Abstract
There is a clinical need for plasma tests to detect and quantify the in vivo destruction of pancreatic β-cells in type 1 diabetes. We previously developed a time-resolved fluorescence immunoassay (TRFIA) to glutamate decarboxylase 65 kDa (GAD65) (GAD65-TRFIA) that was able to detect the synchronous necrotic destruction of transplanted β-cells in the hours after their infusion in the liver. This GAD65-TRFIA, however, lacked sensitivity to detect continued β-cell rejection beyond this acute phase. The aim of present study was to gain at least an order of magnitude in analytical sensitivity by switching to Becton Dickinson cytometric bead array (CBA) (GAD65-CBA) enhanced sensitivity format, using the same couple of monoclonal antibodies. We compared the performances of GAD65-CBA and GAD65-TRFIA using Clinical and Laboratory Standards Institute protocols for linearity, imprecision, specificity, limit of detection, and functional sensitivity. We conducted a method comparison and assessed the biologic potential on samples from human recipients of islet grafts. The GAD65-CBA showed acceptable linearity and imprecision. Switching from TRFIA to CBA lowered functional sensitivity by a factor 35 and lowered limit of detection by a factor 11 with minimal need for method optimization. The enhanced sensitivity greatly expands the application domain of our biomarker and allowed for the first time to detect ongoing β-cell destruction up to at least 1 day after islet transplantation. We conclude that the GAD65-CBA is suitable for biological and clinical assessment of the real-time destruction of β-cells in intraportal transplantation.
Type 1 diabetes mellitus (T1D) arises after the insidious destruction of most insulin-producing β-cells. Though this preclinical phase can be recognized by circulating autoantibodies directed against β-cell-selective epitopes, the triggers, kinetics, duration, and interpatient variability of the underlying T cell-mediated β-cell destruction are enigmatic. This stimulated the search for novel diagnostic solutions for real-time monitoring of β-cell destruction in vivo. This is typically done by the detection of β-cell-selective biomarker molecules discharged after plasma membrane disruption during necrosis. In vivo detection of β-cell injury is extremely challenging, given the limited number of β-cells in healthy humans (∼5 × 108 cells), and the observation that during immune-mediated destruction only a fraction of these cells is actively damaged by cytotoxic T cells (1). β-Cell-selective nucleic acid-type biomarkers, such as microRNA-375 (2) or demethylated insulin DNA (3), might become game changers, but their absolute quantification in blood is still hampered by lack of method robustness and transferability and their interpretation complicated by the presence of baseline levels in healthy controls. The biomarker with the longest track record is glutamate decarboxylase 65 kDa (GAD65), an enzyme selectively expressed in pancreatic islets, neural and reproductive tissues (4, 5), and a classical autoantigen in T1D. GAD65 was found to be released from rat and human β-cells after toxin-induced plasma membrane disruption in vitro (6, 7), in vivo streptozotocin injections in rats (7, 8) and after xeno- or allo-transplantation of human islets (7). This surge was graded according to streptozotocin dose (8) or to the number of infused β-cells (7). Using an in-house time-resolved fluorescence immunoassay (TRFIA), we found that disproportionally elevated plasma GAD65 concentrations (>15.4pM) 60 minutes after implantation of standardized β-cell allografts predicted poor outcome at month 2 in human T1D islet graft recipients (7). However, this GAD65-TRFIA was too insensitive to detect GAD65 surges in all graft recipients and episodes of subtle β-cell destruction as might occur during chronic graft rejection or earlier stages of the natural history of T1D (7, 9). We therefore investigated whether conversion of our GAD65-TRFIA format to the enhanced sensitivity cytometric bead array (CBA) format from Becton Dickinson (10), with use of the same monoclonal antibody pair, could improve assay sensitivity.
Materials and Methods
Monoclonal antibody sandwich
The same mouse monoclonal sandwich antibody couple was used to compare analytical performance of TRFIA and CBA: a capture antibody (clone GAD6, kindly provided by Dr D. Gottlieb, St. Louis, MO) reactive to the C-terminal region of human and rat GAD65 (11), and a detection antibody (clone N-GAD65mAb, kindly provided by Dr C. Hampe, Seattle, WA) reactive to the N-terminal region of human and rodent GAD65 (12). The N-GAD65mAb was biotinylated using EZ Link NHS-Biotin (Thermo Scientific) according to manufacturer's protocol.
GAD65-TRFIA assay
We used an in-house developed TRFIA for human/rat GAD65 as previously reported (13) with 1 minor modification: directly Europium-labeled N-GAD65mAb detection antibody was replaced by the same biotinylated N-GAD65mAb antibody as used in the CBA method. After overnight incubation at 4°C with biotinylated N-GAD65mAb (0.32 μg/mL), wells were washed 4 times, incubated for 1 hour at room temperature with 125 μL of a 0.12-μL/mL solution of Europium-streptavidin (PerkinElmer) in TRFIA assay buffer (50mM Tris, 0.9-g/dL NaCl, 0.05-g/dL bovine γ-globulin, 0.5-g/dL BSA, 0.05% [vol/vol] Tween 20, 2.5-g/dL MgCl2, and 0.05-g/dL NaN3; pH 7.75), and washed 4 times before addition of 200 μL of “enhancement solution” (PerkinElmer) and measurement of fluorescence at 615 nm after 5 minutes on Wallac Victor (PerkinElmer) (2).
Conversion of plate-to-bead-based CBA assay
The GAD6 capture antibody was covalently attached to CBA functional beads (E7; Becton Dickinson) with sulfosuccinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate chemistry according to manufacturer's protocol. Functionalized beads were stored shielded from light at 4°C for no more than 6 months.
For a single measurement, 25 μL of plasma was mixed with 25 μL of Assay Diluent (Becton Dickinson) and 20-μL beads solution at 6000 beads/well in Capture Bead Diluent (Becton Dickinson). This was incubated in a 96-well Multiscreen filtration plate (Millipore) on a shaking plate at room temperature for 4 hours, shielded from light. This was followed by addition of 20 μL of a 5-μg/mL solution of biotinylated N-GAD65mAb without previous washing. After a 2-hour incubation at room temperature on the shaking plate, wells were washed 6 times with 170 μL of Wash buffer (Becton Dickinson) using a Millipore Multiscreen (PerkinElmer) adaptor, followed by addition of 100 μL of Human Enhanced Sensitivity Detection Reagent (Part B) (Becton Dickinson). After incubation for 1 hour at room temperature, on the shaking plate shielded from light, wells were washed 6 times, and content was transferred to 1.5-mL microcentrifuge tubes for analysis on a FACSAria (Becton Dickinson).
As illustrated in Supplemental Figure 1, single beads were gated based on forward scatter, their intrinsic color code defined by allophycocyanine-cyanine dye couple after excitation by a 633-nm laser. Bound biotinylated N-GAD65mAb detection antibody was quantified with streptavidin-bound phycoerythrin (PE) fluorescence at 488 nm. FACS output files (.fcs) were read into Flowing Software v2.5 (University of Turku, Turku, Finland). Frequency distribution histograms of PE fluorescence showed a single high peak in all samples and the median PE fluorescence of this peak was selected for quantification.
Analytical validation algorithm
Linearity was assessed according to Clinical and Laboratory Standards Institute (CLSI) guideline EP06-A (14) by triplicate measurement in human plasma (K3-EDTA, supplemented with 1% of a 0.12-mg/mL solution of aprotinin [Stago] in 0.9% NaCl), spiked with human recombinant GAD65 (Diamyd) over 3 concentration ranges: 0pM–77pM (10 points), 0pM–7.7pM (7 points), and 0pM–1.5pM (5 points). Linearity was assessed by one-way ANOVA, and r2 was determined. Within-run, between-day, and total variation were estimated according to CLSI guideline EP5-A2 (15): 2 concentrations of GAD65 in plasma were run twice in duplicate for 20 different days.
Analytical sensitivity was determined according to CLSI guideline EP7-2A (16); to determine functional sensitivity, plasma samples spiked with ascending amounts of a lysate of human pancreatic β-cells were measured in duplicate over 10 days, and CV% was plotted as function of GAD65 concentration to determine the analyte concentration resulting in 20% CV plus 2 SD (40%) added to this graphically derived amount to achieve 95% confidence. Limit of detection (LoD) (mean of blank plus 3 SD) was determined on repeated (n = 20) measurements on a blank sample from a healthy donor for 3 different days. We also analyzed GAD65 levels in healthy controls (n = 40, 30 ± 5 y of age, male/female = 18/22) and in a routine cohort of diseased controls (n = 33). Method comparison between TRFIA and CBA assays was done by Deming regression analysis on 25 samples, either human β-cell lysate spiked in plasma (n = 6) or samples obtained at various time points after intraportal islet transplantation in T1D human recipients (n = 19). Correlation was assessed by nonparametric Spearman rank correlation.
The GAD65-CBA was applied to measure circulating GAD65 concentrations after islet transplantation: nonuremic T1D patients received intraportal infusion of at least 2.106 β-cells/kg bodyweight, and plasma was sampled at various time points before and up to 24 hours after transplantation. All recipients were negative for GAD65 autoantibodies (GADAs). They show a negative interference starting from titers as low as 90 WHO U/mL (reference value, <23 WHO U/mL) (7), as derived from in-house produced interferograms using our monoclonal antibody couple. Statistical elevations above baseline were determined using 2-tailed Wilcoxon signed-rank test. These studies were approved by our institutional Ethical Committee (protocol NCT00623610, ethical approval 98/059D) and were in accordance with the Helsinki Declaration of 1975 as revised in 2008. All participating subjects gave their informed consent before inclusion. Plasma was collected in K3-EDTA Monovette tubes (Sarstedt) supplemented with 1% of a 0.12-mg/mL solution of aprotinin (Stago) in 0.9% NaCl solution. Samples were stored at −80°C after centrifugation for 15 minutes at 1600g.
Statistical analysis was done with Prism 5 (GraphPad) using the statistical tests specified above.
Results
We converted a sandwich antibody couple from a plate-based TRFIA to a bead-based CBA immunoassay format, with minimal adaptations. The design of the GAD65-CBA assay, the gating of the beads, and the concentration-dependent increase of median bead-bound PE fluorescence are illustrated in Supplemental Figure 1.
Linearity
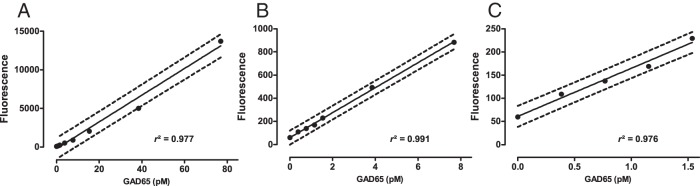
The linearity of the GAD65-CBA was excellent over a concentration range up to 77pM (Figure 1A) and was maintained also in the lower GAD65 concentration range 1.5pM (Figure 1, B and C).
Figure 1. Linearity of the GAD65-CBA assay over 3 different concentration ranges.
Full line represents linear regression and dotted lines represent 95% prediction interval. A, 0pM–77pM; slope (95%CI) = 173 (163–184); y-intercept (95%CI) = −200 (−486–487). B, 0pM–7.7pM; slope (95%CI) = 108 (103–113), y-intercept (95%CI) = 60 (43–77). C, 0pM–1.5pM; slope (95%CI) = 104 (94–114), y-intercept (95%CI) = 61 (52–70).
Imprecision
The GAD65-CBA was more precise at lower analyte levels (Table 1): within-run CV% was 2.48% vs 11.9% at GAD65 concentrations of 1.73pM and 10.0pM, respectively. Between-day imprecisions over 20 consecutive days were comparable at lower (9.80%) and higher (8.53%) GAD65 levels, resulting in total imprecisions of 10.0% and 13.4%, respectively.
Table 1.
Imprecision of the GAD65-CBA
| Imprecision | GAD65 Concentration |
|||
|---|---|---|---|---|
| Low Level (1.73pM) |
High Level (10.0pM) |
|||
| SD, pM | CV, % | SD, pM | CV, % | |
| Within-run | 0.04 | 2.48 | 1.35 | 11.9 |
| Between day | 0.14 | 9.80 | 0.86 | 8.53 |
| Total | 0.14 | 10.0 | 1.35 | 13.4 |
CV, coefficient of variation.
Analytical comparison with GAD65-TRFIA
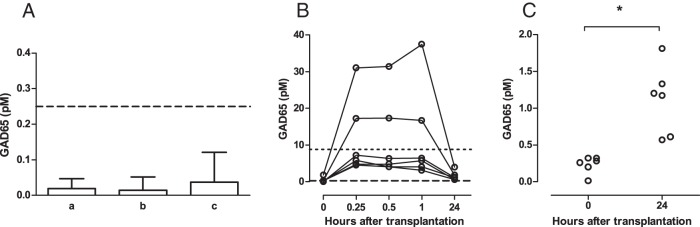
The main objective of this study was to investigate whether conversion from TRFIA to the CBA format improved the analytical sensitivity of our GAD65 immunoassay. We therefore compared their respective functional sensitivities, determined on dilutions of human β-cell lysate spiked in plasma over 10 different days, and graphically determined as the GAD65 concentration for which 20% CV is reached. This value (plus 40% accounting for the upper limit of the 95% CI) amounted to 0.25pM for the GAD65-CBA, vs 8.8pM for the GAD65-TRFIA (Figure 2A), indicating a 35-fold improvement. The CBA showed less variation on blank samples, with LoD (mean plus 3 SD of 20 identical blanks over 3 days of 0.12pM, vs 1.38pM for TRFIA). GAD65 concentrations obtained with CBA and TRFIA after split sample analysis were significantly correlated, r2 = 0.94 in Spearman rank regression analysis (Figure 2B), taking only samples with GAD65 values above the functional sensitivity of either assay format. We additionally studied reference values for the GAD65-CBA on a cohort of healthy controls (n = 40) and on a cohort of diseased controls. The latter cohort included patients with 1 of the next conditions (n = 3 per condition; n = 33 in total): pancreatitis, type 2 diabetes mellitus, long-standing GADA-positive T1D, neurological disease, liver disease, muscle trauma, kidney transplantation, presence of monoclonal antibodies, presence of polyclonal hypergammaglobulinemia, presence of heterophile antibodies, or presence of rheumatoid factor (for antibodies, please see Table 2). In all these samples GAD65 concentration remained invariably below the functional sensitivity of the GAD65-CBA (Figure 3A).
Figure 2. Comparison of functional sensitivities and method comparison between TRFIA and CBA.
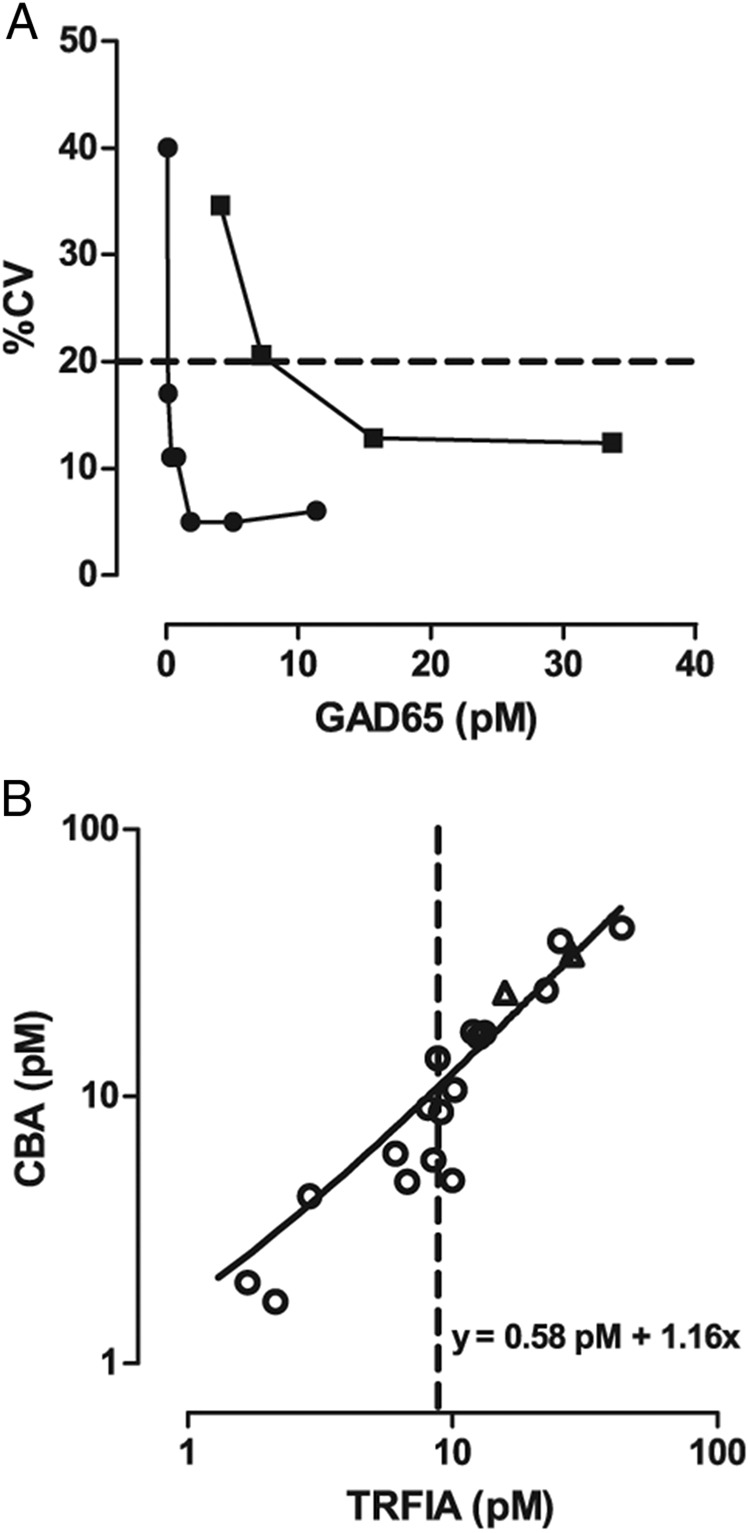
A, Comparison between functional sensitivity of CBA (rounds) and TRFIA (squares), broken line indicates 20% CV. B, Method comparison; circles are patient samples after transplantation and triangles represent results for different concentrations of lysate of human β-cells in plasma; slope (95%CI) = 1.16 (0.88–1.45); y-intercept (95%CI) = 0.58 (−5.00–6.16); P < .0001. Broken line indicates functional sensitivity of TRFIA.
Table 2.
Antibody Table
| Peptide Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | |
|---|---|---|---|---|---|---|
| Glutamate decarboxylase (GAD65) | 65 kDa | AA 521–585 | Clone GAD6 | Dr D. Gottlieb, St. Louis, MO | Monoclonal; mouse | N/A |
| Glutamate decarboxylase (GAD65) | 65 kDa | AA 1–115 | Clone N-GAD65mAb | Dr C. Hampe, Seattle, WA | Monoclonal; mouse | 0.32 μg/mL |
Figure 3. Analysis of biologically relevant samples for GAD65 with CBA.
A, Apparent GAD65 concentration (mean ± SD) observed in test of limit of detection (a), 40 healthy controls (b), and 33 diseased controls (c). B, Time points of 6 intraportal human β-cell transplantations, lines connect points for each patient (C) GAD65 at baseline and 24 hours after transplantation of human β-cells. Functional sensitivity: dotted line, TRFIA; broken line, CBA; *, P = .03.
The biological relevance of improved analytical sensitivity of GAD65-CBA was illustrated in long-standing nonuremic T1D patients after intraportal infusion of islet allografts. Experiments using the GAD65-TRFIA previously showed a surge of graft-derived GAD65 in plasma sampled up to 8 hours after islet infusion (7) in 1 out of 3 graft recipients. Using the more sensitive GAD65-CBA format, we could detect a clear posttransplant surge in GAD65 in all 6 of 6 recipients, whereas the GAD65-TRFIA detected such surge above functional sensitivity in 2 out of 6 (Figure 3B). Twenty-four hours after islet infusion, GAD65 concentrations were still above the functional sensitivity and above baseline values as measured by GAD65-CBA (P = .03) (Figure 3C) but were undetectable with GAD65-TRFIA (Figure 3B).
Discussion
Bead-based immunoassays are attractive, because they allow miniaturization of sample volumes and higher throughput through multiplexing (10). Their main application today is the multiplexed measurement of cytokines. Analytical performance appears adequate for longitudinal studies within the same technology, although standardization is needed to compare results between different commercial platforms (17). In terms of analytical sensitivity, studies thus far indicated that bead-based cytokine assays are not superior. In particular, the original CBA platform was found less sensitive than microtiter plate-based enzyme-linked (18) and electrochemiluminescence-based (19) immunoassays.
This contrasts with the main finding of our study; we converted a plate-based TRFIA (13) to the Becton Dickinson CBA bead format and achieved a 35-fold gain in functional sensitivity. A possible explanation for this was our use of a novel generation of CBA detection reagents, referred to as Becton Dickinson Human Enhanced Sensitivity, which in our hands (data not shown) outperforms the previously reported streptavidin-PE detection reagents (13, 18, 19).
The GAD65-CBA can easily be integrated in any routine laboratory workflow, providing the appropriate FACS platform is available. Sample throughput of current prototype is limited but could be enhanced by using automatic plate counters instead of manual analysis. GAD65 was stable for at least 6 hours at room temperature or 4°C in serum or various types of plasma (Supplemental Table 1). The stability at 37°C is less (20). We recommend collecting the samples in K-EDTA tubes supplemented with aprotinin, particularly if they cannot be handled rapidly or if the measurement of other peptides is envisaged on the same samples.
Improved analytical sensitivity is crucial for the clinical use of low-abundance biomarkers in plasma: despite its good relative β-cell selectivity, GAD65 was not detected among the high-abundance proteins in the rodent or human β-cell (21). In line with this, plasma GAD65 could not be detected in healthy controls, nor in diseased controls. Islet transplantation represents an interesting model for validation studies, because the acute posttransplant phase is characterized by β-cell disruption due to mechanical or inflammatory stress. Using our previously developed GAD65-TRFIA (13), we could detect clear GAD65 surges above baseline shortly after islet transplantation, albeit only in 1 out of 3 GADA-negative islet graft recipients (7), and establish associations with long-term clinical outcome: GAD65 levels above 15pM at 1 hour after transplant predicted poor C-peptide production 2 months after transplantation (7). Pilot data in this study were promising: a clear surge was detected in all graft recipients 1 hour after islet allograft infusion and remained increased at 24 hours (Figure 3). Ongoing experiments in larger cohorts indicate GAD65 above functional sensitivity up to 6 days after transplantation. Our findings indicate 2 obstacles in the integration of GAD65-CBA in standard operating procedures of islet transplantation. First, due to its relatively limited half-life (∼3 h), GAD65 should be measured daily, ideally multiple times a day, to confirm or rule out low-grade β-cell destruction. The way forward is to use sampling methods, which would allow accurate biomarker quantification in a single drop of blood. Second, solutions are needed to circumvent negative interference with GADA by including alternative biomarkers (eg, miR-375). We are currently compiling validation cohorts of recent-onset GADA-negative T1D patients, and will subject them to combinatorial profiling of GAD65 and mir-375, thereby hopefully providing the much needed proof-of-concept data that real-time detection of β-cell injury in the early stages of T1D is within reach.
Acknowledgments
This work was supported by the Agency for Innovation by Science and Technology Flanders Grant IWT-SB-621 (PhD grant to O.C.), by the Research Foundation Flanders Research Grant G.0492.12N and a Senior Clinical Investigator grant (to G.A.M.), by the Juvenile Diabetes Research Foundation Pilot Grant JDRF-PNF-2014-181-A-V (to F.K.G. and G.A.M.), and by the Universitair Ziekenhuis Brussel Wetenschappelijk Fonds Willy Gepts (G.A.M.). This study was part of projects financed by the Flemish Government (IWT130138) and the European Union's Seventh Framework Programme (FP7/2007-2013) under Grant Agreement 241883.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the Agency for Innovation by Science and Technology Flanders Grant IWT-SB-621 (PhD grant to O.C.), by the Research Foundation Flanders Research Grant G.0492.12N and a Senior Clinical Investigator grant (to G.A.M.), by the Juvenile Diabetes Research Foundation Pilot Grant JDRF-PNF-2014-181-A-V (to F.K.G. and G.A.M.), and by the Universitair Ziekenhuis Brussel Wetenschappelijk Fonds Willy Gepts (G.A.M.). This study was part of projects financed by the Flemish Government (IWT130138) and the European Union's Seventh Framework Programme (FP7/2007-2013) under Grant Agreement 241883.
Footnotes
- CBA
- cytometric bead array
- CLSI
- Clinical and Laboratory Standards Institute
- CI
- confidence interval
- CV%
- percentage coefficient of variation
- GAD65
- glutamate decarboxylase 65 kDa
- GADA
- GAD65 autoantibody
- LoD
- limit of detection
- PE
- phycoerythrin
- T1D
- type 1 diabetes mellitus
- TRFIA
- time-resolved fluorescence immunoassay.
References
- 1. In't Veld P. Insulitis in human type 1 diabetes: the quest for an elusive lesion. Islets. 2011;3:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Erener S, Mojibian M, Fox JK, Denroche HC, Kieffer TJ. Circulating miR-375 as a biomarker of β-cell death and diabetes in mice. Endocrinology. 2013;154:603–608. [DOI] [PubMed] [Google Scholar]
- 3. Usmani-Brown S, Lebastchi J, Steck AK, Beam C, Herold KC, Ledizet M. Analysis of β-cell death in type 1 diabetes by droplet digital PCR. Endocrinology. 2014;155:3694–3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Christgau S, Schierbeck H, Aanstoot HJ, et al. . Pancreatic β cells express two autoantigenic forms of glutamic acid decarboxylase, a 65-kDa hydrophilic form and a 64-kDa amphiphilic form which can be both membrane-bound and soluble. J Biol Chem. 1991;266:23516. [PubMed] [Google Scholar]
- 5. Geigerseder C, Doepner R, Thalhammer A, et al. . Evidence for a GABAergic system in rodent and human testis: local GABA production and GABA receptors. Neuroendocrinology. 2003;77:314–323. [DOI] [PubMed] [Google Scholar]
- 6. Smismans A, Ling Z, Pipeleers D. Damaged rat β cells discharge glutamate decarboxylase in the extracellular medium. Biochem Biophys Res Commun. 1996;228:293–297. [DOI] [PubMed] [Google Scholar]
- 7. Ling Z, De Pauw P, Jacobs-Tulleneers-Thevissen D, et al. . Plasma GAD65, marker for early β cell loss following intraportal β cell transplantation in type 1 diabetes mellitus. J. Clin Endocrinol Metab. 2015;100:2314–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Waldrop MA, Suckow AT, Marcovina SM, Chessler SD. Release of glutamate decarboxylase-65 into the circulation by injured pancreatic islet β-cells. Endocrinology. 2007;148:4572–4578. [DOI] [PubMed] [Google Scholar]
- 9. Piemonti L, Guidotti LG, Battaglia M. Modulation of early inflammatory reactions to promote engraftment and function of transplanted pancreatic islets in autoimmune diabetes. Adv Exp Med Biol. 2010;654:725–747. [DOI] [PubMed] [Google Scholar]
- 10. Morgan E, Varro R, Sepulveda H, et al. . Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clin Immunol. 2004;110:252–266. [DOI] [PubMed] [Google Scholar]
- 11. Gottlieb DI, Chang YC, Schwob JE. Monoclonal antibodies to glutamic acid decarboxylase. Proc Natl Acad Sci USA. 1986;83:8808–8812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hampe CS, Lundgren P, Daniels TL, Hammerle LP, Marcovina SM, Lernmark A. A novel monoclonal antibody specific for the N-terminal end of GAD65. J Neuroimmunol. 2001;113:63–71. [DOI] [PubMed] [Google Scholar]
- 13. Rui M, Hampe CS, Wang C, et al. . Species and epitope specificity of two 65 kDa glutamate decarboxylase time-resolved fluorometric immunoassays. J Immunol Methods. 2007;319:133–143. [DOI] [PubMed] [Google Scholar]
- 14. Clinical and Laboratory Standards Institute. Evaluation of the Linearity of Quantitative Measurement Procedures: A Statistical Approach; Approved Guideline. vol CLSI Guideline EP06-A Wayne, PA: Clinical and Laboratory Standards Institute; 2003. [Google Scholar]
- 15. Clinical and Laboratory Standards Institute. Evaluation of Precision Performance of Quantitative Measurement Methods; Approved Guideline. 2nd ed vol CLSI Document EP05-A2 Wayne, PA: Clinical and Laboratory Standards Institute; 2004. [Google Scholar]
- 16. Clinical and Laboratory Standards Institute. Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures; Approved Guideline. 2nd ed vol CLSI Guideline EP17-A2 Wayne, PA: Clinical and Laboratory Standards Institute; 2012. [Google Scholar]
- 17. Dupuy AM, Kuster N, Lizard G, et al. . Performance evaluation of human cytokines profiles obtained by various multiplexed-based technologies underlines a need for standardization. Clin Chem Lab Med. 2013;51:1385–1393. [DOI] [PubMed] [Google Scholar]
- 18. Young SH, Antonini JM, Roberts JR, Erdely AD, Zeidler-Erdely PC. Performance evaluation of cytometric bead assays for the measurement of lung cytokines in two rodent models. J Immunol Methods. 2008;331:59–68. [DOI] [PubMed] [Google Scholar]
- 19. Dabitao D, Margolick JB, Lopez J, Bream JH. Multiplex measurement of proinflammatory cytokines in human serum: comparison of the meso scale discovery electrochemiluminescence assay and the cytometric bead array. J Immunol Methods. 2011;372:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schlosser M, Walschus U, Klöting I, Walther R. Determination of glutamic acid decarboxylase (GAD65) in pancreatic islets and its in vitro and in vivo degradation kinetics in serum using a highly sensitive enzyme immunoassay. Dis Markers. 2008;24:191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martens GA, Jiang L, Verhaeghen K, et al. . Protein markers for insulin-producing β cells with higher glucose sensitivity. PLoS One. 2010;5:e14214. [DOI] [PMC free article] [PubMed] [Google Scholar]