Abstract
Although named “ovarian cancer,” it has been unclear whether the cancer actually arises from the ovary, especially for high-grade serous carcinoma (HGSC), also known as high-grade serous ovarian cancer, the most common and deadliest ovarian cancer. In addition, the tumor suppressor p53 is the most frequently mutated gene in HGSC. However, whether mutated p53 can cause HGSC remains unknown. In this study, we bred a p53 mutation, p53R172H, into conditional Dicer-Pten double-knockout (DKO) mice, a mouse model duplicating human HGSC, to generate triple-mutant (TKO) mice. Like DKO mice, these TKO mice develop metastatic HGSCs originating from the fallopian tube. Unlike DKO mice, however, even after fallopian tubes are removed in TKO mice, ovaries alone can develop metastatic HGSCs, indicating that a p53 mutation can drive HGSC arising from the ovary. To confirm this, we generated p53R172H-Pten double-mutant mice, one of the genetic control lines for TKO mice. As anticipated, these double-mutant mice also develop metastatic HGSCs from the ovary, verifying the HGSC-forming ability of ovaries with a p53 mutation. Our study therefore shows that ovaries harboring a p53 mutation, as well as fallopian tubes, can be a distinct tissue source of high-grade serous ovarian cancer in mice.
As implicit in the name, ovarian cancer is supposed to be a cancer originating in the ovary. Yet, the exact tissue origin of ovarian cancer has been an enigma, in particular, for high-grade serous carcinoma (HGSC), also known as high-grade serous ovarian cancer (1, 2). Although HGSC is the most common and deadliest subtype among ovarian cancers, it is rarely detected at stage I, when tumors are confined to the ovary (<1.5% of HGSC cases) (3–5). Instead, most cases of HGSC present at an advanced stage, including cancers of the ovary, with widespread metastases in the peritoneal cavity (4, 6). Then, there are cases of ovarian cancer in which tumors are limited to the ovary without metastasis (1, 7). These stage I ovarian tumors, however, are mostly nonepithelial tumors—such as granulosa-cell and germ-cell tumors—or epithelial ovarian cancers like endometrioid, clear-cell, mucinous, and borderline ovarian tumors (8). Viewing the stage I ovarian tumor and the advanced-stage cancer, although seen in separate cases, as the same cancer in different stages of progression, ovarian cancer, including HGSC, was generally assumed to arise from the ovary (7).
Recently, this notion was challenged when the fallopian tube, instead of the ovary, had emerged as a likely source of ovarian cancer, especially in women carrying hereditary breast cancer susceptibility gene (BRCA) mutations (9–11). Prone to ovarian cancer later in their life, women with positive BRCA mutations are advised to undergo risk-reducing bilateral salpingo-oophorectomy, a prophylactic surgery removing the ovaries and fallopian tubes. Unexpectedly in these women, a microscopic precursor lesion called serous tubal intraepithelial carcinoma (STIC) was found in the fallopian tube—but not in the ovary—leading to the “fallopian tube hypothesis” of ovarian cancer.
Substantial evidence supporting this fallopian tube hypothesis has since accumulated. Overall, BRCA-mutation-positive cases make up only 10%–15% of all ovarian cancers (2, 11). Yet, these STIC lesions are also found in fallopian tubes of ovarian cancer patients not carrying BRCA mutations (12, 13). In any of these studies, however, one cannot know whether STIC lesions would have progressed to ovarian cancer. Then, a recent study of mouse models has provided such evidence (14). In this study, development of STIC lesions—by disabling or altering Brca1/2, transformation-related protein 53 (Trp53; p53), and phosphatase and tensin homolog (Pten)—in the fallopian tube leads to ovarian and peritoneal metastases of HGSC (14). In addition, another study shows that mice with conditional deletion of Pten and Dicer develop fallopian tube-originated HGSCs, which duplicate the clinical nature of human HGSC (15). Moreover, when symptomatic women are promptly diagnosed, 7 (78%) of 9 HGSC cases have fallopian tubes as their tumor origin, reinforcing the notion that the fallopian tube is indeed a primary starting site of HGSC (16). In the remaining 2 HGSC cases (22%), however, the cancers are traced to the ovary as their origin, suggesting that HGSC can also arise from the ovary in women.
Besides the tissue or cell of origin, another perplexing issue in ovarian cancer is the role of p53 mutations. The cancer genome landscape of HGSC is unambiguous: tumor protein 53 (TP53), the human p53 gene, is mutated in 96%–97% of HGSC cases, inevitably suggesting a causal relationship (5, 17). Yet, it is not clear whether mutated p53 is a driver mutation causing HGSC. Other than p53 being the most frequently mutated gene in human HGSC, there exists little direct evidence that p53 mutations can actually cause HGSC. No human studies or mouse models have convincingly demonstrated the causality between p53 mutations and HGSC (18–23).
Here, our study shows the critical role of a p53 mutant in the formation of HGSC in the ovary. Using mouse models, we provide evidence that ovaries harboring a p53 mutation can develop metastatic HGSC, which resembles human HGSC. Thus, our study also reveals a causal link between a p53 mutation and high-grade serous ovarian cancer.
Materials and Methods
Generation of p53 triple-mutant (TKO) and double-mutant (DMu) mice
Expressed in Müllerian mesenchyme-derived tissues, including the fallopian tube, Amhr2cre/+ induces HGSC in double-knockout (DKO) mice (Dicerflox/flox Ptenflox/flox Amhr2cre/+). Amhr2cre/+ was thus used to generate TKO and DMu mice. Mice carrying p53LSL-R172H/+, a conditional p53 activating mutation, were mated with DKO mice to generate TKO mice (p53LSL-R172H/+ Dicerflox/flox Ptenflox/flox Amhr2cre/+). These TKO mice were further bred with mice carrying only Ptenflox/flox to remove Dicerflox/flox and generate DMu mice (p53LSL-R172H/+ Ptenflox/flox Amhr2cre/+). Similarly, mating TKO mice with Dicerflox/flox mice generated mice carrying double mutations of p53R172H and Dicer (p53LSL-R172H/+ Dicerflox/flox Amhr2cre/+).
Surgical excision of fallopian tubes
After TKO mice were anesthetized with Avertin, both fallopian tubes were surgically removed under dissection microscope.
Histological examinations of tumor
Tumors from TKO mice, fallopian tube-deficient TKO mice, and DMu mice were paraffin embedded, sectioned, and examined by a pathologist (Dr Donna Coffey) specialized in gynecologic oncology.
Immunohistochemistry
Immunohistochemistry was performed as previously described (15). The next primary antibodies were used: keratin 8 (KRT8; TROMA-I [supernatant], rat, 1:20; University of Iowa Hybridoma Bank), KRT14 (rabbit, 1:1000; Covance), cancer antigen 125 (CA125; rabbit, 1:100; gift from Dr Robert Bast Jr), E-cadherin (CDH1; rabbit, 1:100; Cell Signaling Technology), and p53 (rabbit, 1:100; Santa Cruz Biotechnology, Inc) (for antibodies, please see Supplemental Table 1).
Results and Discussion
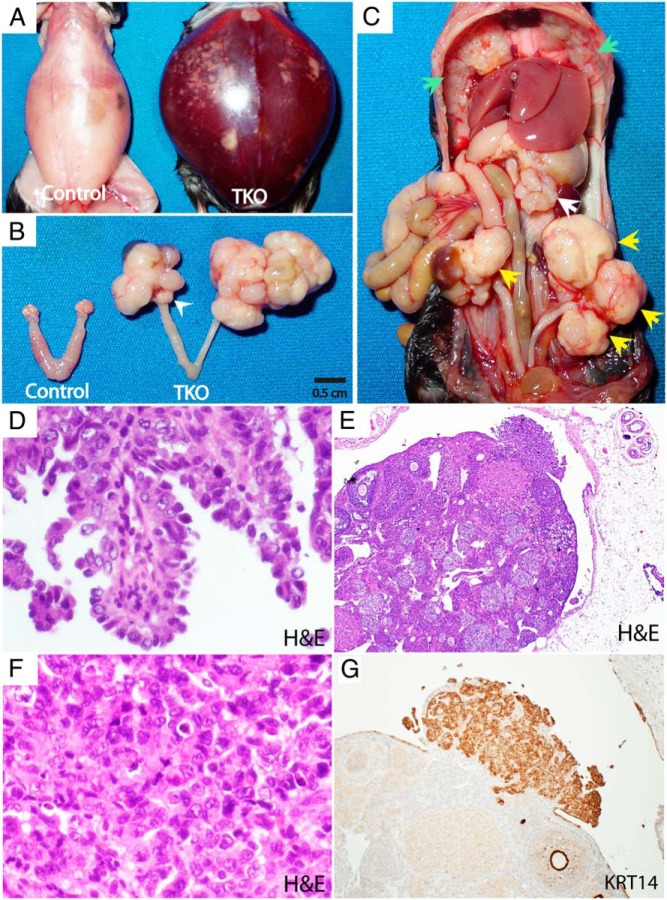
To address the role of a p53 mutation in HGSC, we bred a p53 activating mutant, p53R172H, into conditional Dicer-Pten DKO mice, generating TKO mice (p53LSL-R172H/+ Dicerflox/flox Ptenflox/flox Amhr2cre/+). Like DKO mice, these TKO mice develop massive HGSCs throughout the peritoneal cavity, including primary tumors and metastases in the omentum and diaphragm, which induce ascites and eventually kill these mice (Figure 1, A–D). Also, as in DKO mice, the fallopian tube is the origin of HGSC in TKO mice. After removal of both ovaries in TKO mice (n = 5), these ovary-deficient TKO mice can still develop HGSC from the fallopian tube, as in ovary-deficient DKO mice (15). However, unlike DKO mice, in which tumors arise solely from the fallopian tube (15), TKO mice also have tumors on the surface of the ovary (Figure 1, E–G). Grossly, the ovary appears unaffected in TKO mice (Figure 1B). Microscopically, however, the TKO ovary has an outgrowth of tumors confirmed as HGSC (Figure 1, E–G). Yet, these tumors may have sprouted de novo from the ovary, or could be implants from rampantly growing and spreading neighboring fallopian tube HGSCs.
Figure 1. TKO mice (p53LSL-R172H/+ Dicerflox/flox Ptenflox/flox Amhr2cre/+) develop metastatic HGSCs with primary fallopian tube tumors.
A–C, Gross phenotype. A 6.6-month-old TKO mouse showing massive ascites (A) with bilateral fallopian tube tumors (B; C, yellow arrows) with a grossly normal-looking ovary (a white arrowhead), accompanied by widespread peritoneal metastases, including omentum (a white arrow) and diaphragm (green arrows) tumors (C). D, Papillary architecture with HGSC histology in peritoneal metastasis (H&E, ×40). E–G, HGSC in the ovary. The TKO ovary has an outgrowth of tumors (E) composed of cells displaying nuclear pleiomorphism, irregular chromatin distribution, macronucleoli, increased mitotic activity, and widespread apoptosis—characteristic of HGSC (F) (H&E, ×40). These HGSC cells are abundantly positive for KRT14 (G) (×10). Although the TKO ovary is capable of forming HGSC, the ovarian HGSCs shown in this figure are likely the growth of an implant spread from the neighboring fallopian tube HGSCs.
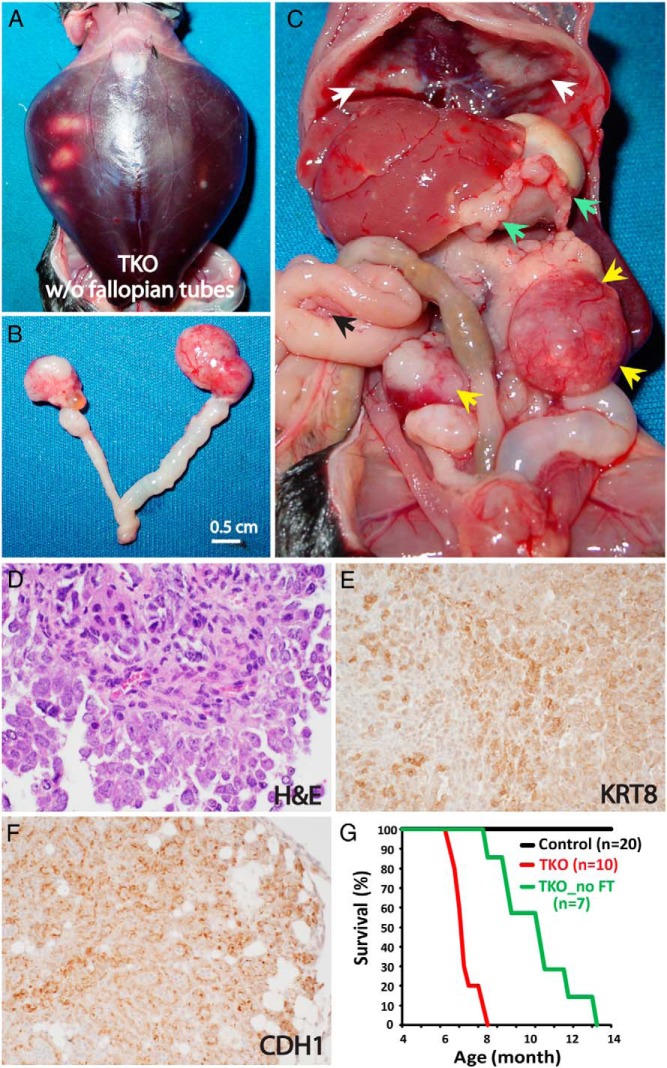
To further investigate whether HGSC can arise de novo from the ovary, both fallopian tubes were surgically removed from TKO mice 2 months after birth, when there was no sign of tumor in the fallopian tube or ovary (Supplemental Figure 1). Surprisingly, these TKO mice lacking fallopian tubes develop HGSCs in the ovary, which metastasize to the peritoneal cavity, including the omentum, diaphragm, and mesentery (35%, 7/20 mice) (Figure 2). Despite the widespread metastases in the peritoneal cavity, however, the overall metastatic tumor burden in fallopian tube-removed TKO mice is less extensive than that of intact TKO mice. Yet, the tumor burden still kills these fallopian tube-deficient TKO mice at 8–14 months of age after inducing ascites in most cases (Figure 2). In stark contrast, when both fallopian tubes are removed from DKO mice, these mice fail to develop any tumors, showing that the fallopian tube is the sole origin of HGSC in the DKO model (15). Thus, the ovarian HGSCs in fallopian tube-deficient TKO mice propose an intriguing possibility: the ovary, as well as the fallopian tube, can be a source of HGSC.
Figure 2. Fallopian tube-deficient TKO mice develop metastatic HGSCs arising from the ovary.
A-C. Gross phenotype. A 13-month-old TKO mouse, with the fallopian tubes surgically excised at 2 months of age, develops hemorrhagic ascites (A) with bilateral ovarian tumors (B; C, yellow arrows) and widespread metastases across the peritoneal cavity, including the omentum (green arrows), diaphragm (white arrows), and mesentery (a black arrow) (C). D–F, HGSC histology. The ovarian tumors (D) exhibit the cellular morphology characteristic of HGSC, as described in Figure 1. In addition, the ovarian (E) and omentum (F) tumors express epithelial markers, E-cadherin (CDH1) and cytokeratin 8 (KRT8), respectively. G, Survival curves. Fallopian tube-deficient TKO mice die significantly later than intact TKO mice (median survival, 11.2 [8.5–14.3] vs 7.3 [6.4–8.4] mo; P < .0001, log-rank test). * TKO_no FT, TKO mice without fallopian tubes.
Our study indicates that TKO mice could have 2 distinct tissue sources for HGSC: the fallopian tube and the ovary. To confirm the ovarian origin of HGSC in TKO mice, we examined genetic mouse control lines of these TKO mice (Table 1). One control line is aforementioned DKO mice (Dicerflox/flox Ptenflox/flox Amhr2cre/+), which develop HGSCs attributable only to the fallopian tube with no involvement of the ovary (15). No single-gene knockout mice—p53LSL-R172H/+ Amhr2cre/+, Dicerflox/flox Amhr2cre/+, and Ptenflox/flox Amhr2cre/+—developed HGSC (Table 1). Neither did mice carrying double mutations of p53R172H and Dicer (p53LSL-R172H/+ Dicerflox/flox Amhr2cre/+). We therefore postulated that the remaining genetic combination, p53R172H and Pten, could drive HGSC of ovarian origin. To test this possibility, p53R172H-Pten DMu mice (p53LSL-R172H/+ Ptenflox/flox Amhr2cre/+) were generated.
Table 1.
HGSC Formation in Genetic Mouse Control Lines of TKO Mice
| Genotype | Percentage of Mice (Number of Mice) |
Age (mo) | |
|---|---|---|---|
| Ovarian HGSC | Fallopian Tube HGSC | ||
| (p53LSL-R172H/+ Dicerflox/flox Ptenflox/flox Amhr2cre/+) | |||
| p53LSL-R172H/+ Amhr2cre/+ | 0% (0/15) | 0% (0/15) | 12–16 |
| Dicerflox/flox Amhr2cre/+ | 0% (0/10) | 0% (0/10) | 12–33 |
| Ptenflox/flox Amhr2cre/+ | 0% (0/20)a | 0% (0/20) | 7–22 |
| Dicerflox/flox Ptenflox/flox Amhr2cre/+ | 0% (0/24)b | 100% (24/24)b | 6.5–13 |
| p53 LSL-R172H/+ Dicerflox/flox Amhr2cre/+ | 0% (0/19) | 0% (0/19) | 12–18 |
| p53 LSL-R172H/+ Ptenflox/flox Amhr2cre/+ | 30% (15/50)c | 0% (0/50) | 7.6–15.3 |
Ptenflox/flox Amhr2cre/+ mice develop granulosa-cell tumors in the ovary (65%, 13/20 mice), as reported previously (29).
See Kim et al (15), in which ovary-deficient mice develop HGSC from the fallopian tube, whereas fallopian tube-deficient mice with ovaries develop no tumors.
While 30% of DMu mice develop metastatic ovarian HGSCs with normal fallopian tubes, the rest of DMu mice (35/50), though not HGSC, develop granulosa-cell tumors in the ovary, leading the mortality.
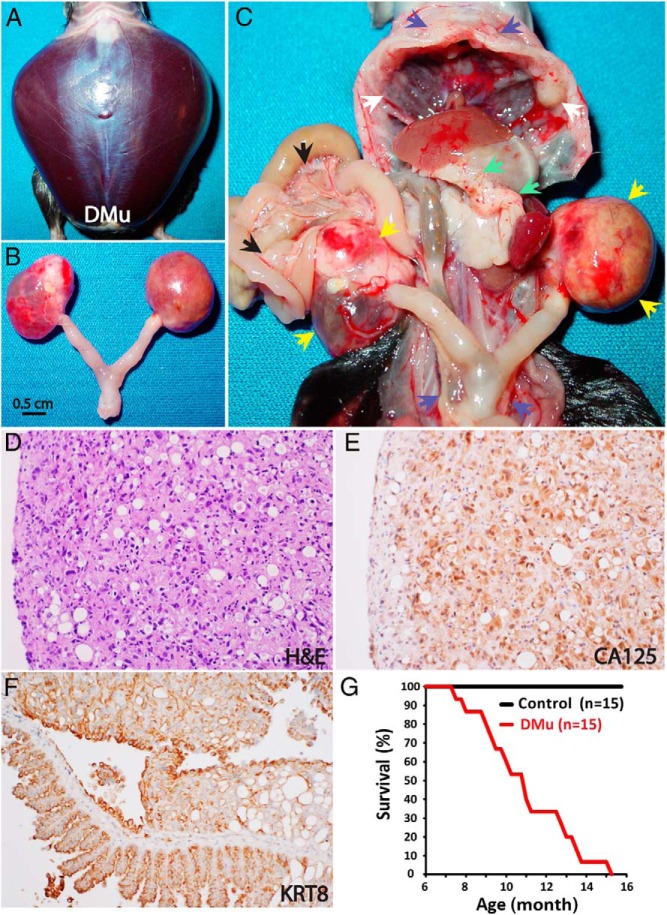
As anticipated, 30% of DMu mice (15/50 mice) develop HGSCs in the ovary, which spread throughout the peritoneal cavity—notably to the omentum and across the peritoneal membrane, including the mesentery and diaphragm—accompanied by ascites, leading to death (Figure 3). These DMu mice, however, show no evidence of tumors in the fallopian tube (Supplemental Figure 2). In addition, as in fallopian tube-deficient TKO mice, although peritoneal metastases are widespread, the ovarian HGSCs in DMu mice accompany less metastatic tumor burden than HGSCs originating from the fallopian tube in TKO and DKO mice. Accordingly, the tumor progression of ovarian HGSC is similar between DMu mice and fallopian tube-deficient TKO mice. The median survival of DMu mice (n = 15) is 11.2 (7.6–15.3) months of age (Figure 3), which is identical to that of fallopian tube-deficient TKO mice (n = 7): 11.2 (8.5–14.3) months (Figure 2). However, the most striking difference between ovarian and fallopian tube-originated HGSCs is the incidence of HGSC. Although ovarian HGSC develops in 30%–35% of DMu mice and fallopian tube-deficient TKO mice, HGSC of fallopian tube origin occurs in 100% of DKO and TKO mice. Collectively, these results suggest that the ovary possess less tumorigenic potential than does the fallopian tube. Nonetheless, DMu mice show that the mutation of 2 genes, p53 and Pten, can drive metastatic HGSCs in the ovary, echoing the phenotype of ovarian HGSCs in fallopian tube-deficient TKO mice. Indeed, high-grade serous ovarian cancer, or HGSC, can arise de novo from the ovary harboring a p53 mutation (Supplemental Figure 3).
Figure 3. p53R172H-Pten DMu mice develop ovary-originated metastatic HGSCs.
Ascites (A)-inducing tumors in the ovary (B; C, yellow arrows) in a 15-month-old DMu mouse with widespread metastases in the abdominal cavity, including the omentum (green arrows), diaphragm (white arrows), mesentery (black arrows), and peritoneal membrane (blue arrows). These tumors are histologically HGSCs (D), which are positive for CA125 (E), a biomarker for ovarian cancer, as well as KRT8 (F), an epithelial marker. G, Survival curves of DMu mice (p53LSL-R172H/+ Ptenflox/flox Amhr2cre/+) with metastatic HGSCs and control mice (p53LSL-R172H/+ Ptenflox/flox). Median survival for DMu mice, 11.2 months (range, 7.6–15.3) (n = 15).
Although the ovary can develop metastatic HGSC, our study also shows that the fallopian tube is still the dominant tissue site for HGSC development. In intact TKO mice, HGSC always arises from the fallopian tube, completely eclipsing HGSC-forming potential of the ovary (Figure 1). Only after fallopian tubes are removed, ovaries then could unleash their tumorigenic potential to form HGSC in TKO mice (Figure 2). Nevertheless, our study shows that the ovary, albeit less dominant, can be an independent tissue source of HGSC.
What remains unresolved in these mouse models of ovarian HGSCs is the cell of origin. It is conceivable that the cancer cells of these ovarian HGSCs may have originated from the fallopian tube. For instance, it was suggested that during ovulation, epithelial cells from the fallopian tube fimbria may shed and implant on the ovary, serving as seeds for HGSC (24). Also, it was shown in mice that transformed epithelial cells in the fallopian tube could spread to form HGSC in the ovary (14). In our study, Amhr2-Cre (Amhr2cre/+) would be active in the fallopian tube at the time of, and before, tube removal in TKO mice, thereby having already inactivated Dicer and Pten and activated a mutant p53 in the fallopian tube. Yet, there was no sign of transformation in the removed fallopian tubes from TKO mice (Supplemental Figure 1). In addition, in the previous study of DKO mice, also harboring Amhr2-Cre, when fallopian tubes are removed at 6–11 weeks of age, these fallopian tube-deficient DKO mice develop no tumors even at 14–16 months of age (15). It is thus unlikely that tubal cells would have been transformed at the time of tube removal, let alone transformed cells would migrate to the ovary. Similarly, DMu mice, although developing metastatic ovarian HGSC, display no abnormalities in the fallopian tube (Supplemental Figure 2), also dimming the likelihood of fallopian tube origin for the ovarian HGSCs. Taken together, the initiating cancer cells for ovarian HGSCs are unlikely to have originated from the fallopian tube. Rather, they are likely to be intrinsic to the ovary.
Another unresolved issue related to the cell of origin is the cell-type specificity of the Amhr2 gene, which directs the expression of Amhr2-Cre (Amhr2cre/+) in our mouse models. Amhr2 is expressed in the reproductive tract derived from the Müllerian duct mesenchyme: the stroma of the fallopian tube, uterus, cervix, and vagina (25–27). It is also expressed in the ovarian stroma, including granulosa cells of ovarian follicles (28, 29). Recently, a lineage-tracing study of Amhr2 in the mouse uterus suggests cell plasticity of Amhr2-expressing cells. In this study, the fate of Amhr2-expressing cells are tracked during endometrial regeneration, which occurs in the mouse uterus after parturition (30). As expected, most Amhr2-positive cells become stromal cells in the uterus. Intriguingly, then, a subset of Amhr2-positive cells differentiates into endometrial epithelium, becoming epithelial cells instead of stromal cells. These results suggest that some Amhr2-positive cells could change their cell fate under a certain condition. In this study, it is parturition, but this may well be the case when certain genes are inactivated or mutated in Amhr2-expressing tissues, such as the fallopian tube and ovary.
Over the last decade, the fallopian tube has been gaining acceptance as the primary tissue origin of HGSC in women (9, 11, 24). However, causal evidence is still lacking. A recent study of mouse models involving Trp53 (p53), Brca1/2, and Pten shows that STICs, preinvasive lesions in the fallopian epithelium, can advance to form HGSCs in the ovary and peritoneal membrane (14). This STIC transformation to HGSC, however, occurs only in the presence of Brca mutations. In the same study, p53 mutation and Pten inactivation, without Brca1 or 2 inactivation, still lead to the formation of STIC lesions in the fallopian tube epithelium. Yet, these STICs remain noninvasive in the fallopian tube and do not progress into HGSCs or metastasize (14). Thus, current evidence supports that STIC is likely a precursor lesion for HGSC in high-risk women carrying BRCA mutations, which constitute 10%–15% of overall ovarian cancer cases (2, 11). It remains to be known, however, whether STIC is indeed a precursor lesion for HGSCs in the general population, which account for most of ovarian cancer cases.
Meanwhile, our finding of the ovary as a source of HGSC may offer a clinical implication in the prevention of ovarian cancer in the general population. A recent epidemiologic study has indicated that even after menopause, keeping the ovary appears to offer survival benefit to women. The overall mortality is lower for women with ovaries than those without ovaries (31). This survival benefit of keeping ovaries, together with supposed fallopian tube origin of HGSC, would favor salpingectomy, removal of fallopian tubes alone, in reducing the risk of ovarian cancer (32, 33). On the other hand, our current mouse study and a recent clinical study have shown that the ovary too can be a potential site of HGSC (16). Thus, these studies still support the removal of ovaries and fallopian tubes together—bilateral salpingo-oophorectomy—to protect women from ovarian cancer sprouting from either source.
The present study also reveals the causal role of a p53 mutation in ovarian HGSC. Unlike DKO ovaries, which fail to develop any tumors (15), TKO ovaries with p53R172H mutation can develop metastatic HGSCs (Figure 2). Similarly, DMu mice carrying p53R172H mutation and Pten deletion can also produce metastatic ovarian HGSCs without affecting the fallopian tube (Figure 3 and Supplemental Figure 2). This ovarian HGSC formation, unique to mice with a p53 mutation, suggests a tumorigenic role of a p53 mutation in the ovary. Although the mechanisms behind a p53 mutation's role in ovarian HGSC require further studies, these findings offer evidence that a p53 mutation is a driver inducing HGSC in the ovary.
Despite the recent advances in elucidating the origin of ovarian cancer, the challenges facing us still loom large. The recent recommendation by a United States government panel against the screening of ovarian cancer, because screening has not led to any reduction in mortality, is a sobering reminder that there is a gaping hole in our understanding of the mechanisms of ovarian cancer (34). Here, our present study of mouse models adds a new dimension to our evolving understanding of the origin of ovarian cancer: high-grade serous ovarian cancer can arise from ovaries harboring a p53 mutation.
Acknowledgments
We thank the National Cancer Institute Mouse Repository for p53R172H/+ mutant mice. We also thank the next investigators for making individual mutant mouse line readily available for our study: Dr Tyler Jacks at the Massachusetts Institute of Technology for p53R172H/+ mutant mice, Dr Hong Wu at the University of California, Los Angeles, for Ptenflox/flox mice, Dr Alexander Tarakhovsky at The Rockefeller University for Dicerflox/flox mice, and Dr Richard Behringer at The University of Texas MD Anderson Cancer Center for Amhr2cre/+ mice. In addition, we thank Dr Robert Bast Jr at the MD Anderson Cancer Center for the CA125 antibody. We also thank the next investigators for the critical reading of our manuscript and their comments: Dr Jeff Rosen, Dr Li Xin, Dr Laising Yen, Dr Choel Kim, Dr Ravi Singh, Dr Oh-Joon Kwon, Dr Wan-Hee Yoon, and Dr Beom-Jun Kim at Baylor College of Medicine; Dr Jaehyuk Lee at the MD Anderson Cancer Center; and Dr Facundo Fernandez at the Georgia Institute of Technology.
This work was supported by the National Cancer Institute, the Ovarian Cancer Research Fund, a Marsha Rivkin Ovarian Cancer Challenge grant, and the Dan L. Duncan Cancer Center at Baylor College of Medicine. J.K. is supported by the Ruth L. Kirschstein National Research Service Award (F32CA159523) and the Pathway to Independence Award (K99CA179137), both from the National Cancer Institute.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the National Cancer Institute, the Ovarian Cancer Research Fund, a Marsha Rivkin Ovarian Cancer Challenge grant, and the Dan L. Duncan Cancer Center at Baylor College of Medicine. J.K. is supported by the Ruth L. Kirschstein National Research Service Award (F32CA159523) and the Pathway to Independence Award (K99CA179137), both from the National Cancer Institute.
Footnotes
- BRCA
- breast cancer susceptibility gene
- CA125
- cancer antigen 125
- CDH1
- E-cadherin
- DKO
- double-knockout
- DMu
- double-mutant
- HGSC
- high-grade serous carcinoma
- KRT8
- keratin 8
- KRT14
- keratin 14
- Pten
- phosphatase and tensin homolog
- STIC
- serous tubal intraepithelial carcinoma
- TKO
- triple-mutant.
References
- 1. Cho KR, Shih IeM. Ovarian cancer. Annu Rev Pathol. 2009;4:287–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bast RC Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9:415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seidman JD, Horkayne-Szakaly I, Haiba M, Boice CR, Kurman RJ, Ronnett BM. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol. 2004;23:41–44. [DOI] [PubMed] [Google Scholar]
- 4. Seidman JD, Zhao P, Yemelyanova A. “Primary peritoneal” high-grade serous carcinoma is very likely metastatic from serous tubal intraepithelial carcinoma: assessing the new paradigm of ovarian and pelvic serous carcinogenesis and its implications for screening for ovarian cancer. Gynecol Oncol. 2011;120:470–473. [DOI] [PubMed] [Google Scholar]
- 5. Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yemelyanova AV, Cosin JA, Bidus MA, Boice CR, Seidman JD. Pathology of stage I versus stage III ovarian carcinoma with implications for pathogenesis and screening. Int J Gynecol Cancer. 2008;18:465–469. [DOI] [PubMed] [Google Scholar]
- 8. Hogg R, Friedlander M. Biology of epithelial ovarian cancer: implications for screening women at high genetic risk. J Clin Oncol. 2004;22:1315–1327. [DOI] [PubMed] [Google Scholar]
- 9. Nik NN, Vang R, Shih IeM, Kurman RJ. Origin and pathogenesis of pelvic (ovarian, tubal, and primary peritoneal) serous carcinoma. Annu Rev Pathol. 2014;9:27–45. [DOI] [PubMed] [Google Scholar]
- 10. Piek JM, van Diest PJ, Zweemer RP, et al. . Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–456. [DOI] [PubMed] [Google Scholar]
- 11. Crum CP, Drapkin R, Kindelberger D, Medeiros F, Miron A, Lee Y. Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res. 2007;5:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kindelberger DW, Lee Y, Miron A, et al. . Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–169. [DOI] [PubMed] [Google Scholar]
- 13. Przybycin CG, Kurman RJ, Ronnett BM, Shih IeM, Vang R. Are all pelvic (nonuterine) serous carcinomas of tubal origin? Am J Surg Pathol. 2010;34:1407–1416. [DOI] [PubMed] [Google Scholar]
- 14. Perets R, Wyant GA, Muto KW, et al. . Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell. 2013;24:751–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim J, Coffey DM, Creighton CJ, Yu Z, Hawkins SM, Matzuk MM. High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc Natl Acad Sci USA. 2012;109:3921–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gilbert L, Basso O, Sampalis J, et al. . Assessment of symptomatic women for early diagnosis of ovarian cancer: results from the prospective DOvE pilot project. Lancet Oncol. 2012;13:285–291. [DOI] [PubMed] [Google Scholar]
- 17. Ahmed AA, Etemadmoghadam D, Temple J, et al. . Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J Pathol. 2010;221:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Donehower LA. The p53-deficient mouse: a model for basic and applied cancer studies. Semin Cancer Biol. 1996;7:269–278. [DOI] [PubMed] [Google Scholar]
- 19. Olive KP, Tuveson DA, Ruhe ZC, et al. . Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell. 2004;119:847–860. [DOI] [PubMed] [Google Scholar]
- 20. Clark-Knowles KV, Senterman MK, Collins O, Vanderhyden BC. Conditional inactivation of Brca1, p53 and Rb in mouse ovaries results in the development of leiomyosarcomas. PLoS One. 2009;4:e8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quinn BA, Brake T, Hua X, et al. . Induction of ovarian leiomyosarcomas in mice by conditional inactivation of Brca1 and p53. PLoS One. 2009;4:e8404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Birch JM, Alston RD, McNally RJ, et al. . Relative frequency and morphology of cancers in carriers of germline TP53 mutations. Oncogene. 2001;20:4621–4628. [DOI] [PubMed] [Google Scholar]
- 23. Varley JM. Germline TP53 mutations and Li-Fraumeni syndrome. Hum Mutat. 2003;21:313–320. [DOI] [PubMed] [Google Scholar]
- 24. Kurman RJ, Shih IeM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arango NA, Kobayashi A, Wang Y, et al. . A mesenchymal perspective of Müllerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev. 2008;75:1154–1162. [DOI] [PubMed] [Google Scholar]
- 26. Tanwar PS, Zhang L, Roberts DJ, Teixeira JM. Stromal deletion of the APC tumor suppressor in mice triggers development of endometrial cancer. Cancer Res. 2011;71:1584–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanwar PS, Kaneko-Tarui T, Zhang L, Tanaka Y, Crum CP, Teixeira JM. Stromal liver kinase B1 [STK11] signaling loss induces oviductal adenomas and endometrial cancer by activating mammalian target of rapamycin complex 1. PLoS Genet. 2012;8:e1002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baarends WM, Uilenbroek JT, Kramer P, et al. . Anti-müllerian hormone and anti-müllerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology. 1995;136:4951–4962. [DOI] [PubMed] [Google Scholar]
- 29. Laguë MN, Paquet M, Fan HY, et al. . Synergistic effects of Pten loss and WNT/CTNNB1 signaling pathway activation in ovarian granulosa cell tumor development and progression. Carcinogenesis. 2008;29:2062–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang CC, Orvis GD, Wang Y, Behringer RR. Stromal-to-epithelial transition during postpartum endometrial regeneration. PLoS One. 2012;7:e44285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parker WH, Broder MS, Chang E, et al. . Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses' health study. Obstet Gynecol. 2009;113:1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dietl J, Wischhusen J, Häusler SF. The post-reproductive Fallopian tube: better removed? Hum Reprod. 2011;26:2918–2924. [DOI] [PubMed] [Google Scholar]
- 33. Tone AA, Salvador S, Finlayson SJ, et al. . The role of the fallopian tube in ovarian cancer. Clin Adv Hematol Oncol. 2012;10:296–306. [PubMed] [Google Scholar]
- 34. Moyer VA. Screening for ovarian cancer: U.S. preventive services task force reaffirmation recommendation statement. Ann Intern Med. 2012;7326:0003–4819. [DOI] [PubMed] [Google Scholar]