Abstract
Background
Inflammatory bowel disease (IBD) is characterized by episodic intestinal injury and repair. Myofibroblasts are gastrointestinal (GI) tract stromal cells that regulate the reparative process, and are known targets of inflammatory mediators including bradykinin (BK). However, the mechanisms through which inflammation regulates myofibroblast-induced wound healing remain incompletely understood. Here, we demonstrate, for the first time, that BK stimulates myofibroblast migration through protein kinase D (PKD)-mediated activation of the COX-2 and Hsp27 pathways.
Materials and Methods
CCD-18Co is a human colonic myofibroblast cell line used from passages 8–14. An in vitro scratch assay assessed the effect of BK (100nM) on myofibroblast migration over 24h in the presence or absence of several inhibitors (CID755673 (10 µM) and NS398 (10 µM)). Hsp27 siRNA evaluated the effect of Hsp27 on colonic myofibroblast migration. Antibodies to pPKD, pHsp27, and COX-2 evaluated expression levels by Western blot.
Results
BK stimulated myofibroblast migration over 24h. BK also led to rapid and sustained phosphorylation of PKD at Ser-916, rapid phosphorylation of Hsp27 at Ser-82, and increased COX-2 expression over 4h. BK-mediated COX-2 expression and Hsp27 phosphorylation were both inhibited by the PKD inhibitor CID755673. Similarly, BK-induced myofibroblast migration was significantly inhibited by CID755673 (p<0.05), by the direct COX-2 inhibitor NS398 (p<0.05), and by Hsp27 siRNA (p<0.05).
Conclusions
BK stimulates myofibroblast migration through PKD-mediated activation of COX-2 and Hsp27. PKD, COX-2 and Hsp27 all appear to regulate myofibroblast cell migration, a stromal population that may play an important role in mucosal healing in the setting of inflammation.
Keywords: bradykinin, protein kinase D, myofibroblast, COX-2, Hsp27
INTRODUCTION
Inflammatory bowel disease (IBD) describes two clinical entities, Crohn’s disease and ulcerative colitis (UC). Though distinct in many ways, they share overlapping characteristics that include repetitive inflammatory injury that is immune-mediated and chronic. Intestinal repair is a complex process that involves stromal-epithelial cell communication to restore the integrity of the bowel wall. Myofibroblasts are stromal cells of the gastrointestinal (GI) tract that are a known target of inflammatory mediator signaling [1–4] and appear to play an important role in restoring intestinal homeostasis [5–7] Myofibroblasts migrate to areas of injury, and interact with the overlying epithelium to regulate cell proliferation as well as deposition and remodeling of the underlying extracellular matrix [6, 8–11].
We have recently demonstrated that myofibroblast migration can be stimulated by TNF-α [12], a potent 17-kDa pro-inflammatory cytokine that is is known to regulate myofibroblast function [1–3] and has been strongly implicated in the pathogenesis of IBD [3, 13]. TNF-α was found to stimulate myofibroblast migration through signaling pathways involving cyclo-oxygenase -2 (COX-2) and heat shock protein 27 (Hsp27) [12]. COX-2, the rate-limiting enzyme in the biosynthesis of prostaglandins (PGs) and thromboxanes, is upregulated in the setting of colitis and plays an important role in mucosal repair [1, 8, 12, 14]. Hsp27 is a molecular chaperone and a member of the small heat shock protein group encoded by the HSPB1 gene that regulates the stress response, wound healing, and cell migration [12, 15–17].
Interestingly, both COX-2 and Hsp27 are known downstream targets of protein kinase D (PKD), a ubiquitous serine-threonine kinase that is involved in biological responses to inflammation and oxidative stress, but is not independently activated by TNF-α [1, 2, 18]. Structurally, PKD is composed of a cysteine rich domain (CRD) and a pleckstrein homology (PH) domain, which keep PKD in a basal, inactive state [2]. PKD can be activated by multiple G protein-coupled receptor (GPCR) agonists, including bradykinin, in the setting of inflammation [1, 2], but the role of PKD in the regulation of myofibroblast migration is unknown. Consequently, the purpose of this study was to determine whether GPCR-mediated PKD activation can regulate colonic myofibroblast migration, and to determine the underlying cell signaling mechanisms that are involved in this process.
Here, we demonstrate that bradykinin-mediated PKD activation stimulates the migration of human colonic myofibroblasts (18Co) through signaling pathways that involve COX-2 and Hsp27. Our results support the notion that PKD signaling may play an important role in myofibroblast migration and intestinal repair. Furthermore, COX-2 and Hsp27 appear to be conserved signaling targets that regulate myofibroblast migration through activation by multiple upstream agonists.
MATERIALS AND METHODS
Cell Culture
The human myofibroblast cell line CCD-18Co was purchased from American Type Culture Collection (Manassas, VA). These cells share structural and functional similarities with primary colonic subepithelial myofibroblasts and have been validated by several previous studies [19–21]. CCD-18Co cells were cultured in DMEM containing 10% FBS, penicillin, streptomycin, fungizone and glutamine at 37 °C in 5% CO2 humidified air. Cells were passaged when confluent and experiments were performed with cells from passages 8 through 14.
SDS-PAGE and Immunoblotting
Cell lysis was performed using Triton buffer (50 mM Tris, pH 7.5, 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 5 mM sodium pyrophosphate, 10 mM sodium glycerophosphate, 1% Triton X-100, 50 mM NaF plus 1% Calbiochem Protease Inhibitor Cocktail) and lysates were assayed for protein using the Bradford protein assay, then diluted with 5× Laemmli loading buffer for SDS-PAGE. Equal amounts of protein were loaded in 4–20% Tris/glycine gels, and electrophoresed for 120 min at 130 Volts constant voltage. The gel was blotted onto a PVDF membrane by electrophoretic transfer at 25 V constant voltage overnight. The membrane was washed, blocked with 5% milk, and probed with primary antibodies. Appropriate secondary antibodies conjugated to horseradish peroxidase (Pierce, Rockford, IL) and a chemiluminescent substrate (SuperSignal, Pierce, Rockford, IL) were used to visualize immunoreactive bands. The primary antibodies against pHsp27, pPKD, and COX-2 were purchased from Cell Signaling (Danvers, MA), antibodies against Hsp27 and GAPDH were purchased from Santa Cruz (Santa Cruz, CA). Donkey anti-mouse and donkey anti-rabbit secondary antibodies were purchased from Pierce (Rockford, IL). Protein expression was analyzed with software Image J and was normalized relative to total protein for each lane (“relative expression”).
Scratch Assay
For migration assays, cells were plated in 35 mm dishes until confluent. Under varying conditions, a scratch gap was then created by scratching the cell monolayer with a sterile pipette tip and the cells were incubated in serum free medium. Three pictures were randomly taken along the scratch gap (time 0 h) and these positions were marked for subsequent re-evaluation (time 24 h). The scratch area was measured with software Image J and normalized to time 0 h control. Cells were treated with CID755673 (10 µM), NS398 (10 µM) or HSP27 siRNA (100 nM) prior to exposure to BK during the migration assay.
SiRNA Transfection
HSP27 siRNA (Cat # 4392420 Ambion, CA) or negative control siRNA (Cat # 4611 Ambion, CA) was used to transfect 80–90% confluent CCD-18Co cells with Lipofectamine 2000 (Invitrogen, CA) at 100 nM according to the manufacturer’s instructions. Western blotting confirmed the silencing effect of siRNA on the expression levels of Hsp27 [12]. Transfected cells were then used for migration experiments.
Materials and Reagents
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin G potassium, streptomycin, fungizone, and glutamine were purchased from Invitrogen (Carlsbad, CA). TNF-α was purchased from R&D Systems (Minneapolis, MN). Hsp27 siRNA was purchased from Ambion (Carlsbad, CA). NS398 was purchased from Calbiochem (San Diego, CA). CID755673 was purchased from Sigma-Aldrich (St. Louis, MO).
RESULTS
Bradykinin stimulates myofibroblast migration via PKD
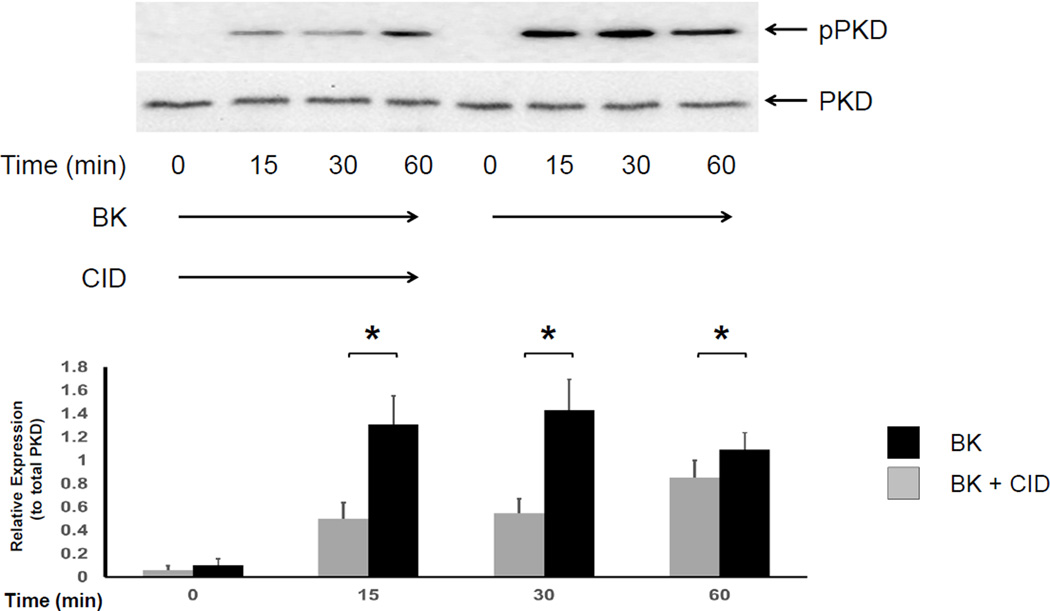
Bradykinin is an endogenous kinin that has been implicated in the pathophysiology of many inflammatory conditions including IBD [4, 14]. Bradykinin binds to one of two cell surface receptors (the kinin B1 and B2 receptors) that belong to the G protein coupled receptor (GPCR) family, leading to the activation of downstream signaling targets including Protein kinase D (PKD) [2, 4]. Exposure of 18Co cells to BK leads to rapid, PKC-mediated phosphorylation of PKD at Ser-744 [2], followed by a rapid auto-phosphorylation of PKD at Ser-916, an effect that is inhibited by the potent and highly specific PKD inhibitor CID755673 [22, 23] (Fig 1). To determine whether bradykinin regulates myofibroblast migration, 18Co cells grown to confluence on a 35mm cell culture plate were scratched with a sterile pipette tip to create a scratch gap, the area of which was measured before and after exposure to bradykinin (100 nM) for 24 h. As demonstrated in Fig 2, untreated myofibroblasts in serum-free media did not significantly migrate after 24 h, with a negligible decrease in the size of the scratch gap area. However, 18Co cells exposed to bradykinin demonstrated enhanced myofibroblast migration, leading to a significant decrease in the scratch gap area compared to untreated cells (p<0.05). Exposure of 18Co cells to 100 nM bradykinin did not stimulate cellular proliferation over 24 h, as measured by [3H] thymidine incorporation(data not shown). Interestingly, pre-treatment with the PKD inhibitor CID755673 blocked BK-induced myofibroblast migration, implicating PKD in this process (Fig 2).
Figure 1. BK-mediated PKD phosphorylation at Ser-916 is inhibited by CID755673.
Confluent 18Co cells were washed and equilibrated in serum-free media for 30 min, then exposed to BK (100nM) for 60 min in the presence or absence of the PKD inhibitor CID755673 (10 µM). Cell lysates were then analyzed by SDS-PAGE and Western blot using an antibody that detects phosphorylated PKD protein (pPKD) at serine residue 916 (Ser-916). BK induced the phosphorylation of PKD at Ser-916, an effect that significantly inhibited by CID755673 (CID) at 15, 30 and 60 min. The results shown are the mean ± S.E. n ≥ 3 and are expressed as a relative expression level of phosphorylated PKD, displayed in graphical form below. Equal protein loading was verified using an antibody that detects total PKD protein. * denotes p < 0.05.
Figure 2. BK-induced myofibroblast migration is inhibited by CID755673.
The surface of a confluent monolayer of 18Co cells was scratched with a sterile pipette tip and incubated in serum free medium. Three pictures were randomly taken along the scratch gap and pictured positions were marked (time 0 h). Cells were then treated with BK (100 nM) for 24 h, in the presence or absence of CID755673 (10 µM), an inhibitor of PKD. Three pictures were taken after 24 h at the same positions as marked previously. The scratch gap area was measured with software Image J and normalized to time 0 control. The results shown are the mean ± S.E. n ≥ 3 and are expressed as a percentage decrease in scratch gap area. * denotes p < 0.05.
Bradykinin leads to enhanced COX-2 expression via PKD
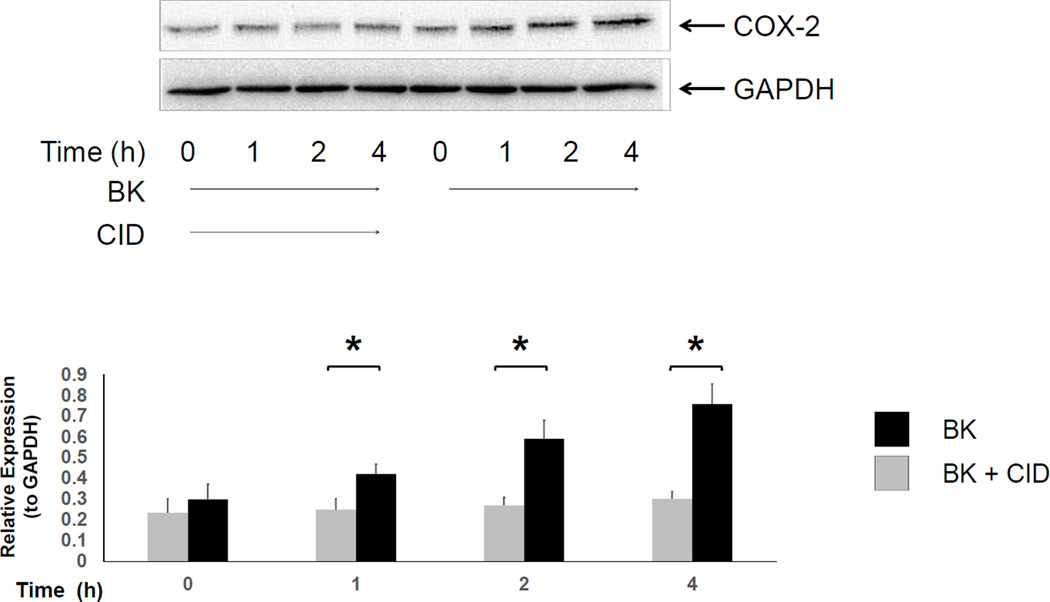
Cyclo-oxygenase-2 (COX-2) is the rate-limiting enzyme that converts arachidonic acid to prostaglandins G2 and H2, the precursors of prostaglandins (PGs) and thromboxanes [24]. To determine whether BK-induced COX-2 expression was involved in myofibroblast migration, 18Co cells were first exposed to bradykinin (100 nM) over 4 h and COX-2 expression was analyzed by Western blot. As shown in Fig 3, exposure of 18Co cells to BK led to a time-dependent increase in COX-2 expression which was statistically significant after 1 hr of exposure and steadily increased over the 4 h time period, consistent with previous results [1, 14]. The increased expression of COX-2 induced by BK was inhibited by the PKD inhibitor CID755673, as was BK-induced myofibroblast migration (Fig 2), suggesting that myofibroblast migration, which has been shown to be partially mediated by COX-2 [12], is also regulated by PKD.
Figure 3. BK-mediated COX-2 expression is inhibited by CID755673.
Confluent 18Co cells were washed and equilibrated in serum-free media for 30 min, then exposed to BK (100nM) for 4 h in the presence or absence of the PKD inhibitor CID755673 (10 µM). Cell lysates were then analyzed by SDS-PAGE and Western blot using an antibody that detects cyclo-oxygenase 2 (COX-2). The results shown are the mean ± S.E. n ≥ 3 and are expressed as a relative expression level of COX-2 protein, displayed in graphical form below. Equal protein loading was verified using an antibody that detects GAPDH protein. * denotes p < 0.05.
BK leads to rapid and sustained Hsp27 phosphorylation via PKD
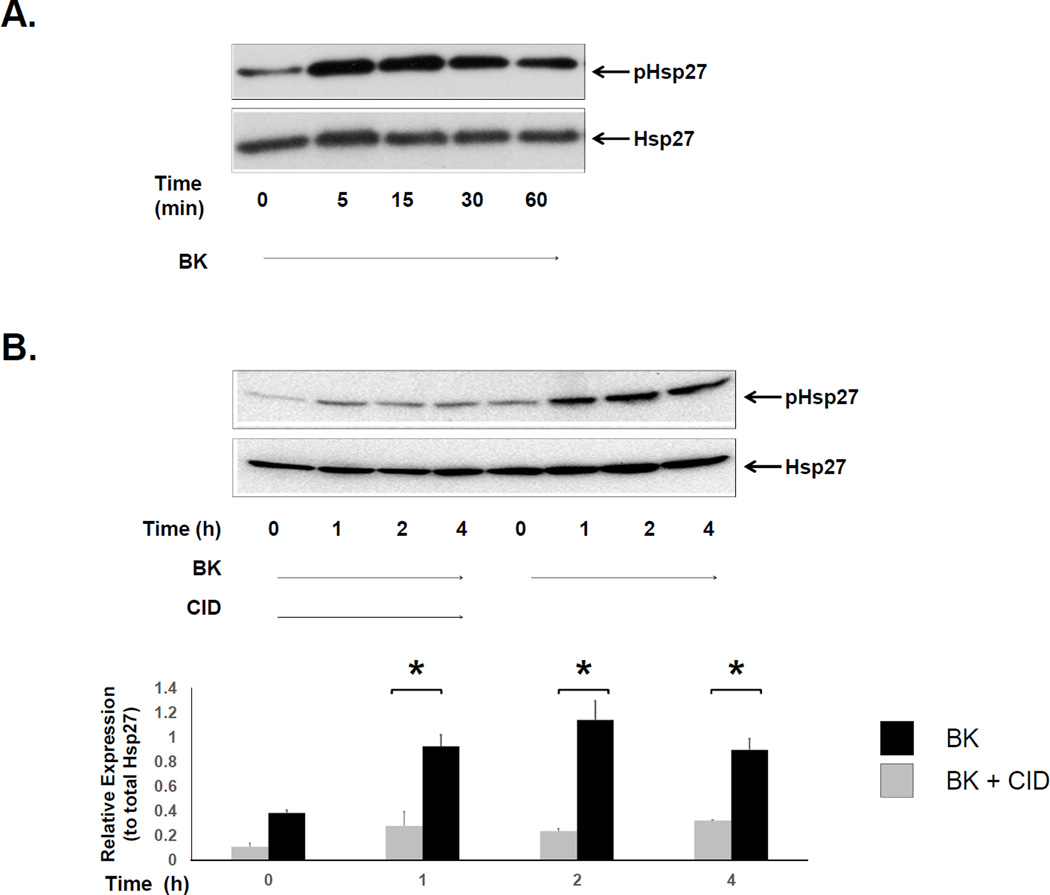
Heat shock protein 27 (Hsp27) is a molecular chaperone that is known to be involved in many cellular processes including fibrosis [15, 16], the response to stress [25], wound healing and cell migration [16, 17], and tumorigenesis [25, 26]. Hsp27 is a known downstream target of PKD [18] and is activated by post-translational phosphorylation at Serine (Ser)-15, -78, -82 and Threonine (Thr-143) residues. Because Hsp27 has been recently implicated in myofibroblast migration [12], we sought to determine whether PKD-mediated Hsp27 phosphorylation could stimulate myofibroblast migration. 18Co cells were incubated with 100nM bradykinin and Hsp27 phosphorylation at Serine residue 82 was assessed by Western blot using a site-specific antibody. As shown in Fig 4A, exposure of 18Co cells to BK induced a rapid phosphorylation of Hsp27, peaking at 5 min followed by a sustained phosphorylation over 4 h (Fig 4B). BK-induced Hsp27 phosphorylation was completely inhibited by pre-treatment with the PKD inhibitor CID755673 (Fig 4B).
Figure 4. BK-mediated Hsp27 phosphorylation at Ser-82 is inhibited by CID755673.
A. Confluent 18Co cells were washed and equilibrated in serum-free media for 30 min, then exposed to BK (100nM) for 60 min. Cell lysates were then analyzed by SDS-PAGE and Western blot using an antibody that detects phosphorylated Hsp27 at serine residue 82 (pHsp27). BK induced the rapid phosphorylation of Hsp27 at Ser-82, evident after 5 min and sustained over the 60 min time period. Equal protein loading was verified using an antibody that detects total Hsp27 protein. B. Confluent 18Co cells were washed and equilibrated in serum-free media for 30 min, then exposed to BK (100nM) for 4 h, in the presence and absence of the PKD inhibitor CID755673. Cell lysates were then analyzed by SDS-PAGE and Western blot using an antibody that detects phosphorylated Hsp27 at serine residue 82 (pHsp27). BK induced the phosphorylation of Hsp27 at Ser-82, an effect that was significantly inhibited by CID755673 at 1, 2 and 4 h. The results shown are the mean ± S.E. n ≥ 3 and are expressed as a relative expression level of phosphorylated Hsp27 protein, displayed in graphical form below. Equal protein loading was verified using an antibody that detects total Hsp27 protein. * denotes p < 0.05.
BK-induced Myofibroblast Migration is Inhibited by Hsp27 siRNA
Exposure to BK led to rapid and sustained phosphorylation of Hsp27 and a striking increase in myofibroblast migration, effects that were both inhibited by the PKD inhibitor CID755673. To determine whether Hsp27 was involved in BK-induced myofibroblast migration, we utilized Hsp27 siRNA. We have previously shown that siRNA targeting Hsp27 produces a significant depletion of Hsp27 protein in 18Co cells with a corresponding decrease in inducible Hsp27 phosphorylation at serine residue 82 [12]. To determine what role Hsp27 may have on BK-mediated colonic myofibroblast migration, 18Co cells transfected with Hsp27 siRNA and non-targeted siRNA were exposed to BK for 24 h and myofibroblast migration was analyzed. Hsp27 siRNA led to a significant reduction in myofibroblast migration following exposure to BK compared to 18Co cells transfected with non-targeted siRNA (Fig 5A). The results indicate that Hsp27 regulates BK-mediated myofibroblast migration.
Figure 5. BK-induced myofibroblast migration is inhibited by Hsp27 siRNA and NS398.
Panel A: 18Co cells were transfected with 100 nM Hsp27 siRNA or with a non-targeting sequence as described in Materials and Methods. The surface of a confluent monolayer of transfected 18Co cells was scratched with a sterile pipette tip. Three pictures were randomly taken along the scratch gap and pictured positions were marked (time 0 h). Cells were then treated with BK (100nM) for 24 h. Three pictures were taken after 24 h at the same positions as marked previously. The scratch gap area was measured with software Image J and normalized to time 0 control. The results shown are the mean ± S.E. n ≥ 3 and are expressed as percentage decrease in scratch gap area. * denotes p < 0.05. Panel B: The surface of a confluent monolayer of 18Co cells was scratched with a sterile pipette tip and incubated in serum free medium. Three pictures were randomly taken along the scratch gap and pictured positions were marked (time 0 h). Cells were then treated with BK (100 nM) for 24 h, in the presence or absence of NS398 (10 µM), a COX-2-specific inhibitor. Three pictures were taken after 24 h at the same positions as marked previously. The scratch gap area was measured with software Image J and normalized to time 0 control. The results shown are the mean ± S.E. n ≥ 3 and are expressed as a percentage decrease in scratch area. * denotes p < 0.05.
BK- induced Myofibroblast Migration is Inhibited by NS398
COX-2 is upregulated in the setting of intestinal inflammation [27, 28], and regulates cell migration in a number of cell types, including the myofibroblast [29–31]. Having demonstrated that BK stimulates both myofibroblast migration and COX-2 expression in a PKD-dependent manner, we sought to determine whether enhanced COX-2 expression contributed to BK-mediated myofibroblast migration. After creation of a scratch gap, confluent 18Co cells were exposed to BK (100 nM) for 24 h in the presence or absence of NS398 (10 µM), a COX-2-specific inhibitor. While NS398 alone had no effect on myofibroblast migration, NS398 significantly inhibited BK-stimulated myofibroblast migration (Fig 5B).
DISCUSSION
Directional cellular migration is a critical aspect of wound healing but remains poorly understood. The myofibroblast may play an important role in the wound healing process by migrating into damaged areas, modifying the extracellular matrix (ECM) through secretion of matrix metalloproteinases [4] and ECM proteins [32, 33], and interacting with the intestinal stem cell niche to restore intestinal wall architecture following inflammatory injury.
Previous work in our laboratory demonstrated that the pro-inflammatory cytokine TNF-α stimulates myofibroblast migration through signaling pathways involving COX-2 and Hsp27 [12, 34]. As known downstream targets of PKD, these findings raised new questions regarding the potential for PKD to also regulate myofibroblast migration. We utilized the inflammatory mediator bradykinin, a PKD agonist, to test whether PKD activation could regulate cell migration in the myofibroblast. In the present study, we demonstrate that BK stimulates robust myofibroblast migration through PKD-mediated activation of COX-2 and Hsp27.
This study suggests that COX-2 and Hsp27 may be critical downstream effector proteins that modulate myofibroblast migration, regardless of the upstream agonist. GPCR-dependent (bradykinin-mediated) and -independent (TNF-α) pathways have now both been shown to stimulate myofibroblast migration through enhanced COX-2 expression and Hsp27 phosphorylation. A growing body of evidence supports the idea that Hsp27 regulates migration of many cell types, possibly through reorganization and polymerization of the actin cytoskeleton [35–37]. COX-2 has been strongly implicated in wound healing, and these findings may partially explain how non-steroidal anti-inflammatory drugs (NSAIDs) may exacerbate IBD [38, 39] or lead to mucosal ulceration [40, 41]. The findings suggest that combination therapies directed at multiple targets may be therapeutically beneficial.
PKD has been implicated in the process of cell migration in other cell types [34, 42–44], though this has not been previously described in the myofibroblast. PKD is upregulated in the myofibroblast in the setting of inflammation, both in experimental models [1, 2] as well as in primary myofibroblasts taken from human colon tissue (data not shown). As a critical regulator of COX-2 expression in the myofibroblast [14, 45] and a target of inflammatory mediator cross talk [46], PKD appears to play an important role in the stromal response to inflammation. PKD may serve as a novel therapeutic target to promote mucosal healing following episodes of colitis.
Acknowledgments
This work was supported by National Institutes of Health Grant 5KO8DK085136-06 to JY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: EC,SS - study conception and design, data acquisition, data analysis, article drafting, article revision, final approval; TL – data acquisition, data analysis, article drafting, article revision, final approval; JY – study conception and design, data analysis, article drafting, article revision, final approval.
Author Disclosure Statement: The authors have no disclosures.
REFERENCES
- 1.Rodriguez Perez CE, et al. TNF-alpha potentiates lysophosphatidic acid-induced COX-2 expression via PKD in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2011;300(4):G637–G646. doi: 10.1152/ajpgi.00381.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoo J, et al. Protein kinase D mediates synergistic expression of COX-2 induced by TNF-{alpha} and bradykinin in human colonic myofibroblasts. Am J Physiol Cell Physiol. 2009;297(6):C1576–C1587. doi: 10.1152/ajpcell.00184.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armaka M, et al. Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J Exp Med. 2008;205(2):331–337. doi: 10.1084/jem.20070906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoo J, et al. Protein kinase D1 mediates synergistic MMP-3 expression induced by TNF-alpha and bradykinin in human colonic myofibroblasts. Biochem Biophys Res Commun. 2011;413(1):30–35. doi: 10.1016/j.bbrc.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayashi Y, et al. Topical implantation of mesenchymal stem cells has beneficial effects on healing of experimental colitis in rats. J Pharmacol Exp Ther. 2008;326(2):523–531. doi: 10.1124/jpet.108.137083. [DOI] [PubMed] [Google Scholar]
- 6.Iwanaga K, et al. Prostaglandin E2 promotes wound-induced migration of intestinal subepithelial myofibroblasts via EP2, EP3, and EP4 prostanoid receptor activation. J Pharmacol Exp Ther. 2012;340(3):604–611. doi: 10.1124/jpet.111.189845. [DOI] [PubMed] [Google Scholar]
- 7.Liu L, et al. Effect of local injection of mesenchymal stem cells on healing of sutured gastric perforation in an experimental model. Br J Surg. 2015;102(2):e158–e168. doi: 10.1002/bjs.9724. [DOI] [PubMed] [Google Scholar]
- 8.Shao J, et al. Roles of myofibroblasts in prostaglandin E2-stimulated intestinal epithelial proliferation and angiogenesis. Cancer Res. 2006;66(2):846–855. doi: 10.1158/0008-5472.CAN-05-2606. [DOI] [PubMed] [Google Scholar]
- 9.Powell DW, et al. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol. 1999;277(2 Pt 1):C183–C201. doi: 10.1152/ajpcell.1999.277.2.C183. [DOI] [PubMed] [Google Scholar]
- 10.Powell DW, et al. Epithelial cells and their neighbors I. Role of intestinal myofibroblasts in development, repair, and cancer. Am J Physiol Gastrointest Liver Physiol. 2005;289(1):G2–G7. doi: 10.1152/ajpgi.00075.2005. [DOI] [PubMed] [Google Scholar]
- 11.Mahida YR, et al. Adult human colonic subepithelial myofibroblasts express extracellular matrix proteins and cyclooxygenase-1 and -2. Am J Physiol. 1997;273(6 Pt 1):G1341–G1348. doi: 10.1152/ajpgi.1997.273.6.G1341. [DOI] [PubMed] [Google Scholar]
- 12.Saini S, Liu T, Yoo J. TNF-alpha stimulates colonic myofibroblast migration via COX-2 and Hsp27. J Surg Res. 2016;204(1):145–152. doi: 10.1016/j.jss.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilhelm SM, et al. A review of infliximab use in ulcerative colitis. Clin Ther. 2008;30(2):223–230. doi: 10.1016/j.clinthera.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Yoo J, et al. TNF-alpha and LPA promote synergistic expression of COX-2 in human colonic myofibroblasts: role of LPA-mediated transactivation of upregulated EGFR. BMC Gastroenterol. 2013;13:90. doi: 10.1186/1471-230X-13-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wettstein G, et al. Inhibition of HSP27 blocks fibrosis development and EMT features by promoting Snail degradation. FASEB J. 2013;27(4):1549–1560. doi: 10.1096/fj.12-220053. [DOI] [PubMed] [Google Scholar]
- 16.Suarez E, et al. Up-regulation of tension-related proteins in keloids: knockdown of Hsp27, alpha2beta1-integrin, and PAI-2 shows convincing reduction of extracellular matrix production. Plast Reconstr Surg. 2013;131(2):158e–173e. doi: 10.1097/PRS.0b013e3182789b2b. [DOI] [PubMed] [Google Scholar]
- 17.Shin KD, et al. Blocking tumor cell migration and invasion with biphenyl isoxazole derivative KRIBB3, a synthetic molecule that inhibits Hsp27 phosphorylation. J Biol Chem. 2005;280(50):41439–41448. doi: 10.1074/jbc.M507209200. [DOI] [PubMed] [Google Scholar]
- 18.Yuan J, Rozengurt E. PKD, PKD2, and p38 MAPK mediate Hsp27 serine-82 phosphorylation induced by neurotensin in pancreatic cancer PANC-1 cells. J Cell Biochem. 2008;103(2):648–662. doi: 10.1002/jcb.21439. [DOI] [PubMed] [Google Scholar]
- 19.Shao J, et al. Roles of Myofibroblasts in Prostaglandin E2-Stimulated Intestinal Epithelial Proliferation and Angiogenesis. Cancer Res. 2006;66(2):846–855. doi: 10.1158/0008-5472.CAN-05-2606. [DOI] [PubMed] [Google Scholar]
- 20.Laurens K, et al. Myofibroblast Matrix Metalloproteinases Activate the Neutrophil Chemoattractant CXCL7 From Intestinal Epithelial Cells. Gastroenterology. 2006;130(1):127. doi: 10.1053/j.gastro.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 21.Pacheco II, MacLeod RJ. CaSR stimulates secretion of Wnt5a from colonic myofibroblasts to stimulate CDX2 and sucrase-isomaltase using Ror2 on intestinal epithelia. Am J Physiol Gastrointest Liver Physiol. 2008;295(4):G748–G759. doi: 10.1152/ajpgi.00560.2007. [DOI] [PubMed] [Google Scholar]
- 22.Sharlow ER, et al. Potent and selective disruption of protein kinase D functionality by a benzoxoloazepinolone. J Biol Chem. 2008;283(48):33516–33526. doi: 10.1074/jbc.M805358200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torres-Marquez E, et al. CID755673 enhances mitogenic signaling by phorbol esters, bombesin and EGF through a protein kinase D-independent pathway. Biochem Biophys Res Commun. 2010;391(1):63–68. doi: 10.1016/j.bbrc.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marnett LJ, DuBois RN. COX-2: A Target for Colon Cancer Prevention. Annual Review of Pharmacology and Toxicology. 2002;42(1):55–80. doi: 10.1146/annurev.pharmtox.42.082301.164620. [DOI] [PubMed] [Google Scholar]
- 25.Katsogiannou M, Andrieu C, Rocchi P. Heat shock protein 27 phosphorylation state is associated with cancer progression. Front Genet. 2014;5:346. doi: 10.3389/fgene.2014.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang RC, et al. Proteomic Characterization of Annexin l (ANX1) and Heat Shock Protein 27 (HSP27) as Biomarkers for Invasive Hepatocellular Carcinoma Cells. PLoS One. 2015;10(10):e0139232. doi: 10.1371/journal.pone.0139232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D, DuBois RN. PPARdelta and PGE signaling pathways communicate and connect inflammation to colorectal cancer. Inflamm Cell Signal. 2014;1(6) doi: 10.14800/ics.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang D, Dubois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2010;29(6):781–788. doi: 10.1038/onc.2009.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayoral R, et al. Prostaglandin E2 promotes migration and adhesion in hepatocellular carcinoma cells. Carcinogenesis. 2005;26(4):753–761. doi: 10.1093/carcin/bgi022. [DOI] [PubMed] [Google Scholar]
- 30.Saalbach A, et al. Fibroblasts support migration of monocyte-derived dendritic cells by secretion of PGE2 and MMP-1. Exp Dermatol. 2015;24(8):598–604. doi: 10.1111/exd.12722. [DOI] [PubMed] [Google Scholar]
- 31.Lu DY, et al. Bradykinin-induced cell migration and COX-2 production mediated by the bradykinin B1 receptor in glioma cells. J Cell Biochem. 2010;110(1):141–150. doi: 10.1002/jcb.22520. [DOI] [PubMed] [Google Scholar]
- 32.van Putten S, Shafieyan Y, Hinz B. Mechanical control of cardiac myofibroblasts. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 33.Mia MM, Bank RA. The pro-fibrotic properties of transforming growth factor on human fibroblasts are counteracted by caffeic acid by inhibiting myofibroblast formation and collagen synthesis. Cell Tissue Res. 2015 doi: 10.1007/s00441-015-2285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durand N, Borges S, Storz P. Protein Kinase D Enzymes as Regulators of EMT and Cancer Cell Invasion. J Clin Med. 2016;5(2) doi: 10.3390/jcm5020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang J, et al. Silencing heat shock protein 27 (HSP27) inhibits the proliferation and migration of vascular smooth muscle cells in vitro. Mol Cell Biochem. 2014;390(1–2):115–121. doi: 10.1007/s11010-014-1962-1. [DOI] [PubMed] [Google Scholar]
- 36.Doshi BM, Hightower LE, Lee J. The role of Hsp27 and actin in the regulation of movement in human cancer cells responding to heat shock. Cell Stress Chaperones. 2009;14(5):445–457. doi: 10.1007/s12192-008-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hedges JC, et al. A role for p38 (MAPK)/HSP27 pathway in smooth muscle cell migration. J Biol Chem. 1999;274(34):24211–24219. doi: 10.1074/jbc.274.34.24211. [DOI] [PubMed] [Google Scholar]
- 38.Guslandi M. Exacerbation of inflammatory bowel disease by nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors: fact or fiction? World J Gastroenterol. 2006;12(10):1509–1510. doi: 10.3748/wjg.v12.i10.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Long MD, et al. Role of Nonsteroidal Anti-Inflammatory Drugs in Exacerbations of Inflammatory Bowel Disease. J Clin Gastroenterol. 2016;50(2):152–156. doi: 10.1097/MCG.0000000000000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maiden L, et al. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128(5):1172–1178. doi: 10.1053/j.gastro.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 41.Graham DY, et al. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3(1):55–59. doi: 10.1016/s1542-3565(04)00603-2. [DOI] [PubMed] [Google Scholar]
- 42.Durand N, Borges S, Storz P. Functional and therapeutic significance of protein kinase D enzymes in invasive breast cancer. Cell Mol Life Sci. 2015;72(22):4369–4382. doi: 10.1007/s00018-015-2011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doppler H, et al. Protein kinase d isoforms differentially modulate cofilin-driven directed cell migration. PLoS One. 2014;9(5):e98090. doi: 10.1371/journal.pone.0098090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ochi N, et al. Protein kinase D1 promotes anchorage-independent growth, invasion, and angiogenesis by human pancreatic cancer cells. J Cell Physiol. 2011;226(4):1074–1081. doi: 10.1002/jcp.22421. [DOI] [PubMed] [Google Scholar]
- 45.Yoo J, et al. TNF-alpha and LPA promote synergistic expression of COX-2 in human colonic myofibroblasts: role of LPA-mediated transactivation of upregulated EGFR. BMC Gastroenterol. 2013;13(1):90. doi: 10.1186/1471-230X-13-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoo J, et al. TNF-alpha induces upregulation of EGFR expression and signaling in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2012;302(8):G805–G814. doi: 10.1152/ajpgi.00522.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]