Insulin-like peptide 3 levels are normal and positively correlated with LH levels in infants with Klinefelter syndrome, in contrast to adult patients.
Abstract
Context:
Klinefelter syndrome (KS) is the most common sex chromosome disorder and a major cause of male infertility. In adult patients, serum inhibin B and anti-Mullerian-hormone (AMH) are undetectable, testosterone secretion is often impaired, and the tubules are depleted of germ cells. Before puberty, inhibin B, AMH, and testosterone levels are within the normal range.
Objective:
Sertoli and Leydig cell secretions, including insulin-like peptide-3 (INSL3), were evaluated in infants with nonmosaic XXY karyotype to assess testicular function soon after birth.
Design:
The study was conducted in four University Pediatric Departments from the United States and France.
Subjects:
Sixty-eight prenatally diagnosed infants aged 2–750 d were enrolled.
Main Outcome Measures:
Serum FSH, LH, inhibin B, AMH, and INSL3 were measured by immunoassay, and testosterone was measured by tandem mass-spectrometry.
Results:
In infants with KS, INSL3 levels transiently increased at 2–3 months of age and were significantly correlated with testosterone (Spearman r = 0.57) and LH (Spearman r = 0.73) levels. They did not differ from controls. Testosterone levels were within the normal range, but most of them were below the median of controls. Inhibin B and AMH levels were also within normal range. Inhibin B was correlated with FSH (Spearman r = 0.49). AMH was not correlated with FSH or testosterone. FSH levels were above normal in 25% of patients, despite normal inhibin B levels.
Conclusions:
In infants with KS, Leydig cells are normally sensitive to the LH proliferative effect. In contrast, the Sertoli cell sensitivity to FSH is questionable, which may be prophetic of the postpubertal Sertoli cell resistance to FSH.
Klinefelter syndrome (KS) is characterized by a specific phenotype related to the abnormal 47,XXY karyotype. It is the most common sex chromosome disorder, affecting about 1 in 500 to 1000 males at birth (1–3). In these subjects, seminiferous tubules degenerate during pubertal maturation (reviewed in Ref. 4). As a result, KS accounts for about 4% of infertile men and 11% of azoospermic men (5).
It has been previously established (6, 7) that the Sertoli cell hormones, inhibin B and anti-Mullerian hormone (AMH), are normally secreted before puberty in KS, including in infants. Both inhibin B and testosterone undergo the postnatal increase characteristic of the so-called minipuberty. However, the degree of testosterone secretion in infants with KS is controversial because it has been reported either as low normal (7–9) or high normal (10).
In adults with KS, testosterone levels have been repeatedly reported as subnormal (11, 12), and these low levels have been considered as a risk factor predisposing to the metabolic syndrome (13). Consequently, testosterone administration has been proposed as a protective tool in adolescent and adult subjects who had testosterone levels below the normal range (11, 12, 14) or at least high LH levels despite low normal testosterone levels (15).
The successful treatment of infertility in adult KS patients by means of testicular sperm extraction followed by intracytoplasmic sperm injection makes it of marked importance to evaluate the factors responsible for successful or failed sperm extraction. Accelerated germ cell depletion has been reported as simultaneous to the onset of puberty (16, 17). Sertoli cell marker secretion and expression have been shown to decrease dramatically at the end of puberty (8, 17, 18). These findings prompted clinical researchers to evaluate the hormonal environment of germ cells before and during puberty in KS patients, with the aim to better understand the decline in tubular functions and to propose future interventions for the preservation of germ cells. Given the clinical questions around testosterone treatment in infancy in KS and the controversy about the status of testosterone production during infancy, we undertook a study of Leydig cell secretion in comparison to Sertoli cell secretion in a large cohort of infants with KS. Our aim was to add new data to our previous study (6) to ascertain the hormone environment of germ cell soon after birth and to help describe the natural history of testis function in boys with nonmosaic XXY karyotype. We measured the Leydig cell peptide hormone insulin-like peptide 3 (INSL3), as well as inhibin B, AMH, and testosterone, in a cohort of 68 KS infants, compared with a control group.
INSL3 is a peptide hormone belonging to the relaxin family, produced in mammals by the testis and ovary (reviewed in Ref. 19). Its biological action is mediated via a receptor currently known as relaxin family peptide receptor 2, a member of the G protein-coupled membrane receptor family. In mammals, its role in testis descent during fetal life is well documented (reviewed in Ref. 20). Some cases of cryptorchidism have been related to a mutation either in the INSL3 gene or the relaxin family peptide receptor 2 gene (21). In adults, INSL3 acts as a germ cell survivor factor (22) and may also be involved in follicle selection and survival (23).
In the male, INSL3 is produced by Leydig cells and depends on LH activity (24). However, its relationship with LH is not an acute regulation similar to that of testosterone (20).
We report in this study that in infants with KS, INSL3 levels do not differ from levels in control infants and are positively correlated with testosterone and with LH levels.
Subjects and Methods
Subjects
Sixty-eight 2- to 750-d-old infants with nonmosaic XXY karyotype were enrolled in the study. Thirty-one were recruited in the United States (Columbia University, New York, NY; and Thomas Jefferson University, Philadelphia, PA) and 37 in France (Hopital Trousseau and Hopital Saint Vincent de Paul, Paris). All of them were prenatally diagnosed in the course of prenatal cytogenetic examination in amniotic fluid from pregnancies at risk for Down syndrome.
At birth, they had normal weight and length: 3.38 ± 0.44 kg and 50.6 ± 1.8 cm, respectively (mean ± sd). None had external genital tract abnormality. At first examination, they were sampled for hormone assays after parents' informed consent or had clinical samples drawn later for evaluation of gonadal function with parents' informed consent. Four subjects were sampled twice within the first 2 yr of life.
The study was approved by the local ethical committees.
Hormone assays
Gonadotropins FSH and LH, inhibin B, and AMH were assayed by immunoassay as previously described (6).
Testosterone was assayed by ultra-pressure liquid chromatography/tandem mass spectrometry, using Quattro Premier XE mass spectrometer (Waters-France, St-Quentin en Yvelines, France). The sensitivity was 0.02 nmol/liter. The intra- and interassay coefficients of variation were at the level 8.9 nmol/liter (115 ng/dl), 3.9 and 4.1%, respectively, and at the level 17.1 nmol/liter (493 ng/dl), 3.2 and 4.4%, respectively.
Reference values were established in 215 infants who were aged 2–750 d, sampled for various reasons, and free of any metabolic or endocrine disorder.
INSL3 was assayed by RIA in 50 infants from the cohort of 68 subjects with KS and in 50 control infants using Phoenix reagents (Phoenix Pharmaceuticals, Belmont, CA). According to the manufacturer, the antiserum did not exhibit any significant cross-reactivity with other members of the relaxin family (INSL4, INSL5, INSL6, INSL7), or with insulin, C-peptide, and inhibin B. In our hands, sensitivity was 11 pg/ml. Intraassay coefficients of variation were at the levels 36 and 115 pg/ml, 9.7 and 5%, respectively.
Statistics
The correlations between hormone levels were computed by means of the Spearman nonparametric method. Partial correlations between hormone levels and age at sampling were also computed. Comparison between KS infants and controls was performed by means of the Wilcoxon nonparametric estimate. A P < 0.05 was considered significant.
Results
INSL3 levels in infants with KS did not differ from controls. They exhibited a marked increase during the first month of life, reaching a maximum at about 2–3 months of age, as in control male infants (Fig. 1). Thereafter, INSL3 levels decreased sharply similarly in control and XXY infants, with some levels being below the detection limit. The median levels (and range) at 16 d to 5 months of age (n = 32 per subgroup) were 92 (26–222) pg/ml in KS and 88 (<11–190) pg/ml in controls (P = 0.59, KS vs. controls). They were at 6 months to 2 yr of age (n = 18 per subgroup) 39 (<11–110) pg/ml in KS and 32 (<11–80) pg/ml in controls (P = 0.30, KS vs. controls).
Fig. 1.
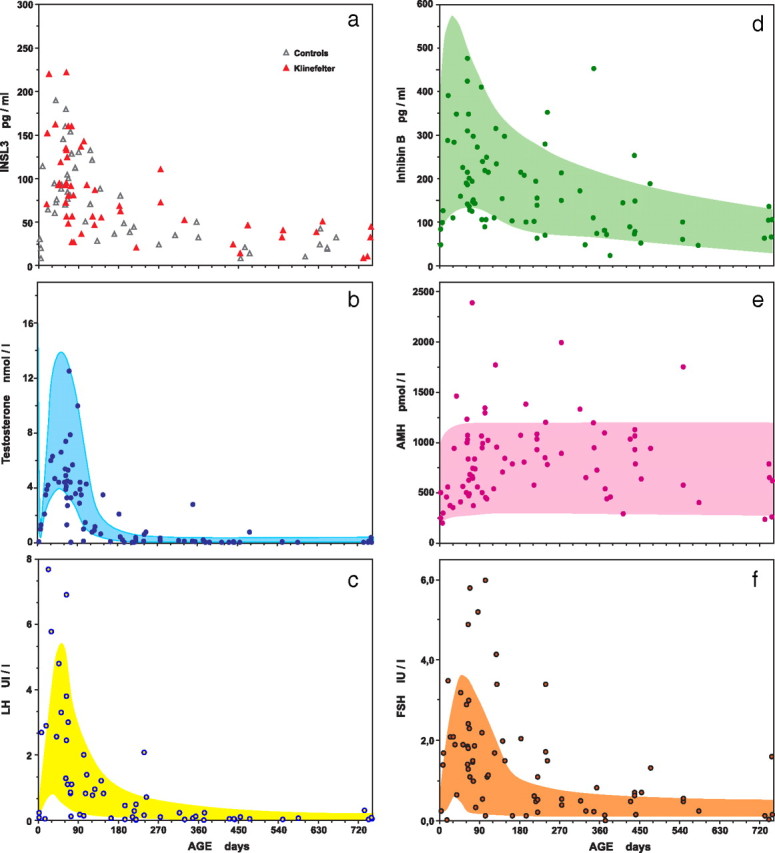
Serum levels of INSL3 (a), testosterone (b), LH (c), inhibin B (d), AMH (e), and FSH (f) in infants with nonmosaic KS, compared with control levels. One nanomole/liter of testosterone = 28.8 ng/dl. The limits of the shaded areas are the smoothed 5th and 95th percentiles of control levels.
INSL3 levels were positively correlated with testosterone levels (Spearman r = 0.57; P < 0.0001) and with LH levels (Spearman r = 0.73; P < 0.0001) (Fig. 2). When computing partial correlation between age, INSL3 and testosterone or LH, INSL3 levels remained significantly correlated with testosterone and with LH levels (r = 0.314, P < 0.05; and r = 0.547, P < 0.0001, respectively).
Fig. 2.
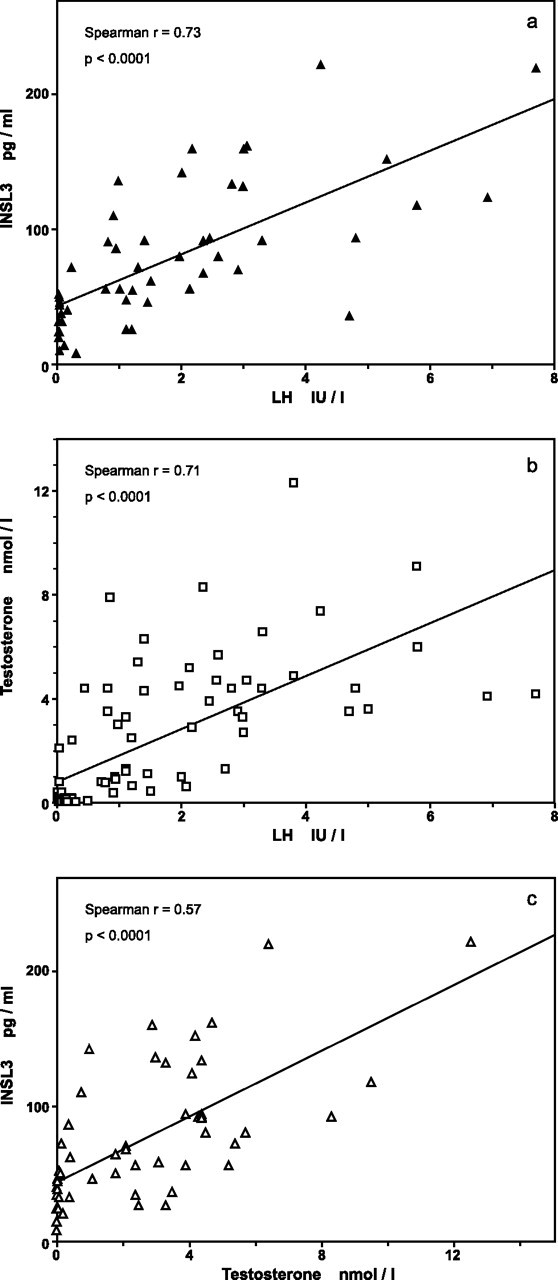
Correlations between serum levels of INSL3 (a), testosterone (b), and LH (c) in infants with nonmosaic KS. One nanomole/liter of testosterone = 28.8 ng/dl. Regression lines are shown, although rank correlations were computed.
Similar correlations were found in the group of control infants: INSL3 vs. LH, Spearman r = 0.666, P < 0.0001; partial correlation, r = 0.404, P < 0.01; and INSL3 vs. testosterone, Spearman r = 0.72, P < 0.0001; partial correlation, r = 0.542, P < 0.001.
Testosterone levels measured by tandem mass spectrometry were within the relevant normal range, but only five of 38 KS patients aged 16 to 120 d had testosterone concentration above the median of control values.
Testosterone levels were highly correlated with LH levels (Fig. 2B) [Spearman r = 0.71 (P < 0.0001)] and remained significantly correlated with LH when computing partial correlations between age at sampling, testosterone, and LH (r = 0.398; P < 0.0001).
Inhibin B and AMH levels were almost all within normal ranges (Fig. 1). Thirteen subjects had inhibin B levels below the 5th percentile of normal range, and 10 subjects had AMH levels above the 95th percentile of normal range.
The majority of FSH levels were within normal limits, but 25% of subjects had FSH levels above the 95th percentile of controls (Fig. 1f). In contrast, all LH levels (Fig. 1c) were within the normal range, except in four subjects (5% of the cohort).
Inhibin B levels were significantly correlated with FSH levels: Spearman r = 0.49; P = 0.0002 (Fig. 3a). Inhibin B levels remained significantly correlated with FSH when computing partial correlation between age at sampling, inhibin B, and FSH (r = 0.244; P < 0.05).
Fig. 3.
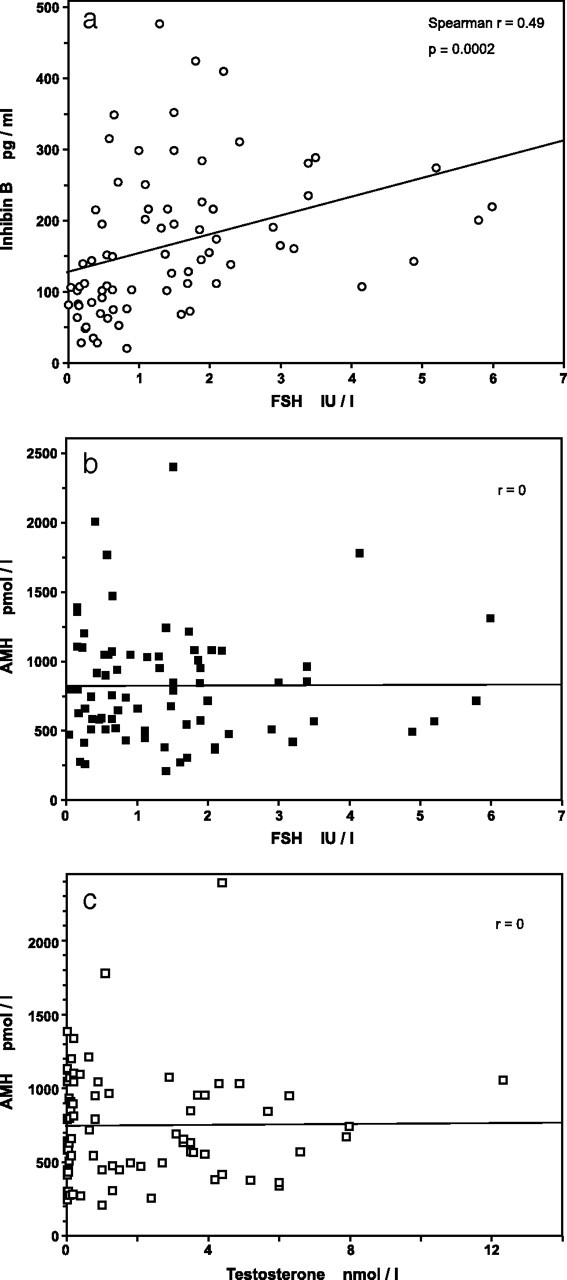
Correlations between serum levels of inhibin B (a), FSH, AMH (b and c), and testosterone in infants with nonmosaic KS. One nanomole/liter of testosterone = 28.8 ng/dl. Regression lines are shown, although rank correlations were computed.
There was no correlation between AMH and FSH levels or between AMH and testosterone levels: r = 0 for both (Fig. 3, b and c), even during the rising phase of hormone levels from birth to 90 d of life.
Discussion
We report here for the first time that INSL3 levels in infants with KS did not differ from the levels in normal male control infants. INSL3 levels, as in control infants, exhibited the typical rise of the so-called minipuberty, as has been described for testosterone and inhibin B levels.
INSL3 secretion and KS
So far, INSL3 levels in KS have been reported previously only in adolescents and adults.
Wikström et al. (25) found normal levels in 14 adolescents with KS aged 10–13 yr, compared with 32 controls aged 9–14 yr. It should be noted, however, that in this study the number of subjects at pubertal stages 2 and 3 was low. Foresta et al. (26) found very low levels in nine untreated adults with KS, with almost no overlap with normal adult levels. Bay et al. (27) reported that in adults with KS, mean INSL3 levels were eight times lower than in normal men, but the majority of them were given testosterone. Of the five untreated men with KS, three had normal lNSL3 levels.
Thus, it seems that the impairment in INSL3 production found in some adults with KS occurs during or after puberty because infants have quite normal secretion as reported here.
INSL3 and the LH-Leydig cell axis: the Leydig cell complement is normal in infants with KS
In adolescent boys with KS, Wikström et al. (25) found a positive correlation between INSL3 and LH levels. However, they did not take into account the effect of age and did not compute the partial correlation between age at sampling, INSL3, and LH levels. No clear data are available in adults with KS. Our study confirmed that, independent of the effect of the simultaneous change in LH and INSL3 secretion with age, INSL3 levels are positively correlated with LH levels in KS infants.
Previous reports in normal adults showed that INSL3 levels were positively correlated with testosterone (27) and LH levels (26). In adolescent boys, Ferlin et al. (28) found a significant correlation of INSL3 with LH levels at all pubertal stages.
Prolonged suppression of LH by means of testosterone administration markedly suppressed INSL3 secretion in normal men (24, 27), as well as in adolescent subjects with KS (25). Administration of a GnRH antagonist + testosterone suppressed INSL3 RNA expression in testicular biopsies from men requesting vasectomy (29). Repeated administration of human chorionic gonadotropin increased INSL3 levels in men with hypogonadotropic hypogonadism (27), as well as in normal men previously suppressed by means of testosterone + medroxyprogesterone (24). These data are consistent with the concept that INSL3 secretion is LH-dependent.
However, a single injection of human chorionic gonadotropin failed to increase INSL3 levels (24, 27), suggesting that INSL3 secretion is constitutive and not acutely regulated. In accordance with this assumption, INSL3 levels are negatively correlated with LH levels in hemiorchidectomized men (30). In these men, a subsequent increase in LH secretion resulted in a compensatory increase in testosterone production by the remaining testis. By contrast, INSL3 levels remained intermediate between men with anorchia and normal men, suggesting that INSL3 levels reflect the size of the Leydig cell complement rather than LH activity. Studies in rodents or in Leydig cell cultures are consistent with such a model (reviewed in Ref. 20), except in the experiments conducted by Tremblay et al. (31) who found a positive effect of testosterone on INSL3 transcription in MA-10 Leydig cells. In addition, it should be noted that three independent sites for the binding of steroidogenic factor-1, a transcription factor involved in gonadal development, are present on the INSL3 gene promoter (32).
In conclusion, it is likely that LH exerts its positive effect on INSL3 secretion via a stimulation of Leydig cell proliferation and differentiation. According to this point of view, INSL3 serum level would be a marker of the Leydig cell complement during the two proliferative phases, i.e. minipuberty and puberty. Indeed, in a large cohort of 3-month-old boys (33), as well as in normal adolescent boys (28), INSL3 levels were reported as being markedly higher than in prepubertal boys aged 4 to 10 yr.
In this setting, our results in infants with KS suggest that the Leydig cell complement is quite normal during the first year of life, and the Leydig cells are quite sensitive to the proliferating and differentiating action of LH.
Inhibin B and the FSH-Sertoli cell axis: is there a relative Sertoli cell resistance to FSH in infants with KS?
FSH levels are above the 95th percentile of controls in 25% of infants with KS. However, all of these subjects had inhibin B levels within the normal range. Conversely, in 13 subjects (19% of the cohort), inhibin B levels are below the 5th percentile of normal range despite within normal range FSH levels. On the other hand, inhibin B levels are positively correlated with FSH as in the dynamic phase of puberty, whereas in the adult steady state there is an inverse relationship between FSH and inhibin B (34). It may be suggested that the increase in FSH production in a significant proportion of infants with KS is a compensatory response to a relative Sertoli cell resistance to FSH, prophetic of the pubertal collapse of Sertoli cell secretions (16, 17). The subnormal inhibin B level in 19% of subjects is consistent with such an assumption.
The Sertoli cell hormone AMH or Mullerian-inhibiting substance plays an important role in sexual differentiation during fetal life by promoting the Mullerian duct regression (35). After birth, AMH secretion has been reported as FSH-dependent both through a direct stimulation of the Sertoli cell by FSH and as a consequence of the proliferative effect of FSH upon the Sertoli cell complement (36). In our subjects, AMH levels were not correlated with FSH levels. This lack of relationship between FSH and AMH levels may give evidence of the independence of AMH secretion from FSH in infancy.
AMH and testosterone in infants with KS
Large amounts of AMH are secreted in prepubertal boys, maximum levels being achieved between 1 month and 1 yr of age (37). Thereafter, AMH levels decrease slowly before puberty, then rapidly as testosterone levels are increasing. The inverse relationship between AMH and testosterone was reported in various conditions, normal puberty, abnormal puberty such as testotoxicosis, and after testosterone suppression by means of GnRH agonists (37). The mechanisms involved in AMH inhibition act mainly at the mRNA levels as shown by experiments in the mouse (38). Surprisingly, there is no inverse relationship between AMH and testosterone in either KS or control infants. In KS infants, as in control infants, AMH levels increased simultaneously with testosterone levels for the first months of life and were not affected by the subsequent decrease in testosterone levels. This suggests a resistance of Sertoli cells to testosterone. In fact, it was recently shown that expression of the androgen receptor mRNA is deeply depressed in normal infants relative to adults (39). As a consequence, Sertoli cells from infants with KS or from normal male infants expressed AMH despite the high testosterone environment during the first 6 months of life, as do Sertoli cells in subjects with the androgen insensitivity syndrome (37).
Conclusion
Our data regarding INSL3 secretion in infants with nonmosaic KS provide evidence that the Leydig cells are normally sensitive to the proliferating and differentiating effect of LH and support the assumption that the Leydig cell complement is normal in size given the similar concentrations found in KS infants and controls. These results point to uncertainties about treating KS infants with testosterone based on their testosterone levels (9). According to reported data, testosterone replacement in KS subjects has positive somatic and behavioral effects (reviewed in Refs. 12 and 15). Lifelong substitution therapy is indicated as soon as serum testosterone levels become low, or if LH levels are above normal even if testosterone levels are in the low end of the normal range (15). The age of 11–12 yr has been proposed as the optimum time for replacement therapy (14). Also, KS infants may benefit from testosterone administration at the age of minipuberty (40). Subjects with micropenis have been treated successfully by means of transcutaneous or im testosterone administration (15). It should be noted, however, that testosterone levels are within the normal range in XXY prenatally diagnosed infants, although most of them were below the median of normal. In addition, testosterone administration leads to LH suppression, and, as a consequence, to INSL3 suppression as mentioned above. INSL3 function in children and adults is not fully understood, but it has been reported that in male rats, its receptor is expressed in germ cells and INSL3 administration suppresses germ cell apoptosis in vivo (22). Given the uncertainty regarding the effects of INSL3 suppression resulting from testosterone administration and the lack of comparative studies, some investigators think that testosterone administration is only indicated in children whose testosterone levels are below normal.
In contrast to the LH-INSL3 axis, the sensitivity of Sertoli cells to FSH may not be intact in infants with KS because a significant proportion do have increased FSH levels despite normal inhibin B concentrations. Such a situation may predict the postpubertal resistance of Sertoli cells to FSH action in subjects with KS. It may prompt clinical investigators to carefully monitor FSH changes in children with KS long before puberty, with the hope of designing future conservative interventions to prevent germ cell degeneration.
Acknowledgments
The skillful assistance of Aurélie Canicio, Catherine Gaillard, Yvette Le Bihan, Nathalie Robert, and Josette Salaün is gratefully acknowledged.
This work was supported in part by Institut de Recherche Endocrinienne et Metabolique (Paris, France).
Disclosure Summary: S.C., J.L.R., C.B., M.R., and N.L. have nothing to declare. I.F. is a chairperson of the Research Committee of the Klinefelter Syndrome Association.
Footnotes
- AMH
- Anti-Mullerian hormone
- INSL3
- insulin-like peptide 3
- KS
- Klinefelter syndrome.
References
- 1. Nielsen J , Wohlert M. 1990. Sex chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Århus, Denmark. Birth Defects Orig Artic Ser 26:209–223 [PubMed] [Google Scholar]
- 2. Bojesen A , Juul S , Gravholt CH. 2003. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab 88:622–626 [DOI] [PubMed] [Google Scholar]
- 3. Morris JK , Alberman E , Scott C , Jacobs P. 2008. Is the prevalence of Klinefelter syndrome increasing? Eur J Hum Genet 16:163–170 [DOI] [PubMed] [Google Scholar]
- 4. Aksglaede L , Wikström AM , Rajpert-De Meyts E , Dunkel L , Skakkebaek NE , Juul A. 2006. Natural history of seminiferous tubule degeneration in Klinefelter syndrome. Hum Reprod Update 12:39–48 [DOI] [PubMed] [Google Scholar]
- 5. Van Assche E , Bonduelle M , Tournaye H , Jorris H , Verheyen G , Devroey P , Van Steirteghem A , Liebaers I. 1996. Cytogenetics of infertile men. Hum Reprod 11(Suppl 4):1–24 [DOI] [PubMed] [Google Scholar]
- 6. Christiansen P , Andersson AM , Skakkebaek NE. 2003. Longitudinal studies of inhibin B levels in boys and young adults with Klinefelter syndrome. J Clin Endocrinol Metab 88:888–891 [DOI] [PubMed] [Google Scholar]
- 7. Lahlou N , Fennoy I , Carel JC , Roger M. 2004. Inhibin B and anti-müllerian hormone, but not testosterone levels, are normal in infants with nonmosaic Klinefelter syndrome. J Clin Endocrinol Metab 89:1864–1868 [DOI] [PubMed] [Google Scholar]
- 8. Sørensen K , Nielsen J , Wohlert M , Bennett P , Johnsen SG. 1981. Serum testosterone of boys with karyotype 47,XXY (Klinefelter's syndrome) at birth. Lancet 2:1112–1113 [DOI] [PubMed] [Google Scholar]
- 9. Ross JL , Samango-Sprouse C , Lahlou N , Kowal K , Elder FF , Zinn A. 2005. Early androgen deficiency in infants and young boys with 47,XXY Klinefelter syndrome. Horm Res 64:39–45 [DOI] [PubMed] [Google Scholar]
- 10. Aksglaede L , Petersen JH , Main KM , Skakkebaek NE , Juul A. 2007. High normal testosterone levels in infants with non-mosaic Klinefelter's syndrome. Eur J Endocrinol 157:345–350 [DOI] [PubMed] [Google Scholar]
- 11. Smyth CM , Bremner WJ. 1998. Klinefelter syndrome. Arch Intern Med 158:1309–1314 [DOI] [PubMed] [Google Scholar]
- 12. Lanfranco F , Kamischke A , Zitzmann M , Nieschlag E. 2004. Klinefelter's syndrome. Lancet 364:273–283 [DOI] [PubMed] [Google Scholar]
- 13. Bojesen A , Kristensen K , Birkebaek NH , Fedder J , Mosekilde L , Bennett P , Laurberg P , Frystyk J , Flyvbjerg A , Christiansen JS , Gravholt CH. 2006. The metabolic syndrome is frequent in Klinefelter's syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care 29:1591–1598 [DOI] [PubMed] [Google Scholar]
- 14. Winter JS. 1990. Androgen therapy in Klinefelter syndrome during adolescence. Birth Defects 26:235–245 [PubMed] [Google Scholar]
- 15. Bojesen A , Gravholt CH. 2007. Klinefelter syndrome in clinical practice. Nat Clin Pract Urol 4:192–204 [DOI] [PubMed] [Google Scholar]
- 16. Wikström AM , Raivio T , Hadziselimovic F , Wikström S , Tuuri T , Dunkel L. 2004. Klinefelter syndrome in adolescence: onset of puberty is associated with accelerated gem cell depletion. J Clin Endocrinol Metab 89:2263–2270 [DOI] [PubMed] [Google Scholar]
- 17. Wikström AM , Hoei-Hansen CE , Dunkel L , Rajpert-De Meyts E. 2007. Immunoexpression of androgen receptor and nine markers of maturation in the testes of adolescent boys with Klinefelter syndrome: evidence for degeneration of germ cells at the onset of puberty. J Clin Endocrinol Metab 92:714–719 [DOI] [PubMed] [Google Scholar]
- 18. Bastida MG , Rey RA , Bergadá I , Bedecarrás P , Andreone L , del Rey G , Boywitt A , Ropelato MG , Cassinelli H , Arcari A , Campo S , Gottlieb S. 2007. Establishment of testicular endocrine function impairment during childhood and puberty in boys with Klinefelter syndrome. Clin Endocrinol (Oxf) 67:863–870 [DOI] [PubMed] [Google Scholar]
- 19. Foresta C , Zuccarello D , Garolla A , Ferlin A. 2008. Roles of hormones, genes, and environment in human cryptorchidism. Endocr Rev 29:560–580 [DOI] [PubMed] [Google Scholar]
- 20. Ivell R , Anand-Ivell R. 2009. Biology of insulin-like factor 3 in human reproduction. Hum Reprod Update 15:463–476 [DOI] [PubMed] [Google Scholar]
- 21. Ferlin A , Zuccarello D , Zuccarello B , Chirico MR , Zanon GF , Foresta C. 2008. Genetic alterations associated with cryptorchidism. JAMA 300:2271–2276 [DOI] [PubMed] [Google Scholar]
- 22. Kawamura K , Kumagai J , Sudo S , Chun SY , Pisarska M , Morita H , Toppari J , Fu P , Wade JD , Bathgate RA , Hsueh AJ. 2004. Paracrine regulation of mammalian oocyte maturation and male germ cell survival. Proc Natl Acad Sci USA 101:7323–7328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spanel-Borowski K , Schäfer I , Zimmermann S , Engel W , Adham IM. 2001. Increase in final stages of follicular atresia and premature decay in corpora lutea in Insl3-deficient mice. Mol Reprod Dev 58:281–286 [DOI] [PubMed] [Google Scholar]
- 24. Bay K , Matthiesson KL , McLachlan RI , Andersson AM. 2006. The effects of gonadotropin suppression and selective replacement on insulin-like factor 3 secretion in normal adult men. J Clin Endocrinol Metab 91:1108–1111 [DOI] [PubMed] [Google Scholar]
- 25. Wikström AM , Bay K , Hero M , Andersson AM , Dunkel L. 2006. Serum insulin-like factor 3 levels during puberty in healthy boys and boys with Klinefelter syndrome. J Clin Endocrinol Metab 91:4705–4708 [DOI] [PubMed] [Google Scholar]
- 26. Foresta C , Bettella A , Vinanzi C , Dabrilli P , Meriggiola MC , Garolla A , Ferlin A. 2004. Insulin-like factor 3: a novel circulating hormone of testis origin in humans. J Clin Endocrinol Metab 89:5952–5958 [DOI] [PubMed] [Google Scholar]
- 27. Bay K , Hartung S , Ivell R , Schumacher M , Jürgensen D , Jorgensen N , Holm M , Skakkebaek NE , Andersson AM. 2005. Insulin-like factor 3 serum levels in 135 normal men and 85 men with testicular disorders: relationship to the luteinizing hormone-testosterone axis. J Clin Endocrinol Metab 90:3410–3418 [DOI] [PubMed] [Google Scholar]
- 28. Ferlin A , Garolla A , Rigon F , Rasi Caldogno L , Lenzi A , Foresta C. 2006. Changes in serum insulin-like factor 3 during normal male puberty. J Clin Endocrinol Metab 91:3426–3431 [DOI] [PubMed] [Google Scholar]
- 29. Bayne RA , Forster T , Burgess ST , Craigon M , Walton MJ , Baird DT , Ghazal P , Anderson RA. 2008. Molecular profiling of the human testis reveals stringent pathway-specific regulation of RNA expression following gonadotropin suppression and progestogen treatment. J Androl 29:389–403 [DOI] [PubMed] [Google Scholar]
- 30. Anand-Ivell R , Wohlgemuth J , Haren MT , Hope PJ , Hatzinikolas G , Wittert G , Ivell R. 2006. Peripheral INSL3 concentrations decline with age in a large population of Australian men. Int J Androl 29:618–626 [DOI] [PubMed] [Google Scholar]
- 31. Tremblay JJ , Robert NM , Laguë E. 2009. Nuclear receptors, testosterone, and posttranslational modifications in human INSL3 promoter activity in testicular Leydig cells. Ann NY Acad Sci 1160:205–212 [DOI] [PubMed] [Google Scholar]
- 32. Sadeghian H , Anand-Ivell R , Balvers M , Relan V , Ivell R. 2005. Constitutive regulation of the Insl3 gene in rat Leydig cells. Mol Cell Endocrinol 241:10–20 [DOI] [PubMed] [Google Scholar]
- 33. Bay K , Virtanen HE , Hartung S , Ivell R , Main KM , Skakkebaek NE , Andersson AM , Toppari J. 2007. Insulin-like factor 3 levels in cord blood and serum from children: effects of age, postnatal hypothalamic-pituitary-gonadal axis activation, and cryptorchidism. J Clin Endocrinol Metab 92:4020–4027 [DOI] [PubMed] [Google Scholar]
- 34. Stewart TM , Liu DY , Garrett C , Jørgensen N , Brown EH , Baker HWG. 2009. Associations between andrological measures, hormones and semen quality in fertile Australian men: inverse relationship between obesity and sperm output. Hum Reprod 24:1561–1568 [DOI] [PubMed] [Google Scholar]
- 35. Josso N , Picard JY , Rey R , di Clemente N. 2006. Testicular anti-Müllerian hormone: history, genetics, regulation, and clinical applications. Pediatr Endocrinol Rev 3:347–358 [PubMed] [Google Scholar]
- 36. Lukas-Croisier C , Lasala C , Nicaud J , Bedecarrás P , Kumar TR , Dutertre M , Matzuk MM , Picard JY , Josso N , Rey R. 2003. Follicle-stimulating hormone increases testicular anti-Mullerian hormone (AMH) production through Sertoli cell proliferation and a non classical cyclic adenosine 5′-monophosphate mediated activation of the AMH gene. Mol Endocrinol 17:550–561 [DOI] [PubMed] [Google Scholar]
- 37. Rey R. 1998. Endocrine, paracrine, and cellular regulation of postnatal anti-Müllerian hormone secretion by Sertoli cells. Trends Endocrinol Metab 9:271–276 [DOI] [PubMed] [Google Scholar]
- 38. Al-Attar L , Noël K , Dutertre M , Belville C , Forest MG , Burgoyne PS , Josso N , Rey R. 1997. Hormonal and cellular regulation of Sertoli cell anti-Müllerian hormone production in the postnatal mouse. J Clin Invest 100:1335–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chemes HE , Rey RA , Nistal M , Regadera J , Musse M , González-Peramato P , Serrano A. 2008. Physiological androgen insensitivity of the fetal, neonatal, and early infantile testis is explained by the ontogeny of the androgen receptor expression in Sertoli cells. J Clin Endocrinol Metab 93:4408–4412 [DOI] [PubMed] [Google Scholar]
- 40. Simpson JL , de la Cruz F , Swerdloff RS , Samango-Sprouse C , Skakkebaek NE , Graham JM , Hassold T , Aylstock M , Meyer-Bahlburg HF , Willard HF , Hall JG , Salameh W , Boone K , Staessen C , Geschwind D , Giedd J , Dobs AS , Rogol A , Brinton B , Paulsen CA. 2003. Klinefelter syndrome: expanding the phenotype and identifying new research directions. Genet Med 5:460–468 [DOI] [PubMed] [Google Scholar]


