Abstract
Frailty is a distinct phenotype that is highly prevalent in chronic kidney disease (CKD) and appears to be more prevalent with declining GFR. Exercise training or intervention to increase physical activity may ameliorate poor physical functioning and frailty, and may even improve survival in patients with CKD. Although exercise interventions improve outcomes across the spectrum of CKD, including patients treated with dialysis, patients treated with dialysis face barriers to exercise that patients with pre-dialysis CKD do not. Rehabilitation at earlier stages of CKD (or “prehabilitation” before dialysis) might be more beneficial than not addressing the declining physical functioning and low physical activity until patients are receiving dialysis. This review summarizes available literature on frailty in the CKD and ESRD population, including exercise interventions and the limited evidence for “prehabilitation” as a strategy.
Frailty in the CKD and ESRD population
Frailty can result from accumulation of many small insults that lead to increasing vulnerability and lack of functional reserve over time.1 Fried et al. defined frailty as a clinical syndrome in which 3 or more of the following were present: unintentional weight loss (10 lbs in the past year), self-reported exhaustion, weakness (grip strength), slow walking speed, and low physical activity. In this construct, frailty is considered as a distinct phenotype in which comorbidity is a potential etiology and disability a possible outcome.2 Frailty lies at the intersection of numerous physiological systems and is an aggregate result of decrements in function, which can include immune dysfunction, inflammation, sarcopenia, cognitive deficits, among others; no single system is responsible for frailty.3
Patients with chronic kidney disease (CKD) have a high prevalence of frailty compared to the general population.4-6 Shlipak et al. studied patients enrolled in the Cardiovascular Health Study, examining whether chronic renal insufficiency (CRI, defined as Cr ≥ 1.5 mg/dL in men and ≥ 1.3 mg/dL in women) was associated with frailty. In this cross-sectional analysis, among the 5,808 patients with measured creatinine, the prevalence of frailty was higher in those with CRI (15%) than those without (6%), and after multivariate adjustment, CRI remained significantly associated with frailty (OR 1.76, 95% CI 1.28 – 2.41).4
Unfortunately, the majority of studies associating CKD with frailty are cross-sectional,7 but frailty does appear to become more prevalent with declining GFR. Roshanravan et al. studied 336 patients with CKD stages 1-4 to determine the prevalence and determinants of frailty in the CKD population as well as its association with a composite outcome of all-cause mortality or renal replacement therapy. They also found that the prevalence of frailty was about 14%, nearly twice as high as in a reference population of controls.5 The higher prevalence appeared to be driven primarily by more physical inactivity and exhaustion in the patients with CKD. After multivariate adjustment, eGFRcys < 30 and eGFRcys of 30-44 ml/min/1.73 m2 were associated with 2.8-fold (95%CI 1.3 – 6.3) and 2.1-fold (95% CI 1.0 – 4.7) higher prevalence of frailty respectively, as compared to those with eGFRcys > 60.
The prevalence has been even higher in cohorts of patients treated with dialysis. The first study to examine frailty among 2275 incident dialysis patients enrolled in the Dialysis Morbidity and Mortality Study (DMMS) Wave 2 found that two-thirds were frail. Although elderly patients were more likely to be frail, 44% of patients under 40 were frail, as well as 61.1% of those aged 40-50. Women were more likely to be frail than men (OR 1.55, 95% CI 1.27 – 1.88). Those with diabetes and history of stroke were also more likely to be frail, as well as those without permanent dialysis access. Peritoneal dialysis (PD) patients were less likely to be frail than hemodialysis (HD) patients, with an OR of 0.80 (95% CI 0.65 – 0.97). Despite its high prevalence, frailty was associated with outcomes in this cohort similar to in community dwelling elders (HR 2.24, 95% CI 1.60 – 3.15 for death in multivariable analysis).6
The prevalence of frailty among patients on dialysis has ranged from 30% to 73%.8 It is important to note that comparing among studies of frailty in the CKD and ESRD population is difficult because many studies use different measurements for frailty, including using BMI cut offs in place of weight loss, using different instruments to measure exhaustion or physical activity, or substituting patients' self-reported physical functioning for objectively measured strength and gait speed.7 Patients' self-reported physical functioning, as reported by the Physical Function (PF) scale of SF-36 in particular, is related to their performance in tests of gait speed and chair rising time,9 and the PF score has been used as a substitute for physical performance measures in several studies of frailty.6,7,10 However, because self-report of difficulty in physical function and objective measurement of performance are fundamentally different, the specific measure of frailty used can significantly influence the prevalence of frailty. In a study of 732 adult patients treated with maintenance hemodialysis, 387, or 53% were found to be frail based on self-reported function. With an objective performance based definition of frailty, only 232, or 32% of the cohort met criteria for frailty.11
Frail patients may be initiated on dialysis earlier than those who do not exhibit the frailty phenotype, perhaps because components of frailty (e.g., exhaustion) may be perceived as symptoms of uremia, or because uremia contributes to the physical dysfunction associated with frailty.12-14 Although there are few longitudinal studies of frailty or physical function before and after initiation of dialysis, available evidence suggests that elderly patients experience further loss of functioning after starting dialysis. Kurella Tamura et al. studied patients who were in nursing homes when dialysis was initiated. They reported that only 39% of patients were able to maintain pre-dialysis functional status in the first three months after initiation, and by one year, 58% had died and only 13% were alive with functional status intact. Initiation of dialysis was associated with a sharp decline on the Minimum Data Set-Activities of Daily Living (ADL) scale even after accounting for the presence or absence of accelerated decline in the three months preceding dialysis.15 Similarly, Jassal et al. studied initiation of dialysis in a cohort of 97 patients over age 80. At the time of dialysis initiation, 78% were living at home with no assistance required for ADLs. However, within 6 months, more than 30% of patients had loss of function such that they required caregiver support or transfer to a nursing home.16 Both of these studies are limited by not including patients who did not initiate dialysis to compare the evolution of functional status with and without renal replacement therapy, and both involve groups at high risk for functional decline. Nevertheless, there was no evidence for improvement in performance after starting dialysis, and these studies suggest that without efforts to improve or preserve functioning before or after the start of dialysis, the trajectory is rapidly downward.15,16
Other studies have examined physical functioning or physical activity directly rather than as contributors to frailty, and these also appear to decline with worsening kidney function.17-19 Hellberg et al. found a statistically significant association between eGFR and the distance walked in 6 minutes after multivariable analysis, such that a 10 ml/min/1.73 m2 lower eGFR was associated with 35 m shorter walking distance. Patients with lower eGFR also had lower quadriceps muscle strength and shorter reach, although eGFR was not significantly associated with several other functional tests including grip strength.20 In a survey that included patients treated with HD and PD and patients with CKD not on dialysis, the physical component summary of the SF-36 (PCS) score was found to be substantially lower in all of these groups than for matched controls (36.0 vs. 48.2 for patients on HD, 37.4 vs. 47.8 for PD patients, and 39.8 vs. 46.9 for CKD patients).19 Although most studies associating GFR with physical function have been cross-sectional, at least one study has shown that exercise capacity in patients with stage 3-4 CKD declined as estimated creatinine clearance (Cockcroft-Gault) declined.21
A cross-sectional study of self-reported physical activity in 100 patients across the spectrum of CKD and of healthy controls also showed a graded association between kidney function and physical activity measured by a questionnaire combining elements from the General Practice Physical Activity Questionnaire and the Human Activity Profile (HAP). The Highest level of activity was among healthy controls (score of 14.7 ± 4.24), with progressively lower activity among transplant recipients, patients with CKD stage 3-5, those on home hemodialysis, peritoneal dialysis, and those receiving in-center hemodialysis (11.4 ± 4.20).22 The Comprehensive Dialysis study assessed physical activity among 1547 ambulatory patients new to dialysis and found that physical activity was extremely low, with scores for all age and gender categories that were below the 5th percentile for healthy individuals.23
Brenner and Bohart demonstrated that among 19 patients treated with dialysis, those who reported higher levels of activity by questionnaire were more likely to have better quality of life and physical function compared to those who report less activity.24 A Japanese study of 202 dialysis patients examined the association between habitual physical activity (as derived from accelerometer data) and mortality and showed that 93.3% of those who had physical activity > 50 minutes/day (time spent with vector magnitude on the accelerometer at grade 1 or higher out of 11 total grades) survived after 7 years vs. 77.2% in those who reported less than 50 min/day of activity. After adjusting for confounders with a multivariate model including use of a propensity score for physical activity, each 10 min/day increment of physical activity was associated with a HR of 0.78 for mortality (95% CI 0.66 – 0.92).25 Two U.S. cohorts of greater than 1,000 patients each also demonstrated a strong link between physical activity and survival among patients treated with dialysis.26,27
Given these strikingly low levels of physical activity and the links between low physical activity and poor physical function and higher mortality, it is logical to consider that exercise training or interventions to increase physical activity could ameliorate poor physical functioning and frailty and might even improve survival in patients with CKD. We will review data on the effects of exercise interventions, starting with studies conducted in the setting of hemodialysis, as this is the most-studied population.
Exercise in patients on dialysis
Numerous studies have shown that exercise interventions are beneficial, feasible, and can be safely implemented in the ESRD population.28-30 Both aerobic and resistance exercise interventions, administered in the dialysis facility or outside of dialysis, have resulted in improvements in physical function.
Aerobic exercise interventions
There have been many studies of aerobic exercise interventions in patients treated with dialysis, as well as several systematic reviews on this topic.31-35 In a systematic review conducted by Barcellos et al., of the 45 randomized controlled trials in patients treated with dialysis, a total of 14 trials measured aerobic capacity as an outcome. All 14 reported an increase in VO2peak with aerobic exercise.31 Similarly, in a meta-analysis from Heiwe and Jacobson, the pooled effect of aerobic exercise intervention in 21 studies of patients treated with dialysis was an improvement in aerobic capacity (standard effect size of about 0.8).33 The majority of these exercise interventions were 8-12 weeks in duration, although some lasted as long as 6 months. Average improvement in VO2peak was around 20%, 31,33,35,36 which may be related to cardiac adaptation to exercise. Specifically, studies have shown better LV systolic function at rest after exercise, as well as higher cardiac output index and stroke volume index following programs of aerobic training.37,38 Importantly, although VO2peak improved after aerobic training or combined aerobic and resistance training, it remained substantially below levels of age-matched controls even after training.32
Both intra- and extradialytic programs can improve aerobic capacity, and in patients with very low aerobic capacity, moderate extradialytic rather than intradialytic exercise may result in greater gains.34 The extent to which this greater degree of improvement in aerobic capacity in extradialytic exercise as compared to intradialytic exercise translates to greater functional changes or survival benefit is unclear. Also, intradialytic exercise programs tend to have higher adherence than home-based or extradialytic programs.39
Improvement in aerobic exercise capacity may also be related to other functional gain in this population. Ouzouni et al. randomized 35 patients to either a rehabilitation program consisting of intradialytic exercise training or a control group receiving usual care. Both groups had measurements of baseline aerobic capacity as well as a psychosocial assessment including Beck's Depression Inventory and questionnaires of HRQoL, as well as erythropoiesis stimulating agents (ESA) administered to achieve a target Hgb of 11 g/dL. At the 10 month follow-up, VO2peak in the exercise group increased by 21.1%. The exercise group also had a decrease of 39.4% in the depression index and improvement in the quality of life (QoL) index, life satisfaction index, and the physical component scale of the SF-36. VO2peak had a positive correlation with the QoL index both at baseline and at follow-up. Multivariate analysis showed that the extent of reduction of depression, increase in aerobic capacity, and adherence to the training program were significant predictors of the extent of improvement in quality of life. There was no significant change in the control group over the course of the study.36
Another study randomized 48 patients treated with HD into four groups that received the following: a 6-month supervised outpatient renal rehabilitation program with three weekly aerobic and strengthening sessions on non-dialysis days; an intradialytic exercise program; an unsupervised moderate exercise program at home; and no exercise (control group). An additional group was composed of healthy sex- and age-matched sedentary individuals. The highest dropout rate was among patients assigned to the extradialytic rehabilitation program, at 24%, as compared to 17% for the intradialytic and home exercise groups. However, for those completing the program, participation in the full supervised (extradialytic) program also resulted in the greatest increases in VO2peak (43% compared to 24% with intradialytic exercise, 17% with home exercise, and a decrease of 3% in the control group that was not statistically significant).40
Although few studies followed patients beyond the relatively short intervention period, there is some evidence that exercise programs can be feasible even on a long-term basis. A 4-year study compared 48 patients treated with hemodialysis who were randomly assigned to either a supervised outpatient exercise program or an in-center, intradialytic bicycling program. There were a total of 8 drop outs in the extradialytic group and 5 in the intradialytic group over the 4 years of the study, and a total of 16 and 18 patients completed the programs, respectively. At one year, exercise capacity, as measured by VO2peak, increased by 47% for the extradialytic exercise group and 36% in the intradialytic exercise group. At 4 years, greater improvements were observed (70% and 50%, respectively).41
In addition to high intensity exercise training interventions, low or moderate intensity exercise also appears to improve walking capacity as measured by the 6-minute walk test (6MWT), even among patients with very low levels of initial performance, and the 6MWT may be easier to perform than maximal treadmill testing. Distance on the 6MWT has also been independently associated with survival and lower risk of hospitalization in the dialysis population.42,43 A home-based exercise program of two daily 10-minute home walking sessions on non-dialysis days at 50% below maximal treadmill speed showed improvements in 6 minute walking distance and health related quality of life for the exercise group vs the control group at the end of 6 months. A follow-up one year after completion of study showed that although the exercise group had some detraining, their 6-minute walk scores remained above baseline, whereas the control group had declined throughout the period of the study and the follow-up.44 Both aerobic and resistance exercise have a dose-response relationship with physical health such that even small doses may be more beneficial than none. In one study comparing intradialytic cycling to a pedometer based intervention, those completing the intervention had similar improvements in sit to stand and sit and reach testing despite no significant change in VO2peak or the 6 minute walk distance between or within study groups either at 12 or 24 weeks.45 Low and moderate intensity programs also appear to result in improved control of blood pressure46,47 and better vascular functioning.48 However, there are no studies adequately powered to assess whether this translates to improved survival or decreased CV risk.34
Resistance Training
One of the key components of frailty is muscle weakness. Isokinetic muscle strength has been shown to correlate with VO2peak,49 and muscle strength has also been shown to correlate with gait speed, another component of frailty.50 Hellberg et al. conducted a retrospective, longitudinal study of 134 patients starting renal replacement therapy between 1998 and 2006. Twenty-two patients died during follow-up. Better grip strength, functional reach, and standing heel rise were associated with lower mortality in univariate analysis. In multivariate analysis adjusting for age and comorbidity, grip strength remained an independent predictor, and a 50% lower grip strength was associated with four times higher risk of mortality.17
A pioneering study of high intensity resistance training including 2 supervised and 1 unsupervised session per week in 16 dialysis patients (10 of whom ultimately completed the study), showed statistically significant improvements in gait speed, peak torque of leg extensors (only at 90 degrees/s), time to complete sit-to-stand, and maximal walking speed. However, there was no change in grip strength. Of the 6 who did not complete the study, one lost motivation, and one was transplanted. No patients stopped due to injury.51
Since then, there have been several trials of progressive resistance training in patients with ESRD designed to improve physical functioning by increasing strength. Of the nine trials of resistance training included in one systematic review, all nine reported an improvement in muscle strength with resistance training.31 Two of the larger trials examined muscle size directly using imaging of the thighs. A randomized controlled trial of exercise training in 79 patients who were receiving maintenance hemodialysis by Johansen et al. demonstrated that a 12 week course of lower extremity resistance training three times per week resulted in an increase in quadriceps muscle cross-sectional area (CSA) and an improvement in self-reported physical function as compared to non-exercising groups.52 Another RCT of high-intensity, progressive resistance training (PRT) in patients treated with hemodialysis resulted in no statistically significant difference in total muscle CSA in the exercise group compared to non-exercising controls based on CT imaging of the thighs. However, there was significant improvement in muscle attenuation, indicating a decrease in intramuscular fat content (and therefore an increase in actual muscle area) with the intervention. Strength and mid-thigh and mid-arm circumference also increased and CRP decreased in the PRT group compared to the controls.53
Combined resistance and aerobic training
DePaul et al. conducted a randomized, placebo-controlled trial of exercise intervention involving progressive resistance training of quadriceps and hamstring as well as cycle ergometer training three times weekly for 12 weeks in 38 patients treated with hemodialysis.54 Those in the control group (18 patients) trained with a non-progressive program of range of motion exercises. The exercise group (20 patients) experienced substantial and statistically significant improvement in scores on the submaximal exercise test and muscle strength, but not in 6MWT or physical components of the SF-36 as compared to the control group. Five months after study completion, those in the exercise group were still stronger, but the differences were no longer statistically significant.
A RCT from the Netherlands involving low-moderate intensity strength training prior to the dialysis session and intradialytic cycling for a total of 12 weeks showed that participation in this exercise program (60 patients out of a total 103 randomized) resulted in a statistically significant increase in lower extremity muscle strength and VO2peak. Scores on the Vitality and General Health scales of the SF-36 and the single question about health change also improved significantly.55
Other smaller studies have also reported substantial benefit with combined interventions.56,57 A program involving progressive aerobic and resistance training may be superior to resistance or aerobic training alone. In one study, patients were randomly assigned to either resistance exercise alone for 10 weeks or a combined program. Total exercise was the same for both groups at 30 minutes performed in the first 2 hours of hemodialysis. Out of 80 patients approached, 22 were excluded and 32 refused to participate, and the remaining 26 patients were randomized with 13 in each arm. Those in the combined program had an improvement of 39.7 ± 61.4 m in the 6MWT, whereas those in resistance alone actually worsened by 19.2 ± 53.9 m. There was a statistically significant difference between the two groups in change over time. However, no pure strength outcomes were assessed.57 Of note, those who refused to participate had longer dialysis vintage, higher hematocrit, and were more likely to be women than patients who participated in the study.
Alternatives to Exercise Interventions
Physical Therapy
There are unique challenges involved in tailoring typical PT and OT programs to patients with ESRD, including barriers related to health and logistics. However, use of comprehensive PT and OT can be beneficial if the specific needs of the individual patient are kept in mind, and intradialytic therapy is also possible.58 A non-randomized trial of 52 patients in either an experimental skilled renal rehabilitation program (including exercise, activities, and neuromuscular re-education) or control showed that patients enrolled in the program had statistically significant improvement in 6MWT, grip strength, and 20′ fast gait speed after 12 weeks.59 There is no reason to expect that referral to traditional PT would not benefit patients on dialysis, and a small, non-randomized study showed improvement in quality of life scores and functional performance after referral to traditional PT among 5 patients treated with dialysis who were enrolled for 9 weeks.60 Inpatient rehabilitation has also been shown to be beneficial for patients treated with dialysis.61,62
Limitations
The vast majority of these studies involved patients receiving hemodialysis rather than peritoneal dialysis. For example, a 2008 of the literature on exercise interventions in dialysis patients found that only 2 of the 17 aerobic interventions included patients on peritoneal dialysis. However, there is no evidence to support that peritoneal dialysis patients do not also benefit from exercise interventions, given that they did improve their aerobic capacity in the 2 studies in which they were included.35
Bohm et al. discussed some of the methodological issues with studies of exercise in patients treated with dialysis, including uncertainty about the optimal modality and dose of exercise, the best time for intervention (intradialytic vs. outside of dialysis), lack of motivation by patients, lack of enthusiasm of treatment teams and providers, safety and health concerns, and frequent hospitalizations and clinical status changes in the dialysis population that can interrupt training. Conclusions are further limited by the heterogeneity of the ESRD population, the presence of multiple comorbid conditions, and difficulty of implementing long-term interventions given the high rate of dropout in the studies to date and the high mortality in the patient population.63
Although most dialysis patients have reported interest in physical exercise when surveyed,64 they also report substantial barriers to participation. A study of 100 patients in California dialysis facilities found that 98% agreed that a sedentary lifestyle was unhealthy and that increasing exercise would be beneficial, but only 8% reported no barriers to exercise.65 The most common barriers were fatigue on dialysis days (67%) and non-dialysis days (40%), as well as shortness of breath (48%). In multivariate analysis, the factors most associated with lower activity level were the total number of barriers endorsed, having too many medical problems, lack of time on hemodialysis days, and lack of motivation.65 In one Italian dialysis center, out of 104 patients enrolled in a study of barriers to physical activity, 96 % reported at least one barrier. After multivariate analysis, having too many medical problems, chest pain, and sadness were all associated with lack of physical activity.66 An additional Canadian study of patients treated with maintenance hemodialysis reported that patients perceived symptoms such as fatigue, health issues such as osteoporosis, time, transportation, and equipment as barriers to exercise.67
Although there are barriers from patients including lack of motivation, there are also iatrogenic barriers. A survey of 505 nephrologists (55% from the U.S., and the rest from other parts of the world) explored provider opinions about and barriers to exercise counseling and found that only 38% offered exercise counseling for inactive patients either “almost always” or “often.” Nephrologists who did not counsel patients routinely were more likely to report lack of confidence in their ability to provide counseling and lack of conviction that patients would respond as a barrier to offering counseling.68 Even among primarily European nephrologists, exercise prescription has been noted to be suboptimal despite better adoption of exercise for patients treated with dialysis in many European countries.69 An Italian study by Fiaccadori et al. reported that the most frequent barriers to counseling about or prescribing exercise from the doctors' and nurses' perspective were lack of time and belief that the patients would not be adherent or had low interest. Of note, less than half (42.3%) of the patients expressed lack of motivation in a study that surveyed patients directly, and lack of motivation was not significantly associated with inactivity in multivariate analysis.66
Taken together, the evidence from exercise studies in patients on dialysis supports the idea that rehabilitation at earlier stages of CKD (or “prehabilitation” before dialysis) might be more beneficial than not addressing the declining physical functioning and low physical activity until patients are receiving dialysis. Specifically, difficulty in recruitment and retention is a common problem in the studies of exercise in patients on dialysis. A higher percentage of patients with earlier stages of CKD may still be capable of undertaking an exercise program, and adherence to exercise may also be less of a problem if patients are less debilitated. For example, Greenwood et al. studied the feasibility of an exercise program for adults including those with CKD 3-4, on maintenance HD, and after receiving a renal transplant. A multidisciplinary team conducted twice-weekly supervised exercise sessions and prescribed once-weekly home-based exercise for a total of 12 weeks.70 Out of the 263 patients referred, 131 commenced the program, and 77 completed it. Patients who completed the program (attended at least 12 of the supervised sessions) had improvements in anxiety, depression, exercise capacity, and physical function. Higher self-reported level of fitness at baseline was associated with better chances of completing the program, and hemodialysis patients had the largest percentage of non-completers (49%).
In addition, the lack of complete restoration of the functioning that appears to be lost during pre-dialysis stages of CKD with exercise after dialysis initiation, as well as dialysis scheduling constraints and provider apathy, also suggest that pre-habilitation could prevent some or all of the frailty and poor physical functioning that develops during advanced CKD and initiation of dialysis and lead to better functioning among incident and prevalent dialysis patients (Figure 1). Few studies have addressed this possibility directly, but in one study of 135 patients on maintenance hemodialysis, patients reported that their participation in physical activity was limited and was primarily in the form of low-intensity, recreational activities; only 10% of participants reported exercise expenditure of greater than 1000 kcal/week. Pre-dialysis exercise habits of at least three sessions a week correlated positively with exercise after dialysis initiation, raising the possibility that increasing activity in the predialysis phases of disease could “carry over” into the dialysis setting.64
Figure 1.
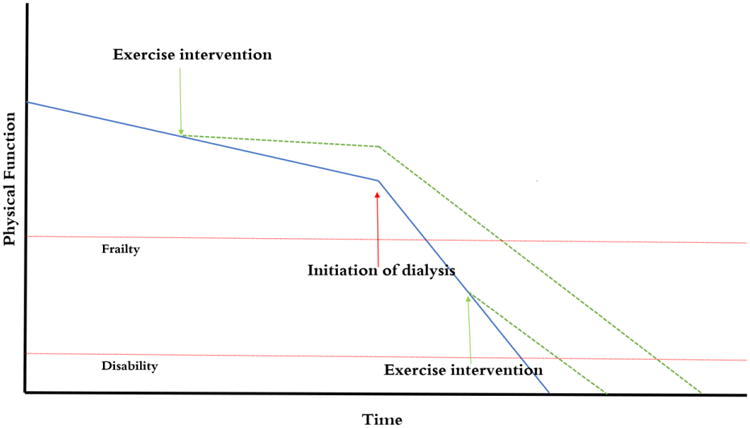
Exercise interventions may be able to blunt the impact of age and declining GFR on loss of physical function. Physical function often deteriorates even more rapidly after initiation of dialysis. An exercise intervention instituted pre-dialysis may provide longer time without frailty than one instituted after dialysis, and may also be easier to perform.
Exercise intervention improves outcomes in pre-dialysis CKD
Although comparative studies are rare, there is evidence that rehabilitation is beneficial among patients with CKD not requiring dialysis.71-73 Gould et al. published a systematic review of the effects of exercise in patients with CKD and found that aerobic exercise improved peak exercise capacity, and aerobic, resistance, and combined aerobic plus resistance exercise interventions improved muscle strength.74 Combination resistance and exercise training may be superior to either aerobic or resistance training alone.74,75
Exercise in the earlier stages of CKD has been relatively understudied compared to in the dialysis population, with fewer randomized controlled trials and significantly more heterogeneity in patient selection.76-78 Many studies of exercise in CKD have excluded patients with significant co-morbidities or known CAD.77 In general, aerobic exercise appears to improve VO2peak in patients with CKD,78 but the effect of exercise on strength, physical function, and quality of life is less consistent in the literature.77,78 Additionally, aerobic exercise need not be of high intensity in order to improve VO2peak.33
Although most exercise interventions have been of relatively short duration, one study of pre-dialysis patients with CKD showed that a year-long aerobic exercise intervention consisting of both supervised and home-based exercise with individualized regimens resulted in improvements to VO2peak and exercise duration as well as augmentation index (a measure of arterial stiffness), which decreased by 11.7% (95% CI -18.79 to -4.61) in the exercise group as compared to the controls.79 This study also showed that improvement with exercise was associated with meaningful improvement in overall quality of life as measured by the EuroQuol five dimensions questionnaire. Although studies have not adequately examined effects of exercise on cardiovascular outcomes, exercise does appear to improve lipid metabolism.80,81
There are also fewer studies of resistance training in the CKD population, but in one study of 10 patients with average eGFR of 15 ± 7 mL/min/1.73 m2, combined aerobic and resistance training of large muscle groups 3 times a week for 3 months was associated with improvement in exercise capacity on bicycle ergometer and decrease in heart rate when exercising at equal loads as compared to controls, likely mediated by improvement in muscle strength and function.82 A group of 9 matched controls did not show any improvement during the same period. Another study of patients randomized to low-protein diet alone or low protein diet plus resistance training for 12 weeks showed improvement in type I and type II muscle fiber CSA in patients who performed resistance training.83
There has been at least one study directly comparing exercise interventions in pre-dialysis patients to patients treated with dialysis. Eighteen pre-dialysis (expected to start dialysis in the next 6-12 months) and 18 dialysis patients were randomly assigned to either 6 months of exercise coaching and rehabilitation counseling or standard of care, with an additional follow-up 6 months after the end of the intervention.84 Both rehabilitation groups (pre-dialysis and dialysis) had increases in their general health ratings over the time of the intervention, whereas both control groups had decreases. The pre-dialysis group walked farther on the 6-minute walk than the dialysis group initially and after the 6-month intervention. Predialysis patients who did and did not exercise had similar time to initiation of RRT, but the study was underpowered for this and other comparisons. Dialysis patients who exercised did not improve their physical functioning significantly compared to controls, and the superiority of rehabilitation in the predialysis vs. dialysis setting was not formally assessed, presumably due to the small number of patients. Although the authors did not perform a statistical comparison of the results in pre-dialysis and dialysis patients, they concluded that rehabilitation services were qualitatively more beneficial in the pre-dialysis patients.84
In one study of 376 patients with and without CKD (defined as eGFR <60 mL/min/1.73 m2) who completed cardiac rehabilitation, 115 (31%) had CKD. Eighty-seven percent of the CKD patients had eGFR >30 mL/min/1.73 m2. The CKD patients tended to be older and to have more cardiac risk factors and comorbidities. Although baseline self-reported physical activity level and distance walked in six minutes were lower both at baseline and after rehabilitation in the CKD group as compared to non-CKD patients, both groups had similar relative improvement in both measures after rehabilitation.85 Shigeta et al. conducted a study of patients undergoing cardiovascular and orthopedic inpatient rehabilitation and found that CKD stage higher than 3b was associated with delayed progress in physical therapy (length of stay more than 2 SD above the mean) with OR of 3.3 (95% CI 1.3 – 9.0) for cardiovascular disease and 3.3 (95% CI 1.3 – 7.9) for orthopedic disorders.86
Exercise interventions in CKD may be as effective as exercise interventions in healthy controls. A study by Heiwe et al. compared the effects of a non-randomly assigned exercise intervention among 37 patients with advanced CKD and 26 healthy elderly controls.87 The exercise program consisted of strength and endurance training three times per week for 12 weeks. At baseline, the patients with CKD had a maximal workload that was 87% (64 – 113%) of age-expected norm, whereas the healthy controls had a maximal workload 80% (83-187%) of norm. Patients with CKD had significantly lower static muscular endurance and performance on the 6MWT than the healthy elderly individuals. Both groups showed similar improvements in muscle strength and dynamic muscular endurance after the intervention, as well as improvements in functional mobility and walking distance compared with their non-exercising counterparts.87
Unfortunately, there is little data directly examining the effect of exercise before dialysis initiation on patient outcomes after transitioning to dialysis. Cheng et al. published a report of their experience with a renal rehabilitation program that included pre-dialysis education and individualized, physiotherapist recommended exercise as core tenets. The specifics of the exercise program delivered within this multidisciplinary program were not discussed beyond Tai Chi three times a week and some of the social and group interaction elements. They reported that those who completed the pre-dialysis program achieved were more likely to choose PD as their dialysis modality than those who did not. They also reported improvements in physical capacity and fewer hospital admissions, although they did not report numbers or magnitude.88
Progression of CKD
When considering exercise interventions in the CKD population, it is important to consider the theoretical possibility of a benefit in slowing progression of kidney disease, which could happen through improvement in inflammation or blood pressure control. Conversely, there is the possibility of risk, or more likely, that a rise in creatinine related to higher muscle mass could be interpreted as a decline in kidney function. A few small studies have reported a beneficial effect of exercise on progression of CKD. A Japanese study of patients with both CVD and CKD (19 patients; eGFR <60 mL/min/1.73 m2) reported that 12 weeks of exercise therapy resulted in statistically significant improvements in anaerobic threshold (AT-VO2) during maximal exercise testing and HDL-C levels, as well as reduced triglyceride level. eGFR was also improved, with change in eGFR correlating significantly with change in AT-VO2 and HDL-C, and negatively with triglyceride levels.81
Another study of a short-term exercise program of moderate intensity in patients with type 2 diabetes reported improvement in eGFR in patients with CKD 2 and 3. Forty-two percent of those with CKD 3 (16 of 38), improved to CKD 1 or 2 after the intervention.80 A third randomized controlled trial of physical training (either 30 minutes of daily bicycling or equivalent physical activity) vs. maintenance of usual lifestyle in 30 patients with median eGFR of 25 mL/min/1.73m2 showed improvement in maximal work capacity, but did not show change in rate of progression of GFR over the 20-month follow-up period.89
Neither delayed progression of CKD nor improvement of GFR has been shown in any large randomized controlled trials or in meta-analysis, which may be due to the lack of large-scale trials with adequate follow-up to examine disease progression.33,74,78 According to one review, assuming a rate of decline in controls of 1.47 – 3.4 mL/min/1.73 m2 per year, a study would require 1870 patients to detect a 30% improvement in eGFR with adequate conventional statistical power, making it unlikely that RCT data will soon be available to address the question.77 However, although it is unclear whether exercise has a significant effect on progression of renal disease, it does not appear to worsen it.
Nevertheless, a large, population-based observational study of 63,257 Chinese men and women followed for a median of 15.3 years for the development of incident ESRD examined risk according to level of physical activity. Compared to those with no regular physical activity, those who performed moderate activity for at least 2 hours a week or strenuous physical activity for at least 30 minutes a week had a 24% lower adjusted risk of ESRD. Strenuous activity was independently associated with a 42% lower adjusted risk of ESRD. Similar results were seen with a composite outcome including death and ESRD.90
Among individuals with established CKD, Robinson-Cohen et al. studied a cohort of 256 patients in the Seattle Kidney Study with eGFR from 15-59 (mean 42) mL/min/1.73 m2. During follow up (median of 3.7 years), participants who reported at least 150 minutes of physical activity per week had the lowest rate of eGFRcystatin c loss (-6.2%/year vs. -9.6%/year among less active participants). After adjustment, each 60-minute increment in weekly activity was associated with a 0.5% slower decline in eGFR. However, after adjusting for eGFR, physical activity was not associated with any difference in the incidence of ESRD.91
Pechter et al. performed a non-randomized study examining the effects of aquatic aerobic exercise on cardiorespiratory reserve and cardiovascular and inflammatory markers in 26 patients with moderate CKD (mean eGFR of the exercise group 62.9 ± 5.9, mean eGFR of the control group 69.8 ± 12.3).92 Seventeen patients were in the exercise group and 9 in the control group. Patients performed aerobic exercises for 30 minutes while immersed in a pool, with a 10 minute warm-up, a 10 minute of gradually increasing exercise, and a 10 minute cool down period and stretches at the end. Those in the exercise group had improved peak oxygen pulse, peak ventilation, and peak load as well as improved peak oxygen consumption at maximal load (although this last outcome did not reach statistical significance). There was a statistically significant improvement in eGFR and decline in proteinuria in the exercise group but not the control group. The exercise group had improved systolic and diastolic blood pressures, a possible mediator of improvement in eGFR. Pechter et al. also reported on the 10-year follow-up from this study. Ten patients from the original study left due to lack of time, and 7 patients continued with regular exercise under supervision of a physiotherapist. At termination of the study, there was no significant difference in time to death or dialysis. However, there did appear to be a difference in occurrence of the endpoint with the exact multinomial test. Fifty-five percent of those in the sedentary group reached the study endpoint (3 death and 2 renal replacement therapy). However, in the exercise group, no patients reached dialysis in 10 years. Among patients who did not reach the endpoint, there was no significant difference in eGFR or proteinuria between the exercisers and the controls.93
Delivering an intervention
With regard to safety, in the extensive literature on exercise in patients with CKD, there have been no serious adverse events documented related to exercise. When considering the physical activity recommendations for elderly adults and patients with sedentary lifestyles such as many patients treated with dialysis, there are no specific recommendations for screening with stress testing prior to exercise. However, recommendations should be individualized and appropriate referrals may be necessary for cardiac rehabilitation or physical therapy. The intensity of the exercise should also be tailored to the participant. Many of these studies prescribed exercise sessions of at least 30 minutes duration, although it is uncertain whether this duration is required for either physiologic or functional improvement. An ideal aerobic exercise program might include 3-7 days per week of exercise, with interval training of shorter duration as necessary to build up to 30 minutes per day. Individual exercise sessions should consist of warm-up, a main phase of exercise, and a cool-down period.32
Although logistics can be a concern with supervised or intradialytic exercise, unsupervised exercise and home activity recommendations also provide benefit.30 Evidence appears to support starting at low intensity and gradually increasing intensity of both aerobic and resistance exercise training in patients with CKD or ESRD. American College of Sports Medicine guidelines recommend that training not be scheduled immediately after dialysis and that if intradialytic exercise is implemented, it should be early in the dialysis session to prevent hypotension.94,95 Although there is significant variability in programs of progressive resistance training, it is likely that at least 8 weeks of duration, typically for three sessions per week are necessary to show substantive changes in outcomes.96 To attain benefit from exercise may not require vigorous intervention or gym-based programs; rather, given the numerous functional barriers to exercise, even smaller increases in activity levels may be beneficial.97
Conclusion
Despite the lack of direct data that “pre-habilitation” in the non-dialysis-dependent stages of CKD improves outcomes in patients treated with dialysis, there is a preponderance of evidence that exercise improves physical function across the spectrum of CKD. Experts have recommended exercise for every stable patient with CKD, regardless of age, gender, comorbidities, or prior exercise, and have highlighted the importance of providing information to patients on the proper conduct of exercise and its benefits.98 There are guidelines for exercise for the general population and in CKD both from American and European groups, but they may be more difficult to achieve for many patients with advanced CKD or treated with dialysis than for patients at earlier stages of CKD.99 Patients face many barriers to exercise that may increase as GFR declines, including lower baseline levels of physical functioning, higher prevalence of frailty, depression, and anxiety, and logistical barriers such as transportation. Exercise interventions may be better delivered before patients develop the debility that sometimes comes with the transition to ESRD, and recommendations for physical activity may best be delivered along with other pre-ESRD care.100 Nephrologists should take an active role in counseling patients on exercise and physical activity prior to treatment with dialysis but should also not ignore exercise prescription for patients who are already being treated with dialysis. 101
Figure 2.
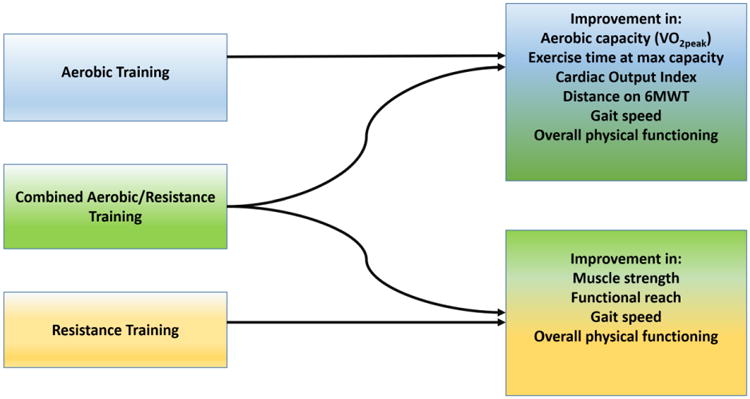
Types of exercise interventions and expected outcomes. Increases in strength are training-specific; grip strength will improve with grip training, leg strength will improve with exercises focusing on the legs, etc.
| Systematic Reviews of Exercise Interventions | Interventions by Stage of CKD | |||||
|---|---|---|---|---|---|---|
| First Author, Year | Type of Studies | Total Number of Studies Included | Pre-dialysis | ESRD | Transplant | Comments |
| Cheema, 2005102 | 13 RCTs, 7 uncontrolled trials, 9 controlled trials | 29 | 29 | |||
| Type of Intervention | ||||||
| Aerobic | 19 | 19 | ||||
| Resistance | 1 | 1 | ||||
| Combined | 9 | 9 | ||||
| Koufaki, 201378 | Pre-dialysis: 5 RCTs, 8 non-random controlled, 4 uncontrolled. Transplant: 6 RCT, 10 uncontrolled | 33 | 17 | 16 | One study with pre-HD, HD, and Tx patients. One study with pre-HD and HD patients. One study with KTx and HD patients. | |
| Type of Intervention | ||||||
| Aerobic | 24 | 13 | 3 | 8 | ||
| Resistance | 7 | 4 | 3 | |||
| Combined | 6 | 1 | 5 | |||
| Heiwe, 201433 | 41 RCTs | 41 | 6 | 33 | 3 | One study enrolled patients both treated with HD and in CKD (aerobic intervention). One study enrolled patients treated with PD alone. One study involved an aerobic, resistance, and combined intervention (in HD). Only one study enrolled patients treated with both HD and CAPD. |
| Type of Intervention | ||||||
| Aerobic | 33 | 5 | 25 | 3 | ||
| Resistance | 7 | 1 | 6 | |||
| Combined | 4 | 0 | 4 | |||
| Barcellos, 201531 | RCTs | 59 | 11 | 46 | 3 | One study included patients who were both on dialysis and pre-dialysis. Only one study included both HD and CAPD patients. One study compared aerobic training to resistance training. |
| Type of Intervention | ||||||
| Aerobic | 43 | 7 | 33 | 3 | ||
| Resistance | 8 | 2 | 6 | |||
| Combined | 9 | 2 | 7 | |||
| Sah, 201596 | 1 uncontrolled trial, 2 non-randomized trials, and 8 RCTs | 11 | 2 | 8 | Two papers from same study | |
| Type of Intervention | ||||||
| Aerobic | 1 | 1 | ||||
| Resistance | 4 | 1 | 3 | |||
| Combined | 5 | 1 | 4 | |||
| Additional Unique Studies of Exercise Intervention in this Review | 3 uncontrolled trials, 2 non-randomized controlled trial, 2 randomized controlled trials | 8 | 2 | 6 | ||
| Type of Intervention | ||||||
| Aerobic | 5 | 1 | 4 | |||
| Resistance | ||||||
| Combined | 3 | 1 | 2 | |||
| Total Independent or Unique Studies (No. of patients) | 26 (913) | 74 (3304) | 18 (734) | |||
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lam M, Jassal SV. The concept of frailty in geriatric chronic kidney disease (CKD) patients. Blood Purif. 2015;39:50–54. doi: 10.1159/000368952. [DOI] [PubMed] [Google Scholar]
- 2.Fried Linda P, et al. Frailty in Older Adults: Evidence for a Phenotype. Journal of Gerontology. 2001;56A:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the Concepts of Disability, Frailty, and Comorbidity: Implications for Improved Targeting and Care. Journal of Gerontology. 2004;59:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 4.Shlipak MG, et al. The presence of frailty in elderly persons with chronic renal insufficiency. American Journal of Kidney Diseases. 2004;43:861–867. doi: 10.1053/j.ajkd.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 5.Roshanravan B, et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. 2012;60:912–921. doi: 10.1053/j.ajkd.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960–2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 7.Walker SR, et al. Association of frailty and physical function in patients with non-dialysis CKD: a systematic review. BMC Nephrol. 2013;14:228. doi: 10.1186/1471-2369-14-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musso CG, Jauregui JR, Macias Nunez JF. Frailty phenotype and chronic kidney disease: a review of the literature. Int Urol Nephrol. 2015;47:1801–1807. doi: 10.1007/s11255-015-1112-z. [DOI] [PubMed] [Google Scholar]
- 9.Johansen KL, et al. Validation of questionnaires to estimate physical activity and functioning in end-stage renal disease. Kidney Int. 2001;59:1121–1127. doi: 10.1046/j.1523-1755.2001.0590031121.x. [DOI] [PubMed] [Google Scholar]
- 10.Painter P, Kuskowski M. A closer look at frailty in ESRD: getting the measure right. Hemodialysis international International Symposium on Home Hemodialysis. 2013;17:41–49. doi: 10.1111/j.1542-4758.2012.00719.x. [DOI] [PubMed] [Google Scholar]
- 11.Johansen KL, et al. Comparison of self-report-based and physical performance-based frailty definitions among patients receiving maintenance hemodialysis. Am J Kidney Dis. 2014;64:600–607. doi: 10.1053/j.ajkd.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172:1071–1077. doi: 10.1001/archinternmed.2012.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansen KL, Delgado C, Bao Y, Kurella Tamura M. Frailty and dialysis initiation. Seminars in dialysis. 2013;26:690–696. doi: 10.1111/sdi.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurella Tamura M, O'Hare AM, McCulloch CE, Johansen KL. Signs and symptoms associated with earlier dialysis initiation in nursing home residents. Am J Kidney Dis. 2010;56:1117–1126. doi: 10.1053/j.ajkd.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurella Tamura M, et al. Functional Status of Elderly Adults before and after Initiation of DIalysis. New England Journal of Medicince. 2009;361:1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jassal SV, Chiu E, Hladunewich M. Loss of Independence in Patients Starting Dialysis at 80 years of Age or Older. New England Journal of Medicince. 2009;361:1612–1613. doi: 10.1056/NEJMc0905289. [DOI] [PubMed] [Google Scholar]
- 17.Hellberg M, Wiberg EM, Höglund P, Simonsen O, Clyne N. Physical Function at Start of Renal Replacement Therapy - Independent Predictor of Survival. Nephrology Dialysis Transplantation. 2012;27:ii121–ii132. doi: 10.1093/ndt/gfs216. [DOI] [Google Scholar]
- 18.Hiraki K, et al. Decreased physical function in pre-dialysis patients with chronic kidney disease. Clinical and experimental nephrology. 2013;17:225–231. doi: 10.1007/s10157-012-0681-8. [DOI] [PubMed] [Google Scholar]
- 19.Molsted S, Prescott L, Heaf J, Eidemak I. Assessment and clinical aspects of health-related quality of life in dialysis patients and patients with chronic kidney disease. Nephron Clin Pract. 2007;106:c24–33. doi: 10.1159/000101481. [DOI] [PubMed] [Google Scholar]
- 20.Hellberg M, Hoglund P, Abdulahi H, Svensson P, Clyne N. Aerobic capacty, strength, balance, and fine motor skills correlate to GFR in patients with CKD 3B-5, not started on RRT. Nephrology Dialysis Transplantation. 2014;29:iii39–iii39. doi: 10.1093/ndt/gfu126. [DOI] [Google Scholar]
- 21.Leikis MJ, et al. Exercise performance falls over time in patients with chronic kidney disease despite maintenance of hemoglobin concentration. Clin J Am Soc Nephrol. 2006;1:488–495. doi: 10.2215/CJN.01501005. [DOI] [PubMed] [Google Scholar]
- 22.Hayhurst WS, Ahmed A. Assessment of physical activity in patients with chronic kidney disease and renal replacement therapy. Springerplus. 2015;4:536. doi: 10.1186/s40064-015-1338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansen KL, et al. Low level of self-reported physical activity in ambulatory patients new to dialysis. Kidney Int. 2010;78:1164–1170. doi: 10.1038/ki.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brenner I, Brohart K. Weekly energy expenditure and quality of life in hemodialysis patients. CANNT J. 2008;18:36–40. [PubMed] [Google Scholar]
- 25.Matsuzawa R, et al. Habitual physical activity measured by accelerometer and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2012;7:2010–2016. doi: 10.2215/CJN.03660412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Hare AM, Tawney K, Bacchetti P, Johansen KL. Decreased survival among sedentary patients undergoing dialysis: results from the dialysis morbidity and mortality study wave 2. Am J Kidney Dis. 2003;41:447–454. doi: 10.1053/ajkd.2003.50055. [DOI] [PubMed] [Google Scholar]
- 27.Johansen KL, et al. Association of physical activity with survival among ambulatory patients on dialysis: the Comprehensive Dialysis Study. Clin J Am Soc Nephrol. 2013;8:248–253. doi: 10.2215/cjn.08560812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johansen KL. Exercise in the End-Stage Renal Disease Population. Journal of the American Society of Nephrology. 2007;18:1845–1854. doi: 10.1681/asn.2007010009. [DOI] [PubMed] [Google Scholar]
- 29.Painter PL. Exercise in end-stage renal disease. Exercise and sport sciences reviews. 1988;16:305–339. [PubMed] [Google Scholar]
- 30.Painter P. Implementing exercise: what do we know? Where do we go? Advances in chronic kidney disease. 2009;16:536–544. doi: 10.1053/j.ackd.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Barcellos FC, Santos IS, Umpierre D, Bohlke M, Hallal PC. Effects of exercise in the whole spectrum of chronic kidney disease: a systematic review. Clinical Kidney Journal. 2015;8:753–765. doi: 10.1093/ckj/sfv099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segura-Orti E, Johansen KL. Exercise in end-stage renal disease. Seminars in dialysis. 2010;23:422–430. doi: 10.1111/j.1525-139X.2010.00766.x. [DOI] [PubMed] [Google Scholar]
- 33.Heiwe S, Jacobson SH. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2014;64:383–393. doi: 10.1053/j.ajkd.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 34.Parsons TL, King-Vanvlack CE. Exercise and end-stage kidney disease: functional exercise capacity and cardiovascular outcomes. Advances in chronic kidney disease. 2009;16:459–481. doi: 10.1053/j.ackd.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Johansen KL. Exercise and Dialysis. Hemodialysis International. 2008;12:290–300. doi: 10.1111/j.1542-4758.2008.00269.x. [DOI] [PubMed] [Google Scholar]
- 36.Ouzouni S, Kouidi E, Sioulis A, Grekas D, Deligiannis A. Effects of intradialytic exercise training on health-related quality of life indices in haemodialysis patients. Clin Rehabil. 2009;23:53–63. doi: 10.1177/0269215508096760. [DOI] [PubMed] [Google Scholar]
- 37.Deligiannis A. Cardiac adaptations following exercise training in hemodialysis patients. Clinical nephrology. 2004;61 Suppl 1:S39–45. [PubMed] [Google Scholar]
- 38.Deligiannis A, et al. Cardiac effects of exercise rehabilitation in hemodialysis patients. International journal of cardiology. 1999;70:253–266. doi: 10.1016/s0167-5273(99)00090-x. [DOI] [PubMed] [Google Scholar]
- 39.Shalom R, Blumenthal JA, Williams RS, McMurray RG, Dennis VW. Feasibility and benefits of exercise training in patients on maintenance dialysis. Kidney Int. 1984;25:958–963. doi: 10.1038/ki.1984.117. [DOI] [PubMed] [Google Scholar]
- 40.Konstantinidou Erasmia, Koukouvou Georgia, Kouidi Evangelia, Deligiannis Asterios, Tourkantonis A. Exercise training in patients with end-stage renal disease on hemodialysis: comparison of three rehabilitation programs. Journal of Rehabilitation Medicine. 2002;34:40–45. doi: 10.1080/165019702317242695. [DOI] [PubMed] [Google Scholar]
- 41.Kouidi E, Grekas D, Deligiannis A, Tourkantonis A. Outcomes of long-term exercise training in dialysis patients: comparison of two training programs. Clinical nephrology. 2004;61 Suppl 1:S31–38. [PubMed] [Google Scholar]
- 42.Torino C, et al. Physical performance and clinical outcomes in dialysis patients: a secondary analysis of the EXCITE trial. Kidney & blood pressure research. 2014;39:205–211. doi: 10.1159/000355798. [DOI] [PubMed] [Google Scholar]
- 43.Kohl LM, et al. Prognostic value of the six-minute walk test in end-stage renal disease life expectancy: a prospective cohort study. Clinics. 2012;67:581–586. doi: 10.6061/clinics/2012(06)06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malagoni Anna Maria. Acute and long-term effects of an exercise program for dialysis patients prescribed in hospital and performed at home. J Nephrol. 2008;21:871–878. [PubMed] [Google Scholar]
- 45.Bohm C, et al. Effects of intradialytic cycling compared with pedometry on physical function in chronic outpatient hemodialysis: a prospective randomized trial. Nephrol Dial Transplant. 2014;29:1947–1955. doi: 10.1093/ndt/gfu248. [DOI] [PubMed] [Google Scholar]
- 46.Miller BW, Cress CL, Johnson ME, Nichols DH, Schnitzler MA. Exercise during hemodialysis decreases the use of antihypertensive medications. Am J Kidney Dis. 2002;39:828–833. doi: 10.1053/ajkd.2002.32004. [DOI] [PubMed] [Google Scholar]
- 47.Anderson JE, Boivin MR, Jr, Hatchett L. Effect of exercise training on interdialytic ambulatory and treatment-related blood pressure in hemodialysis patients. Renal failure. 2004;26:539–544. doi: 10.1081/jdi-200031735. [DOI] [PubMed] [Google Scholar]
- 48.Mustata S, Chan C, Lai V, Miller JA. Impact of an exercise program on arterial stiffness and insulin resistance in hemodialysis patients. J Am Soc Nephrol. 2004;15:2713–2718. doi: 10.1097/01.ASN.0000140256.21892.89. [DOI] [PubMed] [Google Scholar]
- 49.Diesel W, Noakes TD, Swanepoel C, Lambert M. Isokinetic muscle strength predicts maximum exercise tolerance in renal patients on chronic hemodialysis. Am J Kidney Dis. 1990;16:109–114. doi: 10.1016/s0272-6386(12)80563-4. [DOI] [PubMed] [Google Scholar]
- 50.Johansen KL, et al. Muscle atrophy in patients receiving hemodialysis: effects on muscle strength, muscle quality, and physical function. Kidney Int. 2003;63:291–297. doi: 10.1046/j.1523-1755.2003.00704.x. [DOI] [PubMed] [Google Scholar]
- 51.Headley S, et al. Resistance training improves strength and functional measures in patients with end-stage renal disease. American Journal of Kidney Diseases. 2002;40:355–364. doi: 10.1053/ajkd.2002.34520. [DOI] [PubMed] [Google Scholar]
- 52.Johansen KL. Effects of Resistance Exercise Training and Nandrolone Decanoate on Body Composition and Muscle Function among Patients Who Receive Hemodialysis: A Randomized, Controlled Trial. Journal of the American Society of Nephrology. 2006;17:2307–2314. doi: 10.1681/asn.2006010034. [DOI] [PubMed] [Google Scholar]
- 53.Cheema B, et al. Progressive exercise for anabolism in kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol. 2007;18:1594–1601. doi: 10.1681/ASN.2006121329. [DOI] [PubMed] [Google Scholar]
- 54.DePaul V, Moreland J, Eager T, Clase CM. The effectiveness of aerobic and muscle strength training in patients receiving hemodialysis and EPO: a randomized controlled trial. Am J Kidney Dis. 2002;40:1219–1229. doi: 10.1053/ajkd.2002.36887. [DOI] [PubMed] [Google Scholar]
- 55.van Vilsteren MC, de Greef MH, Huisman RM. The effects of a low-to-moderate intensity pre-conditioning exercise programme linked with exercise counselling for sedentary haemodialysis patients in The Netherlands: results of a randomized clinical trial. Nephrol Dial Transplant. 2005;20:141–146. doi: 10.1093/ndt/gfh560. [DOI] [PubMed] [Google Scholar]
- 56.Oh-Park M, et al. Exercise for the dialyzed: aerobic and strength training during hemodialysis. Am J Phys Med Rehabil. 2002;81:814–821. doi: 10.1097/01.PHM.0000030623.81541.DA. [DOI] [PubMed] [Google Scholar]
- 57.Orcy RB, Dias PS, Seus TL, Barcellos FC, Bohlke M. Combined resistance and aerobic exercise is better than resistance training alone to improve functional performance of haemodialysis patients--results of a randomized controlled trial. Physiotherapy research international : the journal for researchers and clinicians in physical therapy. 2012;17:235–243. doi: 10.1002/pri.1526. [DOI] [PubMed] [Google Scholar]
- 58.Nussbaum J, Garcia RK. Restorative physical and occupational therapy: a critical need for patients with chronic kidney and end-stage renal disease. Advances in chronic kidney disease. 2009;16:529–535. doi: 10.1053/j.ackd.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 59.Nussbaum J, Garcia RK. The Effects of a Physical Therapy Intervention on Functional Outcome Measures in Patients with End Stage Renal Disease on Hemodialysis: Preliminary Results of the ProHealth Renal Rehabilitation Program. Blood Purification (Abstracts) 2012;33:212–223. doi: 10.1159/000334571. [DOI] [Google Scholar]
- 60.Nussbaum J. The Effects of a 9 Week Physical Therapy Program on 5 Patients with End Stage Renal Disease on Hemodialysis. Blood Purification (Abstracts) 2011;31:209–223. doi: 10.1159/000322687. [DOI] [Google Scholar]
- 61.Li M, Porter E, Lam R, Jassal SV. Quality improvement through the introduction of interdisciplinary geriatric hemodialysis rehabilitation care. Am J Kidney Dis. 2007;50:90–97. doi: 10.1053/j.ajkd.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Forrest GP. Inpatient rehabilitation of patients requiring hemodialysis. Archives of Physical Medicine and Rehabilitation. 2004;85:51–53. doi: 10.1016/s0003-9993(03)00366-6. [DOI] [PubMed] [Google Scholar]
- 63.Bohm CJ, Ho J, Duhamel TA. Regular physical activity and exercise therapy in end-stage renal disease: how should we “move” forward? J Nephrol. 2010;23:235–243. [PubMed] [Google Scholar]
- 64.Allen K, Gappmaier E. Exercise habits and attitudes of patients undergoing hemodialysis. Cardiopulmonary Physical Therapy Journal. 2001;12 [Google Scholar]
- 65.Delgado C, Johansen KL. Barriers to exercise participation among dialysis patients. Nephrol Dial Transplant. 2012;27:1152–1157. doi: 10.1093/ndt/gfr404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fiaccadori E, et al. Barriers to physical activity in chronic hemodialysis patients: a single-center pilot study in an Italian dialysis facility. Kidney & blood pressure research. 2014;39:169–175. doi: 10.1159/000355793. [DOI] [PubMed] [Google Scholar]
- 67.Kontos PC, et al. Factors influencing exercise participation by older adults requiring chronic hemodialysis: a qualitative study. Int Urol Nephrol. 2007;39:1303–1311. doi: 10.1007/s11255-007-9265-z. [DOI] [PubMed] [Google Scholar]
- 68.Johansen KL, Sakkas GK, Doyle J, Shubert T, Dudley RA. Exercise counseling practices among nephrologists caring for patients on dialysis. Am J Kidney Dis. 2003;41:171–178. doi: 10.1053/ajkd.2003.50001. [DOI] [PubMed] [Google Scholar]
- 69.Krause R. Nephrologists' view on exercise training in chronic kidney disease (results of the questionnaire at the WCN 2003) Clinical nephrology. 2004;61 Suppl 1:S2–4. [PubMed] [Google Scholar]
- 70.Greenwood SA, et al. Evaluation of a pragmatic exercise rehabilitation programme in chronic kidney disease. Nephrol Dial Transplant. 2012;27 Suppl 3:iii126–134. doi: 10.1093/ndt/gfs272. [DOI] [PubMed] [Google Scholar]
- 71.Clyne N. Physical working capacity in uremic patients. Scandinavian journal of urology and nephrology. 1996;30:247–252. doi: 10.3109/00365599609182300. [DOI] [PubMed] [Google Scholar]
- 72.Clyne N. Motion förbättrar arbetsförmågan och muskelstyrkan vid kronisk njursvikt. Läkartidningen. 2004;101:4111–4115. [PubMed] [Google Scholar]
- 73.Clyne N. The importance of exercise training in predialysis patients with chronic kidney disease. Clinical nephrology. 2004;61 Suppl 1:S10–13. [PubMed] [Google Scholar]
- 74.Gould DW, Graham-Brown MP, Watson EL, Viana JL, Smith AC. Physiological benefits of exercise in pre-dialysis chronic kidney disease. Nephrology. 2014;19:519–527. doi: 10.1111/nep.12285. [DOI] [PubMed] [Google Scholar]
- 75.American College of Sports, M et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 76.Johansen KL, Painter P. Exercise in individuals with CKD. Am J Kidney Dis. 2012;59:126–134. doi: 10.1053/j.ajkd.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Howden Erin J, Fassett Robert G, Isbel Nicole M, Coombes JS. Exercise Training in Chronic Kidney Disease Patients. Sports Med. 2012;42:473–488. doi: 10.2165/11630800-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 78.Koufaki P, Greenwood SA, Macdougall IC, Mercer TH. Exercise therapy in individuals with chronic kidney disease: a systematic review and synthesis of the research evidence. Annual review of nursing research. 2013;31:235–275. doi: 10.1891/0739-6686.31.235. [DOI] [PubMed] [Google Scholar]
- 79.Mustata S, et al. Effects of exercise training on physical impairment, arterial stiffness and health-related quality of life in patients with chronic kidney disease: a pilot study. Int Urol Nephrol. 2011;43:1133–1141. doi: 10.1007/s11255-010-9823-7. [DOI] [PubMed] [Google Scholar]
- 80.Nylen ES, Gandhi SM, Kheirbek R, Kokkinos P. Enhanced fitness and renal function in Type 2 diabetes. Diabet Med. 2015;32:1342–1345. doi: 10.1111/dme.12789. [DOI] [PubMed] [Google Scholar]
- 81.Toyama K, Sugiyama S, Oka H, Sumida H, Ogawa H. Exercise therapy correlates with improving renal function through modifying lipid metabolism in patients with cardiovascular disease and chronic kidney disease. J Cardiol. 2010;56:142–146. doi: 10.1016/j.jjcc.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 82.Clyne N, Ekholm J, Jogestrand T, Lins LE, Pehrsson SK. Effects of exercise training in predialytic uremic patients. Nephron. 1991;59:84–89. doi: 10.1159/000186524. [DOI] [PubMed] [Google Scholar]
- 83.Castaneda C, et al. Resistance training to counteract the catabolism of a low-protein diet in patients with chronic renal insufficiency. A randomized, controlled trial. Annals of internal medicine. 2001;135:965–976. doi: 10.7326/0003-4819-135-11-200112040-00008. [DOI] [PubMed] [Google Scholar]
- 84.Fitts SS, Guthrie MR, Blagg CR. Exercise coaching and rehabilitation counseling improve quality of life for predialysis and dialysis patients. Nephron. 1999;82:115–121. doi: 10.1159/000045386. doi:45386. [DOI] [PubMed] [Google Scholar]
- 85.Venkataraman R, Sanderson B, Bittner V. Outcomes in patients with chronic kidney disease undergoing cardiac rehabilitation. Am Heart J. 2005;150:1140–1146. doi: 10.1016/j.ahj.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 86.Shigeta K, et al. Effects of chronic renal dysfunction on rehabilitation progress in patients undergoing inpatient rehabilitation. Physiotherapy. 2015;101:e1385. doi: 10.1016/j.physio.2015.03.1329. [DOI] [Google Scholar]
- 87.Heiwe S, Tollback A, Clyne N. Twelve weeks of exercise training increases muscle function and walking capacity in elderly predialysis patients and healthy subjects. Nephron. 2001;88:48–56. doi: 10.1159/000045959. doi:45959. [DOI] [PubMed] [Google Scholar]
- 88.Cheng YY, Wong YF, Chu BY, Lam WO, Ho YW. Rehabilitating a dialysis patient. Perit Dial Int. 2003;23 Suppl 2:S81–83. [PubMed] [Google Scholar]
- 89.Eidemak I, Haaber AB, Feldt-Rasmussen B, Kanstrup IL, Strandgaard S. Exercise training and the progression of chronic renal failure. Nephron. 1997;75:36–40. doi: 10.1159/000189497. [DOI] [PubMed] [Google Scholar]
- 90.Jafar TH, Jin A, Koh WP, Yuan JM, Chow KY. Physical activity and risk of end-stage kidney disease in the Singapore Chinese Health Study. Nephrology. 2015;20:61–67. doi: 10.1111/nep.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Robinson-Cohen C, et al. Physical activity and change in estimated GFR among persons with CKD. J Am Soc Nephrol. 2014;25:399–406. doi: 10.1681/ASN.2013040392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pechter U, et al. Beneficial effects of water-based exercise in patients with chronic kidney disease. Int J Rehabil Res. 2003;26:153–156. doi: 10.1097/01.mrr.0000070755.63544.5a. [DOI] [PubMed] [Google Scholar]
- 93.Pechter U, Raag M, Ots-Rosenberg M. Regular aquatic exercise for chronic kidney disease patients: a 10-year follow-up study. Int J Rehabil Res. 2014;37:251–255. doi: 10.1097/MRR.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 94.Morishita Y, Nagata D. Strategies to improve physical activity by exercise training in patients with chronic kidney disease. Int J Nephrol Renovasc Dis. 2015;8:19–24. doi: 10.2147/IJNRD.S65702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smart NA, et al. Exercise & Sports Science Australia (ESSA) position statement on exercise and chronic kidney disease. J Sci Med Sport. 2013;16:406–411. doi: 10.1016/j.jsams.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 96.Sah SK, Siddiqui MA, Darain H. Effect of progressive resistive exercise training in improving mobility and functional ability of middle adulthood patients with chronic kidney disease. Saudi J Kidney Dis Transpl. 2015;26:912–923. doi: 10.4103/1319-2442.164571. [DOI] [PubMed] [Google Scholar]
- 97.Smith AC, Burton JO. Exercise in kidney disease and diabetes: time for action. J Ren Care. 2012;38(1):52–58. doi: 10.1111/j.1755-6686.2012.00279.x. [DOI] [PubMed] [Google Scholar]
- 98.Koufaki P, Greenwood S, Painter P, Mercer T. The BASES Expert Statement on Exercise Therapy for People with Chronic Kidney Disease. The Sport and Exercise Scientist. 2014 Summer; doi: 10.1080/02640414.2015.1017733. [DOI] [PubMed] [Google Scholar]
- 99.MacKinnon HJ, Feehally J, Smith AC. A review of the role of exercise and factors affecting its uptake for people with chronic kidney disease (CKD) not requiring renal replacement therapy. MASA. 2015;36:37–46. [PubMed] [Google Scholar]
- 100.Pereira BJ. Optimization of pre-ESRD care: the key to improved dialysis outcomes. Kidney Int. 2000;57:351–365. doi: 10.1046/j.1523-1755.2000.00840.x. [DOI] [PubMed] [Google Scholar]
- 101.Painter P. Physical functioning in end-stage renal disease patients: update 2005. Hemodialysis international International Symposium on Home Hemodialysis. 2005;9:218–235. doi: 10.1111/j.1492-7535.2005.01136.x. [DOI] [PubMed] [Google Scholar]
- 102.Cheema BS, Singh MA. Exercise training in patients receiving maintenance hemodialysis: a systematic review of clinical trials. Am J Nephrol. 2005;25:352–364. doi: 10.1159/000087184. [DOI] [PubMed] [Google Scholar]


