.86) | b | 66.4 (0.73) | c |
| Conn.D (mm−3) | 56.8 (5.52) | 18.8 (4.88) | b | 128 (16.68) | b | 71.4 (6.43) | ns |
| Bone formation | |||||||
| P1NP (ng/ml) | 49.3 (2.84) | 43.9 (6.11) | ns | 67.6 (30.4) | ns | 42.43 (0.76) | ns |
Data are shown as average (sem). ns, Not significant.
P < 0.05 vs. WT.
P < 0.01 vs. WT.
P < 0.001 vs. WT.
P < 0.05 for interaction.
Leptin and ghrelin interaction in the regulation of osteoclastogenesis in vivo
Leptin has been found to antagonize a number of ghrelin's activities. To discover whether leptin could regulate ghrelin's activities in bone, we analyzed leptin-deficient (ob/ob) and combined leptin and GHS-R (ob/ob.Ghsr−/−)-deficient mice. As reported earlier (23), leptin deficiency has no effect on osteoclastogenic potential of bone marrow progenitor cells (Fig. 1A). The increased osteoclastogenesis in ex vivo Ghsr−/− bone marrow cultures was also unaffected by leptin deficiency (Fig. 1A), demonstrating the absence of a direct local leptin effect on ghrelin regulation of osteoclast formation. Additionally, leptin treatment did not prevent inhibition of osteoclast formation by ghrelin (Fig. 1B). Next, we examined osteoclastogenesis in vivo using histological sections of femurs from 3-month-old mice. Leptin deficiency alone increased osteoclast number (Fig. 1C), but, most interestingly, deletion of GHS-R in ob/ob mice normalized osteoclast number to WT levels (Fig. 1C), demonstrating that leptin deficiency-increased osteoclastogenesis is due to unopposed ghrelin stimulation of osteoclastogenesis. Overall, these analyses show that ghrelin has a direct negative effect and an indirect positive effect on osteoclastogenesis that is controlled by leptin signaling.
We then investigated the impact of age on the interaction of ghrelin and leptin by examining bone sections from 6-month-old mice. In contrast to the 3-month-old data (Fig. 1C), leptin deficiency led to a decrease in osteoclast formation at 6 months (Fig. 1D). However, like 3-month-old mice, the number of osteoclasts was normalized to WT levels when both leptin and GHS-R were deleted (Fig. 1D). Overall, these data demonstrate an age-dependent mode of interaction between ghrelin and leptin on osteoclastogenesis.
Ablation of leptin decreases trabecular and cortical bone mass at 3 months of age
Leptin deficiency decreased both trabecular and cortical bone parameters as demonstrated by reduced BV/TV, Tb.Th, Ct.V, Ct.Th, perimeter, and MOI, and none of these effects were modified by additional deletion of GHS-R (Fig. 2, A–D, and Table 3). This demonstrates that leptin deficiency decreases trabecular and cortical bone mass independent of ghrelin signaling at 3 months.
At 6 months, ghrelin and leptin have opposing actions on trabecular bone
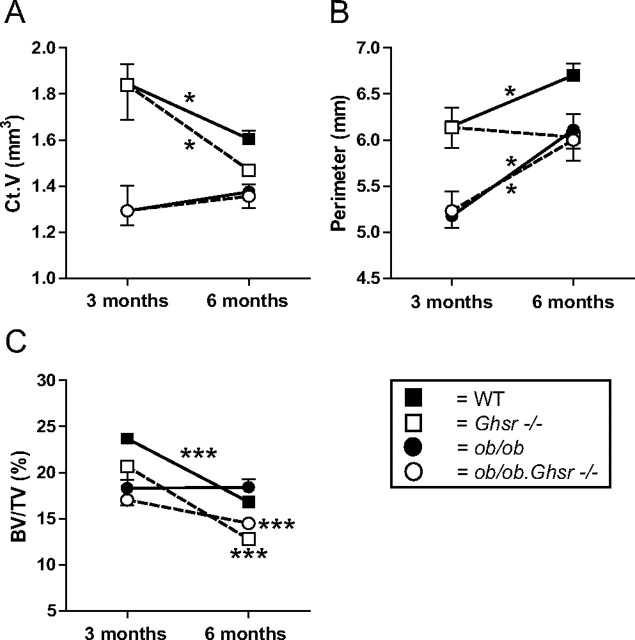
Bone structural analyses demonstrated that deletion of ghrelin signaling leads to a decline in both cortical and trabecular bone mass at 6 months of age (Fig. 2, E–H). As exemplified in Fig. 3 for Ct.V, perimeter, and BV/TV, this revealed that the absence of ghrelin signaling has an age-dependent effect on bone.
Fig. 3.
Effects of ghrelin receptor and leptin deficiency on (A) Ct.V, (B) perimeter, and (C) BV/TV exemplifying age as a determinant of the effects of these hormones and their interaction. * and ***, Significant change from 3 to 6 months.
The impact of leptin deficiency also showed age-related dynamics, but these were different in trabecular and cortical bone. Both trabecular and cortical parameters (Fig. 2, A–D) were reduced at 3 months, although Tb.N (Fig. 2E) was increased, whereas cortical parameters (Fig. 2, G and H) were still decreased at 6 months.
Additional deletion of GHS-R compensated for the increased trabecular parameters Tb.N and Conn.D and the effect on BV/TV in ob/ob mice. This can also be appreciated from Fig. 2I, showing representative slices through femoral head, neck, and lesser trochanter. The effects on Ct.V, MOI (Fig. 2, G and H), and perimeter in ob/ob.Ghsr−/− mice were identical to that of Ghsr−/− or ob/ob mice alone. Additional loss of leptin compensated for the decreased Ec.V caused by GHS-R deficiency (Table 4). At 3 months, Ghsr−/− mice had unaltered levels of the bone formation marker P1NP in their serum, whereas ob/ob mice had significantly decreased levels of P1NP. Additional ablation of GHS-R did not compensate for this effect in ob/ob mice (Table 4). At 6 months, ablation of neither GHS-R nor leptin affected P1NP levels (Table 4).
Discussion
In this study, we have shown for the first time that ghrelin exerts dual effects on osteoclastogenesis, that the orexigenic hormone ghrelin and the anorectic hormone leptin interact in the control of osteoclastogenesis and bone metabolism, and that age is an important determinant of the ghrelin and leptin interaction and their effects on bone. These current observations are placed in the context of previous findings on the regulation of bone metabolism by leptin (5, 23) and effects of leptin on GHS-R expression in the hypothalamus (24). This is summarized in Fig. 4, describing the interaction between ghrelin and leptin in osteoclastogenesis.
Fig. 4.
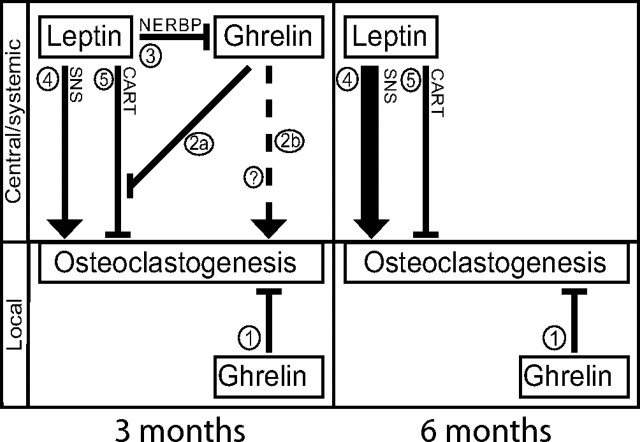
Model of leptin and ghrelin action on osteoclastogenesis. Numbers refer to pathways affecting osteoclastogenesis as described in the article text. Lines ending with an arrow or a flat line indicate stimulation or inhibition of osteoclastogenesis, respectively. Lines 4 and 5 are based on Ducy et al. (23) and Elefteriou et al. (5), and line 3 is based on Komori et al. (24). Question mark indicates unknown systemic/central route to stimulate osteoclastogenesis. See text for detailed description.
Ghrelin and leptin interaction to control osteoclastogenesis
We find that ghrelin directly inhibits osteoclastogenesis in bone marrow cultures of 3-month-old mice (Fig. 4, process 1). The fact that the increase in multinuclear osteoclasts is paralleled by a decrease in mononuclear cells led us to conclude that the direct inhibitory effect of ghrelin on osteoclast is primarily located at the level of differentiation and fusion and not at the level of progenitor/precursor proliferation. The unchanged in vivo osteoclast number and bone mass demonstrates tight regulation of ghrelin activity in vivo by mechanism(s) not intrinsic to bone marrow. Our finding that the osteoclastogenic phenotype of ob/ob mice (23) is completely reversed by Ghsr deletion showed that ghrelin counterregulates its own local negative effects on osteoclasts via a separate, more potent, systemic/central stimulatory pathway (Fig. 4, process 2). Because ob/ob.Ghsr−/− mice do not have decreased numbers of osteoclasts, their phenotype also demonstrates that leptin suppresses this osteoclastogenic ghrelin pathway (Fig. 4, process 3), thus balancing ghrelin's stimulatory and inhibitory pathways. If this inhibition of ghrelin's effect by leptin did not exist, a decrease in osteoclast number would have been observed. The systemic/central nature of this regulatory effect of leptin on ghrelin is based on our observation that leptin treatment does not modulate the effect of ghrelin on osteoclastogenesis in vitro and previous data demonstrating central control of osteoclastogenesis by leptin (5).
The mechanism of ghrelin's positive effect on osteoclastogenesis (Fig. 4, process 2) remains to be determined, although clues can be found in the literature. Leptin stimulation of the SNS has been shown to affect bone metabolism by stimulating osteoclastogenesis (Fig. 4, process 4) and inhibiting osteoblastogenesis (5, 6). It was found that this pathway was modulated by the negative influence of CART, which is also induced by leptin (Fig. 4, process 5) (5). Intriguingly, ghrelin suppresses CART expression in hypothalamic neurons (25). We hypothesize that by suppressing leptin-induced CART signaling (Fig. 4, process 2a) and/or an alternative systemic/central route (Fig. 4, process 2b), ghrelin stimulates osteoclastogenesis.
Leptin's negative effect on ghrelin's systemic/central osteoclastogenic activity may be explained by the recent finding that leptin increases expression of negative regulatory element-binding protein in GHS-R-positive neurons in the hypothalamus (24). Negative regulatory element-binding protein represses GHS-R expression, providing a mechanism by which leptin could control ghrelin's systemic/central actions on osteoclastogenesis (Fig. 4, process 3). Moreover, these effects were localized to the arcuate nucleus, where leptin induces CART (5). However, because at 3 months Ghsr−/− mice have unaltered rather than increased osteoclastogenesis, a component of the CART-mediated effect of leptin on osteoclastogenesis must be ghrelin signaling independent (Fig. 4, process 5).
Ghrelin and leptin effects and their interplay are age-dependent
An important observation is the shift in the weight of ghrelin's impact on bone structure with progressing age, from a systemic/central stimulatory toward a local inhibitory effect. The increased osteoclastogenesis and decreased bone mass in older Ghsr−/− mice demonstrates that with aging, the systemic stimulatory pathway declines, allowing ghrelin's local inhibitory effects to dominate. Several clinical observations support this age-dependent relationship between ghrelin and bone turnover with aging. Ghrelin did not correlate with bone resorption markers in a cohort of pubertal boys (27) or with bone mass in young men (28), whereas a negative correlation with bone resorption was observed in a large study of older men and a positive correlation with bone mass (29).
The effects of leptin and its interaction with ghrelin are also age dependent. Leptin affects osteoclastogenesis via two counterbalancing pathways: the SNS (positive) and CART (negative) (Fig. 4, processes 4 and 5) (5). The increased osteoclast number in older Ghsr−/− mice may be due to a relatively more robust leptin signal through the SNS compared with the CART pathway. Evidence exists for increased sympathetic tone with aging (30), but it is unclear whether CART signaling changes with aging (31).
Age-related changes in the interplay between ghrelin and leptin are clearly demonstrated by comparing the impact of leptin deficiency in the presence of ghrelin signaling. In contrast with the observed increase in osteoclast number at 3 months, leptin deficiency led to a decrease in osteoclast formation at 6 months. In the absence of leptin, the local inhibitory action of ghrelin (Fig. 4, process 1) is dominant over the systemic/central positive ghrelin signal (Fig. 4, process 2). Leptin deficiency probably amplifies this effect, because at 6 months, leptin has a relatively greater osteoclastogenic activity via the SNS (Fig. 4, process 5). Most interestingly, at both ages, osteoclastogenesis is unchanged compared with WT mice, when both ghrelin signaling and leptin are deficient. These observations demonstrate that at 3 months, the increased osteoclastogenesis in the absence of leptin signaling is due to unopposed systemic/central ghrelin stimulation of osteoclastogenesis, whereas at 6 months, increased osteoclastogenesis in the absence of ghrelin signaling is due to unopposed leptin stimulation of osteoclastogenesis. Together, these data strongly demonstrate the significance of an optimal ghrelin and leptin balance for proper control of osteoclast formation and ultimately bone health, especially in relation to aging.
Further evidence for the impact of age on ghrelin and leptin effects and interactions is derived from the bone structural analyses. GHS-R deficiency had no effect on bone structure at 3 months but did decrease both trabecular and cortical parameters at 6 months of age. These age-dependent structural observations parallel the effects on osteoclast number while P1NP levels are unaffected, indicating that ghrelin regulates bone mass primarily through control of osteoclasts. Analyses of the ob/ob mice at both 3 and 6 months showed, firstly, that leptin affects cortical and trabecular bone differently. This is in line with reports in the literature (32) and highlights the site specificity of leptin's effects on bone. Secondly, our analyses showed differences in dynamics in trabecular and cortical bone with aging. The changes in trabecular bone at both ages correspond with the changes in osteoclast number, suggesting that the trabecular bone phenotype is primarily driven by changes in osteoclastogenesis. In support of this is that Tb.N is mainly affected by bone resorption, whereas changes in cortical bone mass are dependent on periosteal bone formation (33, 34).
The interplay of leptin with ghrelin is both age dependent and site specific. It is only observed at 6, but not at 3, months, and at 6 months combined GHS-R leptin deficiency led to normalization of the trabecular bone phenotype. This demonstrates that leptin and ghrelin have opposing activities in their modulation of trabecular bone metabolism that is age dependent. This mimics leptin-ghrelin antagonistic effects observed in other tissues (17, 26).
In conclusion, we demonstrate that two important regulators of energy homeostasis modulate bone metabolism and add to the knowledge-base needed to tackle osteoporosis, which in itself is an age-dependent disorder that is linked with pathologies of energy homeostasis. An appealing aspect of this study is that it suggests that elderly subjects, the group in which osteoporosis develops, would benefit from ghrelin treatment or enhanced GHS-R signaling to inhibit osteoclastogenesis and improve bone structure.
Acknowledgments
We thank Marijke Schreuders-Koedam, Piet Kramer, Bas Karels, Ton de Jong, and Piet van der Heul for technical assistance and Professor R. Baron (Harvard School of Medicine) for critically reading our manuscript.
This work was supported by the Zorgonderzoek Nederland Medische Wetenschappen, The Netherlands, Program Grant 91203022.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ALP
- Alkaline phosphatase
- BV/TV
- bone volume/tissue volume
- CART
- cocaine- and amphetamine-regulated transcript
- Conn.D
- connectivity density
- μCT
- microcomputed tomography
- Ct.Th
- cortical thickness
- Ct.V
- cortical volume
- 3D
- three-dimensional
- Ec.V
- endocortical volume
- GHS-R
- GH secretagogue receptor
- MOI
- moment of inertia
- P1NP
- procollagen type 1 amino-terminal propeptide
- RANKL
- receptor activator of nuclear factor κB ligand
- SNS
- sympathetic nervous system
- Tb.N
- trabecular number
- Tb.Th
- trabecular thickness
- TRAP
- tartrate-resistant acid phosphatase
- WT
- wild type.
References
- 1. Rosen CJ. 2009. Bone: serotonin, leptin and the central control of bone remodeling. Nat Rev Rheumatol 5:657–658 [DOI] [PubMed] [Google Scholar]
- 2. Oury F , Karsenty G. 2011. Towards a serotonin-dependent leptin roadmap in the brain. Trends Endocrinol Metab 22:382–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hamrick MW , Ferrari SL. 2008. Leptin and the sympathetic connection of fat to bone. Osteoporos Int 19:905–912 [DOI] [PubMed] [Google Scholar]
- 4. Welt CK , Chan JL , Bullen J , Murphy R , Smith P , DePaoli AM , Karalis A , Mantzoros CS. 2004. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 351:987–997 [DOI] [PubMed] [Google Scholar]
- 5. Elefteriou F , Ahn JD , Takeda S , Starbuck M , Yang X , Liu X , Kondo H , Richards WG , Bannon TW , Noda M , Clement K , Vaisse C , Karsenty G. 2005. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434:514–520 [DOI] [PubMed] [Google Scholar]
- 6. Takeda S , Elefteriou F , Levasseur R , Liu X , Zhao L , Parker KL , Armstrong D , Ducy P , Karsenty G. 2002. Leptin regulates bone formation via the sympathetic nervous system. Cell 111:305–317 [DOI] [PubMed] [Google Scholar]
- 7. Clemens TL , Karsenty G. 2011. The osteoblast: an insulin target cell controlling glucose homeostasis. J Bone Miner Res 26:677–680 [DOI] [PubMed] [Google Scholar]
- 8. Nogueiras R , Tschöp MH , Zigman JM. 2008. Central nervous system regulation of energy metabolism: ghrelin versus leptin. Ann NY Acad Sci 1126:14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tschöp M , Smiley DL , Heiman ML. 2000. Ghrelin induces adiposity in rodents. Nature 407:908–913 [DOI] [PubMed] [Google Scholar]
- 10. Dezaki K , Damdindorj B , Sone H , Dyachok O , Tengholm A , Gylfe E , Kurashina T , Yoshida M , Kakei M , Yada T. 2011. Ghrelin attenuates cAMP-PKA signaling to evoke insulinostatic cascade in islet β-cells. Diabetes 60:2315–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao TJ , Liang G , Li RL , Xie X , Sleeman MW , Murphy AJ , Valenzuela DM , Yancopoulos GD , Goldstein JL , Brown MS. 2010. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA 107:7467–7472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ma X , Lin L , Qin G , Lu X , Fiorotto M , Dixit VD , Sun Y. 2011. Ablations of ghrelin and ghrelin receptor exhibit differential metabolic phenotypes and thermogenic capacity during aging. PLoS One 6:e16391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Delhanty PJ , van der Eerden BC , van der Velde M , Gauna C , Pols HA , Jahr H , Chiba H , van der Lely AJ , van Leeuwen JP. 2006. Ghrelin and unacylated ghrelin stimulate human osteoblast growth via mitogen-activated protein kinase (MAPK)/phosphoinositide 3-kinase (PI3K) pathways in the absence of GHS-R1a. J Endocrinol 188:37–47 [DOI] [PubMed] [Google Scholar]
- 14. Kim SW , Her SJ , Park SJ , Kim D , Park KS , Lee HK , Han BH , Kim MS , Shin CS , Kim SY. 2005. Ghrelin stimulates proliferation and differentiation and inhibits apoptosis in osteoblastic MC3T3-E1 cells. Bone 37:359–369 [DOI] [PubMed] [Google Scholar]
- 15. Fukushima N , Hanada R , Teranishi H , Fukue Y , Tachibana T , Ishikawa H , Takeda S , Takeuchi Y , Fukumoto S , Kangawa K , Nagata K , Kojima M. 2005. Ghrelin directly regulates bone formation. J Bone Miner Res 20:790–798 [DOI] [PubMed] [Google Scholar]
- 16. Sun Y , Wang P , Zheng H , Smith RG. 2004. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA 101:4679–4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun Y , Asnicar M , Saha PK , Chan L , Smith RG. 2006. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab 3:379–386 [DOI] [PubMed] [Google Scholar]
- 18. van der Eerden BC , Hoenderop JG , de Vries TJ , Schoenmaker T , Buurman CJ , Uitterlinden AG , Pols HA , Bindels RJ , van Leeuwen JP. 2005. The epithelial Ca2+ channel TRPV5 is essential for proper osteoclastic bone resorption. Proc Natl Acad Sci USA 102:17507–17512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Waarsing JH , Day JS , Weinans H. 2004. An improved segmentation method for in vivo microCT imaging. J Bone Miner Res 19:1640–1650 [DOI] [PubMed] [Google Scholar]
- 20. Eijken M , Hewison M , Cooper MS , de Jong FH , Chiba H , Stewart PM , Uitterlinden AG , Pols HA , van Leeuwen JP. 2005. 11β-Hydroxysteroid dehydrogenase expression and glucocorticoid synthesis are directed by a molecular switch during osteoblast differentiation. Mol Endocrinol 19:621–631 [DOI] [PubMed] [Google Scholar]
- 21. Erlebacher A , Derynck R. 1996. Increased expression of TGF-β 2 in osteoblasts results in an osteoporosis-like phenotype. J Cell Biol 132:195–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ke HZ , Brown TA , Qi H , Crawford DT , Simmons HA , Petersen DN , Allen MR , McNeish JD , Thompson DD. 2002. The role of estrogen receptor-β, in the early age-related bone gain and later age-related bone loss in female mice. J Musculoskelet Neuronal Interact 2:479–488 [PubMed] [Google Scholar]
- 23. Ducy P , Amling M , Takeda S , Priemel M , Schilling AF , Beil FT , Shen J , Vinson C , Rueger JM , Karsenty G. 2000. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197–207 [DOI] [PubMed] [Google Scholar]
- 24. Komori T , Doi A , Furuta H , Wakao H , Nakao N , Nakazato M , Nanjo K , Senba E , Morikawa Y. 2010. Regulation of ghrelin signaling by a leptin-induced gene, negative regulatory element-binding protein, in the hypothalamic neurons. J Biol Chem 285:37884–37894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Lartigue G , Dimaline R , Varro A , Dockray GJ. 2007. Cocaine- and amphetamine-regulated transcript: stimulation of expression in rat vagal afferent neurons by cholecystokinin and suppression by ghrelin. J Neurosci 27:2876–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dixit VD , Schaffer EM , Pyle RS , Collins GD , Sakthivel SK , Palaniappan R , Lillard JW , Taub DD. 2004. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest 114:57–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jürimäe J , Pomerants T , Tillmann V , Jürimäe T. 2009. Bone metabolism markers and ghrelin in boys at different stages of sexual maturity. Acta Paediatr 98:892–896 [DOI] [PubMed] [Google Scholar]
- 28. Pomerants T , Tillmann V , Jürimäe J , Jürimäe T. 2007. The influence of serum ghrelin, IGF axis and testosterone on bone mineral density in boys at different stages of sexual maturity. J Bone Miner Metab 25:193–197 [DOI] [PubMed] [Google Scholar]
- 29. Gonnelli S , Caffarelli C , Del Santo K , Cadirni A , Guerriero C , Lucani B , Franci B , Nuti R. 2008. The relationship of ghrelin and adiponectin with bone mineral density and bone turnover markers in elderly men. Calcif Tissue Int 83:55–60 [DOI] [PubMed] [Google Scholar]
- 30. Seals DR , Dinenno FA. 2004. Collateral damage: cardiovascular consequences of chronic sympathetic activation with human aging. Am J Physiol Heart Circ Physiol 287:H1895–H1905 [DOI] [PubMed] [Google Scholar]
- 31. Kmiec Z. 2006. Central regulation of food intake in ageing. J Physiol Pharmacol 57(Suppl 6):7–16 [PubMed] [Google Scholar]
- 32. Hamrick MW , Pennington C , Newton D , Xie D , Isales C. 2004. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone 34:376–383 [DOI] [PubMed] [Google Scholar]
- 33. Aaron JE , Makins NB , Sagreiya K. 1987. The microanatomy of trabecular bone loss in normal aging men and women. Clin Orthop Relat Res 260–271 [PubMed] [Google Scholar]
- 34. Seeman E. 2001. Unresolved issues in osteoporosis in men. Rev Endocr Metab Disord 2:45–64 [DOI] [PubMed] [Google Scholar]