Abstract
All crocodilians and many turtles exhibit temperature-dependent sex determination where the temperature of the incubated egg, during a thermo-sensitive period (TSP), determines the sex of the offspring. Estrogens play a critical role in sex determination in crocodilians and turtles, as it likely does in most nonmammalian vertebrates. Indeed, administration of estrogens during the TSP induces male to female sex reversal at a male-producing temperature (MPT). However, it is not clear how estrogens override the influence of temperature during sex determination in these species. Most vertebrates have 2 forms of nuclear estrogen receptor (ESR): ESR1 (ERα) and ESR2 (ERβ). However, there is no direct evidence concerning which ESR is involved in sex determination, because a specific agonist or antagonist for each ESR has not been tested in nonmammalian species. We identified specific pharmaceutical agonists for each ESR using an in vitro transactivation assay employing American alligator ESR1 and ESR2; these were 4,4′,4′'-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT) and 7-bromo-2-(4-hydroxyphenyl)-1,3-benzoxazol-5-ol (WAY 200070), respectively. Alligator eggs were exposed to PPT or WAY 200070 at a MPT just before the TSP, and their sex was examined at the last stage of embryonic development. Estradiol-17β and PPT, but not WAY 200070, induced sex reversal at a MPT. PPT-exposed embryos exposed to the highest dose (5.0 μg/g egg weight) exhibited enlargement and advanced differentiation of the Müllerian duct. These results indicate that ESR1 is likely the principal ESR involved in sex reversal as well as embryonic Müllerian duct survival and growth in American alligators.
All crocodilian and many turtle species exhibit temperature-dependent sex determination (TSD), where the ambient temperature during a thermo-sensitive period (TSP) of egg incubation (developmental stages 21–24 in the American alligator, Alligator mississippiensis) determines the sex of the offspring (1). Estrogens play a critical role in sex determination in crocodilians and turtles, as it likely does in most nonmammalian vertebrates. Indeed, administration of estrogens during the TSP overrides a male-producing temperature (MPT) in crocodilians and various turtles, amphibians, and teleost fish (2–5). In general, timing of the sensitivity to estrogens, especially 17β-estradiol (E2), coincides with the TSP in 2 turtle species (6, 7).
Embryonic exposure to estrogenic compounds can alter sex determination in nonmammalian species, but does it also lead to other health consequences as it does in mammals? There are clear mechanistic differences between sex determination, differentiation, and maintenance in mammals: sex determination and differentiation are driven by sex chromosomes (eg, sex-determining region Y gene), whereas sex maintenance has more potential to be affected by environmental signals. In contrast, sex determination, differentiation, and maintenance have not been totally distinguished in TSD species due to the lack of sex chromosome, although some studies indicated differences between the temperature- and exogenous estrogen-induced receptor signaling pathway and DNA methylation of cytochrome P450–19 (CYP19) promoter in TSD species (8–10). Although exposure to estrogenic compounds does not influence sex determination in mammals, there is clear evidence from the public health disaster involving embryonic exposure to the pharmaceutical estrogen diethylstilbestrol (DES) that in utero estrogen exposure is associated with health risks such as breast cancer, clear cell adenocarcinoma of vagina and cervix, reproductive tract structural differences, pregnancy complications, and infertility in women as well as noncancerous epididymal cysts in men (11). Some environmental contaminants have been identified as estrogenic in action and included in the endocrine disruptors (12–14). Thus, it is important to understand the mechanisms by which estrogenic compounds influence embryonic development.
The American alligator is a long-lived organism that serves as a top predator in aquatic freshwater and estuarine ecosystems in the Southeastern United States. Individuals exhibit limited geographical ranges. Thus, the alligator is considered a sentinel species to investigate local, long-term environmental health, including issues such as bioaccumulation/biomagnification of environmental contaminates. Indeed, we have evaluated various endocrine and reproductive alterations associated with environmental contaminants and the American alligator is considered an important sentinel of environmental health (15, 16).
Sensitivity to temperature and exogenous chemicals during the TSP could provide a powerful model to investigate the impact of estrogenic endocrine disrupting contaminants (EDCs) as well as climate change due to alter global temperatures. In ovo exposure to exogenous estrogens, pharmaceutical estrogens, or EDCs such as transnonachlor, p,p'-Dichlorodiphenyldichloroethylene (DDE) and Polychlorinated biphenyl (PCB), at environmentally relevant concentration, can induce sexual reverse in red-eared turtles (17–21). Likewise, endogenous or pharmaceutical estrogens as well as in ovo exposure to p,p'-Dichlorodiphenyldichloroethane (DDD), o,p'-DDE, or p,p'-DDE skews sex ratios in the American alligator (18, 22–26). However, it is still not clear how endogenous or pharmaceutical estrogens or even EDCs override or alter TSD. The effect of estrogens during TSD indicates that they act locally and directly on the gonad to induce ovarian differentiation as documented with in vitro studies in turtles (27, 28). Although cell migration from the mesonephros into the gonad is important for the differentiation of functional testicular seminiferous tubules (29), the gonadal tissue itself can “sense” temperature and estrogenic compounds, which can initiate sex determination without mesonephric cell migrations (27, 28).
Estrogen-induced SOX9 suppression during turtle TSP can be delayed by administration of the estrogen receptor (ESR) antagonist, ICI-182780 (7α,17β-[9-[(4,4,5,5,5-Pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol) (30), which has potent antiestrogenic activity and binds to both ESR subtypes in mammals (31–33). These observations indicate that E2 has the potential to regulate the suppression of SOX9 during natural ovarian determination in at least 1 species with TSD. Most vertebrates studied to date, except sharks (34), have 2 forms of the nuclear ESRs, ESR1 (ERα) and ESR2 (ERβ), which have different ligand specificity, distribution, and function (35). In the red-eared turtle, localization and timing of ESRs expression suggests that ESR1 and ESR2 play important roles in sex determination and differentiation, respectively (10, 36). ESR1 mRNA abundance in the turtle gonad peaks late in the TSP, stage 19 in both female-producing temperature (FPT) and MPT, whereas ESR2 mRNA concentration in the gonad gradually increases after TSP, stage 23 at FPT as detected by quantitative real-time PCR (Q-PCR) (10). Although the pattern of ESR localization under differing temperatures regimens was not so clear, differential shifts of each ESR after E2 administration in the turtle indicated potentially different roles at each developmental stage (10). Exogenous E2 exposure rapidly reduced ESR1 in the ovarian medulla and stayed on the boundary of medulla and cortex, whereas ESR2 responded to E2 slowly and was expressed in the cortex during later stages based on visualization with in situ hybridization (10). Thus, ESR1 and ESR2 could be involved in sex determination and differentiation of ovary development in the red-eared slider turtle sequentially, and this suggests that ESR1 could play important roles in exogenous E2-induced sex reversal in TSD amniotes. Further detailed investigation is required to understand estrogen signaling during normal TSD as well as during estrogen-induced sex reversal in crocodilian species.
Here, we report on studies using pharmaceutical estrogen agonists that have specific affinities for each ESR in mammals. We tested each on ESR1 and ESR2 clones from the American alligator using an in vitro transactivation assay. We then exposed eggs exogenously to E2, or the ESR specific agonists before the TSP and examined their potential to sex reverse embryos incubated at a MPT.
Materials and Methods
Transactivation assay
The expression plasmids of the American alligator ESRs in the pcDNA3.1 vector (Life Technologies), and the luciferase reporter plasmid containing 4 estrogen-responsive elements were prepared as described previously (37). To exclude possible differences in affinity of each ESR for a common ERE, a fusion protein of GAL4 and ligand-binding domain (LBD) (including the hinge region and F-domain) of each ESR was also tested. Each partial cDNA was subcloned into pBIND vector (Promega) as describe previous (38), and pG5luc vector (Promega), including 5 GAL4-binging site, was used as the reporter construct. Hormonal transcriptional activity of the HEK293 cells transfected with the expression and reporter constructs using FuGene transfection reagent (Promega) was measured by the luciferase reporter gene assay as previously described (37, 38). E2 was purchased from Sigma-Aldrich, and all ESR agonists, 4,4′,4′'-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT), 7-bromo-2-(4-hydroxyphenyl)-1,3-benzoxazol-5-ol (WAY 200070), 2,3-bis(4-hydroxyphenyl)-propionitrile (DPN), and 2-chloro-3′-fluoro-3,4′-dihydroxy-[1,1-biphenyl]-4-carboxaldehyde oxime (FERb 033), were purchased from Tocris Bioscience. Each estrogenic compound was dissolved in Dimethyl sulfoxide (DMSO) (Sigma-Aldrich), serially diluted with DMSO, and added to the culture media at 0.1% DMSO. Luciferase activity was measured as chemiluminescence activity using the Dual-Luciferase Reporter Assay System (Promega). Luminescence was measured using a Turner Designs Luminometer TD-20/20 (Promega). All transfections were performed in triplicate. Estrogenic activity of each ligand was determined and ranked based on it EC50 calculated in fold inductions from the dose-response curve.
Incubation and treatment of eggs
All field and laboratory work were conducted under permits from the Florida Fish and Wildlife Conservation Commission and Unites States Fish and Wildlife Service. Five clutches of alligator eggs were collected from the Lake Woodruff National Wildlife Refuge, and 2 clutches were collected from the Lake Apopka, Florida in June, 2010 within the first 2 weeks after oviposition. Lake Woodruff has relatively low chemical contamination of persistent organic pollutants, and American alligators exhibit healthy reproductive activity; this lake serves as a reference site for many studies of alligator reproductive biology and development, whereas the Lake Apopka is contaminated with agricultural compounds (15, 39). At least 1 egg from each clutch was examined to determine the developmental stage of the embryo based on criteria described by Ferguson (40). Eggs were treated by applying 0.5 μL of solvent with or without the active compound onto the surface of the eggshell at developmental stage 19; the TSP for sex determination in the American alligator is developmental stages from 21 through 24 (23, 25). Eggs were then incubated at 33.5°C, the temperature at which 100% males (MPT) are produced or at 30.0°C the incubation temperature that produces 100% females (FPT) (1, 23). All treatments were administrated with 0.5 μL of 95% ethanol (EtOH) per gram of egg weight (E.W.): E2 at 0.05, 0.5, 5, 50, and 500 ng/g E.W., PPT or WAY 200070 at 0.5 ng, 50 ng and 5 μg/g E.W., with doses equivalent to 0.05 parts per billion–5 parts per million based on E.W. The highest doses were equivalent to 1.8 × 10−6M E2, 1.3 × 10−5M PPT, and 1.6 × 10−5M WAY 200070, based on E.W. Eggs were incubated in wet sphagnum moss (see details in Refs. 23, 25). Developmental stage was predicted based on an equation developed for the pattern of embryonic development at these temperatures (25). Necropsy was performed at developmental stage 27, which was 1 stage before hatching. The gonad-adrenal-mesonephros (GAM) complexes were dissected free of other tissues and preserved in Bouin's fixative or RNAlater (Life Technologies).
Morphology of Müllerian duct and GAM
The GAM from each animal was removed with the Müllerian duct and fixed in Bouin's fixative overnight. After overnight fixation, the tissue was transferred to 70% ethanol. Gross morphology of Müllerian duct-GAM complex was observed with a stereomicroscope, and pictures of the complex were obtained digitally. The presence or absence of the Müllerian duct was determined. A fixed GAM from each animal was embedded in paraffin, sectioned at 7 μm, and stained with hematoxylin and eosin for microscopic examination and assignment of sex. Testes were identified by the presence of developed medullary sex cords and seminiferous tubules, whereas ovaries were characterized by the presence of germ cells in the cortex and lacunae in the medulla as described previously (41).
RNA isolation and quantitative RT-PCR
Gonadal tissue was dissected free (using a stereomicroscope) from other tissues for each GAM preserved in RNAlater, and gonadal RNA was isolated using the total RNA Isolation system (Promega) as described previously (42). Using the isolated total RNA, cDNA was obtained using a iScript cDNA Synthesis kit (Bio-Rad). Q-PCR was conducted using a CFX96 real-time PCR detection system (Bio-Rad) with homebrew SYBR Green reaction mixture, which contained 20mM Tris-HCl (pH 7.75), 50mM KCl, 3mM MgCl2, 4% DMSO, 0.5× SYBR Green I (Invitrogen), 0.5% glycerol, 0.5% Tween 20 (Sigma-Aldrich), 0.2mM deoxynucleotide mix (Thermo Scientific), 0.01-U/μL AmpliTaq Gold (Applied Biosystems), 0.2μM primer mix, and 1/25 vol of cDNA. The cycle-threshold values were compared with a standard curve obtained from serial dilutions of the plasmid DNA, whose concentration was known and which contained the target cDNA. The plasmids were diluted with TE buffer (10mM Tris-HCl and 1mM EDTA; pH 8.0) plus 5 ng/μL of yeast tRNA as described previously (42, 43). The raw data were obtained as number of copies/μL, and each value was normalized by a normalization factor, which was obtained by using Norm Finder (44). Normalization factor was the geographic mean of eukaryotic elongation factor-1 (EEF1) and ribosomal protein L8 (RPL8) mRNA abundance, whose stability was the best combination of 2 genes in 4 internal control genes, EEF1, RPL8, β-actin, and 18S ribosomal RNA, in Norm Finder (see Supplemental Table 1). PCR primer sequences and annealing temperatures are shown in Supplemental Table 2 below. mRNA abundance was also analyzed using the ΔΔ Ct method with efficiency correction (45). Q-PCR efficiency was estimated by CFX Manager Software (version 3.1; Bio-Rad). Intra- and interassay coefficient of variations for all 3 assays was less than 5% in cycle-threshold values.
Statistical analyses
EC50 values of various estrogenic compounds in the in vitro ESR transactivation assays were estimated by nonlinear best-fit models using 3 parameters for fitting the curve. χ2 tests were used to compare sex ratios among treatment groups and/or egg-incubation temperatures with Bonferroni corrections. Concentrations of mRNA were compared by one-way ANOVA followed by Dennett's comparison, with the EtOH treatment group incubated at MPT serving as the control group. In the cases where groups showed nonnormal distributions, a Kruskal-Wallis test followed by Dunn's comparison was used to evaluate the treatment effects. Effects of egg-incubation temperature on mRNA abundance was evaluated by unpaired t test for normally distributed samples, or by Mann-Whitney for samples without normal distribution or equal deviations. Outliers were detected and removed using the robust regression and outliers removal method at Q = 0.1%. Each value is shown as a mean ± 1 SEM. Statistical analyses were conducted using Prism 6.0b (GraphPad Software) and JMP 9.0.2 (SAS Institute) for Mac. Cluster analysis of ovarian mRNA abundance was conducted with GENE-E software (Broad Institute).
Results and Discussion
Specificities of ESR agonists in vitro
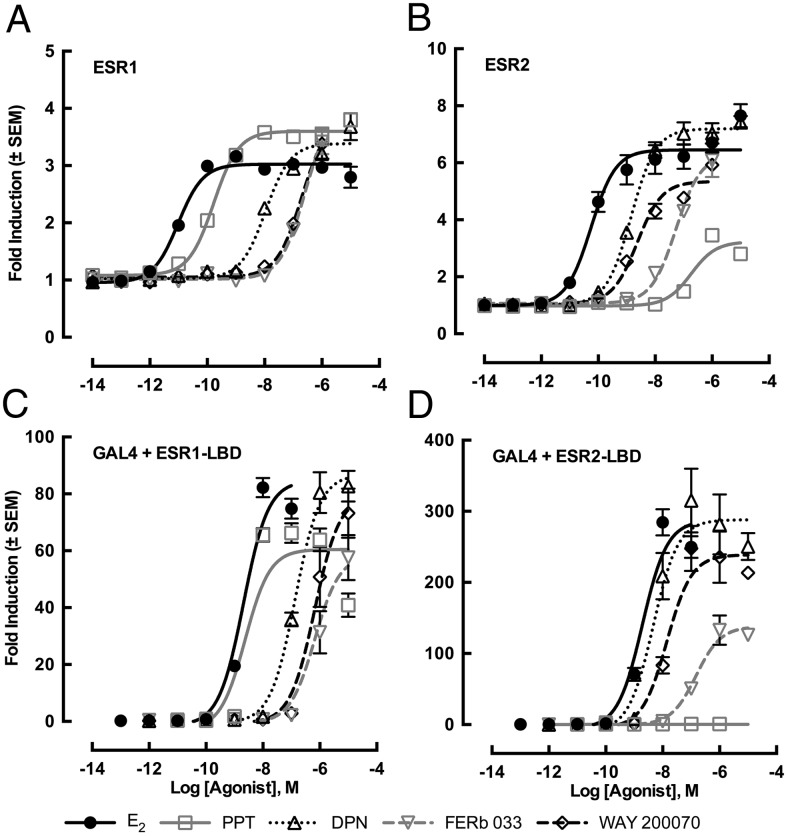
ESR agonists induced transcriptional activity in the in vitro ESR transactivation assay via full-length receptor on ERE with EC50 values ranked as follows: for ESR1, E2 > PPT > DPN > FERb 033 = WAY 200070; and for ESR2, E2 > DPN = WAY 200070 > FERb 033 > PPT (Figure 1, A and B, and Table 1). As seen with the assay using the full-length receptor, each ESR revealed a similar specificity to ESR agonists when the fusion protein of GAL4 and ESR LBD were used, although the sensitivities to each agonist were lowered (Figure 1, C and D, and Table 1). Thus, the specificity of each ligand was dependent on the LBD affinity to the ligand rather than the selectivity of the ERE. This reduction in ligand sensitivity was also observed in a previous study of sex steroid hormone receptors in the slider turtle (38). This shift in sensitivity could be caused due to lack of the activation function 1 domain in the A-B domain of ESR. PPT displayed great specificity for alligator ESR1. Although DPN and WAY 200070 showed similar transactivation activity on alligator ESR2, WAY 200070 exhibited significantly less cross-reactivity on ESR1 than DPN; therefore, we used WAY 200070 as a specific agonist for alligator ESR2 (Figure 1 and Table 1). Studies examining the specificity of pharmaceutical ESR agonists in mammalian rodents showed that DPN exhibited the highest specificity for ESR2 (46, 47), whereas alligator ESR2 displayed better specificity for WAY 200070. Although alligator ESR2 has a predicted amino acid identity similar to 90%–92% with that of the ligand binding region of mammalian ESR2 (48), specific characteristics of ESR2 binding affinity or transactivation are different as noted here.
Figure 1. Transcriptional activities of alligator ESR1 (A and C) or ESR2 (B and D) for various ESR ligands.
HEK293 cells were transiently transfected with the 4 estrogen-responsive elements containing vector together and an alligator ESR expression vector (A and B) or were transfected with pG5luc vector containing 5 GAL4 binding sites and LBD of each ESR in pBIND vector, including the GAL4 DNA-binding domain (C and D). Cells were incubated with serial concentrations of E2, PPT, DPN, FERb 033, or WAY 200070 at 10−14M–10−5M. Data were represented as fold inductions of luciferase signals as compared with vehicle (DMSO) controls. PPT and WAY 200070 showed the highest specificity on ESR1 and ESR2, respectively. Each column represents the mean of triplicate determinations, and vertical bars represent the mean ± 1 SEM.
Table 1.
Specificity of ESR Agonists on Full-Length of ESR or Gal4-Ligand Binding Domain of ESR Fusion Protein in Transactivation
| ESR1 Full Length on ERE |
ESR2 Full Length on ERE |
|||||||
|---|---|---|---|---|---|---|---|---|
| EC50 (M) | RP (%) | RE (%) | ESR1/ESR2 of EC50 | EC50 (M) | RP (%) | RE (%) | ESR2/ESR1 of EC50 | |
| E2 | 9.66 × 10−12 | 100.0 | 100.0 | 0.2 | 5.55 × 10−11 | 100.0 | 100.0 | 5.7 |
| PPT | 1.67 × 10−10 | 5.8 | 128.3 | 0.0 | 1.83 × 10−7 | 0.0 | 41.5 | 1093.0 |
| DPN | 1.08 × 10−8 | 0.1 | 117.6 | 7.6 | 1.42 × 10−9 | 3.9 | 113.6 | 0.1 |
| FERb 033 | 2.53 × 10−7 | 0.0 | 148.9 | 4.7 | 5.38 × 10−8 | 0.1 | 96.9 | 0.2 |
| WAY 200070 | 1.94 × 10−7 | 0.0 | 138.9 | 82.8 | 2.35 × 10−9 | 2.4 | 79.7 | 0.0 |
| ESR1 LBD-GAL4 |
ESR2 LBD-GAL4 |
|||||||
|---|---|---|---|---|---|---|---|---|
| EC50 (M) | RP (%) | RE (%) | ESR1/ESR2 of EC50 | EC50 (M) | RP (%) | RE (%) | ESR2/ESR1 of EC50 | |
| E2 | 2.08 × 10−9 | 100.0 | 100.0 | 1.07 | 1.93 × 10−9 | 100.0 | 100.0 | 0.93 |
| PPT | 2.37 × 10−9 | 87.6 | 71.5 | N/A | N.D. | N/A | N.D. | N/A |
| DPN | 1.36 × 10−7 | 1.5 | 102.5 | 29.12 | 4.66 × 10−9 | 41.5 | 100.5 | 0.03 |
| FERb 033 | 1.13 × 10−6 | 0.2 | 76.0 | 5.84 | 1.49 × 10−7 | 1.3 | 48.1 | 0.13 |
| WAY 200070 | 6.62 × 10−7 | 0.3 | 93.8 | 47.17 | 1.40 × 10−8 | 13.8 | 83.3 | 0.02 |
RP, relative potency = (EC50 of E2/EC50 of other agonist) × 100; RE, relative efficacy = maximum response % of E2 found at any doses.
Gross anatomy. Effects of in ovo exposure to estrogens during the TSP at different incubation temperatures
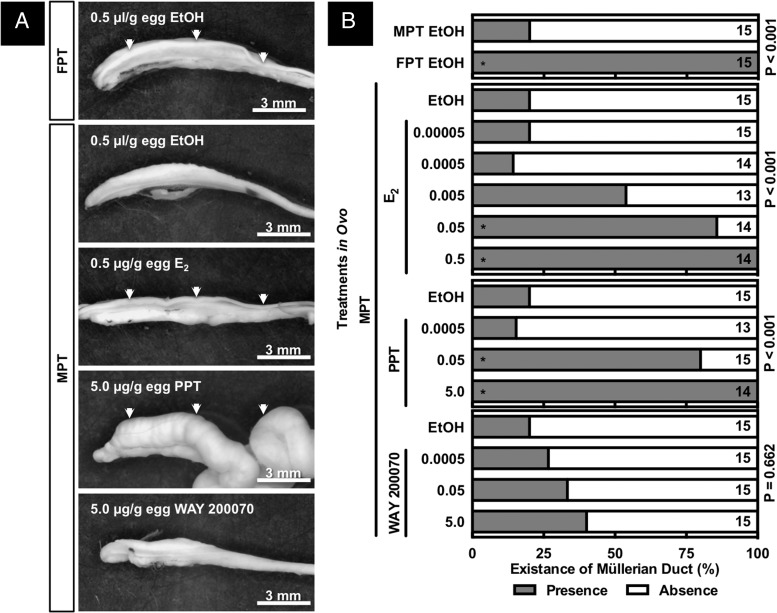
Expanded Müllerian ducts, enlarged cloaca, and a thickened pubis symphysis were observed after exposure to 5-μg PPT/g E.W. at a MPT, whereas these alterations were not seen after exposure to 2 lower doses of PPT, nor after exposure to any of the doses of E2 or WAY 200070 (Figures 2 and 3), although E2 did sex reverse embryos incubated at MPT (see below). In the present study, ovarian anti-Müllerian hormone (AMH) mRNA abundances were not altered by treatments in ovo; thus, it is unlikely that a suppressed AMH signal would relate to the PPT-induced hyperplasia. As expected, Müllerian ducts were identified in the embryos incubated at FPT but not in most of the embryos incubated at MPT (Figure 3). Regression of the Müllerian duct, which is mainly regulated by AMH (49), was interrupted by exposure to PPT (0.5 and 5 μg/g E.W.) or E2 (0.05 and 0.5 μg/g E.W.) but not by WAY 200070 exposure (Supplemental Figure 1). Thus, an estrogenic signal via ESR1 plays a critical role in the survival of the Müllerian duct.
Figure 2. Gross morphology around the abdominal cavity in embryonic American alligator at developmental stage 27.
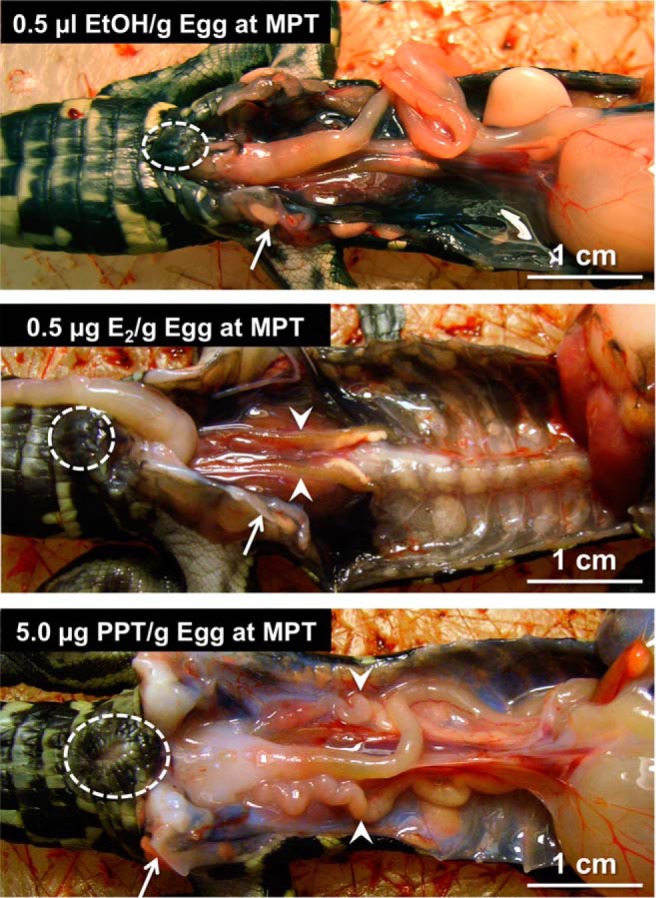
Embryo at developmental stage 19 was exposed to 0.5-μL 95% ethanol/g E.W. (top panel), 0.5-μg E2/g E.W. (E2) (middle panel), or 5.0 μg/g E.W. of PPT (bottom panel) at MPT. PPT induced enlarged cloaca, pelvic bone, and Müllerian duct. Circle, arrow, and arrowhead indicate cloaca, pelvic bone, and Müllerian duct, respectively.
Figure 3. Gross morphology of GAM complex and Müllerian duct (A) isolated from embryonic American alligator at developmental stage 27.
Embryo at developmental stage 19 was exposed to 0.5 μL/g E.W. of 95% ethanol, various doses of E2, PPT, or WAY 200070 at FPT or MPT. Existences of Müllerian duct were observed and counted, and data were shown as percentage of Müllerian ductal presence or absence (B). An exposure to E2 and PPT increased the existence of Müllerian duct at MPT, whereas WAY 200070 exposure did not alter it. Numbers on the graph indicate number of animals. Asterisks indicate significant difference in χ2 test (P < .05).
Gonadal histology. Effects of in ovo exposure to estrogens during the TSP at different incubation temperatures
Based on histological characteristics, sex reversal was significantly induced under 2 of the experimental conditions. Embryos exposed to the higher doses of E2 and PPT (0.05–0.5 μg/g and 0.5–5.0 μg/g E.W., respectively) and incubated at a MPT exhibited ovarian, vs testicular, development in all the exposed embryos (P < .0001 and P = .0027, respectively), when compared with exposure to EtOH (vehicle) control at MPT (P = .0027) (Figure 4). All other treatments, except EtOH at FPT control (P < .0001), showed no significant difference in their gonadal sex ratio within a treatment group when compared with the EtOH vehicle control at MPT (Figure 4). Gonadal size was obviously smaller in the embryos exposed to 5-μg PPT/g E.W. when compared with the other treatment groups. Ovarian medullary lacunae in embryos exposed to 5-μg PPT/g E.W. were more developed than those observed from embryos exposed to E2 treatments (Figure 4C). Developmental exposure to E2 or overexpression of CYP19 was sufficient to induce sex reversal in genetic male chickens, but it did not alter gonadal size or ovarian lacunae development (50, 51). Although sex reversal and hypertrophic Müllerian ducts were induced by DES exposure in male chickens, a smaller gonad also was not observed (52). The smaller gonads and limited development of ovarian lacunae in the alligator neonates observed in this study are hypothesized to be a response to disproportionate activation of ESR1 signaling after PPT exposure.
Figure 4. Gonadal histology of embryonic American alligator at developmental stage 27 (A).
Embryo at developmental stage 19 was exposed to 0.5 μL/g E.W. of 95% ethanol, various doses of E2, PPT, or WAY 200070 at FPT or MPT. Gonadal characteristics were observed and counted, and data were shown as percentage of ovarian or testicular characteristics (B) and score of lacunae (C). An exposure to E2 and PPT induced the ovarian development at MPT, whereas WAY 200070 exposure and control induced the testicular development with seminiferous tubules at MPT. PPT exposure at developmental stage 19 induced higher score of lacunae development. Numbers on the graph indicate number of animals. Asterisks indicate significant difference in χ2 test or (P < .05).
Estrogen stimulates not only nuclear ESRs but also stimulates membrane bound ESRs (53, 54). Although PPT could induce or enhance rapid reaction via cell membrane bound receptors in mammals (48, 55–58), those effects were highly reduced in ESR1 knockout mice (56). Thus, the estrogen-induced sexual reversal observed in the present study was most likely caused through nuclear ESR1, but further investigations are required to clarify whether nuclear or membrane ESR1, or both, are induced when exogenous estrogens are used to induce sex reversal in the American alligator.
mRNA abundance. Effects of in ovo exposure to estrogens during the TSP at different incubation temperatures
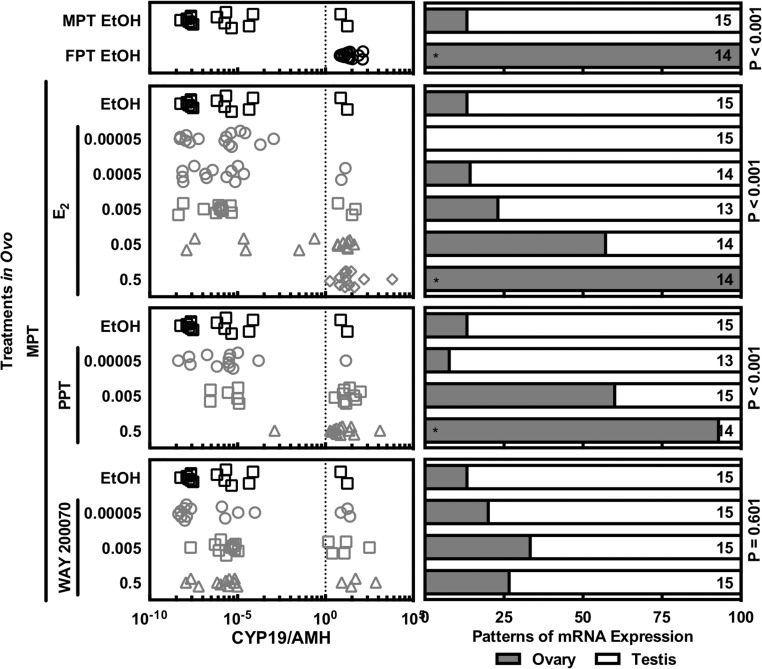
The pattern of mRNA abundance was analyzed by quantitative RT-PCR with known concentrations of serially diluted plasmid DNA as standard samples (see Supplemental Table 2). Gonadal tissues displayed a clear induction of sex-specific mRNA after egg incubation at thermally sensitive temperatures as well as in ovo exposure to the specific agonists for the 2 ESRs. Males showed higher gonadal mRNA abundance of AMH compared with CYP19 (aromatase), whereas female displayed the opposite pattern as observed for other vertebrates (42, 59, 60). Using this sexual dimorphic pattern as a criterion, the sex of embryos was functionally confirmed (Figure 5). Although most embryos displayed consistent results based on the existence of the Müllerian duct, gonadal morphology, and gonadal mRNA pattern, Müllerian duct existence was the least reliable of these 3 sexing criteria as described previously (61). The mRNA expression pattern in 3 embryos did not matched with their gonadal morphology, but they were not observed in a specific treatment group (Figure 5). The gonadal morphology of 1 male displayed an ovarian-like mRNA expression pattern (CYP19 > AMH), whereas 2 females with ovaries exhibited a testicular-like mRNA expression pattern (CYP19 < AMH) (Figure 5). When the mRNA abundances were analyzed by treatment group, it was difficult to evaluate the effects of each treatment due to having both sexes in some treatment groups in which sex reversal occurred in only some of the exposed embryos (Supplemental Figure 1).
Figure 5. Discrimination of gonads by sexual dimorphic pattern of gonadal mRNA abundances of CYP19 (aromatase) and AMH.
Embryo at developmental stage 19 was exposed to 0.5 μL/g E.W. of 95% ethanol, various doses of E2, PPT, or WAY 200070 at FPT or MPT. Gonadal tissue was speared out from GAM complex at developmental stage 27, and their mRNA abundances were quantified by Q-PCR. Male pattern was defined with lower abundance of CYP19 mRNA and higher abundance of AMH, whereas female pattern was defined with high CYP19 and low AMH mRNA abundances. An exposure to E2 and PPT induced the female pattern of mRNA abundance at MPT, whereas WAY 200070 exposure and control induced the male pattern of mRNA abundance at MPT. Numbers on the graph indicate number of animals. Asterisks indicate significant difference in χ2 test (P < .05).
Table 2.
Primers for Q-PCR
| Primer Name | Position | Sequence (5′ to 3′) | Annealing Temperature (°C) | GenBank Accession Number |
|---|---|---|---|---|
| ACTB | 209 | ATGAGGCCCAAAGCAAAAGA | 64.0 | DQ421415 |
| 280 | CCCAGTTGGTGACAATGCC | |||
| AMH | 1483F | AGCAGCTCAACCTCTCTGAGGA | 64.0 | NM_001287280 |
| 1726R | TAGCAGAAAGCCAGAAGGTGC | |||
| CYP19 | 1227F | CAGCCAGTTGTGGACTTGATCA | 66.4 | AY029233 |
| 1305R | TTGTCCCCTTTTTCACAGGATAG | |||
| DAX1 | 618F | TGCTCTTTCCTTGCTGAGATC | 62.0 | AF180295 |
| 692R | ACTGTGCCAATGATAGGCCTA | |||
| DMRT1 | 175F | AGCCCAACTCACTCAACAAG | 62.0 | XM_006261265 |
| 261R | GATGGAAGGAACATCCTGAA | |||
| EEF1 | 321F | CGTTCTGGTAAGAAGCTGGA | 62.0 | XM_006269752 |
| 489R | TGACACCAACAGCAACAGTC | |||
| ESR1 | 410F | AAGCTGCCCCTTCAACTTTTTA | 64.0 | AB115909 |
| 481R | TGGACATCCTCTCCCTGCC | |||
| ESR2 | 34F | AAGACCAGGCGCAAAAGCT | 60.7 | XM_006265650 |
| 105R | GCCACATTTCATCATTCCCAC | |||
| FOXL2 | 111F | CATCAGCAAGTTCCCCTTC | 60.7 | EU848473 |
| 207R | GGGCACCTTGATGAAACAC | |||
| FST | 800F | GCCCTACTGGGCAGATCCAT | 64.0 | DQ010156 |
| 911R | CCTTGAAATCCCACAAGCAT | |||
| GDF9 | 557F | TCAGTTTCCTCCTCTTCTCCAATT | 62.0 | XM_006263403 |
| 634R | ACACACTTGGCTAGAAGGATCATTC | |||
| INHA | 645F | CAACTGCCACCGCGC | 66.4 | XM_006261090 |
| 707R | ACAATCCACTTGTCCCAGCC | |||
| RN18S | 1082F | GAGTATGGTTGCAAAGCTGAA | 66.4 | AF173605 |
| 1303R | AGTCTCGTTCGTTATCGGAAT | |||
| RPL8 | 702F | GGTGTGGCTATGAATCCTGT | 62.0 | XM_006266675 |
| 827R | ACGACGAGCAGCAATAAGAC |
ACTB, β-actin; DMRT1, doublesex and mab-3-related transcription factor 1; INHA, inhibin α; RN18S, 18S ribosomal RNA.
Gonadal mRNA abundances of CYP19, dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1 (DAX1), follistatin (FST), forkhead box L2 (FOXL2), growth and differentiation factor 9 (GDF9), and AMH were compared within the female embryos to evaluate their ovarian function. DAX1 is an orphan member of the nuclear hormone receptor superfamily and plays an important role in ovarian development and regulation of CYP19 (62–64). Fst is produced by granulosa cells in ovary and antagonizes activin signaling, which plays important roles in the feedback loop of the hypothalamic-pituitary-gonadal axis (65). FOXL2 is a transcription factor and master regulator for CYP19 and is critical in ovarian differentiation and maintenance (66, 67). GDF9 is vital for early ovarian follicle development and contributes to testicular germ cell health (68, 69). In previous reports, ovarian FST, GDF9 mRNA expressions were lower in neonates from Lake Apopka (contaminated with agricultural chemicals) as compared with a reference lake, Lake Woodruff (70, 71).
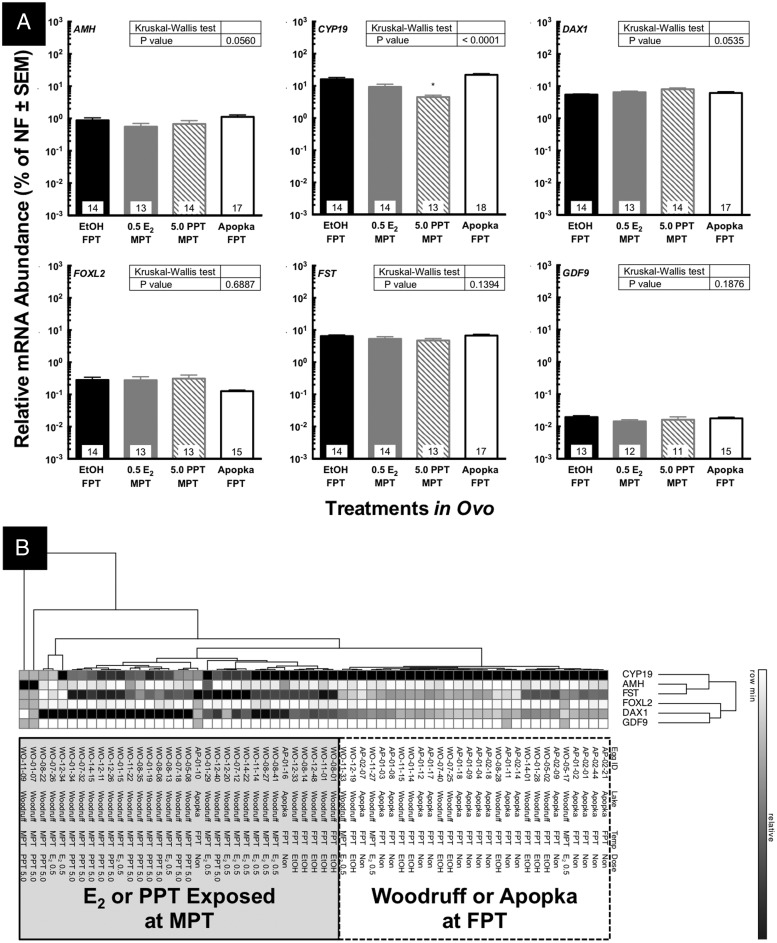
Sex reversed embryos induced by exposure to 5 μg of PPT/g egg showed a significant suppression in gonadal mRNA abundance of CYP19 (Figure 6A). This result in CYP19 mRNA abundance was observed using both methods of analyzing abundance, the standard curve method and ΔΔCt method. DNA methylation of the gonadal SOX9 and CYP19 promoter is involved in TSD in the American alligator (59). Although DNA methylation of the CYP19 promoter plays a critical role in TSD of the European sea bass, E2-induced sex reversal did not altered the DNA methylation level of CYP19 promoter in the female gonad (9). In red-eared slider turtle, DNA methylation of the gonadal CYP19 promoter was associated with TSD, whereas PCB-induced sex-reversed hatchlings showed no difference from normal females in gonadal DNA methylation of the CYP19 promoter (8, 72). We are currently investigating DNA methylation patterns after in ovo PPT or E2 exposure as well as methylation in American alligator from Lake Apopka. AMH, DAX1, FOXL2, FST, and GDF9 mRNA abundance in the embryonic ovary were not altered by either E2 or PPT exposure in ovo in this study (Figure 6A). Gonadal mRNA abundances of CYP19, DAX1, FST, and GDF9, however, did show significant reductions in neonatal or juvenile ovarian tissues obtained from alligators from Lake Apopka when compared with similar tissues from Lake Woodruff (70, 71, 73). Additionally, mRNA abundances of ESR1, ESR2, DAX1, and inhibin-α did not show definitive alteration in the embryonic ovary after PPT exposure in ovo (Supplemental Figures 2 and 3). These differences in mRNA abundances between present and previous study could be due to age differences of animals. Further investigations are required to clarify it.
Figure 6. Alterations of gonadal mRNA abundances at developmental stage 27 in female embryos (A).
Embryo at developmental stage 19 was exposed to 0.5 μL/g E.W. of 95% ethanol, various doses of E2, PPT, or WAY 200070 at FPT or MPT. Gonadal tissue was speared out from GAM complex at developmental stage 27, and their mRNA abundances were quantified by Q-PCR. The embryos from Lake Apopka were also analyzed at stage 27 after incubation at FPT without exogenous exposure. CYP19 (aromatase), DAX1, FST, FOXL2, GDF9, and AMH were quantified. Asterisks indicate significant difference as compared with vehicle controls (EtOH) at FPT (P < .05). Based on the quantified mRNA abundances, the 2-way hierarchical clustering was conducted with GENE-E software (B) and classified the individual embryos into 2 major groups.
The contaminants in Lake Apopka such as DDE and PCBs exhibit very low affinities for the alligator ESR1 as compared with E2 but are present at concentrations (parts per million) that are biologically relevant (L.J.G., unpublished data). Although suppressed CYP19 expression and enzyme function were previously observed in neonatal or juvenile alligators from Lake Apopka (70, 74), the suppression of CYP19 mRNA abundance in PPT-exposed gonads could be due to a different pathway from the observed suppression in Apopka alligator ovaries based on the results of the cluster analysis performed using the current dataset at developmental stage 27 (Figure 6B). Based on the mRNA abundances of the genes examined in this study, female embryos were classified into 3 major clusters (Figure 6B). PPT- or E2-exposed animals made a major cluster, whereas the EtOH vehicle control and Apopka embryos clustered together (Figure 6B). Thus, egg-incubation temperature could induce differences in gonadal function based on the mRNA abundances rather than the developmental exposure to estrogen, PPT, or environmental contaminants in Lake Apopka at developmental stage 27 (Figure 6B). These results indicate that the pattern of mRNA abundance in an individual embryo could be more meaningful than the mean of each treatment group at developmental stage 19. This is coincident with the DNA methylation pattern of CYP19 in sex-reversed gonads of PCB-exposed red-eared slider turtles, which was correlated with temperature rather than exposure to PCB (8). Sea bass also reveal that E2 exposure did not alter DNA methylation levels in the CYP19 promoter in females induced at the same temperature (9). Therefore, temperature could be the primary factor regulating DNA methylation patterns of the gonadal CYP19 gene during development, although further investigation is required to understand the details of the mechanism driving alteration of DNA methylation patterns in sex-reserved animals.
Enlarged Müllerian duct induced by PPT exposure
Although the Müllerian ducts were identified in some male neonates (61), their presence has been used as a secondary characteristic in sexing female American alligators, especially in developing embryos and neonates. We have, however, observed that some embryos exhibit Müllerian ducts even with testicular tissue: this observation was made mostly in the group exposed to 0.05-μg PPT/g E.W. or 0.005-μg E2/g E.W., which are below the dose needed to sex reverse the embryo. Thus, the presence of a Müllerian duct cannot be used as a good indicator for gonadal sex (female) in embryos or hatchlings. A hypertrophic Müllerian duct was previously induced by in ovo exposure to 0.5 mg of norethindrone (19-nor-17α-ethynyltestosterone, a progestin) in the American alligator (75), and the histological characteristics reported were similar to those induced in this study with PPT-induced hyperplasia. Thus, PPT or PPT metabolites might have progestin activity in the American alligator, but this possibility has not been tested. In ovo exposure to estriol or DES (a synthetic estrogen) in chickens (52, 76), and in ovo exposure to estradiol benzoate or testosterone propionate in the snapping turtle, Chelydra serpentina (19), induced hypertrophic Müllerian ducts. In the present study, ovarian AMH mRNA abundances were not altered by treatments in ovo within females; thus, suppression of AMH action on the Müllerian ducts is unlikely to be related to the observed PPT-induced hyperplasia. However, here, we observed not only hyperplasia after in ovo PPT exposure but regional (anterior-posterior axis) differentiation in the enlarged Müllerian duct with both epithelial and stromal expansions (based on histological analysis) (data not shown) in the American alligator. ESR1 is required to induce hyperplasia and hypertrophy in vaginal epithelium and all uterine compartments in E2-stimulated mice (77). Thus, hyperplasia of Müllerian ducts is induced via an ESR1 signal and is consistent with previous studies reporting hypertrophic Müllerian ducts in estradiol benzoate-treated snapping turtles (19). Further investigations are needed to understand the mechanisms inducing oviducal hyperplasia or hypertrophy in reptiles.
In conclusion, we have identified that ESR1 plays a central role in sex reversal induced with an exogenous estrogen in the American alligator. We saw no evidence in this study that ESR2 plays a similar role. Although the abundances of CYP19 and AMH mRNA were reversed by PPT or E2 exposure independent of egg-incubation temperature, ovarian mRNA expression patterns were associated with egg-incubating temperature rather than exposure based on the genes examined in this study and a cluster analysis at developmental stage 19. Further, we have noted that stimulation of ESR1 alone with an exogenous pharmaceutical ligand leads to abnormal maturation and growth (hypertrophy and hyperplasia) of the Müllerian tract of the developing embryo. At this point, we do not know if this phenotype is a response to ESR1 activation above normal stimulation or persistence of the signal with a lack of concomitant ESR2 signaling as would occur if a native endogenous ligand, such as E2, had been used. Further investigations are needed to understand steroid signaling during ovarian development as well as the possible role of EDCs induced sex reversal or skewed sex ratios via alterations in the ESR1 signaling cascade.
Acknowledgments
This work was supported by the Gulf of Mexico Research Initiative (S.K. and L.J.G.); a grant from the National Institute of Standards and Technology (L.J.G. and S.K.); the South Carolina Centers of Economic Excellence for Marine Genomics (L.J.G.); Grants-in-Aid for Scientific Research 17052032 (to Y.K.), 20570064 (to Y.K.), 21510068 (to Y.K.), and 19370027 (to T.I.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; and a grant from the National Institute for Basic Biology (T.I.).
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the Gulf of Mexico Research Initiative (S.K. and L.J.G.); a grant from the National Institute of Standards and Technology (L.J.G. and S.K.); the South Carolina Centers of Economic Excellence for Marine Genomics (L.J.G.); Grants-in-Aid for Scientific Research 17052032 (to Y.K.), 20570064 (to Y.K.), 21510068 (to Y.K.), and 19370027 (to T.I.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; and a grant from the National Institute for Basic Biology (T.I.).
Footnotes
- AMH
- anti-Müllerian hormone
- CYP19
- cytochrome P450–19
- DAX1
- dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1
- DDE
- Dichlorodiphenyldichloroethylene
- DDT
- Dichlorodiphenyldichloroethane
- DES
- diethylstilbestrol
- DMSO
- Dimethyl sulfoxide
- DPN
- 2,3-bis(4-hydroxyphenyl)-propionitrile
- E2
- 17β-estradiol
- EDC
- endocrine disrupting contaminant
- EEF1
- eukaryotic elongation factor-1
- ESR
- estrogen receptor
- EtOH
- ethanol
- E.W.
- egg weight
- FERb 033
- 2-chloro-3′-fluoro-3,4′-dihydroxy-[1,1-biphenyl]-4-carboxaldehyde oxime
- FOXL2
- forkhead box L2
- FPT
- female-producing temperature
- FST
- follistatin
- GAM
- gonad-adrenal-mesonephros
- GDF9
- growth and differentiation factor 9
- ICI-182780
- 7α,17β-[9-[(4,4,5,5,5-Pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol
- LBD
- ligand-binding domain
- MPT
- male-producing temperature
- PCB
- Polychlorinated biphenyl
- PPT
- 4,4′,4′'-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol
- Q-PCR
- quantitative real-time PCR
- RPL8
- ribosomal protein L8
- TSD
- temperature-dependent sex determination
- SOX9
- sex determining region Y-box 9
- TSP
- thermo-sensitive period
- WAY 200070
- 7-bromo-2-(4-hydroxyphenyl)-1,3-benzoxazol-5-ol.
References
- 1. Lang JW, Andrews HV. Temperature-dependent sex determination in crocodilians. J Exp Zool. 1994;270:28–44. [Google Scholar]
- 2. Pieau C. Temperature variation and sex determination in reptiles. Bioessays. 1996;18:19–26. [Google Scholar]
- 3. Devlin RH, Nagahama Y. Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture. 2002;208:191–364. [Google Scholar]
- 4. Smith CA, Sinclair AH. Sex determination: insights from the chicken. Bioessays. 2004;26:120–132. [DOI] [PubMed] [Google Scholar]
- 5. Dournon C, Houillon C, Pieau C. Temperature sex-reversal in amphibians and reptiles. Int J Dev Biol. 1990;34:81–92. [PubMed] [Google Scholar]
- 6. Merchant-Larios H, Ruiz-Ramirez S, Moreno-Mendoza N, Marmolejo-Valencia A. Correlation among thermosensitive period, estradiol response, and gonad differentiation in the sea turtle Lepidochelys olivacea. Gen Comp Endocrinol. 1997;107:373–385. [DOI] [PubMed] [Google Scholar]
- 7. Wibbels T, Bull JJ, Crews D. Synergism between temperature and estradiol: a common pathway in turtle sex determination? J Exp Zool. 1991;260:130–134. [DOI] [PubMed] [Google Scholar]
- 8. Matsumoto Y, Hannigan B, Crews D. Embryonic PCB exposure alters phenotypic, genetic, and epigenetic profiles in turtle sex determination, a biomarker of environmental contamination. Endocrinology. 2014; 55:4168–4177. [DOI] [PubMed] [Google Scholar]
- 9. Navarro-Martin L, Vinas J, Ribas L, et al. . DNA methylation of the gonadal aromatase (cyp19a) promoter is involved in temperature-dependent sex ratio shifts in the European sea bass. PLoS Genet. 2011;7:e1002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramsey M, Crews D. Steroid signaling system responds differently to temperature and hormone manipulation in the red-eared slider turtle (Trachemys scripta elegans), a reptile with temperature-dependent sex determination. Sex Dev. 2007;1:181–196. [DOI] [PubMed] [Google Scholar]
- 11. US-CDC. 2012 CDC's DES Update. http://www.cdc.gov/DES.
- 12. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. . Endocrine-disrupting chemicals: an endocrine society scientific statement. Endocr Rev. 2009;30:293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rooney AA, Guillette LJ Jr. Contaminant interactions with steroid receptors: evidence for receptor binding. In: Guillette LJ Jr, Crain DA, eds. Endocrine Disrupting Contaminants: An Evolutionary Perspective. New York, NY: Francis and Taylor; 2000:82–125. [Google Scholar]
- 14. McLachlan JA. Environmental signaling: what embryos and evolution teach us about endocrine disrupting chemicals. Endocr Rev. 2001;22:319–341. [DOI] [PubMed] [Google Scholar]
- 15. Guillette LJ Jr, Crain DA, Gunderson MP, et al. . Alligators and endocrine disrupting contaminants: a current perspective. Am Zool. 2000;40:438–452. [Google Scholar]
- 16. Hamlin HJ, Guillette LJ Jr. Embryos as targets of endocrine disrupting contaminants in wildlife. Birth Defects Res C Embryo Today. 2011;93:19–33. [DOI] [PubMed] [Google Scholar]
- 17. Willingham E, Crews D. Sex reversal effects of environmentally relevant xenobiotic concentrations on the red-eared slider turtle, a species with temperature-dependent sex determination. Gen Comp Endocrinol. 1999;113:429–435. [DOI] [PubMed] [Google Scholar]
- 18. Bull JJ, Gutzke WH, Crews D. Sex reversal by estradiol in three reptilian orders. Gen Comp Endocrinol. 1988;70:425–428. [DOI] [PubMed] [Google Scholar]
- 19. Crews D, Wibbels T, Gutzke WH. Action of sex steroid hormones on temperature-induced sex determination in the snapping turtle (Chelydra serpentina). Gen Comp Endocrinol. 1989;76:159–166. [DOI] [PubMed] [Google Scholar]
- 20. Crews D, Bull JJ, Wibbels T. Estrogen and sex reversal in turtles: a dose-dependent phenomenon. Gen Comp Endocrinol. 1991;81:357–364. [DOI] [PubMed] [Google Scholar]
- 21. Crews D. Sex determination: where environment and genetics meet. Evol Dev. 2003;5:50–55. [DOI] [PubMed] [Google Scholar]
- 22. Rooney AA. Variation in the endocrine and immune system of juvenile alligators: environmental influence on physiology. Master's Thesis Gainesville, FL: University of Florida; 1998. [Google Scholar]
- 23. Milnes MR, Bryan TA, Medina JG, Gunderson MP, Guillette LJ Jr. Developmental alterations as a result of in ovo exposure to the pesticide metabolite p,p'-DDE in Alligator mississippiensis. Gen Comp Endocrinol. 2005;144:257–263. [DOI] [PubMed] [Google Scholar]
- 24. Matter JM, McMurry CS, Anthony AB, Dickerson RL. Development and implementation of endocrine biomarkers of exposure and effects in American alligators (Alligator mississippiensis). Chemosphere. 1998;37:1905–1914. [Google Scholar]
- 25. Kohno S, Guillette LJ Jr. Endocrine disruption and reptiles: using the unique attributes of temperature-dependent sex determination to assess impacts. In: Matthiessen P, ed. Endocrine Disrupters: Hazard Testing and Assessment Methods. Hoboken, NJ: John Wiley, Sons; 2013:245–271. [Google Scholar]
- 26. Crain DA, Guillette LJ Jr, Rooney AA, Pickford DB. Alterations in steroidogenesis in alligators (Alligator mississippiensis) exposed naturally and experimentally to environmental contaminants. Environ Health Perspect. 1997;105:528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pieau C, Dorizzi M. Oestrogens and temperature-dependent sex determination in reptiles: all is in the gonads. J Endocrinol. 2004;181:367–377. [DOI] [PubMed] [Google Scholar]
- 28. Shoemaker-Daly CM, Jackson K, Yatsu R, Matsumoto Y, Crews D. Genetic network underlying temperature-dependent sex determination is endogenously regulated by temperature in isolated cultured Trachemys scripta gonads. Dev Dyn. 2010;239:1061–1075. [DOI] [PubMed] [Google Scholar]
- 29. Yao HH, Capel B. Temperature, genes, and sex: a comparative view of sex determination in Trachemys scripta and Mus musculus. J Biochem. 2005;138:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barske LA, Capel B. Estrogen represses SOX9 during sex determination in the red-eared slider turtle Trachemys scripta. Dev Biol. 2010;341:305–314. [DOI] [PubMed] [Google Scholar]
- 31. Wakeling AE. Similarities and distinctions in the mode of action of different classes of antioestrogens. Endocr Relat Cancer. 2000;7:17–28. [DOI] [PubMed] [Google Scholar]
- 32. Kuiper GG, Carlsson B, Grandien K, et al. . Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138:863–870. [DOI] [PubMed] [Google Scholar]
- 33. Tremblay GB, Tremblay A, Copeland NG, et al. . Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor β. Mol Endocrinol. 1997;11:353–365. [DOI] [PubMed] [Google Scholar]
- 34. Katsu Y, Kohno S, Narita H, et al. . Cloning and functional characterization of Chondrichthyes, cloudy catshark, Scyliorhinus torazame and whale shark, Rhincodon typus estrogen receptors. Gen Comp Endocrinol. 2010;168:496–504. [DOI] [PubMed] [Google Scholar]
- 35. Thornton JW. Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc Natl Acad Sci USA. 2001;98:5671–5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ramsey M, Crews D. Steroid signaling and temperature-dependent sex determination-Reviewing the evidence for early action of estrogen during ovarian determination in turtles. Semin Cell Dev Biol. 2009;20:283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Katsu Y, Matsubara K, Kohno S, et al. . Molecular cloning, characterization, and chromosome mapping of reptilian estrogen receptors. Endocrinology. 2010;151:5710–5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Katsu Y, Ichikawa R, Ikeuchi T, Kohno S, Guillette LJ Jr, Iguchi T. Molecular cloning and characterization of estrogen, androgen, and progesterone nuclear receptors from a freshwater turtle (Pseudemys nelsoni). Endocrinology. 2008;149:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Milnes MR, Guillette LJ Jr. Alligator tales: new lessons about environmental contaminants from a sentinel species. Bioscience. 2008;58:1027–1036. [Google Scholar]
- 40. Ferguson MWJ. Reproductive biology and embryology of the crocodilians. In: Gans C, Billett FS, Maderson PFA, eds. Biology of the Reptilia: Development. New York, NY: J. Wiley and Sons; 1985:329–491. [Google Scholar]
- 41. Smith CA, Elf PK, Lang JW, Joss JMP. Aromatase enzyme-activity during gonadal sex-differentiation in alligator embryos. Differentiation. 1995;58:281–290. [Google Scholar]
- 42. Kohno S, Katsu Y, Urushitani H, Ohta Y, Iguchi T, Guillette LJ Jr. Potential contributions of heat shock proteins to temperature-dependent sex determination in the American alligator. Sex Dev. 2010;4:73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kohno S, Kamishima Y, Iguchi T. Molecular cloning of an anuran V(2) type [Arg(8)] vasotocin receptor and mesotocin receptor: functional characterization and tissue expression in the Japanese tree frog (Hyla japonica). Gen Comp Endocrinol. 2003;132:485–498. [DOI] [PubMed] [Google Scholar]
- 44. Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–5250. [DOI] [PubMed] [Google Scholar]
- 45. Pfaffl M. Quantification strategies in real time PCR. In: Bustin SA, ed. A-Z of Quantitative PCR. La Jolla, CA: International University Line; 2004:87–112. [Google Scholar]
- 46. Weiser MJ, Wu TJ, Handa RJ. Estrogen receptor-β agonist diarylpropionitrile: biological activities of R- and S-enantiomers on behavior and hormonal response to stress. Endocrinology. 2009;150:1817–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nakamura T, Katsu Y, Watanabe H, Iguchi T. Estrogen receptor subtypes selectively mediate female mouse reproductive abnormalities induced by neonatal exposure to estrogenic chemicals. Toxicology. 2008;253:117–124. [DOI] [PubMed] [Google Scholar]
- 48. Katsu Y, Bermudez DS, Braun EL, et al. . Molecular cloning of the estrogen and progesterone receptors of the American alligator. Gen Comp Endocrinol. 2004;136:122–133. [DOI] [PubMed] [Google Scholar]
- 49. Kobayashi A, Behringer RR. Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet. 2003;4:969–980. [DOI] [PubMed] [Google Scholar]
- 50. Lambeth LS, Cummins DM, Doran TJ, Sinclair AH, Smith CA. Overexpression of aromatase alone is sufficient for ovarian development in genetically male chicken embryos. PLoS One. 2013;8:e68362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Akazome Y, Mori T. Evidence of sex reversal in the gonads of chicken embryos after oestrogen treatment as detected by expression of lutropin receptor. J Reprod Fertil. 1999;115:9–14. [DOI] [PubMed] [Google Scholar]
- 52. Hutson JM, Ikawa H, Donahoe PK. Estrogen inhibition of Mullerian inhibiting substance in the chick embryo. J Pediatr Surg. 1982;17:953–959. [DOI] [PubMed] [Google Scholar]
- 53. Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. [DOI] [PubMed] [Google Scholar]
- 54. Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. [DOI] [PubMed] [Google Scholar]
- 55. Filice E, Recchia AG, Pellegrino D, Angelone T, Maggiolini M, Cerra MC. A new membrane G protein-coupled receptor (GPR30) is involved in the cardiac effects of 17β-estradiol in the male rat. J Physiol Pharmacol. 2009;60:3–10. [PubMed] [Google Scholar]
- 56. Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J Neurosci. 2010;30:12950–12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chimento A, Sirianni R, Delalande C, et al. . 17β-estradiol activates rapid signaling pathways involved in rat pachytene spermatocytes apoptosis through GPR30 and ER α. Mol Cell Endocrinol. 2010;320:136–144. [DOI] [PubMed] [Google Scholar]
- 58. Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol. 2006;102:175–179. [DOI] [PubMed] [Google Scholar]
- 59. Parrott BB, Kohno S, Cloy-McCoy JA, Guillette LJ Jr. Differential incubation temperatures result in dimorphic DNA methylation patterning of the SOX9 and aromatase promoters in gonads of alligator (Alligator mississippiensis) embryos. Biol Reprod. 2014;90:1–11. [DOI] [PubMed] [Google Scholar]
- 60. Urushitani H, Katsu Y, Miyagawa S, et al. . Molecular cloning of anti-Müllerian hormone from the American alligator, Alligator mississippiensis. Mol Cell Endocrinol. 2011;333:190–199. [DOI] [PubMed] [Google Scholar]
- 61. Ferguson MWJ, Joanen T. Temperature-dependent sex determination in Alligator mississippiensis. J Zool. 1983;200:143–177. [Google Scholar]
- 62. Hanley NA, Hagan DM, Clement-Jones M, et al. . SRY, SOX9, and DAX1 expression patterns during human sex determination and gonadal development. Mech Develop. 2000;91:403–407. [DOI] [PubMed] [Google Scholar]
- 63. Jordan BK, Mohammed M, Ching ST, et al. . Up-regulation of WNT-4 signaling and dosage-sensitive sex reversal in humans. Am J Hum Genet. 2001;68:1102–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Swain A, Zanaria E, Hacker A, Lovell-Badge R, Camerino G. Mouse Dax1 expression is consistent with a role in sex determination as well as in adrenal and hypothalamus function. Nat Genet. 1996;12:404–409. [DOI] [PubMed] [Google Scholar]
- 65. Yao HH, Matzuk MM, Jorgez CJ, et al. . Follistatin operates downstream of Wnt4 in mammalian ovary organogenesis. Dev Dyn. 2004;230:210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schmidt D, Ovitt CE, Anlag K, et al. . The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131:933–942. [DOI] [PubMed] [Google Scholar]
- 67. Uhlenhaut NH, Jakob S, Anlag K, et al. . Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139:1130–1142. [DOI] [PubMed] [Google Scholar]
- 68. Pennetier S, Uzbekova S, Perreau C, Papillier P, Mermillod P, Dalbiès-Tran R. Spatio-temporal expression of the germ cell marker genes MATER, ZAR1, GDF9, BMP15, and VASA in adult bovine tissues, oocytes, and preimplantation embryos. Biol Reprod. 2004;71:1359–1366. [DOI] [PubMed] [Google Scholar]
- 69. Zheng P, Dean J. Oocyte-specific genes affect folliculogenesis, fertilization, and early development. Semin Reprod Med. 2007;25:243–251. [DOI] [PubMed] [Google Scholar]
- 70. Moore BC, Hamlin HJ, Botteri NL, Guillette LJ Jr. Gonadal mRNA expression levels of TGFβ superfamily signaling factors correspond with post-hatching morphological development in American alligators. Sex Dev. 2010;4:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Moore BC, Milnes MR, Kohno S, et al. . Altered gonadal expression of TGF-β superfamily signaling factors in environmental contaminant-exposed juvenile alligators. J Steroid Biochem Mol Biol. 2011;127:58–63. [DOI] [PubMed] [Google Scholar]
- 72. Matsumoto Y, Buemio A, Chu R, Vafaee M, Crews D. Epigenetic control of gonadal aromatase (cyp19a1) in temperature-dependent sex determination of red-eared slider turtles. PLoS One. 2013;8:e63599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kohno S, Bermudez DS, Katsu Y, Iguchi T, Guillette LJ. Gene expression patterns in juvenile American alligators (Alligator mississippiensis) exposed to environmental contaminants. Aquat Toxicol. 2008;88:95–101. [DOI] [PubMed] [Google Scholar]
- 74. Milnes MR, Roberts R, Guillette LJ Jr. Effects of incubation temperature and estrogen exposure on aromatase activity in the brain and gonads of embryonic alligators. Environ Health Persp. 2002;110(suppl 3):393–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Austin HB. The effects of norethindrone on the mullerian ducts of the American alligator. Gen Comp Endocrinol. 1991;84:300–307. [DOI] [PubMed] [Google Scholar]
- 76. Biau S, Bayle S, de Santa Barbara P, Roig B. The chick embryo: an animal model for detection of the effects of hormonal compounds. Anal Bioanal Chem. 2007;387:1397–1403. [DOI] [PubMed] [Google Scholar]
- 77. Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. [DOI] [PubMed] [Google Scholar]