Abstract
Context: Sympathetic activation promotes insulin resistance and arterial hypertension with increasing adiposity. A difference in the relationship between adiposity and sympathetic activity between women and men could contribute to the known gender difference in cardiovascular disease risk.
Objective: We tested whether muscle sympathetic nerve activity (MSNA) is correlated differently with waist circumference, waist to hip ratio (WHR), and body mass index (BMI) in women and men.
Design and Setting: We pooled data from two microneurography centers (Berlin, Germany; Gdansk, Poland) for a cross-sectional study.
Participants: We studied 111 normotensive, healthy Caucasian subjects (70 males and 41 females). Age ranged between 19 and 62 yr and BMI ranged between 18 and 40 kg/m2.
Intervention: No intervention was applied during the study.
Measurements: Supine heart rate, blood pressure, and MSNA were recorded after at least 30 min rest.
Results: MSNA in bursts per minute was age dependent in both sexes [r (male) = 0.56, r (female) = 0.34, P < 0.01]. Controlling for waist and hip circumferences, age dependence remained highly significant in men (r = 0.43) and women (r = 0.43). Adjusting for age, in men, waist circumference (r = 0.29), WHR (r = 0.39), and BMI (r = 0.31) were predictive for MSNA and directly correlated (P < 0.01) but not in women. Adjusting for BMI, in men, only WHR (r = 0.40) remained predictive for MSNA.
Conclusion: These data support the hypothesis of a gender difference in the regulation of the sympathetic nervous system, in which MSNA mainly relates to WHR in men but not women. The phenomenon may contribute to the sexual dimorphism in cardiovascular disease risk.
A microneurography study in 111 healthy subjects demonstrates that muscle sympathetic nerve activity relates to waist-to-hip ratio in men but not in women.
Studies in patients fulfilling metabolic syndrome criteria suggest that sympathetic activation promotes insulin resistance and arterial hypertension with increasing adiposity (1). Muscle sympathetic nerve activity (MSNA) is positively correlated with increased body weight (2). Yet some obese patients are normotensive and feature normal sympathetic nerve activity (3, 4). The observation may be explained in part by the fact that sympathetic activity is more closely correlated with abdominal than with sc fat mass (5, 6, 7, 8). Indeed, sympathetic activity is similar in sc obese and nonobese men (5, 6). Another possible explanation for the variable expression of MSNA is that genetic or nongenetic factors affect the interaction between fat tissue and sympathetic nerve activity. Female gender may be such a factor. Even though percent body fat is higher in women than men (9), MSNA tends to be reduced at least before menopause (10, 11). Previous studies in smaller numbers of subjects suggested that gender differences in adipose tissue distribution had an impact on MSNA (12). Possibly fat distribution affects sympathetic responses differently in men and women. However, direct measurements of sympathetic nerve activity addressing this issue in a sufficient number of patients are rare. The issue is clinically relevant, given the known gender difference in cardiovascular disease risk that abates at on older age (13, 14). Therefore, we tested the hypothesis that the relationship between sympathetic nerve activity and waist circumference as a marker of central adiposity may be different between men and women. We analyzed the relationship between adiposity and MSNA in a relatively large cohort of healthy men and women with various degrees of adiposity. We focused our analysis on waist circumference, which closely correlates with visceral fat mass (15).
Subjects and Methods
We studied 111 Caucasian subjects, 70 men and 41 women. Body mass index (BMI) ranged between 18 and 40 kg/m2. Subjects were recruited in Berlin (n = 43) and Gdansk (n = 68). All subjects were normotensive with blood pressure less than 140/90 mm Hg and healthy as judged by a history, physical examination, and routine blood testing. Subjects received no medication or hormone replacement therapies. The Institutional Human Subjects Review Committees approved the study and informed consent was obtained from all subjects.
Subjects were weighed with light clothes after they had emptied the bladder. We measured waist circumference at a level midway between the lower rib margin and iliac crest with the tape all around the body in horizontal position and hip circumference at the level trochanter major while the subject was standing to calculate the waist to hip ratio (WHR).
Cardiovascular and sympathetic measurements were conducted with the subjects supine. Electrocardiogram and beat-by-beat blood pressure (Finapres; Ohmeda, Englewood, CA) were measured continuously (Cardioscreen; Medis GmbH, Ilmenau, Germany). Brachial blood pressure (Dinamap; Critikon, Tampa, FL) was determined. MSNA was recorded from the right peroneal nerve (MSNA unit, Biomedical Engineering Department, University of Iowa, Iowa City, IA) as described previously (16). Nerve activity was amplified with a total gain of 100,000, bandpass filtered (0.7–2 kHz), full-wave rectified, and integrated. After instrumentation, subjects rested for at least 20 min to achieve a stable baseline. Then resting heart rate, blood pressure, and MSNA were recorded.
Data sets from both centers were analog-to-digital converted at 500 Hz using the Windaq pro+ software (Dataq Instruments Inc., Akron, OH). R-R intervals, diastolic blood pressure, systolic blood pressure values, and sympathetic bursts were defined off-line for the complete records using a program written by one of the authors (A.D.) that is based on PV-wave software (Visual Numerics Inc., Houston, TX). The number of bursts per minute (burst frequency), the number of bursts per 100 heart beats (burst incidence), and the mean area under the MSNA bursts per minute were quantified using an automated detection algorithm (17).
All data are expressed as mean ± sem. Interindividual differences were compared by the unpaired t test. Relationships between measurements were assessed by single and multiple linear regressions. In both genders, the estimated slopes of regression lines were calculated. The interaction between parameters was assessed by comparing regression slopes and intercepts in male and female subjects before and after correction for aging (SPSS software, version 14.0 for Windows, SPSS Inc., Chicago, IL). Results of single parameters are reported with nominal significance levels. A value for P < 0.05 was considered significant.
Results
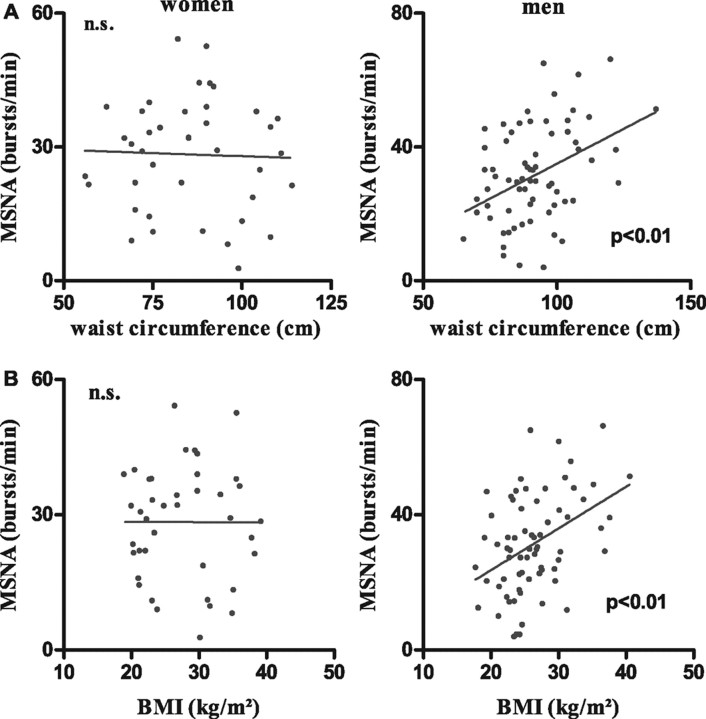
Demographic data of the study population is given in Table 1. Mean age and BMI were similar in women and in men. Systolic blood pressure, diastolic blood pressure, BMI, waist circumference, and WHR were directly correlated with age in both women and men. Sympathetic activity in bursts per minute was also age dependent in both groups. However, in men a larger proportion of the variability in sympathetic activity was explained by an age effect. To test for independent influences of age and body composition on MSNA, we calculated partial correlations for either age or waist and hip circumferences as well as WHR. Controlling for waist and hip circumferences, age dependence remained highly significant in men (r = 0.43) and women (r = 0.43). When we adjusted for age, waist circumference (r = 0.29), WHR (r = 0.39), and BMI (r = 0.31) predicted MSNA in men (P < 0.01). However, after adjustment for age, the relationship between waist circumference or BMI and MSNA was reversed in women. BMI and waist circumference were highly correlated in our study population (women r = 0.95, men r = 0.92). Adjusting for BMI in men, only WHR (r = 0.40) remained predictive for MSNA. The results of the regression analysis of BMI, waist circumference, and WHR vs. age and MSNA in bursts per minute after correction for age and BMI are shown in Table 2. BMI, waist circumference, and WHR did not correlate with blood pressure after correction for the influences of age. Figure 1 illustrates the correlation between MSNA and waist circumference (top panel) and between MSNA and BMI (bottom panel) in women and men. Waist circumference, WHR, and BMI were correlated with sympathetic activity in men but not women. Finally, backward regression analysis with WHR and waist and hip circumference as starting variables revealed independent influences of waist circumference on nerve activity in men only.
TABLE 1.
Baseline characteristics
| Parameter | Men | Statistic | Women |
|---|---|---|---|
| n | 70 | 41 | |
| Age (yr) | 39 ± 1.4 | 39 ± 1.7 | |
| Weight (kg) | 83.6 ± 2.0 | 1 | 75.3 ± 2.8 |
| Height (cm) | 177.5 ± 0.8 | 2 | 165.7 ± 0.9 |
| BMI (kg/m2) | 26.5 ± 0.6 | 27.4 ± 0.9 | |
| Waist circumference (cm) | 92.0 ± 1.7 | 1 | 84.8 ± 2.5 |
| Hip circumference (cm) | 100.2 ± 1.2 | 104.2 ± 2.0 | |
| WHR | 0.91 ± 0.01 | 2 | 0.81 ± 0.01 |
| Heart rate (beats/min) | 64 ± 1.1 | 65 ± 1.2 | |
| RR interval (msec) | 954 ± 15 | 944 ± 18 | |
| Systolic blood pressure (mm Hg) | 124 ± 2 | 2 | 116 ± 2 |
| Diastolic blood pressure (mm Hg) | 74 ± 1 | 70 ± 1 | |
| MSNA frequency (bursts/min) | 31 ± 2 | 28 ± 2 | |
| MSNA incidence (bursts per 100 beats) | 49 ± 3 | 43 ± 3 | |
| MSNA (normal burst area) | 13.1 ± 0.7 | 11.2 ± 0.8 |
P < 0.05.
P < 0.01.
TABLE 2.
Regression analysis
| Parameter | r (men) | r (women) |
|---|---|---|
| BMI vs. | ||
| Age (yr) | 0.3051 | 0.4522 |
| MSNA age corrected (bursts/min) | 0.3071 | −0.296 |
| Waist circumference vs. | ||
| Age (yr) | 0.3051 | 0.4272 |
| MSNA age corrected (bursts/min) | 0.2871 | −0.3171 |
| MSNA BMI corrected (bursts/min) | 0.033 | −0.080 |
| WHR vs. | ||
| Age (yr) | 0.4292 | 0.3791 |
| MSNA age corrected (bursts/min) | 0.3901 | 0.114 |
| MSNA BMI corrected (bursts/min) | 0.3991 | 0.165 |
Relationship of BMI (kilograms per square meter), waist circumference, and WHR vs. age and MSNA (bursts per minute) in men and women after correction for the influences of age and BMI.
P < 0.05.
P < 0.01.
Fig. 1.
A, Linear regression analysis between MSNA (bursts per minute) and waist circumference in women (top left panel) and men (top right panel). B, Linear regression analysis of MSNA in bursts per minute and BMI in women (bottom left panel) and men (bottom right panel). n.s., Not significant.
Discussion
We observed a gender difference in the relationship between sympathetic vasomotor tone and measures of central adiposity. With increasing age, central adiposity and MSNA increased in women and men. In men, MSNA was correlated with BMI, waist circumference, and WHR after we adjusted for age. In contrast, with age adjustment, the correlation between BMI, waist circumference, and WHR with sympathetic activity in women was reversed. In men but not women, WHR was correlated with MSNA, even after adjustment for BMI. An increase in BMI, waist circumference, WHR, blood pressure, and MSNA with aging has been described in numerous studies. With increasing age, women may accumulate fat more rapidly than men (9). The phenomenon has been attributed to a more pronounced decline in physical activity and peak oxygen consumption in women compared with men. Indeed, adjustment for these variables reduced the age-related increase in waist circumference from 2 to 1% per decade in men and from 4 to 1% per decade in women (9).
Adipose tissue generates signals regulating sympathetic activity. The leptinergic system appears to be particularly important in this regard (18, 19). Gender and adiposity may affect brain leptin release (20). In animals, leptin applied into the brain increases sympathetic nerve traffic to peripheral tissues (21). Circulating leptin concentrations are correlated with sympathetic activity in some but not all studies (22). Yet MSNA was not related to BMI in hypertensive women, despite higher leptin levels (23). Sympathetic activity may be more closely correlated with leptin that is bound to a truncated leptin receptor than to free leptin concentrations (18). Our study suggests that the increase in sympathetic activity with age (10, 11) may not be fully explained by increased adiposity.
In any event, total and central body fat was associated with raised catecholamine levels in older men (24). In another study, central obesity was characterized by greater sympathetic activation compared with peripheral obesity. The authors suggested that metabolic factors rather than gender or baroreflex mechanisms explained the sympathetic activation (2). Furthermore, adiposity was associated with sympathetic vasomotor tone in women and men. The regression line may be shifted downward such that at a given degree of adiposity, sympathetic activity is lower in young women (12). Earlier studies suggested that such differences exist only in younger subjects. The mean age of our subjects was 39 yr, which might explain the failure to observe gender differences in MSNA. Waist to thigh ratio was the primary factor related to sympathetic activity in this study. However, a smaller study showed that the correlation between waist to thigh ratio and sympathetic activity was significant only in men (12). Our study confirms and extends the observation. Central fat distribution (WHR) was correlated with sympathetic activity in men, even after adjustment for age and BMI. The observation suggests that fat distribution is an important variable affecting MSNA in men. The relationship was absent in women.
Our study suggests that female gender may be a factor rendering the sympathetic nervous system less sensitive to increased adiposity and central fat distribution. The findings that leptin is associated with the risk of coronary heart disease in men but not older women supports the hypothesis (25). It is tempting to speculate that gender may affect the response of central sympathetic pathways to adipose tissue derived leptinergic signals. The response may also be affected by genetic factors. For example, in Pima Indians, circulating leptin levels and resting energy expenditure increase appropriately with increasing adipose tissue mass (26). Yet sympathetic vasomotor tone and blood pressure fail to increase with increasing obesity (26).
One limitation of our study is that we did not measure visceral adipose tissue directly using imaging techniques. We cannot completely exclude the possibility that our findings result from a gender difference in the relationship between visceral adipose tissue mass and waist circumference. However, several studies showed a good correlation between visceral adipose tissue mass and waist circumference over a wide age range (15, 27). Another possible limitation is that we measured only sympathetic nerve traffic to skeletal muscle in the leg. We cannot exclude a gender difference in the regional distribution of sympathetic activity to target organs that are not accessible to nerve recordings in human subjects (28). Preferential cardiac sympathetic activation in patients with heart failure, coronary artery disease, arterial hypertension, or ventricular arrhythmias are an example for regionalization of sympathetic activity (29).
Despite these issues, we suggest that abdominal fat is an important adipose tissue depot regulating MSNA in men. However, our study also suggests that women may be protected from sympathetic activation through a hitherto unknown mechanism. Thus, sympathetic activation is influenced by the amount of adipose tissue, which is highly correlated with circulating leptin levels and the sensitivity of the central nervous system to adipose tissue derived signals. The sensitivity of the central nervous system to adipose tissue derived signals appears to be modulated by fat distribution and gender. From a metabolic point of view, lower sympathetic activity in women may be a disadvantage as low resting sympathetic activity may predispose to further weight gain (30). On the other hand, raised sympathetic activity contributes to obesity-associated arterial hypertension (31), which may provide a cardiovascular benefit for women.
Footnotes
This work was supported by Deutsche Forschungsgemeinschaft, the Zentrum für Deutsche Luft-und Raumfahrt (DLR), and the European Union (InGenious HyperCare, Grant LSHM-CT-2006-hyphen]037093).
Disclosure Statement: The authors of the manuscript have nothing to declare.
First Published Online September 9, 2008
Abbreviations: BMI, Body mass index; MSNA, muscle sympathetic nerve activity; WHR, waist to hip ratio.
References
- 1.Fagius J 2003. Sympathetic nerve activity in metabolic control—some basic concepts. Acta Physiol Scand 177:337–343 [DOI] [PubMed] [Google Scholar]
- 2.Grassi G, Dell'Oro R, Facchini A, Quarti TF, Bolla GB, Mancia G 2004. Effect of central and peripheral body fat distribution on sympathetic and baroreflex function in obese normotensives. J Hypertens 22:2363–2369 [DOI] [PubMed] [Google Scholar]
- 3.Ribeiro MM, Trombetta IC, Batalha LT, Rondon MU, Forjaz CL, Barretto AC, Villares SM, Negrao CE 2001. Muscle sympathetic nerve activity and hemodynamic alterations in middle-aged obese women. Braz J Med Biol Res 2001 34:475–478 [DOI] [PubMed] [Google Scholar]
- 4.Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK 1998. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation 98:772–776 [DOI] [PubMed] [Google Scholar]
- 5.Alvarez GE, Beske SD, Ballard TP, Davy KP 2002. Sympathetic neural activation in visceral obesity. Circulation 106:2533–2536 [DOI] [PubMed] [Google Scholar]
- 6.Alvarez GE, Ballard TP, Beske SD, Davy KP 2004. Subcutaneous obesity is not associated with sympathetic neural activation. Am J Physiol Heart Circ Physiol 287:H414–H418 [DOI] [PubMed]
- 7.Jones PP, Davy KP, Seals DR 1997. Relations of total and abdominal adiposity to muscle sympathetic nerve activity in healthy older males. Int J Obes Relat Metab Disord 21:1053–1057 [DOI] [PubMed] [Google Scholar]
- 8.Jones PP, Davy KP, Alexander S, Seals DR 1997. Age-related increase in muscle sympathetic nerve activity is associated with abdominal adiposity. Am J Physiol 272(6 Pt 1):E976–E980 [DOI] [PubMed]
- 9.Poehlman ET, Toth MJ, Bunyard LB, Gardner AW, Donaldson KE, Colman E, Fonong T, Ades PA 1995. Physiological predictors of increasing total and central adiposity in aging men and women. Arch Intern Med 155:2443–2448 [PubMed] [Google Scholar]
- 10.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers V 2005. A gender selective interaction between aging, blood pressure and sympathetic nerve activity. Hypertension 45:522–525 [DOI] [PubMed] [Google Scholar]
- 11.Ng AV, Callister R, Johnson DG, Seals DR 1993. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension 21:498–503 [DOI] [PubMed] [Google Scholar]
- 12.Jones PP, Snitker S, Skinner JS, Ravussin E 1996. Gender differences in muscle sympathetic nerve activity: effect of body fat distribution. Am J Physiol 270 (2 Pt 1):E363–E366 [DOI] [PubMed]
- 13.Anand SS, Islam S, Rosengren A, Franzosi MG, Steyn K, Yusufali AH, Keltai M, Diaz R, Rangarajan S, Yusuf S 2008. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J 29:932–940 [DOI] [PubMed] [Google Scholar]
- 14.Narkiewicz K, Kjeldsen SE, Hedner T 2006. Hypertension and cardiovascular disease in women: progress toward better understanding of gender-specific differences? Blood Press 15:68–70 [DOI] [PubMed] [Google Scholar]
- 15.Pouliot MC, Despres JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, Nadeau A, Lupien PJ 1994. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol 73:460–468 [DOI] [PubMed] [Google Scholar]
- 16.Tank J, Schroeder C, Diedrich A, Szczech E, Haertter S, Sharma AM, Luft FC, Jordan J 2003. Selective impairment in sympathetic vasomotor control with norepinephrine transporter inhibition. Circulation 107:2949–2954 [DOI] [PubMed] [Google Scholar]
- 17.Heusser K, Tank J, Diedrich A, Engeli S, Klaua S, Kruger N, Strauss A, Stoffels G, Luft FC, Jordan J 2006. Influence of sibutramine treatment on sympathetic vasomotor tone in obese subjects. Clin Pharmacol Ther 79:500–508 [DOI] [PubMed] [Google Scholar]
- 18.Tank J, Jordan J, Diedrich A, Schroeder C, Furlan R, Sharma AM, Luft FC, Brabant G 2003. Bound leptin and sympathetic outflow in nonobese men. J Clin Endocrinol Metab 88:4955–4959 [DOI] [PubMed] [Google Scholar]
- 19.Rahmouni K, Haynes WG, Morgan DA, Mark AL 2003. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci 23:5998–6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiesner G, Vaz M, Collier G, Seals D, Kaye D, Jennings G, Lambert G, Wilkinson D, Esler M 1999. Leptin is released from the human brain: influence of adiposity and gender. J Clin Endocrinol Metab 84:2270–2274 [DOI] [PubMed] [Google Scholar]
- 21.Rahmouni K, Haynes WG, Morgan DA, Mark AL 2003. Intracellular mechanisms involved in leptin regulation of sympathetic outflow. Hypertension 41(3 Pt 2):763–767 [DOI] [PubMed]
- 22.Correia ML, Rahmouni K 2006. Role of leptin in the cardiovascular and endocrine complications of metabolic syndrome. Diabetes Obes Metab 8:603–610 [DOI] [PubMed] [Google Scholar]
- 23.Lambert E, Straznicky N, Eikelis N, Esler M, Dawood T, Masuo K, Schlaich M, Lambert G 2007. Gender differences in sympathetic nervous activity: influence of body mass and blood pressure. J Hypertens 25:1411–1419 [DOI] [PubMed] [Google Scholar]
- 24.Poehlman ET, Gardner AW, Goran MI, Arciero PJ, Toth MJ, Ades PA, Calles-Escandon J 1995. Sympathetic nervous system activity, body fatness, and body fat distribution in younger and older males. J Appl Physiol 78:802–806 [DOI] [PubMed] [Google Scholar]
- 25.Lawlor DA, Smith GD, Kelly A, Sattar N, Ebrahim S 2007. Leptin and coronary heart disease risk: prospective case control study of British women. Obesity (Silver Spring) 15:1694–1701 [DOI] [PubMed] [Google Scholar]
- 26.Weyer C, Pratley RE, Snitker S, Spraul M, Ravussin E, Tataranni PA 2000. Ethnic differences in insulinemia and sympathetic tone as links between obesity and blood pressure. Hypertension 36:531–537 [DOI] [PubMed] [Google Scholar]
- 27.Onat A, Avci GS, Barlan MM, Uyarel H, Uzunlar B, Sansoy V 2004. Measures of abdominal obesity assessed for visceral adiposity and relation to coronary risk. Int J Obes Relat Metab Disord 28:1018–1025 [DOI] [PubMed] [Google Scholar]
- 28.Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M 1997. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation 96:3423–3429 [DOI] [PubMed] [Google Scholar]
- 29.Esler M 1993. Clinical application of noradrenaline spillover methodology: delineation of regional human sympathetic nervous responses. Pharmacol Toxicol 73:243–253 [DOI] [PubMed] [Google Scholar]
- 30.Spraul M, Ravussin E, Fontvieille AM, Rising R, Larson DE, Anderson EA 1993. Reduced sympathetic nervous activity. A potential mechanism predisposing to body weight gain. J Clin Invest 92:1730–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunz I, Schorr U, Klaus S, Sharma AM 2000. Resting metabolic rate and substrate use in obesity hypertension. Hypertension 36:26–32 [DOI] [PubMed] [Google Scholar]