Abstract
Metabolic malprogramming has been associated with low birth weight; however, the interplay between insulin secretion disruption and adrenal function upon lipid metabolism is unclear in adult offspring from protein-malnourished mothers during the last third of gestation. Thus, we aimed to study the effects of a maternal low-protein diet during the last third of pregnancy on adult offspring metabolism, including pancreatic islet function and morphophysiological aspects of the liver, adrenal gland, white adipose tissue, and pancreas. Virgin female Wistar rats (age 70 d) were mated and fed a protein-restricted diet (4%, intrauterine protein restricted [IUPR]) from day 14 of pregnancy until delivery, whereas control dams were fed a 20.5% protein diet. At age 91 d, their body composition, glucose-insulin homeostasis, ACTH, corticosterone, leptin, adiponectin, lipid profile, pancreatic islet function and liver, adrenal gland, and pancreas morphology were assessed. The birth weights of the IUPR rats were 20% lower than the control rats (P < .001). Adult IUPR rats were heavier, hyperphagic, hyperglycemic, hyperinsulinemic, hyperleptinemic, and hypercorticosteronemic (P < .05) with higher low-density lipoprotein cholesterol and lower high-density lipoprotein cholesterol, adiponectin, ACTH, and insulin sensitivity index levels (P < .01). The insulinotropic action of glucose and acetylcholine as well as muscarinic and adrenergic receptor function were impaired in the IUPR rats (P < .05). Maternal undernutrition during the last third of gestation disrupts the pancreatic islet insulinotropic response and induces obesity-associated complications. Such alterations lead to a high risk of metabolic syndrome, characterized by insulin resistance, visceral obesity, and lower high-density lipoprotein cholesterol.
The growing pandemic of metabolic syndrome has become a great threat to worldwide public health. Low birth weight and glucocorticoid dysfunction, both of which seem to imprint the onset of several chronic metabolic diseases such as type 2 diabetes and obesity in adulthood, have been tightly linked with undernutrition early in life (1, 2). These observations suggested that this fast-growing pandemic is a consequence of “nutritional transition,” in which a poor nutrient supply in gestation is replaced by a normal or even high-energy diet later in life, which is commonly observed in developing countries around the world (3–7). Currently, several studies are focusing on the developmental origins of health and disease, which is based on the thrifty phenotype hypothesis that helps to explain the increasing prevalence of metabolic syndrome (8–10).
A low-protein diet during only the first 2 weeks of lactation affects the autonomic nervous system, which influences pancreatic islet function. In previous studies with this animal model, we have shown that the pancreatic islets in these animals have an insulin secretion impairment even though these animals present with a lean phenotype (11–13). In contrast, the effects of a low-protein diet throughout gestation have been associated with hyperinsulinemia and an obese phenotype (14, 15). In fact, adverse events in the human intrauterine environment induce placental insufficiency that leads newborns to display an intrauterine growth restriction, which alters physiological pathways that induce metabolic syndrome (16–18).
Several studies noted an association between undernutrition and hypothalamic-pituitary-adrenal (HPA) axis hyperactivity, which influences a pattern of metabolic programming early in life (19, 20) by affecting the hypothalamic neuronal connections during a crucial phase of development (20–22). In fact, hypercorticosteronemia has been associated with many metabolic diseases (19, 23).
Experimental low-protein diet models offered early in life have been extensively used to better understand the influences of perinatal and postnatal protein restriction on the development of the central nervous system (24–26). In humans, the development of central nervous system connections takes place mainly during the intrauterine phase; however, in rodents, it occurs mainly in a period from the last third of gestation until approximately the first 2 weeks of the suckling phase (27–29).
Although many studies have tested the effects of undernutrition in different early developmental stages on the offspring's cardiometabolic parameters, such as throughout pregnancy (4, 30, 31), throughout lactation (15, 32), throughout pregnancy and lactation (14, 15, 33–35), throughout part of pregnancy and the entire lactation period (36, 37), during the last half of pregnancy (38), during the first half of lactation (39) and during the first two thirds of the suckling phase (11–13). However, only a few studies have attempted to study only the last third of gestation to determine the malprogramming effects from undernutrition (31, 40–43).
It is well known that individuals born from mothers during the Dutch famine period (between the years 1944 and 1945) displayed a high risk for obesity in adulthood (44). Indeed, glucose intolerance and insulin resistance later in life have been reported, especially in individuals whose mothers were exposed to the Dutch famine during the last trimester of gestation (45). Thus, it is clear that additional studies aimed at exploring malnutrition, especially during the last third of gestation, are necessary to investigate long-term outcomes on metabolic diseases, obesity, and pancreatic complications.
In this study, we aimed to evaluate the effects of an isocaloric low-protein diet (4% protein) that was offered to dams only during the last week of pregnancy on the offspring's HPA axis and its influence on body composition, the lipid profile, glucose homeostasis, and insulin secretion. Furthermore, with this model, we evaluated the ability of pancreatic islets to release insulin under the action of autonomous nervous system insulinotropic and insulinostatic agents, and the morphological aspects of the liver, adrenal gland, adipose tissue, and pancreatic islets, which are important targets of excessive circulating glucocorticoids.
Materials and Methods
Animal model and experimental design
Virgin female 70-day-old Wistar rats were fed a commercial diet (20.5% protein content; Nuvital) and maintained under controlled lighting and temperature (lights on 0700–1900 h and temperature was maintained at 23 ± 2°C). Female rats were mated with proven male breeders, and, afterward, a vaginal smear, which was washed with saline solution (NaCl, 0.9%, w/v), was collected to evaluate the presence of spermatozoa. The day on which spermatozoa were present in a vaginal smear was designated as the day of conception (day 0 of pregnancy). Only rats that were pregnant within 10 days were used for experimental procedures.
On day 14 of pregnancy, one batch of female rats (n = 5) were fed a low-protein diet [containing 4% protein, as previously reported (46)] until the day of delivery; this group was considered the Intrauterine Protein Restriction (IUPR) group. Another batch of female rats (n = 5) were fed a commercial diet (Control group). After delivery, both groups received a standard commercial diet (20.5% of protein). At birth, the litter size was adjusted to six pups per dam (preferentially male) throughout the lactation period. Both offspring groups were weaned at day 21. We used six randomly chosen pups per litter, (IUPR, n = 30 and Control, n = 30).
Throughout the experimental period, the rat offspring were kept under controlled temperature (23 ± 2°C) and photoperiod (0700–1900 h) conditions, with water and food ad libitum. The Ethical Committee for Animal Experiments of the State University of Maringá, which adheres to Brazilian Federal Law, approved the protocol.
Body weight gain and food intake assessment
From weaning until 90 days of age, the body weight (bw) and food intake levels were determined every 2 days. Food intake was quantified by measuring the difference between the weight of the chow given previously and the weight of chow at the end of 2 days, divided by the number of days and the number of rats in the cages. Then, this result was correlated with the mean of the rats' bw in the cages. The area under the curve (AUC) of the entire observation period was also calculated for both bw and food intake measurements.
Intracerebroventricular injection of insulin
To evaluate the central action of insulin on energy balance, a batch of rats (n = 8–10 for each group) underwent surgery to implant a cannula into the right lateral ventricle, as previously described (47). The precise coordinates of the cannula implantation into the right lateral ventricle was performed according to a previous study (48). After the surgery, each rat was kept in individual cages.
A recovery time of 5 days was permitted after the surgical procedure. The cannula placement was evaluated with a water drinking response test, which was induced by angiotensin II (2 mL of 10−6 mol/L solution) infusion. Rats that drank less than 5 mL within 15 minutes after treatment were excluded from the studies (49).
The intracerebroventricular (icv) treatment and food intake measurement after the icv injection were performed in rats deprived of food for 12 hours (0700–1900 h) with free access to water. The icv injection (2 μL) with saline (NaCl, 0.9%) or insulin (10−6 mol/L) was performed at 1900 hours, as previously published (50). Thereafter, standard chow was given, and food intake was determined by measuring the difference between the weight of the chow given and the weight of chow at the end of the two different periods, at 4 hours (2300 h) and 12 hours (0700 h) after the icv injection.
Intravenous glucose tolerance test
A silicone cannula was implanted into the right jugular vein of a batch of rats from both groups, and it was stabilized in the dorsal region of the neck in anesthetized rats (ketamine, 3 mg/100 g bw; and xylazine, 0.6 mg/100 g bw). To avoid blood clotting, the cannula was treated with heparinized saline, (50 IU heparin/mL [0.83 nkat/L]) of saline solution (NaCl, 0.9%, w/v) prior to administration. After a 12-hour fast (2000–0800 h) without anesthesia, a glucose load (1 g/kg bw) was infused through the cannula into the blood stream of the rats (n = 15) from both groups. Blood samples (350–400 mL) were collected immediately before the infusion of the glucose load (0 min) and at 5, 15, 30, and 45 minutes following the infusion.
Subtraction of the fasting plasma glucose and insulin concentration was used to quantify the glycemia (ΔGlycemia) and insulinemia (ΔInsulinemia) increments for each time period of the intravenous glucose tolerance test (ivGTT). The increases in total ΔGlycemia and ΔInsulinemia were calculated using the glycemia and/or insulinemia AUC for the 45 minutes ivGTT time point.
Insulin sensitivity index
The insulin sensitivity index (ISI), which generates a reasonable approximation of whole-body insulin sensitivity (51), was used to measure whole-body insulin sensitivity in our experimental model. To calculate the ISI, we performed the following calculation: ISI = 104/√[(fasting glycemia) × (fasting insulinemia) × (AUCΔGlycemia × AUCΔInsulinemia)].
Biochemical parameters
Plasma lipid measurements
Blood samples collected from 12 hours fasted (2000–0800 h) and conscious rats were removed through the cannula in the right jugular vein for lipid profile assessments. Then, the blood was centrifuged, and the plasma was stored at −20°C. The triglyceride as well as total and high-density lipoprotein (HDL) cholesterol plasma measurements from the blood samples of both groups (n = 8) were detected with a colorimetric method, using commercial kits (Gold Analisa). For circulating very low-density lipoprotein and low-density lipoprotein (LDL) cholesterol level measurements, we used the Friedewald calculation.
To quantify the Castelli indices I and II, the following calculations were used: Castelli index I = total cholesterol/HDL cholesterol and Castelli index II = LDL cholesterol/HDL cholesterol, as previously reported (52).
Plasma glucose, insulin, adiponectin, leptin, corticosterone, and ACTH measurements
Blood samples collected from 12 hours (2000–0800 h) fasted and conscious rats were removed through the cannula that was implanted in the right jugular vein to assess the fasting values of several biochemical parameters (n = 8–15). Plasma obtained from the blood samples was stored at −80°C for the subsequent determination of the following parameters: glucose concentration by the glucose oxidase method, with a commercial kit (Gold Analisa); insulin concentration, which was quantified with a RIA kit and a gamma counter (Wizard2 Automatic Gamma Counter, 2470 Model; PerkinElmer) with 125I-labeled recombinant human (rh) insulin (PerkinElmer); leptinemia and corticosteronemia levels, which were measured with commercial ELISA kits (Enzo Life Sciences); adiponectinemia (AdipoGen); and ACTH (MyBioSource). The intra- and interassay coefficients of variation were 9.8 and 12.2% for insulin, 4.8 and 4.9% for adiponectin, 5.9 and 7.2% for leptin, 7.7 and 9.7% for corticosterone, and 3.7 and 4.7% for ACTH, respectively. The hormone level detection limits were 1.03 pmol/L for insulin, 1.67 pmol/L for adiponectin, 4.20 pmol/L for leptin, 74.46 pmol/L for corticosterone, and 0.22 pmol/L for ACTH.
Pancreatic islet isolation
Pancreatic islets were isolated using a collagenase technique as described previously (53) with some adaptations. For this purpose, 91-day-old rats were decapitated and their abdominal walls were opened. The rats' common bile ducts were injected with 8 mL of Hank's buffered saline solution (composition in mmol/L: NaCl, 136.9; KCl, 5.4; MgSO4.7H2O, 0.81; Na2HPO4, 0.34; KH2PO4, 0.44; CaCl2.2H2O, 1.26; NaHCO3, 4.16; glucose, 0.06; BSA, 15; (v/v): 95% O2 + 5% CO2, mixed/10 min, pH 7.4) containing 0.1% collagenase type XI plus 5% BSA and 0.6% N-(2-hydroxyethylpiperazine)-N′-(2-ethanesulfonic acid), HEPES (w/v; Sigma-Aldrich). The pancreas, which was swollen with the collagenase solution, was quickly excised and incubated at 37°C in a glass beaker for 17–18 minutes. The suspension was then discarded, and the pancreas was washed with Hank's buffered saline solution in three continuous washings. The islets were collected with the aid of a stereomicroscope. Six rats from at least three different litters were used for each experimental procedure group.
Insulin secretion stimulation
To adapt the isolated islets to a baseline glucose concentration (5.6 mmol/L), the islets (four islets per well) were preincubated for 60 minutes in 1 mL of normal Krebs-Ringer solution (composition in mmol/L: NaCl, 115; NaHCO3, 24; KCl, 1.6; MgCl.6H2O, 1; CaCl2.2H2O, 1; BSA, 15), pH 7.4, which contained 5.6 mmol/L of glucose. This solution was gassed with 95% O2 mixed with 5% CO2 to maintain a pH of 7.4. To study the response under the insulinotropic effects of different glucose and acetylcholine doses, after the preincubation, batches of islets were incubated for an additional 60 minutes with different glucose (5.6, 8.3, 11.1, 16.7, 20.0 and 24.0 mmol/L) or acetylcholine concentrations (0.1, 1, 10, 100, 1000 μmol/L).
The supernatants from the incubations were collected and stored at −20°C for further insulin measurements.
Another batch of islets were used to study muscarinic acetylcholine receptor (mAChR) function. Thus, following preincubation (glucose 5.6 mmol/L), islets were incubated for an additional 60 minutes in a Krebs-Ringer solution containing 8.3 mmol/L of glucose and/or 8.3 mmol/L of glucose plus 10 mmol/L of acetylcholine in the presence of neostigmine (10 mmol/L) to prevent acetylcholinesterase action in the islets. To block the mAChR function, one of the following cholinergic antagonists was added into the Krebs-Ringer solution: a nonselective antagonist, atropine (Atr, 10 μmol/L); a selective antagonist for mAChR subtype M2, methoctramine (MTT, 1 mmol/L); or a selective mAChR subtype M3 antagonist, 4-diphenylacetoxy-N-methyl-piperidine methiodide (4-DAMP; 100 mmol/L).
To study adrenoceptor function, another batch of pancreatic islets, after preincubation (glucose 5.6 mmol/L), was incubated with a high-glucose concentration (16.7 mmol/L) either in the presence of epinephrine (1 mmol/L) plus the α2-adrenoceptor antagonist, yohimbine (Yoh; 10 mmol/L), or in the presence of epinephrine and the β2-adrenoceptor antagonist, propranolol (Pro; 1 mmol/L).
Similar to the mAChR studies, the antagonist doses were previously tested, and the concentrations that induced at least 50% inhibition or potentiated insulin secretion following stimulation with 16.7 mmol/L glucose were chosen.
All of the drugs described above for studying the muscarinic and adrenergic functions were purchased from Sigma-Aldrich).
Adipose tissue morphometric analyses
Samples from retroperitoneal, periepididymal, and inguinal adipose tissue were removed and fixed in Carnoy's solution (60% absolute ethanol, 30% chloroform, and 10% acetic acid) and embedded in histological paraffin (BIOTEC). Nonserial histological sections (5 μm thick) were obtained by using a Leica RM2145 semimotorized rotary microtome (Leica Biosystems) and stained with hematoxylin and eosin (H&E). The morphometric analyses were performed using digital images (TIFF 24-bit color, 2560 × 1920 pixels) obtained with light microscopy (Olympus BX41) and a QColor 3 Olympus camera with a 20× objective. An adipocyte area of 1200 cells per group from six rats for each experimental group was measured and analyzed with the Image-Pro Plus 4.5 software (Media Cybernetics).
Liver morphological analyses
Liver samples were removed, fixed in liquid nitrogen, and stored in a −80°C freezer. Subsequently, these samples were embedded in Tissue-Tek (Sakura Finetek) at the optimal cutting temperature. Nonserial histological sections (10 μm thick) were cut with a Leica CM1850 cryostat (Leica Biosystems) and stained with Sudam III (Red Oil). The lipid inclusion percentage was determined by calculating the ratio between the Red Oil marked area and total area of the images × 100 (% = marked area/total area × 100). The morphological analyses were performed using digital images (TIFF 24-bit color, 2560 × 1920 pixels) obtained with light microscopy (Olympus BX41) and a QColor 3 Olympus camera with a 40× objective. The images were acquired randomly from six rats per group (20 images per rat), producing 120 images per group.
Immunohistochemical and morphometric endocrine pancreas analyses
Pancreatic samples were removed and fixed in 10% formalin and embedded in paraffin (BIOTEC). Nonserial histological sections (5 μm thick) of the entire pancreas were made (Leica RM2145 semimotorized rotary microtome; Leica Biosystems) (six sections per group). Then, the sections were deparaffinized and any endogenous peroxidase was blocked with a 3% hydrogen peroxide solution ([v/v]: H2O2 in methanol; 3 mL/97 mL) for 15 minutes. The sections were then washed in phosphate-buffer solution (PBS; 10 mmol/L, pH 7.4) and incubated with blocking solution containing 10% nonimmune goat serum (Histostain-Plus, Invitrogen) for 10 minutes.
The sections were incubated with an anti-insulin antibody (dilution 1:500, Sigma-Aldrich) for 60 minutes at room temperature (Table 1). After washing with PBS, the sections were incubated with a biotinylated secondary antibody (Histostain Plus Kit, Invitrogen) for 10 minutes, washed with PBS, and incubated with a diaminobenzidine chromogenic solution (Histostain-Plus) for 15 minutes. Then, they were washed again with PBS and counterstained with hematoxylin. Morphometric analyses were performed using digital images (TIFF 24-bit color, 2560 × 1920 pixels) obtained with a light microscope (Olympus BX41) and a QColor 3 Olympus camera with 2× and 20× objectives. The pancreatic islet number per area of pancreas from the immunostained islets (insulin positive islets; six rats for each group) was measured. The islet sectional area (40 islets per rat) for each group was analyzed with the Image-Pro Plus 4.5 software (Media Cybernetics).
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of the Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| Insulin | Monoclonal anti-insulin | Product No. I 2018 | Monoclonal anti-insulin (mouse IgG1 isotype) | 1/500 |
Adrenal gland morphometric analyses
Left adrenal gland samples were removed and fixed in 10% formalin and embedded in paraffin (BIOTEC), and nonserial histological sections (5 μm thick) were cut with a Leica RM2145 semimotorized rotary microtome (Leica Biosystems) and stained with H&E. The morphometric analyses were performed using digital images (TIFF 24-bit color, 2560 × 1920 pixels) obtained with a light microscope (Olympus BX41) and a QColor 3 Olympus camera with a 2× objective. Adrenal gland area measurements from 20 images per rat (six rats per group) were analyzed with the Image-Pro Plus 4.5 software (Media Cybernetics).
Fat-pad accumulation measurements
At the end of the experimental procedures, the rats were euthanized and their fat-pad stores (retroperitoneal, periepididymal, inguinal, and mesenteric; n = 20 from five different litters) were removed and weighed. Each of the fat-pad store values were correlated with the bw of each rat and were calculated as g/100 kg bw.
Statistical analyses
The data are presented as SEM, and they were subjected to the D'Agostino Pearson normality test to assess their Gaussian distribution. Then, all data were subjected to Student t tests or one-way ANOVA followed by the Bonferroni test. P < .05 were considered significantly different. The statistical tests were performed using GraphPad Prism version 6.0 for Windows (GraphPad Software, Inc.).
Results
Effects of an IUPR diet
Body weight composition, catch-up growth, food intake, and fat stores
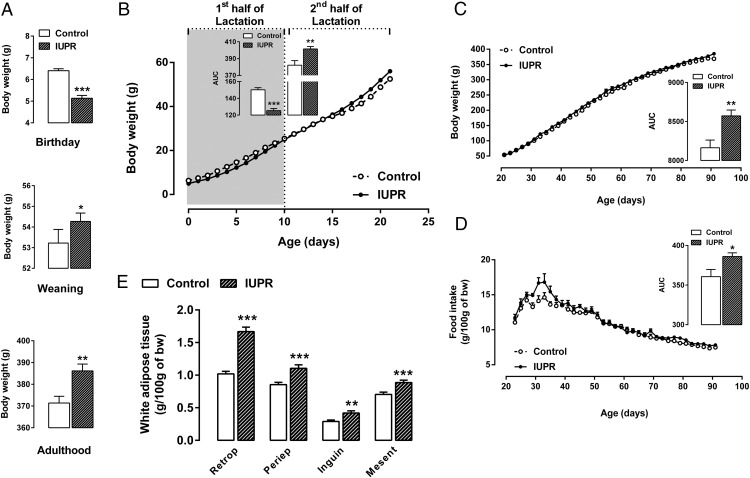
The litter size and the male/female rate per litter between the groups were not significantly different. However, the maternal low-protein diet during the last week of pregnancy induced an approximately 20% reduction in the offspring birth weights (P < .001; n = 30; Figure 1A). However, the IUPR rats caught up with the control-rat bw values at weaning and adulthood (+3%; P < .05; n = 30 and +4%; P < .01; n = 30, respectively; Figure 1A).
Figure 1. Body weights at birth, weaning and at adulthood (A), the bw evolution throughout lactation (B) and from weaning until adulthood (C), food intake levels from weaning until adulthood (D), and white adipose tissue weights at adulthood (E) are shown.
The data are expressed as the means ± SEM and were obtained from 30 rats (A–C), 20 rats (E) or five experimental group litters (D). The inserts on the left (B) and right (C and D) panels provide the AUC values, which were calculated from the first or the second half of the bw curve (B), from the entire bw curve (C) or from the food intake data (D). All the differences were analyzed with Student t tests, where *, P < .05; **, P < .01; ***, P < .001; and ns denoted samples were not significantly different. Abbreviations: Retrop, retroperitoneal fat; Periep, Periepididymal fat; Inguin, inguinal fat; and Mesent, mesenteric fat.
During the first half of lactation, the bw evolution AUCs from the IUPR rats were 16% lower than the control rats (P < .001; n = 30; Figure 1B). In contrast, a small catchup in growth was observed in the period from the second half of the suckling phase to weaning in the IUPR rats, as reflected by the bw AUCs (+5%; P < .01; n = 30; Figure 1B), which was maintained for the experimental period (+3%; P = .062; n = 30; Figure 1C). This catch-up growth could be explained by a higher food intake, after adjusting for bw, which was 7% higher in the IUPR group than in the control group (P < .05; n = 5 litters; Figure 1D).
The fat-pad store assessments showed that the IUPR rats had higher white adipose stores than the control rats (Figure 1E). The retroperitoneal, periepididymal, inguinal, and mesenteric fat-pad increments were, on average, 39% (P < .01; n = 20). In addition to the fat-pad increments, the Lee index in the IUPR rats was also higher when compared with the control rats (P < .001; n = 20; data not shown), even though their body lengths were not significantly different (P = .059; n = 20; data not shown).
Central action of insulin upon food intake
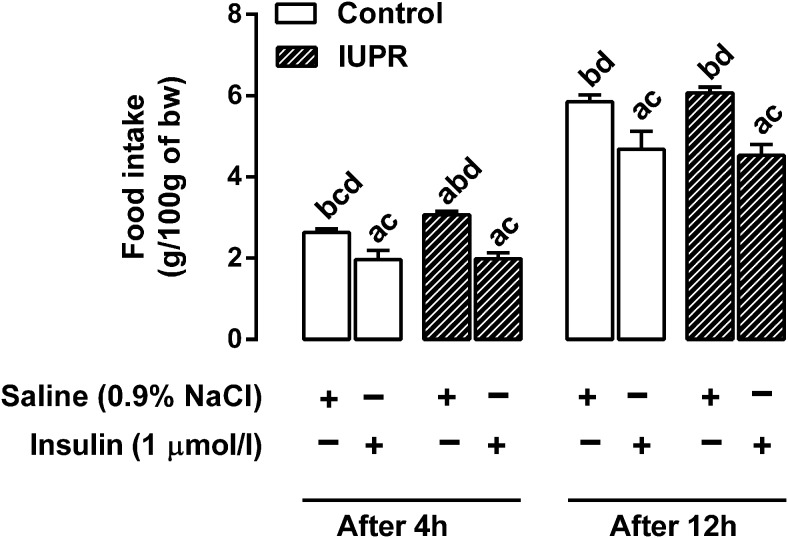
As expected, an insulin icv injection into the right lateral ventricle led to a reduction in food intake in both the control and IUPR groups, and it had acute and prolonged effects (Figure 2). A food ingestion assessment 4 hours after a saline icv injection showed that the IUPR rats were hyperphagic in relation to the control rats (+17%; P < .05; n = 6). The acute action (4 h after) of the insulin icv injection blocked food intake by approximately 25% in the controls and approximately 35% in the IUPR rats (P < .05; n = 6); however, these results were not significantly different between the groups. Twelve hours after the insulin icv injection, food intake was approximately 20% lower in the controls and approximately 25% lower in the IUPR rats when compared with their counterpart, saline-treated rats (P < .05; n = 6); however, no significant difference was found between the groups.
Figure 2. Ad libitum food intake in 12 h fasted (0700–1900 h) rats, 4 h (at 2300 h) and 12 h (at 0700 h) after saline or insulin icv injection.
The data are expressed as the means ± SEM of six rats from each experimental group and analyzed with one-way ANOVA, followed by the Bonferroni test for each batch of data separately (at 4 and 12 h after the icv injection). The letters on the bars denote significant differences between the groups (P < .05). A, Controls treated with saline, B, controls treated with insulin; C, IUPR treated with saline; and D, IUPR treated with insulin.
Biochemical parameters, lipid profile, and glucose homeostasis
As shown in Table 2, under fasting conditions, the IUPR rats displayed hyperglycemia (+21%; P < .001; n = 15) and hyperinsulinemia (+31%; P < .05; n = 15), which were associated with low ISI values (−27%, P < .01; n = 15). In addition, hypoadiponectinemia (−36%; P < .01; n = 8), hyperleptinemia (+80%; P < .05; n = 8), hypercorticosteronemia (+35%; P < .001; n = 8) and low levels of ACTH (−65%; P < .001; n = 8) were also found in the IUPR rats when compared with the controls. Regarding the lipid profile, whereas the triglyceride, total cholesterol, and very low-density lipoprotein cholesterol levels were not significantly different between the IUPR and control rats (P > .05; n = 8; data not shown), the LDL cholesterol was increased by 94% and the HDL cholesterol was reduced by 46% in the IUPR rats (P < .001; n = 8; Table 2). The Castelli indices I and II were higher (+64% and +234%, respectively) in the IUPR rats when compared with the control rats (P < .001; n = 8; Table 2).
Table 2.
Effect of a Low-protein Diet During the Last Week of Pregnancy on Glucose/Insulin Homeostasis, Plasma Hormones and the Lipid Profile of Male Rat Offspring at Age 91 d
| Parameters | Control | IUPR |
|---|---|---|
| Fasting glucose, mmol/L | 5.14 ± 0.12 | 6.22 ± 0.13c |
| Fasting insulin, pmol/L | 26.51 ± 1.32 | 34.74 ± 3.29a |
| ISI | 17.83 ± 0.76 | 13.08 ± 1.37b |
| Fasting adiponectin, nmol/L | 480.10 ± 27.17 | 307.80 ± 38.91b |
| Fasting leptin, pmol/L | 48.73 ± 12.59 | 87.88 ± 7.06a |
| Fasting corticosterone, nmol/L | 1364.0 ± 67.27 | 1846.0 ± 88.82c |
| Fasting ACTH, pmol/L | 57.83 ± 5.86 | 20.01 ± 4.01c |
| LDL cholesterol, μmol/L | 420.40 ± 45.68 | 815.70 ± 31.71c |
| HDL cholesterol, μmol/L | 1624.0 ± 134.0 | 872.0 ± 88.56c |
| Castelli index I | 1.53 ± 0.05 | 2. 52 ± 0.14c |
| Castelli index II | 0.30 ± 0.04 | 1.01 ± 0.10c |
All data are expressed as the means ± sem of 8–15 rats from at least five different litters.
The significant differences between the control and treated groups were obtained by Student t tests:
P < .05;
P < .01,
P < .001.
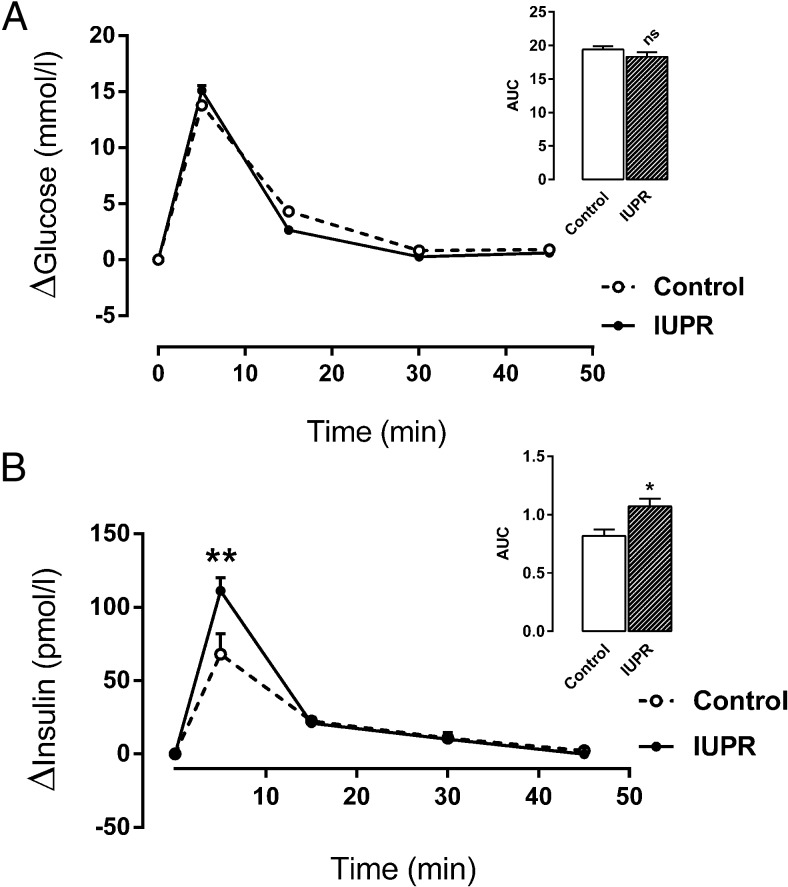
During the ivGTT, the blood glucose level increment was not changed between the groups (P = .202; n = 15; Figure 3A). However, the blood insulin level increment in the IUPR rats was higher in the first 5 minutes of ivGTT, leading to an AUC increment change of approximately 31% compared with the control rats (P < .01; n = 15; Figure 3B).
Figure 3. Plasma glucose and insulin concentrations from ivGTT evaluations.
Glycemia (A) and insulinemia (B) data are expressed as the means ± SEM from 15 rats from each experimental group. The inserts on the upper right panels show the AUC values that were calculated from the ivGTT results. The differences were evaluated by Student t tests where *, P < .05; **, P < .01, and ns denoted samples were not significantly different.
Pancreatic islet function and insulin secretion
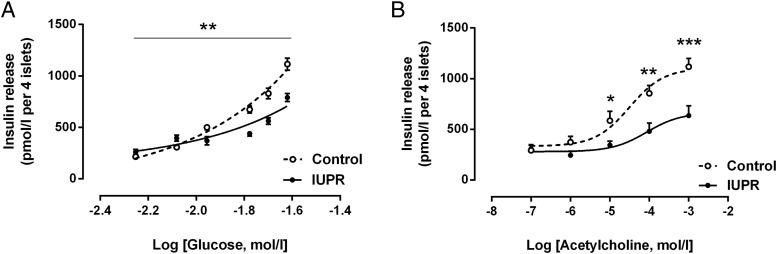
The capacity of the isolated IUPR rat pancreatic islets to secrete insulin was less responsive to high glucose concentrations. The insulin secretion under the basal (5.6 mmol/L glucose) and 8.3 mmol/L glucose conditions was higher in IUPR rat islets when compared with the control rat islets (P < .01; n = 6 rats from three litters; Figure 4A). In contrast, these islets were less responsive to high glucose concentrations (11.1, 16.7, 20.0, and 24.0 mmol/L). An incubation with increasing acetylcholine concentrations showed that the IUPR islets were less responsive to cholinergic action under high concentrations (P < .01, n = 6 rats from three litters; Figure 4B).
Figure 4. Insulin secretion from isolated pancreatic islets under the insulinotropic action of different glucose (A) and acetylcholine (B) concentrations.
The symbols represent the means ± SEM of the pancreatic islet insulin release levels, which were stimulated by glucose (5.6, 8.3, 11.1, 16.7, 20.0, and 24.0 mmol/L; A) or acetylcholine (0.1, 1.0, 10.0, 100.0, 1000.0 μmol/L; B). The pancreatic islets were obtained from six rats from three different litters of each experimental group. The significant differences between the control and IUPR groups for each glucose and acetylcholine concentration were determined by Student t tests, where *, P < .05; **, P < .01; and ***, P < .001.
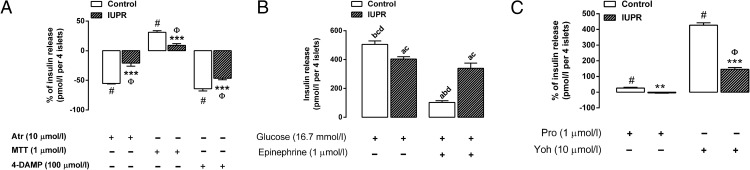
The islets' response to mAChR antagonists was reduced in the IUPR rat pancreatic islets. As depicted in Figure 5A, when the nonselective mAChR antagonist, Atr, was added, it blocked insulin secretion by 55% in the controls and 21% in the IUPR islets (P < .001, n = 6 rats from three litters). Along the same line, MTT, a selective antagonist for the M2mAChR subtype, increased insulin secretion by 31% in the controls and only 9% in the IUPR rat islets. In addition, 4-DAMP, a selective antagonist for the M3mAChR subtype, decreased insulin secretion by 64% in the controls and only 46% in the IUPR islets (P < .001; n = 6 rats from three litters).
Figure 5. Insulin secretion from isolated pancreatic islets under the action of mAChR antagonists (A), epinephrine (B), or adrenoceptor antagonists (C).
The bars represent the means ± SEM of the insulin secretion levels from the pancreatic islets of six rats from three different litters. The line at 0 represents 100% of the glucose-induced insulin release under the effect of 8.3 mmol/L glucose plus 10 μmol/L acetylcholine (A) or 16.7 mmol/L glucose plus 1 μmol/L epinephrine (C). The bars above or below the line at 0 (A and C) represent the increased or decreased glucose-induced insulin release percentages that were altered by the following treatments ([μmol/L]: Atr, 10; MTT, 1; 4-DAMP, 100; Pro, 1; and Yoh, 10). Insulin secretion induced by 16.7 mmol/L glucose plus 1 μmol/L epinephrine (B). The symbols over the bars refer to the significance levels, as follows: #, P < .01 for the control group and Φ, P < .01 for the IUPR group for each indicated antagonist treatment compared with the 0 lines in parts A and C; ***, P < .001; **, P < .01 between the control and IUPR groups for each respective antagonist treatment, as determined by Student t tests. The letters over the bars (Figure B) represent significant differences between the glucose and epinephrine treatments among the control and IUPR groups, as determined by one-way ANOVAs, in which A, controls were treated with glucose; B, IUPR rats were treated with glucose; C, controls were treated with epinephrine; and D, IUPR rats were treated with epinephrine.
In comparison with the control rat islets, the IUPR rat pancreatic islets secreted 20% less insulin (P < .01, n = 6 rats from three litters; Figure 5B) when incubated with 16.7 mmol/L of glucose. The effect of epinephrine on blocking insulin secretion was 80% in the isolated pancreatic islets from the control rats (P < .001; n = 6 rats from three litters; Figure 5B), whereas it was only 16% in the IUPR rat islets. However, there were no significant insulin secretion differences with the glucose alone and glucose plus epinephrine treatments in the IUPR group (P = .089; n = 6 rats from three litters; Figure 5B).
Regarding insulin secretion under the action of adrenoceptor antagonists, a reduction in the IUPR rat pancreatic islet responsiveness was observed. When compared with insulin secretion following epinephrine treatment, Pro (β2-adrenoceptor antagonist) increased the insulin secretion level by 26% in the control islets (P < .01; n = 6 rats from three litters; Figure 5C) and reduced it by 4% in the IUPR islets (P = .083; n = 6 rats from three litters; Figure 5C). These values were significantly different between the control and IUPR groups (P < .01; n = 6 rats from three litters; Figure 5C). In contrast, Yoh (α2-adrenoceptor antagonist) promoted insulin secretion in both the control and IUPR groups compared with insulin secretion following epinephrine treatment in their counterpart islet groups. Nonetheless, insulin secretion was increased approximately 4.3-fold in the control islets, whereas it was increased only 1.5-fold in the IUPR islets (P < .001, n = 6 rats from three litters; Figure 5C). These values were significantly different between the control and IUPR groups (P < .001; n = 6 rats from three litters; Figure 5C).
Adipose tissue, adrenal gland, liver, and pancreatic morphology
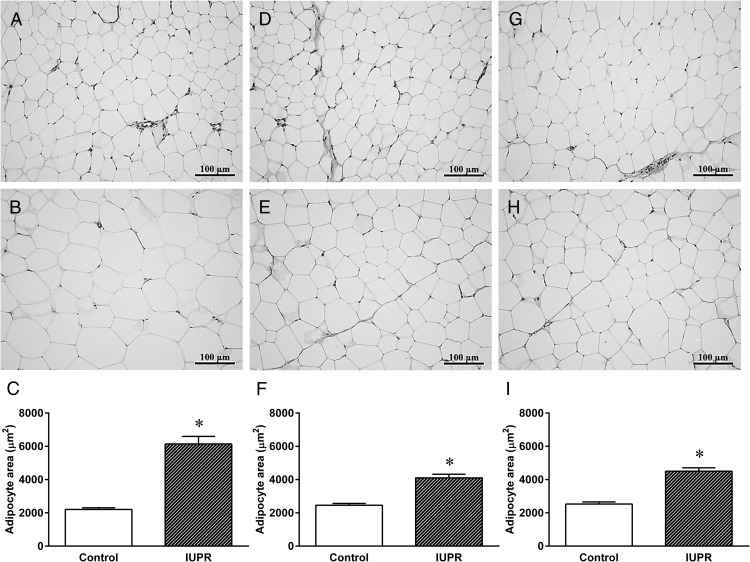
As depicted in Figure 6, H&E staining showed larger adipocytes (retroperitoneal, periepididymal, and inguinal; Figure 6, B, E, and H, respectively) in the IUPR rats when compared with the normal adipocyte histology from the controls (Figure 6, A, D, and G). The IUPR rats displayed bigger adipocyte areas in the retroperitoneal (+179%), periepididymal (+67%), and inguinal fat stores (+78%) compared with the controls (Figure 6, C, F, and I, respectively; P < .001; n = 6).
Figure 6. Adipose tissue morphometric analysis of the rats at adulthood.
Representative photomicrography results of the retroperitoneal (A, B), periepididymal (D, E), and inguinal (G, H) adipose tissues from control (six rats) and IUPR rats (six rats), respectively. Quantitative analyses of the adipocytes areas are shown in (C, F, and I) for the control and IUPR groups. All data are expressed as the means ± SEM from six rats from each experimental group. The differences were analyzed by Student t tests where ***, P < .001. Photomicrography images show adipose tissue stained with H&E (20× objective magnification, bars = 100 μm).
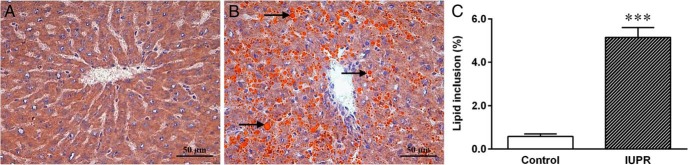
Figure 7 shows that in the hepatic tissue, the Red Oil staining in the IUPR rat tissue (n = 6) was morphologically changed. Qualitative analysis showed that the IUPR rat livers displayed increased amounts of lipid inclusions (Figure 7B) compared with the normal hepatocyte histology from the controls (Figure 7A). A quantitative analysis showed that the lipid inclusion in the liver was approximately 8.8-fold higher in the IUPR rats than in the control rats (P < .001; n = 6; Figure 7C).
Figure 7. Liver morphology from the rats at adulthood.
Representative photomicrography of the normal-seeming control livers (A; six rats) and of the lipid inclusion in the IUPR group livers (B; six rats). The arrows (→) suggest the orange lipid droplets, which show steatosis in the IUPR group livers. The photomicrography shows a liver stained with Red Oil (40× objective magnification, bars = 50 μm). Quantitative liver morphology analysis is shown in Figure 7C. The results are expressed as the means ± SEM of six rats for each experimental group and were analyzed by Student t tests where ***, P < .001.
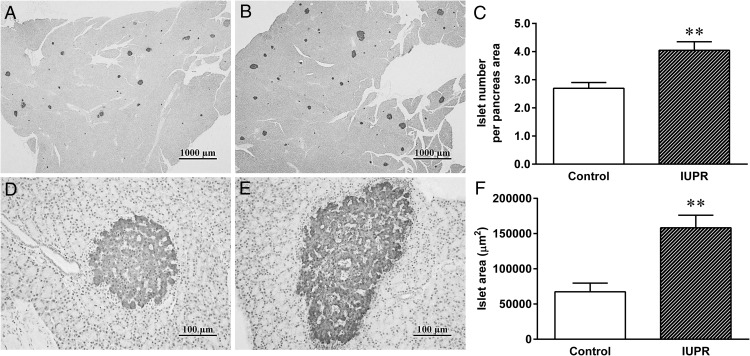
Insulin-immunostaining of the pancreas displayed modifications in the number and area of pancreatic islets in the IUPR rats (n = 6; Figure 8, B and E) when compared with the controls (n = 6; Figure 8, A and D). Quantification of the IUPR rat tissue displayed higher amounts (+50%) and higher areas (+135%) of pancreatic islets than in the controls (P < .01; n = 6; Figure 8, C and F, respectively).
Figure 8. Pancreatic morphometric analysis of the rats at adulthood.
Representative photomicrography of endocrine pancreases that were immunostained for insulin from the control (A, 2×; and D, 20× objective magnification) and IUPR groups (B, 2×; and E 2× objective magnification). Quantitative analyses of the pancreatic islets (number per area and area) are shown in parts C and F, respectively. The results are expressed as the means ± SEM of six rats for each experimental group and were analyzed with Student t tests, **, P < .01. In parts A and B, the bars = 1000 μm, and in the parts D and E, the bars = 100 μm.
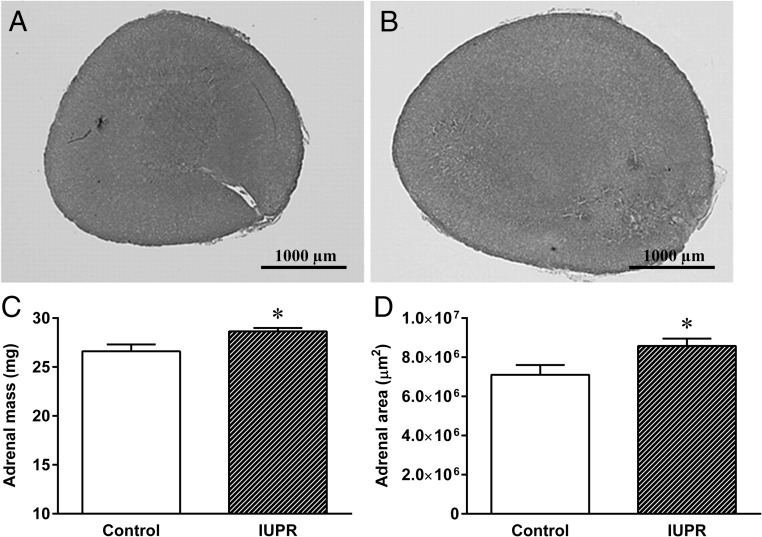
As shown in Figure 9, H&E staining from the left adrenal glands depicted larger gland masses in the IUPR rats (Figure 9B) than in the controls (Figure 9A). A quantitative analysis showed that the IUPR rats displayed a slight increase in the mass (+8%) and an increase in the area (+20%) of the left adrenal gland compared with the controls (P < .05; n = 6; Figure 9, C and D, respectively.
Figure 9. Adrenal gland morphometric analysis from the rats at adulthood.
Representative adrenal gland photomicrographs are shown for the control (A) and IUPR (B) groups. Quantitative adrenal gland mass (C) and adrenal gland area (D) analyses are displayed. The adrenal photomicrography images were stained with H&E (2× objective magnification, bars = 1000 μm). The results are expressed as the means ± SEM of six rats for each experimental group and were analyzed with Student t tests, *, P < .05.
Discussion
To our knowledge, this is the first direct demonstration that maternal undernutrition due to a low-protein diet in the last third of pregnancy induces glucose-insulin homeostasis dysfunction in adult male rat offspring that corroborates the Dutch famine study in which maternal food deprivation, especially in later gestation, was associated with glucose intolerance, insulin resistance, and obesity in young adults (45). In addition, we showed a clear impairment in the pancreatic islets to secrete insulin due to the action of the autonomous nervous system as well as a derangement in other metabolic parameters, implicating a risk of obesity-associated metabolic complications in adult male rat offspring.
The last third of pregnancy is a critical period for fetal growth and organ/tissue maturation (particularly the wiring of the hypothalamic neuronal pathways) and a time of particular sensitivity to environmental stressors (21, 22). Our data show that undernutrition induced by a low-protein diet in pregnant dams during the last third of pregnancy programmed their offspring to be prone to metabolic syndrome development in adulthood. In the current study, the IUPR rats were overweight, hyperphagic, hyperglycemic, and had a higher accumulation of visceral adipose tissue, which is associated with hyperleptinemia, hyperinsulinemia, hypoadiponectinemia, and peripheral insulin resistance. Despite higher insulin secretion levels, the pancreas responsiveness was inadequate to several insulin stimulants and inhibitory agents.
The low pancreatic islet insulin secretion responsiveness in the IUPR rats following acetylcholine treatment suggests an impairment in autonomous nervous system function, especially the insulinotropic receptors, indicating the weak capacity of the M3mAChR subtype.
The insulinotropic effect of MTT (through the inhibition of the M2mAChR subtype) was 71% lower in the IUPR islets, and the insulinostatic effect of 4-DAMP (by the blockade of the M3mAChR subtype) was 28% lower in the IUPR islets than in the control rat islets; both of which implies that M3mAChR had a decreased acetylcholine responsiveness compared with M2mAChR in the IUPR rat islets. This decreased acetylcholine responsiveness contributes to the physiological cholinergic response being drastically reduced, as we found in this study. Indeed, we also observed reduced insulinotropic effects for the β2-adrenoceptor and α2-adrenoceptor. Taken together, these results suggest a disturbance of the nerve terminals of the autonomous nervous system that converges on the pancreatic islets in the IUPR rats.
The normoglycemia exhibited by the IUPR rats during the ivGTT may be explained by their hyperinsulinemia. However, we found higher amounts and larger pancreatic islets in the IUPR rats, and these islets were unable to secrete enough insulin under high-glucose demands in an in vitro experiment. In fact, in basal conditions (5.6 mmol/L) as well as in slightly high glucose conditions (8.3 mmol/L), pancreatic islets exhibited a normal or even higher capacity to secrete insulin. In contrast, these pancreatic islets were unable to secrete enough insulin under high glucose conditions (11.1–24.0 mmol/L). In vivo, the IUPR rats presented a slightly higher glycemic level; therefore, insulin was being secreted in higher amounts. As these mice age, it is possible that these animals develop a higher glycemic level and, consequently, pancreatic β-cell failure, leading to overt diabetes.
In the present study, the higher plasma corticosterone levels may explain the hyperglycemia combined with the insulin resistance in the adult IUPR rats. In addition, we observed hypertrophy of the adrenal gland in the IUPR rats, suggesting a primary adrenal gland dysfunction because the ACTH level was lower (54). In line with this observation, maternal food restriction for a short period during later gestation has been associated with increased cortisol levels without changes in ACTH levels in a guinea pig fetus model (55). In contrast, intrauterine food deprivation during the last half of gestation programmed an HPA axis–associated neuroendocrine malfunction in adult offspring as a long-term consequence, which increased the susceptibility to metabolic diseases when the animals were exposed to a high-fat diet and/or stress conditions (56). In addition, this result suggests that those alterations could be imprinted as soon as these animals are born. Here, the IUPR rats were hyperphagic and displayed higher fat-pad accumulation and hepatic steatosis. It is known that glucocorticoids can induce and/or amplify bw gain and hyperphagia in rats (57). In addition, hyperinsulinemia and peripheral insulin-resistance can contribute to the obese phenotype in IUPR rats. Accordingly, obese and type 2 diabetic patients with a hyperphagic behavioral pattern have been associated with high cortisol and low ACTH blood levels (58).
We did not observe central insulin resistance when injecting insulin via icv in the lateral ventricle. This effect seems to be contradictory to the hyperphagia found in IUPR rats, as the major effect of insulin on the hypothalamic nucleus is to inhibit food intake through the blockage of neuropeptide-Y neurons (59). However, the chronic action of glucocorticoids along with leptin resistance upon central neuronal pathways may play preponderant roles over insulin in food intake regulation. As insulin is higher in the serum when an animal is hyperphagic, it is also possible that there was an impairment in insulin transport through the blood brain barrier.
Regarding the oil red detection of neutral lipid inclusion in the liver samples that were present in large amounts in the IUPR rat livers, we highlight here that a low-protein diet during last third of pregnancy led to the establishment of hepatic steatosis in the IUPR rats' adult offspring. Moreover, our study showed a cholesterol imbalance of high LDL and low HDLcholesterol plasma levels, even though there were not any changes in the triglycerides. A previous study showed that cholesterol dysregulation in offspring whose dams were fed a protein-restricted diet (8%) during pregnancy and lactation could cause early program changes through epigenetic mechanisms by histone modification, which repressed the cholesterol 7α-hydroxylase promoter (60). Along this line, glucocorticoids have been reported to impair DNA methylation and changes in histone modifications in the hepatocytes of rat offspring from dams fed a protein-restricted diet during their entire pregnancy (61). In addition, the higher Castelli index I and II levels in the IUPR rats suggests an increased atherogenesis risk. The Castelli index has been reported as an important indicator for atherosclerosis risk (52). Moreover, the hypoadiponectinemia that was present in the IUPR rats is another risk factor for atherogenesis. Adiponectin, through its vascular action in stimulating nitric oxide production in endothelial cells, has an important role in preventing atherogenesis (62).
In accordance with our data, we can infer that the hormonal changes (hyperinsulinemia, hypercorticosteronemia, hyperleptinemia and hypoadiponectinemia) observed herein are important cross-talking factors in the development and maintenance of obesity and metabolic syndrome in IUPR rats. Alterations in the blood levels of these hormones are closely implicated in metabolic syndrome (63, 64).
In summary, the data described in the current study provide strong evidence that maternal protein malnutrition, at critical periods, especially during the last third of pregnancy, can malprogram the offspring's corticosterone production capacity and increase its susceptibility to hepatic steatosis, endocrine pancreas malfunction/exhaustion and cardiometabolic disease onset as long-term consequences. This evidence is in agreement with studies evaluating Dutch famine subjects, which strengthens the importance of our study and highlights the notion that proper nutrition is essential during pregnancy.
Acknowledgments
P.C.F.M. is a recipient of the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior fellowship at the Institute Polytechnique LaSalle Beauvais, EGEAL-UP (Expression de gènes et régulation épigénétique par l'aliment - Unité Prope, Beauvais Cedex, France).
This work was supported by the Brazilian Federal Foundation, Conselho Nacional de Desenvolvimento Científico e Tecnológico, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Paraná Science Foundation (Fundação Araucária), and the Carlos Chagas Filho Research Foundation of the State of Rio de Janeiro (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro).
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the Brazilian Federal Foundation, Conselho Nacional de Desenvolvimento Científico e Tecnológico, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Paraná Science Foundation (Fundação Araucária), and the Carlos Chagas Filho Research Foundation of the State of Rio de Janeiro (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro).
Footnotes
- 4-DAMP
- 4-diphenylacetoxy-N-methyl-piperidine methiodide
- Atr
- atropine
- AUC
- area under the curve
- bw
- body weight
- H&E
- hematoxylin and eosin
- HDL
- high-density lipoprotein
- HPA
- hypothalamic-pituitary-adrenal axis
- icv
- intracerebroventricular
- ISI
- insulin sensitivity index
- IUPR
- intrauterine protein restricted
- ivGTT
- intravenous glucose tolerance test
- LDL
- low-density lipoprotein
- mAChR
- muscarinic acetylcholine receptor
- MTT
- methoctramine.
References
- 1. O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. Am J Physiol Endocrinol Metab. 2004;287:E863–E870. [DOI] [PubMed] [Google Scholar]
- 2. Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: The thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. [DOI] [PubMed] [Google Scholar]
- 3. Hales CN. Metabolic consequences of intrauterine growth retardation. Acta Paediatr Suppl. 1997;423:184–187; discussion 188. [DOI] [PubMed] [Google Scholar]
- 4. Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab. 2000;279:E83–E87. [DOI] [PubMed] [Google Scholar]
- 5. Barker DJ. Intrauterine programming of adult disease. Mol Med Today. 1995;1:418–423. [DOI] [PubMed] [Google Scholar]
- 6. Brandão AP, Brandão AA, de Magalhães ME, Pozzan R. Management of metabolic syndrome in young population. Am J Ther. 2008;15:356–361. [DOI] [PubMed] [Google Scholar]
- 7. Tavares FG, Coimbra Junior CE, Cardoso AM. [Blood pressure levels of Suruí indigenous adults in Rondônia, Brazil]. Cien Saude Colet. 2013;18:1399–1409. [PubMed] [Google Scholar]
- 8. Vaag AA, Grunnet LG, Arora GP, Brøns C. The thrifty phenotype hypothesis revisited. Diabetologia. 2012;55:2085–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fewtrell MS, Doherty C, Cole TJ, Stafford M, Hales CN, Lucas A. Effects of size at birth, gestational age and early growth in preterm infants on glucose and insulin concentrations at 9–12 years. Diabetologia. 2000;43:714–717. [DOI] [PubMed] [Google Scholar]
- 10. Petry CJ, Ozanne SE, Wang CL, Hales CN. Effects of early protein restriction and adult obesity on rat pancreatic hormone content and glucose tolerance. Horm Metab Res. 2000;32:233–239. [DOI] [PubMed] [Google Scholar]
- 11. Gravena C, Andreazzi AE, Mecabo FT, Grassiolli S, Scantamburlo VM, Mathias PC. Protein restriction during lactation alters the autonomic nervous system control on glucose-induced insulin secretion in adult rats. Nutr Neurosci. 2007;10:79–87. [DOI] [PubMed] [Google Scholar]
- 12. de Oliveira JC, Miranda RA, Barella LF, et al. . Impaired β-cell function in the adult offspring of rats fed a protein-restricted diet during lactation is associated with changes in muscarinic acetylcholine receptor subtypes. Br J Nutr. 2014;111:227–235. [DOI] [PubMed] [Google Scholar]
- 13. de Oliveira JC, Scomparin DX, Andreazzi AE, et al. . Metabolic imprinting by maternal protein malnourishment impairs vagal activity in adult rats. J Neuroendocrinol. 2011;23:148–157. [DOI] [PubMed] [Google Scholar]
- 14. Fernandez-Twinn DS, Wayman A, Ekizoglou S, Martin MS, Hales CN, Ozanne SE. Maternal protein restriction leads to hyperinsulinemia and reduced insulin-signaling protein expression in 21-mo-old female rat offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288:R368–R373. [DOI] [PubMed] [Google Scholar]
- 15. Zambrano E, Bautista CJ, Deás M, et al. . A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J Physiol. 2006;571:221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ross MG, Beall MH. Adult sequelae of intrauterine growth restriction. Semin Perinatol. 2008;32:213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Desai M, Crowther NJ, Ozanne SE, Lucas A, Hales CN. Adult glucose and lipid metabolism may be programmed during fetal life. Biochem Soc Trans. 1995;23:331–335. [DOI] [PubMed] [Google Scholar]
- 18. Desai M, Gayle D, Han G, Ross MG. Programmed hyperphagia due to reduced anorexigenic mechanisms in intrauterine growth-restricted offspring. Reprod Sci. 2007;14:329–337. [DOI] [PubMed] [Google Scholar]
- 19. Laborie C, Molendi-Coste O, Breton C, et al. . Maternal perinatal undernutrition has long-term consequences on morphology, function and gene expression of the adrenal medulla in the adult male rat. J Neuroendocrinol. 2011;23:711–724. [DOI] [PubMed] [Google Scholar]
- 20. Coupé B, Dutriez-Casteloot I, Breton C, et al. . Perinatal undernutrition modifies cell proliferation and brain-derived neurotrophic factor levels during critical time-windows for hypothalamic and hippocampal development in the male rat. J Neuroendocrinol. 2009;21:40–48. [DOI] [PubMed] [Google Scholar]
- 21. Bouret SG, Simerly RB. Developmental programming of hypothalamic feeding circuits. Clin Genet. 2006;70:295–301. [DOI] [PubMed] [Google Scholar]
- 22. Plagemann A, Waas T, Harder T, Rittel F, Ziska T, Rohde W. Hypothalamic neuropeptide Y levels in weaning offspring of low-protein malnourished mother rats. Neuropeptides. 2000;34:1–6. [DOI] [PubMed] [Google Scholar]
- 23. Sebaai N, Lesage J, Breton C, Vieau D, Deloof S. Perinatal food deprivation induces marked alterations of the hypothalamo-pituitary-adrenal axis in 8-month-old male rats both under basal conditions and after a dehydration period. Neuroendocrinology. 2004;79:163–173. [DOI] [PubMed] [Google Scholar]
- 24. Stern WC, Forbes WB, Resnick O, Morgane PJ. Seizure susceptibility and brain amine levels following protein malnutrition during development in the rat. Brain Res. 1974;79:375–384. [DOI] [PubMed] [Google Scholar]
- 25. Resnick O, Miller M, Forbes W, Hall R, Kemper T, Bronzino J, Morgane PJ. Developmental protein malnutrition: Influences on the central nervous system of the rat. Neurosci Biobehav Rev. 1979;3:233–246. [DOI] [PubMed] [Google Scholar]
- 26. Morgane PJ, Mokler DJ, Galler JR. Effects of prenatal protein malnutrition on the hippocampal formation. Neurosci Biobehav Rev. 2002;26:471–483. [DOI] [PubMed] [Google Scholar]
- 27. Smart JL, Dobbing J. Vulnerability of developing brain. VI. Relative effects of foetal and early postnatal undernutrition on reflex ontogeny and development of behaviour in the rat. Brain Res. 1971;33:303–314. [DOI] [PubMed] [Google Scholar]
- 28. Morgane PJ, Austin-LaFrance R, Bronzino J, et al. . Prenatal malnutrition and development of the brain. Neurosci Biobehav Rev. 1993;17:91–128. [DOI] [PubMed] [Google Scholar]
- 29. Markakis EA. Development of the neuroendocrine hypothalamus. Front Neuroendocrinol. 2002;23:257–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chamson-Reig A, Thyssen SM, Hill DJ, Arany E. Exposure of the pregnant rat to low protein diet causes impaired glucose homeostasis in the young adult offspring by different mechanisms in males and females. Exp Biol Med. 2009;234:1425–1436. [DOI] [PubMed] [Google Scholar]
- 31. Chamson-Reig A, Thyssen SM, Arany E, Hill DJ. Altered pancreatic morphology in the offspring of pregnant rats given reduced dietary protein is time and gender specific. J Endocrinol. 2006;191:83–92. [DOI] [PubMed] [Google Scholar]
- 32. Fagundes AT, Moura EG, Passos MC, et al. . Maternal low-protein diet during lactation programmes body composition and glucose homeostasis in the adult rat offspring. Br J Nutr. 2007;98:922–928. [DOI] [PubMed] [Google Scholar]
- 33. Breton C, Lukaszewski MA, Risold PY, et al. . Maternal prenatal undernutrition alters the response of POMC neurons to energy status variation in adult male rat offspring. Am J Physiol Endocrinol Metab. 2009;296:E462–E472. [DOI] [PubMed] [Google Scholar]
- 34. Plagemann A, Harder T, Rake A, Melchior K, Rohde W, Dörner G. Hypothalamic nuclei are malformed in weanling offspring of low protein malnourished rat dams. J Nutr. 2000;130:2582–2589. [DOI] [PubMed] [Google Scholar]
- 35. Orozco-Sólis R, Lopes de Souza S, Barbosa Matos RJ, et al. . Perinatal undernutrition-induced obesity is independent of the developmental programming of feeding. Physiol Behav. 2009;96:481–492. [DOI] [PubMed] [Google Scholar]
- 36. Delahaye F, Breton C, Risold PY, et al. . Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology. 2008;149:470–475. [DOI] [PubMed] [Google Scholar]
- 37. Molendi-Coste O, Grumolato L, Laborie C, et al. . Maternal perinatal undernutrition alters neuronal and neuroendocrine differentiation in the rat adrenal medulla at weaning. Endocrinology. 2006;147:3050–3059. [DOI] [PubMed] [Google Scholar]
- 38. Khorram O, Han G, Bagherpour R, et al. . Effect of maternal undernutrition on vascular expression of micro and messenger RNA in newborn and aging offspring. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1366–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Garcia-Souza EP, da Silva SV, Félix GB, et al. . Maternal protein restriction during early lactation induces GLUT4 translocation and mTOR/Akt activation in adipocytes of adult rats. Am J Physiol Endocrinol Metab. 2008;295:E626–636. [DOI] [PubMed] [Google Scholar]
- 40. Inoue T, Kido Y, Asahara S, et al. . Effect of intrauterine undernutrition during late gestation on pancreatic beta cell mass. Biomed Res. 2009;30:325–330. [DOI] [PubMed] [Google Scholar]
- 41. Bertin E, Gangnerau MN, Bailbé D, Portha B. Glucose metabolism and beta-cell mass in adult offspring of rats protein and/or energy restricted during the last week of pregnancy. Am J Physiol. 1999;277:E11–E17. [DOI] [PubMed] [Google Scholar]
- 42. Bertin E, Gangnerau MN, Bellon G, Bailbé D, Arbelot De Vacqueur A, Portha B. Development of beta-cell mass in fetuses of rats deprived of protein and/or energy in last trimester of pregnancy. Am J Physiol Regul Integr Comp Physiol. 2002;283:R623–R630. [DOI] [PubMed] [Google Scholar]
- 43. Léonhardt M, Lesage J, Croix D, Dutriez-Casteloot I, Beauvillain JC, Dupouy JP. Effects of perinatal maternal food restriction on pituitary-gonadal axis and plasma leptin level in rat pup at birth and weaning and on timing of puberty. Biol. Reprod. 2003;68:390–400. [DOI] [PubMed] [Google Scholar]
- 44. Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70:811–816. [DOI] [PubMed] [Google Scholar]
- 45. Ravelli AC, van der Meulen JH, Michels RP, et al. . Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351:173–177. [DOI] [PubMed] [Google Scholar]
- 46. Malta A, de Moura EG, Ribeiro TA, et al. . Protein-energy malnutrition at mid-adulthood does not imprint long-term metabolic consequences in male rats [published online July 2, 2015]. Eur J Nutr. Available online at: http://link.springer.com/article/10.1007/s00394-015-0960-8/fulltext.html. [DOI] [PubMed]
- 47. Camargo RL, Torrezan R, de Oliveira JC, et al. . An increase in glucose concentration in the lateral ventricles of the brain induces changes in autonomic nervous system activity. Neurol Res. 2013;35:15–21. [DOI] [PubMed] [Google Scholar]
- 48. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed. ed Burlington, MA: Elsevier Academic Press, 2005. [Google Scholar]
- 49. Stoppa GR, Cesquini M, Roman EA, et al. . Intracerebroventricular injection of citrate inhibits hypothalamic AMPK and modulates feeding behavior and peripheral insulin signaling. J Endocrinol. 2008;198:157–168. [DOI] [PubMed] [Google Scholar]
- 50. Ropelle ER, Pauli JR, Prada P, et al. . Inhibition of hypothalamic Foxo1 expression reduced food intake in diet-induced obesity rats. J Physiol. 2009;587:2341–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. [DOI] [PubMed] [Google Scholar]
- 52. Fagundes AT, Moura EG, Passos MC, et al. . Temporal evaluation of body composition, glucose homeostasis and lipid profile of male rats programmed by maternal protein restriction during lactation. Horm Metab Res. 2009;41:866–873. [DOI] [PubMed] [Google Scholar]
- 53. de Oliveira JC, Lisboa PC, de Moura EG, et al. . Poor pubertal protein nutrition disturbs glucose-induced insulin secretion process in pancreatic islets and programs rats in adulthood to increase fat accumulation. J Endocrinol. 2013;216:195–206. [DOI] [PubMed] [Google Scholar]
- 54. Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab. 2006;291:E965–E973. [DOI] [PubMed] [Google Scholar]
- 55. Lingas R, Dean F, Matthews SG. Maternal nutrient restriction (48 h) modifies brain corticosteroid receptor expression and endocrine function in the fetal guinea pig. Brain Res. 1999;846:236–242. [DOI] [PubMed] [Google Scholar]
- 56. Zhang L, Xu D, Zhang B, et al. . Prenatal food restriction induces a hypothalamic-pituitary-adrenocortical axis-associated neuroendocrine metabolic programmed alteration in adult offspring rats. Arch Med Res. 2013;44:335–345. [DOI] [PubMed] [Google Scholar]
- 57. Smart JL, Tolle V, Low MJ. Glucocorticoids exacerbate obesity and insulin resistance in neuron-specific proopiomelanocortin-deficient mice. J Clin Invest. 2006;116:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Benbaibeche H, Haffaf el M, Kacimi G, Oudjit B, Khan NA, Koceir EA. Implication of corticotropic hormone axis in eating behaviour pattern in obese and type 2 diabetic participants. Br J Nutr. 2015;113:1237–1243. [DOI] [PubMed] [Google Scholar]
- 59. Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. [DOI] [PubMed] [Google Scholar]
- 60. Sohi G, Marchand K, Revesz A, Arany E, Hardy DB. Maternal protein restriction elevates cholesterol in adult rat offspring due to repressive changes in histone modifications at the cholesterol 7alpha-hydroxylase promoter. Mol Endocrinol. 2011;25:785–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lillycrop KA, Slater-Jefferies JL, Hanson MA, Godfrey KM, Jackson AA, Burdge GC. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br J Nutr. 2007;97:1064–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Han SH, Quon MJ, Kim JA, Koh KK. Adiponectin and cardiovascular disease: Response to therapeutic interventions. J Am Coll Cardiol. 2007;49:531–538. [DOI] [PubMed] [Google Scholar]
- 63. la Fleur SE, Akana SF, Manalo SL, Dallman MF. Interaction between corticosterone and insulin in obesity: Regulation of lard intake and fat stores. Endocrinology. 2004;145:2174–2185. [DOI] [PubMed] [Google Scholar]
- 64. Yadav A, Jyoti P, Jain SK, Bhattacharjee J. Correlation of adiponectin and leptin with insulin resistance: A pilot study in healthy north Indian population. Indian journal of clinical biochemistry : IJCB. 2011;26:193–196. [DOI] [PMC free article] [PubMed] [Google Scholar]