Abstract
Adult pancreatic stem and progenitor cells may serve as an alternative source of insulin-secreting endocrine cells in cell replacement therapy for type 1 diabetes, but much remained unknown about these cells. We previously identified adult murine pancreatic progenitor-like cells that displayed in vitro self-renewal and tri-lineage differentiation activities in a three-dimensional colony/organoid assay containing 1% methylcellulose and 5% Matrigel. However, the presence of other undefined culture components, such as serum and conditioned medium, has prevented a complete understanding of the signals required for progenitor cell growth. Here, we have established a serum-free, conditioned medium-free colony assay with the inclusion of seven defined factors: epidermal growth factor (EGF), R-Spondin 1 (RSPO1), Noggin, nicotinamide, exendin-4, activin B, and vascular endothelial growth factor (VEGF)-A. The requirements for colony growth were characterized and we found that EGF and nicotinamide were necessary and sufficient for the colony growth and long-term self-renewal of these progenitors. However, the seven factor (7F) culture medium better induced colony size and self-renewal in long-term culture than EGF plus nicotinamide alone. Individual 3-week-old colonies grown in the 7F culture medium expressed ductal, acinar, and endocrine lineage markers, suggesting that tri-lineage differentiation of the tri-potent progenitors was occurring without genetic manipulation. A delayed inhibition of Notch signaling using small molecules in 2-week-old cultures enhanced endocrine gene expression in 3-week-old colonies. This better-defined colony assay system will enable our and other laboratories for in-depth mechanistic studies on the biology of these progenitor cells.
Keywords: : differentiation, epidermal growth factor, nicotinamide, Notch signaling pathway, pancreatic colony-forming units, self-renewal
Introduction
Pancreatic colony-forming units (PCFUs) are progenitor-like cells capable of giving rise to colonies (also named organoids) in three-dimensional culture systems employing semisolid media [1–6]. Using the culture system that our laboratory developed (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd), adult murine PCFUs can self-renew long term and differentiate into the three major pancreatic lineages, that is, duct, acinar, and endocrine cells, including the insulin-expressing beta cells [1]. The in vitro activities of self-renewal and tri-lineage differentiation displayed by the adult PCFUs suggest that these cells are stem-like cells. However, the existence of adult pancreatic progenitors is hotly debated in the scientific community [7], and much remained unknown about the biology of adult PCFUs.
One of the significant hurdles to effectively study adult PCFUs in our culture system is the presence of undefined growth factors, for example, conditioned medium from murine embryonic stem cell (mESC)-derived pancreatic-like cells [8] and fetal calf serum (FCS). In addition, production of conditioned medium involves multiple steps [9], which is difficult in laboratories that do not routinely culture mESCs and thus prevents a wider adaptation of our culture system. Therefore, in this study we sought to establish a better-defined culture condition for adult murine PCFUs.
Materials and Methods
Mice
Two to 4-month-old Sox9/EGFP transgenic mice (CD-1 background; both sexes) [10] were used. All mice were maintained under specific pathogen-free conditions, and animal experiments were conducted using protocols approved by the Institutional Animal Care and Use Committee at City of Hope.
Additional methods are described in Supplementary Data.
Results
Instead of FCS, we used KnockOut Serum Replacement that is known to maintain fetal pancreatic organoids in culture [3]. To replace the conditioned medium, epidermal growth factor (EGF), R-Spondin 1 (RSPO1), and Noggin, which support the in vitro growth of adult pancreatic bi-potent [2] and intestinal [11] progenitors, were tested. Other ingredients in our standard culture remained unchanged (Supplementary Fig. S1). This new culture system is referred to as “seven factor (7F).”
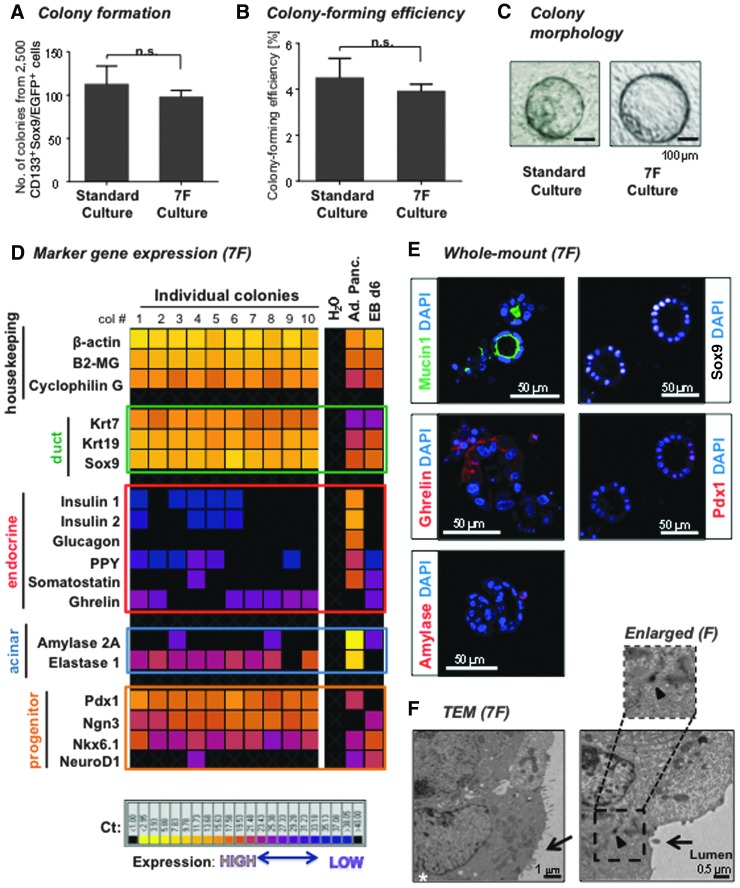
Adult murine PCFUs, which are enriched in the sorted CD133+Sox9/EGFP+ ductal cells [1], were plated into either the standard or 7F condition and the resulting colonies were examined after 3 weeks. The number of colonies formed (Fig. 1A) and the colony-forming efficiency (Fig. 1B) supported by the 7F condition was similar to the standard culture system. The morphology of the 7F-grown colonies also resembled the cystic colonies derived from the standard culture system (Fig. 1C).
FIG. 1.
The serum-free, conditioned medium-free, 7F culture system allows formation of colonies (organoids) composed of the three major pancreatic lineages. (A) Colony number and (B) colony-forming efficiency are shown for the standard (n = 11) and 7F (n = 27) culture systems after 3 weeks in culture. n.s., not significant (P > 0.05) determined by t-test. Data represent mean ± SEM. (C) Representative photomicrograph of 3-week-old cystic colonies grown in the standard or 7F culture system. Bar: 100 μm. (D) Single-colony microfluidic qRT-PCR analysis for ductal, progenitor, acinar, and endocrine markers. Each column is from a single colony. cDNA from adult pancreas (Ad. Panc.) and day-6 embryoid bodies from a mouse embryonic stem cell line (EB d6) [29] were used as positive controls. H2O was used as negative control. Shown is one representative experiment of three with the same trend. (E) Representative photomicrographs of whole-mount immunofluorescent staining for ductal (Mucin1, Sox9), acinar (Amylase), endocrine (Ghrelin), and pancreatic progenitor (Pdx1) markers in colonies grown in 7F. Bar: 50 μm. (F) Representative TEM images showing ductal cell characteristics such as a multi-lobed nucleus (white asterisk), microvilli (black arrow) facing the lumen and desmosomes (black arrowhead) at cell–cell junctions. The dotted box is shown enlarged on top. Bars: 1 and 0.5 μm. 7F, seven factor; cDNA, complementary DNA; qRT-PCR, quantitative reverse transcription–polymerase chain reaction; SEM, standard error of the mean; TEM, transmission electron microscopy.
Three-week-old colonies from 7F culture were examined one-by-one for lineage marker expression using microfluidic quantitative reverse transcription–polymerase chain reaction (qRT-PCR) analysis. All colonies expressed markers for ductal (Krt7, Krt19, and Sox9 [12,13]) and progenitor cells (Pdx1 [14], Nkx6.1 [15] , and Ngn3 [16]) (Fig. 1D). All colonies also expressed at least one of the six markers for pancreatic endocrine hormones (insulin1, insulin2, glucagon, pancreatic polypeptide, somatostatin, and ghrelin) [17], with ghrelin being the most frequently expressed among colonies (Fig. 1D). All colonies except for No. 9 expressed acinar markers (amylase2A and elastase1) (Fig. 1D), suggesting that majority of the PCFUs examined were tri-potent, consistent to our previous report using the standard culture system [1]. Among individual 3-week-old colonies grown in 7F, the expression patterns of the six endocrine markers were different, suggesting that PCFUs are heterogeneous in their potentials to differentiate into the five subtypes of the endocrine lineage.
Whole-mount immunostaining confirmed protein expression of Sox9, Pdx1, ghrelin, and amylase (Fig. 1E). Additionally, Mucin1 (a ductal cell marker) was detected at the lumen side toward the center of cysts, suggesting polarized ductal cells. Transmission electron microscopy analyses further confirmed the characteristics of ductal epithelium: the presence of microvilli (black arrows) that face the lumen, lobular nuclei (white asterisk), and desmosomes at cell–cell junctions (black arrow head) (Fig. 1F).
To determine which culture components were necessary for colony growth, we omitted individual components one at a time. Omission of serum replacement completely prevented colony formation (data not shown), and omission of either EGF (Supplementary Fig. S2A) or nicotinamide (Supplementary Fig. S2B) nearly prevented colony formation, demonstrating that serum replacement, EGF, and nicotinamide are essential for colony growth.
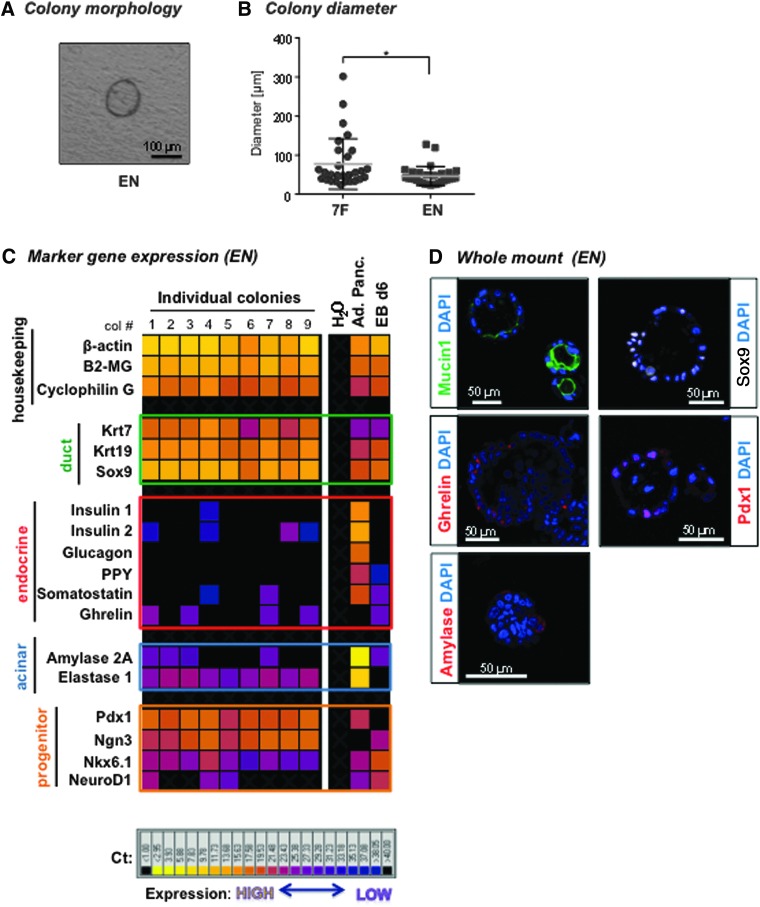
EGF and nicotinamide, but not the other five factors, were included in the culture system referred to as the “EN” condition (Supplementary Fig. S1B). Colonies did form in EN after 3 weeks (Fig. 2A), but they were smaller than those grown in 7F (Fig. 2B). Gene expression pattern of individual colonies from EN was similar to those grown in 7F condition (Fig. 2C). Whole-mount immunofluorescent staining confirmed the protein expression of Mucin1, Sox9, Pdx1, ghrelin and amylase (Fig. 2D). These results demonstrate that EN is sufficient to support the growth and differentiation of PCFUs in vitro.
FIG. 2.
EGF and nicotinamide are sufficient for colony formation. (A) Representative photomicrograph of a 3-week-old colony grown in EN condition. Bar: 100 μm. (B) Colony diameter for the EN (n = 30) and 7F (n = 30) culture systems. Data are mean ± SD. Shown is one representative experiment of three with the same trend. (C) Single-colony microfluidic qRT-PCR analysis of cystic colonies grown in EN for ductal, progenitor, acinar, and endocrine markers. Each column is from a single colony. cDNA from adult pancreas (Ad. Panc.) and day-6 embryoid bodies from a mouse embryonic stem cell line (EB d6) were used as positive controls. H2O was used as negative control. Shown is one representative experiment of three with the same trend. (D) Representative whole-mount immunofluorescent micrographs show expression of ductal (Mucin1, Sox9), acinar (Amylase), endocrine (Ghrelin) and pancreatic progenitor (Pdx1) markers in colonies grown in EN condition. Bar: 50 μm. The colonies were evaluated after 3 weeks in culture. *P < 0.05 was determined by t-test. EGF, epidermal growth factor; SD, standard deviation.
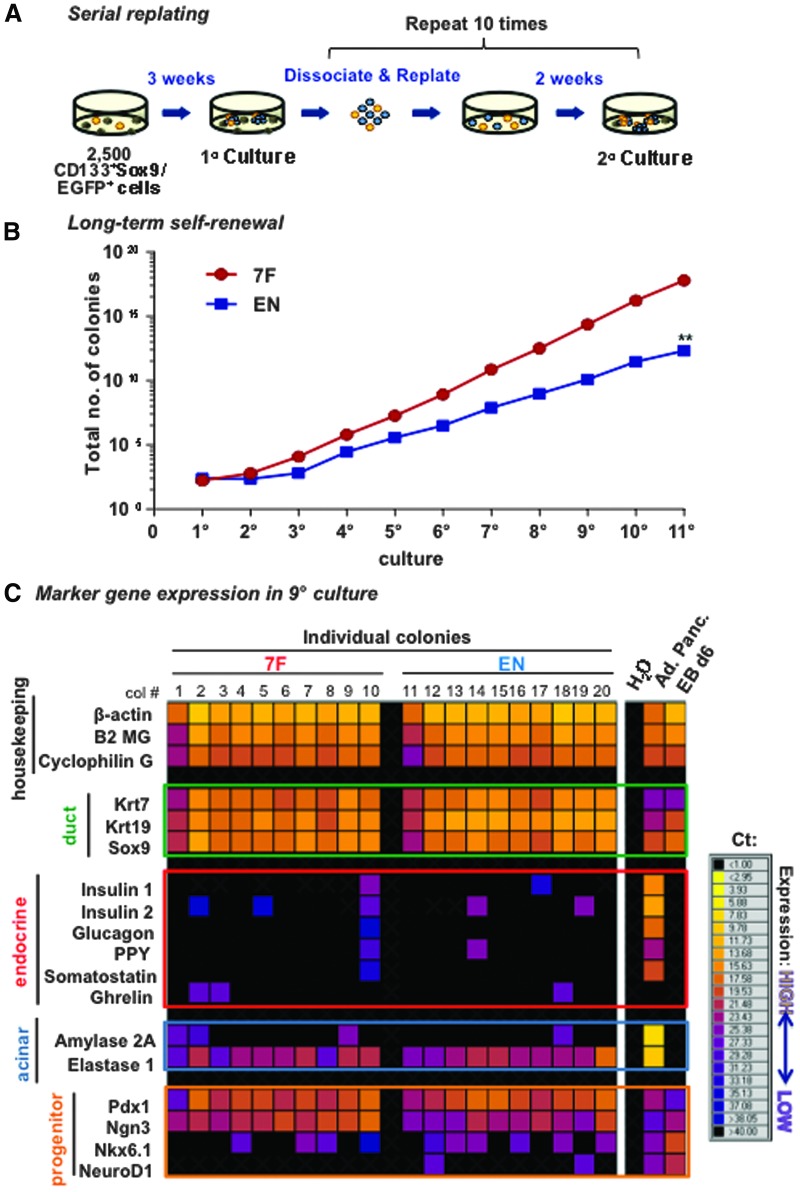
To determine whether PCFUs can self-renew in EN or 7F condition, the 3-week-old colonies were dissociated into single cells, and serially replated 10 times at 2-week intervals (Fig. 3A). Both the 7F and EN culture conditions allowed long-term self-renewal of PCFUs (Fig. 3B). However, EN produced a lower net increase in total PCFUs compared to 7F (Fig. 3B). To test whether PCFUs retain the capacity for tri-lineage differentiation after serial replating, individual colonies from the ninth culture were analyzed by microfluidic qRT-PCR analysis. All colonies exposed to 7F or EN condition long-term maintained expression of markers for ductal, acinar, and progenitor cells (Fig. 3C). Four out of 10 colonies from either 7F or EN condition expressed at least one of the endocrine lineage markers (Fig. 3C), suggesting that the originating PCFUs for these colonies remain tri-potent after long-term culture. Because 7F supported the expansion of PCFUs better than EN (Fig. 3B), the 7F condition was therefore selected for subsequent experiments.
FIG. 3.
7F condition supports self-renewal of adult progenitors better than EN. (A) Schematic of a serial replating experiment. Sorted pancreatic CD133+Sox9/EGFP+ ductal cells are plated into the 7F or EN culture systems. After 3 weeks in the 1° culture, the colonies are counted, dissociated, and replated in the same culture condition as before. After 2 weeks, the 2° colonies are again counted, dissociated, and replated, and the process is repeated. (B) The number of total colonies (adjusted for the fraction of cells replated in each passage) from the 7F and EN culture systems. **P < 0.005 was determined by t-test at 11° culture (n = 3). Shown is one representative experiment of three with the same trend. (C) Microfluidic qRT-PCR analysis for individual colonies lifted from the ninth cultures after serial replating. Each column is from a single colony. cDNA from adult pancreas (Ad. Panc.) and day-6 embryoid bodies from a mouse embryonic stem cell line (EB d6) [29] were used as positive controls. H2O was used as negative control. Shown is one representative experiment of two with the same trend.
To demonstrate the utility of the 7F culture system, we sought to determine whether signaling pathways important for development, such as Notch, influence in vitro differentiation of adult PCFUs. During pancreas development, downregulation of Notch signaling is required for endocrine cell differentiation by de-repressing Ngn3, a transcription factor required for endocrine lineage commitment [18,19]. Freshly sorted CD133+Sox9/EGFP+ ductal cells and 3-week-old colonies grown in 7F condition expressed Notch signaling molecules (Supplementary Fig. S3), suggesting a readiness for these cells to respond to Notch inhibition.
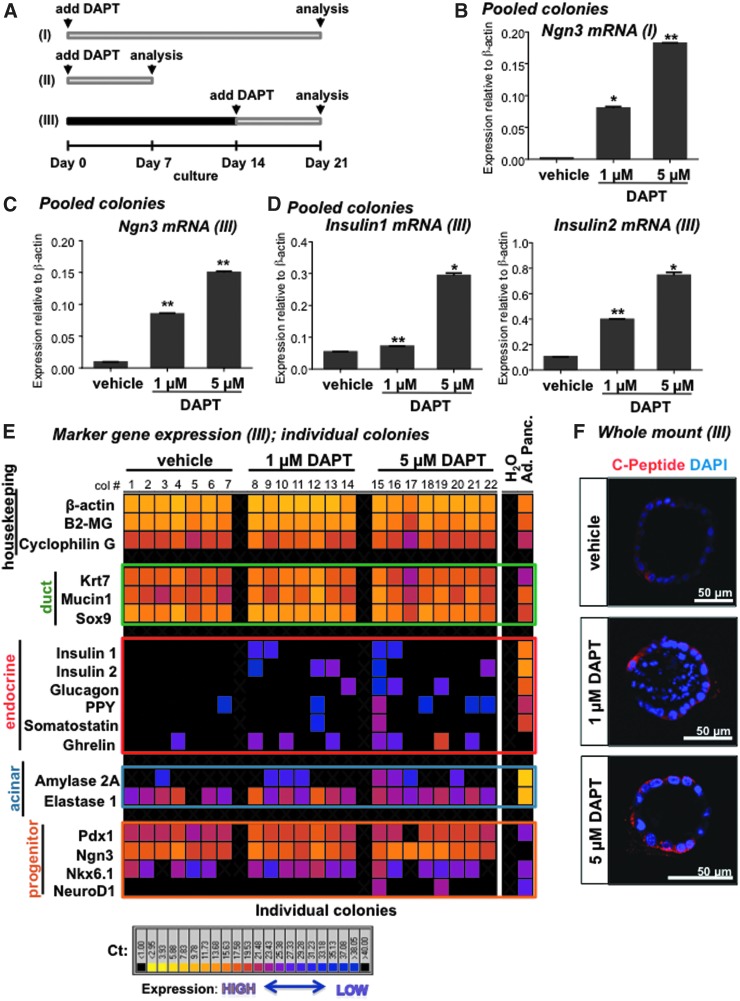
DAPT (N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-gutyl ester), a small molecule that inhibits Notch signaling by inhibiting a processing enzyme, γ-secretase [20], was added to 7F condition at day 0 (Fig. 4A; condition I), which, as expected, caused a downregulation of the Notch target genes Hes1, Hey1, and Hey2 (Supplementary Fig. S4A), and an increase of Ngn3 in pooled 3-week-old colonies (Fig. 4B). L-685,458, another γ-secretase small-molecule inhibitor, was also effective (Supplementary Fig. S5A, B). Interestingly, when DAPT was added on day 0, expression of insulin1 and insulin2 was elevated only in the pooled colonies of 1-week-old (Fig. 4A; condition II and Supplementary Figs. S4C and S5C for L-685,458) but not in the 3-week-old individual colonies (Fig. S4B).
FIG. 4.
Notch signaling regulates endocrine differentiation of colonies in 7F condition. (A) Overview of the different time points of DAPT treatment and colony analysis used in this study. DAPT added on (B) day 0 or (C) day 14 to the 7F culture system increased Ngn3 mRNA expression in 3-week-old colonies. (D) DAPT added on day 14 increased insulin1 and insulin2 mRNA expression in 3-week-old colonies. (E) Single-colony microfluidic qRT-PCR analysis of DAPT-treated cultures. Addition of DAPT on day 14 increased endocrine gene expression in individual 3-week-old colonies. Each column is from a single colony. cDNA from adult pancreas (Ad. Panc.) was used as positive control. H2O was used as negative control. (F) Representative whole-mount immunofluorescence images showing C-Peptide in delayed DAPT-treated colonies compared to vehicle control. Bar: 50 μm. (B–D) *P < 0.05 and **P < 0.005 were determined by t-test compared to vehicle control (n = 2). Data represent mean ± SD. Shown are representative experiments of two independent experiments with the same trend. DAPT, N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-gutyl ester; mRNA, messenger RNA.
Subsequently, DAPT was administered to 2-week-old colonies, and 1 week thereafter colonies were pooled and examined (Fig. 4A, condition III). Again, a decrease of Hes1, Hey1, and Hey2 (Supplementary Fig. S6A, B for L-685,458) and an increase of Ngn3 expression (Fig. 4C and Supplementary Fig. S6C for L-685,458) were observed. Conventional qRT-PCR analysis revealed increased expression of insulin1 and insulin2 in 3-week-old colonies that received DAPT (Fig. 4D) or L-685,458 (Supplementary Fig. S6D) at week 2, compared to those treated with vehicle controls. This was verified by microfluidic qRT-PCR of single colonies; more 3-week-old colonies expressed endocrine hormone genes, including insulin 1 and 2, after treatment with DAPT at week 2 (Fig. 4E). Again, the expression patterns of the six endocrine gene markers examined were different among individual 3-week-old colonies, suggesting that PCFUs are heterogeneous and that they respond to DAPT differently. Whole-mount immunofluorescent staining revealed that C-peptide, a surrogate marker for de novo synthesized insulin, was detectable in the delayed DAPT-treated colonies (Fig. 4F). Together, these data demonstrate that timing of Notch inhibition is important.
Discussion
Here, we eliminated FCS and conditioned media from our standard culture system using serum replacement, defined factors, and small molecules. This new culture system supports adult murine PCFUs to self-renew and differentiate in vitro into cells that resemble ductal, acinar, and endocrine cells in 3-week-old colonies.
Both EGF and nicotinamide were required for colony formation (Supplementary Fig. S2), suggesting that the survival of PCFUs or their immediate progenies was dependent on these factors. EGF is a known survival factor for epithelial cells [21]. Nicotinamide belongs to the vitamin B group and is a precursor for nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP). Both NAD and NADP are critical cofactors for many enzymes involved in metabolism, including enzymes that use oxidation-reduction (redox) reactions to generate energy and those that cause post-translational modification of proteins [22]. Therefore, the requirement of EGF and nicotinamide for the survival of adult PCFUs may not be surprising.
Additionally, we discovered that EGF and nicotinamide were sufficient for the long-term self-renewal of adult PCFUs in vitro (Fig. 3). The roles of EGF in self-renewal of neuronal [23] and mammary [24] stem cells have been documented. The roles of nicotinamide on self-renewal are recently emerging in liver cancer stem cells [25] or hematopoietic stem cells [26]. Our previous work [1] demonstrates that RSPO1 enhances long-term self-renewal of murine adult PCFUs in the standard culture condition. In serial-replating experiments addition of RSPO1 to the EN condition enhanced the expansion of PCFUs that was comparable to the 7F condition (not shown). Together, these studies demonstrate that self-renewal of adult PCFUs in vitro can be influenced by multiple signaling molecules.
EGF and nicotinamide are present in the culture medium devised by Huch et al. [2] to grow adult murine pancreatic organoids. However, using larger ductal cell clusters rather than dissociated single cells, omission of EGF, nicotinamide or RSPO1 only affected colony formation in the long-term culture but not in the initial primary culture [2]. As mentioned, freshly sorted CD133+Sox9/EGFP+ ductal cells failed to survive when nicotinamide or EGF was omitted (Supplementary Fig. S2). Together, these findings may suggest that cell clusters provide paracrine survival signals. Alternatively, differences in other media components may affect colony formation of progenitor cells (more discussion below).
Interestingly, fetal pancreatic progenitor cells of mice form organoids in certain culture conditions [3,27] but not in our standard culture system (not shown). The survival of the fetal organoids requires FGF and a small molecule inhibitor against ROCK, but not EGF [3]. EGF has an effect on proliferation and differentiation but not survival of fetal pancreatic progenitor cells [27]. Collectively, these studies suggest that fetal and adult pancreatic progenitor cells have different signaling requirements for survival.
Our previous [1] and present culture systems allow the detection of tri-potent progenitor cells of adult mice, whereas the Huch condition [2] supports bi-potent progenitor cells having duct and endocrine lineage potentials. This discrepancy could be due to four possibilities. First, adult PCFUs are heterogeneous and different sub-populations may respond to dissimilar culture conditions. Second, culture systems may lack certain intrinsic or extrinsic cues to permit lineage expression [3]. For example, it is necessary to overexpress three transcription factors (Pdx1, Ngn3, and MafA) in adult pancreatic organoids grown in the Huch-like condition to detect insulin gene expression [4,5]. The 2-week-old colonies in our 7F condition required inhibition of Notch signaling for an enhanced endocrine differentiation (Fig. 4). Third, inhibitory signals maybe present in the Huch condition. We recently showed that Matrigel at 1%, compared to no addition, increased ductal cells and decreased endocrine and acinar cells in colonies derived from PCFUs of 1-week-old mice in our standard culture condition supplemented with a laminin hydrogel [28]. Therefore, the high concentrations (>90%) of Matrigel used in the Huch culture system may discourage the growth of endocrine and acinar cells. Fourth, the conventional qRT-PCR analysis used in the Huch study is less sensitive than the microfluidic qRT-PCR employed in our laboratory. These possibilities require further investigation.
In summary, our serum-free, conditioned medium-free culture system has allowed (1) the identification of EGF and nicotinamide as essential and sufficient factors for colony formation, (2) the discernment of Notch signaling having impacts on adult PCFU differentiation similar to pancreas development, and (3) the tri-lineage differentiation of adult PCFUs without genetic manipulations. We anticipate that this culture system will accelerate the knowledge gains and the potential use of adult pancreatic progenitors for regenerative medicine in type-1 diabetes.
Supplementary Material
Acknowledgments
We thank Lucy Brown and Alexander Spalla from the Analytical Cytometry Core, and Ricardo Zerda and Zhuo Li from the Electron Microscopy Core at City of Hope for assistance in sorting and transmission electron microscopy, respectively. Nancy Linford provided editorial assistance for the article. This work is supported in part by the National Institutes of Health (R01DK099734 to H.T.K., P30CA33572); Office of Naval Research (ONR-N00014-02-1 0958); and National Science Foundation (NSF-DBI-9970143). Support from the Oxnard Foundation and Ella Fitzgerald Foundation to H.T.K. are also gratefully acknowledged. L.W. is supported by a postdoctoral fellowship as part of an institutional grant to City of Hope from the California Institute for Regenerative Medicine (CIRM).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Jin L, Feng T, Shih HP, Zerda R, Luo A, Hsu J, Mahdavi A, Sander M, Tirrell DA, Riggs AD. and Ku HT. (2013). Colony-forming cells in the adult mouse pancreas are expandable in Matrigel and form endocrine/acinar colonies in laminin hydrogel. Proc Natl Acad Sci U S A 110:3907–3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huch M, Bonfanti P, Boj SF, Sato T, Loomans CJ, van de Wetering M, Sojoodi M, Li VS, Schuijers J, et al. (2013). Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J 32:2708–2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greggio C, De Franceschi F, Figueiredo-Larsen M, Gobaa S, Ranga A, Semb H, Lutolf M. and Grapin-Botton A. (2013). Artificial three-dimensional niches deconstruct pancreas development in vitro. Development 140:4452–4462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorrell C, Tarlow B, Wang Y, Canaday PS, Haft A, Schug J, Streeter PR, Finegold MJ, Shenje LT, Kaestner KH. and Grompe M. (2014). The organoid-initiating cells in mouse pancreas and liver are phenotypically and functionally similar. Stem Cell Res 13:275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J, Sugiyama T, Liu Y, Wang J, Gu X, Lei J, Markmann JF, Miyazaki S, Miyazaki J, et al. (2013). Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells. Elife 2:e00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin L, Gao D, Feng T, Tremblay J, Quijano J, Wedeken L, Luo A, Hsu J, Mahdavi A, et al. (2016). Cells with surface expresion of CD133 high CD71 low are enriched for tripotent colony-forming progenitor cells in adult murine pancreas. Stem Cell Res 16:40–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang FX. and Morahan G. (2014). Pancreatic stem cells remain unresolved. Stem Cells Dev 23:2803–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ku HT, Chai J, Kim YJ, White P, Purohit-Ghelani S, Kaestner KH. and Bromberg JS. (2007). Insulin-expressing colonies developed from murine embryonic stem cell-derived progenitors. Diabetes 56:921–929 [DOI] [PubMed] [Google Scholar]
- 9.Winkler M, Trieu N, Feng T, Jin L, Walker S, Singh L. and Ku HT. (2011). A quantitative assay for insulin-expressing colony-forming progenitors. J Vis Exp 57:e3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME. and Heintz N. (2003). A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425:917–925 [DOI] [PubMed] [Google Scholar]
- 11.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ. and Clevers H. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459:262–265 [DOI] [PubMed] [Google Scholar]
- 12.Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G. and Sander M. (2007). SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci U S A 104:1865–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, et al. (2011). Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet 43:34–41 [DOI] [PubMed] [Google Scholar]
- 14.Jonsson J, Carlsson L, Edlund T. and Edlund H. (1994). Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 371:606–609 [DOI] [PubMed] [Google Scholar]
- 15.Schaffer AE, Freude KK, Nelson SB. and Sander M. (2010). Nkx6 transcription factors and Ptf1a function as antagonistic lineage determinants in multipotent pancreatic progenitors. Dev Cell 18:1022–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu G, Dubauskaite J. and Melton DA. (2002). Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129:2447–2457 [DOI] [PubMed] [Google Scholar]
- 17.Muraro MJ, Dharmadhikari G, Grun D, Groen N, Dielen T, Jansen E, van Gurp L, Engelse MA, Carlotti F, de Koning EJ. and van Oudenaarden A. (2016). A single-cell transcriptome atlas of the human pancreas. Cell Syst 4:385–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U. and Edlund H. (1999). Notch signalling controls pancreatic cell differentiation. Nature 400:877–881 [DOI] [PubMed] [Google Scholar]
- 19.Murtaugh LC, Stanger BZ, Kwan KM. and Melton DA. (2003). Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A 100:14920–14925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shih HP, Kopp JL, Sandhu M, Dubois CL, Seymour PA, Grapin-Botton A. and Sander M. (2012). A Notch-dependent molecular circuitry initiates pancreatic endocrine and ductal cell differentiation. Development 139:2488–2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang L. and Baker NE. (2003). Cell cycle withdrawal, progression, and cell survival regulation by EGFR and its effectors in the differentiating Drosophila eye. Dev Cell 4:359–369 [DOI] [PubMed] [Google Scholar]
- 22.Opitz CA. and Heiland I. (2015). Dynamics of NAD-metabolism: everything but constant. Biochem Soc Trans 43:1127–1132 [DOI] [PubMed] [Google Scholar]
- 23.Craig CG, Tropepe V, Morshead CM, Reynolds BA, Weiss S. and van der Kooy D. (1996). In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J Neurosci 16:2649–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snedeker SM, Brown CF. and DiAugustine RP. (1991). Expression and functional properties of transforming growth factor alpha and epidermal growth factor during mouse mammary gland ductal morphogenesis. Proc Natl Acad Sci U S A 88:276–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu C, Liu L, Shan J, Shen J, Xu Y, Zhang Q, Yang Z, Wu L, Xia F, et al. (2013). Histone deacetylase 3 participates in self-renewal of liver cancer stem cells through histone modification. Cancer Lett 339:60–69 [DOI] [PubMed] [Google Scholar]
- 26.Ludin A, Gur-Cohen S, Golan K, Kaufmann KB, Itkin T, Medaglia C, Lu XJ, Ledergor G, Kollet O. and Lapidot T. (2014). Reactive oxygen species regulate hematopoietic stem cell self-renewal, migration and development, as well as their bone marrow microenvironment. Antioxid Redox Signal 21:1605–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonfanti P, Nobecourt E, Oshima M, Albagli-Curiel O, Laurysens V, Stange G, Sojoodi M, Heremans Y, Heimberg H. and Scharfmann R. (2015). Ex vivo expansion and differentiation of human and mouse fetal pancreatic progenitors are modulated by epidermal growth factor. Stem Cells Dev 24:1766–1778 [DOI] [PubMed] [Google Scholar]
- 28.Ghazalli N, Mahdavi A, Feng T, Jin L, Kozlowski MT, Hsu J, Riggs AD, Tirrell DA. and Ku HT. (2015). Postnatal pancreas of mice contains tripotent progenitors capable of giving rise to duct, acinar, and endocrine cells in vitro. Stem Cells Dev 24:1995–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ku HT, Zhang N, Kubo A, O'Connor R, Mao M, Keller G. and Bromberg JS. (2004). Committing embryonic stem cells to early endocrine pancreas in vitro. Stem Cells 22:1205–1217 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.