Abstract
Nonalcoholic fatty liver disease, particularly its more aggressive form, nonalcoholic steatohepatitis (NASH), is associated with hepatic insulin resistance. Osteocalcin, a protein secreted by osteoblast cells in bone, has recently emerged as an important metabolic regulator with insulin-sensitizing properties. In humans, osteocalcin levels are inversely associated with liver disease. We thus hypothesized that osteocalcin may attenuate NASH and examined the effects of osteocalcin treatment in middle-aged (12-mo-old) male Ldlr−/− mice, which were fed a Western-style high-fat, high-cholesterol diet for 12 weeks to induce metabolic syndrome and NASH. Mice were treated with osteocalcin (4.5 ng/h) or vehicle for the diet duration. Osteocalcin treatment not only protected against Western-style high-fat, high-cholesterol diet-induced insulin resistance but substantially reduced multiple NASH components, including steatosis, ballooning degeneration, and fibrosis, with an overall reduction in nonalcoholic fatty liver disease activity scores. Further, osteocalcin robustly reduced expression of proinflammatory and profibrotic genes (Cd68, Mcp1, Spp1, and Col1a2) in liver and suppressed inflammatory gene expression in white adipose tissue. In conclusion, these results suggest osteocalcin inhibits NASH development by targeting inflammatory and fibrotic processes.
With the global increase in obesity and metabolic syndrome, the associated complications such as nonalcoholic steatohepatitis (NASH) are presenting an enormous burden on United States healthcare and healthcare expenditures (1, 2). Nonalcoholic fatty liver disease (NAFLD), an excessive accumulation of fat in the liver due to diet or genetics, triggers a cascade of molecular and cellular reactions that result in increased inflammation, fibrosis, and eventual hepatocyte degeneration, cirrhosis, and end-stage liver disease. NASH-induced cirrhosis is the third most common cause for liver transplantation, but there are no approved therapies for its treatment or prevention (3). Recently, increased levels of osteocalcin, a hormone secreted by osteoblasts, have been associated with enhanced insulin action and secretion resulting in improved glucose metabolism in humans and mice with obesity-induced insulin resistance or type 2 diabetes (4–7). Further, insulin resistance due to long-term glucocorticoid treatment is associated with reduced osteocalcin secretion and is mitigated by transgenic osteocalcin expression (8).
Clinical studies have highlighted an association between osteocalcin and liver disease. Patients with liver cirrhosis have decreased serum levels of osteocalcin (9, 10), which negatively correlates with liver enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST), inflammatory markers of liver disease (11). In fact, serum osteocalcin was found to be the only independent predictor of hepatocyte ballooning in patients with NAFLD (12). Despite these intriguing clinical associations, few studies have investigated osteocalcin effects on NASH development and progression.
Ferron et al (5) showed that daily injection with a supraphysiological dose of osteocalcin efficiently reduced fat mass and prevented liver steatosis in wild-type mice fed a high-fat diet, but did not examine its effect on NASH development. Therefore, in the current study, we tested the impact of osteocalcin on NASH in Western-style high-fat, high-cholesterol diet (WHFD)-fed middle-aged (12-mo-old) Ldlr−/− male mice, which develop 4 of 5 metabolic syndrome criteria and classical NASH similar to that seen in humans, including hepatic fat infiltration, rampant inflammation, fibrosis, and ballooning degeneration (13–15). We confirmed the insulin-sensitizing effects of osteocalcin and further found that osteocalcin protects against development of NASH in these mice.
Materials and Methods
Animals and treatments
Middle-aged (12-mo-old) Ldlr−/− male mice were fed a chow diet (14% kcal fat; Harlan Teklad 8904) or WHFD (41% kcal fat, 1.5% cholesterol; Research Diets D12079Bi) for 12 weeks, and a subset of animals from each diet group was treated with osteocalcin (4.5 ng/h) for the study duration. Body weights and percent body fat were determined on a weekly basis throughout the study. After 12 weeks of treatment, a fasting blood sample was collected for lipid analyses, mice were killed, and liver and white adipose tissues were frozen for gene expression or paraformaldehyde-fixed for histology. All protocols were approved by the Institutional Animal Care and Use Committee of the Houston Methodist Research Institute and carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Osteocalcin treatment
Both human and bovine decarboxylated osteocalcin were used in this study in order to confirm that were not species-specific osteocalcin effects. Human osteocalcin was purchased from AnaSpec (catalog number 22830) and administered at a rate of 4.5 ng/h via 90-day release pellets from Innovative Research of America sc-implanted between the neck and left shoulder. Control mice received a vehicle-infused placebo pellet. Bovine osteocalcin was purified from bovine tibial cortical bone as previously described (16) and was administered at a 4.5 ng/h rate via sc osmotic pumps (Alzet pumps) on the neck of the mouse. Decarboxylated bovine osteocalcin was obtained by heating of 1 mg of purified lyophilized osteocalcin in vacuo at 110°C for 4 hours (17). The osteocalcin preparation was checked for purity and decarboxylation state by native gel electrophoresis with Coomassie blue staining or by Western blotting to nylon 66+ membranes followed by staining with diazobenzene sulfonic acid, which produces a red color reaction with γ-carboxyglutamic acid-containing proteins (18, 19). The purified decarboxylated osteocalcin was resuspended to 1 mg/mL in water, aliquoted into microfuge tubes, and stored frozen at −20°C. Osteocalcin solutions were lyophilized and resuspended to a working stock concentration in PBS and stored at −20°C until use.
Measurement of systemic and muscle-specific insulin sensitivity
Mice were fasted for 6 hours in the early morning, injected with 0.75-U/kg insulin, and blood glucose levels in response to an insulin tolerance test (ITT) levels were determined at 0, 15, 30 45, 60, and 90 minutes after injection. To test ostecalcin effects on insulin signaling, mice were fasted overnight and then killed 10 minutes after ip injection with PBS or 0.75-U insulin/kg body weight to dissect and flash freeze liver and tibialis anterior muscle biopsies. Insulin-induced Akt-phosphorylation in these samples was assessed using standard Western blotting techniques using Akt, phosphorylated-Akt, and antirabbit horse radish peroxidase-labeled secondary antibodies (Cell Signaling Technology).
Body and liver fat measurements
An EchoMRI-3in1 small animal quantitative magnetic resonance (QMR) machine was used to determine percent body fat in the whole animal. Percent liver fat was determined by QMR after killing the mouse and dissection of the liver.
Plasma lipids
Plasma triglycerides, phospholipids, cholesterol, nonesterified fatty acids (NEFA), AST and ALT levels were determined by the Mouse Phenotyping Core at the University of Cincinnati.
Liver histology
Formalin-fixed liver samples were embedded, sectioned, and hematoxylin and eosin stained for general histology or stained with Masson's trichrome stain for collagen content by the pathology department of the Houston Methodist Research Institute. Steatosis, inflammation, ballooning degeneration, and fibrosis were quantified, and NAFLD activity scores were assigned by Dr Lillian Gaber, an expert pathologist, following the guidelines described by Kleiner et al (20). Group assignment of the mice was not provided to the pathologist.
RNA purification, cDNA synthesis, and qRT quantitative real-time-PCR
Liver and white adipose tissue RNA was isolated using RNeasy kits (QIAGEN), reversed transcribed into cDNA with High Capacity cDNA Reverse Transcription kits (Applied Biosystems), and analyzed for candidate gene expression using TaqMan PCR Core reagent kits and gene-specific primer/probe sets (Applied Biosystems). Candidate gene expression was normalized to Ppia (cyclophilin A) expression.
Liver hydroxyproline
Liver tissue (10 mg) was homogenized in water, and hydroxyproline levels were determined by a colorimetric assay (Sigma-Aldrich). Briefly, oxidized hydroxyproline reacts with 4-(dimethylamino)benzaldehyde, which results in a colorimetric (560 nm) product proportional to the amount of hydroxyproline in the sample.
Statistics
GraphPad Prism 5 software was used for all statistical analyses. Differences between groups were analyzed using two-way ANOVA. Most sample sizes were too small for Shapiro-Wilks normality tests, and thus all samples were assumed to have normal distributions to allow two-way ANOVA. ITT data are presented as percent change from fasting baseline glucose and analyzed by repeated measures ANOVA. Differences between means were determined using Tukey's multiple comparison tests. Differences were determined between the next comparisons: Chow+Veh and Chow+OST, WHFD+Veh and WHFD+OST, Chow+Veh and WHFD+Veh, Chow+OST and WHFD+OST, Chow+Veh and WHFD+OST, and Chow+OST and WHFD+Veh. A two-tailed α = 0.05 was used as the significance cut-off for all tests. All data are presented as mean ± SE. Significance was defined as P < .05, and sample sizes are indicated in each figure legend.
Results
Osteocalcin improves insulin sensitivity and attenuates liver injury markers in a mouse model of metabolic syndrome
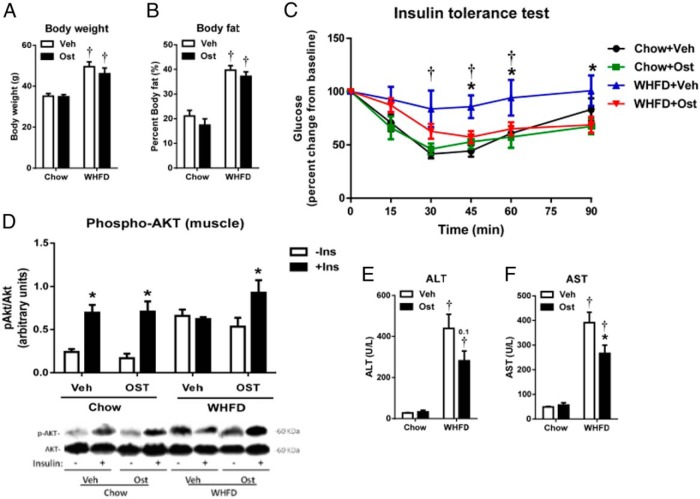
Middle-aged male Ldlr−/− mice fed WHFD for 12 weeks developed obesity and systemic insulin resistance (Figure 1, A–C). However, treatment with decarboxylated human osteocalcin preserved insulin sensitivity (Figure 1C) without change in body weight and body fat, consistent with previous studies using various forms of osteocalcin (5, 21, 22). Insulin-induced Akt phosphorylation, a measure of skeletal muscle insulin sensitivity, revealed similar robust responses in osteocalcin-treated and untreated chow-fed mice (Figure 1D). However, WHFD-fed mice revealed markedly increased fasting Akt phosphorylation, which responded to insulin only in osteocalcin treated mice, consistent with a known osteocalcin effect to improve insulin sensitivity (Figure 1D). Osteocalcin treatment did not increase insulin-induced Akt phosphorylation in livers of WHFD-fed mice (data not shown) but significantly attenuated WHFD-induced AST and trended to decrease ALT increases associated with liver injury (Figure 1, E and F), suggesting that osteocalcin may have protective effects on diet-induced liver disease.
Figure 1. Osteocalcin improves insulin sensitivity and liver function in metabolic syndrome mice.
Body weight (A) and percent body fat (B) of middle-aged Ldlr-/- mice fed chow or WHFD and treated with vehicle or human osteocalcin are shown. Systemic insulin sensitivity was measured with an ITT (C), and skeletal muscle-specific (D) and liver-specific (E) insulin sensitivity was measured with insulin-stimulated phosphorylation of Akt. Liver enzymes indicative of liver function, ALT and AST were measured in plasma (F and G). Data shown as mean ± SEM; n = 6 chow+Veh, n = 5 chow+OST, n = 7 WHFD+Veh, n = 8 WHFD+OST for A–C; n = 3 for all chow data, n = 4 for all WHFD data for D and E; n = 3 chow+Veh, n = 4 chow+OST, n = 6 WHFD+Veh, n = 6 WHFD+OST for F and G. *, P < .05 vs vehicle;  , P < .05 vs chow by two-way ANOVA and Tukey's test. −Ins, without insulin; +Ins, with insulin.
, P < .05 vs chow by two-way ANOVA and Tukey's test. −Ins, without insulin; +Ins, with insulin.
Osteocalcin attenuates inflammatory gene expression in adipose tissue of mice with NASH
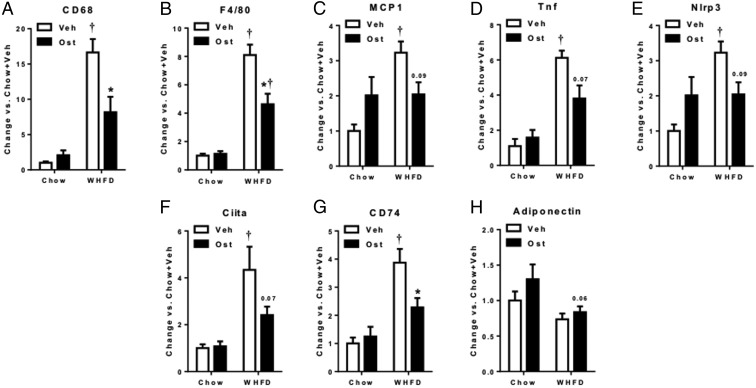
Adipose tissue inflammation contributes to insulin resistance and NASH. To investigate the effects of osteocalcin on adipose tissue, we determined the expression of key inflammatory genes in adipose tissue of WHFD-fed mice. Macrophage marker and migration genes Cd68, F4/80, and Monocyte Chemoattractant Protein 1 (Mcp-1) were robustly increased with WHFD, but these increases were significantly attenuated (Cd68 and F4/80) or trended to decrease (Mcp-1) with osteocalcin treatment (Figure 2, A–C). Similarly, WHFD-induced proinflammatory increases in cytokine (Tnf), inflammasome (Nlrp3), and class II major histocompatibility complex (Ciita and Cd74) genes also repressed with osteocalcin treatment (Figure 2, D and E). Osteocalcin, however, had no effect on the expression of adiponectin, an antiinflammatory adipose-derived factor. Thus, osteocalcin has systemic effects to reduce inflammation in liver and adipose tissue, which may contribute to improved insulin sensitivity during high-fat ingestion.
Figure 2. Osteocalcin suppresses expression of inflammatory genes in white adipose tissue.
Gene expression of inflammatory genes Cd68 (A), F4/80 (B), Mcp1(C), Tnf (D), Nlrp3 (E), Ciita (F), Cd74 (G), and expression of Adiponectin (H) were determined, normalized to Ppia gene expression and expressed as change vs chow+veh. Data shown as mean ± SEM; n = 6 chow+Veh, n = 7 chow+OST, n = 5 WHFD+Veh, n = 7 WHFD+OST. *, P < .05 vs vehicle for matched diet;  , P < .05 vs chow for matched treatment by two-way ANOVA and Tukey's test.
, P < .05 vs chow for matched treatment by two-way ANOVA and Tukey's test.
Osteocalcin decreases hepatic fat content
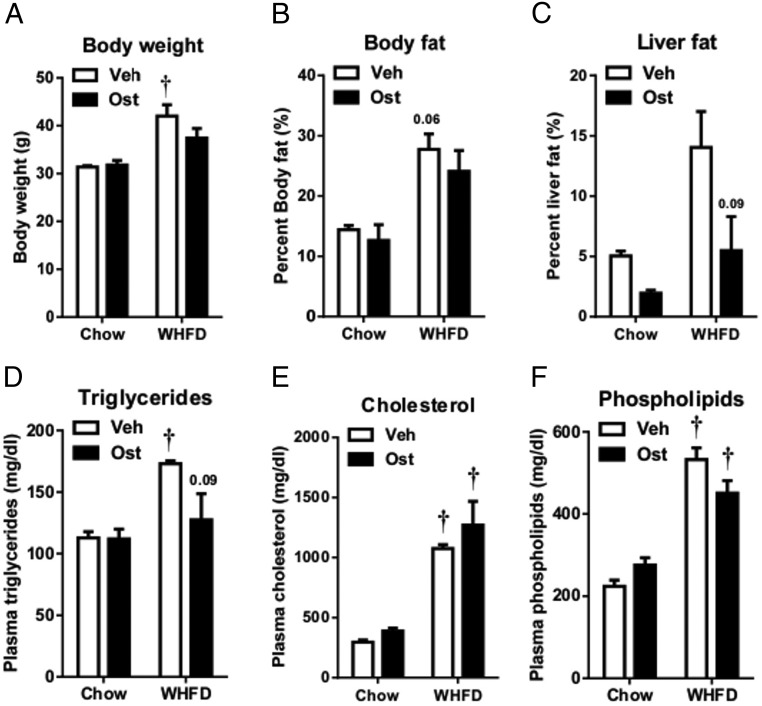
To further investigate osteocalcin effects on liver metabolism and NASH, we treated WHFD-fed and chow-fed Ldlr−/− mice with decarboxylated bovine osteocalcin, chosen for its greater availability at reduced cost. Osteocalcin treatment did not affect body weight or percent body fat (Figure 3, A and B) but reduced liver fat content in both chow- and WHFD-fed mice (Figure 3C). Plasma triglyceride and phospholipid decreases in osteocalcin- vs PBS-treated WHFD-fed mice did not reach significance, although the triglycerides trended to drop with osteocalcin treatment (P = .09) (Figure 3, D–F). Thus, it is not clear whether osteocalcin attenuates plasma lipid concentrations to attenuate liver steatosis.
Figure 3. Osteocalcin reduces liver fat content in mice fed a WHFD for 12 weeks.
Body weight (A), percent body fat (B), percent liver fat (C), and plasma triglycerides (D), total cholesterol (E), and phospholipids (F) were collected on the day of killing, after 12 weeks of diet and treatment. Data shown as mean ± SEM; n = 3 chow+Veh, n = 3 chow+OST, n = 7 WHFD+Veh, n = 7 WHFD+OST. *, P < .05 vs vehicle for matched diet;  , P < .05 vs chow for matched treatment by two-way ANOVA and Tukey's test.
, P < .05 vs chow for matched treatment by two-way ANOVA and Tukey's test.
Osteocalcin reduces pathological manifestation of NASH
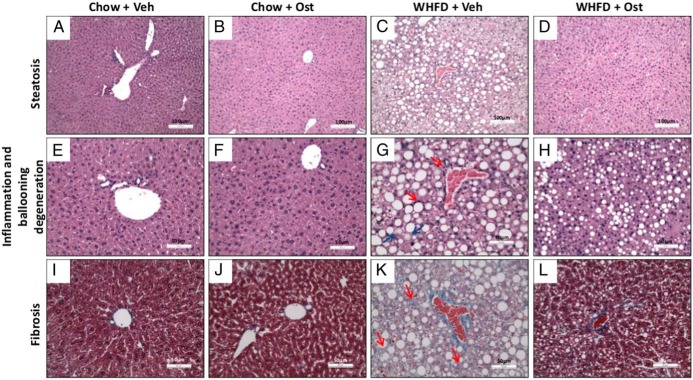
We have previously shown that by 12-week WHFD middle-aged Ldlr−/− mice develop NASH, characterized by extensive steatosis, inflammation, fibrosis, and ballooning degeneration (13). Osteocalcin had no apparent effect on the normal liver histology of chow-fed mice (Figure 4), However, osteocalcin attenuated characteristic pathological changes of NASH in WHFD-fed mice (Figure 4), including steatosis, inflammation, ballooning degeneration, and fibrosis (Figure 4 and Table 1), so that fewer osteocalcin- vs Vehicle-treated WHFD-fed mice scored in the highest class for each measurement. Thus, osteocalcin treatment substantially alleviated WHFD-induced NASH development.
Figure 4. Osteocalcin improves NASH.
Representative images showing steatosis, inflammation, and fibrosis for chow+veh (A, E, and I), chow+Ost (B, F, and J), WHFD+Veh (C, G, and H), WHFD+Ost (D, H, and L) are shown. All pictures are centered on a portal vein, and inflammation and fibrosis are indicated by arrows. Magnification is indicated by size bars in the figures.
Table 1.
Histological Scoring for NASH
| NAFLD activity scores | Chow+Veh | Chow+Ost | HFD+Veh | HFD+Ost |
|---|---|---|---|---|
| %N(0 to 2) | 100 | 100 | 37.5 | 42.85 |
| %N(3 to 4) | 0 | 0 | 25 | 42.85 |
| %N(5 to 8) | 0 | 0 | 37.5 | 14.3 |
| N | 3 | 4 | 8 | 7 |
| Inflammation score | Chow + Veh | Chow + Ost | HFD + Veh | HFD + Ost |
| %N(0) | 66.7 | 50.0 | 50.0 | 57.1 |
| %N(1) | 33.3 | 50.0 | 12.5 | 0.0 |
| %N(2) | 0.0 | 0.0 | 37.5 | 42.9 |
| N | 3 | 4 | 8 | 7 |
| Steatosis score | Chow + Veh | Chow + Ost | HFD + Veh | HFD + Ost |
| %N(0) | 100 | 100 | 0 | 14.3 |
| %N(1) | 0 | 0 | 25 | 28.6 |
| %N(2) | 0 | 0 | 37.5 | 28.6 |
| %N(3) | 0 | 0 | 37.5 | 28.6 |
| N | 3 | 4 | 8 | 7 |
| Ballooning score | Chow + Veh | Chow + Ost | HFD + Veh | HFD + Ost |
| %N(class 0) | 100 | 100 | 50 | 85.7 |
| %N(class 1) | 0 | 0 | 50 | 14.3 |
| %N(class 2 | 0 | 0 | 0 | 0 |
| N | 3 | 4 | 8 | 7 |
| Fibrosis score | Chow + Veh | Chow + OST | HFD + Veh | HFD + OST |
| %N(0) | 100 | 75 | 0 | 14 |
| %N(1) | 0 | 25 | 13 | 43 |
| %N(2) | 0 | 0 | 25 | 29 |
| %N(3) | 0 | 0 | 63 | 14 |
| N | 3 | 4 | 8 | 7 |
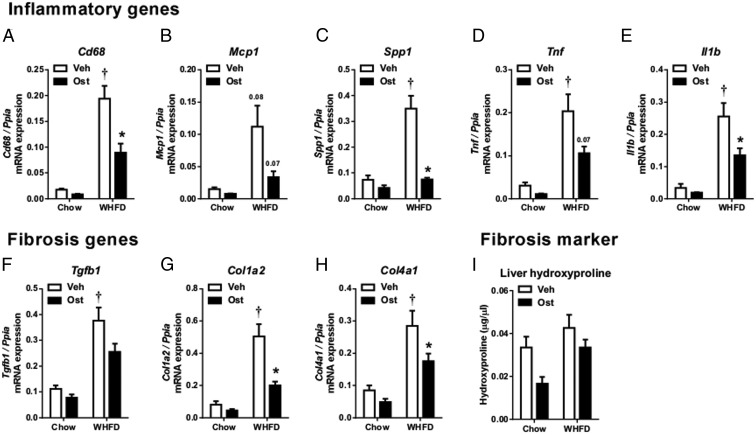
Osteocalcin reduces expression of inflammatory and fibrosis genes in the livers of mice with NASH
To determine the mechanism by which osterocalcin alleviates NASH in WHFD-fed Ldlr−/− mice, we examined gene expression of pathways implicated in NASH progression, including inflammation, fibrosis, mitochondrial structure and function, fatty acid metabolism, and insulin signaling. Livers of WHFD-fed Ldlr−/− mice had robust increases in expression of the proinflammatory genes Cd68, Mcp1, Spp1, Tnf, and Il1b (Figure 5, A–E) and the profibrotic genes Tgfb1, Col1a2, and Col4a1 (Figure 5, F–H). Treatment with osteocalcin significantly suppressed Cd68, Spp1, and Il1b and trended to decrease Mcp1 and Tnf (Figure 5, A–E). Osteocalcin also significantly suppressed Col1a2 and Col4a1 and trended to decrease Tgfb1 expression (Figure 5, F–H). Liver tissue hydroxyproline, a marker of fibrosis, trended to decrease with osteocalcin treatment in both chow-fed and WHFD-fed mice but was not statistically significant (Figure 5I). Taken together, these data suggest that osteocalcin has both antiinflammatory and antifibrotic effects, which protect the liver from development of NASH.
Figure 5. Osteocalcin improves expression of inflammatory and fibrosis genes.
Gene expression of inflammatory genes Cd68 (A), Mcp1 (B), Spp1 (C), Tnf (D), Il1β (E), and fibrosis genes Tgfb1 (F), Col1a2 (G), Col4a1 (H) were determined and normalized to Ppia gene expression. Liver hydroxyproline (I) was determined by colorimetric assay. Data shown as mean ± SEM; n = 3 chow+Veh, n = 3 chow+OST, n = 7 WHFD+Veh, n = 7 WHFD+OST. *, P < .05 vs vehicle for matched diet;  , P < .05 vs chow for matched treatment by two-way ANOVA and Tukey's test.
, P < .05 vs chow for matched treatment by two-way ANOVA and Tukey's test.
Liver steatosis can result from increased plasma lipid uptake, increased lipid synthesis or storage, or decreased fatty acid oxidation. We therefore examined liver expression of genes involved in mitochondrial biogenesis and function and fatty acid metabolism (Supplemental Figures 1 and 2). WFHD increased liver expression of the mitochondrial biogenesis regulator, Ppargc1a but not Tfam, and increased or trended to increase mitochondrial complex II (Sdha), III (Uqcrc1), IV (Cox5a), and V (Atp5a) subunits but not the complex I subunit NdufsS6. Conversely, osteocalcin tended to reverse most of these WHFD-induced changes (Supplemental Figure 1) and had no effect on WFD-induced increases in fatty acid oxidation (Cpt1a and Hadha) and synthesis (Srebp1c) genes (Supplemental Figure 2), suggesting that osteocalcin does not attenuate steatosis by increasing mitochondrial function.
Discussion
Our results suggest a promising role for osteocalcin in mitigating liver injury in a mouse model of metabolic syndrome and NASH. Liver steatosis triggers a cascade of molecular and cellular reactions that increase liver inflammation, fibrosis, and hepatocyte degeneration that can ultimately lead to cirrhosis. Preventing the elevated liver steatosis of NAFLD from progressing to NASH, a more aggressive inflammatory state, is considered an important target to prevent end-stage liver disease in obesity (23). Preventing fibrosis also has important therapeutic implications (24). We find that osteocalcin prevents the development of multiple NASH-associated traits, including systemic insulin resistance, hepatic steatosis, fibrosis, ballooning degeneration, and elevated liver enzyme levels.
NAFLD and NASH susceptibility increases with age (13, 25), and NASH is more prevalent and severe in the elderly (26). Similarly, we find that aging increases hepatic steatosis in chow-fed mice and that aging is required for the development of NASH in our WHFD-fed mouse model (13), suggesting that this model is particularly relevant for investigation of factors that promote NASH. Osteocalcin trended to suppress steatosis in WHFD- as well as chow-fed mice, the latter evident only by QMR analyses but not histology, likely due to moderate changes in microsteatosis in chow-fed mice that were difficult to quantitate with histology. Furthermore, our results with 2 different osteocalcin preparations confirm its reported effect to maintain insulin action during diet-induced obesity and further reveal effects to attenuate liver and adipose tissue inflammation, which are implicated in NASH development. Osteocalcin improves insulin sensitivity (4), glucose metabolism (5), and insulin secretion (6). We found that low-dose osteocalcin treatment attenuated systemic and skeletal muscle insulin resistance but did not improve liver insulin signaling. Residual steatosis and inflammation may explain this hepatic insulin resistance, so that further attenuation of these phenotypes with higher doses of osteocalcin may improve liver insulin sensitivity.
Clinical results indicate that circulating osteocalcin negatively correlates with plasma levels of the liver injury markers ALT and AST, whereas subjects with the greatest osteocalcin increases during weight loss also reveal the greatest ALT decreases (11). Serum osteocalcin levels in patients with NAFLD are also lower than healthy controls, inversely associated with ALT, AST, homeostatic model assessment-insulin resistance, and the only independent predictor of hepatocyte ballooning degeneration (12). These observations lead us to test whether osteocalcin treatment could prevent NASH in a WHFD-fed mouse model, where NASH is driven by obesity, aging, and oxidative stress, all of which are common risk factors for human NASH development (27–29).
Hepatic steatosis is the initial event in NALFD development that can ultimately lead to NASH, and osteocalcin treatment markedly attenuates the accumulation of liver fat in WHFD-fed mice (Figure 3C). Hepatic steatosis derives from de novo lipogenesis (26%), dietary fat (15%), and adipose tissue lipolysis (59%) (30). Osteocalcin treatment modestly decreased genes related to mitochondrial biogenesis (Supplemental Figure 1) but did not alter liver expression of genes involved in fatty acid oxidation and de novo lipogenesis (Supplemental Figure 2), whereas dietary fat intake remained constant. This suggests that an effect to reduce inflammation-driven lipolysis in adipose tissue may explain the steatosis effect. However, although osteocalcin reduced multiple inflammation markers in adipose tissue, its effects on plasma lipids were inconclusive. Further studies are therefore required to address this issue.
Chronic liver inflammation, which can lead to fibrosis and eventually cirrhosis, is a key step in the progression of NAFLD to NASH. We found that osteocalcin treatment attenuated liver expression of markers of macrophage infiltration and migration, including Cd68 and Mcp1. Osteocalcin also decreased liver expression of Spp1, a key mediator of macrophage adhesion and migration (31), which correlates with disease severity in NAFLD patients (32, 33), and which is implicated in diet-induced insulin resistance (34, 35). Thus, Spp1 inhibition may partially mediate the effects of osteocalcin. Osteocalcin also decreased adipose expression of the Nlrp3 inflammasome, a major pathway of Il1-β generation (36) and Ciita, both of which stimulate adipose inflammation (36, 37), which promotes insulin resistance and secretion of factors implicated in NASH development (28). Our data indicate that osteocalcin inhibits multiple pathways of tissue inflammation. Specific mechanisms underlying the antiinflammatory effects of osteocalcin in liver and adipose tissue are unclear, as are the contribution of these effects to NASH attenuation. Osteocalcin signaling in the liver may directly inhibit critical proinflammatory and profibrotic genes to attenuate NASH, or act indirectly through antiinflammatory effects, action to improve insulin sensitivity, because improved glucose metabolism can attenuate liver oxidative stress, steatosis, and inflammation. Notably, osteocalcin treatment is reported to inhibit endoplasmic reticulum stress (38), which regulates several key proinflammatory processes.
In summary, this investigation demonstrates that osteocalcin treatment can attenuate age- and diet-driven NASH development in a mouse model that mimics human disease. Results from this study suggest that these effects arise from actions to inhibit proinflammatory mechanisms that promote hepatic steatosis, inflammation, and fibrosis. However, further studies, including identification of the osteocalcin receptor(s), are required to better define the exact mechanisms involved and their potential for therapeutic interventions to prevent or attenuate NASH in at-risk patients.
Acknowledgments
This work was supported by grants from Houston Methodist Research Institute (O.M.S.), American Heart Association (A.A.G.), Vivian Smith Foundation (A.O.G.), and MacDonald Foundation and the Patel Family Foundation (W.A.H.).
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by grants from Houston Methodist Research Institute (O.M.S.), American Heart Association (A.A.G.), Vivian Smith Foundation (A.O.G.), and MacDonald Foundation and the Patel Family Foundation (W.A.H.).
Footnotes
- ALT
- alanine aminotransferase
- AST
- aspartate aminotransferase
- ITT
- insulin tolerance test
- Mcp1
- Monocyte Chemoattractant Protein 1
- NAFLD
- nonalcoholic fatty liver disease
- NASH
- nonalcoholic steatohepatitis
- NEFA
- nonesterified fatty acids
- OST
- osteocalcin
- QMR
- quantitative magnetic resonance
- Veh
- vehicle
- WHFD
- Western-style high-fat, high-cholesterol diet.
References
- 1. Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ. 2012;31:219–230. [DOI] [PubMed] [Google Scholar]
- 2. Mainous AG 3rd, Baker R, Koopman RJ, et al. Impact of the population at risk of diabetes on projections of diabetes burden in the United States: an epidemic on the way. Diabetologia. 2007;50:934–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tilg H, Moschen AR. Evolving therapies for non-alcoholic steatohepatitis. Expert Opin Drug Discov. 2014;9:687–696. [DOI] [PubMed] [Google Scholar]
- 4. Aller R, Castrillon JL, de Luis DA, et al. Relation of osteocalcin with insulin resistance and histopathological changes of non alcoholic fatty liver disease. Ann Hepatol. 2011;10:50–55. [PubMed] [Google Scholar]
- 5. Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone. 2012;50:568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gower BA, Pollock NK, Casazza K, Clemens TL, Goree LL, Granger WM. Associations of total and undercarboxylated osteocalcin with peripheral and hepatic insulin sensitivity and β-cell function in overweight adults. J Clin Endocrinol Metab. 2013;98:E1173–E1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Q, Zhang B, Xu Y, Xu H, Zhang N. The relationship between serum osteocalcin concentration and glucose metabolism in patients with type 2 diabetes mellitus. Int J Endocrinol. 2013;2013:842598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brennan-Speranza TC, Henneicke H, Gasparini SJ, et al. Osteoblasts mediate the adverse effects of glucocorticoids on fuel metabolism. J Clin Invest. 2012;122:4172–4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pietschmann P, Resch H, Müller C, Woloszczuk W, Willvonseder R. Decreased serum osteocalcin levels in patients with liver cirrhosis. Bone Miner. 1990;8:103–108. [DOI] [PubMed] [Google Scholar]
- 10. Liu JJ, Chen YY, Mo ZN, et al. Relationship between serum osteocalcin levels and non-alcoholic fatty liver disease in adult males, South China. Int J Mol Sci. 2013;14:19782–19791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernández-Real JM, Ortega F, Gómez-Ambrosi J, Salvador J, Frühbeck G, Ricart W. Circulating osteocalcin concentrations are associated with parameters of liver fat infiltration and increase in parallel to decreased liver enzymes after weight loss. Osteoporos Int. 2010;21:2101–2107. [DOI] [PubMed] [Google Scholar]
- 12. Yilmaz Y, Kurt R, Eren F, Imeryuz N. Serum osteocalcin levels in patients with nonalcoholic fatty liver disease: association with ballooning degeneration. Scand J Clin Lab Invest. 2011;71:631–636. [DOI] [PubMed] [Google Scholar]
- 13. Gupte AA, Liu JZ, Ren Y, et al. Rosiglitazone attenuates age- and diet-associated nonalcoholic steatohepatitis in male low-density lipoprotein receptor knockout mice. Hepatology. 2010;52:2001–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Collins AR, Lyon CJ, Xia X, et al. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res. 2009;104:e42–e54. [DOI] [PubMed] [Google Scholar]
- 15. Gupte AA, Minze LJ, Reyes M, et al. High-fat feeding-induced hyperinsulinemia increases cardiac glucose uptake and mitochondrial function despite peripheral insulin resistance. Endocrinology. 2013;154:2650–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nishimoto SK, Price PA. Proof that the γ-carboxyglutamic acid-containing bone protein is synthesized in calf bone. Comparative synthesis rate and effect of coumadin on synthesis. J Biol Chem. 1979;254:437–441. [PubMed] [Google Scholar]
- 17. Nishimoto SK, Price PA. The vitamin K-dependent bone protein is accumulated within cultured osteosarcoma cells in the presence of the vitamin K antagonist warfarin. J Biol Chem. 1985;260:2832–2836. [PubMed] [Google Scholar]
- 18. Nishimoto SK, Araki N, Robinson FD, Waite JH. Discovery of bone γ-carboxyglutamic acid protein in mineralized scales. The abundance and structure of Lepomis macrochirus bone γ-carboxyglutamic acid protein. J Biol Chem. 1992;267:11600–11605. [PubMed] [Google Scholar]
- 19. Nishimoto SK. A colorimetric assay specific for γ-carboxyglutamic acid-containing proteins: its utility in protein purification procedures. Anal Biochem. 1990;186:273–279. [DOI] [PubMed] [Google Scholar]
- 20. Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. [DOI] [PubMed] [Google Scholar]
- 21. Hill HS, Grams J, Walton RG, Liu J, Moellering DR, Garvey WT. Carboxylated and uncarboxylated forms of osteocalcin directly modulate the glucose transport system and inflammation in adipocytes. Horm Metab Res. 2014;46:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou B, Li H, Liu J, et al. Intermittent injections of osteocalcin reverse autophagic dysfunction and endoplasmic reticulum stress resulting from diet-induced obesity in the vascular tissue via the NFκB-p65-dependent mechanism. Cell Cycle. 2013;12:1901–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mehta SR. Advances in the treatment of nonalcoholic fatty liver disease. Ther Adv Endocrinol Metab. 2010;1:101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schuppan D, Schattenberg JM. Non-alcoholic steatohepatitis: pathogenesis and novel therapeutic approaches. J Gastroenterol Hepatol. 2013;28(suppl 1):68–76. [DOI] [PubMed] [Google Scholar]
- 25. Sheedfar F, Sung MM, Aparicio-Vergara M, et al. Increased hepatic CD36 expression with age is associated with enhanced susceptibility to nonalcoholic fatty liver disease. Aging (Albany NY). 2014;6:281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. [DOI] [PubMed] [Google Scholar]
- 27. Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol. 2009;51:371–379. [DOI] [PubMed] [Google Scholar]
- 28. Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. [DOI] [PubMed] [Google Scholar]
- 29. Sheedfar F, Di Biase S, Koonen D, Vinciguerra M. Liver diseases and aging: friends or foes? Aging Cell. 2013;12:950–954. [DOI] [PubMed] [Google Scholar]
- 30. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Giachelli CM, Lombardi D, Johnson RJ, Murry CE, Almeida M. Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Am J Pathol. 1998;152:353–358. [PMC free article] [PubMed] [Google Scholar]
- 32. Bertola A, Deveaux V, Bonnafous S, et al. Elevated expression of osteopontin may be related to adipose tissue macrophage accumulation and liver steatosis in morbid obesity. Diabetes. 2009;58:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yilmaz Y, Ozturk O, Alahdab YO, et al. Serum osteopontin levels as a predictor of portal inflammation in patients with nonalcoholic fatty liver disease. Dig Liver Dis. 2013;45:58–62. [DOI] [PubMed] [Google Scholar]
- 34. Nomiyama T, Perez-Tilve D, Ogawa D, et al. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J Clin Invest. 2007;117:2877–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bruemmer D, Collins AR, Noh G, et al. Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J Clin Invest. 2003;112:1318–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deng T, Cui J, Lyon CJ, et al. Inflammasomes and obesity. Obesity, Inflammation and Cancer Springer Science+Business Media New York. 2013;2013:25–60. [Google Scholar]
- 37. Deng T, Lyon CJ, Minze LJ, et al. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab. 2013;17:411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou B, Li H, Xu L, Zang W, Wu S, Sun H. Osteocalcin reverses endoplasmic reticulum stress and improves impaired insulin sensitivity secondary to diet-induced obesity through nuclear factor-κB signaling pathway. Endocrinology. 2013;154:1055–1068. [DOI] [PubMed] [Google Scholar]