Abstract
Pigment epithelium-derived factor (PEDF) is an antiinflammatory protein that circulates at high levels in the metabolic syndrome. Metabolic studies of PEDF knockout (KO) mice were conducted to investigate the relationship between PEDF, inflammatory markers, and metabolic homeostasis. Male PEDF KO mice demonstrated a phenotype consisting of increased adiposity, glucose intolerance, and elevated serum levels of metabolites associated with the metabolic syndrome. Genome expression analysis revealed an increase in IL-1β signaling in the livers of PEDF KO mice that was accompanied by impaired IRS and Akt signaling. In human hepatocytes, PEDF blocked the effects of an IL-1β challenge by suppressing activation of the inflammatory mediator c-Jun N-terminal kinase while restoring Akt signaling. RNA interference of PEDF in human hepatocytes was permissive for c-Jun N-terminal kinase activation and decreased Akt signaling. A metabolomics profile identified elevated circulating levels of tricarboxyclic acid cycle intermediates including succinate, an inducer of IL-1β, in PEDF KO mice. Succinate-dependent IL-1β expression was blocked by PEDF in PEDF KO, but not wild-type hepatocytes. In vivo, PEDF restoration reduced hyperglycemia and improved hepatic insulin signaling in PEDF KO mice. These findings identify elevated PEDF as a homeostatic mechanism in the human metabolic syndrome.
Obesity is a central feature of the metabolic syndrome and is associated with low-grade inflammation and impaired insulin signaling (1). Cytokines such as IL-1β are implicated, and its blockade improves insulin signaling (2–5). The mechanisms that link obesity with IL-1β release are increasingly recognized (6, 7). The counterregulatory mechanisms that maintain glucose homeostasis, however, are less appreciated.
Pigment epithelium-derived factor (PEDF) is a 50-kDa secreted pleiotropic glycoprotein that is an endogenous inhibitor of angiogenesis and inflammation (8, 9). Clinical studies have identified PEDF as a biomarker of the metabolic syndrome with higher circulating levels corresponding to components of the metabolic syndrome including adiposity, insulin resistance, and nonalcoholic fatty liver disease (10–15). In diabetic patients treated with metformin, PEDF levels increased, raising the possibility of an insulin-sensitizing role (16). Thus, clinical studies have determined PEDF's association with the metabolic syndrome, but its function is unclear.
One mechanism by which PEDF may act in a protective manner is through its antiinflammatory activity (9). Kidney cells exposed to high-glucose media released inflammatory cytokines such as MCP-1, which were blocked with PEDF (9). Retinas in PEDF-overexpressing mice demonstrated reduced injury and inflammatory cytokines in response to oxygen deprivation (17). Moreover, PEDF injection into the eyes of diabetic rats decreased IL-1β levels (18). These studies demonstrated PEDF's antiinflammatory effects in 2 organs typically affected by diabetes.
Other experimental studies have provided conflicting data on PEDF's role in metabolism. PEDF infusion in lean wild-type (WT) mice led to activation of the inflammatory mediator c-Jun N-terminal kinase (JNK) resulting in insulin resistance (19, 20). These studies focused on acute (hours) and short-term (5 days) effects, however (20, 21). We previously showed that PEDF decreases hepatocyte lipid accumulation, suggesting a homeostatic role (22, 23). Recently, our group and others identified PEDF's ability to modulate Wnt signaling (24, 25). Although best known as a developmental pathway, Wnt signaling also regulates metabolic and inflammatory pathways, with polymorphisms in the Wnt target gene TCF7L2 emerging as among the strongest risk factors for type 2 diabetes development (26–28). Wnt signaling occurs through a canonical pathway with β-catenin translocation to the nucleus, or a noncanonical pathway that converges on JNK as its central effector (29). Because PEDF-mediated JNK activation was previously reported to decrease insulin signaling (20), genomic, metabolic phenotyping, and metabolomic studies were performed in PEDF KO mice and in human hepatocytes to investigate this functional interaction.
Here, we report that PEDF knockout (KO) mice recapitulate features of the metabolic syndrome with increased adiposity, liver steatosis, impaired glucose tolerance, and a serum metabolomic profile that consisted of increased proinflammatory metabolites. Elevated circulating succinate, a recently identified inducer of IL-1β expression (6), and other tricarboxyclic acid (TCA) cycle intermediates were identified in the sera of PEDF KO mice, indicating enhanced metabolic flux as the source of succinate. Succinate challenge could induce IL-1β expression in PEDF KO hepatocytes that could be blocked with PEDF. Genome-wide expression analysis confirmed induction of IL-1β expression in the livers of PEDF KO mice. In murine and human hepatocytes, PEDF significantly suppressed IL-1β-mediated JNK activation while improving insulin signal transduction. These findings indicate that PEDF functions to counter the inflammatory consequences of the metabolic syndrome.
Materials and Methods
Animals
PEDF KO mice were bred with WT C57BL/6J mice to generate heterozygous breeding pairs, which were used to generate KO and WT breeding pairs (22). Mice were backcrossed for greater than 10 generations. Male mice (2–7 months of age) were used for experiments. Mice were maintained in normal SPF conditions and fed a standard mouse chow diet ad libitum. Mice undergoing insulin stimulation were given insulin 1 mU/g body weight and euthanized after 15 minutes. After cervical dislocation, liver and white adipose tissues were harvested, snap frozen in liquid nitrogen, and blood was collected by cardiac puncture. Glucose measurements were done from a tail vein puncture using Accu-Check (Bayer) after 6 hours fast. A high-fat diet (45% kJ from fat; Research Diets, catalog no. D12451) was used for 1 week for specific experiments. All experiments were done in accordance to the Veterans Affairs and Yale University Institutional Animal Use and Care Committees.
Metabolic testing
Glucose tolerance tests were performed as described elsewhere (30). Mice (7 month-old) were fasted for 6 hours and injected with 1.5 g/kg glucose ip, and glucose and insulin measurements were obtained at 30-minute intervals for 2 hours. Magnetic resonance spectroscopy (MRS) to measure total body fat content was assessed using 1H MRS (Bruker BioSpin). Metabolic phenotyping and insulin clamp studies (3 and 7 month-old) were performed at the Yale Mouse Metabolic Phenotyping Center (MMPC). Liver triglycerides were quantified using a commercial assay (Wako Diagnostics). Prolonged fasting (18 hours) was done in 2-month-old mice to assess gluconeogenic enzymes.
RNA analysis, gene microarray, and quantitative PCR
Total RNA from livers was isolated using Trizol (Invitrogen) for gene microarray analysis and quantitative RT-PCR. Integrity and purity of RNA was assessed by a BioAnalyzer 2100 (Agilent Technologies) at Yale's Center for Genome Analysis. Primer sets (Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org) were commercially obtained (Applied Biosystems). Quantitative RT-PCR of the target genes was performed on a TaqMan ABI 7500 system (Applied Biosystems) (31).
Metabolomics profiling
Serum biochemical alterations related to the absence of PEDF were assessed from 16-week old PEDF KO and age-matched control mice on a normal chow diet (n = 6/group) using a commercial vendor (Metabolon). Samples were processed for gas chromatography/mass spectrometry and liquid chromatography/tandem mass spectrometry platforms as described elsewhere (32).
Cell culture and primary hepatocyte/Kupffer cell isolation
Primary murine hepatocytes were obtained as described previously (23). Livers were perfused with Hanks' A and then Hanks' B medium containing 0.05% collagenase (Boehringer Mannheim Biochemicals) and 0.8 U trypsin inhibitor (Sigma). Primary murine Kupffer cells were obtained as described elsewhere (33) and plated onto 6-well plates, and supernatants were collected for IL-1β measurements after palmitate exposure. Primary human hepatocytes were obtained from the Yale Liver Center through a consortium agreement with the University of Pittsburgh. Informed consent in writing was obtained from each patient, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Human hepatocytes were obtained either from unused donor livers or from normal liver parenchyma in patients undergoing resection. Primary hepatocytes were incubated for 24 hours for acclimatization and assessed for viability. Cells were maintained in 10% DMEM + insulin for the next 24 hours until experiments were performed. Primary murine hepatocytes were isolated from age-matched WT and PEDF KO mice (age 2 months). Briefly, isolated primary hepatocytes were plated in Williams' Medium E (Invitrogen) and supplemented with 10% fetal bovine serum (Invitrogen), 10 mM HEPES, 2 mM L-Glutamine (Sigma), 1 μM dexamethasone (Sigma), 4 mg/L insulin (Sigma), 1% penicillin, and streptomycin. Media were changed after 24 hours, and experiments were conducted 48 hours after isolation. In experiments studying insulin signaling, cells were treated with 100 nM recombinant human insulin (Novo Nordisk) for 15 minutes. IL-1β (10 ng/mL, R&D Systems) and PEDF (500 ng/mL) were added 15 minutes and 2 hours before completion of experiments, respectively. Studies involving primary cells were performed at least 3 separate times.
For succinate experiments, murine hepatocytes were isolated as above. Media was changed to 10% DMEM with glutamine + insulin for 24 hours and then challenged with 5 mM succinate (diethyl succinate, Sigma) for 12–16 hours.
Stromovascular fraction isolation
Subcutaneous fat pads were dissected from age-matched WT and PEDF KO mice and digested in Hanks balanced salt solution medium containing 3% BSA (American Bioanalytical), 0.8 mg/mL of type 2 collagenase (Worthington Chemicals), 1.2 mM CaCl2, 1.0 mM MgCl2, and 0.8 mM ZnCl2 for 1 hour. Stromovascular fractions were obtained after differential centrifugation. Adipogenic differentiation cocktail was added on day 0 for 3 days. Cells were harvested after differentiation into adipocytes which was confirmed by Oil Red O staining. IL-1β levels were assessed using an ELISA (R&D Systems) 18 hours after palmitate challenge, and lysates were obtained from the same cells for insulin signal transduction assessments.
Palmitate-BSA coupling
Sodium palmitate (Sigma) was dissolved in 150 mM NaCl at 70°C. This solution was added to fatty acid-free BSA solution at 37°C at a molar ratio of 6:1. BSA was conjugated with palmitate for 1 hour at 37°C. Concentrations were confirmed by Fatty Acid kit (Wako Diagnostics). For all experiments palmitate was used at 0.5 mM.
Immunoblotting
Proteins were separated by 10% SDS-PAGE on gradient gels (Bio-Rad Laboratories). Antibodies against phospho-tyrosine (Millipore Corp.), p-IRS-1 S307, insulin receptor substrate (IRS)-2, p-JNK Ty183/185, p-P38 MAPK, p-c-Jun Ser63, p-NF-kβ p65, p-Iκκ-β S176/180, total Akt, p-Akt S473, p-Akt Thr308, phosphoenol pyruvate carboxykinase (PEPCK), forkhead box O-1 (FoxO-1) thr24 (Cell Signaling Technologies), pyruvate carboxylase (Santa Cruz Biotechnology), succinyl lysine residues (PTM Biolab, PTM-401,), IL-1β (R&D), and β-actin (Sigma) were used. For immunoprecipitation experiments, cell lysates were incubated with Dynabeads (Invitrogen) at 4°C for 2 hours. Rabbit IgG isotype (Cell Signaling Technologies) control was used.
PEDF protein and PEDF restoration
Recombinant human PEDF was generated and integrity confirmed by silver staining. For in vitro experiments PEDF was added at 500 ng/mL (10 nM) for 2 hours unless otherwise specified. PEDF (25 μg/kg body weight) was injected ip on alternate days for a period of 4 weeks.
PEDF microspheres
Recombinant PEDF protein was packaged into alginate microspheres as described elsewhere (34). PEDF microspheres were assessed for their ability to release protein for up to 3 weeks. PEDF microspheres (10 μg) were implanted in the epididymal fat pads of PEDF KO mice. Blood glucose was monitored weekly by tail vein puncture. At 4 weeks, mice were euthanized for tissue and serum collection.
Statistics
Student's t test was used to compare 2 groups and one-way ANOVA with Bonferroni post hoc test was used for comparisons between more than 2 groups and expressed as means ± SEM. After analysis, results were normalized to the mean of the control group for ease of comparison.
Results
PEDF KO mice display features of the human metabolic syndrome
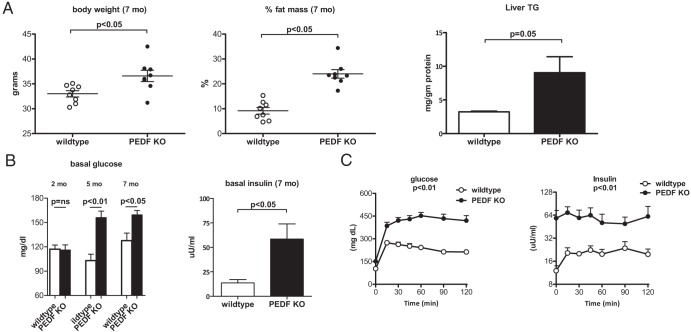
Metabolic parameters were assessed in male PEDF KO mice and controls at 2, 5, and 7 months of age. No differences in body weights were seen in 2-month-old mice (data not shown). At 7 months of age, body weights were modestly higher in PEDF KO mice (Figure 1A). Despite similar body weights, PEDF KO mice had a more than 2-fold increase in total body adiposity at 7 months by H1 MRS that was also reflected in increased liver triglyceride content (Figure 1A). Under 6-hour fasting conditions, young (2 months of age) PEDF KO mice had glucose levels similar to controls. Older (5 and 7 months of age) KO mice demonstrated significantly higher fasting glucose and insulin levels compared with controls, indicating an age-dependent onset of fasting hyperglycemia (Figure 1B). In older mice, PEDF deficiency led to markedly abnormal glucose tolerance tests without a compensatory decrease in glucose 2 hours after glucose challenge (Figure 1C). Insulin levels remained relatively constant in PEDF KO mice at all time points during the study, suggesting already maximal insulin output in PEDF KO mice (Figure 1C). Thus, PEDF KO mice display a metabolic phenotype with increased fat mass and impaired glucose homeostasis that worsens with age.
Figure 1.
PEDF KO mice have increased total body adiposity and impaired glucose tolerance testing. A, Seven-month old PEDF KO mice display slightly higher body weights, a more than 2-fold higher % fat mass by 1H MRS, and modestly elevated hepatic triglyceride (TG) content than controls. B, Serum glucose levels at 2, 5, and 7 months of age with elevated insulin levels in older KO mice. C, Impaired glucose tolerance tests with elevated insulin levels in PEDF KO mice. (n = 8 mice/group).
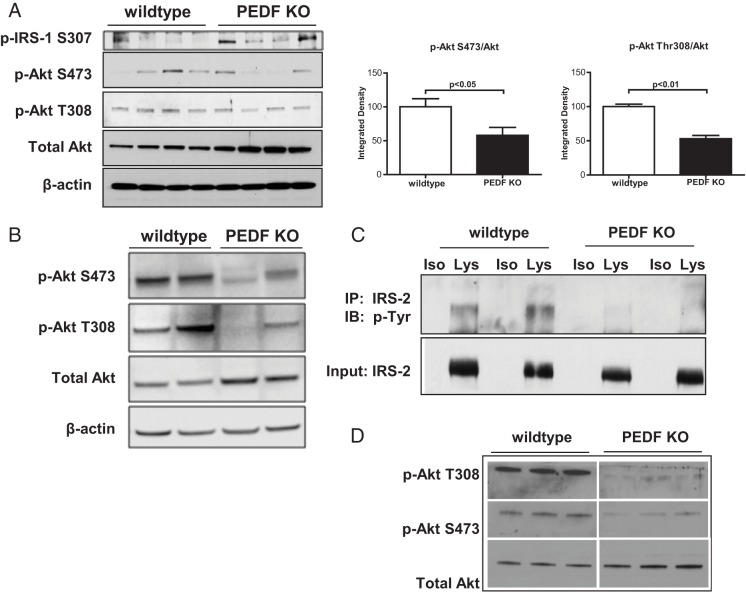
Because basal glucose and insulin levels were higher in PEDF KO mice, hepatic insulin signaling was interrogated. PEDF KO livers from 7-month old mice demonstrated significantly higher p-IRS1 S307 (Figure 2A) indicative of defective proximal insulin signaling. Immunoblots revealed lower p-Akt T308 to total Akt and p-Akt S473 to total Akt ratios in PEDF KO compared with control livers, consistent with reduced insulin signal transduction in the absence of insulin (Figure 2A). In insulin-stimulated mice, PEDF KO liver lysates also demonstrated defects in Akt signal transduction that was driven in part by increased levels of total Akt (Figure 2B). Furthermore, insulin-stimulated primary PEDF KO hepatocytes demonstrated diminished levels of phosphotyrosine IRS-2, the main mediator of proximal insulin signaling in hepatocytes (Figure 2C), and lower p-Akt to total Akt ratios (Figure 2D). These findings indicate that absence of PEDF results in diminished insulin signaling in PEDF KO livers and in isolated primary hepatocytes.
Figure 2.
PEDF KO mice show decreased hepatocyte insulin signaling. A, Decreased insulin signaling in total liver lysates as reflected by increased IRS-1 Ser307 phosphorylation and diminished pAkt/total Akt levels. B, Diminished pAkt/total Akt signaling in PEDF KO liver lysates after insulin stimulation in mice. C, Reduced tyrosine phosphorylation of IRS-2 in primary KO hepatocytes compared with WT controls. D, Decreased pAkt/total Akt in KO hepatocytes compared with WT controls after insulin (100 nM) stimulation for 15 minutes. IP, immunoprecipitation; IB, immunoblot; Iso, isotype control; Lys, lysate. (n = 6–10 mice for whole livers; n = 3 wells for primary hepatocytes)
Despite diminished Akt signaling in KO murine livers and hepatocytes, insulin sensitivity as measured by hyperinsulinemic-euglycemic clamp studies on 3-month (Supplemental Figure 1) and 7-month old mice (data not shown) did not reveal differences in global insulin sensitivity. Hepatic glucose production was significantly higher in KO mice, and a trend toward higher insulin levels was apparent in the basal state. However, hepatic glucose production could be suppressed equally upon insulin infusion, and glucose infusion rate during clamp studies was not different between groups (Supplemental Figure 1). Thus, PEDF KO mice display glucose intolerance and diminished hepatic insulin signaling but respond to the levels of insulin used during hyperinsulinemic clamp studies.
PEDF deficiency results in increased IL-1β-mediated inflammation in the liver
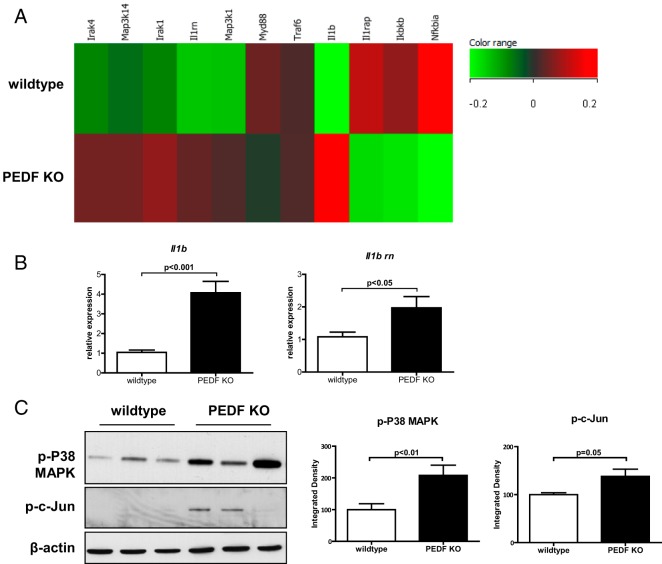
Because systemic insulin resistance parameters (Supplemental Figure 1) did not reflect the reduced insulin transduction seen in KO livers and hepatocytes (Figure 2), we investigated potential modifiers of insulin signaling in the liver using genomic microarrays on whole-liver RNA. Gene set enrichment analysis (http://www.broadinstitute.org) showed that genes from inflammatory processes (chemokine receptor binding, chemokine, and cytokine activities) were among the top up-regulated gene ontology categories. Specifically, a prominent up-regulation in Il1b was noted with hierarchical clustering of genes in the IL-1 receptor pathway in KO livers (Figure 3A). Downstream of IL-1 receptor, the expression of stress mediators such as JNK, and c-jun were significantly elevated in PEDF KO livers compared with controls (data not shown). Genomic profiling therefore highlights the IL-1β pathway and stress mediators such as JNK and c-Jun as potential links between PEDF deficiency and impaired glucose homeostasis.
Figure 3.
Gene expression analysis of PEDF KO livers identifies induction of IL-1β signaling. A, Gene Set Enrichment Analysis analysis reveals an increase in Il1b-related genes. B, Confirmation of elevated Il1b gene and its antagonist Il1rn expression by quantitative PCR. C, Downstream inflammatory signaling mediators of IL-1β, p-cJun, and p-P38, were increased in hepatic lysates. (n = 4–5 mice for GSEA; n = 8 mice for other experiments).
We confirmed the gene array findings by showing a corresponding increase in Il1b and Il1rn expression by quantitative PCR (Figure 3B). Downstream mediators of IL-1β, the active phosphorylated forms of P38 MAPK and c-Jun, were increased in PEDF KO livers (Figure 3C). We failed, however, to find elevated circulating IL-1β or other inflammatory cytokines in the sera of KO animals (data not shown). Instead, increased hepatic IL-1β expression and inflammatory mediator activation are suggestive of a localized effect at the level of hepatocytes.
A metabolomic profile identifies succinate as a metabolite inducer of IL-1β in PEDF KO mice
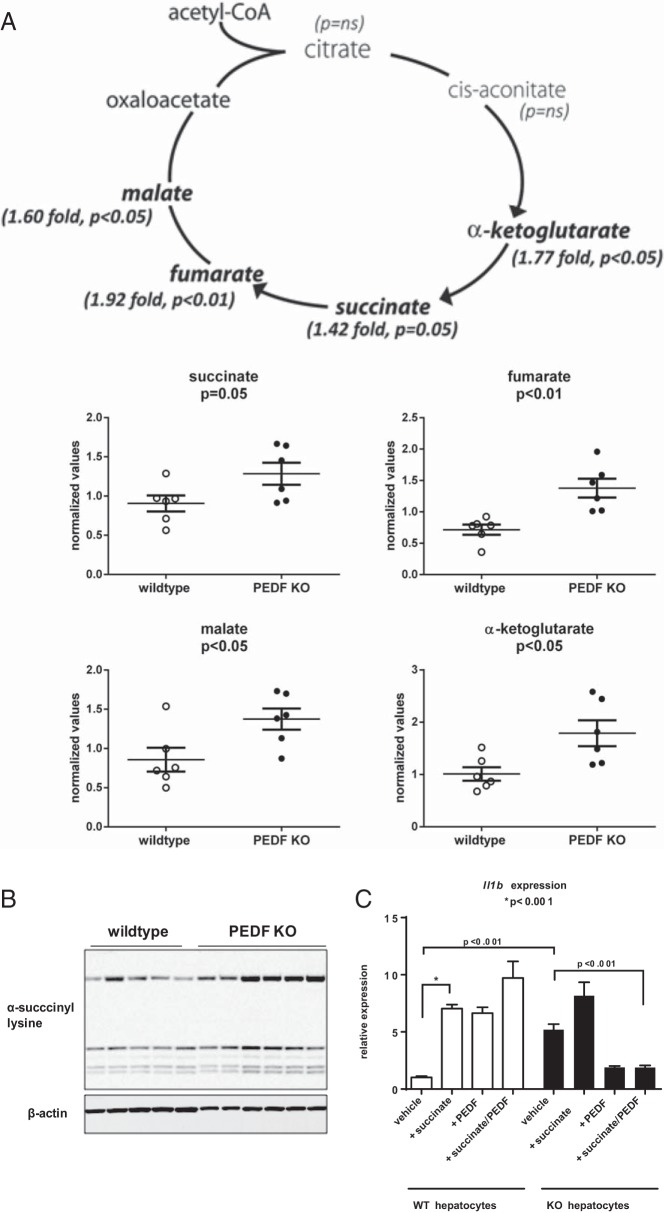
The lack of systemic insulin resistance suggested other tissue-specific inducers of IL-1β expression in PEDF KO mice. A metabolomics screen identified 44 of 298 biochemicals that were differentially expressed in PEDF KO mice with P ≤ .05 (Figure 4 and Supplemental Figure 2). In particular, PEDF KO mice had significantly higher levels of the TCA cycle intermediates α-ketoglutarate, succinate, fumarate, and malate compared with controls (Figure 4A). Of the TCA intermediates that could be linked to IL-1β induction, succinate was chosen as the most likely (6). In fact, we observed increased succinylation of proteins in PEDF KO liver lysates using an antibody directed against succinyl lysine residues (Figure 4B). Consistent with the gene expression analysis, PEDF KO hepatocytes had a 5-fold increase in IL-1β compared with WT hepatocytes at baseline (Figure 4C). In WT hepatocytes, succinate challenge increased IL-1β expression more than 6-fold with the surprising observation that PEDF itself could similarly raise expression levels (Figure 4C). In marked contrast, PEDF markedly suppressed IL-1β expression in response to succinate in PEDF KO hepatocytes (Figure 4C). These results identify succinate-dependent IL-1β expression in hepatocytes, with PEDF capable of suppressing this response.
Figure 4.
Metabolomic profiling identifies a preferential increase in tricarboxylic acid (TCA) cycle intermediates known to increase IL-1β. A, Sera from control and PEDF KO mice demonstrates elevated levels of specific TCA cycle intermediates in PEDF KO mice (bold). B, Succinyl lysine residues are increased in lysates from PEDF KO livers. C, Succinate results in enhanced Il1b expression in WT and PEDF KO hepatocytes with PEDF pretreatment inhibiting Il1b expression in KO hepatocytes (n = 6/group for metabolomics study; n = 5–8 for succinyl lysine residues; n = 3/group for Il1b expression).
The metabolomics profile identified other markers in PEDF KO mice that have been associated with the metabolic syndrome (Supplemental Figure 2). PEDF KO mice demonstrated a highly significant elevation of α-hydroxyvalerate, an insulin secretagogue (35). Other biomarkers of early insulin resistance in patients such as α-hydroxybutyrate trended toward higher levels but did not reach statistical significance (32, 36). Common laboratory parameters seen in the metabolic syndrome, such as elevated serum uric acid and cholesterol, were also higher in PEDF KO mice (Supplemental Figure 2). Thus, PEDF deficiency leads to a serum metabolic profile that is commonly seen in the human metabolic syndrome.
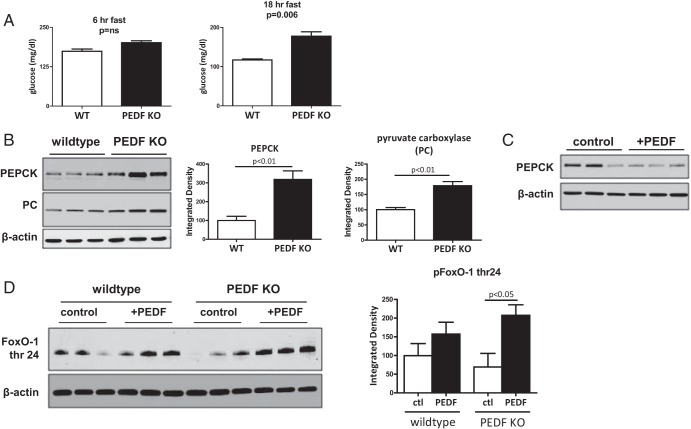
Increased gluconeogenic enzymes in PEDF KO livers can be suppressed by PEDF
Because elevated levels of TCA cycle intermediates and succinylated liver proteins were identified in conjunction with increased basal hepatic glucose output, we assessed whether increased gluconeogenic enzymes may be contributing to the hyperglycemia in PEDF KO mice. In young (2 months of age) mice, increasing fasting times to 18 hours revealed differences in glucose levels that were not apparent with shorter (6 hours) fasting times (Figure 5A). The rate-limiting gluconeogenic enzymes, PEPCK and pyruvate carboxylase, were significantly higher in the livers of young PEDF KO mice compared with controls under prolonged fasting conditions (Figure 5B). Levels of PEPCK, moreover, could be reduced with PEDF reconstitution in vivo (Figure 5C). FoxO-1 is the key transcriptional regulator of gluconeogenic enzymes (26). PEDF treatment of primary hepatocytes increased phosphorylation (inactive form) of FoxO-1, demonstrating that PEDF could negatively regulate FoxO-1 (Figure 5D). Thus, PEDF's ability to negatively regulate gluconeogenic enzymes likely contributes to the hyperglycemia seen in PEDF KO mice.
Figure 5.
Increased fasting times accentuate differences in glycemic control in young PEDF KO mice and reveal elevated levels of gluconeogenic enzymes. A, Fasting for 18 hours accentuates differences in serum glucose in young (2 months of age) PEDF KO mice. B, Elevated levels of rate-limiting gluconeogenic enzymes, PEPCK, and pyruvate carboxylase (PC) in hepatic lysates from young PEDF KO mice. C, Reconstitution of PEDF in PEDF KO mice reduces PEPCK levels. D, PEDF treatment of primary WT and PEDF KO hepatocytes demonstrated significantly increased levels of phosphorylated (inactive) FoxO-1 thr24 after PEDF treatment.ctl, control.
PEDF dampens IL-1β-mediated effects on IRS and Akt signaling in human hepatocytes
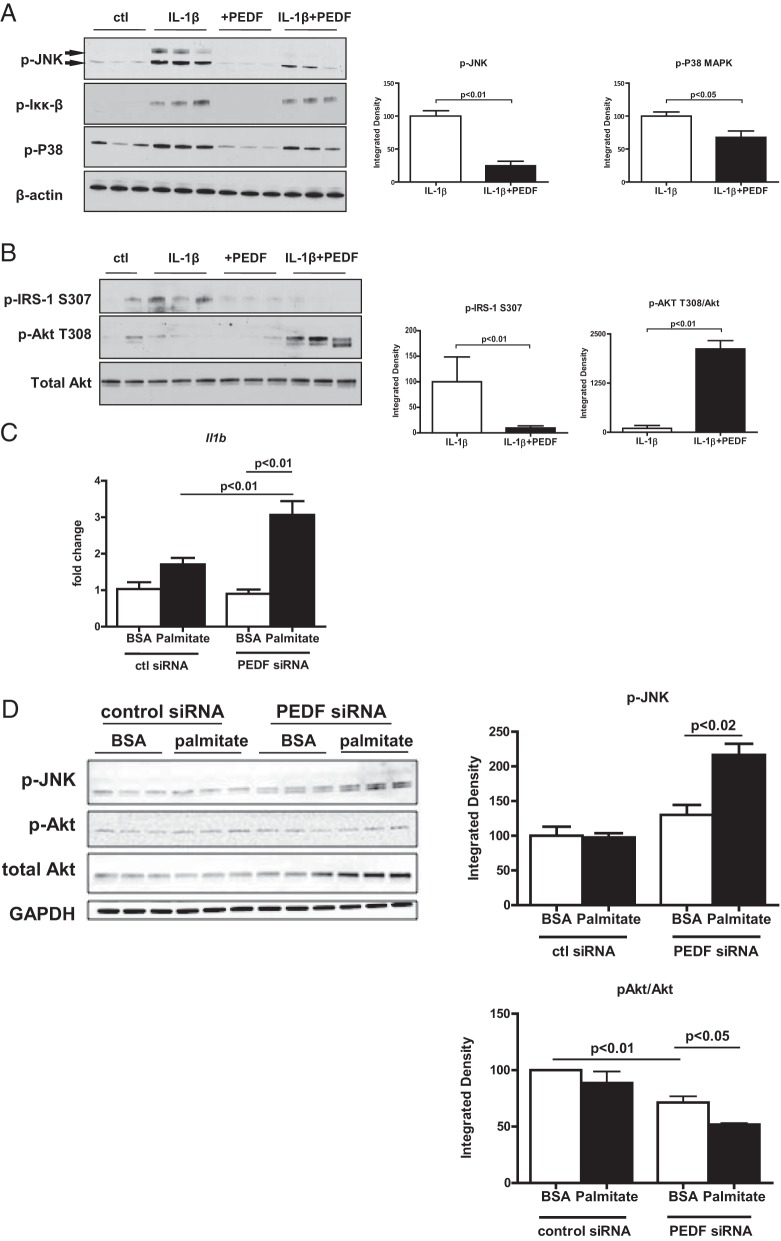
Given concerns regarding the suitability of using mice to model human immune responses (37), primary human hepatocytes were utilized to test whether PEDFs could suppress IL-1β and normalize IRS and Akt signaling in human cells. The activation status of the intracellular kinases JNK and Iκκβ was assessed given their role in impeding insulin signal transduction and the previously reported role of PEDF in promoting JNK activation (20, 38, 39). Adding IL-1β increased activation of p-JNK, p-Iκκβ, and p-P38 (Figure 6A). PEDF had the most pronounced effect on inhibiting JNK phosphorylation (Figure 6A). These findings indicate that PEDF blunts the activation of intracellular stress mediators in human hepatocytes within the context of an IL-1β challenge.
Figure 6.
In human hepatocytes, PEDF suppresses IL-1β-mediated JNK activation whereas PEDF knockdown is permissive for IL-1β release, JNK activation, and decreased Akt signaling. A, PEDF (10 nM) ameliorated the effects of IL-1β (10 ng/mL) on p-JNK, p-Iκκβ, and p-P38. B, PEDF blunted the effects of IL-1β on IRS1 and Akt signaling. C, IL-1β expression was significantly increased in hepatocytes after RNA interference (RNAi) of PEDF expression. D, Knockdown of PEDF was permissive for JNK activation and a diminished p-Akt to total Akt ratio under palmitate challenge. Representative results from hepatocyte isolations derived from human livers (n = 3 separate isolations). ctl, control.
PEDF's ability to maintain hepatocyte insulin signaling was next assessed. In the presence of IL-1β alone, p-IRS-1 S307 levels increased (Figure 6B), consistent with a proximal defect in insulin signaling that was previously seen in PEDF KO livers and hepatocytes (Figure 2). With PEDF reconstitution, p-IRS-1 S307 levels decreased and p-Akt T308 levels increased, consistent with improved insulin signal transduction (Figure 6B). In contrast, PEDF knockdown with small interfering RNA in human hepatocytes was permissive for enhanced Il1b gene expression under conditions of palmitate challenge (Figure 6C and Supplemental Figure 3). Palmitate led to JNK activation and a diminished p-Akt to total Akt ratio (Figure 6D) in hepatocytes with PEDF knockdown. PEDF alone had no effects on Akt signaling, emphasizing that PEDF's role in metabolic homeostasis occurred within the context of an inflammatory stimulus. PEDF's effects on human hepatocyte insulin signaling in the setting of IL-1β therefore were analogous to those seen in mice.
PEDF deficiency is permissive for IL-1β expression and release from Kupffer cells and adipocytes
We next investigated whether PEDF deficiency was permissive for IL-1β release from Kupffer cells and adipocytes because the former mediates multiple inflammatory responses in the liver whereas the latter is a site of PEDF expression and a source of inflammation in the metabolic syndrome (20, 40). Primary PEDF KO Kupffer cells secreted more than 2-fold higher levels of IL-1β under palmitate exposure than WT controls (Supplemental Figure 4). PEDF KO adipocytes also demonstrated significantly higher secreted IL-1β compared with WT adipocytes at baseline and after palmitate challenge (Supplemental Figure 4) and is consistent with the inflammatory changes seen in the liver. Similarly, adipose tissue from PEDF KO similarly demonstrated modestly elevated IL-1β expression under basal conditions that was accentuated with 1 week of high-fat feed feeding (Supplemental Figure 4). We next evaluated whether JNK activation using palmitate was accentuated in the absence of PEDF. PEDF KO adipocytes had higher p-JNK levels than WT cells under control (BSA) conditions that increased with palmitate exposure (Supplemental Figure 4). These results indicate that absence of PEDF is permissive for IL-1β induction in primary Kupffer cells and adipose tissue in vitro and in vivo.
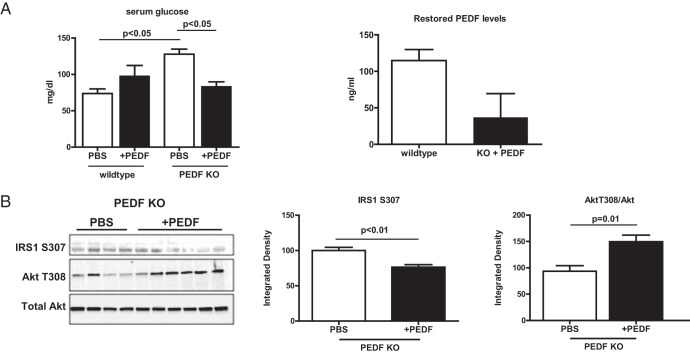
PEDF restoration in vivo ameliorates fasting hyperglycemia and improves hepatocyte insulin signaling
PEDF was injected ip for 4 weeks to determine whether glucose intolerance and Akt signaling could be corrected in vivo. PEDF treatment of WT mice led to a nonsignificant increase in fasting glucose (Figure 7A). In contrast, PEDF KO animals treated with either ip PEDF or PEDF-containing microspheres demonstrated a significant decrease in fasting glucose (Figure 7A and Supplemental Figure 5). This occurred despite the fact that restored PEDF levels in KO mice were one-third of those found in WT mice (Figure 7A), indicating redundancy in serum PEDF levels that has been noted elsewhere (41). Correction of hyperglycemia with PEDF coincided with improved hepatic insulin signal transduction as manifested by decreased IRS1 S307 phosphorylation and enhanced Akt T308 phosphorylation (Figure 7B and Supplemental Figure 5).
Figure 7.
Restoration of PEDF to PEDF KO mice corrects hyperglycemia and improves insulin signal transduction. A, Correction of fasting hyperglycemia in KO mice treated with PEDF and serum PEDF levels in WT and KO mice with PEDF delivery. B, PEDF decreased IRS1 S307 phosphorylation and increased Akt T308 phosphorylation.
Discussion
Clinical studies highlight circulating PEDF as a biomarker of the metabolic syndrome, but its physiological role remains incompletely understood (20, 40, 42). In this study, PEDF's ability to mitigate the effects of IL-1β and JNK activation were defined in a murine model of PEDF deficiency and in human hepatocytes. PEDF markedly suppressed JNK activation, normalizing IRS and Akt signal transduction in the setting of IL-1β exposure. RNA interference of PEDF in human hepatocytes led to enhanced IL-1β expression, indicating that human hepatocyte inflammatory responses are also PEDF responsive. Finally, PEDF reconstitution in vivo corrected hyperglycemia and the diminished IRS1 and Akt signal transduction seen in PEDF KO mice. The sum of our data points to a protective role for PEDF in countering the inflammatory changes seen in the metabolic syndrome.
A multifaceted role for PEDF in metabolism emerged from the analyses done in the PEDF KO mice. In addition to suppressive effects on IL-1β and JNK activation, PEDF had other notable metabolic effects. Hyperglycemia in young PEDF KO mice could be unmasked with prolonged fasting times and was associated with significantly elevated levels of the gluconeogenic enzymes, PEPCK and pyruvate carboxylase. In combination with increased levels of TCA cycle intermediates in the setting of diminished insulin signal transduction, these findings point to an anaplerotic cycle whereby nonoxidative flux of intermediates occurs to generate glucose precursors, a condition seen in experimental and human fatty liver disease (43–45). As a consequence, succinate, an inducer of IL-1β expression (6), was elevated. In turn, succinate-induced IL-1β expression could be suppressed by PEDF, thereby supporting its role as a protective mechanism in the metabolic syndrome.
Unidentified from our studies, however, was how the absence of PEDF in mice could initially trigger enhanced TCA flux. One possible target is PEDF's interaction with Wnt signaling. Park et al (24) first identified PEDF's ability to inhibit Wnt signaling in the eye, and we subsequently showed that PEDF modulates Wnt signaling to reciprocally regulate adiposity vs bone mass in mice (25). In the current study, PEDF inactivated hepatocyte FoxO-1, a key regulator of gluconeogenic enzymes, and decreased its downstream targets. Regulation of FoxO-1 and gluconeogenic enzymes strongly suggests convergence with canonical Wnt signaling (26). However, PEDF's ability to dampen JNK signaling also suggests its ability to inhibit noncanonical Wnt signaling. This complexity will require investigation of PEDF interactions with Wnt receptor subtypes that can regulate the pathways pertinent to the current study (24, 25).
Contrary to the homeostatic role for PEDF proposed in the current study, Crowe et al (20) reported that PEDF contributes to insulin resistance by activating JNK. In their study, PEDF activated JNK acutely, whereas 5-day infusion increased lipid deposition and insulin resistance (20). The supraphysiologic PEDF levels (1000-fold) used in their study may have induced opposite effects of those in the physiological range. These seemingly divergent results indicate that PEDF is highly regulated and emphasizes the usefulness of using the PEDF KO mice to discern its contribution to inflammation and metabolism because many tissue sites express PEDF, with the liver and adipose tissues being sites of highest expression (20, 46). Thus, studies in the PEDF KO model allowed us to investigate whether PEDF promotes or inhibits features of the metabolic syndrome.
Elevated PEDF in the metabolic syndrome likely has other functions. Because PEDF stimulates osteoblast differentiation, elevated PEDF may represent a mechanism to couple bone mass with BMI in the metabolic syndrome (25, 47). Related to osteoblast maturation, null mutations in the PEDF gene were recently identified to be the cause of osteogenesis imperfecta (OI) type VI (48, 49). Preliminary studies have demonstrated that metabolic derangements are present in these patients because BMI was significantly higher in OI type VI patients compared with other OI subtypes (I, III, and IV) and healthy controls (50). Additional investigation of metabolic parameters in OI type VI patients will likely provide insight into PEDF's role in metabolism.
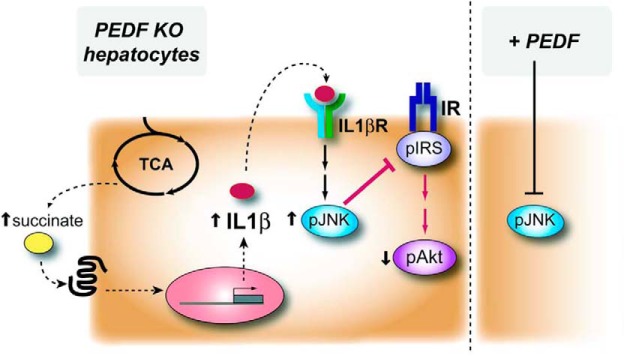
In summary, the metabolic phenotype of PEDF KO mice captures features of the human metabolic syndrome. Prominent among these was diminished glucose tolerance and the presence of hepatic IL-1β responses that appear driven by a complex interaction consisting of increased gluconeogenic enzymes, enhanced TCA flux, and the production of proinflammatory metabolites such as succinate. These changes led to JNK activation and decreased insulin signaling as evidenced by IRS1 phosphorylation and diminished Akt signaling (Figure 8). PEDF countered these effects in vitro and in vivo. PEDF mitigated IL-1β-induced JNK activation and suppressed the IL-1β release that occurred with metabolic stressors such as palmitate or succinate. Because the liver and adipose tissues are major sites of its production, PEDF likely represents an endogenous mechanism to curtail inflammatory responses that occur with obesity. Our findings support this view, and thus, elevated PEDF levels in the metabolic syndrome represent a protective mechanism to counter the effects of cytokines on insulin signaling.
Figure 8.
Schematic diagram of metabolic effects in PEDF KO hepatocytes where enhanced TCA intermediates including succinate enhanced IL-1β expression. IL-1β activates the inflammatory mediator JNK, which can reduce insulin signal transduction as evidenced by increased IRS-1 phosphorylation, decreased IRS-2, and diminished Akt signaling. PEDF treatment of murine and human hepatocytes markedly suppressed JNK activation in response to IL-1β exposure.
Acknowledgments
We thank Dr. Fred Gorelick (Yale University School of Medicine) for insightful comments and Kathy Harry (Yale Liver Center) for expert technical assistance.
This work was supported by Digestive Diseases Research Core Centers–5P30DK034989 (to C.C.); Yale Core Center for Musculoskeletal Disorders P30-AR46032; Veterans Affairs Merit Grant (to C.C.); Metabolomic studies funded by a Pilot and Research CDA grant, NorthShore University HealthSystem (to J.A.D.); German Research Foundation (BI1292/5-1) and the German Diabetes Association (Hellmut Mehnert Project Promotion) (to A.L.B.).
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by Digestive Diseases Research Core Centers–5P30DK034989 (to C.C.); Yale Core Center for Musculoskeletal Disorders P30-AR46032; Veterans Affairs Merit Grant (to C.C.); Metabolomic studies funded by a Pilot and Research CDA grant, NorthShore University HealthSystem (to J.A.D.); German Research Foundation (BI1292/5-1) and the German Diabetes Association (Hellmut Mehnert Project Promotion) (to A.L.B.).
Footnotes
- FoxO-1
- forkhead box O-1
- IRS
- insulin receptor substrate
- JNK
- c-Jun N-terminal kinase
- KO
- knockout
- OI
- osteogenesis imperfecta
- PEPCK
- phosphoenol pyruvate carboxykinase
- PEDF
- pigment epithelium-derived factor
- TCA
- tricarboxyclic acid
- WT
- wild type.
References
- 1. Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. [DOI] [PubMed] [Google Scholar]
- 2. Spranger J, Kroke A, Möhlig M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. [DOI] [PubMed] [Google Scholar]
- 3. Lagathu C, Yvan-Charvet L, Bastard JP, et al. Long-term treatment with interleukin-1beta induces insulin resistance in murine and human adipocytes. Diabetologia. 2006;49:2162–2173. [DOI] [PubMed] [Google Scholar]
- 4. Nov O, Kohl A, Lewis EC, et al. Interleukin-1beta may mediate insulin resistance in liver-derived cells in response to adipocyte inflammation. Endocrinology. 2010;151:4247–4256. [DOI] [PubMed] [Google Scholar]
- 5. Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. [DOI] [PubMed] [Google Scholar]
- 6. Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wen H, Gris D, Lei Y, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. [DOI] [PubMed] [Google Scholar]
- 9. Zhang SX, Wang JJ, Gao G, Shao C, Mott R, Ma JX. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. Faseb J. 2006;20:323–325. [DOI] [PubMed] [Google Scholar]
- 10. Yamagishi S, Adachi H, Abe A, et al. Elevated serum levels of pigment epithelium-derived factor in the metabolic syndrome. J Clin Endocrinol Metab. 2006;91:2447–2450. [DOI] [PubMed] [Google Scholar]
- 11. Sabater M, Moreno-Navarrete JM, Ortega FJ, et al. Circulating pigment epithelium-derived factor levels are associated with insulin resistance and decrease after weight loss. J Clin Endocrinol Metab. 2010;95:4720–4728. [DOI] [PubMed] [Google Scholar]
- 12. Chen C, Tso AW, Law LS, et al. Plasma level of pigment epithelium-derived factor is independently associated with the development of the metabolic syndrome in Chinese men: a 10-year prospective study. J Clin Endocrinol Metab. 2010;95:5074–5081. [DOI] [PubMed] [Google Scholar]
- 13. Gattu AK, Birkenfeld AL, Jornayvaz F, et al. Insulin resistance is associated with elevated serum pigment epithelium-derived factor (PEDF) levels in morbidly obese patients. Acta Diabetol. 2012;49(Suppl 1):S161–S169. [DOI] [PubMed] [Google Scholar]
- 14. Hyogo H, Yamagishi SI, Maeda S, Kimura Y, Ishitobi T, Chayama K. Serum levels of pigment epithelium-derived factor (PEDF) are independently associated with procollagen III N-terminal peptide levels in patients with nonalcoholic fatty liver disease. Clin Biochem. 2012;45(18):1554–1557. [DOI] [PubMed] [Google Scholar]
- 15. Franck N, Gummesson A, Jernas M, et al. Identification of adipocyte genes regulated by caloric intake. J Clin Endocrinol Metab. 2011;96:E413–E418. [DOI] [PubMed] [Google Scholar]
- 16. Akin S, Aksoy DY, Cinar N, et al. Pigment epithelium-derived factor (PEDF) increases in type 2 diabetes after treatment with metformin. Clin Endocrinol (Oxf). 2012;77:852–856. [DOI] [PubMed] [Google Scholar]
- 17. Park K, Jin J, Hu Y, Zhou K, Ma JX. Overexpression of pigment epithelium-derived factor inhibits retinal inflammation and neovascularization. Am J Pathol. 2011;178:688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shen X, Xie B, Cheng Y, Jiao Q, Zhong Y. Effect of pigment epithelium derived factor on the expression of glutamine synthetase in early phase of experimental diabetic retinopathy. Ocul Immunol Inflamm. 2011;19:246–254. [DOI] [PubMed] [Google Scholar]
- 19. Borg ML, Andrews ZB, Duh EJ, Zechner R, Meikle PJ, Watt MJ. Pigment epithelium-derived factor regulates lipid metabolism via adipose triglyceride lipase. Diabetes. 2011;60:1458–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crowe S, Wu LE, Economou C, et al. Pigment epithelium-derived factor contributes to insulin resistance in obesity. Cell Metab. 2009;10:40–47. [DOI] [PubMed] [Google Scholar]
- 21. Apte RS, Barreiro RA, Duh E, Volpert O, Ferguson TA. Stimulation of neovascularization by the anti-angiogenic factor PEDF. Invest Ophthalmol Vis Sci. 2004;45:4491–4497. [DOI] [PubMed] [Google Scholar]
- 22. Chung C, Doll JA, Gattu AK, et al. Anti-angiogenic pigment epithelium-derived factor regulates hepatocyte triglyceride content through adipose triglyceride lipase (ATGL). J Hepatol. 2008;48:471–478. [DOI] [PubMed] [Google Scholar]
- 23. Chung C, Shugrue C, Nagar A, et al. Ethanol exposure depletes hepatic pigment epithelium-derived factor, a novel lipid regulator. Gastroenterology. 2009;136:331–340.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park K, Lee K, Zhang B, et al. Identification of a novel inhibitor of the canonical Wnt pathway. Mol Cell Biol. 2011;31:3038–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gattu AK, Swenson ES, Iwakiri Y, et al. Determination of mesenchymal stem cell fate by pigment epithelium-derived factor (PEDF) results in increased adiposity and reduced bone mineral content. Faseb J. 2013;27:4384–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu H, Fergusson MM, Wu JJ, et al. Wnt signaling regulates hepatic metabolism. Sci Signal. 2011;4:ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grant SF, Thorleifsson G, Reynisdottir I, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320–323. [DOI] [PubMed] [Google Scholar]
- 28. Liu W, Singh R, Choi CS, et al. Low density lipoprotein (LDL) receptor-related protein 6 (LRP6) regulates body fat and glucose homeostasis by modulating nutrient sensing pathways and mitochondrial energy expenditure. J Biol Chem. 2012;287:7213–7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. [DOI] [PubMed] [Google Scholar]
- 30. Birkenfeld AL, Lee HY, Guebre-Egziabher F, Alves TC, et al. Deletion of the mammalian INDY homolog mimics aspects of dietary restriction and protects against adiposity and insulin resistance in mice. Cell Metab. 2011;14:184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schmitz JC, Protiva P, Gattu AK, et al. Pigment epithelium-derived factor regulates early pancreatic fibrotic responses and suppresses the profibrotic cytokine thrombospondin-1. Am J Pathol. 2011;179:2990–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gall WE, Beebe K, Lawton KA, et al. α-Hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One. 2010;5:e10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ. Superoxide generation by neutrophils and Kupffer cells during in vivo reperfusion after hepatic ischemia in rats. J Leukoc Biol. 1992;52:377–382. [DOI] [PubMed] [Google Scholar]
- 34. Jay SM, Saltzman WM. Controlled delivery of VEGF via modulation of alginate microparticle ionic crosslinking. J Control Release. 2009;134:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Panten U, Willenborg M, Schumacher K, Hamada A, Ghaly H, Rustenbeck I. Acute metabolic amplification of insulin secretion in mouse islets is mediated by mitochondrial export of metabolites, but not by mitochondrial energy generation. Metabolism. 2013;62:1375–1386. [DOI] [PubMed] [Google Scholar]
- 36. Ferrannini E, Natali A, Camastra SF, et al. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes. 2013;62:1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seok J, Warren HS, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013;110:3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem. 2000;275:9047–9054. [DOI] [PubMed] [Google Scholar]
- 39. Gao Z, Hwang D, Bataille F, et al. Serine phosphorylation of insulin receptor substrate 1 by inhibitor κ B kinase complex. J Biol Chem. 2002;277:48115–48121. [DOI] [PubMed] [Google Scholar]
- 40. Famulla S, Lamers D, Hartwig S, et al. Pigment epithelium-derived factor (PEDF) is one of the most abundant proteins secreted by human adipocytes and induces insulin resistance and inflammatory signaling in muscle and fat cells. Int J Obes (Lond). 2011;35:762–772. [DOI] [PubMed] [Google Scholar]
- 41. Petersen SV, Valnickova Z, Enghild JJ. Pigment-epithelium-derived factor (PEDF) occurs at a physiologically relevant concentration in human blood: purification and characterization. Biochem J. 2003;374:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oberbach A, Blüher M, Wirth H, et al. Combined proteomic and metabolomic profiling of serum reveals association of the complement system with obesity and identifies novel markers of body fat mass changes. J Proteome Res. 2011;10:4769–4788. [DOI] [PubMed] [Google Scholar]
- 43. Satapati S, Sunny NE, Kucejova B, et al. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J Lipid Res. 2012;53:1080–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 2011;14:804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jitrapakdee S, Vidal-Puig A, Wallace JC. Anaplerotic roles of pyruvate carboxylase in mammalian tissues. Cell Mol Life Sci. 2006;63:843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tombran-Tink J, Mazuruk K, Rodriguez IR, et al. Organization, evolutionary conservation, expression and unusual Alu density of the human gene for pigment epithelium-derived factor, a unique neurotrophic serpin. Mol Vis. 1996;2:11. [PubMed] [Google Scholar]
- 47. Li F, Song N, Tombran-Tink J, Niyibizi C. Pigment epithelium derived factor enhances differentiation and mineral deposition of human mesenchymal stem cells [published online August 13, 2013]. Stem Cells. doi:10.1002/stem.1505. [DOI] [PubMed] [Google Scholar]
- 48. Becker J, Semler O, Gilissen C, et al. Exome sequencing identifies truncating mutations in human SERPINF1 in autosomal-recessive osteogenesis imperfecta. Am J Hum Genet. 2011;88:362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Homan EP, Rauch F, Grafe I, et al. Mutations in SERPINF1 cause osteogenesis imperfecta type VI. J Bone Miner Res. 2011;26:2798–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rauch F, Husseini A, Roughley P, Glorieux FH, Moffatt P. Lack of circulating pigment epithelium-derived factor is a marker of osteogenesis imperfecta type VI. J Clin Endocrinol Metab. 2012;97:E1550–E1556. [DOI] [PubMed] [Google Scholar]