Abstract
Context: Male hormonal contraceptive methods require effective suppression of sperm output.
Objective: The objective of the study was to define the covariables that influence the rate and extent of suppression of spermatogenesis to a level shown in previous World Health Organization-sponsored studies to be sufficient for contraceptive purposes (≤1 million/ml).
Design: This was an integrated analysis of all published male hormonal contraceptive studies of at least 3 months' treatment duration.
Setting: Deidentified individual subject data were provided by investigators of 30 studies published between 1990 and 2006.
Participants: A total of 1756 healthy men (by physical, blood, and semen exam) aged 18–51 yr of predominantly Caucasian (two thirds) or Asian (one third) descent were studied. This represents about 85% of all the published data.
Intervention(s): Men were treated with different preparations of testosterone, with or without various progestins.
Main Outcome Measure: Semen analysis was the main measure.
Results: Progestin coadministration increased both the rate and extent of suppression. Caucasian men suppressed sperm output faster initially but ultimately to a less complete extent than did non-Caucasians. Younger age and lower initial blood testosterone or sperm concentration were also associated with faster suppression, but the independent effect sizes for age and baseline testicular function were relatively small.
Conclusion: Male hormonal contraceptives can be practically applied to a wide range of men but require coadministration of an androgen with a second agent (i.e. progestin) for earlier and more complete suppression of sperm output. Whereas considerable progress has been made toward defining clinically effective combinations, further optimization of androgen-progestin treatment regimens is still required.
Hormonal methods that exploit negative feedback suppression of pituitary gonadotropin secretion, analogous to ovulation inhibition by combined estrogen-progestin contraceptives, are effective and reversible (1, 2, 3, 4, 5, 6, 7). Androgen or androgen-progestin treatment combinations can inhibit spermatogenesis to azoospermia (no sperm in ejaculate) or near-azoospermia (≤1 million/ml semen), with some regimens being more effective than others (8, 9, 10, 11, 12, 13, 14, 15, 16). This degree of suppression of sperm output provides effective contraception with efficacy rates of 97–100% (1, 2, 3, 4). Recovery of sperm output to levels consistent with normal male fertility can also be expected in all men (5), and short-term safety parameters have been identified (17).
Understanding the factors responsible for suppression of sperm output to levels that provide reliable contraception is essential for designing optimal regimens for male hormonal contraceptives. In particular, understanding which factors predict rapid suppression of sperm output is especially important for a practical method that couples may wish to switch to without delay. Conversely, identifying which men are likely to suppress sperm output more slowly is equally important to allow antecedent counseling.
Physiological studies in humans and other primates suggest biphasic effects are plausible because gonadotropin withdrawal impairs different stages of spermatogenesis both early (spermatogonial proliferation) and late (spermiation) in the spermatogenic cycle (18). Different early, compared with late, rates of suppression of sperm output could thereby result. Previous work has implicated Asian (compared with Caucasian) ethnicity (19), lower baseline FSH concentration and greater gonadotropin suppression (4, 20), progestin coadministration (21, 22), and lower testosterone dose (22, 23) in more complete eventual suppression of sperm output. However, available studies were limited by relatively small sample sizes, which precluded adequately powered multivariate analyses. An integrated analysis, combining data from all available sources, would allow assessment of the relative and independent importance of putative predictors.
We therefore conducted an integrated analysis of individual participant data to examine factors that may influence the extent and the rate of spermatogenic suppression, to define predictors. Such an analysis was feasible because of the inherent consistency of study design and the standardized methods used to assess the primary endpoint (semen analysis) arising from a long history of collaboration and information sharing among investigators active in the field, with regular meetings sponsored for three decades by the World Health Organization (WHO) and more recently by international summit meetings. These groups have, in addition, developed comprehensive international advisory recommendations related to the development of hormonal regimens of male fertility regulation (6, 24, 25). We also recently analyzed the rate of recovery of spermatogenesis after hormonal methods are ceased (see Subjects and Methods) (5). In the current analysis, we examined both the rate and extent of spermatogenic suppression stratifying on a time-dependent variable to distinguish early from later influences using data largely derived previously (5).
Subjects and Methods
Data collection, subjects, and interventions
Studies in which men were administered an androgen or androgen-progestin regimen for at least 3 months were initially identified by PubMed search using key terms “androgen” or “testosterone” with “contraception.” An exposure period of at least 3 months was chosen because the spermatogenic cycle requires about 70 d to complete (26, 27). Additional studies were located by scrutinizing reference lists and surveying members of the international hormonal male contraception summit group, which includes representatives of virtually all active clinical research groups studying male hormonal methods. All identified studies recruited healthy, eugonadal men on the basis of unremarkable reproductive history; physical examination (including testicular examination); blood hormonal, biochemical, and hematological parameters; and semen analysis (requiring two semen analysis with sperm concentration of at least 20 million/ml). Semen was consistently assessed by contemporaneous WHO-recommended methods (28) before and at least monthly (every 2–4 wk) during treatment in all studies. Baseline sperm concentration was defined as the mean sperm concentration from at least two individual semen analyses.
Thirty-four studies were identified at the time of data collection in 2005 (1, 2, 3, 4, 23, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57); however, four studies were excluded from the analysis because the necessary data were not available from study investigators (54, 55, 56, 57). Since the time of data collection, two additional studies have been published (58, 59) (Tables 1 and 2). Data from these two recent studies were not included in the current analysis. Deidentified individual subject data were supplied by investigators of 20 single- and 10 multicenter studies spanning five continents (North and South America, Europe, Asia, and Australia) through a standardized worksheet that was approved by the Harbor-UCLA Medical Center Institutional Review Board (Table 1). Hence, about 85% of all individual subject data from all 36 published studies identified in 2007 were available for analysis, and the 30 included studies were similar to the six studies not included (Table 2). In all clinical studies, prior written informed consent was obtained from each subject after approval from the relevant human subjects research ethics committee. Men were aged 18–51 yr, were treated for 16–78 wk with im testosterone (T), T undecanoate (TU), T decanoate (TD), T enanthate (TE); oral T capsule (TU); transdermal T patch; biodegradable T pellets; or 7-α methyl-19-nor-testosterone (MENT) acetate implants with or without oral cyproterone acetate (CPA), oral or implants of levonorgestrel (LNG), oral or injections of norethisterone (NET), oral desogestrel (DSG), implants of etonogestrel (ENG), or injections of depot medroxyprogesterone acetate (DMPA). As shown, studies were either efficacy studies (in which pregnancies/contraceptive failures was the end point) or suppression studies (in which the end point was sperm output). For efficacy studies, treatment ceased if suppression of sperm concentration to azoospermia, 1 million/ml or less or 3 million/ml or less did not occur by 6 months (1, 2, 3, 4), and data were then censored statistically. For suppression studies, some of these were exploratory to determine best dose, delivery systems, or androgen-progestin combinations and hence were not optimized to ensure uniform suppression of spermatogenesis.
TABLE 1.
Included studies
| Study (Ref.) | n | Study centers | Androgen | Progestin | No. of groups | Random allocation | Treatment duration (wk) | Age (yr) | Semen sampling interval (wk) |
|---|---|---|---|---|---|---|---|---|---|
| WHO (2 ) | 399 | 15 | TE 200 mg/wk | None | 1 | N/A | 52–781,b | 21–45 | 4 |
| Gu et al. (3 ) | 308 | 6 | TU 500 mg per 4 wk (1000 mg loading dose) | None | 1 | N/A | 26–521,d | 20–45 | 4 |
| WHO (1 ) | 271 | 10 | TE 200 mg/wk | None | 1 | N/A | 52–781,d | 21–45 | 4 |
| Hay et al. (29 ) | 112 | 6 | TD 400 mg per 4–6 wk | Oral ENG 300 μg/d | 2 | Yes | 48 | 18–45 | 4 |
| Wang et al. (30 ) | 80 | 2 | T pellet 800 mg per 15–18 wk | LNG 0–4 rods2 | 2 | Yes | 30–36 | 24–50 | 3 |
| Wang et al. (31 ) | 72 | 3 | MENT 2–3 rods3 | LNG 0–4 rods2 | 4 | Yes | 52 | 20–45 | 4 |
| Gonzalo et al. (32 ) | 68 | 1 | T patch (10 mg/d) or TE4 | LNG 4 rods or oral LNG 0–125 μg/d2 | 4 | Yes | 24 | 18–50 | 3 |
| Kinniburgh et al. (33 ) | 66 | 2 | T pellet5 | DSG6 | 2 | Yes | 24 | 22–41 | 4 |
| Turner et al. (4 ) | 55 | 2 | T pellet 800 mg per 16–24 wk | DMPA 300 mg/12 wk | 2 | No | 52–781,c | 18–50 | 4 |
| Amory et al. (34 ) | 51 | 1 | TE 25–300 mg/wk | None | 5 | Yes | 24 | 18–50 | 2 |
| Meriggiola et al. (35 ) | 50 | 1 | TU 1000 mg per 6–12 wk | NET 0–200 mg/6–12 wk | 5 | Yes | 48 | 18–50 | 2 |
| Kamischke et al. (36 ) | 42 | 1 | TU 1000 mg per 6 wk | NET 200–400 mg/6 wk or oral NET 10 mg/d | 3 | Yes | 24 | 18–45 | 4 |
| Anawalt et al. (37 ) | 41 | 1 | TE4 | Oral LNG 31.25–62.5 μg/d | 2 | Yes | 24 | 18–51 | 2 |
| Qoubaitary et al. (38 ) | 40 | 2 | TU 750-1000 mg per 8 wk | NET 0–200 mg/8 wk | 4 | Yes | 24 | 18–50 | 4 |
| Bebb et al. (39 ) | 36 | 1 | TE4 | Oral LNG 0–500 μg/d | 2 | Yes | 24 | 20–42 | 2 |
| Anawalt et al. (40 ) | 36 | 1 | TE4 | Oral LNG 125–250 μg/d | 2 | Yes | 24 | 20–46 | 2 |
| Von Eckardstein et al. (41 ) | 35 | 3 | MENT 1–4 rods7 | None | 3 | Yes | 26–52 | 20–45 | 4 |
| Gu et al. (42 ) | 30 | 1 | TU 1000 mg per 8 wk | DMPA 0–300 mg/ 8 wk | 3 | Yes | 24 | 20–45 | 4 |
| Kamischke et al. (43 ) | 28 | 1 | TU 1000 mg per 6 wk | Oral LNG 0–250 μg/d | 2 | Yes | 24 | 18–45 | 4 |
| Anderson et al. (44 ) | 28 | 1 | T pellet5 | ENG 1–2 rods8 | 2 | Yes | 24 | 21–39 | 4 |
| Wu et al. (45 ) | 24 | 1 | TE 50–100 mg/wk | DSG6 | 3 | Yes | 24 | 18–50 | 4 |
| Meriggiola et al. (46 ) | 24 | 1 | TU 1000 mg per 6–8 wk | Oral CPA 0–20 mg/d | 3 | Yes | 44 | 18–45 | 4 |
| Anawalt et al. (47 ) | 24 | 1 | TE 50–100 mg/wk | DSG6 | 3 | Yes | 24 | 20–49 | 2 |
| Hair et al. (48 ) | 23 | 1 | T patch 5 mg/d | DSG 75–300 μg/d | 3 | Yes | 24 | 20–43 | 4 |
| Meriggiola et al. (23 ) | 18 | 1 | TE 100–200 mg/wk | Oral CPA 5 mg/d | 2 | Yes | 16 | 21–45 | 2 |
| Brady et al. (49 ) | 15 | 1 | T pellet5 | ENG 3 rods8 | 1 | N/A | 24–48 | 18–37 | 4 |
| Meriggiola et al. (50 ) | 15 | 1 | TE4 | Oral CPA 0–100 mg/d | 3 | Yes | 16 | 22–44 | 2 |
| Kamischke et al. (51 ) | 14 | 1 | TU 1000 mg per 6 wk | NET 200 mg/6 wk | 1 | N/A | 24 | 18–45 | 4 |
| Meriggiola et al. (52 ) | 10 | 1 | TE4 | Oral CPA 12.5–25 mg/d | 2 | Yes | 16 | 19–42 | 2 |
| Meriggiola et al. (53 ) | 8 | 1 | T capsule 160 mg/d | Oral CPA 25 mg/d | 1 | N/A | 16 | 25–42 | 2 |
Efficacy studies (end point being contraceptive failures) or suppression studies (end point being suppression of spermatogenesis). For these efficacya studies, treatment was ceased after 6 months if sufficient suppression of sperm concentration to azoospermia,b less than 1 million/mlc or less than 3 million/ml,d had not occurred. For all studies, volunteers (n = 2023) were enrolled only after a normal physical examination, normal blood electrolytes, hematology and hormones, and normal semen analysis (i.e. sperm concentration > 20 million/ml). N/A, Not applicable.
Each LNG rod contains 75 mg and releases 36–49 μg/d.
Each MENT rod releases 500 μg/d.
TE 100 mg/wk.
T pellet 400 mg per12 wk.
Oral DSG 150–300 μg/d.
Each MENT rod releases 400 μg/d.
Each ENG rod contains 68 mg and releases about 50 μg/d.
TABLE 2.
Excluded studies
| Study (Ref.) | n | Study centers | Androgen | Progestin | No. of groups | Random allocation | Treatment duration (wk) | Age (yr) | Semen sampling interval (wk) |
|---|---|---|---|---|---|---|---|---|---|
| Brady et al. (59 ) | 130 | 6 | TD 400–600 mg per 4–6 wk | ENG 2 rods (each rod contains 102 mg) | 3 | Yes | 48 | 18–45 | 4–61 |
| WHO (55 ) | 96 | 5 | TE (200 mg per 1–3 wk) or 19 nor T (200 mg per 1–3wk) | DMPA 250 mg per 6 wk | 2 | Yes | 24 | 21–45 | 3 |
| Gui et al. (56 ) | 62 | 1 | TU 500-1000 mg per 8 wk | LNG 0–4 rods2 | 3 | Yes | 24 | 22–35 | 2 |
| Page et al. (58 ) | 44 | 1 | T gel 100 mg/d | DMPA 300 mg per 12 wk | 2 | Yes | 24 | 18–55 | 2 |
| Pollanen et al. (57 ) | 43 | 1 | DHT gel 250 mg/d | LNG 0–4 rods or oral LNG 30 μg/d2 | 5 | Yes | 26 | 21–45 | 4 |
| Anderson et al. (54 ) | 31 | 2 | T pellet 200–400 mg per 12 wk | DSG3 | 2 | Yes | 24 | 19–39 | 4 |
All studies are suppression studies (end point being suppression of spermatogenesis).
Semen sampled every 4 wk for first 24 wk and then 4 or 6 wk for 24–48 wk, depending on group allocation. For all studies, volunteers (n = 406) were enrolled only after a normal physical examination, normal blood electrolytes, hematology and hormones, and normal semen analysis (i.e. sperm concentration > 20 million/ml).
Each LNG rod contains 75 mg and releases 36–49 μg/d.
Oral DSG 150–300 μg/d.
Information was collected for the present analysis from 1756 men (from a potential group of 2023 recruited into all 30 studies) who were exposed to a hormonal regimen for fertility control for at least 3 months. Not all data were obtained from all recruited men. As previously mentioned, this report includes data from a subgroup of men in which recovery of spermatogenesis after cessation of hormonal exposure was additionally documented (5). The primary end point (focal point) for the present study was time to suppress sperm output to a concentration of 1 million/ml or less. This degree of suppression is sufficient for reliable contraception (6). Subjects were censored when they dropped out of the study. These times were extracted by one investigator (P.Y.L.) from complete databases containing all individual semen data supplied by investigators from Sydney, Australia; Münster, Germany; Los Angeles, CA; Seattle, WA; and WHO. These data comprise about half of all sperm threshold data. Extracted data were provided by the investigators for the remaining studies.
Baseline subject and treatment data that might influence suppression times a priori were also collected (Tables 3 and 4). All covariate data collected and used for this analysis are listed in these tables.
TABLE 3.
Participant characteristics at baseline
| Variables | Parameter (mean ± sem) (n = 1756) |
|---|---|
| Continuous | |
| Baseline subject parameters | |
| Age (yr) | 31.69 ± 0.14 |
| Height (cm) | 175.31 ± 0.20 |
| Weight (kg) | 74.50 ± 0.33 |
| BMI (kg/m2) | 24.12 ± 0.08 |
| Testis volume (ml) | 22.03 ± 0.13 |
| FSH (IU/liter) | 3.70 ± 0.06 |
| LH (IU/liter) | 4.04 ± 0.06 |
| Te (nm) | 18.87 ± 0.16 |
| Baseline sperm parameters | |
| Concentration (million/ml) | 77.35 ± 1.16 |
| Total motility (%) | 65.27 ± 0.37 |
| Normal morphology (%) | 49.21 ± 0.70 |
| Volume (ml) | 3.22 ± 0.03 |
| Treatment parameters | |
| Effective T dose (mg/d) | 14.37 ± 0.14 |
| Categorical | n (% of total) (n = 1756) |
| Androgen used | |
| MENT | 71 (4%) |
| T capsule/patch | 60 (3%) |
| Intramuscular TE | 841 (48%) |
| Intramuscular TD | 105 (6%) |
| Intramuscular TU | 494 (28%) |
| T pellet | 186 (11%) |
| Androgen dose | |
| Low | 1612 (92%) |
| High | 144 (8%) |
| Race | |
| Asian | 581 (33%) |
| Non-Asian1 | 1175 (67%) |
| Caucasian | 1108 (63%) |
| Hispanic | 31 (2%) |
| African | 25 (1%) |
| Other | 11 (1%) |
| Progestin use | |
| Not used | 1067 (61%) |
| Used | 689 (39%) |
Blood T concentration can be converted from SI (nm) to conventional units (nanograms per deciliters) by dividing by 0.0347.
Comprises Caucasian, Hispanic, African, and other men.
TABLE 4.
Univariate analysis of predictors of suppression of spermatogenesis to 1 million/ml
| Variables | Univariate analysis hazard ratio (95% CI) | P |
|---|---|---|
| Continuous | ||
| Baseline subject parameters | ||
| Age (yr) | 0.990 (0.981–0.998) | 0.0156 |
| Height (cm) | 1.009 (1.003–1.015) | 0.0046 |
| Weight (kg) | 1.002 (0.998–1.006) | 0.3277 |
| BMI (kg/m2) | 0.996 (0.981–1.011) | 0.6040 |
| Testis volume (ml) | 0.997 (0.988–1.006) | 0.4519 |
| FSH (IU/liter) | 1.016 (0.993–1.039) | 0.1750 |
| LH (IU/liter) | 0.973 (0.952–0.994) | 0.0140 |
| T (nm) | 0.990 (0.982–0.998) | 0.0159 |
| Baseline sperm parameters | ||
| Concentration (million/ml) | 0.997 (0.996–0.998) | <0.0001 |
| Total motility (%) | 0.997 (0.994–1.001) | 0.0965 |
| Normal morphology (%) | 0.994 (0.992–0.996) | <0.0001 |
| Volume (ml) | 1.016 (0.981–1.052) | 0.3730 |
| Treatment parameters | ||
| Effective T dose (mg/d) | 0.972 (0.964–0.981) | <0.0001 |
| Categorical | ||
| Androgen used | ||
| MENT | Referent | |
| T capsule/patch | 0.547 (0.358–0.838) | 0.0055 |
| Intramuscular TE | 1.097 (0.847–1.421) | 0.48 |
| Intramuscular TD | 1.722 (1.259–2.356) | 0.0007 |
| Intramuscular TU | 1.201 (0.923–1.563) | 0.17 |
| T pellet | 1.608 (1.206–2.145) | 0.0012 |
| Androgen dose | ||
| Low | Referent | |
| High | 1.580 (1.325–1.884) | 0.0004 |
| Race | ||
| Asian | Referent | |
| Non-Asian | 1.285 (1.155–1.430) | <0.0001 |
| Progestin use | ||
| Not used | Referent | |
| Used | 1.624 (1.465–1.800) | <0.0001 |
Hazard ratio greater than 1 indicates higher suppression rate, less than 1 lower suppression rate. Blood T concentration can be converted from SI (nanomoles) to conventional units (nanograms per deciliter) by dividing by 0.0347. CI, Confidence interval.
Calculations
We calculated the effective daily T dose according to known pharmacokinetics of testosterone preparations (60, 61, 62, 63). Calculation of daily effective dose is based on the following assumptions and mean values recognizing that there is considerable individual variation in pharmacokinetics. This was 5% of the administered dose for oral TU, 5 mg per patch for transdermal T and 1.3 mg per 200 mg T pellet. For im testosterone esters, the exact amount of T injected was calculated (using the known molecular weight of testosterone discounting the ester side chain) from which the total T dose administered over the entire nominal treatment period based on the injection frequency could be determined. From this the effective daily T dose was calculated.
Analysis
We examined the rate of suppression by means of an integrated reanalysis of individual subject data (64), not a metaanalysis of pooled summary study data. Hence, the unit of analysis was the individual subject, not the 30 identified studies. Univariate analyses of continuous (Cox) and categorical (Kaplan-Meier) variables were initially performed and analogously to a recently applied analytical strategy (5). A parsimonious model was identified by stepwise selection and confirmed by best subset selection of all possible covariate combinations. The best subset selection method chooses a sequence of models with increasing number of predictors, each of which is most predictive among competitors with the same number of predictors. We examined suppression of sperm concentration from baseline to 1 million/ml or less. Finally, we examined whether early or late effects on sperm suppression might differ by creating a time-dependent covariate to dichotomize and on which to stratify the extended Cox analysis (64).
As a separate analytical strategy, we also examined the extent of suppression of sperm output by dichotomizing men into those who did or did not ever suppress to a sperm concentration of 1 million/ml and assessed how they differed in baseline parameters (by two-sample Student t test or Fisher test for continuous and categorical variables, respectively). Significant variables were then analyzed simultaneously by stepwise multivariate logistic regression to identify independent predictive covariables.
All tests were two sided, with P < 0.05 considered significant. Data are shown as median (range) or mean ± sem. These analyses were executed with SAS proc phreg, lifetest, and logistic (version 9.1; SAS Institute, Inc., Cary, NC). Proportional hazards assumptions were confirmed by graphical examination of Schoenberg residuals and supremum and related tests (64).
Results
Rate of suppression
Tables 3 and 4 show baseline characteristics of the 1756 men identified from 30 studies and the univariate (unadjusted) utility of these characteristics as predictors of spermatogenic suppression. For categorical variables, these inferences are similar to those obtained with Kaplan-Meier log-rank tests: androgen, ethnicity, and progestin use (each P < 0.001).
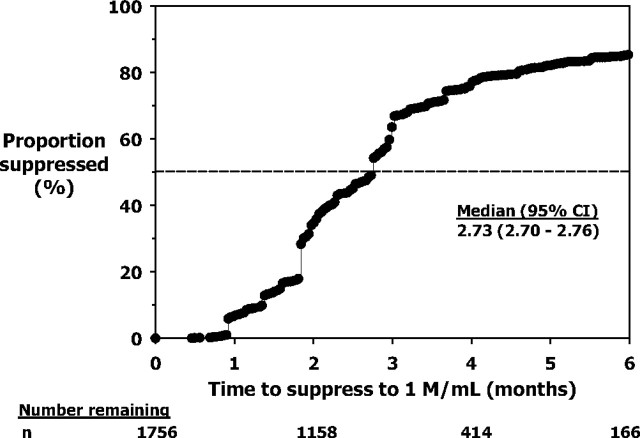
A Kaplan-Meier (unadjusted) curve showing the proportion of men who suppressed their sperm concentrations to 1 million/ml or less was constructed (Fig. 1). This indicated that sperm concentration first falls to 1 million/ml or less after a median time of 2.73 (2.70–2.76 95% confidence interval) months.
Fig. 1.
Kaplan-Meier observed suppression plots showing the proportion of men who suppressed their sperm concentration to 1 million/ml. The number of men (n) remaining at 0, 2, 4, and 6 months are shown below. CI, Confidence interval.
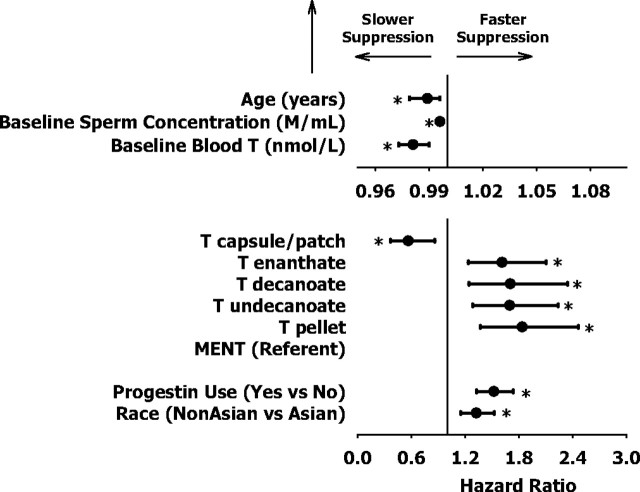
Figure 2 shows the multivariately adjusted hazard ratios and 95% confidence intervals of the final multivariate models selected after stepwise Cox regression from the potential set of all univariate variables (shown in Tables 3 and 4). Hazard ratios exceeding 1.0 indicate faster, whereas those less than 1.0 signify slower, rates of suppression. Considerable overlap of 95% confidence intervals for hazards ratios indicate the comparability among the different T preparations (P < 0.009 vs. MENT for each), except for oral and transdermal T which, as a group, is associated with slower suppression. Faster rates of suppression were observed in Caucasian men or with concurrent progestin administration. Slower suppression occurred with older age, higher baseline blood testosterone, or higher baseline sperm concentration (each significant P < 0.001); however, these adjusted effect sizes were relatively small (hazard ratio > 0.98, for each) in comparison with the expected range of each of these parameters.
Fig. 2.
Final multivariate variables selected after stepwise Cox regression. Hazard ratios and 95% confidence intervals, with multivariate adjustment to exclude effects of other variables, are shown. Hazard ratios exceeding 1 indicate faster, whereas those less than 1 signify slower, rates of suppression for each unit increase in the corresponding variable. *, Hazard ratios that significantly differ from unity (vertical line). Note magnified hazard ratio scale for age, baseline sperm concentration, and baseline T.
Next, we repeated the same analysis but allowed for the rate of suppression of sperm output to differ early or late in time. Early epoch of time was defined as earlier than 3 months, based on the Kaplan-Meier analysis, which showed that the median time to suppression was 2–3 months, and on the 2.5-month duration of the spermatogenic cycle. Similar results were obtained if the early epoch of time is defined as earlier than 2 months. These data (not shown) all indicate that predictors, except for ethnicity, are invariant across time. The hazard ratio for ethnicity, in contrast, was significantly greater than 1 [hazard ratio 1.8430 (1.5330–2.2170), P < 0.0001] during the early time period but was significantly less than 1 [hazard ratio 0.5280 (0.4100–0.6810), P < 0.0001] during the late time period. This means that the rate of suppression of sperm output in Caucasian men is faster, compared with Asian men, during the first 2–3 months of exposure but slower, compared with Asian men thereafter.
Extent of suppression
Table 5 shows the univariate analyses examining differences between men who suppressed their sperm concentration to 1 million/ml (n = 1513) from those who did not (n = 243). Table 6 shows which of these variables are independently important by stepwise multivariate logistic regression. These analyses confirm the extended Cox analysis showing that the most important predictors of incomplete suppression based on effect size are Caucasian (vs. Asian) ethnicity and the use of androgen alone (vs. androgen with progestin) regimens. In addition, increasing obesity and exposure to a higher effective dose of testosterone increases the odds of nonsuppression, but these effects were numerically less important.
TABLE 5.
Univariate analysis of men who suppress spermatogenesis, compared with those who do not
| Variables | Suppressed to 1 million/ml or less (n = 1513) | Did not Suppress to 1 million/ml or less (n = 243) | P (Student t test or Fisher test) |
|---|---|---|---|
| Continuous | Mean ± sem | Mean ± sem | |
| Subject parameters | |||
| Age (yr) | 31.7 ± 0.2 | 31.7 ± 0.4 | 0.98 |
| Height (cm) | 175.3 ± 0.2 | 175.4 ± 0.5 | 0.85 |
| Weight (kg) | 74.2 ± 0.4 | 76.4 ± 0.9 | 0.02 |
| BMI (kg/m2) | 24.0 ± 0.1 | 24.7 ± 0.2 | 0.01 |
| Testis volume (ml) | 21.8 ± 0.1 | 23.1 ± 0.4 | <0.01 |
| FSH (IU/liter) | 3.8 ± 0.1 | 3.3 ± 0.1 | 0.01 |
| LH (IU/liter) | 4.0 ± 0.1 | 4.5 ± 0.3 | <0.01 |
| T (nm) | 18.7 ± 0.2 | 19.8 ± 0.5 | 0.01 |
| Sperm parameters | |||
| Concentration (million/ml) | 75.5 ± 1.2 | 89.2 ± 3.5 | <10−4 |
| Motility (%) | 65.6 ± 0.4 | 63.0 ± 1.1 | 0.01 |
| Morphology (%) | 48.4 ± 0.8 | 54.5 ± 1.9 | <0.01 |
| Volume (ml) | 3.2 ± 0.0 | 3.1 ± 0.0 | 0.32 |
| Treatment parameters | |||
| Effective T dose (mg/d) | 14.0 ± 0.2 | 16.9 ± 0.4 | <10−11 |
| Categorical | n (%) | n (%) | |
| Progestin use | Yes: 642 (37%) | Yes: 47 (3%) | <10−6 |
| No: 871 (50%) | No: 196 (11%) | ||
| Asian race | Yes: 516 (29%) | Yes: 65 (4%) | 0.027 |
| No: 997 (57%) | No: 178 (10%) |
Blood T concentration can be converted from SI (nanomoles) to conventional units (nanograms per deciliter) by dividing by 0.0347.
TABLE 6.
Odds ratio of nonsuppression of spermatogenesis by multivariate logistic regression
| Odds of nonsuppression to 1 million/ml (95% confidence interval) | |
|---|---|
| Caucasian race | 1.965 (1.367–2.826) |
| Concurrent progestin use | 0.299 (0.180–0.497) |
| Effective T dose (mg/d) | 1.039 (1.002–1.078) |
| BMI (kg/m2) | 1.057 (1.013–1.102) |
Discussion
In this analysis, the most comprehensive of its kind, only six factors were independently identified as predicting the rate of suppression of sperm output, and only two factors (concurrent progestin use and ethnicity) are likely to be clinically important because of large effect size. The detection of these small effects (with hazard ratios of no less than 0.98 and therefore close to a no effect hazard ratio of 1) underscores the statistical power of the current data set and the methods of analysis. Furthermore, the highly significant inferences unveiled (often P < 10−3) highlights that these relationships were not due to multiple comparisons. These findings also suggest that it is unlikely that conventional clinical or laboratory variables could be used to identify men a priori who are more or less likely to suppress sperm output to levels consistent with reliable male contraception.
We conclusively show by both an extended Cox regression approach (to allow for time varying covariants) and multivariate logistic regression that progestin coadministration enhances both the rate and extent of spermatogenic suppression. For these reasons, a practical male hormonal method will require an androgen and progestin combination. An important caveat to this finding is that all progestins were considered equivalent for the purposes of this analysis to obtain an average progestin effect. In reality, progestins are likely to differ in their effects on the rate of suppression, but our data set had insufficient power for such a refined analysis because only about one third of the men received progestins, and only a few of the possible androgen-progestin combinations were applied. Progestins are known to differ according to rodent antiovulatory potency, ability to support pregnancy, and binding and activation of progesterone (and other steroid) receptors in various cell systems (65). How these differences translate to suppression of sperm output will ultimately require direct comparison through future experimentation among leading androgen and progestin combination candidate regimens to resolve this question.
The various T preparations examined exerted remarkably similar effects on spermatogenic suppression; increasing effective testosterone dose was a minor but significant predictor of nonsuppression, independent of concurrent progestin use. Although highly statistically significant, the actual dosage differences are relatively small. Nevertheless, this finding has been observed previously by others (23, 66). Increasing androgen administration should theoretically increase intratesticular T, provided the maximal limit to T-dependent feedback inhibition of gonadotropin secretion is reached. Such minor increases in intratesticular testosterone are sufficient to promote spermatogenesis in rodents or primates (67, 68, 69), although direct human evidence is lacking. We speculate that this mechanism may also partly explain why progestin coadministration, which allows lower androgen doses to be administered, is also associated with more complete spermatogenic suppression.
An unusual finding of the present analysis is that ethnicity exerts a time-differential effect on the rate of suppression of sperm output. Such an effect has been observed once before (20). More information concerning the impact of androgen-progestin regimens on sperm output in other non-Asian and non-Caucasian ethnicities (particularly Africans and Hispanics) is needed. An optimized androgen-progestin combination might also overwhelm any ethnic differences in suppression of sperm output, but this question cannot be answered by the current data set.
Increasing age, increasing baseline T, and increasing baseline sperm concentration each decreases the rate of suppression, but effect sizes were small in relation to the expected range for each of these parameters and very close to a no-effect hazard ratio of unity. This assertion is especially true for age, wherein an interval of only two to three decades was included in the current data set. However, clinically important differences in suppression rates may occur when comparing extreme combinations of age, sperm concentration, and blood testosterone. Nevertheless, in general, these factors are less important than ethnicity and concurrent progestin administration.
Increasing body mass index (BMI) was also associated with nonsuppression, and this effect may be clinically important with marked obesity. Morbid obesity is associated with increased metabolic clearance of androgens in women, which is presumed to be due to reduced SHBG (70) because SHBG directly alters metabolic clearance of steroids in primates (71). Reduced SHBG is also observed in obese men, and therefore, testosterone clearance is also likely to be increased. However, extrapolation to weight extremes is inadvisable because the vast majority of men included in this analysis were not markedly overweight (high body mass index was an exclusion criterion in most studies). Whether male hormonal methods are just as effective in completely suppressing sperm output in frankly obese men requires evaluation.
An important caveat in interpreting the present data are the comprehensive scope of this integrated analysis, which included exploratory studies in which the dose and/or methods of delivery of the androgen or progestin were suboptimal for spermatogenic suppression. For this reason, although 50% of men were observed to suppress their sperm output to 1 million/ml or less by 2–3 months, this is most likely an underestimate of the proportion of men who can achieve this degree of suppression of sperm output by this time. Indeed, modern androgen and androgen-progestin combinations report suppressibility in 80–95%, not 50%, of men within 2–3 months (3, 4), and these data compare favorably with the disappearance of sperm after vasectomy (72). Therefore, the inclusion of exploratory studies limits interpretation of absolute suppressibility and attempting to summarize these mostly nonefficacy studies for this purpose is flawed (73). However, we reasoned that a complete data set would enhance detection of more subtle predictors of the relative rate of suppression of sperm output, as was our goal here. Furthermore, our analysis suggests that reliable and timely contraception is a reasonable expectation for a wide range of couples of differing ethnicity, age, and other characteristics. Future research to improve timely suppression of sperm output may require initial coadministration of GnRH antagonists (31), optimization of progestin type and dose, or use of new synthetic androgen receptor modulators.
We conclude that androgen-progestin administration can suppress sperm output in a timely fashion to concentrations that are compatible with reliable contraception in most, but not all, men. The rate of suppression is comparable with that achieved after vasectomy. All available T preparations are broadly equivalent, although excessive T exposure may result in less complete spermatogenic suppression, presumably in the context of already maximal feedback suppression of gonadotropin output. Progestin coadministration is essential for timely and reliable suppression of spermatogenesis. Predicting which men will fail to adequately suppress sperm output is unlikely to be possible with conventional clinical or laboratory data. Whether pharmacogenetic factors may be helpful to determine responsiveness to hormonal contraceptive agents remains speculative.
Acknowledgments
This manuscript is dedicated to Geoff Waites (1928–2005), a distinguished andrologist and a champion of male contraception. We thank the study participants, personnel, and investigators of the original clinical trials that formed the data for this report and Associate Professor Val Gebski, Ph.D., for invaluable statistical advice regarding extended Cox modeling. This focused report necessarily omits many primary references because of editorial constraints.
In a study of Caucasian and Asian men, androgen-progestin administration can suppress sperm output in a time frame comparable to that achieved after vasectomy in most, but probably not all men. Younger men and those with lower serum testosterone or sperm concentration have slightly faster sperm suppression.
Footnotes
Author Contributions and Declaration Statement: P.Y.L., R.S.S., D.J.H., and C.W. are the writing group and were responsible for study design, data analysis, and initial interpretation and preparation of the manuscript. All authors participated in data acquisition and interpretation, administrative and material support, manuscript revision, and approval of the final version of the manuscript. P.Y.L. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis and was supported by Career Development Award 511929 from the National Health and Medical Research Council of Australia. There were no funding sources with an interest in this analysis. J.E. is an employee of Bayer-Schering, and W.M.K. is an employee of N.V. Organon, a part of Schering-Plough Corporation; however, neither of these pharmaceutical companies supported this study analysis. All other authors (P.Y.L., R.S.S., B.D.A., R.A.A., W.J.B., Y.-Q.G., R.I.M., M.C.M., E.N., R.S.-W., K.V., X.-H.W, M.Z., D.J.H., C.W.) have no conflicts of interest with entities directly related to the material being published; however, R.S.S. and F.C.W.W. both declare that they have consulted for Bayer-Schering and N.V. Organon, a part of Schering-Plough Corporation, and W.J.B. holds a patent (without licensing or royalties) for oral testosterone administration.
First Published Online February 26, 2008
Abbreviations: BMI, Body mass index; CPA, cyproterone acetate; DMPA, depot medroxyprogesterone acetate; DSG, desogestrel; ENG, etonogestrel; LNG, levonorgestrel; MENT, 7-α methyl-19-nor-testosterone; NET, norethisterone; T, testosterone; TD, T decanoate; TE, T enanthate; TU, T undecanoate.
References
- 1.WHO Task Force on Methods for the Regulation of Male Fertility 1990. Contraceptive efficacy of testosterone-induced azoospermia in normal men. Lancet 336:955–959 [PubMed] [Google Scholar]
- 2.WHO Task Force on Methods for the Regulation of Male Fertility 1996. Contraceptive efficacy of testosterone-induced azoospermia and oligozoospermia in normal men. Fertil Steril 65:821–829 [PubMed] [Google Scholar]
- 3.Gu YQ, Wang XH, Xu D, Peng L, Cheng LF, Huang MK, Huang ZJ, Zhang GY 2003. A multicenter contraceptive efficacy study of injectable testosterone undecanoate in healthy chinese men. J Clin Endocrinol Metab 88:562–568 [DOI] [PubMed] [Google Scholar]
- 4.Turner L, Conway AJ, Jimenez M, Liu PY, Forbes E, McLachlan RI, Handelsman DJ 2003. Contraceptive efficacy of a depot progestin and androgen combination in men. J Clin Endocrinol Metab 88:4659–4667 [DOI] [PubMed] [Google Scholar]
- 5.Liu PY, Swerdloff RS, Christenson PD, Handelsman DJ, Wang C, Hormonal Male Contraception Summit Group 2006. Rate, extent and modifiers of spermatogenic recovery after hormonal male contraception: an integrated analysis. Lancet 367:1412–1420 [DOI] [PubMed] [Google Scholar]
- 6.Anonymous 2007. Tenth Summit Meeting Consensus: recommendations for regulatory approval for hormonal male contraception. Int J Androl 30:63–64 [DOI] [PubMed] [Google Scholar]
- 7.Liu PY, Veldhuis JD 2008. The hypothalamo-pituitary unit, testis and male accessory organs. In: Strauss JF, Barbieri RL, eds. Yen and Jaffe's reproductive endocrinology: physiology, pathophysiology and clinical management. 6th ed. Philadelphia: W. B. Saunders, in press
- 8.Anderson RA, Baird DT 2002. Male contraception. Endocr Rev 23:735–762 [DOI] [PubMed] [Google Scholar]
- 9.Handelsman DJ 2005. Male contraception. In: DeGroot LJ, Jameson JL eds. Endocrinology. 5th ed. Philadelphia: Elsevier Saunders; 3247–3256
- 10.Wang C, Swerdloff RS 2004. Male hormonal contraception. Am J Obstet Gynecol 190:S60–S68 [DOI] [PubMed]
- 11.Meriggiola MC, Farley TM, Mbizvo MT 2003. A review of androgen-progestin regimens for male contraception. J Androl 24:466–483 [DOI] [PubMed] [Google Scholar]
- 12.Nieschlag E, Henke A 2005. Hopes for male contraception. Lancet 365:554–556 [DOI] [PubMed] [Google Scholar]
- 13.Amory JK, Page ST, Bremner WJ 2006. Drug insight: recent advances in male hormonal contraception. Nat Clin Pract Endocrinol Metab 2:32–41 [DOI] [PubMed] [Google Scholar]
- 14.Matthiesson KL, McLachlan RI 2006. Male hormonal contraception: concept proven, product in sight? Hum Reprod Update 12:463–482 [DOI] [PubMed] [Google Scholar]
- 15.Wu FC 2006. Hormonal approaches to male contraception: approaching reality. Mol Cell Endocrinol 250:2–7 [DOI] [PubMed] [Google Scholar]
- 16.Meriggiola MC, Pelusi G 2006. Advances in male hormonal contraception. Expert Opin Investig Drugs 15:389–397 [DOI] [PubMed] [Google Scholar]
- 17.Wu FC, Farley TM, Peregoudov A, Waites GM 1996. Effects of testosterone enanthate in normal men: experience from a multicenter contraceptive efficacy study. World Health Organization Task Force on Methods for the Regulation of Male Fertility. Fertil Steril 65:626–636 [PubMed] [Google Scholar]
- 18.McLachlan RI, O'Donnell L, Meachem SJ, Stanton PG, De K, Pratis K, Robertson DM 2002. Hormonal regulation of spermatogenesis in primates and man: insights for development of the male hormonal contraceptive. J Androl 23:149–162 [PubMed] [Google Scholar]
- 19.World Health Organization Task Force on Methods for the Regulation of Male Fertility 1995. Rates of testosterone-induced suppression to severe oligozoospermia or azoospermia in two multinational clinical studies. World Health Organization Task Force on Methods for the Regulations of Male Fertility. Int J Androl 18:157–165 [DOI] [PubMed] [Google Scholar]
- 20.Handelsman DJ, Farley TM, Peregoudov A, Waites GM 1995. Factors in nonuniform induction of azoospermia by testosterone enanthate in normal men. World Health Organization Task Force on Methods for the Regulation of Male Fertility. Fertil Steril 63:125–133 [PubMed] [Google Scholar]
- 21.Ly LP, Liu PY, Handelsman DJ 2005. Rates of suppression and recovery of human sperm output in testosterone-based hormonal contraceptive regimens. Hum Reprod 20:1733–1740 [DOI] [PubMed] [Google Scholar]
- 22.McLachlan RI, Robertson DM, Pruysers E, Ugoni A, Matsumoto AM, Anawalt BD, Bremner WJ, Meriggiola C 2004. Relationship between serum gonadotropins and spermatogenic suppression in men undergoing steroidal contraceptive treatment. J Clin Endocrinol Metab 89:142–149 [DOI] [PubMed] [Google Scholar]
- 23.Meriggiola MC, Costantino A, Bremner WJ, Morselli-Labate AM 2002. Higher testosterone dose impairs sperm suppression induced by a combined androgen-progestin regimen. J Androl 23:684–690 [PubMed] [Google Scholar]
- 24.Anonymous 2002. Sixth Summit Meeting Consensus: recommendations for regulatory approval for hormonal male contraception. Int J Androl 25:375 [DOI] [PubMed]
- 25.Waites GM 2003. Development of methods of male contraception: impact of the World Health Organization Task Force. Fertil Steril 80:1–15 [DOI] [PubMed] [Google Scholar]
- 26.Misell LM, Holochwost D, Boban D, Santi N, Shefi S, Hellerstein MK, Turek PJ 2006. A stable isotope-mass spectrometric method for measuring human spermatogenesis kinetics in vivo. J Urol 175:242–246; discussion 246 [DOI] [PubMed]
- 27.Clermont Y 1972. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev 52:198–236 [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization 1999. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed. Cambridge, UK: Cambridge University Press
- 29.Hay CJ, Brady BM, Zitzmann M, Osmanagaoglu K, Pollanen P, Apter D, Wu FC, Anderson RA, Nieschlag E, Devroey P, Huhtaniemi I, Kersemaekers WM 2005. A multicenter phase IIb study of a novel combination of intramuscular androgen (testosterone decanoate) and oral progestogen (etonogestrel) for male hormonal contraception. J Clin Endocrinol Metab 90:2042–2049 [DOI] [PubMed] [Google Scholar]
- 30.Wang C, Wang XH, Nelson AL, Lee KK, Cui YG, Tong JS, Berman N, Lumbreras L, Leung A, Hull L, Desai S, Swerdloff RS 2005. Levonorgestrel implants enhanced the suppression of spermatogenesis by testosterone implants: comparison between Asian and non-Asian men. J Clin Endocrinol Metab 91:460–470 [DOI] [PubMed] [Google Scholar]
- 31.Swerdloff RS, Bagatell CJ, Wang C, Anawalt BD, Berman N, Steiner B, Bremner WJ 1998. Suppression of spermatogenesis in man induced by Nal-Glu gonadotropin releasing hormone antagonist and testosterone enanthate (TE) is maintained by TE alone. J Clin Endocrinol Metab 83:3527–3533 [DOI] [PubMed] [Google Scholar]
- 32.Gonzalo IT, Swerdloff RS, Nelson AL, Clevenger B, Garcia R, Berman N, Wang C 2002. Levonorgestrel implants (Norplant II) for male contraception clinical trials: combination with transdermal and injectable testosterone. J Clin Endocrinol Metab 87:3562–3572 [DOI] [PubMed] [Google Scholar]
- 33.Kinniburgh D, Zhu H, Cheng L, Kicman AT, Baird DT, Anderson RA 2002. Oral desogestrel with testosterone pellets induces consistent suppression of spermatogenesis to azoospermia in both Caucasian and Chinese men. Hum Reprod 17:1490–1501 [DOI] [PubMed] [Google Scholar]
- 34.Amory JK, Anawalt BD, Bremner WJ, Matsumoto AM 2001. Daily testosterone and gonadotropin levels are similar in azoospermic and nonazoospermic normal men administered weekly testosterone: implications for male contraceptive development. J Androl 22:1053–1060 [DOI] [PubMed] [Google Scholar]
- 35.Meriggiola M, Costantino A, Saad F, D'Emidio L, Labate AM, Bertaccini A, Bremner W, Rudolph I, Ernst M, Kirsch B, Martorana G, Pelusi G 2005. Norethisterone enanthate plus testosterone undecanoate for male contraception: effects of various injection intervals on spermatogenesis, reproductive hormones, testis, and prostate. J Clin Endocrinol Metab 90:2005–2014 [DOI] [PubMed] [Google Scholar]
- 36.Kamischke A, Heuermann T, Kruger K, von Eckardstein S, Schellschmidt I, Rubig A, Nieschlag E 2002. An effective hormonal male contraceptive using testosterone undecanoate with oral or injectable norethisterone preparations. J Clin Endocrinol Metab 87:530–539 [DOI] [PubMed] [Google Scholar]
- 37.Anawalt BD, Amory JK, Herbst KL, Coviello AD, Page ST, Bremner WJ, Matsumoto AM 2005. Intramuscular testosterone enanthate plus very low dosage oral levonorgestrel suppresses spermatogenesis without causing weight gain in normal young men: a randomized clinical trial. J Androl 26:405–413 [DOI] [PubMed] [Google Scholar]
- 38.Qoubaitary A, Meriggiola C, Ng CM, Lumbreras L, Cerpolini S, Pelusi G, Christensen PD, Hull L, Swerdloff RS, Wang C 2006. Pharmacokinetics of testosterone undecanoate injected alone or in combination with norethisterone enanthate in healthy men. J Androl 27:853–867 [DOI] [PubMed] [Google Scholar]
- 39.Bebb RA, Anawalt BD, Christensen RB, Paulsen CA, Bremner WJ, Matsumoto AM 1996. Combined administration of levonorgestrel and testosterone induces more rapid and effective suppression of spermatogenesis than testosterone alone: a promising male contraceptive approach. J Clin Endocrinol Metab 81:757–762 [DOI] [PubMed] [Google Scholar]
- 40.Anawalt BD, Bebb RA, Bremner WJ, Matsumoto AM 1999. A lower dosage levonorgestrel and testosterone combination effectively suppresses spermatogenesis and circulating gonadotropin levels with fewer metabolic effects than higher dosage combinations. J Androl 20:407–414 [PubMed] [Google Scholar]
- 41.Von Eckardstein S, Noe G, Brache V, Nieschlag E, Croxatto H, Alvarez F, Moo-Young A, Sivin I, Kumar N, Small M, Sundaram K 2003. A clinical trial of 7α-methyl-19-nortestosterone implants for possible use as a long-acting contraceptive for men. J Clin Endocrinol Metab 88:5232–5239 [DOI] [PubMed] [Google Scholar]
- 42.Gu YQ, Tong JS, Ma DZ, Wang XH, Yuan D, Tang WH, Bremner WJ 2004. Male hormonal contraception: effects of injections of testosterone undecanoate and depot medroxyprogesterone acetate at eight-week intervals in chinese men. J Clin Endocrinol Metab 89:2254–2262 [DOI] [PubMed] [Google Scholar]
- 43.Kamischke A, Ploger D, Venherm S, von Eckardstein S, von Eckardstein A, Nieschlag E 2000. Intramuscular testosterone undecanoate with or without oral levonorgestrel: a randomized placebo-controlled feasibility study for male contraception. Clin Endocrinol (Oxf) 53:43–52 [DOI] [PubMed] [Google Scholar]
- 44.Anderson RA, Kinniburgh D, Baird DT 2002. Suppression of spermatogenesis by etonogestrel implants with depot testosterone: potential for long-acting male contraception. J Clin Endocrinol Metab 87:3640–3649 [DOI] [PubMed] [Google Scholar]
- 45.Wu FC, Balasubramanian R, Mulders TM, Coelingh-Bennink HJ 1999. Oral progestogen combined with testosterone as a potential male contraceptive: additive effects between desogestrel and testosterone enanthate in suppression of spermatogenesis, pituitary-testicular axis, and lipid metabolism. J Clin Endocrinol Metab 84:112–122 [DOI] [PubMed] [Google Scholar]
- 46.Meriggiola MC, Costantino A, Cerpolini S, Bremner WJ, Huebler D, Morselli-Labate AM, Kirsch B, Bertaccini A, Pelusi C, Pelusi G 2003. Testosterone undecanoate maintains spermatogenic suppression induced by cyproterone acetate plus testosterone undecanoate in normal men. J Clin Endocrinol Metab 88:5818–5826 [DOI] [PubMed] [Google Scholar]
- 47.Anawalt BD, Herbst KL, Matsumoto AM, Mulders TM, Coelingh-Bennink HJ, Bremner WJ 2000. Desogestrel plus testosterone effectively suppresses spermatogenesis but also causes modest weight gain and high-density lipoprotein suppression. Fertil Steril 74:707–714 [DOI] [PubMed] [Google Scholar]
- 48.Hair WM, Kitteridge K, O'Connor DB, Wu FCW 2001. A novel male contraceptive pill-patch combination: oral desogestrel and transdermal testosterone in the suppression of spermatogenesis in normal men. J Clin Endocrinol Metab 86:5201–5209 [DOI] [PubMed] [Google Scholar]
- 49.Brady BM, Walton M, Hollow N, Kicman AT, Baird DT, Anderson RA 2004. Depot testosterone with etonogestrel implants result in induction of azoospermia in all men for long-term contraception. Hum Reprod 19:2658–2667 [DOI] [PubMed] [Google Scholar]
- 50.Meriggiola MC, Bremner WJ, Paulsen CA, Valdiserri A, Incorvaia L, Motta R, Pavani A, Capelli M, Flamigni C 1996. A combined regimen of cyproterone acetate and testosterone enanthate as a potentially highly effective male contraceptive. J Clin Endocrinol Metab 81:3018–3023 [DOI] [PubMed] [Google Scholar]
- 51.Kamischke A, Venherm S, Ploger D, von Eckardstein S, Nieschlag E 2001. Intramuscular testosterone undecanoate and norethisterone enanthate in a clinical trial for male contraception. J Clin Endocrinol Metab 86:303–309 [DOI] [PubMed] [Google Scholar]
- 52.Meriggiola MC, Bremner WJ, Costantino A, Di Cintio G, Flamigni C 1998. Low dose of cyproterone acetate and testosterone enanthate for contraception in men. Hum Reprod 13:1225–1229 [DOI] [PubMed] [Google Scholar]
- 53.Meriggiola MC, Bremner WJ, Costantino A, Pavani A, Capelli M, Flamigni C 1997. An oral regimen of cyproterone acetate and testosterone undecanoate for spermatogenic suppression in men. Fertil Steril 68:844–850 [DOI] [PubMed] [Google Scholar]
- 54.Anderson RA, Van Der Spuy ZM, Dada OA, Tregoning SK, Zinn PM, Adeniji OA, Fakoya TA, Smith KB, Baird DT 2002. Investigation of hormonal male contraception in African men: suppression of spermatogenesis by oral desogestrel with depot testosterone. Hum Reprod 17:2869–2877 [DOI] [PubMed] [Google Scholar]
- 55.World Health Organization Task Force on Methods for the Regulation of Male Fertility 1993. Comparison of two androgens plus depot-medroxyprogesterone acetate for suppression to azoospermia in Indonesian men. Fertil Steril 60:1062–1068 [PubMed] [Google Scholar]
- 56.Gui YL, He CH, Amory JK, Bremner WJ, Zheng EX, Yang J, Yang PJ, Gao ES 2004. Male hormonal contraception: suppression of spermatogenesis by injectable testosterone undecanoate alone or with levonorgestrel implants in chinese men. J Androl 25:720–727 [DOI] [PubMed] [Google Scholar]
- 57.Pollanen P, Nikkanen V, Huhtaniemi I 2001. Combination of subcutaneous levonorgestrel implants and transdermal dihydrotestosterone gel for male hormonal contraception. Int J Androl 24:369–380 [DOI] [PubMed] [Google Scholar]
- 58.Page ST, Amory JK, Anawalt BD, Irwig MS, Brockenbrough AT, Matsumoto AM, Bremner WJ 2006. Testosterone gel combined with depomedroxyprogesterone acetate is an effective male hormonal contraceptive regimen and is not enhanced by the addition of a GnRH antagonist. J Clin Endocrinol Metab 91:4374–4380 [DOI] [PubMed] [Google Scholar]
- 59.Brady BM, Amory JK, Perheentupa A, Zitzmann M, Hay CJ, Apter D, Anderson RA, Bremner WJ, Pollanen P, Nieschlag E, Wu FC, Kersemaekers WM 2006. A multicentre study investigating subcutaneous etonogestrel implants with injectable testosterone decanoate as a potential long-acting male contraceptive. Hum Reprod 21:285–294 [DOI] [PubMed] [Google Scholar]
- 60.Handelsman DJ, Conway AJ, Boylan LM 1990. Pharmacokinetics and pharmacodynamics of testosterone pellets in man. J Clin Endocrinol Metab 71:216–222 [DOI] [PubMed] [Google Scholar]
- 61.Nieschlag E, Mauss J, Coert A, Kicovic P 1975. Plasma androgen levels in men after oral administration of testosterone or testosterone undecanoate. Acta Endocrinol 79:366–374 [DOI] [PubMed] [Google Scholar]
- 62.Tauber U, Schroder K, Dusterberg B, Matthes H 1986. Absolute bioavailability of testosterone after oral administration of testosterone-undecanoate and testosterone. Eur J Drug Metab Pharmacokinet 11:145–149 [DOI] [PubMed] [Google Scholar]
- 63.Wilson DE, Meikle AW, Boike SC, Fairless AJ, Etheredge RC, Jorkasky DK 1998. Bioequivalence assessment of a single 5 mg/day testosterone transdermal system versus two 2.5 mg/day systems in hypogonadal men. J Clin Pharmacol 38:54–59 [DOI] [PubMed] [Google Scholar]
- 64.Therneau TM, Grambsch PM 2000. Modeling survival data: extending the Cox model. 1st ed. New York: Springer-Verlag
- 65.Sitruk-Ware R 2006. New progestagens for contraceptive use. Hum Reprod Update 12:169–178 [DOI] [PubMed] [Google Scholar]
- 66.Michel E, Bents H, Akhtar FB, Honigl W, Knuth UA, Sandow J, Nieschlag E 1985. Failure of high-dose sustained release luteinizing hormone releasing hormone agonist (buserelin) plus oral testosterone to suppress male fertility. Clin Endocrinol (Oxf) 23:663–675 [DOI] [PubMed] [Google Scholar]
- 67.Weinbauer GF, Gockeler E, Nieschlag E 1988. Testosterone prevents complete suppression of spermatogenesis in the gonadotropin-releasing hormone antagonist-treated nonhuman primate (Macaca fascicularis). J Clin Endocrinol Metab 67:284–290 [DOI] [PubMed] [Google Scholar]
- 68.Weinbauer GF, Khurshid S, Findscheidt U, Nieschlag E 1989. Sustained inhibition of sperm production and inhibin secretion by a gonadotrophin-releasing hormone antagonist and delayed testosterone substitution in non-human primates (Macaca fascicularis). Acta Endocrinol 123:303–310 [DOI] [PubMed] [Google Scholar]
- 69.Handelsman DJ, Spaliviero JA, Simpson JM, Allan CM, Singh J 1999. Spermatogenesis without gonadotropins: maintenance has a lower testosterone threshold than initiation. Endocrinology 140:3938–3946 [DOI] [PubMed] [Google Scholar]
- 70.Samojlik E, Kirschner MA, Silber D, Schneider G, Ertel NH 1984. Elevated production and metabolic clearance rates of androgens in morbidly obese women. J Clin Endocrinol Metab 59:949–954 [DOI] [PubMed] [Google Scholar]
- 71.Plymate SR, Namkung PC, Metej LA, Petra PH 1990. Direct effect of plasma sex hormone binding globulin (SHBG) on the metabolic clearance rate of 17 β-estradiol in the primate. J Steroid Biochem 36:311–317 [DOI] [PubMed] [Google Scholar]
- 72.Marwood RP, Beral V 1979. Disappearance of spermatozoa from the ejaculate after vasectomy. Br J Med 1:87 [DOI] [PMC free article] [PubMed]
- 73.Grimes DA, Lopez LM, Gallo MF, Halpern V, Nanda K, Schulz KF 2007. Steroid hormones for contraception in men. Cochrane Database Syst Rev: CD004316 [DOI] [PubMed]