Abstract
Background: The contribution of multi-drug–resistant gram-negative bacilli infections (MDRGN-I) in patients with trauma is not well described. We present characteristics of MDRGN-Is among military personnel with deployment-related trauma (2009–2014).
Patients and Methods: Data from the Trauma Infectious Disease Outcomes Study were assessed for infectious outcomes and microbial recovery. Infections were classified using standardized definitions. Gram-negative bacilli were defined as multi-drug–resistant if they showed resistance to ≥3 antibiotic classes or were producers of extended-spectrum β-lactamase or carbapenemases.
Results: Among 2,699 patients admitted to participating U.S. hospitals, 913 (33.8%) experienced ≥1 infection event, of which 245 (26.8%) had a MDRGN-I. There were 543 MDRGN-I events (24.6% of unique 2,210 infections) with Escherichia coli (48.3%), Acinetobacter spp. (38.6%), and Klebsiella pneumoniae (8.4%) as the most common MDRGN isolates. Incidence of MDRGN-I was 9.1% (95% confidence interval [CI]: 8.0–10.2). Median time to MDRGN-I event was seven days with 75% occurring within 13 days post-trauma. Patients with MDRGN-Is had a greater proportion of blast injuries (84.1% vs. 62.5%; p < 0.0001), traumatic amputations (57.5% vs. 16.3%; p < 0.0001), and higher injury severity (82.0% had injury severity score ≥25 vs. 33.7%; p < 0.0001) compared with patients with either no infections or non-MDRGN-Is. Furthermore, MDRGN-I patients were more frequently admitted to the intensive care unit (90.5% vs. 48.5%; p < 0.0001), colonized with a MDRGN before infection (58.0% vs. 14.7%; p < 0.0001), and required mechanical ventilation (78.0% vs. 28.8% p < 0.0001). Antibiotic exposure before the MDRGN-I event was significantly higher across antibiotic classes except first generation cephalosporins and tetracyclines, which were very commonly used with all patients. Regarding outcomes, patients with MDRGN-Is had a longer length of hospitalization than the comparator group (53 vs. 18 days; p < 0.0001).
Conclusions: We found a high rate of MDRGN-I in our population characterized by longer hospitalization and greater injury severity. These findings inform treatment and infection control decisions in the trauma patient population.
Keywords: : gram-negative bacilli, multi-drug–resistant organisms, trauma-related infections
Infection with multi-drug–resistant organisms (MDROs) is of growing concern as development of resistance appears to be outpacing new antibiotic development [1–3]. These MDRO infections include organisms across the microbial spectrum and involve a myriad of sites and co-morbid conditions. Organisms of concern include methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci, Acinetobacter baumannii-calcoaceticus complex, and typical Enterobacteriaceae classified as extended-spectrum β-lactamase and carbapenemase producers.
Researchers have concentrated analyses primarily on intensive care unit (ICU) level populations and specific infection events, with few prospective studies focusing on patients with trauma [4–9]. It is commonly regarded that in addition to the aforementioned risk factors, outcomes such as length of hospital stay, cost, and death are all adversely affected by MDRO infections, particularly when empiric therapy is not appropriate for pathogen susceptibility. Identified risk factors for MDRO infections include colonization, states of immune compromise (e.g., hemodialysis and transplant recipients), previous antibiotic exposure ranging from three to six months pre-infection, previous hospitalization, and admission from long-term–care facilities [10–14]. The epidemiology behind MDRO infections in both combat- and noncombat-related trauma is poorly understood, given the challenges behind patient enrollment and study design.
The Department of Defense (DoD) has been engaged in combat operations for more than a decade in support of Operation Enduring Freedom (OEF) in Afghanistan and Operations Iraqi Freedom (OIF) and New Dawn in Iraq. Injured deployed personnel have experienced the lowest reported case fatality rate associated with battlefield trauma [15]. This trauma population benefited from advanced trauma management on the battlefield and an unprecedented medevac system through Landstuhl Regional Medical Center (LRMC; Germany) before transitioning to a military hospital in the United States [16]. In general, patients arrive at LRMC within 2.5 days of injury and are transported to the United States frequently within three days [17,18].
Although multi-drug–resistant gram-negative infections (MDRGN-I) were common during combat operations and complicated trauma management, initial research focused on surveillance of environmental sources and patient colonization to aid in infection control practices rather than describing the infected population's characteristics [19–21]. In our analysis, we use the largest cohort of military trauma patients ever studied to explore the epidemiologic characteristics associated with MDRGN-Is.
Patients and Methods
Study design
The DoD and Department of Veterans Affairs Multicenter Cohort Study evaluating Infection-Associated Clinical Outcomes in Hospitalized Medical Evacuees following Traumatic Injury (Trauma Infectious Diseases Outcomes Study [TIDOS]) was initiated in 2009 and continued through 2014 to collect standardized infection data prospectively on military personnel with deployment-related injuries [22]. Participating military hospitals in the United States include San Antonio Military Medical Center and Walter Reed National Military Medical Center (Walter Reed Army Medical Center and National Naval Medical Center before merge in September 2011).
Study population
Inclusion in the study population required medical evacuation from the combat theater to LRMC (Germany) for initial care before being transferred to a participating military hospital in the United States. Eligible patients were classified into two groups: (1) Non-MDRGN-I patients who experienced no infection events or infections with either non-resistant organisms or non-gram-negative organisms (e.g., vancomycin-resistant enterococci or MRSA) and (2) MDRGN-I patients who experienced an infection event associated with a MDRGN pathogen.
Clinical data collected from day of injury to discharge from the U.S. military hospital were assessed. General demographic data such as age, gender, branch of service, combat versus non-combat injury, rank, and operational theater (i.e., Iraq or Afghanistan) where trauma occurred were examined in the descriptive analysis. Variables related to trauma history, injury patterns, and clinical characteristics were collected through the DoD Trauma Registry [23] and include injury severity score (ISS; a standardized trauma assessment tool for grading aggregate anatomic severity of injury [24]), type of injury, mechanism of injury (e.g., gunshot and blast), ventilator use, ICU admission, and length of hospitalization. Information specific to infection diagnoses and microbiology were collected through the supplemental TIDOS Infectious Disease module [22].
Isolates were defined as MDRGN in accordance with Centers for Disease Control and Prevention (CDC) criteria as multi-drug–resistant if they showed resistance to ≥3 of four antibiotic classes or were producers of extended-spectrum β-lactamase or carbapenemases [25]. Clinical infection diagnoses were classified based on the standardized definitions of the CDC National Healthcare Safety Network [26]. In cases where the a priori criteria were not met, an infection was still included in the analysis if there was a record of a clinical diagnosis associated with directed antimicrobial treatment continued for a minimum of five days for skin and soft-tissue infections (SSTIs) and 21 days for osteomyelitis. Infectious disease events were excluded if there was documentation of an alternate diagnosis as well as discontinuation of antimicrobial therapy. Colonization was defined as MDRGN isolation on dedicated surveillance culture from axillary, rectal, or groin sampling. Isolates were classified as infecting if they were recovered from clinical infection workups.
Duration of antibiotic exposure required receipt of an antibiotic agent over continuous days. Receiving one or multiple doses within one day (24-hour period) was classified as one day of exposure, with follow-on doses accounting for additional days of exposure. Antibiotic agents were grouped by established classes as follows: Penicillin (PCN), PCN plus β-lactamase inhibitor, anti-pseudomonal PCN, cephalosporins (first, second, third, and fourth generation), fluoroquinolone, tetracyclines (includes doxycycline anti-malarial prophylaxis), clindamycin, carbapenems, aminoglycosides, and vancomycin. Combination exposure required meeting the defined duration criteria of any two or more classes during the period of study, but did not require concomitant dosing. Anti-parasitic (i.e., anti-malarial specific medication), anti-fungal, and topical agents were not included in the exposure analysis.
The end point of interest was the first MDRGN-I event as established by positive culture isolate collection date with distinction of clinical or microbiology diagnosis during analysis. A patient may have multiple MDRGN isolates associated with the same initial MDRGN diagnosis; however, follow-on MDRGN isolates and related diagnoses in the patient were not included in the analysis. Unless otherwise stated in the medical records, discharge from the U.S. military hospital served as the end of analysis for the systemic exposure of antibiotic agents.
Statistical analysis
All data were analyzed using SAS version 9.4 (SAS, Cary, NC). A descriptive analysis was performed using chi-square and Fisher exact tests for bivariable testing of categoric variables. The non-parametric Wilcoxon text was used to compare continuous variables. Antibiotic exposure duration criteria were defined as ≥2 days of continuous exposure.
Results
Characteristics and incidence
Between June 2009 and December 2014, 6,087 wounded military personnel were admitted to LRMC, of which 2,699 transferred to a participating U.S. military hospital and met criteria for inclusion in the analysis. A total of 913 (34%) patients received a diagnosis of an infection, of which 245 (27%) were identified to have an MDRGN-I event (Fig. 1). The overall incidence of MDRGN-Is among the trauma population was 9.1% (95% confidence interval [CI]: 8.0–10.2) with an incidence density of 0.65 (95% CI: 0.6–0.7) per 100 person-days. After transfer to a U.S. military hospital, 1,018 patients with trauma were admitted to the ICU, and a MDRGN-I developed in 193 (19.0% of 1,018; 78.8% of MDRGN-I patients). At LRMC, 391 were admitted to the ICU, of which 28 (11.4% of MDRGN-I patients) had a diagnosis of MDRGN-I. Among the 913 patients experiencing any type of infection, there were 2,210 unique infections diagnosed. For the analysis, patients with MDRGN-Is were compared with 2,454 patients with either no infection (n = 1786) or a non-MDRGN-I (n = 668).
FIG. 1.
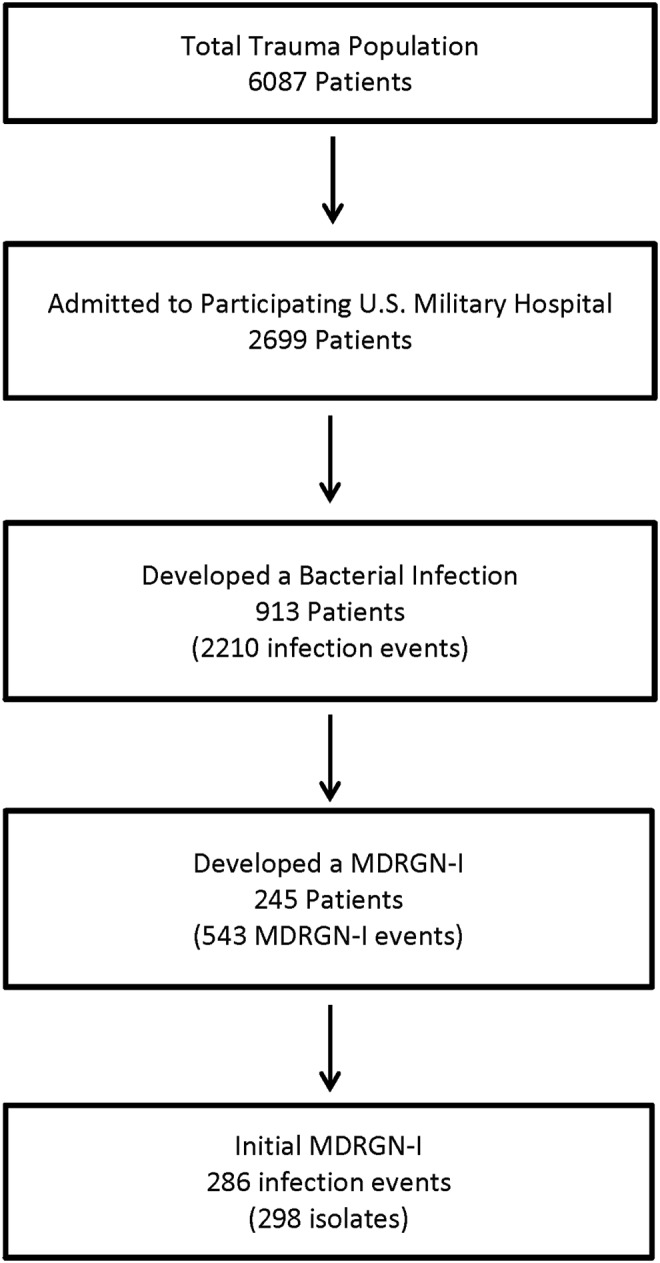
Flow diagram of study population and infection incidence. MDRGN-I = multi-drug–resistant gram-negative infection.
Patients in the analysis were young men with a median age of 23.7 years (interquartile range [IQR]: 21.8–27.2) for MDRGN-I patients and 24.7 years (IQR: 22.1–29.2) for patients in the comparator group. The majority of patients sustained combat-related trauma (85.7%) and were predominantly wounded in support of OEF in Afghanistan (87.9%; Table 1). In addition, 88.5% of the study population were enlisted personnel, and 64.0% served in the U.S. Army.
Table 1.
Demographic Characteristics, No. (%), of Wounded Military Personnel with and without Multi-drug–Resistant Gram-Negative Infections
| Infection type | ||||
|---|---|---|---|---|
| Characteristic | Total (N = 2,699) | None/Non-MDRGN-I (n = 2,454)a | MDRGN-I (n = 245) | p |
| Gender | 0.016 | |||
| Female | 56 (2.1) | 56 (2.3) | 0 | |
| Male | 2587 (95.8) | 2345 (95.6) | 242 (98.8) | |
| Missing | 56 (2.1) | 53 (2.2) | 3 (1.2) | |
| Military operationb | 0.009 | |||
| Iraqi Freedom | 132 (4.9) | 127 (5.2) | 5 (2.0) | |
| New Dawn | 63 (2.3) | 60 (2.4) | 3 (1.2) | |
| Enduring Freedom | 2373 (87.9) | 2141 (87.2) | 232 (94.7) | |
| Other | 1 (<0.1) | 1 (<0.1) | 0 | |
| Missing | 130 (4.8) | 125 (5.1) | 5 (2.0) | |
| Mounted status | <0.0001 | |||
| Dismounted (foot patrol) | 940 (34.8) | 802 (32.7) | 138 (56.3) | |
| Mounted | 738 (27.3) | 701 (28.6) | 37 (15.1) | |
| Missing | 1021 (37.8) | 951 (38.7) | 70 (28.6) | |
| Combat | <0.0001 | |||
| Non-combat | 329 (12.2) | 326 (13.3) | 3 (1.2) | |
| Combat | 2314 (85.7) | 2075 (84.6) | 239 (97.5) | |
| Missing | 56 (2.1) | 53 (2.2) | 3 (1.2) | |
| Rank | 0.328 | |||
| Civilian | 30 (1.1) | 30 (1.2) | 0 | |
| Enlisted | 2390 (88.6) | 2171 (88.5) | 219 (89.4) | |
| Officer | 174 (6.4) | 157 (6.4) | 17 (6.9) | |
| Warrant | 16 (0.6) | 15 (0.6) | 1 (0.4) | |
| Missing | 89 (3.3) | 81 (3.3) | 8 (3.3) | |
| Branch of Service | NA | |||
| Air Force | 107 (4.0) | 104 (4.2) | 3 (1.2) | |
| Army | 1728 (64.0) | 1584 (64.5) | 144 (58.8) | |
| Marine | 675 (25.0) | 591 (24.1) | 84 (34.3) | |
| Navy | 100 (3.7) | 89 (3.6) | 11 (4.5) | |
| Civilian | 1 (<0.1) | 1 (<0.1) | 0 | |
| Other | 32 (1.2) | 32 (1.3) | 0 | |
| Missing | 56 (2.1) | 53 (2.2) | 3 (1.2) | |
Comparator population includes 1,786 patients with no infection and 668 patients with a non-MDRGN-I event.
Operations Iraqi Freedom and New Dawn were based in Iraq. Operating Enduring Freedom was based in Afghanistan.
MDRGN-I = multi-drug–resistant gram-negative infections.
A greater proportion of MDRGN-I patients were treated in the ICU (90.5% vs. 48.5%; p < 0.0001; Table 2) and had MDRGN colonization before infection (58.0% vs. 14.7%; p < 0.0001) compared with the patients with no infection or non-MDRGN-Is. In addition, MDRGN-I patients had greater injury severity (82.0% with critical injuries vs. 33.7%; p < 0.0001), sustained more blast-related trauma (84.1% vs. 62.5%; p < 0.0001), and experienced a larger proportion of traumatic or early surgical amputations (57.5% vs. 16.3%; p < 0.0001). Among the MDRGN-I patients, 78.0% required mechanical ventilation during their hospitalization, which is higher compared with the patients with no infection or non-MDRGN-Is (28.8%; p < 0.0001). Patients with a MDRGN-I event had a significantly longer period of hospitalization when compared with patients with no infections or non-MDRGN-I events (median of 53 d vs. 18 d; p < 0.0001).
Table 2.
Clinical Characteristics, No. (%), of Military Trauma Patients with and without Multi-drug–Resistant Gram-Negative Infections
| Infection type | ||||
|---|---|---|---|---|
| Characteristics | Total (N = 2,699) | None/Non-MDRGN-I (n = 2,454)a | MDRGN-I (n = 245) | p |
| ICU Admission | <0.0001 | |||
| LRMC only | 391 (14.5) | 363 (14.8) | 28 (11.4) | |
| U.S. hospital ± LRMC | 1018 (37.7) | 825 (33.6) | 193 (78.8) | |
| None | 1284 (47.6) | 1261 (51.4) | 23 (9.4) | |
| Missing | 6 (0.2) | 5 (0.2) | 1 (0.4) | |
| Injury Severity Score | <0.0001 | |||
| 0–9 (mild) | 728 (27.0) | 724 (29.5) | 4 (1.6) | |
| 10–14 (moderate) | 363 (13.4) | 352 (14.3) | 11 (4.5) | |
| 15–24 (severe) | 523 (19.4) | 497 (20.2) | 26 (10.6) | |
| ≥ 25 (critical) | 1029 (38.1) | 828 (33.7) | 201 (82.0) | |
| Missing | 56 (2.1) | 53 (2.2) | 3 (1.2) | |
| Blast injury | 1740 (64.5) | 1534 (62.5) | 206 (84.1) | <0.0001 |
| Improvised explosive device (IED) | 1399 (51.8) | 1212 (49.4) | 187 (76.3) | <0.0001 |
| Non-IED | 341 (12.6) | 322 (13.1) | 19 (7.8) | |
| Non-blast injury | 0.058 | |||
| Gunshot wound | 448 (16.6) | 424 (17.3) | 24 (9.8) | |
| Other | 511 (18.9) | 496 (20.2) | 15 (6.1) | |
| Early surgical/traumatic amputation | 541 (20.0) | 400 (16.3) | 141 (57.5) | <0.0001 |
| Any fracture | 2034 (75.4) | 1815 (74.0) | 219 (89.4) | <0.0001 |
| Inpatient ventilation | <0.0001 | |||
| LRMC only | 406 (15.0) | 351 (14.3) | 55 (22.4) | |
| U.S. hospital only | 16 (0.6) | 13 (0.5) | 3 (1.2) | |
| 1–2 week duration | 2 (0.1) | 2 (0.1) | 0 | |
| LRMC and U.S. hospital | ||||
| ≤1week duration | 465 (17.2) | 335 (13.6) | 130 (53.1) | |
| ≥2 week duration | 8 (0.3) | 5 (0.2) | 3 (1.2) | |
| None | 1802 (66.8) | 1748 (71.2) | 54 (22.0) | |
| Death | 29 (1.1) | 20 (0.8) | 9 (3.7) | 0.001 |
| MDRGN colonization | 504 (18.7) | 362 (14.7) | 142 (58.0) | <0.0001 |
| Antibiotic use for ≥2 d | ||||
| Fluoroquinolone | 940 (34.8) | 826 (33.7) | 114 (46.5) | <0.0001 |
| First generation cephalosporins | 1872 (69.4) | 1692 (68.9) | 180 (73.5) | 0.143 |
| Tetracycline | 2230 (82.6) | 2038 (83.0) | 192 (78.4) | 0.065 |
| Aminoglycosides | 242 (9.0) | 204 (8.3) | 38 (15.5) | <0.001 |
| Anti-pseudomonal PCN | 293 (10.9) | 245 (10.0) | 48 (19.6) | < 0.0001 |
| Vancomycin | 713 (26.4) | 609 (24.8) | 104 (42.4) | <0.0001 |
| Carbapenem | 537 (19.9) | 446 (18.2) | 91 (37.1) | <0.0001 |
| Cephalosporin + fluoroquinolone | 747 (27.7) | 657 (26.8) | 90 (36.7) | <0.001 |
| Anti-pseudomonal PCN + aminoglycoside | 66 (2.4) | 54 (2.2) | 12 (4.9) | 0.009 |
| Vancomycin + anti-pseudomonal PCN | 199 (7.4) | 167 (6.8) | 32 (13.1) | <0.001 |
| Vancomycin + carbapenem | 448 (16.6) | 370 (15.1) | 78 (31.8) | <0.0001 |
Comparator population includes 1,786 patients with no infection and 668 patients with a non-MDRGN-I event.
MDRGN-I = multi-drug–resistant gram-negative infections; ICU = intensive care unit; LRMC = Landstuhl Regional Medical Center; PCN = penicillin.
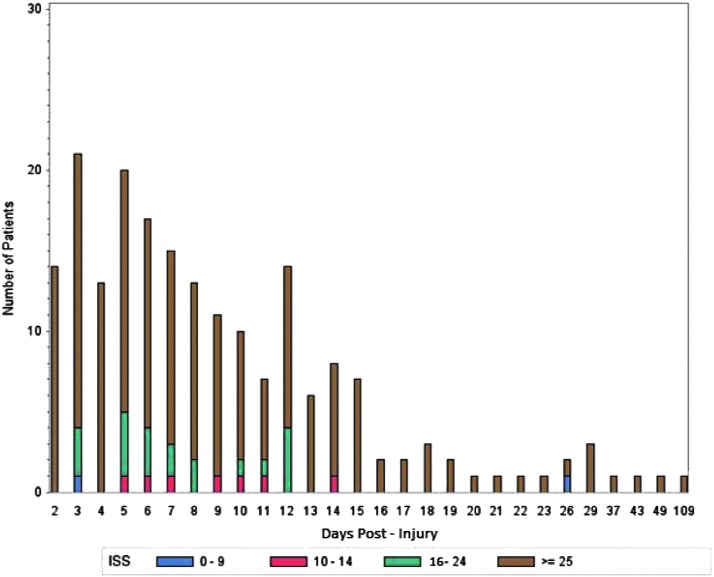
The median time to MDRGN-I event was seven days post-injury (IQR: 4–13 d). As stated, injury severity was high for MDRGN-I patients (Fig. 2) with a relative risk for MDRGN-I of 10.6 (95% CI: 6.3–17.8) associated with an ISS >15. When admission unit (ICU vs. ward) at LRMC before transfer to a participating U.S. military hospital was examined, admission to the ICU carried a relative risk of 8.7 (95% CI: 5.7–13.4) for MDRGN-I.
FIG. 2.
Time (days) from injury to first multi-drug–resistant gram-negative culture per patient, stratified by injury severity score (ISS), which is a standardized trauma assessment tool for grading aggregate anatomic severity of injury. ISS was calculated within 15 days of injury. ISS 0–9 are mild injuries, 10–14 are moderate injuries, 15–24 are severe injuries, and ≥25 are critical injuries.
Antibiotic exposure
Among the patients with MDRGN-Is, 238 (97.1%) received at least one antibiotic agent with the majority sustaining critical injuries (81.9% with ISS ≥25), while a lesser number had severe injuries (10.9% with ISS 15–24). In addition, 38.5% of patients with severe injuries received antibiotic agents from 1–2 classes followed by 30.8% with antibiotic agents from 3–4 classes. Patients with MDRGN-Is and critical injuries primarily received antibiotic agents from 3–4 classes (37.8%), while 24.4%, 23.4%, and 9.5% were prescribed antibiotic agents from 5–6, 1–2, and 7–8 classes, respectively.
The distribution of antibiotic classes received by the two groups during the first two weeks post-injury was examined (Fig. 3). Among patients with MDRGN-I events, the use of tetracycline and first generation cephalosporins decreased from 91% during the first week after injury to 45%–48% in the second week. A similar decrease was observed with the no infection/non-MDRGN-I event patients (tetracycline: 80%–32%; first generation cephalosporins: 78%–39%). In addition, 49% of MDRGN-I patients received carbapenems during the first week compared with 62% during the second, while there was no change among patients in the comparator group. Use of fluoroquinolones, clindamycin, and PCN plus inhibitors also decreased during the period for both the MDRGN-I and no infection/non-MDRGN-I patients; however, use of vancomycin increased only in the MDRGN-I group.
FIG. 3.
Proportion of antibiotic classes received by patients with multi-drug–resistant Gram-negative infections (MDRGN-I; n = 245) and either no infection or a non-MDRGN-I (n = 2,454). Data are restricted to antibiotic classes prescribed within the first two weeks post-injury: (A) Days 0–7 post-injury and (B) days 8–14 post-injury. Gen = generation; PCN = penicillin.
Bivariable testing showed significant differences in distribution of antibiotic agents prescribed for ≥2 days for both antibiotic agents prescribed alone and in clinically relevant combinations (Table 2), except for first generation cephalosporins and tetracyclines, both commonly used for peri-operative prophylaxis and anti-malarial prophylaxis, respectively. Broad-spectrum and other selected potent antibiotic agents prescribed to patients with MDRGN-Is and those with no infection or a non-MDRGN-Is included vancomycin (42.4% vs. 24.8%; p < 0.0001), anti-pseudomonal PCN (19.6% vs. 10.0%; p < 0.0001), aminoglycosides (15.5% vs. 8.3%; p < 0.001), carbapenems (37.1% vs. 18.2%; p < 0.0001), and fluoroquinolones (46.5% vs. 33.7%; p < 0.0001).
Examination of common or selected antibiotic class combinations of interest used for two or more days resulted in the following distributions: First generation cephalosporin and fluoroquinolone (36.7% vs. 26.8%; p < 0.001), anti-pseudomonal PCN and aminoglycoside (4.9% vs. 2.2%; p < 0.009), vancomycin and anti-pseudomonal PCN (13.1% vs. 6.8%; p < 0.001), and vancomycin and carbapenem (31.8% vs. 15.1%; p < 0.0001) for MDRGN-I compared with patients with no infection or non-MDRGN-I. Assessing cumulative class exposure of antibiotic at two or more days demonstrated a trend (p < 0.001) for greater class exposure for MDRGN-I patients (Fig. 4).
FIG. 4.
Distribution of number of antibiotic class combinations that were received for at least two days by patients with a multi-drug–resistant gram-negative infection (MDRGN-I) or no infection/non-MDRGN-I (columns). Antifungal agents, anti-parasitic agents, and topical agents are excluded. For each antibiotic class distribution, the percentage of patients out of the total number of MDRGN-I (n = 245) and no-infection/non-MDRGN-I patients (n = 2,454) is presented (lines).
Clinical diagnoses
Among the 913 patients who had at least one infecting event, 245 (26.8%) patients experienced an MDRGN-I with a total of 543 unique infections (24.6% of 2,210 unique infection events). Specifically, 286 (53% of 543) were initial MDRGN-I events with diagnoses of SSTIs (47.9%) being predominant, followed by pneumonia (19.6%), blood stream infections (BSI) (10.8%), sepsis/other (7.3%), and osteomyelitis (7.7%; Fig. 5A). One central nervous system MDRGN-I event was also recorded.
FIG. 5.
Distribution of infection syndrome. (A) Stratified by multi-drug–resistant gram-negative (MDRGN) status. Proportion of infection events with either a MGRGN infection (MDRGN-I) or a non-MDRGN-I is listed next to each column. (B) Stratified by bacterial organism. SSTI = skin and soft-tissue infections; BSI = blood stream infections; UTI = urinary tract infections; CNS = central nervous system infections; PNA = pneumonia; osteo = osteomyelitis.
Microbiology
A total of 298 MDRGN isolates were obtained from 286 initial unique infection events. Escherichia coli (n = 144; 48.3%), Acinetobacter spp. (n = 115; 38.6%), and Klebsiella pneumoniae (n = 25; 8.4%) (Fig. 5B) were most commonly isolated. Pseudomonas spp. (n = 11; 3.7%) and Enterobacter spp. (n = 3; 1.0%) were the least common isolates meeting the criteria for MDRGN.
Discussion
The TIDOS project represents the largest prospective collection of data dedicated to evaluating infections and outcomes in a unique trauma population of 2,699 U.S. service members injured during deployment-related operations. This population affords a unique opportunity to date to study the epidemiology and predictors of MDRGN-I events in a trauma population.
The overall incidence of MDRGN-I events among wounded military personnel over the five-year study period was 9.1%. Not only does this study include ICU patients, but also, unlike previous analyses, we assessed patients admitted to non-critical care wards with less severe traumatic injuries. When restricted to patients admitted to the ICU in the United States, the proportion with an MDRGN-I was high at 19.0%. In comparison, a retrospective patient review in trauma patients with pneumonia determined a multi-drug–resistant incidence for primary infection at 30.6%; however, the authors were not clear in their multi-drug–resistant definitions and their estimate includes multi-drug–resistant gram-positive organisms in their trauma ICU population [27]. A French retrospective cohort of 28 combat trauma patients experienced an MDRGN-I rate of 25% with 57.1% MDRGN colonization over an estimated pre-deployment baseline of 2% [28]. Furthermore, one center in Johannesburg reported a 63% multi-drug–resistant infection rate as part of a prospective, single center, 16-month cohort consisting of 73 trauma ICU patients with high composite ISS [7].
When looking more closely at our subpopulation of patients with any infection event (n = 913), an MDRGN-I occurred in 26.8% of patients and for 18.9% (n = 173) of patients, this was their initial infection event. This high proportion of MDRGN-I within the population may inform approaches to antibiotic selection when a patient has clinical signs of evolving infection and high injury severity.
Illustrating the effect of complex polytrauma, 92.7% of MDRGN-I patients were categorized with an ISS ≥15, and 82% of these with an ISS ≥25 compared with 54.0% and 33.7%, respectively, among the patients with no infections or non-MDRGN-Is. Furthermore, 92.9% of the MDRGN-I patients who received an antibiotic agent had an ISS ≥15 (81.9% with ISS ≥25). These findings match smaller analyses previously published linking mangled extremities as being at high risk for infection, as well as an association with injury severity [29,30].
The epidemiology of MDRGN colonization has been examined early on during combat operations as part of infection control measures, as well as through previous analyses [31]. In our analysis, antecedent MDRGN colonization was higher in MDRGN-I patients compared with those with no infections or non-MDRGN-I events (58% and 14.7%, respectively). These surveillance cultures were obtained via axillary or groin swabs. Previous analysis found that 6.6% of U.S. wounded personnel admitted to LRMC between 2009 and 2012 were colonized with MDRGN bacilli [32]. Among patients who transferred to participating hospitals in the United States, the proportion colonized at admission with MDRGN bacilli was reported between 12.4% and 14% [31,32].
The DoD, along with foreign military partners, has pursued epidemiologic studies of MDRO surveillance data as it involves integrated care and a multi-faceted approach to prevention of infection after battlefield trauma [20,21,33,34]. Early wound bacteriology studies revealed minimal presence of MDROs, and follow-up epidemic investigations support a nosocomial basis for MDRO infections that complicate wound management and influenced infection control efforts in DoD facilities [21,35–39]. This work has led to discussions of appropriate post-trauma prophylaxis and updates to the Joint Trauma System DoD clinical practice guidelines for prevention of combat trauma-related infections [40]. Our findings support a correlation between MDRGN colonization and subsequent infection and, given that this population had minimal to no previous contact with the health system before injury, these organisms were acquired in the combat casualty care system, which consists of medical evacuation and transition through multiple military treatment facilities/hospitals.
The frequent use of systemic antibiotic agents, including combination therapy in our trauma population, is readily apparent with significant differences in exposure for each class by study subgroup. Figures 3 and 4 illustrate higher use of several antibiotic agents (e.g., anti-pseudomonal PCN and carbapenems) and that multi-class exposure is common. Poole et al. [41] highlight the risk of wound colonization with resistant bacteria in surgical patients after 48 hours of prophylactic antibiotic agents, likely a common circumstance in our patient population. In addition, an analysis of injured service members found that 14% of 2,079 trauma admissions were colonized with MDRGN bacilli at admission to participating hospitals in the United States, of which 98% received antibiotic agents before collection of surveillance swabs (≤2 d of hospital admission). The majority of colonized patients received more than one antibiotic agent with cefazolin (with/without other antibiotic agents) being predominant (93%). When examined in a logistic regression model, use of cefazolin (with/without fluoroquinolones) was independently associated with risk of MDRGN bacilli colonization [31].
With this analysis, we are unable to answer questions behind the risk associated with specific antibiotic agents because our study only begins to describe antibiotic exposure as a risk for MDRGN-I, either as a single class or in combination. It is worth noting that 75% of MDRGN-I occurred by day 13, representing a short period of rapid transfer of care between facilities and multiple providers further complicating assessment of clinical variables.
The clinical diagnoses and related isolates within our trauma population (Fig. 5A,B) are congruent with the types of trauma experienced on the battlefield. Specifically, 64.5% of injuries were tied to a blast event with SSTIs, pneumonia, BSIs, osteomyelitis, and urinary tract infections being the most common infection events. In addition, pneumonia (n = 58 MDRGN-I, 19.5%), osteomyelitis (n = 23 MDRGN-I; 7.7%), and SSTIs (n = 143 MDRGN-I; 48.0%) contributed the largest proportion of MDRGN isolates. The most frequently isolated organisms affecting patients were E. coli and Acinetobacter spp. Pseudomonas spp., a common etiologic agent, was underrepresented in the population because of a low proportion of multi-drug resistance.
Research on trauma-related MDRO infections is limited frequently by small numbers, involves a single center, and is retrospective in design. A recent review compared general battlefield trauma epidemiology from OEF and OIF to the Vietnam experience and discussed risk factors for infection, but not the circumstances surrounding MDRO infections [16]. Summarizing earlier analyses, authors cite predictors for infection as: Operation(s) before U.S. hospital admission, higher ISS, blast injuries, abdominal soft-tissue wounds, ≥3 injury locations, and loss of limb(s) [19,22,30,42]. No analyses prospectively describing injury circumstances, clinical course of illness, or predictors for MDRO infections have been performed either within or outside of the U.S. military.
Data are emerging from developing countries on predictors of MDRO infection where increased incidence of trauma correlates with civil unrest. Researchers from South Africa recently reported on polytrauma patients with a larger percent of injuries occurring in middle-aged adults as a result of motor vehicle or motorbike accidents. Initial analysis shows older patients being at greatest risk of MDRO infection with associated increase in length of stay and death [7]. Younger patient populations have been assessed in cross-sectional studies in trauma centers located in areas of conflict including Syria, Iraq, and Libya. Similar epidemiologic patterns were found in MDRO infections and polymicrobial infections and associated with delayed surgical closure of wounds and use of indwelling medical devices [6,9,43].
Our data support previous published findings that MDRGN organisms are acquired during the continuum of casualty care. Research has also demonstrated that patients who had not received antibiotic agents largely had no growth at all and those who received early field management with or without antibiotic agents grew skin commensals with no reported drug resistance [38]. This finding was further reinforced by a wound surveillance study published by Sheppard et al. [37]. The acquisition of MDRGN organisms as a nosocomial process early in the combat casualty care system is supported by infection control work assessing both potential patient reservoirs and environmental contamination [18,20].
Our analysis identified a higher prevalence of MDRGN carriage in severely injured patients before the initial MDRGN-I event with the median time to MDRGN event being seven days, with 75% of MDRGN-I events occurring by 13 days post-trauma. The resultant adverse impact of these MDRGN-I events is seen by the significantly longer period of hospitalization compared with the patients with no infections or non-MDRGN-I events (53 d vs. 18 d, respectively). These prolonged hospitalizations ostensibly allow for opportunities of hospital-associated transmission. The length of hospitalization is intuitively tied to escalating injury severity and, as the complexity of clinical care needs also increases (i.e., ventilation and repeated operations), these confounding variables likewise add to the risk of MDRGN-I.
Our study population, while benefiting from a multi-center, prospective design and large population, is a post hoc analysis that has some limitations. Nevertheless, our population is younger and with minimal to no co-morbid conditions because of health requirements to make a service member eligible for combat deployments, limiting confounding factors. Furthermore, the comparator group includes patients with non-MDRGN-I events as well as no infections. Based on the findings of our analysis, further analysis and modeling using a comparator population of patients with non-MDRGN-I events are warranted.
Conclusion
Trauma patients have a significant burden of MDRGN-Is, particularly those with high injury severity. For many patients, the MDRGN-I was their first or only infection after trauma, which is interesting from the aspect that we are studying a population whose exposure and previous healthcare contact is limited with exposure risks occurring during the medevac and early hospitalization period. Furthermore, the impact of MDRGN-Is is noticeable in significantly longer hospitalization; however, other factors (e.g., injury severity) may also be a factor in the length of hospitalization.
The MDRGN-I patients have significantly higher injury severity as evidenced by ISS (≥15), traumatic amputations, mechanical ventilation, and ICU admission. The MDRGN-I patients also have a higher proportion of MDRGN colonization before infection and greater exposure to all classes of antibiotic agents. When specifically looking at previous published predictors for MDRGN-Is in the medical literature, including non-trauma patients, the definition of previous antibiotic exposure is based on patient recall and has definitions of within the last 3–6 months while our cohort of patients' exposure occurred during the immediate post-trauma period. We cannot speak to whether these variables are indeed predictors of MDRGN-Is, but these findings lay the groundwork for more detailed modeling. This initial analysis clearly describes the characteristics of a trauma patient who is likely to experience an MDRGN-I and inform infection control measures and antibiotic use considerations.
Contributor Information
Collaborators: The Infectious Disease Clinical Research Program Trauma Infectious Disease Outcomes Study Group
Acknowledgments
We are indebted to the Infectious Disease Clinical Research Program TIDOS study team of clinical coordinators, microbiology technicians, data managers, clinical site managers, and administrative support personnel for their tireless hours to ensure the success of this project.
This work (IDCRP-024) was supported by the Infectious Disease Clinical Research Program, a Department of Defense program executed through the Uniformed Services University of the Health Sciences, Department of Preventive Medicine and Biostatistics. This project has been funded by the National Institute of Allergy and Infectious Diseases, National Institute of Health [Inter-Agency Agreement Y1-AI-5072], and the Department of the Navy under the Wounded, Ill, and Injured Program [HU001-10-1-0014].
Author Disclosure Statement
No competing financial interests exist.
The views expressed are those of the authors and does not necessarily reflect the official views of the Uniformed Services University of the Health Sciences, Henry M. Jackson Foundation for the Advancement of Military Medicine, National Institutes of Health or the Department of Health and Human Services, Walter Reed National Military Medical Center, Landstuhl Regional Medical Center, Brooke Army Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of Defense or the Departments of the Army, Navy or Air Force. Mention of trade names, commercial products, or organization does not imply endorsement by the U.S. Government.'
References
- 1.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Atlanta, GA: www.cdc.gov/drugresistance/threat-report-2013 2013. (Last accessed December12, 2016) [Google Scholar]
- 2.The President's Council of Advisors on Science and Technology. Report to the President on Combatting Antibiotic Resistance. www.whitehouse.gov/sites/default/files/microsites/ostp/PCAST/pcast_carb_report_sept2014.pdf 2014. (Last accessed December12, 2016)
- 3.Nathan C, Cars O. Antibiotic resistance—problems, progress, and prospects. N Engl J Med 2014;371:1761–1763 [DOI] [PubMed] [Google Scholar]
- 4.Dortch MJ, Fleming SB, Kauffmann RM, et al. Infection reduction strategies including antibiotic stewardship protocols in surgical and trauma intensive care units are associated with reduced resistant gram-negative healthcare-associated infections. Surg Infect (Larchmt) 2011;12:15–25 [DOI] [PubMed] [Google Scholar]
- 5.Jean SS, Ko WC, Xie Y, et al. Clinical characteristics of patients with community-acquired complicated intra-abdominal infections: A prospective, multicentre, observational study. Int J Antimicrob Agents 2014;44:222–228 [DOI] [PubMed] [Google Scholar]
- 6.Zorgani A, Abofayed A, Glia A, et al. Prevalence of device-associated nosocomial infections caused by gram-negative bacteria in a trauma intensive care unit in Libya. Oman Med J 2015;30:270–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pillai J, Yazicioglu C, Moeng S, et al. Prevalence and patterns of infection in critically ill trauma patients admitted to the trauma ICU, South Africa. J Infect Dev Ctries 2015;9:736–742 [DOI] [PubMed] [Google Scholar]
- 8.Duane TM, Kikhia RM, Wolfe LG, et al. Understanding gram-negative central line-associated blood stream infection in a surgical trauma ICU. Am Surg 2015;81:816–819 [PubMed] [Google Scholar]
- 9.Teicher CL, Ronat JB, Fakhri RM, et al. Antimicrobial drug-resistant bacteria isolated from Syrian war-injured patients, August 2011-March 2013. Emerg Infect Dis 2014;20:1949–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papst L, Beovic B, Seme K, Pirs M. Two-year prospective evaluation of colonization with extended-spectrum beta-lactamase-producing Enterobacteriaceae: Time course and risk factors. Infect Dis (Lond) 2015;47:618–624 [DOI] [PubMed] [Google Scholar]
- 11.Qureshi ZA, Paterson DL, Pakstis DL, et al. Risk factors and outcome of extended-spectrum beta-lactamase-producing Enterobacter cloacae bloodstream infections. Int J Antimicrob Agents 2011;37:26–32 [DOI] [PubMed] [Google Scholar]
- 12.Rottier WC, Bamberg YR, Dorigo-Zetsma JW, et al. Predictive value of prior colonization and antibiotic use for third-generation cephalosporin-resistant Enterobacteriaceae bacteremia in patients with sepsis. Clin Infect Dis 2015;60:1622–1630 [DOI] [PubMed] [Google Scholar]
- 13.Saely S, Kaye KS, Fairfax MR, et al. Investigating the impact of the definition of previous antibiotic exposure related to isolation of extended spectrum beta-lactamase-producing Klebsiella pneumoniae. Am J Infect Control 2011;39:390–395 [DOI] [PubMed] [Google Scholar]
- 14.Kaye KS, Pogue JM. Infections caused by resistant gram-negative bacteria: Epidemiology and management. Pharmacotherapy 2015;35:949–962 [DOI] [PubMed] [Google Scholar]
- 15.Holcomb JB, Stansbury LG, Champion HR, et al. Understanding combat casualty care statistics. J Trauma 2006;60:397–401 [DOI] [PubMed] [Google Scholar]
- 16.Blyth DM, Yun HC, Tribble DR, Murray CK. Lessons of war: Combat-related injury infections during the Vietnam War and Operation Iraqi and Enduring Freedom. J Trauma Acute Care Surg 2015;79(Suppl 2):S227–S235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manring MM, Hawk A, Calhoun JH, Andersen RC. Treatment of war wounds: A historical review. Clin Orthop Relat Res 2009;467:2168–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yun HC, Murray CK, Roop SA, et al. Bacteria recovered from patients admitted to a deployed U.S. military hospital in Baghdad, Iraq. Mil Med 2006;171:821–825 [DOI] [PubMed] [Google Scholar]
- 19.Petersen K, Riddle MS, Danko JR, et al. Trauma-related infections in battlefield casualties from Iraq. Ann Surg 2007;245:803–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ake J, Scott P, Wortmann G, et al. Gram-negative multidrug-resistant organism colonization in a US military healthcare facility in Iraq. Infect Control Hosp Epidemiol 2011;32:545–552 [DOI] [PubMed] [Google Scholar]
- 21.Hospenthal DR, Crouch HK, English JF, et al. Multidrug-resistant bacterial colonization of combat-injured personnel at admission to medical centers after evacuation from Afghanistan and Iraq. J Trauma 2011;71(Suppl):S52–S57 [DOI] [PubMed] [Google Scholar]
- 22.Tribble DR, Conger NG, Fraser S, et al. Infection-associated clinical outcomes in hospitalized medical evacuees after traumatic injury: Trauma Infectious Disease Outcome Study. J Trauma 2011;71(Suppl):S33–S42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eastridge BJ, Jenkins D, Flaherty S, et al. Trauma system development in a theater of war: Experiences from Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma 2006;61:1366–1372 [DOI] [PubMed] [Google Scholar]
- 24.Linn S. The injury severity score—importance and uses. Ann Epidemiol 1995;5:440–446 [DOI] [PubMed] [Google Scholar]
- 25.Division of Healthcare Quality Promotion. Multidrug-Resistant Organism and Clostridium difficile Infection (MDRO/CDI) Module. www.cdc.gov/nhsn/PDFs/pscManual/12pscMDRO_CDADcurrent.pdf 2016. (Last accessed December12, 2016)
- 26.Centers for Disease Control and Prevention. CDC/NHSN Surveillance Definitions for Specific Types of Infections. www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf 2014. (Last accessed December12, 2016)
- 27.Becher RD, Hoth JJ, Neff LP, et al. Multidrug-resistant pathogens and pneumonia: Comparing the trauma and surgical intensive care units. Surg Infect (Larchmt) 2011;12:267–272 [DOI] [PubMed] [Google Scholar]
- 28.Bousquet A, Martinaud C, MacNab C. Multidrug-resistant bacteria from personnel with combat injury at a French military medical center. J Trauma Acute Care Surg 2012;72:1723–1724 [DOI] [PubMed] [Google Scholar]
- 29.Brown KV, Murray CK, Clasper JC. Infectious complications of combat-related mangled extremity injuries in the British military. J Trauma 2010;69(Suppl 1):S109–S115 [DOI] [PubMed] [Google Scholar]
- 30.Murray CK, Wilkins K, Molter NC, et al. Infections complicating the care of combat casualties during operations Iraqi Freedom and Enduring Freedom. J Trauma 2011;71(Suppl 1):S62–S73 [DOI] [PubMed] [Google Scholar]
- 31.Gilbert LJ, Li P, Murray CK, et al. Multidrug-resistant gram-negative bacilli colonization risk factors among trauma patients. Diagn Microbiol Infect Dis 2016;84:358–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weintrob AC, Murray CK, Lloyd B, et al. Active surveillance for asymptomatic colonization with multidrug-resistant gram negative bacilli among injured sevice members—a three year evaluation. MSMR 2013;20:17–22 [PMC free article] [PubMed] [Google Scholar]
- 33.Chandrasekera RM, Lesho EP, Chukwuma U, et al. The state of antimicrobial resistance surveillance in the Military Health System: A review of improvements made in the last 10 years and remaining surveillance gaps. Mil Med 2015;180:145–150 [DOI] [PubMed] [Google Scholar]
- 34.Merens A, Rapp C, Delaune D, et al. Prevention of combat-related infections: Antimicrobial therapy in battlefield and barrier measures in French military medical treatment facilities. Travel Med Infect Dis 2014;12:318–329 [DOI] [PubMed] [Google Scholar]
- 35.Hospenthal DR, Crouch HK, English JF, et al. Response to infection control challenges in the deployed setting: Operations Iraqi and Enduring Freedom. J Trauma 2010;69(Suppl 1):S94–S101 [DOI] [PubMed] [Google Scholar]
- 36.Lloyd BA, Weintrob AC, Hinkle MK, et al. Adherence to published antimicrobial prophylaxis guidelines for wounded service members in the ongoing conflicts in Southwest Asia. Mil Med 2014;179:324–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheppard FR, Keiser P, Craft DW, et al. The majority of US combat casualty soft-tissue wounds are not infected or colonized upon arrival or during treatment at a continental US military medical facility. Am J Surg 2010;200:489–495 [DOI] [PubMed] [Google Scholar]
- 38.Murray CK, Roop SA, Hospenthal DR, et al. Bacteriology of war wounds at the time of injury. Mil Med 2006;171:826–829 [DOI] [PubMed] [Google Scholar]
- 39.Tribble DR, Lloyd B, Weintrob A, et al. Antimicrobial prescribing practices following publication of guidelines for the prevention of infections associated with combat-related injuries. J Trauma 2011;71(Suppl 2):S299–S306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hospenthal DR, Murray CK, Andersen RC, et al. Guidelines for the prevention of infections associated with combat-related injuries: 2011 update: Endorsed by the Infectious Diseases Society of America and the Surgical Infection Society. J Trauma 2011;71(Suppl 2):S210–S234 [DOI] [PubMed] [Google Scholar]
- 41.Poole D, Chieregato A, Langer M, et al. Systematic review of the literature and evidence-based recommendations for antibiotic prophylaxis in trauma: Results from an Italian consensus of experts. PLoS One 2014;9:e113676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murray CK. Epidemiology of infections associated with combat-related injuries in Iraq and Afghanistan. J Trauma 2008;64(Suppl):S232–S238 [DOI] [PubMed] [Google Scholar]
- 43.Othman N, Babakir-Mina M, Noori CK, Rashid PY. Pseudomonas aeruginosa infection in burn patients in Sulaimaniyah, Iraq: Risk factors and antibiotic resistance rates. J Infect Dev Ctries 2014;8:1498–1502 [DOI] [PubMed] [Google Scholar]