Abstract
Graves' disease is an autoimmune disease that results in and is the most common cause of hyperthyroidism, and the reactivation of persisting Epstein–Barr virus (EBV) in B lymphocytes induces the differentiation of host B cells into plasma cells. We previously reported that some EBV-infected B cells had thyrotropin receptor antibodies (TRAbs) as surface immunoglobulins (Igs), and EBV reactivation induced these TRAb+EBV+ cells to produce TRAbs. EBV reactivation induces Ig production from host B cells. The purpose of the present study was to examine total Ig productions from B cell culture fluids and to detect activation-induced cytidine deaminase (AID), nuclear factor kappa B (NF-κB), and EBV latent membrane protein (LMP) 1 in culture B cells during EBV reactivation induction and then we discussed the mechanisms of EBV reactivation-induced Ig production in relation to autoimmunity. We showed that the EBV reactivation induces the production of every isotype of Ig and suggested that the Ig production was catalyzed by AID through LMP1 and NF-κB. The results that the amount of IgM was significantly larger compared with IgG suggested the polyclonal B cell activation due to LMP1. We proposed the pathway of EBV reactivation induced Ig production; B cells newly infected with EBV are activated by polyclonal B cell activation and produce Igs through plasma cell differentiation induced by EBV reactivation. LMP1-induced AID enabled B cells to undergo class-switch recombination to produce every isotype of Ig. According to this mechanism, EBV rescues autoreactive B cells to produce autoantibodies, which contribute to the development and exacerbation of autoimmune diseases.
Keywords: : Epstein–Barr virus, Graves' disease, reactivation, polyclonal B cell activation, activation-induced cytidine deaminase, autoreactive B cells
Introduction
Graves' disease is an autoimmune disease that results in and is the most common cause of hyperthyroidism. Patients with Graves' disease have serum thyrotropin receptor antibodies (TRAbs) that stimulate thyroid follicular epithelial cells to produce excessive thyroid hormones (12,27,29).
Epstein–Barr virus (EBV) is a human herpes virus, with primary infections occurring in most adults in childhood (11). EBV mainly persists in B lymphocytes and shows four phases (types) of latency (latency 0–3) based on viral antigen expression. EBV occasionally reactivates to switch its replication mode from latent to lytic and produces a large number of infectious virions with the lysis of host cells (7).
EBV was previously suggested to be related to autoimmune diseases, because several autoantibodies were detected during the course of infectious mononucleosis, the symptomatic primary infection of EBV (4,20,26), and this was sometimes found to be accompanied by the development of autoimmune diseases (11,15,23). EBV nuclear antigen (EBNA) 1 has been reported to appear before the onset of systemic lupus erythematosus and shows cross-reactivity to the myelin antigen of patients with multiple sclerosis (11,15,23).
We previously found a positive relationship between serum levels of TRAbs and the EBV-early antigen (EA) antibody, a marker for EBV reactivation, in 66 patients with Graves' disease (17). TRAb titers represent the activity of Graves' disease, and the EBV-EA antibody recognizes the product of the BMRF1 gene expressed in the early phase of lytic reactivation. Even EBV-persisting B cells terminally differentiate into plasma cells and produce a large number of antibodies. If B cells are autoreactive, EBV latent infection or reactivation may affect autoantibody production.
Therefore, we hypothesized that the reactivation of persistent EBV in TRAb-producing B cells may stimulate TRAb production and induce or exacerbate Graves' disease. Then we showed that patients with Graves' disease and healthy controls had EBV-infected B cells with TRAbs on their surface (TRAb+EBV+ cells) (18). We also demonstrated that peripheral blood mononuclear cells (PBMCs) containing these double-positive cells released TRAbs during the induction of EBV reactivation (19).
Thyroid-stimulating TRAbs are IgG1 class immunoglobulins (Igs) (12). Therefore, class-switch recombination (CSR) catabolized by activation-induced cytidine deaminase (AID, encoded by AICDA) should be necessary for B cells to produce thyroid-stimulating TRAb (16). EBV-latent membrane protein (LMP)1 is known to activate mature naive B cells by mimicking the CD40 signal without specific antigen and cognate CD4 T cells (3,11,28). The CD40 signal promotes AICDA transcription through nuclear factor kappa B (NF-κB). Therefore, EBV reactivation could induce the production of class-switched Igs, as well as IgM.
In the present study, we induced EBV reactivation on PBMCs, detected every class of Ig secretion and AID expression, in vitro, and discussed the mechanisms underlying EBV reactivation-induced Ig production in relation to autoimmunity.
Materials and Methods
Subjects
Ten patients with Graves' disease and 14 healthy controls participated in this study (Table 1). All subjects provided written informed consent for participation in the study, and the study protocol was approved by the Medical Ethics Committee for Human Subject Research (No. 707, 707-1-12) at the Faculty of Medicine, Tottori University, Yonago, Japan.
Table 1.
Profiles of the Subjects, Flow cytometry for Plasma Cell Proportion, and Immunohistochemistry for AID, LMP1, and NF-κB
| Flow cytometry | Immunohistochemistry | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD38+ cells (%) | CD138+ cells (%) | AID | LMP1 | NF-κB | |||||||||
| No | Age | Allergy | Treatment | Day 0 | Day 0 | Day 12 | Day 0 | Day 12 | Day 0 | Day 12 | Day 0 | Day 12 | |
| Controls | 1 | 25 | Asthma | 9.87 | 0.95 | 2.15 | 2+ | 3+ | 1+ | 1+ | 3+ | 2+ | |
| 2 | 41 | Atopic dermatitis | NT | NT | NT | NT | NT | NT | NT | NT | NT | ||
| 3 | 53 | NT | NT | NT | NT | NT | NT | NT | NT | NT | |||
| 4 | 32 | NT | NT | NT | NT | NT | NT | NT | NT | NT | |||
| 5 | 24 | Allergic rhinitis | NT | NT | NT | NT | NT | NT | NT | NT | NT | ||
| 6 | 57 | 6.68 | 16.04 | NT | 1+ | 3+ | 2+ | 2+ | 2+ | 1+ | |||
| 7 | 34 | 11.17 | 13.61 | 57.35 | 1+ | 3+ | 1+ | 1+ | 2+ | 2+ | |||
| 8 | 52 | Asthma | 29.17 | 37.22 | 3.44 | NT | NT | 3+ | 1+ | 2+ | 2+ | ||
| 9 | 36 | Atopic dermatitis | 9 | 2.3 | 10.24 | 1+ | 2+ | 1+ | 1+ | 2+ | 1+ | ||
| 10 | 23 | NT | NT | NT | NT | NT | NT | NT | NT | NT | |||
| 11 | 23 | NT | NT | NT | NT | NT | NT | NT | NT | NT | |||
| 12 | 34 | Atopic dermatitis | 14.4 | 24.12 | 47.49 | 3+ | 3+ | 1+ | 1+ | 2+ | 1+ | ||
| 13 | 24 | 4.69 | 1.31 | 2.27 | 1+ | 1+ | 1+ | 3+ | 3+ | 3+ | |||
| 14 | 24 | 2.5 | 1.46 | 15.47 | 1+ | 3+ | 1+ | 1+ | 2+ | 2+ | |||
| Mean | 34.43 | 10.94 | 12.13 | 18.26 | |||||||||
| Patients | 1 | 32 | Asthma, atopic dermatitis | PTU 1.5T | 18.61 | 3.39 | 3.14 | 2+ | 2+ | 1+ | 3+ | 2+ | 3+ |
| 2 | 39 | Asthma, atopic dermatitis | PTU 3T | 10.09 | 13.36 | 5.72 | 1+ | 3+ | 1+ | 1+ | 3+ | 2+ | |
| 3 | 47 | Atopic dermatitis | MMI 2T | 3.88 | 0.17 | 31.46 | NT | NT | NT | NT | 2+ | 1+ | |
| 4 | 51 | MMI 2T | 1.46 | 8.19 | 22.32 | 2+ | 3+ | 1+ | 2+ | 2+ | 2+ | ||
| 5 | 47 | MMI 1T, LT4 50 μg | NT | NT | NT | 1+ | 3+ | 2+ | 2+ | 2+ | 2+ | ||
| 6 | 58 | Allergic rhinitis | MMI 1T, LT4 50 μg | 1.7 | 0.55 | 1.42 | NT | NT | 1+ | 3+ | 2+ | 2+ | |
| 7 | 48 | PTU 2.5T | 2.45 | 0.87 | 2.24 | 1+ | 3+ | 1+ | 2+ | 2+ | 2+ | ||
| 8 | 39 | LT4 50 μg | NT | NT | NT | NT | NT | NT | NT | NT | NT | ||
| 9 | 35 | PTU 1T | 4.31 | 1.5 | 6.12 | 2+ | 3+ | 1+ | 1+ | 2+ | 1+ | ||
| 10 | 29 | Allergic rhinitis | PTU 2T | 8.35 | 0.86 | 17.36 | 1+ | 3+ | 2+ | 2+ | 2+ | 3+ | |
| Mean | 42.5 | 6.36 | 3.61 | 11.22 | |||||||||
Controls Nos. 1–11 and Patients Nos. 1–9 participated in examination of Ig secretion and AID real-time PCR.
AID, activation-induced cytidine deaminase; Ig, immunoglobulin; LMP1, latent membrane protein 1; LT4, levothyroxine; MMI, methylmercaptoimidazole; NT, not enough sample available; NF-κB, nuclear factor kappa B; PTU, propylthiouracil.
The mean ages (±SD) of the Graves' disease patients and healthy controls were 42.50 (±9.17) years and 34.43 (±12.06) years, respectively.
At the time of their diagnosis, patients exhibited symptoms and had laboratory data that included at least one of the following: (1) signs of thyrotoxicosis such as tachycardia, weight loss, finger tremors, and sweating; (2) diffuse enlargement of the thyroid gland; and (3) exophthalmos and/or specific ophthalmopathy. All patients met the following criteria: (1) elevated serum levels of free T4 and/or free T3; (2) the suppression of serum thyrotropin (thyroid stimulating hormone: TSH) (<0.1 μU/mL); and (3) positivity for TRAbs or thyroid-stimulating antibody.
Nine out of the 10 patients were receiving treatments with antithyroid drugs (methylmercaptoimidazole or propylthiouracil). Control subjects were enrolled voluntarily. Their thyroid functions were normal, and they had no family history of thyroid disease.
PBMC preparation
Peripheral blood samples were obtained from Graves' disease patients and healthy controls. PBMCs were separated using a Ficoll-Conray density gradient and stored at −80°C until used (18,19).
Sampling protocol
PBMCs were cultured for 2 days in RPMI 1640/10% FBS with 0.1 μg/mL cyclosporin A at 37°C, as described in our previous study (18,19), to suppress T cell function and enrich the B cell population. They were then transferred to a culture at 33°C to induce EBV reactivation and were regarded as day 0 samples. Culture at 33°C is a physiological way to induce EBV reactivation (6,19,24,25).
On days 0, 5, 10, and 12, half of the culture fluid was sampled and replaced by fresh medium. Culture cells were collected at the same time for total RNA purification. PBMCs for cell population analyses and immunohistochemistry (IHC) were prepared using the same procedure and collected on days 0 and 12.
Flow cytometry for cell population analyses and LMP1 expressions
The PBMCs collected were fixed by 2% paraformaldehyde, and cell population changes, plasma cell populations, and LMP1 expressions were assessed by flow cytometry. We analyzed frequencies of T lymphocytes with CD3 antibody, B lymphocytes with CD79a antibody, and plasma cells with CD138 and CD38 antibodies.
The PBMCs of three subjects (Controls Nos. 6 and 8, Patient No. 3) were analyzed for cell population changes during the culture period. PBMCs of two subjects (Controls Nos. 6 and 8) were analyzed for LMP1 expression variations during the culture period. PBMCs of eight controls and eight patients were analyzed for plasma cell populations.
The primary antibodies (1 μg/1 × 106 cells) used for immunofluorescent staining were as follows, and secondary antibody for CD38 and EBV-LMP was anti-mouse IgG (H+L) goat IgG Alexa Fluor 488 conjugated (Invitrogen, Camarillo, CA).
anti-humanCD3 mouse IgG2a, k (clone HIT3a) APC conjugated (BioLegend, San Diego, CA),
anti-human CD79a mouse IgG1 Alexa Fluor 647 conjugated (AbD Serotec, Oxford, United Kingdom),
anti-human CD138 mouse IgG1 Alexa Fluor 647 conjugated (AbD Serotec),
anti-human CD38 mouse IgG1 (Beckman Coulter, Pasadena, CA),
anti-EBV-LMP mouse IgG (Dako, Glostrup, Denmark).
AICDA mRNA expression
AICDA mRNA was quantified by real-time PCR with a 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster city, CA). The primers (14) and probes used for AICDA and β-actin were as follows:
AICDA
forward primer: 5′-aaatgtccgctgggctaagg-3′
reverse primer: 5′-ggaggaagagcaattccacgt-3′
fluorescent probe: 5′-FAM-tgacagtgctacatcct-MGB-3′(Applied Biosystems).
β-actin
forward primer: 5′-cctggcacccagcacaatg-3′
reverse primer: 5′-gccgatccacacggagtact-3′
fluorescent probe: 5′-VIC-atcaagatcattgctcctcctgagcgc-MGB-3′ (Applied Biosystems).
The results obtained were analyzed using the ΔΔCT method with β-actin as the reference and Raji cells as the calibrator. The amplification efficiencies of the primers of AICDA and β-actin were equal.
Immunohistochemistry
Two percent paraformaldehyde-fixed PBMCs were washed by phosphate-buffered solution (PBS) and permeabilized by 0.5% Tween20/PBS for 10 min at room temperature. After washing by distilled water (DW), PBMCs were smeared on silane-coated slide, washed twice by DW, and air dried.
The proteins of AID, LMP1, and human NF-κB p65 were detected using IHC with the following primary antibodies and Dako EnVision+ System/HRP (Dako) and Dako Liquid DAB+ Substrate Chromogen System (Dako).
AID: anti-AID mouse IgG1-κ (Invitrogen), dilution 1: 200
LMP1: anti-EBV-LMP mouse IgG (Dako), dilution 1: 100
NF-κB: anti-NF-κB p65 (phospho S536) antibody (Abcam, Cambridge, United Kingdom), dilution 1: 100
We semiquantified the positive cell percentage,
+1: 10% and under
+2: over 10% and under 50%
+3: 50% and over
Concerning the positivity of NF-κB, we adopted only nuclear staining, because nuclear expression of NF-κB should be activated form, while cytoplasmic expression would be inactive form.
Ig concentration in the culture medium
The quantities of IgG, IgM, and IgE in the culture medium were measured by ELISA (No. E80-104, E80-100, E80-108; Bethyl, Montgomery, TX), according to the manufacturer's instructions. On the sampling day, we took half of the culture medium for examination and added the same amount of fresh medium. The values at each sampling point represent increases obtained by subtracting half the previous values.
Statistical analyses
Statistical analyses were performed using SPSS Statistics 21 (IBM, Armonk, NY).
Friedman's test was adopted to analyze time course variations in AICDA mRNA expression and Ig concentrations. The Wilcoxon rank sum test was used for comparisons of the concentrations between IgG and IgM.
Results
The B cell population is enriched by a preculture and plasma cell population increases by the EBV reactivation induction
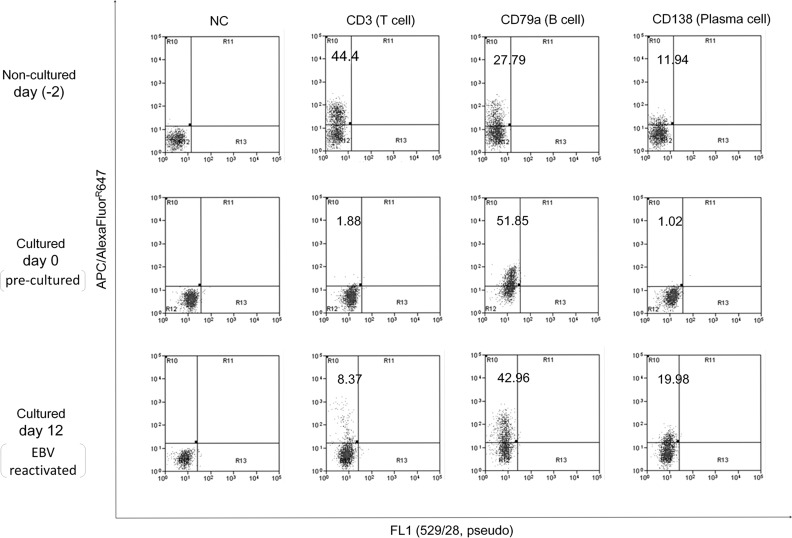
We confirmed in analyses of cell population changes that a preculture suppressed the CD3-positive T cell population and enriched CD79a-positive B cells (Fig. 1 and Table 1).
FIG. 1.
Cell population changes during the culture protocol. PBMCs were cultured at 33°C for the induction of EBV reactivation after a preculture for 2 days. The B cell population was enriched by the preculture, and EBV reactivation was accompanied by an increase in the plasma cell frequency. APC, allophycocyanin; EBV, Epstein–Barr virus; FL, fluorescence; NC, nonstain control; PBMC, peripheral blood mononuclear cell.
During the EBV reactivation induction, the CD138-positive plasma cell population increased. However, even in day 0 samples, the percentages of CD138-positive and CD38-positive plasma cells were 3.61 and 6.36, respectively, in patients and 12.13 and 10.94, respectively, in controls.
AICDA mRNA and protein were expressed on day 5 or later
We detected the expression of AID and AICDA mRNA during the EBV reactivation induction.
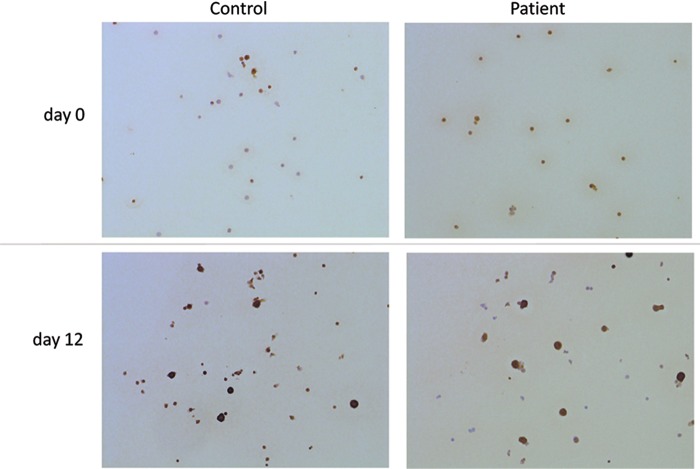
In IHC, the expression of the AID protein in nucleus and cytoplasm was weak in day 0 cells just after the preculture; however, on day 12 of EBV reactivation, we noticed the intensely stained expression of AID in nucleus and cytoplasm of large cells (Fig. 2).
FIG. 2.
Immunohistochemistry for AID of PBMCs in vitro. A few cells were stained by immunohistochemistry for AID in day 0 samples, just after a preculture. In day 12 samples, many cells were positive, particularly large cells that stained intensely. AID, activation-induced cytidine deaminase.
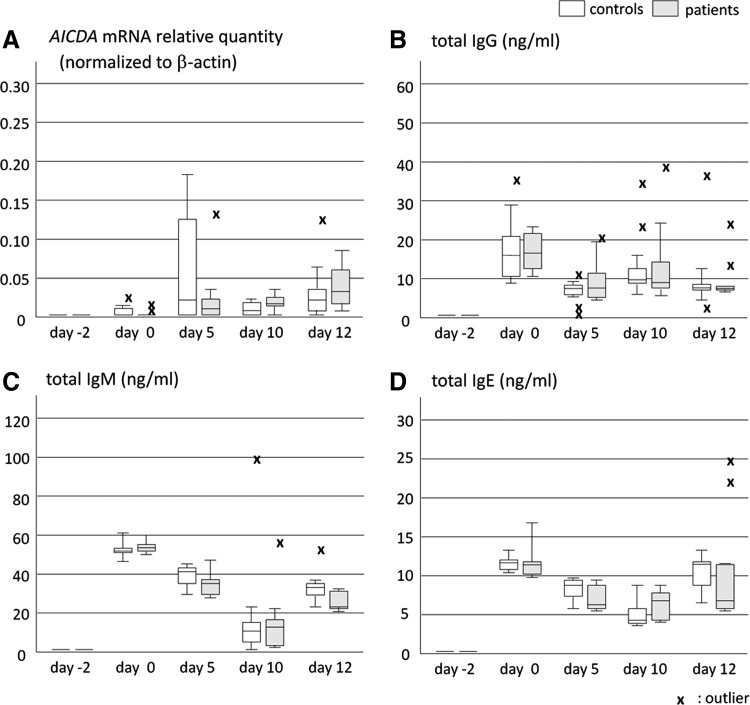
Variations in AICDA mRNA expression from days 0 to 12 were not significant.
However, AICDA mRNA was negligible in day 0 samples and increased on day 5 or later (Fig. 3A).
FIG. 3.
Time course changes in AICDA mRNA and Igs produced. We sampled culture fluid and cells on days 0, 5, 10, and 12 and then examined AICDA mRNA in these cells, as well as Igs in culture fluids. AICDA mRNA was expressed on day 5 or later (A), and Igs was detected with peaks on days 0 and 10–12 (B–D). Variations in IgG, IgM, and IgE were significant according to Friedman's test. “x” represents outlier. AICDA, gene of activation-induced cytidine deaminase; Igs, immunoglobulins.
LMP1 and NF-κB
LMP1 and NF-κB were positive in every cell sample detected by IHC on days 0 and 12 (Table 1). LMP1 was positive in cytoplasm, and we decided positivity of NF-κB by nuclear stain.
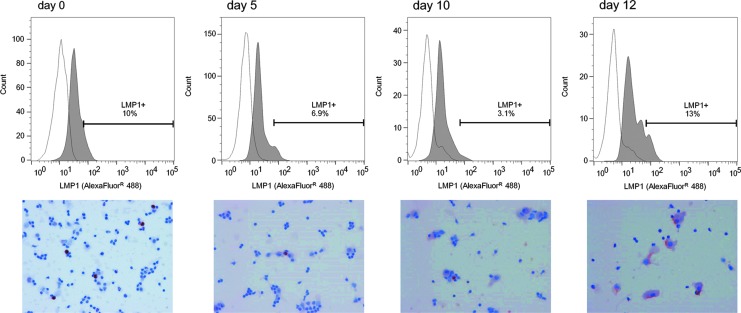
We examined time course changes in LMP1 in two subjects (Control Nos. 6 and 8) by flow cytometry and IHC. LMP1 was expressed throughout the reactivation period with peaks at days 0 and 12 (Fig. 4).
FIG. 4.
Expression of the LMP1 protein during the induction of EBV reactivation. LMP1 expression was detected by IHC and flow cytometry throughout the examination period. A quantitative analysis by flow cytometry revealed two peaks on days 0 and 12. LMP1, latent membrane protein 1.
Biphasic Ig production during the EBV reactivation induction
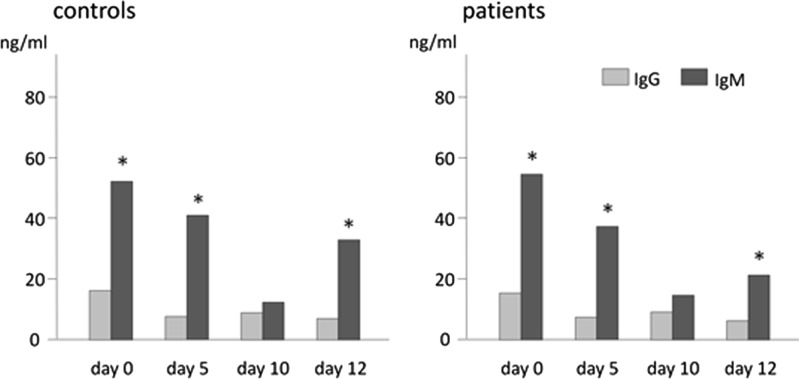
Total IgG, IgM, and IgE production showed biphasic increases on days 0 and 10 or 12. These variations were found to be significant by Friedman's test (Fig. 3B–D). IgM concentrations were significantly higher than those of IgG on days 0, 5, and 12 (Fig. 5).
FIG. 5.
Comparison of the total-IgG and total-IgM produced during the induction of EBV reactivation. The medians of Ig concentrations are shown. In contrast to the proportion of serum Igs, total-IgM concentrations in the culture were significantly higher than those of total-IgG on days 0, 5, and 12. This observation suggests that the proportion of Ig isotypes produced by EBV reactivation-induced mechanisms depends on the proportion of circulating B cell surface globulin isotypes. *p < 0.05.
In the present study, we measured total Ig, regardless of specificity, but were unable to detect any significant differences between patients and controls.
Discussion
EBV reactivation and plasma cell differentiation occur in the same period (10,18,19). We examined Ig secretion during EBV reactivation induction and observed significant biphasic variations in IgG, IgM, and IgE with peaks on days 0 and 10 or 12 (Fig. 3B–D).
Since no stimulation for reactivation functioned on day 0, we speculated that Igs in the day 0 peak secreted from plasma cells already existed in the sample PBMCs. Sample PBMCs on day 0 contained 4–12% plasma cells (Table 1). In contrast, the peak of IgG, IgM, and IgE on day 10 or 12 represents Ig secretion stimulated by EBV reactivation.
EBV can infect both naive and memory B cells (11), but most circulating B cells are mature naive B cells that have IgM on their surface (8,22). Therefore, AID is required for EBV-infected mature naive B cells to produce IgG class-switched antibodies.
During the induction of EBV reactivation on B cell-enriched precultured PBMCs, we detected the expression of AICDA mRNA and AID protein. The expression of AID protein was negligible on day 0, but strongly expressed in large cells on day 12 (Fig. 2), and the expression of AICDA mRNA was initiated on day 5 at its maximum (Fig. 3A). These results are consistent with the expression of AID in activated B cells (30) and suggest that the EBV reactivation induces the production of every isotype of Ig catalyzed by AID in vitro.
When EBV is reactivated, many infectious virions are released and infect the surrounding cells. Most newly infected B cells become latency 3 and express viral antigens, including the LMPs and EBNAs (2,11). LMP1 is thought to promote AICDA transcription through NF-κB (11,14).
We detected LMP1 and NF-κB in culture cells on days 0 and 12 of reactivation using IHC (Table 1). A time course examination by flow cytometry and IHC revealed that LMP1 was expressed throughout the observation period with peaks on days 0 and 12 (Fig. 4). Although the expression of LMP1 on day 0 may be the result of the preculture, its expression on and after day 5 was induced by EBV reactivation.
We suggest that the EBV reactivation induces the production of every isotype of Ig catalyzed by AID in vitro through LMP1 and NF-κB.
This mechanism might have the relevance to allergic diseases. That is, IgE production induced by EBV reactivation may explain some findings showing that the frequency of allergic rhinitis is high in patients with Graves' disease (1,5).
The amount of serum IgG is 10-fold that of serum IgM (13,22), but our results showed that the amount of IgM was significantly larger compared with IgG in vitro (Fig. 5). This result suggests that the proportion of Igs induced by EBV reactivation depends on the proportion of the surface globulins of surrounding culture cells that means the proportion of circulating peripheral B cell surface globulins (8,22).
LMP1 has the ability to induce B cell activation (3,11,29). This polyclonal B cell activation nonspecifically activates any B cells present because it does not require stimulation from specific antigens or cognate CD4 T cells. Consequently, the proportion of Igs produced reflects the proportion of surface Igs of B cells present. We suggest that polyclonal B cell activation due to LMP1 works on composing the proportion of Igs induced by EBV reactivation.
In our previous study, TRAb secretion from EBV-reactivated PBMCs was significantly higher in patients with Graves' disease than in healthy controls (19). In the present study, we measured total Ig, regardless of specificity, but did not find any significant difference between patients and healthy controls (Table 2). This may be because TRAb-producing B cells are only a small fraction of B cells with numerous specificities.
Table 2.
AICDA mRNA Expression and Ig-Isotype Secretion of Peripheral Blood Mononuclear Cells from Graves' Disease Patients and Controls During Epstein–Barr Virus-Reactivation Induction
| Day 0 | Day 5 | Day 10 | Day 12 | |
|---|---|---|---|---|
| AICDA mRNAa | ||||
| Controls | 0.005 | 0.062 | 0.011 | 0.029 |
| Patients | 0.003 | 0.025 | 0.018 | 0.041 |
| p | 0.522 | 0.331 | 0.230 | 0.131 |
| Total IgG (ng/mL) | ||||
| Controls | 17.696 | 7.310 | 13.614 | 10.118 |
| Patients | 16.769 | 9.911 | 14.367 | 9.948 |
| p | 0.882 | 0.882 | 0.552 | 0.71 |
| Total IgM (ng/mL) | ||||
| Controls | 53.502 | 39.181 | 20.87 | 33.546 |
| Patients | 54.562 | 35.704 | 18.195 | 26.275 |
| p | 0.603 | 0.152 | 0.824 | 0.025b |
| Total IgE (ng/mL) | ||||
| Controls | 12.993 | 8.200 | 4.511 | 10.478 |
| Patients | 11.424 | 7.215 | 5.526 | 11.491 |
| p | 0.201 | 0.080 | 0.112 | 0.261 |
AICDA mRNA relative quantity (normalized to β-actin).
Significant.
EBV, Epstein–Barr virus.
These results also suggest that B cells in the culture had been activated nonspecifically and differentiated to produce polyclonal Igs. In other words, the mechanisms of EBV reactivation-induced Ig production are not selective to autoreactive B cells; they work on every B cell in the culture.
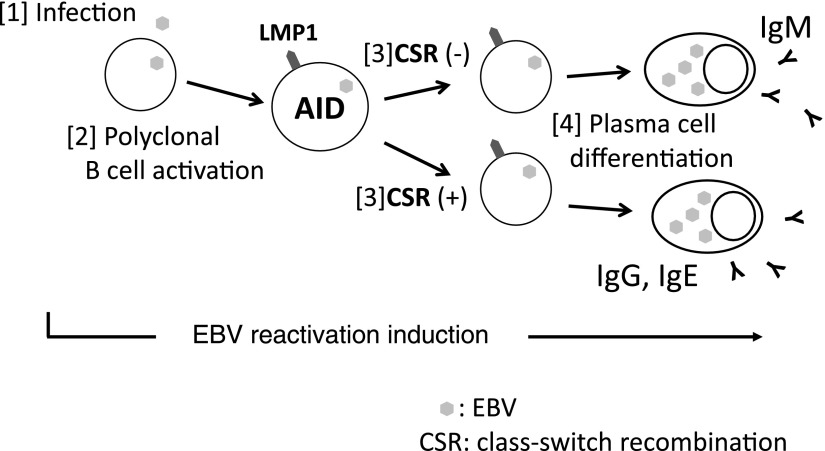
We herein propose a pathway for EBV reactivation-induced Ig production based on the results of the present study (Fig. 6). The following are pathway steps in order:
FIG. 6.
The pathway for EBV reactivation-induced Ig production. [1] B cells are infected by EBV released by preexisted plasma cells. [2] Newly infected cells become latency 3 and express LMP1. LMP1 activates host cells (polyclonal B cell activation). [3] LMP1 stimulates AID expression through NF-κB, which enables to produce class-switched Ig. [4] EBV reactivation induces plasma cell differentiation and Ig production. CSR, class-switch recombination; NF-κB, nuclear factor kappa B.
1. B cells are infected by new EBV virions released from preexisted plasma cells.
2. Newly infected cells become latency 3 and express LMP1.
LMP1 activates host cells (polyclonal B cell activation).
3. LMP1 stimulates AID expression through NF-κB, which enables to produce class-switched Ig.
4. EBV reactivation induces plasma cell differentiation and Ig production.
The specific antigens of autoreactive B cells are self-components. Since difficulties are associated with encountering self-antigens packed inside cells, most autoreactive B cells are purged from lymphoid tissue and die. In our model, even autoreactive B cells that were not activated are rescued and produce Igs with EBV reactivation, which suggests the contribution of the mechanisms underlying EBV reactivation to autoimmune diseases.
Nakamura et al. reported that the frequency of circulating autoreactive IgM-B cells was higher compared with autoreactive IgG-B cells in patients with autoimmune diseases and healthy controls (21). Kumata et al. recently showed that serum levels of TRAb-IgM were higher than those of TRAb-IgG in patients with Graves' disease and healthy controls, in contrast to serum levels of total-IgG being higher than those of total-IgM (9). Both of these findings support the rescue of autoreactive B cells by polyclonal B cell activation due to EBV reactivation.
Conclusions
We showed the production of every class of Ig and detected the expression of AID and EBV-LMP1 during the induction of EBV reactivation. These results indicate that B cells newly infected with EBV are activated by polyclonal B cell activation and produce Igs through plasma cell differentiation induced by EBV reactivation. EBV-LMP1 induced AID enabled B cells to undergo CSR to produce every isotype of Ig. According to this mechanism, EBV rescues autoreactive B cells to produce autoantibodies, which contribute to the development and exacerbation of autoimmune diseases.
Acknowledgments
The authors are grateful to Dr. Shuji Fukata (Kuma Hospital) and former professor Takeshi Sairenji (Tottori University). The authors also thank Medical English Service (Kyoto, Japan) for proofreading this article. This work was supported by Tottori University Faculty of Medicine Research Grant (2015) and Discretionary funds of the director of Tottori University Hospital (2016).
Authors' Contributions
K.N. designed this study and carried out most of the experiments, including cell culture, and then drafted the article. K.K. and S.H. carried out ELISA analyses. Y.N. cooperated with K.N. in flow cytometric analyses. Y.S. and S.K. guided analyses of quantitative PCR. H.S. and M.M. participated in immunohistochemistry. M.K. and I.M. participated in interpretation of the results. K.H. participated in the design and coordination of the study and helped to draft the article.
Disclosure Statement
No competing financial interests exist.
References
- 1.Amino N, Hidaka Y, Takano T, Izumi Y, Tatsumi KI, and Nakata Y. Association of seasonal allergic rhinitis is high in Graves' disease and low in painless thyroiditis. Thyroid 2003;13:811–814 [DOI] [PubMed] [Google Scholar]
- 2.Cohen JI. Epstein-Barr virus infection. N Engl J Med 2000;343:481–492 [DOI] [PubMed] [Google Scholar]
- 3.He B, Raab-Traub N, Casali P, and Cerutti A. EBV-encoded latent membrane protein 1 cooperates with BAFF/BLyS and APRIL to induce T cell-independent Ig heavy chain class switching, J Immunol 2003;171:5215–5224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henle W, Henle G, and Horwitz C. Epstein-Barr virus specific diagnostic tests in infectious mononucleosis. Hum Pathol 1974;5:551–565 [DOI] [PubMed] [Google Scholar]
- 5.Hidaka Y, Amino N, Iwatani Y, Itoh E, Matsunaga M, and Tamaki H. Recurrence of thyrotoxicosis after attack of allergic rhinitis in patients with Graves' disease, J Clin Endocrinol Metab 1993;77:1667–1670 [DOI] [PubMed] [Google Scholar]
- 6.Hinuma Y, Konn M, Yamaguchi J, Wuderski DJ, Blakeslee JR, Jr., and Grace JT, Jr., Immunofluorescence and herpes-type virus particles in the P3HR-1 Burkitt lymphoma cell line. J Virol 1967;1:1045–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kenney SC, and Mertz JE. Regulation of the latent-lytic switch in Epstein-Barr virus, Semin Cancer Biol 2014;26:60–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein U, Küppers R, and Rajewsky K. Evidence for a large compartment of IgM-expressing memory B cells in humans. Blood 1997;89:1288–1298 [PubMed] [Google Scholar]
- 9.Kumata K, Nagata K, Matsushita M, et al. Thyrotropin receptor antibody (TRAb)-IgM levels are markedly higher than TRAb-IgG levels in Graves' disease patients and controls, and TRAb-IgM production is related to Epstein-Barr virus reactivation. Viral Immunol 2016;29:459–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laichalk LL, and Thorley-Lawson DA. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J Virol 2005;79:1296–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Longnecker RM, Kieff E, and Cohen J. Epstein–Barr virus. In: Knipe DM, and Howley PM, eds. Fields Virology. 6th ed. Philadelphia: Lippincott Williams & Wilkins, 2013:1898–1959 [Google Scholar]
- 12.Mandel SJ, Larsen PR, and Davies TF. Thyrotoxicosis. In: Melmed S, Polonsky KS, Larsen PR, and Kronenberg HM, eds. Williams Textbook of Endocrinology, 12th ed. Philadelphia: Saunders, 2011:362–405 [Google Scholar]
- 13.Manz RA, Thiel A, and Radbruch A. Lifetime of plasma cells in the bone marrow. Nature 1997;388:133–134 [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto Y, Marusawa H, Kinoshita K, et al. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med 2007;13:470–476 [DOI] [PubMed] [Google Scholar]
- 15.Münz C, Lünemann JD, Getts MT, and Miller SD. Antiviral immune responses: triggers of or triggered by autoimmunity? Nat Rev Immunol 2009;9:246–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, and Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 2000;102:553–563 [DOI] [PubMed] [Google Scholar]
- 17.Nagata K, Fukata S, Kanai K, et al. The influence of Epstein-Barr virus reactivation in patients with Graves' disease. Viral Immunol 2011;24:143–149 [DOI] [PubMed] [Google Scholar]
- 18.Nagata K, Higaki K, Nakayama Y, et al. Presence of Epstein-Barr virus-infected B lymphocytes with thyrotropin receptor antibodies on their surface in Graves' disease patients and in healthy individuals, Autoimmunity 2014;47:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagata K, Nakayama Y, Higaki K, et al. Reactivation of persistent Epstein-Barr virus (EBV) causes secretion of thyrotropin receptor antibodies (TRAbs) in EBV-infected B lymphocytes with TRAbs on their surface. Autoimmunity 2015;48:328–335 [DOI] [PubMed] [Google Scholar]
- 20.Nagata K, Okuno K, Ochi M, et al. Production of thyrotropin receptor antibodies in acute phase of infectious mononucleosis due to Epstein-Barr virus primary infection: a case report of a child. Springerplus 2015;4:456 eCollection [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura M, Burastero SE, Ueki Y, Larrick JW, Notkins AL, and Casali P. Probing the normal and autoimmune B cell repertoire with Epstein-Barr virus. Frequency of B cells producing monoreactive high affinity autoantibodies in patients with Hashimoto's disease and systemic lupus erythematosus. J Immunol 1988;141:4165–4172 [PubMed] [Google Scholar]
- 22.Parham P. The immune system. 3rd ed. New York: Garland Science, 2009 [Google Scholar]
- 23.Pender M. Preventing and curing multiple sclerosis by controlling Epstein-Barr virus infection. Autoimmunity Rev 2009;8:563–568 [DOI] [PubMed] [Google Scholar]
- 24.Sairenji T, Bertoni G, Medveczky MM, Medveczky PG, and Humphreys RE. Inhibition of Epstein-Barr virus (EBV) release from P3HR-1 and B95-8 cell lines by monoclonal antibodies to EBV membrane antigen gp350/220. J Virol 1988;62:2614–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sairenji T, and Hinuma Y. Re-evaluation of a transforming strain of Epstein-Barr virus from the Burkitt lymphoma cell line, Jijoye. Int J Cancer 1980;26:337–342 [DOI] [PubMed] [Google Scholar]
- 26.Sutton RN, Emond RT, Thomas DB, and Doniach D. The occurrence of autoantibodies in infectious mononucleosis. Clin Exp Immunol 1974;17:427–436 [PMC free article] [PubMed] [Google Scholar]
- 27.Tomer Y, and Huber A. The etiology of autoimmune thyroid disease: a story of genes and environment. J Autoimmun 2009;32:231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uchida J, Yasui T, Takaoka-Shichijo Y, et al. Mimicry of CD40 signals by Epstein-Barr virus LMP1 in B lymphocyte responses. Science 1999;286:300–303 [DOI] [PubMed] [Google Scholar]
- 29.Weetman AP, and McGregor AM. Autoimmune thyroid disease: further developments in our understanding. Endocr Rev 1994;15:788–830 [DOI] [PubMed] [Google Scholar]
- 30.Zhenming X, Pone EJ, Al-Qahtani A, Park SR, Zan H, and Casali P. Regulation of aicda expression and AID activity: relevance to somatic hypermutation and class switch DNA recombination. Crit Rev Immunol 2007;27:367–397 [DOI] [PMC free article] [PubMed] [Google Scholar]