Abstract
Emerging evidence suggests that aging is associated with a reduced ability to distinguish perceptually similar stimuli in one’s environment. As the ability to accurately perceive and encode sensory information is foundational for explicit memory, understanding the neurobiological underpinnings of discrimination impairments that emerge with advancing age could help elucidate the mechanisms of mnemonic decline. To this end, there is a need for preclinical approaches that robustly and reliably model age-associated perceptual discrimination deficits. Taking advantage of rodents’ exceptional olfactory abilities, the present study applied rigorous psychophysical techniques to the evaluation of discrimination learning in young and aged F344 rats. Aging did not influence odor detection thresholds or the ability to discriminate between perceptually distinct odorants. In contrast, aged rats were disproportionately impaired relative to young on problems that required discriminations between perceptually similar olfactory stimuli. Importantly, these disproportionate impairments in discrimination learning did not simply reflect a global learning impairment in aged rats, as they performed other types of difficult discriminations on par with young rats. Among aged rats, discrimination deficits were strongly associated with spatial learning deficits. These findings reveal a new, sensitive behavioral approach for elucidating the neural mechanisms of cognitive decline associated with normal aging.
Keywords: Olfaction, Olfactory discrimination, Aging, Spatial learning, Odor, Water maze, Rat
1. Introduction
The ability to accurately perceive and encode sensory stimuli forms the foundation for normal episodic and other types of explicit memories that critically depend on the hippocampus (Morris et al., 1982; Scoville and Milner, 1957; Squire, 1992). Specifically, during memory formation, the hippocampus and interconnected medial temporal lobe structures receive highly specific input from each of the sensory modalities that is integrated with information about time and space to form a detailed representation of a to-be-remembered event or episode (Felleman and Van Essen, 1991; Moscovitch et al., 2006; Squire et al., 2004). Importantly, explicit memory is among the most vulnerable to decline in aging (Gallagher et al., 1993; Ronnlund et al., 2005; Uttl and Graf, 1993). Substantial evidence indicates that such age-associated mnemonic decline is attributable in part to impaired neuroplasticity mechanisms required for memory maintenance (Barnes, 1979; Burke and Barnes, 2006; Foster and Kumar, 2007). In addition, a growing literature demonstrates that across species, aging is associated with an inability to discriminate among similar features in one’s environment, which could interfere with the accurate encoding of new representations (Burke et al., 2011; Gracian et al., 2013; Reagh et al., 2014; Stark et al., 2010, 2013; Yassa et al., 2011). Although the majority of this research to date has focused on the perception and discrimination of visual stimuli, age-associated alterations in the top-down influence of sensory processing would be expected to contribute to perceptual deficits that span sensory modalities (Burke et al., 2012a, 2014; Ryan et al., 2012). Sensitive preclinical models that facilitate investigation of the neurobiological basis for perceptual and discrimination deficits in aging could offer significant utility for developing and testing interventions to maximize cognitive capacities across the lifespan.
Many individuals over the age of 65 experience significant declines in olfactory function, and such declines have been linked to cognitive impairments and age-related neurodegenerative disease (e.g., Dulay and Murphy, 2002; Segura et al., 2013; Wilson et al., 2011). Moreover, as the primary sensory modality for rodents, olfaction is ideal for both investigating natural animal behaviors and as a means for preclinical investigation of neurocognitive processes under both normal and pathological conditions (Eichenbaum and Robitsek, 2009; Lu et al., 1993). Indeed, previous work from our laboratory reported that aged Fischer 344 (F344) rats are impaired in their ability to discriminate between 2 odors and that these olfactory discrimination deficits are strongly associated with impaired hippocampal-dependent spatial learning (LaSarge et al., 2007).
Olfactory perception and discrimination are strongly influenced by a number of stimulus characteristics including chemical structure and concentration. In particular, a strong relationship exists between odorant chemical structure and activation of glomerular maps in the olfactory bulb that renders this modality well-suited for the systematic manipulation of perceptual similarity between 2 stimuli (Falasconi et al., 2012; Mori et al., 2006). Within a homologous chemical series, the overlap between glomerular activation associated with the detection of 2 odorants increases as the difference in the number of carbon atoms in the carbon chain backbone between the 2 odorants decreases (Falasconi et al., 2012). Thus, by varying carbon chain length between odorants, olfactory stimulus sets can be designed in which perceptual similarity is systematically varied (Cleland et al., 2002; Ho et al., 2006; Weeden et al., 2014). Previously, our laboratory has used a series of aliphatic alcohols to demonstrate that decreasing the difference in carbon chain length between 2 odorants from 5 to 1 produces systematic decrements in discriminability in young rats (Yoder et al., 2014). It remains unknown, however, whether performance across such perceptual gradients is sensitive to alterations in aging, and in particular, may have utility for identifying cognitive vulnerability.
The present study was designed to determine if aging preferentially impairs the ability to discriminate between perceptually similar odorants and if such deficits associate with hippocampal-dependent spatial learning abilities in a rat model of normal cognitive aging. Young and aged F344 rats were cross-characterized on a hippocampal-dependent water maze task and a go/no-go olfactory learning task (Eichenbaum et al., 1983) in which they discriminated between pairs of odors from homologous chemical series of aliphatic compounds (alcohols, aldehydes, or acids, Yoder et al., 2014). Within a chemical class, the difference in the length of the carbon chain between odorants (i.e., the difference in chemical structure) was varied across discrimination problems to systemically manipulate perceptual similarity. The findings indicate that aging interacts with perceptual similarity such that aged rats are disproportionately impaired in their ability to effectively discriminate between perceptually similar olfactory stimuli while maintaining the ability to discriminate dissimilar odorants and without an overall decrement in odor detection threshold. Notably, the deficits on perceptually similar discrimination problems are unlikely to reflect global learning impairments that emerge in aging as the aged rats performed other difficult discrimination problems on par with young subjects. Interestingly, discrimination deficits among aged rats were strongly associated with impaired spatial learning abilities. These results offer a novel rodent behavioral model that should be useful for uncovering the neurobiological underpinnings of perceptual discrimination deficits in aging and their contributions to mnemonic decline.
2. Methods
2.1. Subjects
F344 rats (n = 20 young [6 months]; n = 14 aged [24 months]) were obtained from the National Institute on Aging colony maintained by Charles River. All rats were individually housed in the vivarium in the McKnight Brain Institute at the University of Florida for at least 2 weeks before behavioral testing. Rats were trained in 3 separate groups with Table 1 indicating group sizes and experimental design for each. The rats were maintained on a 12:12 hours light/dark cycle (lights on at 0800), and behavioral testing was conducted during the light cycle. Rats had ad libitum access to dry LabDiet rat chow (Purina Mills) and restricted access to water during periods of olfactory discrimination testing. This regimen resulted in the rats stabilizing at 85%–90% of their free-feeding body weight, which facilitated the use of a nutritional liquid food reward during training and testing procedures (Ensure, Abbott Laboratories). During a typical session, rats received ~10 mL of Ensure per day, followed by 2 hours of unrestricted access to water after daily testing. Rats were tested once daily, 5–7 days per week. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication no. 86–23, revised 1985) and were approved by the University of Florida Institutional Animal Care and Use Committee.
Table 1.
Experimental design
| Experiment | Group size | Chemical classes tested | Olfactory discrimination | Olfactory detection threshold | Water maze | |
|---|---|---|---|---|---|---|
| Experiment 1 | n = 8 (6 mo at start) | Alcohols Δ5, Δ3, Δ1 |
Longitudinal testing, stimuli repeated | Not assessed | Not assessed | |
| Experiment 2 (cohort A) |
n = 4, 6 mo n = 6, 24 mo |
Alcohols Δ5, Δ3, Δ1 |
Single test, stimuli used only once | Three alcohols and 1 unrelated odorant | After olfactory testing | |
| Experiment 2 (cohort B) |
n = 8, 6 mo n = 8, 24 mo |
Alcohols Δ5, Δ3, Δ1 Aldehydes Δ5, Δ3, Δ1 Acids Δ5, Δ3, Δ1 Unrelated odorants |
Single test, stimuli used only once | One odorant in each chemical class and 1 unrelated odorant | Before olfactory testing | |
2.2. General olfactory testing procedures
2.2.1. Olfactory discrimination apparatus
An 8-channel, custom liquid-dilution rodent olfactometer (Tel-Test Inc, Gainesville, FL, USA) was used to assess odor discrimination. The behavioral apparatus and methods employed in this study are identical to those used in our previous work (Yoder et al., 2014, 2015), and detailed descriptions of the training and testing techniques can be found in those previous publications. The olfactometer was connected to a 21-cm deep, 30.5-cm wide, and 24.1-cm tall, ventilated Plexiglas operant chamber. The chamber was fitted with a floor made of conductive stainless steel rods and a polyvinyl chloride odor sampling port containing a metal lick tube through which the liquid reward could be delivered. Contact with the lick tube completed an electrical circuit with the metal floor, which was recorded via computer. The ventilation system provided a steady stream of fresh room air in the chamber, maintaining positive pressure and ensuring that the odorant remained within the sampling port air stream. A photobeam was broken when the rat inserted its head into the sampling port, initiating a trial sequence. Rats were required to keep their noses within the port and sample the stimulus air stream for a minimum of 200 ms, at which time an olfactory stimulus, either the S+ (target stimulus) or S− (control stimulus), as defined below, was introduced into the air stream through the bottom of the sampling port. The air stream and odorant were drawn through the sampling port in which the rat positioned its nose and were then exhausted out of the top by an inline exhaust fan and fed into a central room evacuation system. Stimulus delivery and behavioral responses (sampling port entries and licks) were controlled and monitored by a computer running custom-designed software.
2.2.2. Olfactory discrimination training procedures
Rats were initially shaped to respond at the sampling port to obtain reward. Shaping procedures followed those described by Yoder et al. (2014). Briefly, rats were initially rewarded for contacting the lick tube with their tongue, followed by nose pokes into the sampling port, and finally for remaining in the sampling port during odorant presentation. During the last stage of training, the final valve was introduced, gradually requiring rats to sample the odorant for intervals up to 1 second. A 10% v/v solution of coconut extract (Gordon Food Service) served as the initial training stimulus. Reliable performance during this initial training stage was achieved within 2 sessions (45–90 minutes) for all rats. Before the discrimination task, rats therefore acquired an association between the target odorant and delivery of liquid reward. Once the rats successfully completed shaping, they were transferred to a 2-odorant discrimination program.
Rats were next trained to discriminate dilutions of a target (S+) odorant (coconut extract) in a diluent from the diluent alone (S−). The diluent used for this and all subsequent experiments was near-odorless diethyl phthalate (Laska, 2014). Reinforcement was contingent on the rat reporting detection of the S+ odorant by licking the metal tube (i.e., a correct detection). A correct detection was followed by presentation of ~5 μL of Ensure through the lick tube. Failures to lick in the presence of the S+ or inhibit licking the response tube during presentation of the S− were recorded as incorrect responses and required rats to withdraw their nose from the sampling port for 5 seconds before reinserting their nose to initiate a new trial. Consistent with previous olfactometer studies in rodents (Bodyak and Slotnick, 1999; Yoder et al., 2014), rats were required to respond to the target (S+) odor, coconut extract, by maintaining contact with the lick tube for at least 7 of 10 time bins (each spanning 100 ms) during a 1-second odor presentation. From these calculated lick intervals, the go/no-go criterion was set to 7. Hence, if the rat licked for ~700 ms (7 × 100 ms bins) of the total ~1000 ms (10 × 100 ms bins) response interval, the rat would receive the 5-μL liquid reinforcement. Conversely, if the rat refrained from licking or licked fewer than 7 bins on the control odorant (S−), the trial would be recorded as a correct rejection, thereby allowing the rat to initiate the next trial. Note that the rat was not required to lick during the control (S−) trials and therefore was free to leave the odor port once the decision was made.
Trials were presented in blocks of 20 (10 S+ and 10 S−). Within each block, the sequence of the 20 trials was quasi-random such that each stimulus was limited to 3 consecutive presentations. The percent correct was calculated (for both correct detection and correct rejection) individually for each block. Initial discrimination training consisted of 10 blocks (200 trials). Rats achieved criterion performance (85% or greater) within 2–4 blocks. The following training session consisted of a new target odorant (1% v/v vanilla extract). During this training session, rats were required to respond to the new target odorant, while ignoring the control odorant (1% v/v coconut). A final training session consisted of 1 part-per-million (ppm; 10−4% v/v) orange extract as the target (S+) odorant and 1 ppm (10−4% v/v) vanilla extract as the control (S−) odorant. These additional sessions were incorporated to ensure that the rats would have sufficient experience with the behavioral paradigm to begin testing on monomolecular discrimination pairs, rather than to anticipate an odorless control. Once rats successfully completed all steps of the training procedures, they began testing.
2.2.3. Olfactory discrimination control procedures
To minimize possible detection of subtle airflow or auditory cues, unused odorant valves on the olfactometer apparatus (i.e., those not controlling delivery of either S+ or S− stimuli) were randomly activated across conditions to provide “masking” noise. Locations of saturation bottles were pseudorandomized across sessions. Two control tests were used to confirm that there were no inadvertent odorant or nonodorant cues available to the rats that could serve as discriminative cues. First, the target (S+) odorant bottle was replaced with the diluent alone such that both the S+ and S− saturation bottles contained identical volumes of the control (S−) stimulus. Second, the S+ saturation bottle tubes were pinched off by the experimenter to occlude delivery of the odorant during an S+ trial. Under both control conditions, trained rats performed at chance levels, indicating a lack of inadvertent discrimination cues. Finally, to ensure that the rats were responding only to the presence of airflow (odor), an additional control measure consisted of disconnecting the stimulus stream. Under this condition, rats would initiate a trial, but receive no airflow. In the absence of airflow, rats reliably refrained from responding.
2.3. Experiment 1: longitudinal olfactory discrimination performance using repeated presentations of the same stimuli that vary in perceptual similarity
One cohort of rats (6-month old) was tested longitudinally at 6-month intervals (i.e., testing was conducted at 6, 12, and 18 months of age) for discrimination abilities using the same 3 pairs of aliphatic alcohols at each test point (see Tables 1 and 2). Across pairs, the odorants differed systematically with respect to the length of their carbon chains (i.e., pair 1 differed by 5 carbon atoms [Δ5]; pair 2 differed by 3 carbon atoms [Δ3]; and pair 3 differed by a single carbon atom [Δ1]). The difference in carbon chain length between each pair has been shown to yield systematic variations in perceptual similarity (Can Güven and Laska, 2012; Laska et al., 2006, 2008; Yoder et al., 2014), corresponding to overlapping glomerular activation patterns in the olfactory bulb (Uchida et al., 2000). For each testing time point, 1 discrimination problem was tested each day, and the order of problems presented was randomized across rats. All odorants were presented at 1 ppm liquid concentration [equivalent to 0.0001% v/v; (Slotnick, 2007)]. This concentration was selected on the basis of previous studies (Laska et al., 2008; Yoder et al., 2014) showing that it is sufficiently high to yield similar intensities across stimuli and to account for normal variability in odor sensitivity across rodents (Laska et al., 2006, 2008). Stock odorants (obtained from Sigma-Aldrich at a nominal purity of at least 95%) were stored in glass under inert gas (nitrogen) and refrigerated to limit oxidation (see Table S1 for chemical details of all odorants). The target or control stimulus consisted of 10 mL of the liquid-phase odorant, placed in a 500-mL glass saturation jar. The olfactometer functioned by use of digitally controlled solenoid pinch valves, which briefly bubbled the stimulus air stream through a tube submerged in the liquid-phase odorant to produce a volatilized stimulus that filled the headspace before introduction into the carrier stream and presentation to the rat. Within each pair, 1 odorant was randomly designated as S+ and the other as S−, and rats were reinforced for licking in the presence of the target odorant. During each testing session, rats were required to complete a total of 200 trials (100 S+ trials and 100 S− trials). The identical odor pairs were tested at 6, 12, and 18 months of age.
Table 2.
Odor pairs used in discrimination problems
| Experiment | Chemical class | Odorants | Carbon chain difference |
|---|---|---|---|
| 1 | Aliphatic alcohols | C3 versus C8/1-propanol versus 1-octanol | Δ5 |
| C6 versus C9/1-hexanol versus 1-nonanol | Δ3 | ||
| C4 versus C5/1-butanol versus 1-pentanol | Δ1 | ||
| 2 (cohort A) | Aliphatic alcohols | C3 versus C8/1-propanol versus 1-octanol | Δ5 |
| C6 versus C9/1-hexanol versus 1-nonanol | Δ3 | ||
| C4 versus C5/1-butanol versus 1-pentanol | Δ1 | ||
| 2 (cohort B) | Aliphatic alcohols | C3 versus C8/1-propanol versus 1-octanol | Δ5 |
| C6 versus C9/1-hexanol versus 1-nonanol | Δ3 | ||
| C4 versus C5/1-butanol versus 1-pentanol | Δ1 | ||
| Aliphatic aldehydes | C4 versus C9/1-butanal versus 1-nonanal | Δ5 | |
| C8 versus C11/1-octanal versus 1-undecanal | Δ3 | ||
| C5 versus C6/1-pentanal versus 1-hexanal | Δ1 | ||
| Aliphatic carboxylic acids | C3 versus C8/propanoic versus octanoic | Δ5 | |
| C6 versus C9/hexanoic versus nonanoic | Δ3 | ||
| C4 versus C5/butanoic versus pentanoic | Δ1 | ||
| Structurally unrelated | Citral versus 2-heptanone | n/a | |
| Methyl salicylate versus guaiacol | n/a | ||
| 2-octanone versus benzaldehyde | n/a |
2.3.1. Statistical analysis
For each 20 trial block, accuracy was calculated for individual rats, with incorrect responses including both failure to lick on S+ presentations and failure to withhold the response on S− presentations. Data were analyzed using a 3-factor repeated-measures analysis of variance (ANOVA), with testing time point, carbon chain difference, and trial block as within-subjects factors. In this and all subsequent experiments, p values <0.05 were considered significant.
2.4. Experiment 2: olfactory discrimination performance in young and aged rats using novel stimuli that vary in perceptual similarity
2.4.1. Olfactory discrimination testing
Experiment 2 was designed to better isolate how age affects rats’ abilities to discriminate among perceptually similar stimuli. A between-subjects design was employed, and each stimulus was used only once, thereby minimizing the impact of stimulus familiarity on discrimination performance. In this experiment, 2 naive cohorts of rats (cohort A: n = 4 young, n = 6 aged; cohort B: n = 8 young, n = 8 aged) were first tested on the aliphatic alcohol series described in Experiment 1 (Tables 1 and 2). To confirm that age differences in discrimination abilities were generalized and were not specific to a single chemical class, olfactory discrimination testing in cohort B was then expanded to include discrimination problems using stimuli from 2 additional chemical classes. Specifically, rats were tested using a series of 3 aliphatic aldehyde pairs and a series of 3 aliphatic acid pairs, which differed in carbon chain length by Δ1, Δ3, or Δ5 carbon atoms (Tables 1 and 2). As a control measure, rats in cohort B were also tested for their ability to discriminate between 3 pairs of structurally unrelated odorants (Tables 1 and 2).
2.4.2. Odor detection threshold testing
Following completion of odor discrimination testing, rats in both cohort A and B were tested for their ability to detect and respond to decreasing concentrations of 4 odorants (Tables 1 and 3). Similar to the training sessions described previously, rats were reinforced for licking only in the presence of the target odorant. The percent correct was calculated for both correct detection and correct rejection for each block. When the percent correct reached 85% on 3 successive blocks, the concentration of the S+ stimulus was decreased 10-fold for the following block (odorant dilutions were prepared using diethyl phthalate as the diluent). “Threshold” was defined as the lowest concentration at which a rat achieved 85% or higher on 3 consecutive blocks.
Table 3.
Odorants used for detection threshold testing in Experiment 2
| Cohort | Odorant | Chemical class |
|---|---|---|
| Cohort A | Propanol | Alcohol |
| Pentanol | Alcohol | |
| Octanol | Alcohol | |
| Carvone | Unrelated | |
| Cohort B | Heptanol | Alcohol |
| Heptanal | Aldehyde | |
| Heptanoic acid | Carboxylic acid | |
| Isoamyl acetate | Unrelated |
2.4.3. Water maze testing
Young and aged rats in both cohorts used in Experiment 2 were also assessed for spatial learning abilities on the Morris water maze task using a protocol modified from Gallagher et al. (1993) and Bizon et al. (2009). The order of water maze and odor testing was counterbalanced across the 2 cohorts (see Table 1).
The maze consisted of a circular tank (diameter 183 cm, wall height 58 cm) painted white and filled with water (27 °C) made opaque with the addition of nontoxic white tempera paint. A retractable escape platform (12 cm diameter, HVS Image, UK) was submerged 2 cm below the water’s surface near the center of one quadrant of the maze. The maze was surrounded by black curtains, to which were affixed large white geometric designs that provided extramaze cues. Data were analyzed using a computer-based video tracking system (Water, 2020, HVS Image, UK).
Rats received 3 swim trials/day over 8 consecutive days, with a 60-second intertrial interval. On each training trial, rats were placed in the water facing the wall and permitted to swim until finding the escape platform (which remained in the same quadrant for all 8 days) or until 90 seconds elapsed, at which time they were guided to the platform by the experimenter. Rats remained on the platform for 30 seconds before removal from the maze and the start of the intertrial interval. The starting position for each trial varied pseudorandomly among 4 equally spaced positions around the perimeter of the maze (north, south, east, or west). Every sixth trial was a probe trial on which the escape platform was retracted to the bottom of the tank for the first 30 seconds of the 90-second trial. Training and probe trials assessed spatial acquisition and search strategy, respectively.
To assess rats’ sensorimotor abilities and motivation to escape the water independent of spatial learning ability, rats received 1 session with 6 trials of cue training following the last day of spatial training. In this session, rats were trained to escape to a visible black platform, which protruded 2 cm above the water’s surface and which was moved to a different maze quadrant on each trial. On each trial, rats were given 30 seconds to reach the platform and were allowed to remain there briefly before a 30-second intertrial interval. Performance on this task was assessed as the path length swum to reach the platform.
In the hidden platform (spatial) task, accuracy of performance was assessed using 2 proximity measurements. A cumulative search error measurement was computed from training trials, and a learning index score was calculated from probe trials. For both measures, the rat’s distance from the platform was sampled 10 times/s during each trial and these distances were averaged into 1-second bins. Cumulative search error is the sum of these 1-second averages across training trials, minus the optimal path from the start position to the platform location. The spatial learning index score was calculated from the average proximity (cumulative search error divided by the duration of the probe trial) on the second, third, and fourth probe trials. Scores from these probe trials were weighted and summed to provide an overall measure of spatial learning ability (Bizon et al., 2009; Gallagher et al., 1993). Lower spatial learning indices indicate a more accurate search.
2.4.4. Statistical analyses
2.4.4.1. Olfactory performance
For each 20 trial block, accuracy was calculated for individual rats, with incorrect responses including both misses and false alarms. Data were analyzed using repeated-measures ANOVA conducted separately in each chemical class, with carbon chain difference and age as between-subjects variables, and trial block as a within-subjects variable. Performance on discrimination problems in the alcohol class of odorants (conducted identically in cohorts A and B) was combined for data analysis and presentation. Detection thresholds were analyzed separately for each odorant in cohorts A and B. Age groups were compared using unpaired t tests.
2.4.4.2. Water maze performance
Two-factor ANOVAs were used to compare cumulative search errors on training trials (age × trial block) and probe trials (age × probe trial) in young and aged rats. Aged rats were then assigned to cognitive subgroups on the basis of their spatial learning index calculated as described in Section 2.4.3. Using a median split, the worst aged rats (highest SLIs) were assigned to the “aged spatially impaired” subgroup, and the best aged rats (lowest SLIs) were assigned to the “aged-spatially unimpaired” subgroup. To confirm that these group designations reflected statistically reliable differences in performance between subgroups, probe trial performance was compared between young, aged-unimpaired, and aged-impaired rats using a 2-factor repeated-measures ANOVA (cognitive subgroup × trial). Age effects on cue training were assessed by a t test performed on mean path length data averaged across all test trials.
2.4.4.3. Comparisons of spatial learning and olfactory performance
Two approaches were used to compare spatial learning and olfactory discrimination performance. First, a 3-factor repeated measures ANOVA (cognitive subgroup × carbon chain difference × trial block) was used to compare olfactory discrimination performance between young and aged rats subgrouped on the basis of their spatial learning ability (i.e., aged spatially unimpaired and aged spatially impaired). Second, Pearson’s correlations between spatial learning index and mean percent accuracy on the olfactory discrimination problems were calculated for each level of carbon chain length, as well as for structurally unrelated odor discrimination problems. Only the alcohol series was used for these correlations as this chemical class was tested identically in cohorts A and B. Moreover, young and aged correlations were performed separately. Finally, odor detection threshold was compared across cognitive groups using a 1-factor ANOVA.
3. Results
3.1. Experiment 1: longitudinal olfactory discrimination performance using repeated presentations of the same stimuli that vary in perceptual similarity
In the first experiment, a single group of rats (beginning at 6 months of age) was evaluated at 6-month intervals for their ability to discriminate between the same 3 pairs of aliphatic alcohols that differed in their perceptual similarity. A 3-factor ANOVA (time point [6, 12, and 18 months] carbon chain difference [Δ5, Δ3, and Δ1] × trial block [1–10]) indicated that performance varied as a function of carbon chain difference [F(2,14) = 283.37, p < 0.001], such that rats showed less accuracy in discrimination between more perceptually similar stimuli (Fig. 1). It is also notable, that, across all discrimination problems (Δ5, Δ3, Δ1), accuracy significantly improved as a function of testing time point [F(2,14) = 26.03, p < 0.001]. This result demonstrates that rats show savings of previously learned olfactory discrimination problems, even across long (6 months) intervals and against the background of advancing age.
Fig. 1.
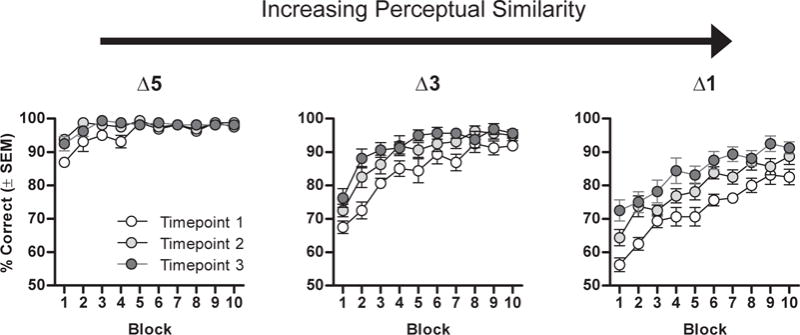
Effects of age on olfactory discrimination learning involving odorants varying in perceptual similarity using a longitudinal design. Graphs show the percentage of correct trials (in 20-trial blocks) on 3 odor discrimination problems in which the odors (selected from a series of aliphatic alcohols) varied in the difference between the numbers of carbon atoms and hence their perceptual similarity. Δ5, Δ3, and Δ1 refer to differences of 5, 3, and 1 carbon atoms between the 2 odorants in each problem. Rats were tested on the same discrimination problems at 3 different time points. Performance was worse on problems with more perceptually similar stimuli but improved across time points with repeated testing. Abbreviation: SEM, standard error of the mean.
Subsequent analyses were performed to determine if this memory for the previously learned discrimination problems influenced the systematic reductions in performance that are typically observed as a function of varying perceptual similarity (Yoder et al., 2014). To do this analysis, performance across each testing time point was compared on only the initial block of trials, as this first block should largely reflect memory for prior testing time points rather than within-session learning. Even when restricting the analysis to the first block of trials, a systematic reduction in accuracy was observed as stimuli became more similar [time point 2, F(2,14) = 80.84, p < 0.001; time point 3, F(2,14) = 14.20, p < 0.001]. This analysis indicates that the effects of perceptual similarity on performance are independent of memory for discrimination information observed across time points. Nevertheless, the overall shift upward in performance (i.e., savings) that was observed at all levels of perceptual similarity, as a result of prior experience with the odor pairs, confounded the ability to accurately evaluate interactions between aging and perceptual similarity. To better distinguish the effects of aging from prior learning, Experiment 2 employed a between-subjects design in which young and aged rats were tested on novel olfactory discrimination problems that varied with respect to carbon chain difference.
3.2. Experiment 2: olfactory performance in young and aged rats using novel stimuli that vary in perceptual similarity
3.2.1. Odor discrimination
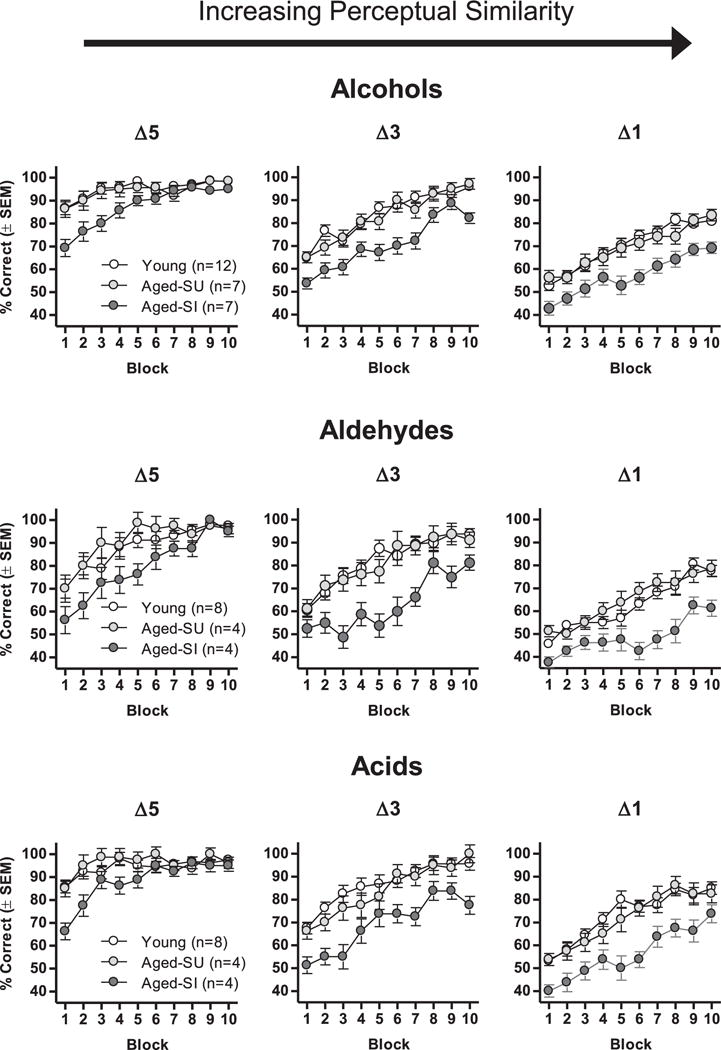
Young and aged rats were tested for their ability to discriminate between odorant pairs that differed in their degree of perceptual similarity (carbon chain difference). Fig. 2 shows performance on discriminations between odorants in each of the 3 chemical classes (alcohols, aldehydes, and acids) that differed by 5, 3, or 1 carbon atoms. Analyses of these data via 3-factor repeated-measures ANOVAs (age × carbon chain difference × trial block) performed separately for each chemical class revealed remarkably similar patterns of results (see Table 4 for ANOVA results). In all 3 chemical classes, performance improved across blocks of trials (main effect of trial block), and performance was worse on more perceptually similar problems (main effect of carbon chain difference). Aged rats performed worse than young across chemical classes, although this difference reached significance only for alcohols and acids. Most importantly, a significant age × carbon chain difference × trial block interaction across all 3 chemical classes indicated that aged rats were disproportionately impaired in their ability to acquire the discrimination problems involving more perceptually similar stimuli.
Fig. 2.
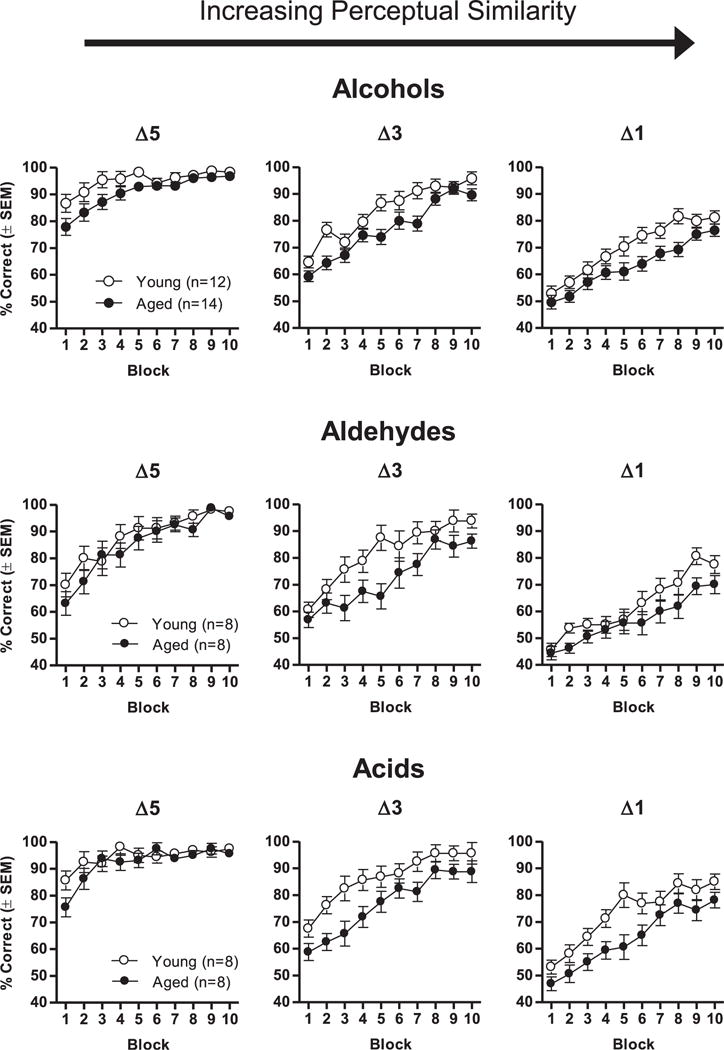
Effects of age on learning of olfactory discrimination problems involving odorants from multiple chemical classes varying in perceptual similarity. Graphs show the percentage of correct trials (in 20-trial blocks) on discrimination problems across 3 classes of odorants, in which the odors varied in the difference between the numbers of carbon atoms and hence their perceptual similarity. Δ5, Δ3, and Δ1 refer to differences of 5, 3, and 1 carbon atoms between the 2 odorants in each problem. Rats were tested on each discrimination problem once. Performance was worse on problems with more perceptually similar stimuli, and aged rats were disproportionally impaired relative to young on these more difficult problems. Abbreviation: SEM, standard error of the mean.
Table 4.
Main effects and 3-way interactions from age × carbon chain difference × trial block 3-factor analyses of variance in Experiment 2
| Chemical class | Age | Carbon chain difference | Trial block | Age × carbon chain difference × trial block |
|---|---|---|---|---|
| Alcohols | F(1,24) = 6.01, p = 0.02 | F(2,48) = 373.75, p < 0.001 | F(9,216) = 113.92, p < 0.001 | F(18,432) = 2.31, p = 0.002 |
| Aldehydes | F(1,14) = 2.51, p = 0.14 | F(2,28) = 280.52, p < 0.001 | F(9,126) = 99.04, p < 0.001 | F(18,252) = 2.21, p = 0.004 |
| Acids | F(1,14) = 4.62, p = 0.05 | F(2,28) = 158.20, p < 0.001 | F(9,126) = 96.10, p < 0.001 | F(18,252) = 1.78, p = 0.03 |
Values in bold indicate statistically significant effects.
Consistent with this interpretation, young and aged rats generally showed more comparable acquisition of discrimination problems involving structurally dissimilar odorants (Fig. 3). No main effects or interactions involving age were observed on 2 of the 3 discrimination problems tested (see Table 5 for ANOVA results). A main effect of age was observed on the citral versus 2-heptanone discrimination problem; however, both young and aged rats performed at greater than 90% accuracy on this problem after the first trial block.
Fig. 3.
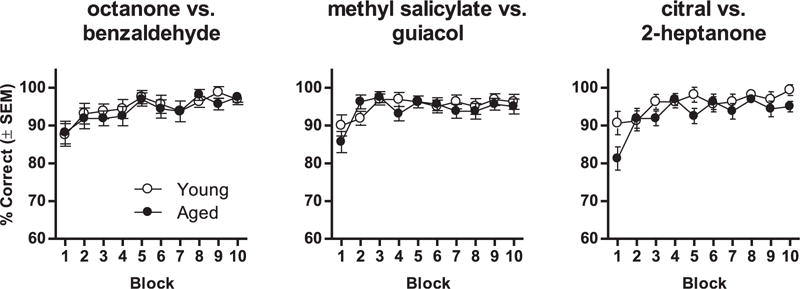
Effects of age on learning of olfactory discrimination problems involving unrelated odorants. Graphs show the percentage of correct trials (in 20-trial blocks) on 3 discrimination problems involving dissimilar odorants. Rats were tested on each discrimination problem once. Aged rats were impaired relative to young on only 1 of the 3 discrimination problems (citral vs. 2-heptanone). Abbreviation: SEM, standard error of the mean.
Table 5.
Comparisons of discrimination problems involving structurally unrelated odorants
| Discrimination | Age | Trial block | Age × trial block |
|---|---|---|---|
| 2-octanone versus benzaldehyde | F(1,14) = 0.48, p = 0.50 |
F(9,126) = 3.72, p < 0.001 |
F(9,126) = 0.23, p = 0.99 |
| Methyl salicylate versus guaiacol | F(1,14) = 1.21, p = 0.29 |
F(9,126) = 3.65, p < 0.001 |
F(9,126) = 0.85, p = 0.57 |
| Citral versus 2-heptanone |
F(1,14) = 5.15, p = 0.04 |
F(9,126) = 6.11, p < 0.001 |
F(9,126) = 1.34, p = 0.22 |
Values in bold indicate statistically significant effects.
One interpretation of the data described in the preceding paragraphs is that the disproportionally worse performance of aged rats may not be specific to perceptual similarity but instead may broadly reflect a learning impairment in aged rats associated with problem difficulty. To address this possibility, we took advantage of the fact that aldehyde discrimination problems were more difficult for even young rats to learn in comparison to the other 2 chemical classes, as evident by lower accuracy on aldehyde discrimination problems relative to alcohols or acids. If aged rats show global learning impairments on more difficult problems, one would expect a disproportionate impairment in their ability to learn the more difficult aldehyde problems. To test this possibility, data were collapsed across trial blocks (Fig. 4), and a three-factor (age × carbon chain difference × chemical class) repeated-measures ANOVA was conducted on data from cohort B (i.e., young and aged rats that were tested across all 3 chemical classes). This analysis revealed a significant main effect of chemical class on task performance [F(2,28) = 66.48, p < 0.001]. Importantly, however, no significant interactions were observed between age and chemical class [chemical class × age: F(2,28) = 0.30, p = 0.74; chemical class × age × carbon chain difference: F(4,56) = 0.79, p = 0.54]. These data indicate that the disproportionate deficits in aged rats on perceptually similar problems are not attributable to global learning impairments, as aged rats perform comparably to young on other types of problems that differ in difficulty.
Fig. 4.
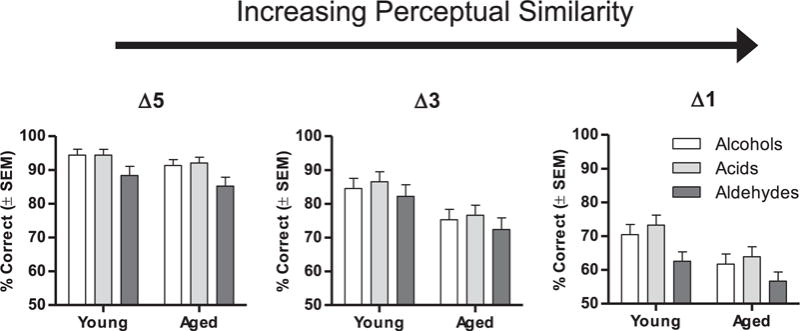
Effects of chemical class and carbon chain difference on olfactory discrimination performance. Graphs show the percentage of correct trials (averaged across blocks) from each of the 3 chemical classes across discrimination problems differing in perceptual similarity, in young and aged rats. Δ5, Δ3, and Δ1 refer to differences of 5, 3, and 1 carbon atoms between the 2 odorants in each problem. Note that only data from rats in cohort B (n = 8 young, n = 8 aged, which were tested on all 3 chemical classes) are shown. Performance was worse on problems in the aldehyde series compared with alcohols and acids, but the magnitude of this difference did not vary as a function of age. Abbreviation: SEM, standard error of the mean.
3.2.2. Odor detection threshold
To determine whether the olfactory discrimination deficits in aged rats were influenced by reductions in the thresholds for detecting olfactory stimuli, rats were tested for their ability to detect and respond to decreasing concentrations of odorants in each of the chemical classes used in the 2 cohorts. As shown in Table 6, there were no age differences in detection threshold for any of the odorants tested (ts < 1.22, ps > 0.24), demonstrating that odor detection abilities were intact in aged rats.
Table 6.
Odor detection thresholds in Experiment 2 (by age)
| Cohort | Odorant | Young | Aged | t test |
|---|---|---|---|---|
| Cohort A | Propanol | −8.50 (1.19) | −9.17 (1.19) | t(8) = 0.38, p = 0.72 |
| Pentanol | −9.25 (1.75) | −9.67 (1.33) | t(8) = 0.19, p = 0.85 | |
| Octanol | −10.25 (2.43) | −9.83 (1.33) | t(8) = 0.16, p = 0.87 | |
| Carvone | −9.25 (2.10) | −8.83 (1.14) | t(8) = 0.19, p = 0.85 | |
| Cohort B | Heptanol | −9.25 (1.03) | −10.88 (0.91) | t(14) = 1.18, p = 0.26 |
| Heptanal | −10.38 (1.55) | −9.88 (1.20) | t(14) = 0.26, p = 0.80 | |
| Heptanoic acid | −8.88 (0.40) | −7.88 (0.72) | t(14) = 1.22, p = 0.24 | |
| Isoamyl acetate | −8.63 (0.91) | −8.13 (0.93) | t(14) = 0.38, p = 0.71 |
3.2.3. Water maze performance
As shown in Fig. 5A, all rats improved across blocks of training trials on the hidden platform version of the Morris water maze task [main effect of trial block, F(3,72) = 36.95, p < 0.001], but aged rats were significantly impaired relative to young [main effect of age, F(1,24) = 12.27, p = 0.002]. Similarly, both young and aged rats showed improved spatial biases across the probe trials that were interpolated through the training protocol [main effect of probe trial: F(3,72) = 16.72, p < 0.001]; however, aged rats showed a significantly less targeted search strategy than young [main effect of age, F(1,24) = 11.53, p = 0.002]. Young and aged rats did not differ in their performance on visible cue training [t(24) = 1.62, p = 0.12].
Fig. 5.
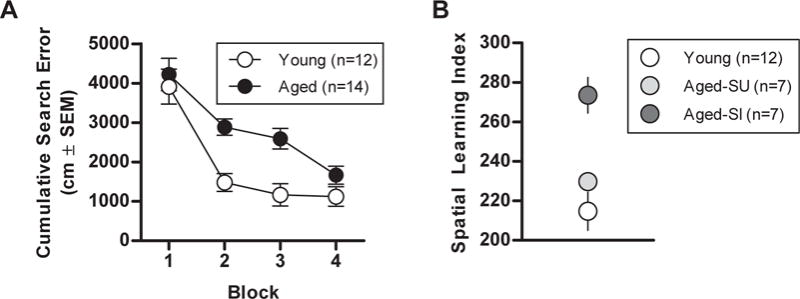
Effects of age on performance in the Morris water maze. Rats received 8 days of training to swim to a hidden platform in the water maze. (A) Performance on acquisition trials, grouped into 5-trial blocks. Aged rats performed significantly worse than young (greater cumulative search error). (B) Performance on interpolated probe trials was used to calculate a “learning index” (see text for details), with higher values indicating less accurate search for the platform location. The learning index was used to divide rats into aged spatially unimpaired (Aged-SU, which performed comparably to young) and aged spatially impaired (Aged-SI, which performed significantly worse than young) subgroups for subsequent analyses of olfactory performance. Abbreviation: SEM, standard error of the mean.
To compare spatial learning and olfactory discrimination abilities, probe trial performance was used to calculate a spatial learning index for each subject (Bizon et al., 2009; Gallagher et al., 1993). On the basis of these spatial learning indices, aged rats were evenly divided into subgroups and designated as either “aged spatially unimpaired” (n = 7) or “aged spatially impaired” (n = 7; Fig. 5B). Comparison of probe trial performance in these cognitive subgroups (cognitive subgroup × probe trial) confirmed that the aged spatially impaired subgroup was significantly impaired relative to both young and aged spatially unimpaired subgroups (main effect of cognitive subgroup, F(2,23) = 12.68, p < 0.001; Tukey’s post hoc, aged spatially impaired vs. young and aged spatially unimpaired, ps < 0.05). In contrast, probe trial performance did not differ between young and aged spatially unimpaired subgroups (p = 0.30).
3.2.4. Relationships between odor discrimination and water maze performance
The cognitive subgroups described in the previous section were used to compare rats’ olfactory discrimination abilities. Comparisons were initially conducted on the alcohol discrimination problems as all rats across cohorts A and B completed this series (Δ5, Δ3, and Δ1; Fig. 6). A 3-factor repeated-measures ANOVA (cognitive subgroup × carbon chain difference × trial block) showed that performance improved across blocks of trials [main effect of trial block, F(9,207) = 112.96, p < 0.001] and was less accurate on more perceptually similar odor discrimination problems [main effect of carbon chain difference, F(2,46) = 393.87, p < 0.001]. Furthermore, there was a main effect of cognitive subgroup [F(2,23) = 12.53, p < 0.001] with post hoc comparisons indicating that the aged spatially impaired subgroup performed significantly worse than both the young and aged spatially unimpaired subgroup (ps < 0.05). The young and aged spatially unimpaired subgroup, however, did not differ in their discrimination abilities (p = 0.93). Further, an interaction between cognitive subgroup × carbon chain difference × trial block was consistent with the disproportionate discrimination impairment in the aged spatially impaired subgroup [F(36,414) = 73.31, p < 0.001]. Similar patterns of results were evident on the aldehyde and acid discrimination problems. Importantly, there were no effects of subgroup on odor detection threshold (Table 7), suggesting that the discrimination deficits in the aged spatially impaired subgroup were not secondary to more fundamental olfactory impairments.
Fig. 6.
Effects of age and spatial learning ability on learning of olfactory discrimination problems varying in perceptual similarity. Graphs show data from Fig. 2 (Experiment 2), with aged rats replotted as a function of their water maze learning index subgroup (aged spatially unimpaired or aged spatially impaired). Δ5, Δ3, and Δ1 refer to differences of 5, 3, and 1 carbon atoms between the 2 odorants in each problem. Across chemical classes, rats classified as aged spatially impaired (Aged-SI) on the basis of their water maze performed worse than young and aged spatially unimpaired (Aged-SU) rats, and the magnitude of this impairment was greater on problems involving more perceptually similar stimuli. Abbreviation: SEM, standard error of the mean.
Table 7.
Odor detection thresholds in Experiment 2 (by cognitive subgroup)
| Cohort | Odorant | Young | Aged unimpaired | Aged impaired | ANOVA |
|---|---|---|---|---|---|
| Cohort A | Propanol | −8.50 (1.19) | −10.33 (0.67) | −8.00 (2.31) | F(2,7) = 0.63, p = 0.56 |
| Pentanol | −9.25 (1.75) | −11.67 (0.88) | −7.67 (2.03) | F(2,7) = 1.29, p = 0.33 | |
| Octanol | −10.25 (2.43) | −10.33 (1.76) | −9.33 (2.33) | F(2,7) = 0.06, p = 0.95 p | |
| Carvone | −9.25 (2.10) | −10.67 (0.88) | −7.00 (1.53) | F(2,7) = 1.01, p = 0.41 | |
| Cohort B | Heptanol | −9.25 (1.03) | −12.00 (1.22) | −9.75 (1.25) | F(2,13) = 1.40, p = 0.28 |
| Heptanal | −10.38 (1.55) | −9.50 (1.55) | −10.25 (2.06) | F(2,13) = 0.07, p = 0.94 | |
| Heptanoic acid | −8.88 (0.40) | −7.25 (1.31) | −8.50 (0.65) | F(2,13) = 1.34, p = 0.30 | |
| Isoamyl acetate | −8.63 (0.91) | −8.50 (1.44) | −7.75 (1.38) | F(2,13) = 0.15, = 0.86 |
To further test the relationships identified in the subgroup analysis, Pearson’s correlations were conducted on individual values for olfactory discrimination (percent accuracy averaged across all trial blocks on alcohol problems) and spatial learning (spatial learning index) performance. Among aged rats, significant relationships were observed between discrimination ability and spatial learning across all problems (Δ5, Δ3, and Δ1), with the strength of these correlations increasing as a function of perceptual similarity (Fig. 7). Significant relationships were also observed among young rats at the Δ3 and Δ1 but not the Δ5 discrimination problems (Table 8). As can be observed in Fig. 7, the spatial learning index of 1 rat in each of the young and aged groups could be considered an outlier relative to the rest of their respective groups (with spatial learning indices more than twice the standard deviations of their respective age group means). To determine whether the relationships between spatial learning and olfactory discrimination performance were driven by these outlying values, the correlations were also calculated with these values removed. As shown in Table 8, none of the correlations in the young group remained significant (ps > 0.12), whereas the correlations in the aged group all remained reliable (ps < 0.05), supporting the robustness of the relationship between olfactory discrimination and spatial learning among aged rats. Finally, a comparison of olfactory discrimination and performance on the visible cue version of the water maze revealed no significant relationships in either age group (Table 8).
Fig. 7.
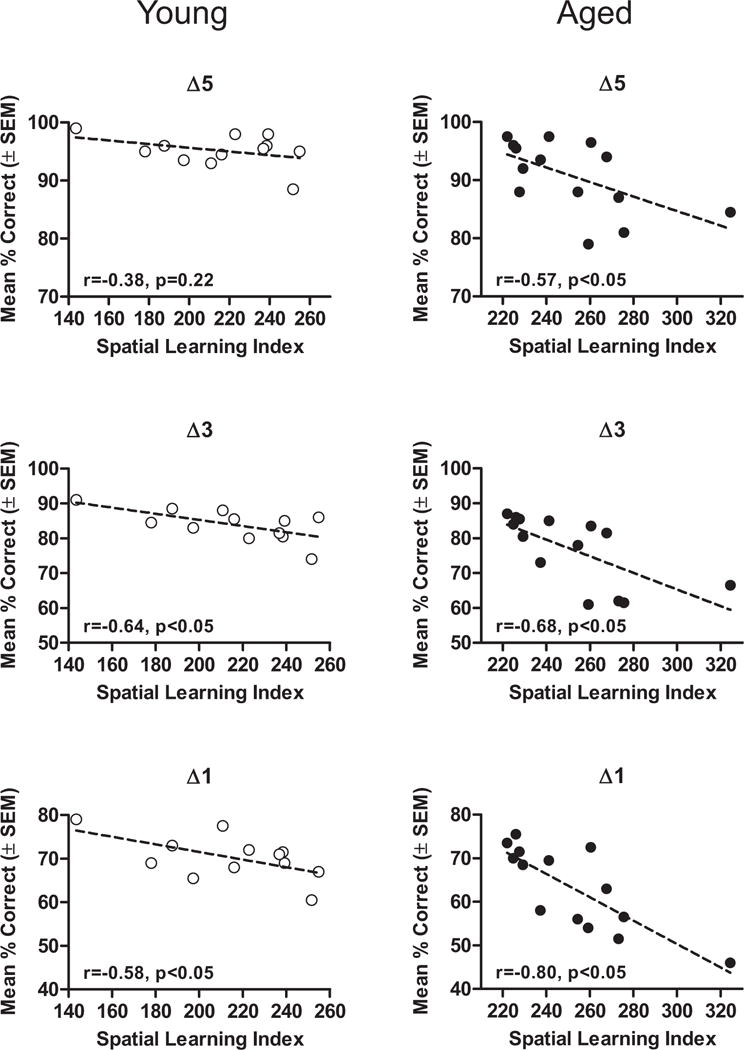
Relationships between spatial and olfactory discrimination learning across multiple chemical classes in young and aged rats. Scatterplots show young and aged rats’ olfactory discrimination learning (percent correct averaged across all trial blocks from the alcohol series) on problems varying in perceptual similarity, plotted as a function of spatial learning index in the water maze. Δ5, Δ3, and Δ1 refer to differences of 5, 3, and 1 carbon atoms between the 2 odorants in each problem. Relationships between olfactory discrimination and spatial learning in the 2 tasks were stronger in aged compared with young rats, and greater on olfactory discrimination problems involving more perceptually similar stimuli (see also Table 8). Abbreviation: SEM, standard error of the mean.
Table 8.
Correlations between water maze and olfactory discrimination learning
| Task | Age group | Δ5 | Δ3 | Δ1 |
|---|---|---|---|---|
| Spatial water maze (all rats) | Young | r = −0.38, p = 0.22 | r = −0.64, p= 0.02 | r = −0.58, p= 0.049 |
| Aged | r =−0.57, p = 0.03 | r = −0.68, p = 0.008 | r = −0.80, p = 0.001 | |
| Spatial water maze (outliers removed) | Young | r = −0.13, p = 0.70 | r = −0.49, p= 0.13 | r = −0.34, p= 0.31 |
| Aged | r =−0.56, p= 0.048 | r = −0.71, p= 0.007 | r = −0.72, p= 0.006 | |
| Visible cue water maze | Young | r = 0.19, p= 0.55 | r = 0.24, p= 0.45 | r = 0.19, p= 0.55 |
| Aged | r = −0.38, p= 0.18 | r = −0.25, p= 0.39 | r = −0.18, p= 0.55 |
Values in bold indicate statistically significant effects.
As previously mentioned in Section 3.2.1, it is possible that the disproportionately worse performance observed in the aged-SI rats as a function of perceptual similarity reflects a more global learning impairment (i.e., this subgroup of rats may be slower to learn overall, and a difference in learning rate could be driving the differences in discrimination performance observed as a function of varying perceptual similarity). To address this issue, learning rates of the cognitive subgroups were assessed by comparing performance on the first and last block of trials in each discrimination problem. This analysis was conducted on data from the acid class of odorants, on which all cognitive subgroups reached asymptotic performance (defined as no main effect of trial block or cognitive subgroup × trial block interaction across the final 3 blocks; Fs < 2.29, ps > 0.09) on each problem type (Δ5, Δ3, and Δ1). Main effects of both cognitive subgroup (Fs > 6.38, ps < 0.01) and trial block (Fs > 182.65, ps < 0.001) were observed on both Δ3 and Δ1 discrimination problems; however, there were no interactions between cognitive subgroup and trial block on these problems (Fs < 0.93, ps > 0.42). This analysis indicates that although aged-SI rats performed disproportionately worse than young and aged-SU groups as a function of perceptual similarity, their learning rate, even on the most difficult problem (i.e., Δ1) did not differ from the other 2 cognitive subgroups (Fig. 8). These results argue against the interpretation that global learning impairments in aged-SI rats account for the performance differences observed as a function of perceptual similarity. It is notable that a cognitive subgroup × trial block interaction was observed on the Δ5 discrimination problem (F = 6.44, p = 0.01); however, as shown in Fig. 6, this interaction appeared to be carried by a ceiling effect on discrimination performance (i.e., all 3 subgroups performed at nearly 100% accuracy by the second half of the session). It is notable that across Δ5 discrimination problems, the improvement between blocks 1 and 2 was nearly identical across groups.
Fig. 8.
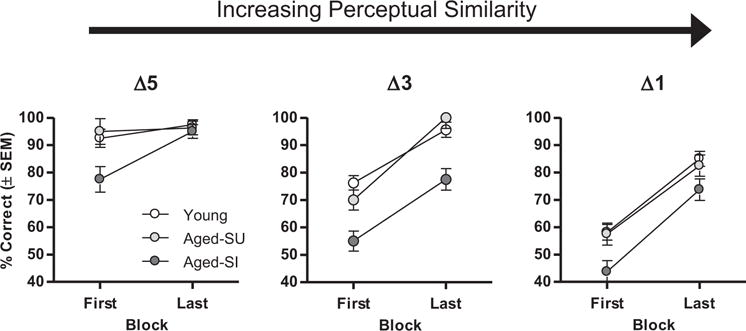
Effects of perceptual similarity and cognitive subgroup on olfactory discrimination learning rate. Graphs show data from the first and last blocks of trials in the acid chemical series in Experiment 2, plotted as a function of cognitive subgroup (young, aged spatially unimpaired (Aged-SU), and aged spatially impaired (Aged-SI)). Δ5, Δ3, and Δ1 refer to differences of 5, 3, and 1 carbon atoms between the 2 odorants in each problem. Aged spatially impaired rats performed worse than the other 2 groups, but on Δ3 and Δ1 discriminations, their rates of acquisition (slopes of the lines from first to last block) did not differ. Acquisition rates did differ on the Δ5 problem, likely due to a ceiling effect on task performance. Abbreviation: SEM, standard error of the mean.
4. Discussion
4.1. Age-related deficits in olfactory discrimination learning
The present findings extend previous work from our laboratories demonstrating age-associated deficits in olfactory discrimination learning. Specifically, using rigorous psychophysical approaches that offer a high degree of stimulus control and the ability to systemically vary perceptual similarity, the results show that aging disproportionately influences the ability to discriminate similar olfactory stimuli. Importantly, these impairments do not appear to be driven by sensory deficits in the aged rats, as aged spatially impaired rats could detect and respond to decreasing concentrations of odorants on par with young subjects. Moreover, these deficits do not appear to reflect a global learning impairment in aged rats such that they are simply less able to learn more “difficult” problems. Two lines of evidence support this contention. First, the aldehyde class of odorants was more difficult to learn, as even young rats performed less accurately on aldehyde problems relative to the other 2 chemical classes. If aged rats simply were impaired in discriminating “difficult” problems, aged rats should also have been disproportionately impaired relative to young in learning aldehyde problems and this was not observed. Second, the learning rates of aged spatially impaired rats were not different from young and aged spatially unimpaired rats, demonstrating that disproportionate deficits on perceptually similar problems in the aged spatially impaired rats were specific to perceptual similarity and not attributable to slower learning. Considered together, these findings provide a novel behavioral model for investigation of the neurobiological underpinnings of perceptual discrimination deficits in aging and their contributions to cognitive dysfunction.
The finding that age-related deficits in olfactory discrimination are most robust with perceptually similar stimuli may explain some discrepant findings in the olfactory and aging literature. Several studies report intact olfactory discrimination abilities in aged rats (Barense et al., 2002; Kraemer and Apfelbach, 2004; Schoenbaum et al., 2002), whereas others have reported deficits (Prediger et al., 2005; Roman et al., 1996). In fact, our own laboratories previously reported an age-related decline in olfactory discriminations using a task in which rats learned to dig in small pots to obtain food rewards on the basis of structurally unrelated odor cues (LaSarge et al., 2007). Comparing these previous results to those in the present study, it is important to note that our previous study only assessed “trials to criterion”, with the total number of trials completed by each subject ranging from 10 to 40. In contrast, in the current experiments, rats completed 200 trials on each problem, regardless of choice accuracy. Although both young and aged rats in the present study ultimately achieved a high level of accuracy (>90%) on problems that included structurally dissimilar odors, close examination of performance reveals that aged rats did lag modestly behind young rats early in training on some discrimination problems (e.g., in the first block of trials on the citral vs. 2-heptanone discrimination problem; see Fig. 3). This modest deficit in acquisition observed on some discrimination problems with dissimilar odors may also be more evident in F344 rats than in other strains, as discrimination between structurally unrelated odors using a trials to criterion measure has been evaluated in Long-Evans rats with no age differences reported (Barense et al., 2002; Schoenbaum et al., 2002).
A clear result of the present study is that comparisons of performance across structurally unrelated, Δ5, and Δ3 problems revealed progressively more robust age-related impairments. Although aged rats were also consistently impaired relative to young on Δ1 problems, the greatest differences between young and aged rat performance were observed on Δ3 problems. The failure to observe greater deficits with the even more structurally similar Δ1 problems is likely attributable to the overall increased difficulty of these discriminations, with even young rats nearing chance performance in some chemical classes (e.g., aldehydes). As such, although age differences were still evident, a “floor effect” prohibited detecting progressively greater deficits. These data suggest that the optimal parametric space for maximizing detection of age-related deficits in discrimination abilities in F344 rats is achievable with problems in which olfactory stimuli differ by 3 carbon atoms.
The age-related discrimination deficits were not evident using a longitudinal design (Experiment 1), in which performance on the same discrimination problems (Δ5, Δ3, and Δ1) was assessed at 3 different time points (every 6 months). Although at all time points, significant differences in performance were observed as a function of perceptual similarity, rats showed overall improved discrimination performance across time points, even against the context of advancing age (i.e., at 12 and 18 months). These findings highlight several preserved features of cognition in aging and define important parameters for maximizing the detection of cognitive decline using olfactory cues in aged rats. Specifically, findings from the longitudinal experiment demonstrate that F344 rats are not impaired in procedural aspects of the discrimination learning task with advancing age. Moreover, the fact that performance in this cohort was better at 18 months in comparison to 6 months indicates that, once learned, olfactory information is retained with a high degree of accuracy across the lifespan. The fact that despite the improvements in discrimination performance across testing time points, rats still showed systematic reductions in accuracy as a function of perceptual similarity, suggests that the performance differences resulting from perceptual similarity are independent of memory for the discriminations across time points. Considered together, data from both Experiments 1 and 2 are supportive of the interpretation that deficits in olfactory discrimination as a function of perceptual similarity in aged rats reflect perceptual rather than sensory or learning/mnemonic deficits. These data further indicate that longitudinal experiments that are designed to detect such deficits will require the use of novel stimuli at each testing time point. To that end, data from Experiment 2 define a range of stimulus sets that could be useful in future longitudinal studies.
4.2. Relationships between olfactory discrimination and spatial learning in aging
A primary goal of this work was to determine the extent to which age-related olfactory discrimination learning deficits associate with well-described deficits in spatial learning (Bizon et al., 2009; Burke and Barnes, 2006; Gallagher et al., 1993). In accord with the extant literature, aged rats in the present study were impaired relative to young on the spatial (hidden platform) but not the cued (visible platform) version of the water maze. Notably, however, a range of performance was observed among aged rats such that some performed on par with young whereas others performed outside this range, demonstrating impairment (Bizon et al., 2009; Gallagher et al., 1993). Consistent with a previous study from our laboratory that employed the “digging” olfactory discrimination task described in Section 4.1 (LaSarge et al., 2007), olfactory discrimination and spatial learning abilities were strongly associated among aged rats in the present study. The converging results obtained with 2 very different olfactory discrimination learning procedures highlight the robust nature of the relationship between olfactory discrimination and spatial learning. These findings are also consistent with a large literature demonstrating associations between olfactory impairments and age-related cognitive decline in humans (Devanand et al., 2010; Doty and Kamath, 2014; Graves et al., 1999; Mobley et al., 2014; Wilson et al., 2007). Unlike in rats, however, the nature of the olfactory deficits in humans is more difficult to define purely in terms of discrimination learning. Specifically, deficits in odor detection can emerge from a variety of environmental factors (e.g., smoking, disease, or cumulative exposure to environmental pollution) and can be difficult to dissociate from discrimination learning impairments (Doty and Kamath, 2014).
In the present study, the strength of the relationship between olfactory discrimination and spatial learning deficits in aged rats increased as a function of perceptual similarity (see Fig. 7 and Table 8). Although it is possible that this relationship reflects independent but parallel declines in neural circuitry mediating these 2 aspects of cognition, a more parsimonious explanation is that they reflect dysfunction within a single neural system. One possibility lies in the cholinergic innervation of the olfactory bulb and hippocampus that arises in the basal forebrain (Woolf et al., 1984; Záborszky et al., 1986). Cholinergic signaling within these structures modulates olfactory discrimination and water maze learning. Although not extensively examined in the olfactory bulb, age-associated changes in hippocampal cholinergic markers are strongly correlated with impaired water maze learning (Kenney and Gould, 2008; Linster and Cleland, 2016; Mandairon et al., 2011; Parent and Baxter, 2004; Schliebs and Arendt, 2011; Zhang et al., 2007). Interestingly, lesions of its cholinergic afferents impair the ability of hippocampus to form stable representations of novel but perceptually similar environments (Ikonen et al., 2002), and blockade of nicotinic cholinergic receptors within olfactory bulb impairs discrimination between perceptually similar odors (Devore et al., 2014). These data could suggest that an age-related loss of cholinergic afferents/signaling mediates the inability to discriminate among perceptually similar stimuli. A caveat to this idea, however, is that cholinergic denervation of hippocampus does not typically impair water maze performance [see (Parent and Baxter, 2004) for review], and age-associated reductions in basal forebrain cholinergic neuron number are not associated with impaired water maze performance in Fischer 344 rats using the same water maze task design employed in the present study (Bañuelos et al., 2013).
With respect to the neural circuits that may be mediating the age-associated deficits reported herein, it is notable that aging is accompanied by impairments in discriminating among similar stimuli across sensory modalities. Indeed, the pattern of olfactory discrimination deficits reported in the present study parallels those from studies of visual object discrimination learning in aged subjects (Stark et al., 2013). Aged rats and monkeys are able to discriminate distinct visual objects that have minimal feature overlap on par with young subjects. In contrast, aged subjects are impaired relative to young in their ability to discriminate between objects that have substantial feature overlap (Burke et al., 2011). Similarly, aged human subjects are impaired in discriminating between perceptually similar visual stimuli (Ryan et al., 2012; Stark et al., 2010; Toner et al., 2009; Yassa et al., 2011). These deficits across sensory modalities may reflect age-related dysfunction in higher order cortical regions involved in multisensory perception and encoding. The perirhinal cortex is a likely candidate for such a brain region, as it receives highly processed input from all sensory modalities, including the olfactory system. Via reciprocal projections with both piriform cortex and hippocampus, the perirhinal cortex can influence how olfactory cues are perceived and encoded and can relay highly processed olfactory representations integral to memory. Indeed, perirhinal cortical neurons are selectively active during both olfactory recognition (Otto and Eichenbaum, 1992; Young et al., 1997) and object exploration tasks (Burke et al., 2012b; Deshmukh et al., 2012).
In addition to perceptual discrimination, the perirhinal cortex is also critical for explicit memory (Buffalo et al., 1999), and lesions of this structure can produce impairments in water maze learning, particularly under conditions that place high demands on visual/spatial cue differentiation (Aggleton et al., 2004). These data would suggest that the perirhinal cortex is a bridge between perceptual processing and mnemonic function (Bussey et al., 2005). Along these lines, more recent data have also shown that the perirhinal cortex is critical for resolving the interference that accumulates over long delays between encoding and retrieval, as well as the interference that occurs when an animal must discriminate between 2 stimuli that share features without a delay [for review, see (Kent et al., 2016)]. In fact, perirhinal cortical lesions produce false memories for novel objects following delays because animals are unable to disambiguate the novel stimulus from the sensory features of irrelevant stimuli encountered during the delay (McTighe et al., 2010). Perirhinal cortical lesions in humans (Barense et al., 2005), monkeys (Bussey et al., 2002), and rats (Bartko et al., 2007) also produce deficits in the ability to distinguish between stimuli that share features, even for simultaneous presentations. Notably, aged rats also show an increased incidence of false memories for novel objects following delays (Burke et al., 2010), as well as a reduced ability to discriminate between similar objects (Burke et al., 2011). The present finding that aged rats are selectively impaired at discriminating between odorants with similar structure suggests that decreasing the difference in the length of the carbon chain backbone between 2 odorants within a chemical class may be comparable to increasing the number of shared features for visual stimuli. Together these data as a whole might suggest that a common mechanism helps resolve interference introduced by either lengthening delays or increasing the perceptual similarity between stimuli and that this mechanism becomes compromised with age. Such a common mechanism may account for the similar relationships among aged rats between water maze performance and both delayed odor recall (Robitsek et al., 2008) and discrimination between perceptually similar odors (present study).
In addition to the behavioral data that implicate perirhinal cortical dysfunction as a mechanism for age-related deficits in perceptual discrimination, there are several neurobiological changes within this brain region that could explain the current data. The perirhinal cortex of aged animals undergoes numerous changes that include reduced neural activity in response to stimuli (Burke et al., 2012a, 2014; Ryan et al., 2012) and alterations in protein expression (Liu et al., 2008a,b, 2009; Moyer et al., 2011), which could account for age-related deficits in perceptual discrimination. A next step in this line of research will be to determine if the individual differences in olfactory discrimination deficits reported here are paralleled in other sensory modalities, as one would expect as a consequence of age-related alterations in perirhinal cortical encoding. Irrespective, the behavioral model (which offers robust stimulus control and translational potential) and data described in the present study should provide an important foundation for better elucidating the contribution of perceptual discrimination deficits to memory decline as well as revealing the underlying circuitry that mediates such decline.
Supplementary Material
Acknowledgments
The authors thank Ryan Gilbert, Michelle Lyman, Olivia Munizza, and Ethan Windham for technical assistance, and Dr. Barry Ache for providing some of the chemical odorants. This work was supported by NIH grant R01AG029421 and the McKnight Brain Research Foundation to J. L. B.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neurobiolaging.2017.01.023.
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose.
References
- Aggleton JP, Kyd RJ, Bilkey DK. When is the perirhinal cortex necessary for the performance of spatial memory tasks? Neurosci Biobehav Rev. 2004;28:611–624. doi: 10.1016/j.neubiorev.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Bañuelos C, LaSarge CL, McQuail JA, Hartman JJ, Gilbert RJ, Ormerod BK, Bizon JL. Age-related changes in rostral basal forebrain cholinergic and GABAergic projection neurons: relationship with spatial impairment. Neurobiol Aging. 2013;34:845–862. doi: 10.1016/j.neurobiolaging.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Bussey TJ, Lee AC, Rogers TT, Davies RR, Saksida LM, Murray EA, Graham KS. Functional specialization in the human medial temporal lobe. J Neurosci. 2005;25:10239–10246. doi: 10.1523/JNEUROSCI.2704-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Fox MT, Baxter MG. Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learn Mem. 2002;9:191–201. doi: 10.1101/lm.48602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, Bussey TJ. Perceptual functions of perirhinal cortex in rats: zero-delay object recognition and simultaneous oddity discriminations. J Neurosci. 2007;27:2548–2559. doi: 10.1523/JNEUROSCI.5171-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, LaSarge CL, Montgomery KS, McDermott AN, Setlow B, Griffith WH. Spatial reference and working memory across the lifespan of male Fischer 344 rats. Neurobiol Aging. 2009;30:646–655. doi: 10.1016/j.neurobiolaging.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodyak N, Slotnick B. Performance of mice in an automated olfactometer: odor detection, discrimination and odor memory. Chem Senses. 1999;24:637–645. doi: 10.1093/chemse/24.6.637. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Ramus SJ, Clark RE, Teng E, Squire LR, Zola SM. Dissociation between the effects of damage to perirhinal cortex and area TE. Learn Mem. 1999;6:572–599. doi: 10.1101/lm.6.6.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Burke SN, Hartzell AL, Lister JP, Hoang LT, Barnes CA. Layer V perirhinal cortical ensemble activity during object exploration: a comparison between young and aged rats. Hippocampus. 2012a;22:2080–2093. doi: 10.1002/hipo.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Maurer AP, Hartzell AL, Nematollahi S, Uprety A, Wallace JL, Barnes CA. Representation of three-dimensional objects by the rat perirhinal cortex. Hippocampus. 2012b;22:2032–2044. doi: 10.1002/hipo.22060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Maurer AP, Nematollahi S, Uprety A, Wallace JL, Barnes CA. Advanced age dissociates dual functions of the perirhinal cortex. J Neurosci. 2014;34:467–480. doi: 10.1523/JNEUROSCI.2875-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Wallace JL, Hartzell AL, Nematollahi S, Plange K, Barnes CA. Age-associated deficits in pattern separation functions of the perirhinal cortex: a cross-species consensus. Behav Neurosci. 2011;125:836–847. doi: 10.1037/a0026238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Wallace JL, Nematollahi S, Uprety AR, Barnes CA. Pattern separation deficits may contribute to age-associated recognition impairments. Behav Neurosci. 2010;124:559–573. doi: 10.1037/a0020893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. Perirhinal cortex resolves feature ambiguity in complex visual discriminations. Eur J Neurosci. 2002;15:365–374. doi: 10.1046/j.0953-816x.2001.01851.x. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. The perceptual-mnemonic/feature conjunction model of perirhinal cortex function. Q J Exp Psychol B. 2005;58:269–282. doi: 10.1080/02724990544000004. [DOI] [PubMed] [Google Scholar]
- Can Güven S, Laska M. Olfactory sensitivity and odor structure-activity relationships for aliphatic carboxylic acids in CD-1 mice. PLoS One. 2012;7:e34301. doi: 10.1371/journal.pone.0034301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland TA, Morse A, Yue EL, Linster C. Behavioral models of odor similarity. Behav Neurosci. 2002;116:222–231. doi: 10.1037//0735-7044.116.2.222. [DOI] [PubMed] [Google Scholar]
- Deshmukh SS, Johnson JL, Knierim JJ. Perirhinal cortex represents nonspatial, but not spatial, information in rats foraging in the presence of objects: comparison with lateral entorhinal cortex. Hippocampus. 2012;22:2045–2058. doi: 10.1002/hipo.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanand DP, Tabert MH, Cuasay K, Manly JJ, Schupf N, Brickman AM, Andrews H, Brown TR, DeCarli C, Mayeux R. Olfactory identification deficits and MCI in a multi-ethnic elderly community sample. Neurobiol Aging. 2010;31:1593–1600. doi: 10.1016/j.neurobiolaging.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devore S, de Almeida L, Linster C. Distinct roles of bulbar muscarinic and nicotinic receptors in olfactory discrimination learning. J Neurosci. 2014;34:11244–11260. doi: 10.1523/JNEUROSCI.1499-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, Kamath V. The influences of age on olfaction: a review. Front Psychol. 2014;5:20. doi: 10.3389/fpsyg.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulay MF, Murphy C. Olfactory acuity and cognitive function converge in older adulthood: support for the common cause hypothesis. Psychol Aging. 2002;17:392–404. [PubMed] [Google Scholar]
- Eichenbaum H, Clegg RA, Feeley A. Reexamination of functional subdivisions of the rodent prefrontal cortex. Exp Neurol. 1983;79:434–451. doi: 10.1016/0014-4886(83)90224-8. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Robitsek RJ. Olfactory memory: a bridge between humans and animals in models of cognitive aging. Ann N Y Acad Sci. 2009;1170:658–663. doi: 10.1111/j.1749-6632.2009.04012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falasconi M, Gutierrez-Galvez A, Leon M, Johnson BA, Marco S. Cluster analysis of rat olfactory bulb responses to diverse odorants. Chem Senses. 2012;37:639–653. doi: 10.1093/chemse/bjs045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Foster TC, Kumar A. Susceptibility to induction of long-term depression is associated with impaired memory in aged Fischer 344 rats. Neurobiol Learn Mem. 2007;87:522–535. doi: 10.1016/j.nlm.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gracian EI, Shelley LE, Morris AM, Gilbert PE. Age-related changes in place learning for adjacent and separate locations. Neurobiol Aging. 2013;34:2304–2309. doi: 10.1016/j.neurobiolaging.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves AB, Bowen JD, Rajaram L, McCormick WC, McCurry SM, Schellenberg GD, Larson EB. Impaired olfaction as a marker for cognitive decline: interaction with apolipoprotein E epsilon4 status. Neurology. 1999;53:1480–1487. doi: 10.1212/wnl.53.7.1480. [DOI] [PubMed] [Google Scholar]
- Ho SL, Johnson BA, Leon M. Long hydrocarbon chains serve as unique molecular features recognized by ventral glomeruli of the rat olfactory bulb. J Comp Neurol. 2006;498:16–30. doi: 10.1002/cne.20973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen S, McMahan R, Gallagher M, Eichenbaum H, Tanila H. Cholinergic system regulation of spatial representation by the hippocampus. Hippocampus. 2002;12:386–397. doi: 10.1002/hipo.1109. [DOI] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol Neurobiol. 2008;38:101–121. doi: 10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent BA, Hvoslef-Eide M, Saksida LM, Bussey TJ. The representational-hierarchical view of pattern separation: not just hippocampus, not just space, not just memory? Neurobiol Learn Mem. 2016;129:99–106. doi: 10.1016/j.nlm.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Kraemer S, Apfelbach R. Olfactory sensitivity, learning and cognition in young adult and aged male Wistar rats. Physiol Behav. 2004;81:435–442. doi: 10.1016/j.physbeh.2004.01.012. [DOI] [PubMed] [Google Scholar]
- LaSarge CL, Montgomery KS, Tucker C, Slaton GS, Griffith WH, Setlow B, Bizon JL. Deficits across multiple cognitive domains in a subset of aged Fischer 344 rats. Neurobiol Aging. 2007;28:928–936. doi: 10.1016/j.neurobiolaging.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Laska M. Olfactory sensitivity and odor structure-activity relationships for aliphatic ketones in CD-1 mice. Chem Senses. 2014;39:415–424. doi: 10.1093/chemse/bju011. [DOI] [PubMed] [Google Scholar]
- Laska M, Rivas Bautista RM, Hernandez Salazar LT. Olfactory sensitivity for aliphatic alcohols and aldehydes in spider monkeys (Ateles geoffroyi) Am J Phys Anthropol. 2006;129:112–120. doi: 10.1002/ajpa.20252. [DOI] [PubMed] [Google Scholar]
- Laska M, Rosandher A, Hommen S. Olfactory discrimination of aliphatic odorants at 1 ppm: too easy for CD-1 mice to show odor structure-activity relationships? J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008;194:971–980. doi: 10.1007/s00359-008-0370-y. [DOI] [PubMed] [Google Scholar]
- Linster C, Cleland TA. Neuromodulation of olfactory transformations. Curr Opin Neurobiol. 2016;40:170–177. doi: 10.1016/j.conb.2016.07.006. [DOI] [PubMed] [Google Scholar]
- Liu P, Chary S, Devaraj R, Jing Y, Darlington CL, Smith PF, Tucker IG, Zhang H. Effects of aging on agmatine levels in memory-associated brain structures. Hippocampus. 2008a;18:853–856. doi: 10.1002/hipo.20448. [DOI] [PubMed] [Google Scholar]
- Liu P, Gupta N, Jing Y, Zhang H. Age-related changes in polyamines in memory-associated brain structures in rats. Neuroscience. 2008b;155:789–796. doi: 10.1016/j.neuroscience.2008.06.033. [DOI] [PubMed] [Google Scholar]
- Liu P, Jing Y, Zhang H. Age-related changes in arginine and its metabolites in memory-associated brain structures. Neuroscience. 2009;164:611–628. doi: 10.1016/j.neuroscience.2009.08.029. [DOI] [PubMed] [Google Scholar]
- Lu XC, Slotnick BM, Silberberg AM. Odor matching and odor memory in the rat. Physiol Behav. 1993;53:795–804. doi: 10.1016/0031-9384(93)90191-h. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Peace ST, Boudadi K, Boxhorn CE, Narla VA, Suffis SD, Cleland TA. Compensatory responses to age-related decline in odor quality acuity: cholinergic neuromodulation and olfactory enrichment. Neurobiol Aging. 2011;32:2254–2265. doi: 10.1016/j.neurobiolaging.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTighe SM, Cowell RA, Winters BD, Bussey TJ, Saksida LM. Paradoxical false memory for objects after brain damage. Science. 2010;330:1408–1410. doi: 10.1126/science.1194780. [DOI] [PubMed] [Google Scholar]
- Mobley AS, Rodriguez-Gil DJ, Imamura F, Greer CA. Aging in the olfactory system. Trends Neurosci. 2014;37:77–84. doi: 10.1016/j.tins.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Takahashi YK, Igarashi KM, Yamaguchi M. Maps of odorant molecular features in the mammalian olfactory bulb. Physiol Rev. 2006;86:409–433. doi: 10.1152/physrev.00021.2005. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum RS. The cognitive neuroscience of remote episodic, semantic and spatial memory. Curr Opin Neurobiol. 2006;16:179–190. doi: 10.1016/j.conb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Furtak SC, McGann JP, Brown TH. Aging-related changes in calcium-binding proteins in rat perirhinal cortex. Neurobiol Aging. 2011;32:1693–1706. doi: 10.1016/j.neurobiolaging.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto T, Eichenbaum H. Complementary roles of the orbital prefrontal cortex and the perirhinal-entorhinal cortices in an odor-guided delayed-nonmatching-to-sample task. Behav Neurosci. 1992;106:762–775. doi: 10.1037//0735-7044.106.5.762. [DOI] [PubMed] [Google Scholar]
- Parent MB, Baxter MG. Septohippocampal acetylcholine: involved in but not necessary for learning and memory? Learn Mem. 2004;11:9–20. doi: 10.1101/lm.69104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prediger RD, Batista LC, Takahashi RN. Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats. Involvement of adenosine A1 and A2A receptors. Neurobiol Aging. 2005;26:957–964. doi: 10.1016/j.neurobiolaging.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Reagh ZM, Roberts JM, Ly M, DiProspero N, Murray E, Yassa MA. Spatial discrimination deficits as a function of mnemonic interference in aged adults with and without memory impairment. Hippocampus. 2014;24:303–314. doi: 10.1002/hipo.22224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitsek RJ, Fortin NJ, Koh MT, Gallagher M, Eichenbaum H. Cognitive aging: a common decline of episodic recollection and spatial memory in rats. J Neurosci. 2008;28:8945–8954. doi: 10.1523/JNEUROSCI.1893-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman FS, Alescio-Lautier B, Soumireu-Mourat B. Age-related learning and memory deficits in odor-reward association in rats. Neurobiol Aging. 1996;17:31–40. doi: 10.1016/0197-4580(95)02030-6. [DOI] [PubMed] [Google Scholar]
- Ronnlund M, Nyberg L, Backman L, Nilsson LG. Stability, growth, and decline in adult life span development of declarative memory: cross-sectional and longitudinal data from a population-based study. Psychol Aging. 2005;20:3–18. doi: 10.1037/0882-7974.20.1.3. [DOI] [PubMed] [Google Scholar]
- Ryan L, Cardoza JA, Barense MD, Kawa KH, Wallentin-Flores J, Arnold WT, Alexander GE. Age-related impairment in a complex object discrimination task that engages perirhinal cortex. Hippocampus. 2012;22:1978–1989. doi: 10.1002/hipo.22069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav Brain Res. 2011;221:555–563. doi: 10.1016/j.bbr.2010.11.058. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent S, Saddoris MP, Gallagher M. Teaching old rats new tricks: age-related impairments in olfactory reversal learning. Neurobiol Aging. 2002;23:555–564. doi: 10.1016/s0197-4580(01)00343-8. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurochem. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura B, Baggio HC, Solana E, Palacios EM, Vendrell P, Bargallo N, Junqué C. Neuroanatomical correlates of olfactory loss in normal aged subjects. Behav Brain Res. 2013;246:148–153. doi: 10.1016/j.bbr.2013.02.025. [DOI] [PubMed] [Google Scholar]
- Slotnick B. Odor-sampling time of mice under different conditions. Chem Senses. 2007;32:445–454. doi: 10.1093/chemse/bjm013. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Lacy JW, Stark CE. A task to assess behavioral pattern separation (BPS) in humans: data from healthy aging and mild cognitive impairment. Neuropsychologia. 2013;51:2442–2449. doi: 10.1016/j.neuropsychologia.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Stark CE. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learn Mem. 2010;17:284–288. doi: 10.1101/lm.1768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learn Mem. 2009;16:338–342. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- Uchida N, Takahashi YK, Tanifuji M, Mori K. Odor maps in the mammalian olfactory bulb: domain organization and odorant structural features. Nat Neurosci. 2000;3:1035–1043. doi: 10.1038/79857. [DOI] [PubMed] [Google Scholar]
- Uttl B, Graf P. Episodic spatial memory in adulthood. Psychol Aging. 1993;8:257–273. doi: 10.1037//0882-7974.8.2.257. [DOI] [PubMed] [Google Scholar]
- Weeden CS, Hu NJ, Ho LU, Kesner RP. The role of the ventral dentate gyrus in olfactory pattern separation. Hippocampus. 2014;24:553–559. doi: 10.1002/hipo.22248. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry. 2007;64:802–808. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Yu L, Bennett DA. Odor identification and mortality in old age. Chem Senses. 2011;36:63–67. doi: 10.1093/chemse/bjq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf NJ, Eckenstein F, Butcher LL. Cholinergic systems in the rat brain: I. projections to the limbic telencephalon. Brain Res Bull. 1984;13:751–784. doi: 10.1016/0361-9230(84)90236-3. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CE. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011;21:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder WM, Gaynor L, Windham E, Lyman M, Munizza O, Setlow B, Bizon JL, Smith DW. Characterizing olfactory binary mixture interactions in Fischer 344 rats using behavioral reaction times. Chem Senses. 2015;40:325–334. doi: 10.1093/chemse/bjv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder WM, Setlow B, Bizon JL, Smith DW. Characterizing olfactory perceptual similarity using carbon chain discrimination in Fischer 344 rats. Chem Senses. 2014;39:323–331. doi: 10.1093/chemse/bju001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BJ, Otto T, Fox GD, Eichenbaum H. Memory representation within the parahippocampal region. J Neurosci. 1997;17:5183–5195. doi: 10.1523/JNEUROSCI.17-13-05183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Záborszky L, Carlsen J, Brashear HR, Heimer L. Cholinergic and GABAergic afferents to the olfactory bulb in the rat with special emphasis on the projection neurons in the nucleus of the horizontal limb of the diagonal band. J Comp Neurol. 1986;243:488–509. doi: 10.1002/cne.902430405. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Watson ML, Gallagher M, Nicolle MM. Muscarinic receptor-mediated GTP-Eu binding in the hippocampus and prefrontal cortex is correlated with spatial memory impairment in aged rats. Neurobiol Aging. 2007;28:619–626. doi: 10.1016/j.neurobiolaging.2006.02.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


