Abstract
Androgenic hormones regulate many aspects of animal social behavior, including the elaborate display routines on which many species rely for advertisement and competition. One way that this might occur is through peripheral effects of androgens, particularly on skeletal muscles that control complex movements and postures of the body and its limbs. However, the specific contribution of peripheral androgen-muscle interactions to the performance of elaborate behavioral displays in the natural world has never been examined. We study this issue in one of the only natural physiological models of animal acrobatics: the golden-collared manakin (Manacus vitellinus). In this tropical bird, males compete with each other and court females by producing firecracker-like wing- snaps and by rapidly dancing among saplings over the forest floor. To test how activation of peripheral androgen receptors (AR) influences this display, we treat reproductively active adult male birds with the peripherally selective antiandrogen bicalutamide (BICAL) and observe the effects of this manipulation on male display performance. We not only validate the peripheral specificity of BICAL in this species, but we also show that BICAL treatment reduces the frequency with which adult male birds perform their acrobatic display maneuvers and disrupts the overall structure and fine-scale patterning of these birds' main complex wing-snap sonation. In addition, this manipulation has no effect on the behavioral metrics associated with male motivation to display. Together, our findings help differentiate the various effects of peripheral and central AR on the performance of a complex sociosexual behavioral phenotype by indicating that peripheral AR can optimize the motor skills necessary for the production of an elaborate animal display.
In many species throughout the animal kingdom, individuals perform elaborate social displays to attract mates and defend territories (1–6). These phenotypes more than likely rely on robust muscular systems that provide not only great strength but also superior motor agility for sustained and highly coordinated physical activity. The mechanisms through which these muscle systems are maintained for such remarkable display behavior are virtually unknown. Here we explore this issue by testing how androgenic signaling systems specifically within the periphery help maintain acrobatic display behavior.
Androgenic hormones act via intracellular androgen receptors (AR) to modulate animal social behavior. A principal target of androgens is the central nervous system (CNS), which controls how and when individuals interact with each other in a top-down manner (7). In many vertebrates, however, AR are also expressed peripherally, and specifically in the skeletal muscles (8–10). Prior studies presume that androgens influence simplistic sexual reflexes and movements in part by acting on discrete muscular targets that have strong sex-specific functions (11–15). Thus, it seems that androgens may also act via AR more broadly throughout the periphery, particularly on skeletal muscles, to facilitate complex movements associated with elaborate, whole-body social signaling behavior. Whether this occurs is not known because no studies have measured the effect of experimentally manipulated peripheral or muscular AR on behavioral output, let alone elaborate behavioral output necessary for physically complex social displays.
Songbirds have emerged as one of the most widely used groups of vertebrates to study how androgenic steroids affect social signals. Much of the work in this area focuses on the brain, and thus explores how androgens guide the sensorimotor and premotor programs of song learning and production (16–19). At the same time, androgens can also act on the avian vocal organ, the syrinx, to presumably influence song (15). This, in turn, implies that androgens act on multiple levels of the motor systems that underlie the acoustic signaling abilities of songbirds. However, not all birds are capable of singing complex vocalizations, and instead many avian species communicate and/or advertise by performing intricate and strenuous flying, jumping, and dancing routines (4–6). To optimize the movement and balance of the wings, legs, and tail that are required for such behavior, peripheral muscular systems throughout the entire body must be fully engaged and coordinated (5). Thus, given the important roles of androgens in modulating birdsong, it is possible that androgenic hormones also help facilitate musculoskeletal systems that guide the physicality behind the signaling traits of nonsinging birds.
Currently one of the only animal models used to study the mechanisms of natural animal acrobatics and fine motor skill is the golden-collared manakin (Manacus vitellinus). In this tropical bird, males compete and attract mates by producing loud wing-snap sonations and by performing elaborate, high-speed dances among saplings near the forest floor (20, 21). Their display is both visual and acoustic, and its extraordinary physicality and complexity requires remarkable strength and neuromuscular control (20). Moreover, male golden-collared manakins express abundant AR in their skeletal muscles compared to other birds that do not exhibit complex courtship displays (22), and nearly all components of the males' display repertoire depend on activation of AR (23). Based on this work, we hypothesize that peripheral AR in the musculoskeletal system facilitates the complex, acrobatic elements of the male display repertoire.
In the current study, we examine this idea by developing a method for manipulating AR exclusively in the periphery and measuring how this manipulation impacts the golden-collared manakin's display. We achieved the first goal by administering bicalutamide (BICAL) to a group of captive manakins. BICAL is a potent antiandrogen that is thought to block AR in a peripherally selective manner (24, 25); thus, we validated that this drug indeed inhibits the functional effects of avian AR in this manner. We achieved the second goal by treating wild breeding males with BICAL and recording how this drug impacts their display. Prior work shows that antiandrogens, like BICAL, are ideal for testing how AR influences avian behavior, because estrogen receptors, not AR, provide negative feedback of androgen secretion in birds (26, 27); as such, antiandrogens [ie, BICAL (28)] inhibit avian AR without changing circulating levels of testosterone (29). Together, these studies allow us to evaluate and measure the role of peripheral androgens in modulating the performance and fine motor skills of the golden-collared manakin's elaborate signaling repertoire.
Materials and Methods
Animals
This work was conducted at the Smithsonian Tropical Research Institute (STRI) in Gamboa, Panama. All experiments were performed during the breeding season from February to April. Golden-collared manakins were collected using passive mist netting from active leks in the nearby forest. The Animal Care and Use Committees at the University of California, Los Angeles, and STRI approved the methods described herein.
Experiment 1: validation studies
We validated the efficacy and peripheral selectivity of BICAL using juvenile male and female golden-collared manakins captured at or near active leks. Juvenile males and females (ie, green birds) have dull green plumage and are distinguishable from adult males, but not each other. These birds maintain low testosterone levels (30, 31), although AR levels in their skeletal muscles are as high as breeding adult males (22). Moreover, testosterone administration to both male and female green birds induces wing-snap behavior similar to that of adult males (30, 31). These features make green birds ideal for testing whether BICAL is peripherally selective on AR in this species.
Upon capture, all green birds were quickly returned to our STRI facility. Individuals were housed singly in small cages (29 × 32 cm) in one room, giving them constant visual and acoustic contact. Birds received fresh papaya ad libitum. After 2-3 days of captivity, each bird was implanted at the base of the neck with a 12-mm SILASTIC brand tube (0.76 mm inner diameter, 1.65 mm outer diameter; Dow Corning Inc, Midland, Michigan) that contained crystalline testosterone. An ice chip was gently pressed against the implantation site to numb the skin before implantation. Details of this procedure have been described previously (23, 32). Prior work verifies that such implants not only increase testosterone to levels typical of breeding males (32), but also induces physiological and behavioral states typical of breeding males (30, 31). At this same time, birds were randomly assigned to receive either an additional time-release BICAL implant (see below for BICAL details; n = 3 males) or an additional control implant (n = 3 males, n = 1 female). The second implant was administered in the same manner as the first implant (23, 32). No bird responded adversely to either the implantation surgeries or the testosterone/BICAL treatments.
Because testosterone induces maximal behavioral and physiological effects in female and juvenile male manakins after 8–18 days of implantation (30, 31), we recorded behavior in this experiment during the same time window. Birds were videotaped with a Sony camcorder (New York, New York) for approximately 8 h/d (between 6:30 am and 4:00 pm). An observer randomly selected a subset of these videos and recorded the rate at which birds fed on papaya and the total number of times birds hopped on or between perches within their cage. On postimplantation day 19, each bird was weighed and killed by rapid decapitation. Three wing muscles (pectoralis, supracoracoideus, and scapulohumeralis caudalis) and 2 areas of the brain (telencephalon and hypothalamus) were quickly dissected from the birds, flash frozen on dry ice, and stored at −80°C for mRNA analysis (see below).
Experiment 2: peripheral AR manipulation in wild adult male birds
We used reproductively active adult male golden-collared manakins to test the effects of peripheral AR on display performance and motor skills. Each bird in this study was captured at its own courtship arena between 6:30 and 9:00 am. Once taken from the mist net, birds were weighed, color banded, and randomly assigned to receive either a time-release BICAL implant (see below for BICAL details; n = 5) or a blank control implant (n = 6). Implants were administered sc on the bird's back and at the base of its neck. Implantation took approximately 2 minutes, and the details of this procedure are described elsewhere (23). No bird was adversely affected by either the implantation or treatment of BICAL, and some birds were observed displaying within minutes or hours after their release.
Birds in this experiment were taken from 7 different leks in geographically distinct locations; each lek contained between 2 and 10 actively breeding adult males. Five of these 7 leks contained at least 4 displaying males, and we included 2 birds from each of these leks in this experiment (one of these birds was assigned to the control group, whereas the other was assigned to the BICAL group). The other 2 leks contained 3 or fewer displaying males; only one bird from each of these leks was used in this experiment, with one individual assigned to the BICAL group and the other individual assigned to the control group.
We recorded the behavior of treated and untreated birds during multiple 30-minute observation sessions. Upon arriving at a male's display site for one of these observation sessions, we recorded whether the male was present as an index of his ability to hold his own arena in the face of likely male-male competition (33). After a 15-minute habituation period, we then recorded the frequency of 5 unambiguous behaviors: 1) chee-poos, 2) wing- snaps, 3) roll-snaps, 4) jump-snap displays, and 5) the number of snaps per jump-snap display (see full descriptions in Results). A total of 4–5 sessions was recorded for each bird during both postimplantation days 1–5 and postimplantation days 6-10. Observation sessions occurred between 7:00 and 9:00 am and between 12:00 and 4:30 pm, which correspond to peak display activity in this species (23). Observation times were balanced, such that each individual was watched an equal number of times throughout the morning and afternoon observation windows. The observers sat approximately 10 m from the arena so that they were close enough to watch the bird without disturbing him. Data were recorded in a notebook.
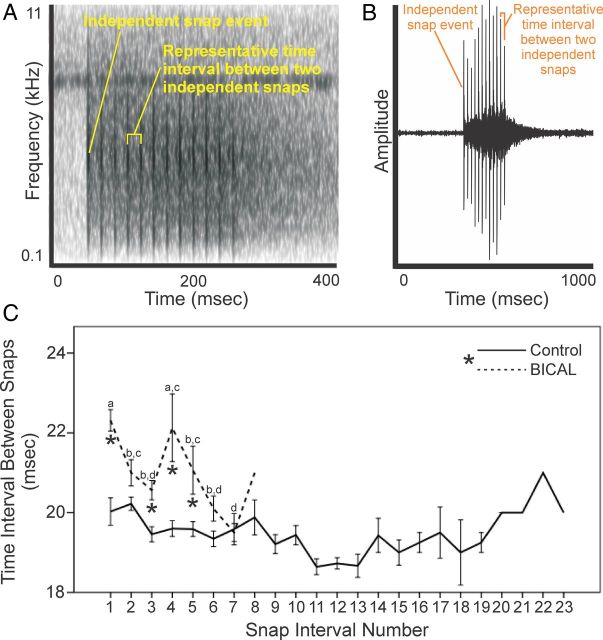
In a randomly selected subset of observation sessions, male roll-snap sonations were tape-recorded (Sony TC-D5M professional tape recorder). Audio files from these recordings were digitized (sample rate = 48 KHz; 16 bit dynamic range) and Audacity Audio Editor (version 1.3.14) was used to produce spectrograms of each male's roll snaps [average 4.7 (±0.7 SEM) roll snaps per bird]. Time intervals between the independent snaps within a given roll snap were measured on spectrograms, and these measurements were verified using waveform diagrams of the same roll snap generated with Praat Phonetics Software (see Figure 4, A and B). Accuracy between measurement techniques was greater than 95%.
Figure 4.
Effects of peripheral AR on the fine motor pattern of the male's roll-snap signal. A, Spectrogram of a control bird's roll snap. Each black broadband line represents an independent snap event within the series of snaps, which ultimately makes up the roll-snap sonation. The space between independent snap events represents the duration between snaps within a roll snap. B, Waveform diagram of the same roll snap on which between-snap interval measurements were confirmed. Each spike represents a single snap event, within the entire roll-snap signal. The space between spikes represents the time interval between snap events. C, Average time intervals between snap events within a roll-snap for BICAL and control birds. Asterisk in the legend denotes a significant effect of treatment (P < .05). Asterisks below the lower error bars of the BICAL bird data points represent significant differences in the time interval at the specific snap interval compared to controls (post hoc contrasts, P < .05). Differences in letters above error bars represent significant differences between snap interval durations within BICAL birds (post hoc ANOVA with between group comparisons, P < .05). All data represent means ± 1 SEM. Solid line represents controls, whereas dashed line represents BICAL treated birds.
BICAL treatment
BICAL (also known as Casodex) is a potent antiandrogen that blocks AR in a peripherally selective manner, and it has been used with success to antagonize AR in both small mammals and birds (24, 25, 28). Custom-made BICAL implants (1.6 × 5 mm diameter pellets) were purchased from Innovative Research of America (Sarasota, Florida), and they were designed to release BICAL at a rate of 0.25 mg/d for up to 21 days. Given the average weight of an adult male manakin during the breeding season, this daily amount of BICAL equates to a dose of 12.5 mg/kg·d. In birds, prior results suggest that this dose is sufficient to induce nontoxic, antiandrogenic effects (28). The manufacturer provided control (blank) implants of the same size as BICAL implants and made from the same materials.
Quantitative PCR (qPCR)
Expression of mRNA in muscle and brain tissues was conducted using qPCR. Procedures for Trizol RNA extractions (Invitrogen, Carlsbad, California), Superscript II reverse transcriptase (Promega, Madison, Wisconsin) reactions, and qPCR analysis are outlined in great detail elsewhere (22, 32, 34). Briefly, reactions were performed in an ABI 7300 sequence detection system (Applied Biosystems Inc, Foster City, California) using 5 ng of template. Primers for parvalbumin (forward, 5′-TTGTCCTGAAGGGCTTTACC; reverse, 5′-TACCATCACCGTCCTTATCTCC), IGF-I (forward, 5′-AACCAGTTCTGTTGCTGCTG; reverse, 5′-AAAGCCTCTGTCTCCACACAC), aromatase (forward, 5′-GGATGAGCACATGGATTTTGC; reverse, 5′-GCAGTCAGATCCCCTCTGTTC), AR (forward, 5′-ATGAGTACCGCATGCACAAA; reverse, 5′-AACTCCTGGGGTGTGATCTG), sorting nexin 2 (forward, 5′-GCAGTAAAAGGTGTGTTTGACCAT; reverse-5′-CTTGCCACTTCTGCCAGCAT), and the housekeeping control gene GAPDH (forward, 5′-TGACCTGCCGTCTGGAAAA; reverse, 5′-CCATCAGCAGCAGCCTTCA) were designed from the zebra finch genome. Many of these primers have been used successfully on avian tissues, including those of manakins (22, 32, 34, 35). Primer concentrations were determined by primer optimization. Reactions were run at 50°C for 2 minutes, 95°C for 10 minutes, and then 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. A final dissociation stage was added to the end of the reaction, which ran at 95°C for 15 seconds, 60°C for 30 seconds, and 95°C for 15 seconds. Reaction efficiencies were near 100%, and dissociation curves verified the absence of contamination. Samples were run in duplicate. The standard curve method was used to measure relative expression of each gene of interest (ie, quantity gene of interest/quantity GAPDH).
Statistical analyses
In the validation experiments, we used 2-tailed t tests to compare weights, feeding behavior, and activity (hop rate) between treatment groups. We used 2-way ANOVAs to compare mRNA levels in wing muscles, with treatment group as one factor and muscle as the other factor. We used t tests to compare mRNA levels in the brain.
In analyses of adult behavior, we used a generalized linear mixed model with a binomial distribution and a logit link function to determine whether BICAL treatment and observation number influenced the probability of detecting males at their respective display arenas. Bird identity was included in this model as a random variable.
Next, we used 2-way mixed-design ANOVAS to assess effects of BICAL on the different behaviors (each behavior was run in its own model). In these models, BICAL treatment was the between-subjects factor, and postimplantation time (days 1–5 or days 6–10) was the within-subjects factor. Significant interactions were followed with post hoc pair-wise contrasts, with applied least significant difference corrections. However, because jump-snap display behavior in the BICAL birds was infrequent, the sample sizes for the snaps/jump-snap display in the BICAL birds were also relatively low. We therefore pooled the snaps/jump-snap display across the postimplantation time, and we used a 2-tailed t test to measure the effect of BICAL on this variable.
For a detailed assessment of the roll-snap motor program, we used a general linear mixed model, with BICAL treatment and the between-snap intervals as the fixed factors and the number of roll snaps nested within bird identity as the random variable. Significant interactions were followed with post hoc ANOVA and pair-wise comparison tests, with applied least significant difference corrections.
All continuous data were log transformed [log(1+X)] to meet the conditions of normality.
Results
Efficacy and validation of peripheral AR manipulation
We first examined whether BICAL influenced the health and activity of captive golden-collared manakins. Compared with controls, birds treated with BICAL showed no difference in body weight (t5 = −1.02, P = .36), feeding time (t5 = 1.10, P = .32), or number of perch hops (t5 = 1.08, P = .33). Thus, BICAL treatment itself did not induce toxic-like effects that might otherwise suppress physical condition, alertness, and levels of activity.
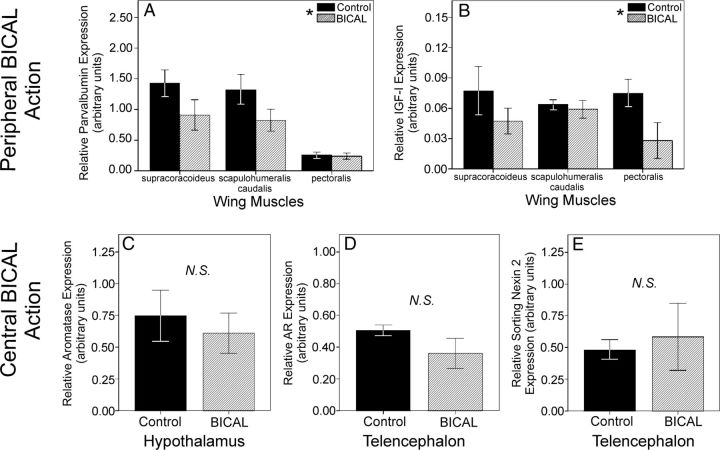
In these same individuals, we validated that BICAL blocks AR in a peripherally selective manner by assessing this drug's effect on mRNA expression of known androgen-dependent genes in both the periphery and the brain (Figure 1). BICAL treatment down-regulated the expression of parvalbumin (F1,15 = 4.71, P = .046) and IGF-I (F1,15 = 4.62, P = .048) in the wing musculature. Past work shows that both of these genes are under strong AR-dependent regulation in manakin skeletal muscles (34). In contrast, BICAL treatment had no effect on expression of hypothalamic aromatase (t5 = 0.43, P = .68) or telencephalic AR (t5 = 1.57, P = .18) and sorting nexin 2 (t5 = −0.25, P = .82). Past work in birds and mammals shows that such neuronal expression of these 3 genes depends on androgen action (36–38). These results collectively show that BICAL blunts the functional effects of AR outside the CNS, like in skeletal muscles, but not inside the CNS itself. Our findings therefore provide some of the first validation that BICAL blocks avian AR in a peripherally specific way.
Figure 1.
Functional effects of BICAL on mRNA expression of known androgen-dependent genes in the periphery and brain. Relative mRNA levels of parvalbumin (A) and IGF-I (B) in 3 skeletal muscles that control wing movement (pectoralis, supracoricoideus, scapulohumeralis caudalis). Relative mRNA levels of aromatase in the hypothalamus (C) as well as AR (D) and sorting nexin 2 (E) in the telencephalon are shown. In A and B, asterisks within a graph's legend depict significant overall effects of BICAL treatment on gene expression. Although some muscles appear to vary in transcriptional responsiveness to BICAL, the actual cause of this variation is unclear considering that AR is robustly expressed in all 3 muscles (22), and prior studies show that these same muscles produce parvalbumin and IGF-I in response to androgenic stimulation (34). In C–E, the abbreviation N.S. denotes no significant difference (P > .05) in gene expression between control and BICAL birds. In all graphs, bars represent means ± 1 SEM. In all graphs, black bars represent controls, whereas hatched bars represent BICAL treated birds.
Peripheral AR and male acrobatic performance
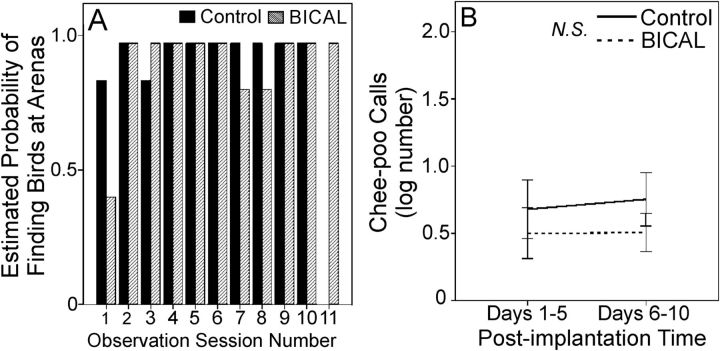
We next explored the effects of peripheral AR on the display repertoire of wild, reproductively active male golden-collared manakins in their natural habitat. We began this analysis by assessing whether BICAL influences the motivation of male birds to socially engage with conspecifics and display (Figure 2). Within a group of breeding males (ie, a lek), individuals usually signal to each other in various social contexts by producing simple chee-poo vocalizations (39). Breeding males also exhibit extreme fidelity for their own display arenas on the forest floor and consequently compete with each other to maintain these sites (33). Indeed, blocking peripheral AR with BICAL had no impact on either of these behaviors, because treated and untreated birds showed no difference in the number of chee-poos they broadcast (F1,9 = 0.27, P = .62) or the probability that they were found around their arenas (F1,74 = 0.10, P = .76). Neither of these variables changed over the course of the study (chee-poo: F1,9 = 0.04, P = .84; arena maintenance: F10,74 = 0.75, P = .68), and both of these variables were unaffected by treatment × time-course interactions (chee-poo: F1,9 = 0.10; P = .77; arena maintenance: F9,74 = 0.25, P = .97). These data are therefore consistent with the notion that BICAL administration did not disrupt social motivation, since treated individuals exhibited a similar ability and willingness to interact with others and maintain their display arena.
Figure 2.
Effects of peripheral AR antagonism on behavior associated with male social arousal and motivation. A, Estimated probability that the individual observing adult male behavior located the target male at its respective display arena. Values at 1.0 represent 100% estimated probability of relocation, whereas values at 0.0 represent 0% estimated probability of relocation. Each bar represents the estimate probability as calculated by our statistical model (see Materials and Methods), and there was no difference in the probability of relocating BICAL-treated birds during the experiment compared with controls (P > .05). B, Average number of chee-poo calls adult male birds broadcast at or near their arena. The N.S. notation within the legend denotes no effect of BICAL treatment (P > .05). Chee-poo data represent means ± 1 SEM. In both graphs, black bars and solid lines represent controls, whereas hatched columns and dashed lines represent BICAL treated birds.
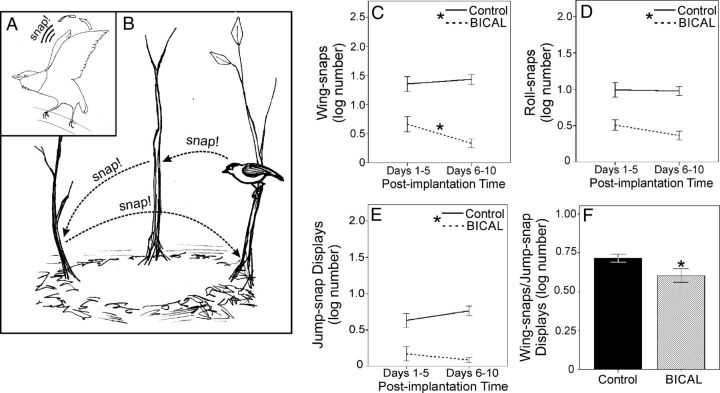
To determine whether peripheral AR influenced the acrobatic elements of the wild birds' display, we analyzed the effects of BICAL treatment on 4 unambiguous display maneuvers that demand exquisite agility and motor performance (Figure 3, A and B): 1) wing-snaps, 2) roll-snaps, 3) jump-snap displays, and 4) the number of wing-snaps per jump-snap display (20, 21, 40). Wing-snaps are loud mechanical sonations produced when males powerfully hit their wings together above their head. Similarly, roll- snaps are produced when males successively snap their wings together at a rate of approximately 50–60 snaps/sec (Hz). Jump-snap displays are the birds' main courtship dance; they are performed when males leap among saplings over a small arena on the forest floor at speeds of approximately 2.5 m/sec, and during each of these leaps, males can produce a single wing-snap in midair. All of these behaviors, including the number of snaps incorporated into jump-snap routines, are greatly reduced in response to BICAL treatment (Figure 3, C–F; wing-snaps: F1,9 = 37.77, P < .001; roll-snaps: F1,9 = 107.95, P < .001; jump-snap displays: F1,9 = 32.74, P < .001; snaps/jump snap display: t9 = 2.33, P = .045). Overall, the frequency with which most of these behaviors were produced did not change over time (wing snap: F1,9 = 2.42; P = .15; roll snap: F1,9 = 0.31; P = .59; jump snap display: F1,9 = 0.05; P = .83), and only wing-snap behavior was affected by a significant treatment × time-course interaction (wing-snap: F1,9 = 6.26; P = .034; roll-snap: F1,9 = 0.17; P = .69; jump-snap display: F1,9 = 2.51; P = .15). Analysis of this interaction showed that BICAL's suppressive effect on wing-snap behavior increased as the study progressed (Figure 3C; P < .05).
Figure 3.
Impact of peripheral AR antagonism on wild, adult male golden-collared manakin display behavior. A, Schematic of a reproductively active male hitting his wings together above his head to produce either a wing-snap sonation (single hit) or a roll-snap sonation (multiple, rapid hits). B, Schematic of a reproductively active bird performing his jump-snap display, in which he rapidly leaps from sapling to sapling around an arena that he has cleared for himself on the forest floor. During each of these leaps, the male snaps his wings together in midair. Average number of wing-snaps (C), roll-snaps (D), jump-snap displays (E), and average number of snaps per jump-snap display (F) is shown. Due to infrequent jump-snap displaying in BICAL birds (see panel E), sample sizes for snaps per jump-snap display in BICAL birds (F) were low; thus, snaps per jump-snap display data were pooled across postimplantation time. Data represent means ± 1 SEM. In all line graphs, asterisks within the legend denote a significant effect of BICAL treatment (P < .05). Asterisks above specific lines denote significant changes in behavior within individuals of the same treatment group (P < .05). Solid lines and black bars represent controls, whereas dashed lines and hatched lines represent BICAL treated birds.
A remarkable feature of wing-snap and jump-snap maneuvers is that they are performed at speeds faster than the human eye can detect. The roll-snap, for example, requires rapid and precise wing elevation and retraction, because the sonation is the product of approximately 6–20 independent wing-snap events that are repeated within a time span of about 100–350 milliseconds (Figure 4, A and B; see also Supplemental Movie 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). The average interval between snap events is roughly 19 milliseconds, and during this time, the wings are lifted above the head from a half-raised position, hit together above the head, and then retracted back to the half-raised position. As such, we investigated whether peripheral AR influenced these fine motor skills by assessing the impact of BICAL on the acoustic patterning of the roll-snap sonation (Figure 4C). Treatment with BICAL not only shortened the number of snaps included in a given roll and thus the overall duration of the roll-snap itself (F22,385 = 2.50, P < .001), but also increased the average time interval between independent snap events by around 2 milliseconds (∼10%–15%) (F1,86 = 14.49, P < .001). Furthermore, measures of the between-snap interval were affected by a significant treatment × snap-interval number interaction (F7,386 = 3.98, P < .001). This, in effect, means that BICAL caused birds to snap their wings together more slowly at the onset of a roll-snap but then speed up the rate of snapping as the roll-snap progressed (F7,132 = 3.71, P = .001; post hoc tests, P < .05). In contrast, the control birds snapped their wings together at a relatively constant rate throughout an entire roll-snap (F22,311 = 1.28, P = .181).
Discussion
Here, we show that the AR in the periphery supports the performance and fine motor coordination of the golden-collared manakin's acrobatic social display. This occurs in a manner that is independent of central AR activation, because blocking AR outside the CNS changes both the frequency with which male birds perform elaborate components of their display repertoire and certain components of the display moves themselves. Our validation studies confirm that BICAL antagonizes AR in a peripherally selective manner and does not impede an individuals' health or activity. Moreover, BICAL does not appear to impact behavior associated with the motivation to display, since treated males were found on their display areas with equal probability to controls and readily produced chee-poo vocalizations that are strongly associated with social arousal and engagement (39). Thus, our results collectively suggest that peripheral AR maintains the capacity for acrobatic performance by acting in a bottom-up manner. This may ultimately occur through some form of AR-induced gating of the processes through which instruction from the brain and spinal cord is communicated to and/or translated into intricate muscular contraction patterns throughout the body.
Androgenic control of elaborate display behavior
We hypothesize that the effects of peripheral AR on display output originate in part from androgenic action on skeletal muscles. Golden-collared manakins express high levels of muscular AR compared with other passerine species that do not exhibit elaborate social displays, and manakin wing muscles have a greater capacity to convert androgens to their more potent androgenic metabolites (22). Moreover, gene expression data from previous studies show that the androgen testosterone increases muscular mRNA expression of parvalbumin and IGF-I (34), and work presented here confirms that this action occurs via AR. The products of these two genes play important roles in regulating neuromuscular function. For example, parvalbumin enhances calcium buffering in muscle cells, which in turn increases the speed of muscle contraction cycling (41, 42). IGF-I, on the other hand, not only increases the size and strength of muscle fibers (43, 44) but also travels from the muscle to the spinal cord in a retrograde fashion in which it then acts to maintain spinal circuitry (45, 46). In light of these findings, it appears as though muscular AR influences how male birds produce complex display behavior by modulating either the functional abilities of the muscle tissue itself and/or the spinal circuits (or spinal central pattern generators) that underlie the motor programs of rapid wing and leg movement. Notably, any of these changes to the muscle and spinal cord may also adjust somatosensory processing and integration, which in theory may also affect display performance.
The effects of BICAL on display output occurred relatively rapidly, with males showing significant decreases in acrobatic display behavior within the first five days of treatment. This implies that AR inhibition influences display output by affecting muscle contractility, rather than overall muscle size. For example, a precipitous decrease in the production of parvalbumin might quickly increases the speed of muscle contraction cycling by reducing muscle relaxation time (42). On the other hand, the immediate impact of IGF-I on muscles may be more subtle (44), because changes in IGF-I production can precede corresponding modifications to muscle growth and hypertrophy (47). We can only speculate about these ideas, given that the time frame in which we assessed how BICAL influences muscular gene expression did not directly overlap with the time frame in which we assessed how BICAL influences adult male behavior. Future studies are needed to examine how variation in AR-dependent genes in muscle tissues is related to different aspects of breeding male display routines and motor skills.
Even as our results underscore the significance of peripheral AR on display output, they obviously do not exclude the central effects of AR action on normal display exhibition. For example, prior work in golden-collared manakins shows that the antiandrogen flutamide, which blocks AR both peripherally and centrally, influences all aspects of male display performance, including the number of times males produce chee-poo vocalizations (23). Given that our peripherally selective manipulation of AR did not impact the rates of chee-poo production and that flutamide blocks AR in the CNS, it appears likely that centralized populations of AR govern how frequently male birds broadcast these calls while they maintain their display sites. In addition, neuroanatomical studies of AR expression patterns within the golden-collared manakin CNS suggest that the spinal cord is an especially critical site at which androgens act to influence motor control of the wings and legs (32, 48). Thus, in the context of these past experiments, our current work suggests that androgens tightly modulate motor control of manakin social behavior at multiple levels of the neuromuscular system.
Building further on this idea, it is also possible that peripheral androgenic action influences other organ systems that either directly or indirectly impact male display behavior. Cardiovascular activity, respiration, and digestion are just a few of these processes that obviously contribute to the males' acrobatic routines, and thus preventing androgenic modulation of these systems might in theory alter display output. How androgens potentially integrate these discrete organs simultaneously for elaborate reproductive behavior is not understood and is a current interest of our laboratory.
Functional significance
Prior studies show that individual adult male golden-collared manakins vary not only in the number of times they normally produce different display moves around their arenas, but also in the speed with which they actually perform these maneuvers (30, 40, 49). This latter finding is revealed by high-speed videography of males performing their main jump-snap display routines, and it turns out that individual differences in display speeds occur on the order of milliseconds (40). Studies that focus on male-female interactions indicate that females prefer to mate with males that perform acrobatic maneuvers more frequently and fractions of a second faster than other conspecifics in the population (49). Although this past work suggests that female golden-collared manakins are able to perceive minuscule differences in male performance speed, it also implies that females can detect the changes to male behavior we induced through BICAL treatment, including the subtle millisecond-scale changes to the temporal patterning of the roll snap. Thus, peripheral AR action may enhance male motor performance in a way that affects the courtship skills and mating success of breeding males.
Evolutionary considerations
In the context of studies on animal vocal communication, our current work implies a degree of convergence in the androgenic mechanisms that modulate adaptive acoustic signal repetition. The roll snap is a repetitive, broad-frequency acoustic signal (Figure 4, A and B) and is used by females to assess male motor skills when making mate choice decisions (49). In this way, roll snaps are reminiscent of trills, or rapidly repeated notes, that are part of many avian (50, 51) and mammalian vocal displays (52, 53). In these species, performance limits constrain the production of trills (54), and thus trill rate can signal an individual's competitive ability and attractiveness (55, 56). The few studies that investigate the effects of androgens on trill production suggest that the activation of AR influences the length and temporal patterning of trill bouts within a song (53); however, this work does not tease apart peripheral and central effects of androgens on trill performance. This past work and our current study imply that AR plays a role in modulating mechanisms that control signal repetition, regardless of whether the signal is vocal, and thus originates from vocal organs (ie, syrinx or larynx), or whether the signal is mechanical, and thus originates from specialized body and limb movement. Our results therefore also provide broader insight into the evolution of elaborate and unusual display routines, such as roll snaps and trills.
Finally, it is notable that androgens likely act within the golden-collared manakins' wing musculature to refine complex reproductive display behavior, because these muscles also are crucial for day-to-day nonreproductive activities (57). As indicated above, in most other research examining androgen-muscle interactions, the muscles under examination serve predominantly sex-specific functions (11–15), such that androgenic effects on these tissues most likely have little influence on the ability to perform basic locomotory activities. In golden-collared manakins, however, the tissues on which AR acts regulate the lifting and retraction of the bird's wings (57), and thus modifications to the basic anatomy and/or physiology of these tissues for reproductive behavior may uniquely impose a cost on the level of optimal flight performance that is otherwise necessary for daily living. Further research is needed to explore these possible trade-offs between different muscular needs for sociosexual behavior and survival.
Conclusions
In summary, we show that activation of peripheral AR sustains the acrobatic abilities and fine motor skills that are necessary for male golden-collared manakins to perform their elaborate sociosexual displays. Given that these effects are coupled with motor control and coordination and that this species expresses high levels of AR in its muscle tissues (22), we conclude that peripheral AR modulates the output of complex behavior by acting within the musculoskeletal system itself.
Acknowledgments
We thank Andy Bass for helpful conversations about this manuscript and data. We also thank Michelle Rensel, Kyla Davidoff, Joy Eaton, and Mital Shah for assistance collecting and analyzing data. We also thank STRI and its administrative staff for their assistance with this project as well as Autoridad Nacional del Ambiente and the Autoridad del Canal de Panama for permission to conduct these studies. M.J.F. was supported in part by National Institutes of Health Training Grant T32 HD007228 awarded to the Laboratory of Neuroendocrinology at the University of California, Los Angeles.
This work was supported by National Science Foundation Grant IOS-0646459 (to B.A.S.).
Disclosure Summary: The authors have nothing to declare.
Footnotes
- AR
- androgen receptor
- BICAL
- bicalutamide
- CNS
- central nervous system
- qPCR
- quantitative PCR
- STRI
- Smithsonian Tropical Research Institute.
References
- 1. Hogg JT , Forbes SH. Mating in bighorn sheep: frequent male reproduction via a high-risk “unconventional” tactic. Behav Ecol Sociobiol. 1997;41:33–48. [Google Scholar]
- 2. Thorbjarnarson JB , Hernandez G. Reproductive ecology of the Orinoco crocodile (Crocodylus intermedius) in Venezuela. II. Reproductive and social behavior. J Herpetol. 1993;27:371–379. [Google Scholar]
- 3. Masonjones HD , Lewis SM. Courtship behavior in the dwarf seahorse, Hippocampus zosterae. Copeia. 1996;634–640. [Google Scholar]
- 4. Nuechterlein GL , Storer RW. The pair formation displays of the western grebe. Condor. 1982;84:350–369. [Google Scholar]
- 5. Bostwick KS , Prum RO. High-speed video analysis of wing-snapping in two manakin clades (Pipridae: Aves). J Exp Biol. 2003;206:3693–3706. [DOI] [PubMed] [Google Scholar]
- 6. Frith CB , Cooper WT. Courtship display and mating of Victoria's riflebird, Ptiloris victoriae, with notes on the courtship displays of congeneric species. Emu. 1996;96:102–113. [Google Scholar]
- 7. Adkins-Regan E. Hormones and Animal Social Behavior. Princeton, NJ: Princeton University Press; 2005. [Google Scholar]
- 8. Regnier M , Herrera AA. Differential sensitivity to androgens within a sexually dimorphic muscle of male frogs (Xenopus laevis). J Neurobiol. 1993;24:1215–1228. [DOI] [PubMed] [Google Scholar]
- 9. Michel G , Baulieu EE. Androgen receptor in rat skeletal muscle: characterization and physiological variations. Endocrinology. 1980;107:2088–2098. [DOI] [PubMed] [Google Scholar]
- 10. Kadi F , Bonnerud P , Eriksson A , Thornell LE. The expression of androgen receptors in human neck and limb muscles: effects of training and self-administration of androgenic-anabolic steroids. Histochem Cell Biol. 2000;113:25–29. [DOI] [PubMed] [Google Scholar]
- 11. Rand MN , Breedlove SM. Androgen locally regulates rat bulbocavernosus and levator ani size. J Neurobiol. 1992;23:17–30. [DOI] [PubMed] [Google Scholar]
- 12. Regnier M , Herrera AA. Changes in the contractile properties by androgen hormones in sexually dimorphic muscles of male frogs (Xenopus laevis). J Physiol (Camb). 1993;461:565–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brantley RK , Marchaterre MA , Bass AH. Androgen effects on vocal muscle structure in a teleost fish with intersexual and intrasexual dimorphism. J Morphol. 1993;216:305–318. [DOI] [PubMed] [Google Scholar]
- 14. Fischer L , Catz D , Kelley D. An androgen receptor messenger-RNA isoform associated with hormone induced cell proliferation. Proc Nat Acad Sci USA. 1993;90:8254–8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Veney SL , Wade J. Steroid receptors in the adult zebra finch syrinx: a sex difference in androgen receptor mRNA, minimal expression of estrogen receptor alpha and aromatase. Gen Comp Endocrinol. 2004;136:192–199. [DOI] [PubMed] [Google Scholar]
- 16. Brenowitz EA , Lent K. Act locally and think globally: Intracerebral testosterone implants induce seasonal-like growth of adult avian song control circuits. Proc Nat Acad Sci USA. 2002;99:12421–12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Remage-Healey L , Joshi NR. Changing neuroestrogens within the auditory forebrain rapidly transform stimulus selectivity in a downstream sensorimotor nucleus. J Neurosci. 2012;32:8231–8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barclay SR , Harding CF. Differential modulation of monoamine levels and turnover rates by estrogen and/or androgen in hypothalamic and vocal control nuclei of male zebra finches. Brain Res. 1990;523:251–262. [DOI] [PubMed] [Google Scholar]
- 19. Soma KK , Wissman AM , Brenowitz EA , Wingfield JC. Dehydroepiandrosterone (DHEA) increases territorial song and the size of an associated brain region in a male songbird. Horm Behav. 2002;41:203–212. [DOI] [PubMed] [Google Scholar]
- 20. Schlinger BA , Day LB , Fusani L. Behavior, natural history and neuroendocrinology of a tropical bird. Gen Comp Endocrinol. 2008;157:254–258. [DOI] [PubMed] [Google Scholar]
- 21. Chapman FM. The courtship of Gould's manakin (Manacus vitellinus vitellinus) on Barro Colorado Island, Canal Zone. Bull Am Museum Nat Hist. 1935;68:472–521. [Google Scholar]
- 22. Feng NY , Katz A , Day LB , Barske J , Schlinger BA. Limb muscles are androgen targets in an acrobatic tropical bird. Endocrinology. 2010;151:1042–1049. [DOI] [PubMed] [Google Scholar]
- 23. Fusani L , Day LB , Canoine V , Reinemann D , Hernandez E , Schlinger BA. Androgen and the elaborate courtship behavior of a tropical lekking bird. Horm Behav. 2007;51:62–68. [DOI] [PubMed] [Google Scholar]
- 24. Freeman SN , Mainwaring WIP , Furr BJA. A possible explanation for the peripheral selectivity of a novel non-steroidal pure antiandrogen, Casodex (ICI 176,334). Br J Cancer. 1989;60:664–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Furr BJA. Casodex (ICI-176,334)—a new, pure, peripherally selective anti-androgen: preclinical studies. Horm Res. 1989;32:69–76. [DOI] [PubMed] [Google Scholar]
- 26. Soma KK , Sullivan K , Tramontin AD , Saldanha CJ , Schlinger BA , Wingfield JC. Acute and chronic effects of an aromatase inhibitor on territorial aggression in breeding and nonbreeding male song sparrows. J Comp Physiol A. 2000;186:759–769. [DOI] [PubMed] [Google Scholar]
- 27. Fusani L , Gahr M , Hutchison JB. Aromatase inhibition reduces specifically one display of the ring dove courtship behavior. Gen Comp Endocrinol. 2001;122:23–30. [DOI] [PubMed] [Google Scholar]
- 28. Fennell MJ , Radecki SJ , Proudman JA , Scanes CG. The suppressive effects of testosterone on growth in young chickens appears to be mediated via peripheral androgen receptor; studies of the anti-androgen ICI 176,334. Poult Sci. 1996;75:763–766. [DOI] [PubMed] [Google Scholar]
- 29. Sperry TS , Wacker DW , Wingfield JC. The role of androgen receptors in regulating territorial aggression in male song sparrows. Horm Behav. 2010;57:86–95. [DOI] [PubMed] [Google Scholar]
- 30. Day LB , Fusani L , Hernandez E , et al. . Testosterone and its effects on courtship in golden-collared manakins (Manacus vitellinus): seasonal, sex, and age differences. Horm Behav. 2007;51:69–76. [DOI] [PubMed] [Google Scholar]
- 31. Day LB , McBroom JT , Schlinger BA. Testosterone increases display behaviors but does not stimulate growth of adult plumage in male golden-collared manakins (Manacus vitellinus). Horm Behav. 2006;49:223–232. [DOI] [PubMed] [Google Scholar]
- 32. Fuxjager MJ , Schultz JD , Barske J , et al. . Spinal motor and sensory neurons are androgen targets in an acrobatic bird. Endocrinology. 2012;153:3780–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McDonald JB , Clay RP , Brumfield RT , Braun MJ. Sexual selection on plumage and behavior in an avian hybrid zone: experimental tests of male-male interactions. Evolution. 2001;55:1443–1451. [DOI] [PubMed] [Google Scholar]
- 34. Fuxjager MJ , Barske J , Du S , Day L , Schlinger BA. Androgens regulate gene expression in avian skeletal muscles. PLoS One. 2012;7:e51482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tomaszycki ML , Peabody C , Replogle K , Clayton DF , Tempelman RJ , Wade J. Sexual differentiation of the zebra finch song system: potential roles for sex chromosome genes. BMC Neurosci. 2009;10:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harada N , Abedohmae S , Loeffen R , Foidart A , Balthazart J. Synergism between androgens and estrogens in the induction of aromatase and its messenger RNA in the brain. Brain Res. 1993;622:243–256. [DOI] [PubMed] [Google Scholar]
- 37. Wu D , Tang YP , Wade J. Co-localization of Sorting Nexin 2 and androgen receptor in the song system of juvenile zebra finches. Brain Res. 2010;1343:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lu S , Simon NG , Wang Y , Hu S. Neural androgen receptor regulation: effects of androgen and antiandrogen. J Neurobiol. 1999;41:505–512. [DOI] [PubMed] [Google Scholar]
- 39. Schlinger BA , Schultz JD , Hertel F. Neuromuscular and endocrine control of an avian courtship behavior. Horm Behav. 2001;40:276–280. [DOI] [PubMed] [Google Scholar]
- 40. Fusani L , Giordano M , Day LB , Schlinger BA. High-speed video analysis reveals individual variability in the courtship displays of male golden-collared manakins. Ethology. 2007;113:964–972. [Google Scholar]
- 41. Heizmann CW , Berchtold MW , Rowlerson AM. Correlation of parvalbumin concentration with relaxation speed in mammalian muscle. Proc Natl Acad Sci USA. 1982;79:7243–7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Muntener M , Kaser L , Weber J , Berchtold MW. Increase of skeletal muscle relaxation speed by direct injection of parvalbumin cDNA. Proc Natl Acad Sci USA. 1995;92:6504–6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Musaro A , McCullagh KJA , Naya FJ , Olson EN , Rosenthal N. IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature. 1999;400:581–585. [DOI] [PubMed] [Google Scholar]
- 44. Adams GR , McCue SA. Localized infusion of IGF-I results in skeletal muscle hypertrophy. J Appl Physiol. 1998;84:1716–1722. [DOI] [PubMed] [Google Scholar]
- 45. Kaspar BK , Llado J , Sherkat N , Rothstein JD , Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. [DOI] [PubMed] [Google Scholar]
- 46. Dobrowolny G , Giacinti C , Pelosi L , et al. . Muscle expression of a local Igf-1 isoform protects motor neurons in an ALS mouse model. J Cell Biol. 2005;168:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Adams GR , Haddad F , Baldwin KM. Time course of changes in markers of myogenesis in overloaded rat skeletal muscles. J Appl Physiol. 1999;87:1705–1712. [DOI] [PubMed] [Google Scholar]
- 48. Schultz JD , Schlinger BA. Widespread accumulation of [H-3]testosterone in the spinal cord of a wild bird with an elaborate courtship display. Proc Natl Acad Sci USA. 1999;96:10428–10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Barske J , Schlinger BA , Wikelski M , Fusani L. Female choice for male motor skills. Proc R Soc Lond Ser B Biol Sci. 2011;278:3523–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. de Kort SR , Eldermire ERB , Valderrama S , Botero CA , Vehrencamp SL. Trill consistency is an age-related assessment signal in banded wrens. Proc R Soc B Biol Sci. 2009;276:2315–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sockman KW. Annual variation in vocal performance and its relationship with bill morphology in Lincoln's sparrows, Melospiza lincolnii. Anim Behav. 2009;77:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Behr O , von Helversen O. Bat serenades—complex courtship songs of the sac-winged bat (Saccopteryx bilineata). Behav Ecol Sociobiol. 2004;56:106–115. [Google Scholar]
- 53. Pasch B , George AS , Hamlin HJ , Guillette LJ , Phelps SM. Androgens modulate song effort and aggression in neotropical singing mice. Horm Behav. 2011;59:90–97. [DOI] [PubMed] [Google Scholar]
- 54. Podos J. A performance constraint on the evolution of trilled vocalizations in a songbird family (Passeriformes: Emberizdae). Evolution. 1997;51:537–551. [DOI] [PubMed] [Google Scholar]
- 55. Pasch B , George AS , Campbell P , Phelps SM. Androgen-dependent male vocal performance influences female preference in neotropical singing mice. Anim Behav. 2011;82:177–183. [Google Scholar]
- 56. Illes AE , Hall ML , Vehrencamp SL. Vocal performance influences male receiver response in the banded wren. Proc R Soc B Biol Sci. 2006;273:1907–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dial KP. Activity patterns of the wing muscles of the pigeon (Columbia livia) during different modes of flight. J Exp Zool. 1992;262:357–373. [Google Scholar]