Abstract
Unconventional oil and gas operations using hydraulic fracturing can contaminate surface and groundwater with endocrine-disrupting chemicals. We have previously shown that 23 of 24 commonly used hydraulic fracturing chemicals can activate or inhibit the estrogen, androgen, glucocorticoid, progesterone, and/or thyroid receptors in a human endometrial cancer cell reporter gene assay and that mixtures can behave synergistically, additively, or antagonistically on these receptors. In the current study, pregnant female C57Bl/6 dams were exposed to a mixture of 23 commonly used unconventional oil and gas chemicals at approximately 3, 30, 300, and 3000 μg/kg·d, flutamide at 50 mg/kg·d, or a 0.2% ethanol control vehicle via their drinking water from gestational day 11 through birth. This prenatal exposure to oil and gas operation chemicals suppressed pituitary hormone concentrations across experimental groups (prolactin, LH, FSH, and others), increased body weights, altered uterine and ovary weights, increased heart weights and collagen deposition, disrupted folliculogenesis, and other adverse health effects. This work suggests potential adverse developmental and reproductive health outcomes in humans and animals exposed to these oil and gas operation chemicals, with adverse outcomes observed even in the lowest dose group tested, equivalent to concentrations reported in drinking water sources. These endpoints suggest potential impacts on fertility, as previously observed in the male siblings, which require careful assessment in future studies.
We have recently reported that chemicals used in and/or produced by unconventional oil and natural gas (UOG) operations can act as endocrine-disrupting chemicals (EDCs) both in vitro and in vivo (1, 2). EDCs are exogenous chemicals or mixtures of chemicals that are able to interfere with any aspect of hormone action (3) through direct interaction with hormone receptors (4, 5) or indirect interactions such as enhancement or suppression of response to endogenous hormones (6–8), modulation of endogenous hormone levels (9, 10), or other mechanisms (11, 12). As many as 1000 synthetic and naturally occurring EDCs have been identified (13), and are often able to act at environmentally relevant concentrations (realistic exposure levels below those traditionally examined in toxicological risk assessments), exhibit nonmonotonic dose-response curves (quantitatively and/or qualitatively different outcomes across a wide dose-response range), and often exert greater effects during critical periods of development when exposure can disrupt normal development and lead to later disease (14–17).
Hydraulic fracturing involves the high-pressure underground injection of up to several million gallons of water mixed with chemicals and suspended solids to increase production from both unconventional (nonporous shale or coal bed layers) and conventional fossil fuel deposits (18, 19). As such, wastewater from the process can be heavily laden with naturally occurring radioactive compounds, heavy metals from the target geologic layer, and chemicals used in extraction operations or liberated during the process such as polycyclic aromatic hydrocarbons, alkenes, alkanes, and other volatile and semivolatile organics (19–26). Oil and natural gas operations can contaminate drinking water, with recent studies suggesting well casing failures (27) and surface spills (28) may be the main causative mechanisms. More than 1000 different chemicals are used in UOG operations throughout the United States (18–20, 29, 30), at least 130 of which are known or suspected EDCs (1, 18, 31). Work in our laboratory has further described increased antagonist receptor activities in surface and ground water near fracturing fluid spill sites (1) and a wastewater injection disposal well (32). These data suggest that oil and natural gas operations may increase EDC activity in nearby surface and ground water, although insufficient baseline water quality data often exists to make a causative link (29, 33).
Health outcomes after exposure to UOG operation chemicals are poorly understood (2, 34, 35), although adverse human and animal health outcomes are more frequently self-reported in UOG regions (36–38) and inpatient hospital utilization rates can be higher (39). Furthermore, Bamberger and Oswald have reported many adverse health effects linked to UOG activity specifically; in cases where residents/animals moved away from activities or local production decreased, certain of these health concerns were alleviated (40). Limited epidemiological studies have assessed adverse health outcomes in potentially exposed populations, reporting that increased risks of congenital heart defects (41), low birth weight/small for gestational age children (42), preterm birth, and high risk pregnancies (43) are associated with increased density and/or proximity to UOG development, outcomes also associated with gestational EDC exposure (44–47). Numerous studies have reported elevated concentrations of specific contaminants in water and/or animals near these operations, including benzene, toluene, ethylbenzene, and xylenes (48–51), 2-butoxyethanol (52), diesel range organics (28), heavy metals (24, 53), and other compounds (25, 54–56). These and other individual chemicals used throughout the UOG process have been associated with increased rates of adverse reproductive outcomes such as miscarriage, preterm birth, and decreased fertility, reviewed previously (2, 35).
Based on mechanistic in vitro work performed in our laboratory (57), a range of endpoints previously demonstrated to be susceptible to estrogen, androgen, progesterone, glucocorticoid, and/or thyroid receptor inhibition were selected for evaluation after gestational exposure, including anogenital distance, body weight, organ weights, pubertal development, serum hormone levels, and folliculogenesis. Specifically, exposure to antiandrogens such as flutamide can alter anogenital distance (58, 59), FSH, LH, and other hormones (60, 61) and disrupt folliculogenesis (62–66) in females of several species. Inhibition of other receptor pathways examined here can decrease uterine and ovary weights (67–71), alter LH, FSH, and other pituitary hormone levels (57, 72), disrupt folliculogenesis (73–75), increase body weights (76, 77), and contribute to cardiac abnormalities (44, 45, 78) and increased collagen content (79). As such, we hypothesized that prenatal exposure to this mixture of UOG EDCs at likely environmentally relevant concentrations would impact hormone-sensitive endpoints in prepubertal and adult female mice.
As of yet, no controlled in vivo experiments have been performed to assess female health outcomes after developmental exposure to environmentally relevant concentrations of UOG chemicals. Recent work has highlighted this deficit, finding that for 1021 UOG chemicals, reproductive or developmental toxicity data existed for only 126 and 192 chemicals, respectively (80). We recently reported adverse reproductive and developmental health outcomes in C57Bl/6 male mice (siblings to the females reported here) after prenatal exposure to likely environmentally relevant concentrations of a mixture of 23 hydraulic fracturing chemicals (57). The goals of this study were to continue to address this major data gap by assessing the effects of developmental exposure to this laboratory-created mixture of oil and gas chemicals on reproductive and developmental health in female C57Bl/6 mice.
Materials and Methods
Chemicals
Twenty-three oil and natural gas operation chemicals (all ≥97% purity) were selected (Supplemental Table 1) based on previous work in our lab demonstrating endocrine activity for 5 receptors (57). All hydraulic fracturing chemicals were purchased from Sigma-Aldrich Co. Stock solutions were prepared in absolute 200 proof ethanol and stored at −20°C between uses.
Animal experiment study design
Reported here are data on female offspring from a pairing of virgin C57Bl/6J mice (sires and dams of mated mice were purchased from Jackson labs); data on male sibling offspring was reported previously (57). Briefly, mice were housed in polysulfone microisolator cages under temperature and light-controlled (12-h light, 12-h dark cycle) conditions in a barrier facility. Mice were fed sterilized LabDiet 5053 and received sterilized, acidified water ad libitum from glass bottles that was found to contain no activity for the estrogen, androgen, progesterone, thyroid, or glucocorticoid receptors (data not shown). All procedures were performed according to approved University of Missouri Animal Care and Use Committee protocol and were in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals.
Ten-week-old mice were time-mated and gestation day 0 was denoted as the day of vaginal plug visualization. Dams were randomized into treatment groups (n = 11, 10, 10, 11, 10, and 10), respectively and, beginning on gestation day 11 and ending at birth, had their drinking water supplemented with experimental treatments (Supplemental Figure 1) as described previously (57). Treatments included a 0.2% ethanol vehicle, 166.67-μg/mL flutamide antiandrogen control (50-mg/kg·d estimated dam exposure based on body weights), and 4 concentrations of a mixture of 23 UOG chemicals shown previously to inhibit the estrogen, androgen, progesterone, glucocorticoid, and/or thyroid receptor (Supplemental Table 1), with each of the 23 chemicals at 0.01, 0.10, 1.0, and 10 μg/mL (3-, 30-, 300-, and 3000-μg/kg·d estimated dam exposure; Mix3, 30, 300, and 3000, respectively). Chemical concentrations were selected to mimic environmentally relevant concentrations as available; concentrations for most chemicals in the 2 lowest doses were equivalent to those reported in drinking water in drilling regions and in the highest dose were equivalent to those reported in industry wastewater samples (35, 57), although analytical measurements on several of the 23 constituent chemicals have not yet been assessed in wastewater and/or drinking water. Litters with less than 2 males and 2 females were completely removed due to concerns of skewed gestational hormone exposure (81, 82); litters with at least 2 males and 2 females were left unaltered, as culling individual pups within litters can alter feeding status, behavior, and physiology (83). Water intake was monitored by weighing the drinking bottle each day of the treatment window, and no significant differences were noted between groups relative to the vehicle control (data not shown). Food intake was not monitored.
As described previously (57), anogenital distance was assessed on postnatal day (PND)7 and body weights were measured through PND21 for all pups (Supplemental Figure 1). One randomly selected female pup from each litter was necropsied on PND21. Each animal was euthanized by carbon dioxide asphyxiation and cardiac puncture, and tissues were excised. Tissues were weighed and then either formalin fixed for 24 hours for histological evaluation or snap frozen in liquid nitrogen. All remaining females were assessed for age of vaginal opening daily beginning on PND25, after which vaginal cytologies were performed for a minimum of 3 weeks to assess age of first vaginal estrus. A second randomly selected female from each litter was collected on approximately PND85 at estrus, based on vaginal cytologies performed daily and for 1 week leading up to collection, as described previously (84, 85). Remaining pups were used in pilot studies to assess other endpoints of interest.
Ovarian follicle assessment
Ovaries used to assess follicle distribution were collected from pups at PND21 and PND85. After fixation, ovaries were embedded in paraffin and every fourth 5-μm section was mounted and stained with hematoxylin & eosin (IDEXX). Slides were randomly coded according to treatment groups and follicle counting was performed using the fractionator approach (86). Briefly, only follicles with a visible oocyte nucleus were counted to ensure no duplication of follicle counts. Follicles were classified as 1) primordial: single layer of squamous granulosa cells; 2) primary: single layer of cuboidal granulosa cells; 3) secondary: more than 1 layer of cuboidal granulosa cells and no antrum; 4) antral: multiple layers of cuboidal granulosa cells and a visible antrum; or 5) atretic: follicles with collapsed basement membrane, zona pellucida remnants, and a majority of picnotic nuclei in any remaining granulosa cells. Because representative follicles of the whole ovary were counted, data are expressed as total follicle numbers at each follicular stage and compared with vehicle control animals.
Serum hormone measurements
Blood was collected from mice via cardiac puncture at time of necropsy and stored on ice. Serum was separated by centrifugation and stored at −80°C until shipment to Dr Wolfe's laboratory at Johns Hopkins University on dry ice, where serum hormone assays were performed as described previously (87). Briefly, pituitary hormone concentrations were simultaneously measured in serum samples using a Milliplex Mouse Pituitary Magnetic Bead Panel (catalog number RPTMAG-49K; Millipore) and measured on a Luminex 200 system (Life Technologies). Serum estradiol (E2) was measured in duplicate using a mouse/rat E2 ELISA kit (catalog number ES180S-100; Calbiotech). Interassay coefficient of variation (CV) for the Luminex pituitary panel was between 3% and 12%, and intraassay CV % was between 1.75% and 6.23%. Limits of sensitivity were as follows: brain-derived neurotrophic factor, 1.6 pg/mL; LH, 1.9 pg/mL; FSH, 9.5 pg/mL; TSH, 1.9 pg/mL; ACTH, 1.7 pg/mL; GH, 1.7 pg/mL; and prolactin (PRL), 46.2 pg/mL. The intraassay CV for the E2 ELISA was 0.87%–8.3%, and the limit of sensitivity was 3 pg/mL.
Heart assessments
Whole hearts from PND21 and PND85 animals were excised from 1 female mouse per litter and were fixed as described above. Complete details on heart staining and analysis are provided in the Supplemental Materials and Methods. Briefly, hearts were paraffin-embedded and 2 5-μm midsagittal sections were cut and placed on slides. For collagen assessments, slides were stained with Picrosirius red, and collagen was visualized using bright-field microscopy. MetaMorph was used to create a tiled image of the whole heart, and areas of heart and collagen were assessed using ImageJ. The collagen deposition was then calculated by taking the percentage of collagen area divided by the heart area. For cardiac myocyte immunostaining, 1 slide per heart was sequentially heated in sodium citrate buffer for antigen retrieval, incubated with a blocking solution, and then incubated overnight with 2 primary mouse antibodies. The next day, the sections were incubated with donkey antimouse secondary antibody and 4′,6-diamidino-2-phenylindole DNA dye, then assessed with an Olympus IX70 fluorescence microscope for troponin I (green), wheat germ agglutinin (red), and cell nuclei (blue). Thirty longitudinal cardiac myocytes were randomly selected, and diameters across the middle of the myofiber nuclei were measured and averaged using ImageJ. One-way ANOVA using SPSS was used to assess significance.
Statistical analysis
Linear models were used to analyze the results from single-point-per-litter data (follicle development stages, sex ratio, litter size, organ weights, body weights postweaning, liver gene expression, cardiac myocyte diameter). Linear mixed models were used to analyze the results from multiple-point-per-litter data (anogenital distance, body weights preweaning, pubertal development, follicle numbers per developmental stage), and incorporated random effects to account for dependence among repeated measurements from litters. Fixed effects included treatment, sex ratio, litter size, body weight, birth weight (PND7), and/or date of measurement or collection when appropriate. Variables were transformed for normal distributions when necessary and adjusted means back transformed for presentation. Least-squares means were used to determine 95% confidence intervals for differences to vehicle control and for planned contrasts. Diagnostic plots were used to assess the fit and check distributional assumptions. Proc GLM and GLIMMIX in SAS 9.4 (SAS, Inc) were used for all analysis, unless specified elsewhere.
Results
Serum hormones and gene expression in developmentally exposed female mice
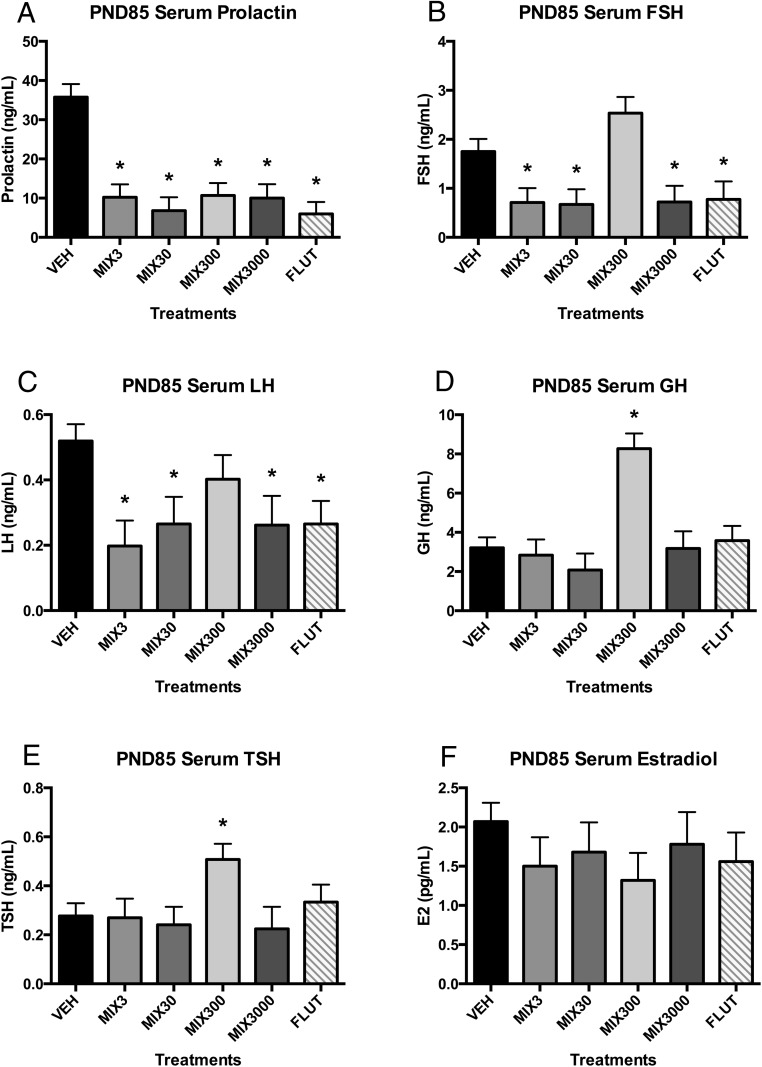
Serum hormone concentrations were assessed at PND85 (Figure 1). Serum concentrations of PRL were suppressed (≥70%) in all Mix groups and in the flutamide control (Figure 1A). Serum FSH and LH were suppressed (≥49%) in all experimental groups relative to control animals, except for Mix300 (Figure 1, B and C). Serum GH and TSH were elevated in the Mix300 group (157% and 83% increases, respectively) (Figure 1, D and E), which was the only experimental group that did not exhibit FSH and LH suppression. Serum E2 was not different between groups (Figure 1F). Serum brain-derived neurotropic factor and ACTH were below the limits of the detection for most animals (data not shown).
Figure 1. Developmental exposure to oil and gas chemicals alters serum hormones in adulthood.
Estimated marginal mean ± SEM of PRL (A), FSH (B), LH (C), GH (D), TSH (E), and E2 (F) for developmentally exposed C57Bl/6 mice collected at PND85. *, different than untreated controls (vehicle) alone at P ≤ .05; n = 9, 9, 7, 8, 6, and 10 litters for vehicle (VEH), Mix3, Mix30, Mix300, Mix3000, and flutamide (FLUT), respectively. Abbreviations: E2, estradiol; FSH, follicle stimulating hormone; GH, growth hormone; LH, luteinizing hormone; PND, postnatal day; TSH, thyroid stimulating hormone.
Thyroid-regulated gene expression was not affected in the liver at PND21 (Supplemental Figure 2). However, Mix300, which had the highest TSH levels relative to the vehicle control at PND85, exhibited the highest expression of both malic enzyme and Thrsp at PND21 (Supplemental Figure 2).
Folliculogenesis in developmentally exposed female mice
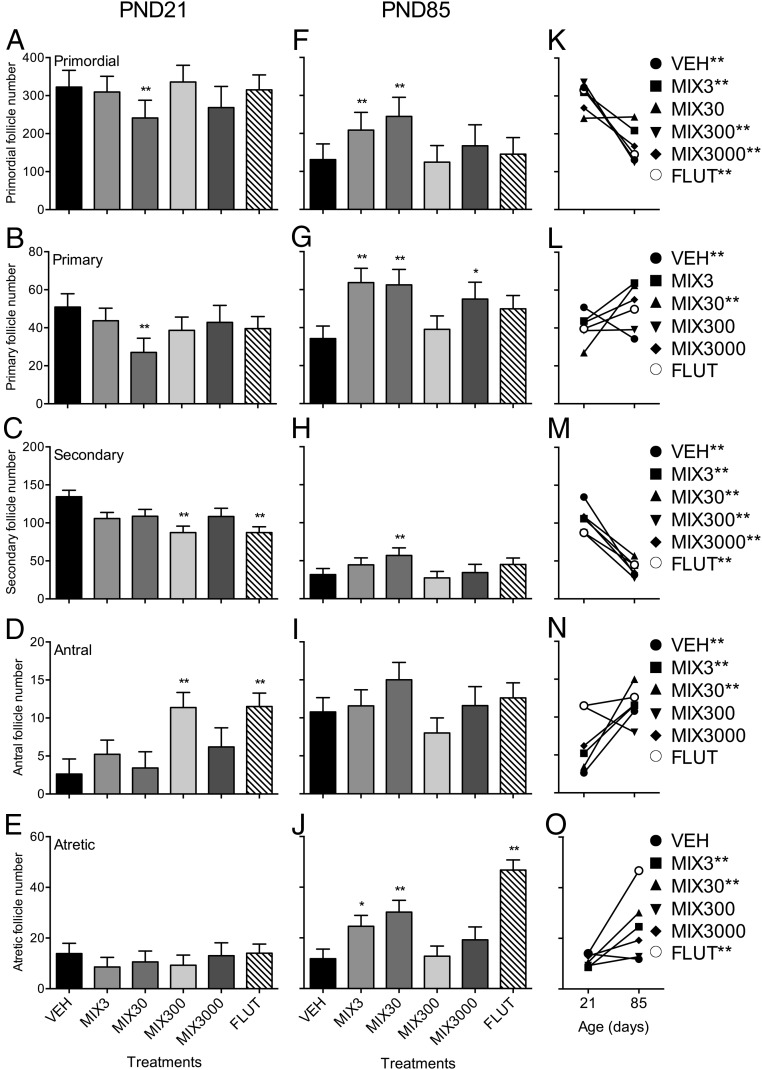
Because EDCs have been shown to alter folliculogenesis in rodents (63), we assessed ovaries at both PND21 and PND85. Each treatment had an effect on at least 1 follicle stage. At PND21 (Figure 2, A–E), primordial and primary follicles were reduced in mice exposed to Mix30 compared with vehicle controls (25% and 49% respectively) (Figure 2, A and B). At PND21, secondary follicles were reduced in Mix300- and flutamide-exposed animals (35% in both treatments) (Figure 2C). Conversely, both Mix300 and flutamide exposure increased antral follicles at PND21 (70% and 71%, respectively) (Figure 2D). No effect of treatment was observed on atretic follicle number at PND21 (Figure 2E). At PND85, primordial follicle number was increased in animals exposed to Mix3 and Mix30 compared with vehicle controls (37% and 46%, respectively) (Figure 2F). Similarly, primary follicle number was increased in animals exposed to Mix3, Mix30, and Mix3000 (46%, 44%, and 37%, respectively) (Figure 2G). Secondary follicle number was increased in PND85 animals exposed to Mix30 by 43% (Figure 2H). The number of atretic follicles was increased in PND85 animals exposed to Mix3, Mix30, and flutamide compared with vehicle animals (52%, 59%, and 75%, respectively) (Figure 2J).
Figure 2. Developmental exposure to oil and gas chemicals alters folliculogenesis.
Least squared mean ± SEM for each follicle type are presented at PND21 (A–E) and PND85 (F–J). The developmental progression from PND21 to PND85 for each follicle type is presented in K–O. **, different than untreated controls (vehicle) alone at P ≤ .05 (A–J); **, different between PND21 and PND85 for a single follicle stage at P ≤ .05 (K–O); n = 8, 9, 7, 8, 5, and 10 litters for vehicle (VEH), Mix3, 30, 300, 3000, and flutamide (FLUT), respectively, at PND21; n = 9, 7, 6, 8, 5, and 8 litters for vehicle, Mix3, 30, 300, 3000, and flutamide, respectively, at PND85. Abbreviation: PND, postnatal day.
Follicle numbers in each stage changed with age in vehicle control animals (Figure 2, K–O). However, Mix30 did not display this normal reduction in primordial follicle numbers over time (Figure 2K). Primary follicles were reduced in vehicle controls over time, whereas numbers of primary follicles actually increased in animals from the Mix30 treatment group (Figure 2L). In addition, no increase in antral follicle numbers was observed in animals exposed to Mix300, Mix3000, or flutamide as compared with the normal increase over time observed in vehicle mice (Figure 2N). Importantly, there was an increase in atretic follicle numbers over time in animals exposed to Mix3, Mix30, and flutamide, whereas no increase was observed in vehicle controls.
Statistical analysis revealed a treatment by age interaction (P < .01). Treatment by follicle stage interaction and an age by treatment by follicle stage interaction (P < .001) were also observed, indicating a significant impact of these hydraulic fracturing chemicals on folliculogenesis, dependent on both dose and age of the animal.
Body and organ weights and puberty in developmentally exposed female mice
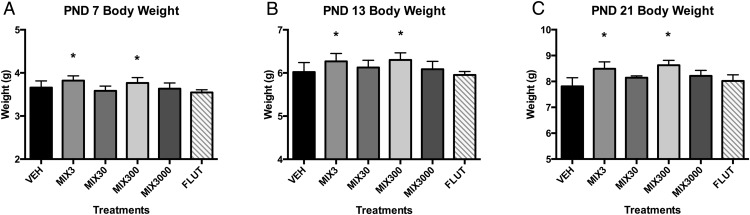
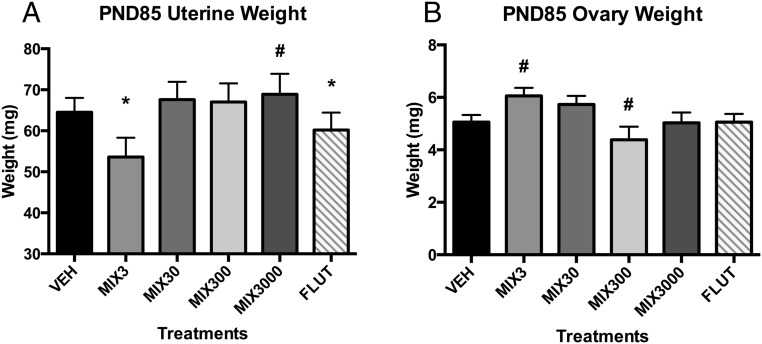
Body weights were elevated in the Mix3 and Mix300 groups at PND7, PND13, and PND21 (Figure 3). Weights were approximately 4% greater at PND7 (Figure 3A), 5% at PND13 (Figure 3B), and 9%–10% at PND21 (Figure 3C and Supplemental Table 2), although were not significantly different at PND85 (Supplemental Table 3). Developmental exposure appeared to alter reproductive organ development; Mix3 and flutamide significantly reduced blotted uterine weight at PND85, with a trend for increase observed in Mix3000 animals (P < .10) (Figure 4A). Further, exposure to Mix3 tended to increase ovary weight at PND85 (P < .10), whereas exposure to Mix300 tended to decrease it (P < .10) (Figure 4B). Spleen weights also tended to be greater in the Mix300 and flutamide groups (P < .10) at PND21 only (Supplemental Tables 2 and 3).
Figure 3. Developmental exposure to oil and gas chemicals increases body weights throughout development.
Estimated marginal mean ± SEM of body weights at PND7 (A), PND13 (B), and PND21 (C) for developmentally exposed C57Bl/6 female mice. All female pups within each litter were weighed at both PND7 and PND13, and only 1 randomly selected female from each litter at PND21. *, different than untreated controls (vehicle) alone at P ≤ .05; n = 9, 9, 7, 8, 6, and 10 litters for vehicle (VEH), Mix3, Mix30, Mix300, Mix3000, and flutamide (FLUT), respectively. Abbreviation: PND, postnatal day.
Figure 4. Developmental exposure to oil and gas chemicals alters uterine and ovary weights in adulthood.
Estimated marginal mean ± SEM of blotted uterine weights (A) and paired ovary weights (B) for developmentally exposed C57Bl/6 female mice. *, different than untreated controls (vehicle) alone at P ≤ .05. #, different than untreated controls (vehicle) alone at .05 < P ≤ .10; n = 9, 9, 7, 8, 6, and 10 litters for vehicle (VEH), Mix3, Mix30, Mix300, Mix3000, and flutamide (FLUT), respectively. Abbreviation: PND, postnatal day.
No significant differences in litter size, sex ratio, and cannibalization rate were observed for any treatments (Supplemental Table 4). However, cannibalization of entire litters before PND7 occurred with 1, 2, and 3 cases in the Mix3, Mix30, and Mix3000 groups, respectively, as reported previously (57). Age of vaginal opening, age of first vaginal estrus, and the length of the interval between these ages were also assessed, although no significant differences were noted between experimental groups (Supplemental Table 4).
Heart assessment in developmentally exposed female mice
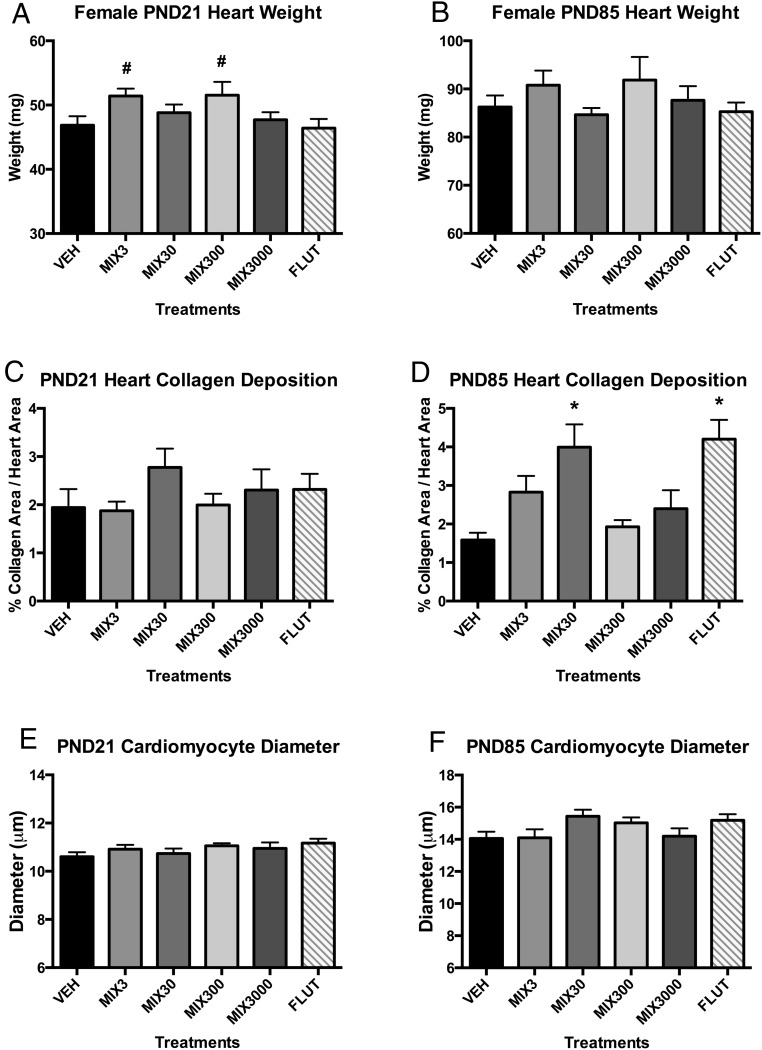
Heart weights tended to be elevated at PND21 in Mix3 and Mix300 groups (P < .10) (Figure 5A), a trend that was still evident but less pronounced at PND85 (Figure 5B), similar to what was observed in males (57). Hearts were assessed for cardiac myocyte size and collagen content. At PND85, Mix3, Mix30, and flutamide hearts exhibited 78% (P < .15), 152% (P < .05), and 165% (P < .05) increased collagen deposition, respectively, than controls (Figure 5D). At PND21, collagen deposition tended to be elevated in the Mix30 group (Figure 5C). Cardiac myocyte size was not found to differ between treatment groups at either PND21 (Figure 5E) or PND85 (Figure 5F).
Figure 5. Developmental exposure to oil and gas chemicals alters heart development in adulthood.
Estimated marginal mean ± SEM of heart weights collected at PND21 (A) and PND85 (B) for developmentally exposed C57Bl/6 female mice. Estimated marginal mean ± SEM of percent collagen deposition in heart sections at PND21 (C) and PND85 (D). Estimated marginal mean ± SEM of cardiac myocyte diameters (μm) at PND21 (E) and PND85 (F). *, different than untreated controls (vehicle) alone at P ≤ .05; #, different than untreated controls (vehicle) alone at .05 < P ≤ .10; n = 9, 9, 7, 8, 6, and 10 litters for vehicle (VEH), Mix3, Mix30, Mix300, Mix3000, and flutamide (FLUT), respectively. Abbreviation: PND, postnatal day.
Discussion
We report for the first time, potential adverse reproductive and developmental health outcomes in female mice after prenatal exposure to a laboratory-created mixture of 23 UOG chemicals, including disrupted folliculogenesis, body weights, uterine weights, and serum hormone concentrations. Exposure via drinking water occurred at environmentally relevant concentrations with the 2 lowest doses equivalent to concentrations reported in drinking water in drilling regions and the highest dose tested equivalent to those concentrations reported in industry wastewater samples (35, 57). We have previously reported adverse health outcomes in the sibling male mice; these experienced increased testis weights, serum testosterone, body weights, heart weights, cardiomyocyte size, and decreased sperm counts (57). Based on assessment of disruption for 5 nuclear receptors by these chemicals in vitro (1, 57), likely causative mechanisms for adverse male health outcomes included antagonism of the estrogen, androgen, glucocorticoid, and thyroid receptors (57). This study expanded this work to developmentally exposed females. Reproductive or developmental effects have been previously reported for several of the UOG chemicals used here, although only at high doses typical of occupational exposure (2).
Pituitary hormone production was strongly altered in animals from all treatment groups (Figure 1). Notably, these mechanisms were hypothesized as a potential causative mechanism for the greatly enhanced serum testosterone observed in the male siblings (57). Serum PRL was suppressed in all mixture groups and in the antiandrogen flutamide control relative to the vehicle. Serum FSH and LH were suppressed in the flutamide group and all mixture groups except for Mix300. Serum GH and TSH both exhibited the opposite trend, with significantly greater concentrations than vehicle in the Mix300 treatment. Suppressed PRL concentrations (≥70% decrease in experimental groups) may result in profound reproductive effects in these animals; PRL is critical for lactation, female receptivity, and parenting behavior, as well as immune function, angiogenesis, metabolism, and more (88). Suppressed FSH (≥55%, all but Mix300) may result in impaired fertility; FSH receptors are localized to ovarian granulosa cells and activation is considered essential for folliculogenesis (89). Suppressed LH (≥49%, all but Mix300) may also impact fertility; LH is critical for folliculogenesis, ovulation, and for maintenance of luteal function (90). Suppressed FSH, LH, and other hormones after flutamide exposure have been reported previously in several species (60, 61), and this may thus reflect an antiandrogenic effect of the mixture treatment on these animals. Elevated GH and TSH in Mix300 animals (157% and 83% increases, respectively) may result in disruption of normal growth and development; GH drives increased muscle mass, growth of internal organs, etc (91), whereas TSH stimulates the release of T4, which after conversion to T3, regulates metabolism, growth and development, and other functions (92). The elevated body weights observed in animals from the Mix300 treatment are consistent with the increases in GH and TSH in this group (Figures 1 and 3), although body weights were not significantly different by PND85 when hormone concentrations were measured. This may be due to compensatory mechanisms for controlling metabolic outcomes in these animals that should be evaluated more comprehensively in future studies.
Decreased uterine and ovary weights were observed in our mice and have also been observed after developmental inhibition of several receptor pathways examined here (67–71). Interestingly, FSH receptor knockout mice also exhibit increased testosterone levels and body weights (68), both observed in male siblings (57). However, these various receptor inhibition models also result in elevated LH, FSH, and/or E2 levels, none of which were observed in our study animals. Maternal hypothyroidism has also been shown to reduce uterine weights, LH, and FSH levels in offspring (72), although also reduces testosterone in males, contrary to what we observed previously (57). Because hypothalamic releasing hormones were not measured in the current study, we could not determine whether the programming target was hypothalamic, pituitary, or a combination. Determining the causative mechanism for the observed effects should be targeted in future research.
Gestational exposure to the UOG mixture appeared to alter folliculogenesis in these animals. The hypothalamic-pituitary-gonadal axis and normal steroid hormone synthesis is integral to normal postpubertal follicular development from the primordial follicle until ovulation (75). As described above, it appears that gonadotropin secretion is disrupted in exposed animals, however it is not clear whether this disruption is the cause or effect of altered ovarian morphology. Interestingly, peripubertal (PND21) ovarian morphology is only mildly altered in exposed animals, before the cyclic secretion of the gonadotropins FSH and LH. Examination of neonatal ovaries after gestational exposure may help shed light on the true extent of chemical exposure on the resultant ovarian pool of oocytes. However, the total number of primordial and primary follicles was reduced in Mix30-exposed animals at PND21, suggesting depletion of the ovarian reserve at this dose. At PND85, the number of primordial follicles remained unchanged, whereas primary follicle number increased 2.3-fold accompanied by a 2.9-fold increase in follicle atresia. This altered transition is suggestive of inappropriate follicle activation (or accumulation) and ultimate follicle death. The ovarian follicle is responsible for housing the finite population of oocytes required for propagation of future generations. In addition, the somatic cells of the follicle (theca and granulosa cells) and corpus luteum (luteal cells) are key to the postpubertal production of the sex steroids progesterone, testosterone, and E2 that are required for normal estrous (or menstrual) cyclicity via feedback control of the hypothalamus. The process of folliculogenesis during fetal development sets the number of total follicles that will be available for activation and potential ovulation during the entire reproductive lifespan of an animal (reviewed in Ref. 93). Thus, although we have not tested the fertility of these offspring, it is likely that they would be fertile, but this fertility may be shorter lived in some animals (based on this altered temporal transition of follicles through the developmental stages).
We reported increased body weights for female offspring at PND7 through PND21 in the Mix3 and Mix300 groups, although limited direct evidence exists regarding the adipogenic potential of the chemicals tested here (76). Nonylphenol and octylphenol (metabolites of the alkylphenol ethoxylates included here) can promote adipogenesis in vitro and in vivo (94, 95), and naphthalene has been associated with increased child obesity rates (96). 2-Ethylhexanol (97) and several oil dispersants and constituent chemicals tested here (98, 99) can activate peroxisome proliferator-activated receptor γ, often considered a master regulator of adipogenesis. To the best of our knowledge, none of these 23 chemicals have directly been tested for adipogenic potential in the 3T3-L1 or similar preadipocyte cell lines. Given that 20 and 6 of the chemicals included here antagonized the androgen and thyroid receptors (57), respectively, this may be the underlying mechanism that drives the increased weight in these animals (100–102). Epidemiological studies have reported disparate findings on birth weight and maternal proximity to unconventional natural gas development during pregnancy; similar study designs reported increased birth weights in Colorado children (41) and decreased birth weights in the Marcellus Shale region (42). Further work should elucidate the adipogenic potential of these chemicals more directly. It should be noted that Mix300 animals exhibited elevated serum GH and TSH, whereas Mix3 did not, suggesting that the mechanism for increased weight in Mix3 and Mix300 animals may be driven via different mechanisms.
We previously found indication for cardiac abnormalities after developmental exposure to the UOG mixture in the male siblings (57) and hypothesized a possible hypertrophic phenotype. In the current analysis of female offspring, we found increased cardiac fibrosis (2.4-fold increase in collagen deposition in Mix30 and flutamide groups) as well as trends for increased heart weights and enlarged cardiomyocytes (P = .12), endpoints that can be indicative of cardiac hypertrophy (103–105). Excess fibrosis can cause stiffness, resulting in a reduction in the diastolic and systolic function (106). Cardiac remodeling, commonly achieved through hypertrophy, is an attempt to ensure appropriate output to meet increased demands. Persistent cardiac remodeling is associated with various cardiomyopathies including myocardial infarction, arrhythmia, and sudden death (107). Importantly, an increased rate of congenital heart defects in human babies has been reported in association with maternal proximity to natural gas development during pregnancy in Colorado (41), and also has been associated with gestational exposure to EDCs and bioactive polycyclic aromatic hydrocarbons (44, 45, 78). Recent work reported increased collagen deposition in female mouse hearts after prenatal and postnatal exposure to bisphenol A (79). Prenatal exposures to glucocorticoids (108), androgens (109), estrogens, and progestins (110) have been associated with increased ventricular hypertrophy. In total, the increased collagen deposition, heart weights, and cardiomyocyte sizes in females and/or males after prenatal exposure to UOG chemicals is suggestive of a programmed change in the dynamic nature of the ventricle that may result in increased risk of cardiac dysfunction. Future work should assess functional effects on heart integrity in a more comprehensive manner.
A trend for increased splenic weights was noted in the Mix300 and flutamide groups at PND21, an endpoint that has been previously shown with androgen deprivation in C57Bl/6 mice (111, 112). This was also observed in the male siblings, and given the antiandrogenic mechanism of action for the mixture in vitro (57), the similar mechanism for many in vivo outcomes and for the flutamide control here, this suggests an antiandrogenic mechanism for this endpoint.
This was the first study to describe the consequences of developmental programming by a mixture of UOG chemicals in female mice; this work has identified many adverse endpoints for future, more comprehensive research. In addition, future research is needed to test the many additional chemicals used in and produced by this process, better characterize environmental presence and concentrations of these and other contaminants, and assess reproductive and developmental outcomes via other exposure routes and developmental windows. Exposure may occur from UOG operations in combination with other personal and/or industrial sources (as described in Supplemental Table 1). Inhalation and dermal absorption are also potential routes of exposure to these chemicals and were not examined in this study. Analytical limitations have prevented complete characterization of all 23 in vivo-tested chemicals in industry wastewater and community drinking water, limiting knowledge on realistic environmental concentrations for several constituents of the UOG chemical mixture used here and for most of the not yet assessed 1021 other chemicals used throughout this process. Given the vast complexity of environmental samples, in vivo work assessing dilutions of UOG wastewater samples side-by-side with this laboratory mixture to assess the relative contribution of various contaminants in adverse health outcomes should be pursued. Given the pulsatile nature of several pituitary hormones examined, more comprehensive hormone assessments over time should be performed in future studies to better characterize this apparent disruption.
In conclusion, we report for the first time that prenatal exposure to graded doses of a laboratory mixture of UOG chemicals at environmentally relevant concentrations can cause adverse reproductive and developmental health outcomes in female C57Bl/6 mice. Coupled with previous in vitro mechanistic data on these chemicals, we further report tentative mechanistic information for the observed adverse health effects. Our results suggest numerous potential threats to fertility and reproductive success in these animals, including altered pituitary hormone levels, reproductive organ weights, and disrupted ovarian follicle development. Notably, increased body weights, disrupted heart development, altered hormone levels, and impacted fertility endpoints were also observed in the male siblings (57), suggesting similar mechanisms of action. Future studies should examine fertility and the other adverse endpoints discussed here in a more comprehensive manner, using both laboratory experiments and epidemiological studies to better characterize the complex mixtures of EDCs used in and produced by oil and natural gas operations and their potential threats to human and animal health.
Acknowledgments
We thank Professor Bill Thatcher for his significant contribution in statistical analysis of follicle count data; Annie Maas, Katelyn Cinnamon, and Michelle Williams for their help in each of the mouse collections; Lisa Pinatti for her help with the thyroid-regulated gene expression analyses; Lada Micheas and Dr J. Wade Davis for their invaluable advice and feedback on the SAS code used here; Professor Richard E. Gilbert at University of Toronto, Canada for generously sharing the Picro Sirius Red collagen staining protocol and Dr Maike Krenz at the Department of Medical Pharmacology and Physiology, University of Missouri, for sharing the cardiac myocyte double staining protocol; and undergraduate student Jiahao Hu for his assistance in the analysis of heart collagen deposition quantification.
This work was supported by the University of Missouri Research Council, a crowd funding campaign on Experiment.com, and the Science To Achieve Results Fellowship Assistance Agreement FP-91747101 awarded by the United States Environmental Protection Agency (to C.D.K.).
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the University of Missouri Research Council, a crowd funding campaign on Experiment.com, and the Science To Achieve Results Fellowship Assistance Agreement FP-91747101 awarded by the United States Environmental Protection Agency (to C.D.K.).
Footnotes
- CV
- coefficient of variation
- E2
- estradiol
- EDC
- endocrine-disrupting chemical
- PND
- postnatal day
- PRL
- prolactin
- UOG
- unconventional oil and natural gas.
References
- 1. Kassotis CD, Tillitt DE, Davis JW, Hormann AM, Nagel SC. Estrogen and androgen receptor activities of hydraulic fracturing chemicals and surface and ground water in a drilling-dense region. Endocrinology. 2014;155(3):897–907. [DOI] [PubMed] [Google Scholar]
- 2. Webb E, Bushkin-Bedient S, Cheng A, Kassotis CD, Balise V, Nagel SC. Developmental and reproductive effects of chemicals associated with unconventional oil and natural gas operations. Rev Environ Health. 2014;29(4):307–318. [DOI] [PubMed] [Google Scholar]
- 3. Zoeller RT, Brown TR, Doan LL, et al. . Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology. 2012;153(9):4097–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fang H, Tong W, Branham WS, et al. . Study of 202 natural, synthetic, and environmental chemicals for binding to the androgen receptor. Chem Res Toxicol. 2003;16(10):1338–1358. [DOI] [PubMed] [Google Scholar]
- 5. Fang M, Webster TF, Ferguson PL, Stapleton HM. Characterizing the peroxisome proliferator-activated receptor (PPARγ) ligand binding potential of several major flame retardants, their metabolites, and chemical mixtures in house dust. Environ Health Perspect. 2015;123(2):166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jansen MS, Nagel SC, Miranda PJ, Lobenhofer EK, Afshari CA, McDonnell DP. Short-chain fatty acids enhance nuclear receptor activity through mitogen-activated protein kinase activation and histone deacetylase inhibition. Proc Natl Acad Sci USA. 2004;101(18):7199–7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen J, Ahn KC, Gee NA, et al. . Triclocarban enhances testosterone action: a new type of endocrine disruptor? Endocrinology. 2008;149(3):1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rajapakse N, Silva E, Kortenkamp A. Combining xenoestrogens at levels below individual no-observed-effect concentrations dramatically enhances steroid hormone action. Environ Health Perspect. 2002;110(9):917–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taxvig C, Elleby A, Sonne-Hansen K, et al. . Effects of nutrition relevant mixtures of phytoestrogens on steroidogenesis, aromatase, estrogen, and androgen activity. Nutr Cancer. 2010;62(1):122–131. [DOI] [PubMed] [Google Scholar]
- 10. Zoeller RT, Bansal R, Parris C. Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology. 2005;146(2):607–612. [DOI] [PubMed] [Google Scholar]
- 11. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. . Endocrine-disrupting chemicals: an Endocrine Society Scientific Statement. Endocr Rev. 2009;30(4):293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walsh LP, McCormick C, Martin C, Stocco DM. Roundup inhibits steroidogenesis by disrupting steroidogenic acute regulatory (StAR) protein expression. Environ Health Perspect. 2000;108(8):769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. The Endocrine Disruption Exchange. TEDX list of potential endocrine disruptors. 2015. Available from http://endocrinedisruption.org/endocrine-disruption/tedx-list-of-potential-endocrine-disruptors/overview Accessed July 29, 2016
- 14. Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect. 2003;111(8):994–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Myers JP, Zoeller RT, vom Saal FS. A clash of old and new scientific concepts in toxicity, with important implications for public health. Environ Health Perspect. 2009;117(11):1652–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. vom Saal FS, Akingbemi BT, Belcher SM, et al. . Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007;24(2):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vandenberg LN, Colborn T, Hayes TB, et al. . Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33(3):378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Waxman HA, Markey EJ, DeGette D. Chemicals used in hydraulic fracturing. The Committee on Energy Commerce U.S. House of Representatives. 2011. Available from http://www.conservation.ca.gov/dog/general_information/Documents/Hydraulic%20Fracturing%20Report%204%2018%2011.pdf Accessed July 29, 2016
- 19. Wiseman HJ. Untested waters: the rise of hydraulic fracturing in oil and gas production and the need to revisit regulation. Fordham Environ Law Rev. 2008;20:115–169. [Google Scholar]
- 20. Deutch J, Holditch S, Krupp F, et al. . The Secretary of the Energy Board Shale Gas Production Subcommittee Ninety-Day Report. 2011. Available from http://www.shalegas.energy.gov/resources/081811_90_day_report_final.pdf Accessed July 29, 2016
- 21. Lee DS, Herman JD, Elsworth D, Kim HT, Lee HS. A critical evaluation of unconventional gas recovery from the Marcellus Shale, Northeastern United States. KSCE J Civil Eng. 2011;15(4):679–687. [Google Scholar]
- 22. Maule AL, Makey CM, Benson EB, Burrows IJ, Scammell MK. Disclosure of hydraulic fracturing fluid chemical additives: analysis of regulations. New Solut. 2013;23(1):167–187. [DOI] [PubMed] [Google Scholar]
- 23. Warner NR, Jackson RB, Darrah TH, et al. . Geochemical evidence for possible natural migration of Marcellus Formation brine to shallow aquifers in Pennsylvania. Proc Natl Acad Sci USA. 2012;109(30):11961–11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fontenot BE, Hunt LR, Hildenbrand ZL, et al. . An evaluation of water quality in private drinking water wells near natural gas extraction sites in the Barnett Shale Formation. Environ Sci Technol. 2013;47(17):10032–10040. [DOI] [PubMed] [Google Scholar]
- 25. Harkness JS, Dwyer GS, Warner NR, Parker KM, Mitch WA, Vengosh A. Iodide, bromide, and ammonium in hydraulic fracturing and oil and gas wastewaters: environmental implications. Environ Sci Technol. 2015;49(3):1955–1963. [DOI] [PubMed] [Google Scholar]
- 26. Harvey TG, Matheson TW, Pratt KC. Chemical class separation of organics in shale oil by thin-layer chromatography. Anal Chem. 1984;56(8):1277–1281. [Google Scholar]
- 27. Darrah TH, Vengosh A, Jackson RB, Warner NR, Poreda RJ. Noble gases identify the mechanisms of fugitive gas contamination in drinking-water wells overlying the Marcellus and Barnett Shales. Proc Natl Acad Sci USA. 2014;111(39):14076–14081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Drollette BD, Hoelzer K, Warner NR, et al. . Elevated levels of diesel range organic compounds in groundwater near Marcellus gas operations are derived from surface activities. Proc Natl Acad Sci USA. 2015;112(43):13184–13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. United States Environmental Protection Agency. Assessment of the Potential Impacts of Hydraulic Fracturing for Oil and Gas on Drinking Water Resources. External Review Draft. Available from http://ofmpub.epa.gov/eims/eimscomm.getfile?p_download_id=523539 Accessed July 29, 2016
- 30. Riedl J, Rotter S, Faetsch S, Schmitt-Jansen M, Altenburger R. Proposal for applying a component-based mixture approach for ecotoxicological assessment of fracturing fluids. Environ Earth Sci. 2013;70:3907–3920. [Google Scholar]
- 31. Colborn T, Kwiatkowski C, Schultz K, Bachran M. Natural gas operations from a public health perspective. Int J Hum Ecol Risk Assess. 2011;17(5):1039–1056. [Google Scholar]
- 32. Kassotis CD, Iwanowicz LR, Akob DM, et al. . Endocrine disrupting activities of surface water associated with a West Virginia oil and gas industry wastewater disposal site. Sci Total Environ. 2016;557–558:901–910. [DOI] [PubMed] [Google Scholar]
- 33. Mauter MS, Alvarez PJ, Burton A, et al. . Regional variation in water-related impacts of shale gas development and implications for emerging international plays. Environ Sci Technol. 2014;45(15):8298–8306. [DOI] [PubMed] [Google Scholar]
- 34. Werner AK, Vink S, Watt K, Jagals P. Environmental health impacts of unconventional natural gas development: a review of the current strength of evidence. Sci Total Environ. 2015;505C:1127–1141. [DOI] [PubMed] [Google Scholar]
- 35. Kassotis CD, Tillitt DE, Lin CH, McElroy JA, Nagel SC. Endocrine-disrupting chemicals and oil and natural gas operations: potential environmental contamination and recommendations to assess complex environmental mixtures. Environ Health Perspect. 2016;124(3):256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bamberger M, Oswald RE. Impacts of gas drilling on human and animal health. New Solut. 2012;22(1):51–77. [DOI] [PubMed] [Google Scholar]
- 37. Rabinowitz PM, Slizovskiy IB, Lamers V, et al. . Proximity to natural gas wells and reported health status: results of a household survey in Washington County, PA. Environ Health Perspect. 2015;123(1):21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Slizovskiy IB, Conti LA, Trufan SJ, et al. . Reported health conditions in animals residing near natural gas wells in southwestern Pennsylvania. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2015;50(5):473–481. [DOI] [PubMed] [Google Scholar]
- 39. Jemielita T, Gerton GL, Neidell M, et al. . Unconventional gas and oil drilling is associated with increased hospital utilization rates. PLoS One. 2015;10(7):e0131093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bamberger M, Oswald RE. Long-term impacts of unconventional drilling operations on human and animal health. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2015;50(5):447–459. [DOI] [PubMed] [Google Scholar]
- 41. McKenzie LM, Guo R, Witter RZ, Savitz DA, Newman LS, Adgate JL. Birth outcomes and maternal residential proximity to natural gas development in rural Colorado. Environ Health Perspect. 2014;122(4):412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stacy SL, Brink LL, Larkin JC, et al. . Perinatal outcomes and unconventional natural gas operations in southwest pennsylvania. PLoS One. 2015;10(6):e0126425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Casey JA, Savitz DA, Rasmussen SG, et al. . Unconventional natural gas development and birth outcomes in Pennsylvania, USA. Epidemiology. 2016;27(2):163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Janerich DT, Dugan JM, Standfast SJ, Strite L. Congenital heart disease and prenatal exposure to exogenous sex hormones. Br Med J. 1977;1(6068):1058–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Snijder CA, Vlot IJ, Burdorf A, et al. . Congenital heart defects and parental occupational exposure to chemicals. Hum Reprod. 2012;27(5):1510–1517. [DOI] [PubMed] [Google Scholar]
- 46. Facemire CF, Gross TS, Guillette LJ Jr. Reproductive impairment in the Florida panther: nature or nurture? Environ Health Perspect. 1995;103(4):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rull RP, Ritz B, Shaw GM. Neural tube defects and maternal residential proximity to agricultural pesticide applications. Am J Epidemiol. 2006;163(8):743–753. [DOI] [PubMed] [Google Scholar]
- 48. Gross SA, Avens HJ, Banducci AM, Sahmel J, Panko JM, Tvermoes BE. Analysis of BTEX groundwater concentrations from surface spills associated with hydraulic fracturing operations. J Air Waste Manag Assoc. 2013;63(4):424–432. [DOI] [PubMed] [Google Scholar]
- 49. Ziemkiewicz PF, Quaranta JD, Darnell A, Wise R. Exposure pathways related to shale gas development and procedures for reducing environmental and public risk. J Nat Gas Sci Eng. 2014;16:77–84. [Google Scholar]
- 50. Akob DM, Cozzarelli IM, Dunlap DS, Rowan EL, Lorah MM. Organic and inorganic composition and microbiology of produced waters from Pennsylvania shale gas Wells. Appl Geochem. 2015;60:116–125. [Google Scholar]
- 51. Esswein EJ, Snawder J, King B, Breitenstein M, Alexander-Scott M, Kiefer M. Evaluation of some potential chemical exposure risks during flowback operations in unconventional oil and gas extraction: preliminary results. J Occup Environ Hyg. 2014;11(10):D174–D184. [DOI] [PubMed] [Google Scholar]
- 52. Llewellyn GT, Dorman F, Westland JL, et al. . Evaluating a groundwater supply contamination incident attributed to Marcellus Shale gas development. Proc Natl Acad Sci USA. 2015;112(20):6325–6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Latta SC, Marshall LC, Frantz MW, Toms JD. Evidence from two shale regions that a riparian songbird accumulates metals associated with hydraulic fracturing. Ecosphere. 2015;6(9):10. [Google Scholar]
- 54. Parker KM, Zeng T, Harkness J, Vengosh A, Mitch WA. Enhanced formation of disinfection byproducts in shale gas wastewater-impacted drinking water supplies. Environ Sci Technol. 2014;48(19):11161–11169. [DOI] [PubMed] [Google Scholar]
- 55. Thurman EM, Ferrer I, Blotevogel J, Borch T. Analysis of hydraulic fracturing flowback and produced waters using accurate mass: identification of ethoxylated surfactants. Anal Chem. 2014;86:9653–9661. [DOI] [PubMed] [Google Scholar]
- 56. Hladik ML, Focazio MJ, Engle M. Discharges of produced waters from oil and gas extraction via wastewater treatment plants are sources of disinfection by-products to receiving streams. Sci Total Environ. 2014;466–467:1085–1093. [DOI] [PubMed] [Google Scholar]
- 57. Kassotis CD, Klemp KC, Vu DC, et al. . Endocrine-disrupting activity of hydraulic fracturing chemicals and adverse health outcomes after prenatal exposure in male mice. Endocrinology. 2015;156(12):4458–4473. [DOI] [PubMed] [Google Scholar]
- 58. Clemens LG, Gladue BA, Coniglio LP. Prenatal endogenous androgenic influences on masculine sexual behavior and genital morphology in male and female rats. Horm Behav. 1978;10(1):40–53. [DOI] [PubMed] [Google Scholar]
- 59. Hotchkiss AK, Lambright CS, Ostby JS, Parks-Saldutti L, Vandenbergh JG, Gray LE Jr. Prenatal testosterone exposure permanently masculinizes anogenital distance, nipple development, and reproductive tract morphology in female Sprague-Dawley rats. Toxicol Sci. 2007;96(2):335–345. [DOI] [PubMed] [Google Scholar]
- 60. Wilson ME, Handa RJ. Direct actions of gonadal steroid hormones on FSH secretion and expression in the infantile female rat. J Steroid Biochem Mol Biol. 1998;66(1–2):71–78. [DOI] [PubMed] [Google Scholar]
- 61. Place NJ, Coscia EM, Dahl NJ, et al. . The anti-androgen combination, flutamide plus finasteride, paradoxically suppressed LH and androgen concentrations in pregnant spotted hyenas, but not in males. Gen Comp Endocrinol. 2011;170(3):455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Murray AA, Gosden RG, Allison V, Spears N. Effect of androgens on the development of mouse follicles growing in vitro. J Reprod Fertil. 1998;113(1):27–33. [DOI] [PubMed] [Google Scholar]
- 63. Knapczyk-Stwora K, Durlej-Grzesiak M, Ciereszko RE, Koziorowski M, Slomczynska M. Antiandrogen flutamide affects folliculogenesis during fetal development in pigs. Reproduction. 2013;145(3):265–276. [DOI] [PubMed] [Google Scholar]
- 64. Manikkam M, Steckler TL, Welch KB, Inskeep EK, Padmanabhan V. Fetal programming: prenatal testosterone treatment leads to follicular persistence/luteal defects; partial restoration of ovarian function by cyclic progesterone treatment. Endocrinology. 2006;147(4):1997–2007. [DOI] [PubMed] [Google Scholar]
- 65. Robinson JE, Forsdike RA, Taylor JA. In utero exposure of female lambs to testosterone reduces the sensitivity of the gonadotropin-releasing hormone neuronal network to inhibition by progesterone. Endocrinology. 1999;140(12):5797–5805. [DOI] [PubMed] [Google Scholar]
- 66. Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology. 2005;146(7):3185–3193. [DOI] [PubMed] [Google Scholar]
- 67. Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol. 2001;15(1):172–183. [DOI] [PubMed] [Google Scholar]
- 68. Danilovich N, Babu PS, Xing W, Gerdes M, Krishnamurthy H, Sairam MR. Estrogen deficiency, obesity, and skeletal abnormalities in follicle-stimulating hormone receptor knockout (FORKO) female mice. Endocrinology. 2000;141(11):4295–4308. [DOI] [PubMed] [Google Scholar]
- 69. Walters KA, McTavish KJ, Seneviratne MG, et al. . Subfertile female androgen receptor knockout mice exhibit defects in neuroendocrine signaling, intraovarian function, and uterine development but not uterine function. Endocrinology. 2009;150(7):3274–3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vallet JL, Christenson RK. Effect of progesterone, mifepristone, and estrogen treatment during early pregnancy on conceptus development and uterine capacity in Swine. Biol Reprod. 2004;70(1):92–98. [DOI] [PubMed] [Google Scholar]
- 71. Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90(23):11162–11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kirkland JL, Gardner RM, Mukku VR, Akhtar M, Stancel GM. Hormonal control of uterine growth: the effect of hypothyroidism on estrogen-stimulated cell division. Endocrinology. 1981;108(6):2346–2351. [DOI] [PubMed] [Google Scholar]
- 73. Niermann S, Rattan S, Brehm E, Flaws JA. Prenatal exposure to di-(2-ethylhexyl) phthalate (DEHP) affects reproductive outcomes in female mice. Reprod Toxicol. 2015;53:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Armenti AE, Zama AM, Passantino L, Uzumcu M. Developmental methoxychlor exposure affects multiple reproductive parameters and ovarian folliculogenesis and gene expression in adult rats. Toxicol Appl Pharmacol. 2008;233(2):286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang W, Hafner KS, Flaws JA. In utero bisphenol A exposure disrupts germ cell nest breakdown and reduces fertility with age in the mouse. Toxicol Appl Pharmacol. 2014;276(2):157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Grun F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147(6 suppl):S50–S55. [DOI] [PubMed] [Google Scholar]
- 77. Heindel JJ, vom Saal FS, Blumberg B, et al. . Parma consensus statement on metabolic disruptors. Environ Health. 2015;14:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Incardona JP, Gardner LD, Linbo TL, et al. . Deepwater Horizon crude oil impacts the developing hearts of large predatory pelagic fish. Proc Natl Acad Sci USA. 2014;111(15):E1510–E1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Belcher SM, Gear RB, Kendig EL. Bisphenol A alters autonomic tone and extracellular matrix structure and induces sex-specific effects on cardiovascular function in male and female CD-1 mice. Endocrinology. 2015;156(3):882–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Elliott EG, Ettinger AS, Leaderer BP, Bracken MB, Deziel NC. A systematic evaluation of chemicals in hydraulic-fracturing fluids and wastewater for reproductive and developmental toxicity [published online ahead of print January 6, 2016]. J Expo Sci Environ Epidemiol. doi: 10.1038/jes.2015.81. [DOI] [PubMed] [Google Scholar]
- 81. Nonneman DJ, Ganjam VK, Welshons WV, Vom Saal FS. Intrauterine position effects on steroid metabolism and steroid receptors of reproductive organs in male mice. Biol Reprod. 1992;47(5):723–729. [DOI] [PubMed] [Google Scholar]
- 82. Vom Saal FS. Variation in phenotype due to random intrauterine positioning of male and female fetuses in rodents. J Reprod Fertil. 1981;62:18. [DOI] [PubMed] [Google Scholar]
- 83. Suvorov A, Vandenberg LN. To cull or not to cull? Considerations for studies of endocrine disrupting chemicals. Endocrinology. 2016;157:2586–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Byers SL, Wiles MV, Dunn SL, Taft RA. Mouse estrous cycle identification tool and images. PLoS One. 2012;7(4):e35538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009;Appendix 4:Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004;127(5):569–580. [DOI] [PubMed] [Google Scholar]
- 87. Yin W, Maguire SM, Pham B, et al. . Testing the critical window hypothesis of timing and duration of estradiol treatment on hypothalamic gene networks in reproductively mature and aging female rats. Endocrinology. 2015;156(8):2918–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80(4):1523–1631. [DOI] [PubMed] [Google Scholar]
- 89. Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15(2):201–204. [DOI] [PubMed] [Google Scholar]
- 90. Westergaard LG, Laursen SB, Andersen CY. Increased risk of early pregnancy loss by profound suppression of luteinizing hormone during ovarian stimulation in normogonadotrophic women undergoing assisted reproduction. Hum Reprod. 2000;15(5):1003–1008. [DOI] [PubMed] [Google Scholar]
- 91. Amato G, Carella C, Fazio S, et al. . Body composition, bone metabolism, and heart structure and function in growth hormone (GH)-deficient adults before and after GH replacement therapy at low doses. J Clin Endocrinol Metab. 1993;77(6):1671–1676. [DOI] [PubMed] [Google Scholar]
- 92. Szkudlinski MW, Fremont V, Ronin C, Weintraub BD. Thyroid-stimulating hormone and thyroid-stimulating hormone receptor structure-function relationships. Physiol Rev. 2002;82(2):473–502. [DOI] [PubMed] [Google Scholar]
- 93. Abbott DH, Padmanabhan V, Dumesic DA. Contributions of androgen and estrogen to fetal programming of ovarian dysfunction. Reprod Biol Endocrinol. 2006;4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Masuno H, Okamoto S, Iwanami J, et al. . Effect of 4-nonylphenol on cell proliferation and adipocyte formation in cultures of fully differentiated 3T3-L1 cells. Toxicol Sci. 2003;75(2):314–320. [DOI] [PubMed] [Google Scholar]
- 95. Hao CJ, Cheng XJ, Xia HF, Ma X. The endocrine disruptor 4-nonylphenol promotes adipocyte differentiation and induces obesity in mice. Cell Physiol Biochem. 2012;30(2):382–394. [DOI] [PubMed] [Google Scholar]
- 96. Kim HW, Kam S, Lee DH. Synergistic interaction between polycyclic aromatic hydrocarbons and environmental tobacco smoke on the risk of obesity in children and adolescents: the U.S. National Health and Nutrition Examination Survey 2003–2008. Environ Res. 2014;135:354–360. [DOI] [PubMed] [Google Scholar]
- 97. Cornu MC, Lhuguenot JC, Brady AM, Moore R, Elcombe CR. Identification of the proximate peroxisome proliferator(s) derived from di (2-ethylhexyl) adipate and species differences in response. Biochem Pharmacol. 1992;43(10):2129–2134. [DOI] [PubMed] [Google Scholar]
- 98. Judson RS, Martin MT, Reif DM, et al. . Analysis of eight oil spill dispersants using rapid, in vitro tests for endocrine and other biological activity. Environ Sci Technol. 2010;44(15):5979–5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Temkin AM, Bowers RR, Magaletta ME, et al. . Effects of Crude Oil/Dispersant Mixture and Dispersant Components on PPARγ activity and identification of dioctyl sodium sulfosuccinate (DOSS; CAS #577–11-7) as a probable obesogen. Environ Health Perspect. 2016;124:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Baxter JD, Webb P. Thyroid hormone mimetics: potential applications in atherosclerosis, obesity and type 2 diabetes. Nat Rev Drug Discov. 2009;8(4):308–320. [DOI] [PubMed] [Google Scholar]
- 101. Bryzgalova G, Effendic S, Khan A, et al. . Anti-obesity, anti-diabetic, and lipid lowering effects of the thyroid receptor β subtype selective agonist KB-141. J Steroid Biochem Mol Biol. 2008;111(3–5):262–267. [DOI] [PubMed] [Google Scholar]
- 102. Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril. 2006;85(5):1319–1340. [DOI] [PubMed] [Google Scholar]
- 103. Fulton RM, Hutchinson EC, Jones AM. Ventricular weight in cardiac hypertrophy. Br Heart J. 1952;14(3):413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hangartner JR, Marley NJ, Whitehead A, Thomas AC, Davies MJ. The assessment of cardiac hypertrophy at autopsy. Histopathology. 1985;9(12):1295–1306. [DOI] [PubMed] [Google Scholar]
- 105. de Vries WB, van der Leij FR, Bakker JM, et al. . Alterations in adult rat heart after neonatal dexamethasone therapy. Pediatr Res. 2002;52(6):900–906. [DOI] [PubMed] [Google Scholar]
- 106. Keen AN, Fenna AJ, McConnell JC, Sherratt MJ, Gardner P, Shiels HA. The dynamic nature of hypertrophic and fibrotic remodeling of the fish ventricle. Front Physiol. 2015;6:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128(1):191–227. [DOI] [PubMed] [Google Scholar]
- 108. Dodic M, Samuel C, Moritz K, et al. . Impaired cardiac functional reserve and left ventricular hypertrophy in adult sheep after prenatal dexamethasone exposure. Circ Res. 2001;89(7):623–629. [DOI] [PubMed] [Google Scholar]
- 109. Thum T, Borlak J. Testosterone, cytochrome P450, and cardiac hypertrophy. FASEB J. 2002;16(12):1537–1549. [DOI] [PubMed] [Google Scholar]
- 110. Valenzuela-Alcaraz B, Crispi F, Bijnens B, et al. . Assisted reproductive technologies are associated with cardiovascular remodeling in utero that persists postnatally. Circulation. 2013;128(13):1442–1450. [DOI] [PubMed] [Google Scholar]
- 111. Olsen NJ, Watson MB, Henderson GS, Kovacs WJ. Androgen deprivation induces phenotypic and functional changes in the thymus of adult male mice. Endocrinology. 1991;129(5):2471–2476. [DOI] [PubMed] [Google Scholar]
- 112. Viselli SM, Stanziale S, Shults K, Kovacs WJ, Olsen NJ. Castration alters peripheral immune function in normal male mice. Immunology. 1995;84(2):337–342. [PMC free article] [PubMed] [Google Scholar]