Lower bone density in GH-deficient adults is associated with lower IGF-1 SD scores, unreplaced sex steroid deficiency, and corticotropin deficiency.
Abstract
Context:
GH deficiency (GHD) is associated with low bone mineral density (BMD). Risk factors for lower BMD in this GHD population have not been fully elucidated. In particular, there are limited published data in GH-naïve subjects.
Objective:
The objective of the study was to identify endocrine correlates of low BMD in treatment-naïve adult GHD subjects.
Design:
This was a retrospective analysis of data extracted from the (Pfizer International Metabolic Study) KIMS database.
Setting:
The study was an international epidemiological survey of more than 15,000 adult GHD patients from 31 countries.
Patients:
A total of 1218 subjects with stringently defined GHD of adult onset (641 women and 577 men) who were GH naïve and had BMD measured in the posterior anterior lumbar spine and femoral neck by dual-energy X-ray absorptiometry.
Main Outcome Measures:
Variables associated with standardized BMD (sBMD) in adult-onset GHD were examined.
Results:
In the LS, body mass index (r = 0.13, P < 0.01), unreplaced sex steroid deficiency (r = −0.17, P < 0.0001), and corticotropin deficiency (r = −0.11, P < 0.01) were independently associated with sBMD. In the FN, age (r = −0.19, P < 0.0001), female gender (r = −0.18, P < 0.0001), body mass index (r = 0.21, P < 0.0001), and decreased IGF-I sd scores (r = 0.10, P < 0.001) were independently associated with sBMD.
Conclusions:
Hormone variables associated with lower sBMD in patients with adult-onset GHD include unreplaced sex steroid deficiency and corticotropin deficiency in the LS and lower IGF-I SDS in the FN.
GH deficiency (GHD) is associated with low bone mineral density (BMD) and decreased bone turnover (1–3). Patients with untreated GHD have an increased fracture risk (4–7).
The mechanisms underlying the effects of GHD on BMD are complex. GH affects bone both directly and indirectly by stimulating IGF-I synthesis from paracrine or endocrine (hepatic) sources (8–13). In addition to GH, several other hormones have significant effects on BMD, including thyroid hormone, cortisol, and sex steroids (3, 14).
Because patients with GHD may have multiple pituitary hormone deficiencies, leading to hypogonadism, hypothyroidism, or hypoadrenalism, deficiencies of additional pituitary hormones or excess (as a result of overreplacement) may influence BMD in this population. Risk factors for low BMD in patients with GHD have not been thoroughly elucidated and may also include younger age at onset, male gender, decreased GH peak on stimulation testing, and hypogonadism (15–18). However, there are limited published data in GH-naïve subjects.
The present study hypothesis was that the severity of GHD, lower IGF-I levels, and the presence of additional pituitary hormone deficiencies may be associated with lower BMD in patients with untreated GHD (15).
To further characterize possible correlates of lower BMD in patients with untreated, stringently defined GHD, a group of GH-naïve adult patients with GHD of adult-onset (AOGHD) was studied, using data extracted from KIMS (Pfizer International Metabolic Study). This is an international, phase IV, observational, epidemiological survey of more than 15,000 adult GHD patients from 31 countries, being treated with GH replacement, according to local practice. In addition, the association between BMD and previous fracture was examined.
Subjects and Methods
Subjects
The entire KIMS database was searched to identify subjects meeting all the following inclusion criteria: AOGHD, GHD diagnosed based on stringent criteria (Table 1), including the consensus guidelines of the GH Research Society (19), and BMD measurements [by dual energy x-ray absorptiometry on a Hologic (Waltham, MA), Lunar/GE (Madison, WI), or Norland (Ft. Atkinson, WI) densitomer] available at the entry of the KIMS study (within 6 months before to 1 month after beginning GH replacement). The majority of subjects were from western European countries.
Table 1.
Criteria used for the diagnosis of GHD in the study population (19)
| Diagnostic test | Diagnostic cut point | |
|---|---|---|
| Insulin tolerance test | Peak GH <3 ng/ml | |
| Glucagon stimulation test | Peak GH <3 ng/ml | |
| GHRH + arginine stimulation test | If BMI <25 kg/m2 | Peak GH <11.5 ng/ml |
| If BMI 25–30 kg/m2 | Peak GH <8 ng/ml | |
| If BMI >30 kg/m2 | Peak GH <4.2 ng/ml | |
| Arginine stimulation test | Peak GH <0.4 ng/ml | |
| IGF-I | IGF-I SDS <−2 and ≥ three additional pituitary hormone deficiencies | |
The following exclusion criteria were applied, using information from subjects' medical history: any prior use of GH, known end-stage renal disease, organ transplant, celiac disease, primary hyperparathyroidism, hyperthyroidism, osteomalacia, and any known vitamin D deficiency.
Study subjects had provided written informed consent at each participating clinical center at the time of the KIMS entry. The study was conducted according to the principles of the Declaration of Helsinki (20).
Methods
Demographic and clinical data extracted included gender; body weight and body mass index (BMI); age at diagnosis of pituitary disease; age at the diagnosis of GHD; age at KIMS entry; estimated duration of GHD (taken as the difference between age at entry into KIMS minus the age at diagnosis of GHD); cause of GHD (including secretory status of pituitary adenomas); previous radiation therapy to the sella; number and type of other pituitary hormone deficits; sex steroid replacement status; a lifetime history of clinical fracture (excluding fracture of skull, fingers, and toes), or a history of clinical fracture during the estimated duration of GHD. With regard to sex steroid replacement therapy, patients were classified into one of the following: deficient but unreplaced, or deficient but replaced with sex steroids, or endogenously sufficient.
Demographic and clinical data were obtained based on information provided by each participating clinical center. Laboratory data extracted from the database included peak GH levels on stimulation testing (performed at each referring center) and IGF-I sd scores (SDS), calculated based on serum IGF-I levels (measured centrally in a single laboratory).
Between 1994 and October 1997, serum IGF-1 was assayed at Kabi Pharmacia (Stockholm, Sweden) and thereafter at Sahlgrenska University Hospital (Gothenburg, Sweden). Between 1994 and November 2002, a RIA was used to measure IGF-I in serum samples after acid/ethanol precipitation of IGF binding proteins (Nichols Institute, San Juan Capistrano, CA) (21). Subsequently (until September 2006), a chemiluminescence immunoassay was used (Nichols Advantage system). The currently used method is Immulite 2500 (Diagnostic Products Corp. Siemens, Deerfield, IL). To calculate IGF-I SDS, the following formulae were used (between 1994 and 1997): SDS − [ln (IGF-I) − (5.95 − 0.0197 × age)]/0.282; (between 1997 and 2002): SDS = [ln (IGF-I) − (15.92 − 0.0146 × age)]/0.272; and (from 2002 onward) based on data from Brabant et al. (22).
Bone density data (BMD values and z scores) from two skeletal regions [including posterior anterior lumbar spine (LS) and femoral neck (FN)] were also extracted. At any given skeletal region, measured BMD values vary between different densitometers because manufacturers use diverse bone edge detection algorithms and calibration standards. To facilitate direct comparisons between BMD data obtained on different densitometers in population studies, predictive equations have been developed, which yield standardized BMD (sBMD) values (23, 24). In the present study, the sBMD data were calculated using BMD values and equations for the LS and FN as described previously (23, 24).
Statistical analysis
The Student's t test was used to compare normally distributed variables, and the Wilcoxon rank sum test was used to compare nonnormally distributed variables. Comparisons of proportions were conducted using the Fisher's exact test or the χ2 test as appropriate.
Stepwise, multivariate regression analyses were conducted to examine variables independently associated with sBMD (LS and FN) in subjects with AOGHD, with sBMD used as the dependent variable. Independent variables introduced in the model included subject age; gender; body weight (or BMI); peak GH on stimulation testing; IGF-I SDS; estimated GHD duration; sex steroid status (deficiency vs. replacement vs. sufficiency); and presence or absence of deficiencies of corticotropin, TSH, or antidiuretic hormone. Nominal variables were assigned numerical codes for the purpose of regression analyses. Additional multivariate regression analyses were conducted in a prespecified subgroup of women with AOGHD who were younger than 50 yr (n = 404).
The Statistical Analysis System (SAS Institute, Inc., Cary, NC) was used to perform statistical procedures. Data are expressed as means ± sd or median (10th percentile, 90th percentile) as appropriate. Statistical significance was accepted at a P < 0.05 level.
Results
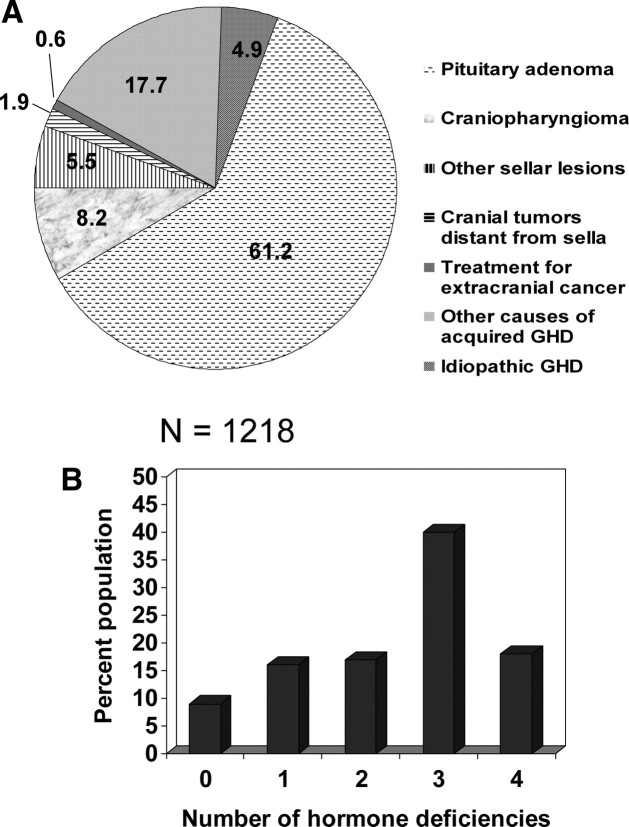
There were 1218 subjects, all diagnosed with AOGHD. There were 641 women (52.6%) and 577 men (47.4%). The majority of subjects were Caucasian from western European countries. All were GH naïve and had severe GHD based on stringent inclusion criteria, as shown in Table 1. Among subjects who underwent insulin tolerance testing, peak GH was 0.50 ng/ml (0.10, 1.80); among subjects who underwent any other stimulation test, peak GH was 0.30 ng/ml (0.10, 2.50). Data on the causes of GHD in the study population are shown in Fig. 1A. The most common cause of GHD was pituitary adenoma. Data on the number of additional pituitary hormone deficiencies are shown in Fig. 1B.
Fig. 1.
A, Causes of GHD in the study population. B, Number of additional pituitary hormone deficiencies in the study population.
Additional characteristics of the study population are shown in Table 2. There were 552 subjects (45.3%) examined on a Hologic densitometer, 601 subjects (49.4%) examined on a Lunar/GE densitometer, and 65 subjects (5.3%) examined on a Norland densitometer.
Table 2.
Characteristics of the study population
| Variable | |
|---|---|
| Number (n) | 1218 |
| Age at diagnosis of pituitary disease (yr) | 40.7 (22.1, 59.6) |
| Age at diagnosis of GHD (yr) | 46.6 (28.2, 63.0) |
| Age at KIMS entry (yr) | 48.3 (31.2, 65.2) |
| Estimated GHD duration (yr) | 0.7 (0.0, 5.5) |
| Gender [females/males (%/%)] | 641/577 (53/47) |
| History of radiation therapy (n (%)) | 348 (28.6) |
| GH stimulation test | |
| Insulin tolerance test [n (%)] | 880 (72%) |
| All others [n (%)] | 338 (28%) |
| IGF-I SDS | −1.5 (−3.5, 0.1) |
| Gonadotropin deficiency [n (%)] | 948 (78) |
| Sex steroid substitution status (def/repl/suff) [n (%)] | 212/736/270 (17/61/22) |
| Corticotropin deficiency [n (%)] | 834 (68) |
| Thyrotropin deficiency [n (%)] | 870 (71) |
| Central diabetes insipidus [n (%)] | 286 (23) |
| History of fracture [n (%)] | 277 (23) |
| Fracture site [spine and pelvis/ribs/femur/lower extremity excluding femur/upper extremity (n (%)] | 20/23/14/70/147 (7.3/8.4/5.1/25.6/53.6) |
| LS sBMD (mg/cm2) | 1033 (847, 1285) |
| LS z score | −0.5 (−2.1, 1.5) |
| FN sBMD (mg/cm2) | 878 (691, 1066) |
| FN z score | −0.3 (−1.6, 1.2) |
Data are shown as number of patients, percentages, mean ± sd, or median (10th and 90th percentiles) as appropriate. def, Sex steroid deficient; repl, sex steroid replaced; suff, endogenously sex steroid sufficient.
Subject characteristics for the study population stratified by sex steroid status are shown in Table 3. Sex steroid-deficient subjects were older than subjects in the other two subgroups. In addition, sex steroid-deficient (even if replaced) subjects had significantly lower IGF-I SDS in comparison with endogenously sex steroid-sufficient subjects. Both sex steroid-deficient and -replaced subjects were more likely to have deficiencies of corticotropin, TSH, or antidiuretic hormone in comparison with endogenously sufficient subjects. Subjects who were sex steroid deficient had significantly lower sBMD in the LS and FN in comparison with subjects who were either sex steroid replaced or sex steroid sufficient. There was no difference in fracture rate between the three subgroups.
Table 3.
Characteristics of the study population stratified by sex steroid status
| Variable | Def | Repl | Suff | Def vs. repl P value | Repl vs. suff P value | Def vs. suff P value |
|---|---|---|---|---|---|---|
| Number (n) | 212 | 736 | 270 | |||
| Age at diagnosis of pituitary disease (yr) | 44.6 (26.9, 62.9) | 39.1 (21.0, 57.5) | 42.1 (23.7, 60.4) | <0.001 | <0.05 | NS |
| Age at diagnosis of GHD (yr) | 51.7 (34.3, 67.9) | 45.3 (26.4, 61.5) | 46.5 (27.9, 64.0) | <0.001 | NS | <0.001 |
| Age at KIMS entry (yr) | 53.6 (35.0, 69.1) | 47.3 (31.0, 63.9) | 47.5 (29.2, 65.0) | <0.001 | NS | <0.001 |
| Estimated GHD duration (yr) | 0.8 (0.0, 5.1) | 0.8 (0.0, 7.1) | 0.6 (0.0, 3.7) | NS | <0.01 | <0.05 |
| Gender (females/males [%/%)] | 184/28 (87/13) | 299/437 (41/59) | 158/112 (59/41) | <0.001 | <0.001 | <0.001 |
| IGF-I SDS | −1.8 (−3.8, 0.1) | −1.7 (−3.6, −0.2) | −1.0 (−2.7, 0.4) | NS | <0.001 | <0.001 |
| Gonadotropin deficiency [n (%)] | 212 (100) | 736 (100) | 0 (0) | NS | <0.001 | <0.001 |
| Corticotropin deficiency [n (%)] | 153 (72) | 567 (77) | 116 (43) | NS | <0.001 | <0.001 |
| Thyrotropin deficiency [n (%)] | 157 (74) | 611 (83) | 105 (39) | <0.01 | <0.001 | <0.001 |
| Central diabetes insipidus [n (%)] | 53 (25) | 206 (28) | 27 (10) | NS | <0.001 | <0.001 |
| History of fracture [n (%)] | 40 (19) | 169 (23) | 73 (27) | NS | NS | NS |
| LS sBMD (mg/cm2) | 989 (800, 1209) | 1054 (839, 1314) | 1038 (885, 1269) | <0.001 | NS | <0.001 |
| LS z score | −0.8 (−1.9, 1.3) | −0.4 (−2.3, 1.5) | −0.5 (−1.7, 1.7) | NS | NS | NS |
| FN sBMD (mg/cm2) | 822 (644, 1029) | 888 (700, 1094) | 888 (691, 1047) | <0.001 | NS | <0.001 |
| FN z score | −0.4 (−2.0, 1.1) | −0.3 (−1.7, 1.2) | −0.1 (−1.6, 1.3) | NS | NS | <0.05 |
Data are shown as number of patients, percentages, mean ± sd, or median (10th and 90th percentiles) as appropriate. def, Sex steroid deficient; NS, not significant; repl, sex steroid replaced; suff, endogenously sex steroid sufficient.
Subject characteristics for the study population stratified by the presence of deficiencies of either corticotropin or TSH are shown in Table 4. At KIMS entry, subjects with TSH deficiency were older than subjects who were sufficient. In addition, subjects with either corticotropin or TSH deficiency had significantly lower IGF-I SDS in comparison with sufficient subjects. Subjects with corticotropin deficiency had significantly lower sBMD in the LS as well as lower z scores in the LS and FN in comparison with sufficient subjects. There were no differences in fracture rate between subgroups of subjects with either corticotropin or TSH deficiency in comparison with respective sufficient subjects.
Table 4.
Characteristics of the study population stratified by the presence or absence of corticotropin or thyrotropin deficiency
| Variable | Corticotropin deficiency vs. sufficiency |
Thyrotropin deficiency vs. sufficiency |
||||
|---|---|---|---|---|---|---|
| Def | Suff | P value | Def | Suff | P value | |
| Number (n) | 834 | 383 | 870 | 346 | ||
| Age at diagnosis of pituitary disease (yr) | 40.1 (22.3, 58.9) | 43.2 (21.3, 60.7) | NS | 40.4 (21.9, 59.1) | 41.4 (22.7, 60.5) | NS |
| Age at diagnosis of GHD (yr) | 46.8 (28.5, 63.7) | 46.4 (27.9, 62.4) | NS | 46.9 (28.3, 63.7) | 45.8 (27.9, 62.4) | NS |
| Age at KIMS entry (yr) | 48.8 (31.4, 65.7) | 47.6 (29.6, 64.2) | NS | 49.1 (31.5, 66.0) | 46.9 (29.1, 63.8) | <0.05 |
| Estimated GHD duration (yr) | 0.7 (0.0, 6.9) | 0.7 (0.0, 4.0) | NS | 0.7 (0.0, 7.1) | 0.7 (0.0, 3.7) | NS |
| Gender [females/males (%/%)] | 429/405 (51/49) | 212/171 (55/45) | NS | 461/409 (53/47) | 180/166 (52/48) | NS |
| IGF-I SDS | −1.7 (−3.7, −0.1) | −1.1 (−3.0, 0.2) | <0.001 | −1.8 (−3.7, −0.1) | −1.1 (−2.7, 0.3) | <0.001 |
| History of fracture [n (%)] | 200 (24) | 80 (21) | NS | 200 (23) | 79 (23) | NS |
| LS sBMD (mg/cm2) | 1028 (828, 1285) | 1055 (885, 1295) | <0.01 | 1032 (838, 1295) | 1038 (869, 1285) | NS |
| LS z score | −0.8 (−2.2, 1.3) | −0.3 (−1.8, 1.7) | <0.01 | −0.5 (−2.1, 1.5) | −0.3 (−1.9, 1.3) | NS |
| FN sBMD (mg/cm2) | 871 (691, 1063) | 883 (704, 1066) | NS | 878 (691, 1076) | 878 (700, 1057) | NS |
| FN z score | −0.4 (−1.7, 1.1) | 0 (−1.5, 1.2) | <0.01 | −0.3 (−1.7, 1.2) | −0.1 (−1.6, 1.1) | NS |
Data are shown as number of patients, percentages, mean ± sd, or median (10th and 90th percentiles) as appropriate. Def, Deficient; NS, not significant; suff, sufficient.
Subject characteristics of those with history of Cushing's disease or prolactinoma are shown in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org. Subjects with history of Cushing's disease were younger at the time of diagnosis of pituitary disease or GHD or at the time of study entry and were more likely to be female. In addition, subjects with Cushing's disease had lower IGF-I SDS, sBMD, and z scores in the FN in comparison with the rest of the AOGHD cohort and were more likely to have a history of fracture. Subjects with a history of prolactinoma showed a trend toward higher sBMD and z scores in the FN.
There were 23 subjects in the study population with a diagnosis of acromegaly who had been rendered GH deficient by prior therapy. They showed no difference in sBMD or z scores (in LS or FN) in comparison with the rest (data not shown). In addition, there was no difference in sBMD or z scores (in LS or FN) between subjects with previous history of radiation therapy (n = 348) in comparison with those who were not irradiated (n = 868; data not shown).
Subject characteristics for the study population stratified by the presence or absence of previous clinical fracture are shown in Supplemental Table 2. Subjects with a history of fracture were more likely to be male, had a longer estimated GHD duration, and showed trends toward lower sBMD in the LS and FN.
Using multivariate regression analysis in the entire AOGHD cohort, the following variables were found to be independently associated with sBMD in LS: BMI (r = 0.13, P < 0.01); unreplaced sex steroid deficiency (r = −0.17, P < 0.0001); and corticotropin deficiency (r = −0.11, P < 0.01). Using body weight instead of BMI in the model did not affect these results (data not shown). In the prespecified subgroup of women younger than 50 yr (n = 404), the following variables were found to be independently associated with sBMD in LS: BMI (r = 0.14, P < 0.05); estimated GHD duration (r = −0.18, P < 0.05); and TSH deficiency (r = −0.23, P < 0.01). Using body weight instead of BMI in the model did not affect these results (data not shown).
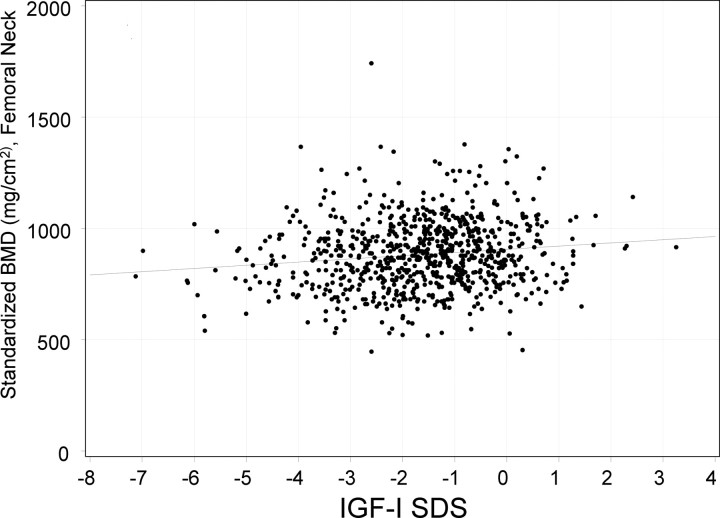
Using multivariate regression analysis in the entire AOGHD cohort, the following variables were found to be independently associated with sBMD in FN: age (r = −0.19, P < 0.0001); female gender (r = −0.18, P < 0.0001); BMI (r = 0.21, P < 0.0001); and IGF-I SDS (r = 0.10, P < 0.001, Fig. 2). Using body weight instead of BMI in the model, age, weight, and IGF-I SDS remained significant independent predictors of sBMD in FN. However, in this model, unreplaced sex steroid deficiency (r = −0.08, P < 0.05), but not gender, was an independent predictor of sBMD in FN. In the prespecified subgroup of women younger than 50 yr (n = 404), the following variables were found to be independently associated with sBMD in FN: BMI (r = 0.35, P < 0.0001); estimated GHD duration (r = −0.13, P < 0.05); and TSH deficiency (r = −0.16, P < 0.05). Using body weight instead of BMI in the model, weight and TSH deficiency remained significant independent predictors of sBMD in FN. However, in this model, estimated GHD duration was no longer an independent predictor of sBMD.
Fig. 2.
Association between sBMD in the FN and IGF-I SDS in subjects with AOGHD.
Discussion
Risk factors associated with lower BMD in GHD have not been fully elucidated. We hypothesized that endocrine variables are associated with lower BMD in this population and examined whether GHD severity, IGF-I SDS, and the presence of additional pituitary hormone deficiencies are associated with lower BMD. Converting BMD values to sBMD data facilitated direct comparisons between BMD values obtained on densitometers from different manufacturers (23, 24).
The study population was a large cohort of GH-naïve adult subjects with stringently defined AOGHD, wherein multivariate predictors of sBMD were examined. In the present study, lower BMI (or weight), unreplaced sex steroid deficiency (central hypogonadism), and corticotropin deficiency (central hypoadrenalism) were independently associated with lower sBMD in the LS of AOGHD subjects. In the FN of AOGHD subjects, older age, female gender (or unreplaced sex steroid deficiency, depending on the model), lower BMI (or weight), and lower IGF-I SDS independently predicted lower sBMD. In addition, in the prespecified subgroup of premenopausal-aged women, lower BMI (or weight), longer estimated GHD duration, and TSH deficiency (central hypothyroidism) were independently associated with lower sBMD in the LS or FN.
These data affirm the important role of sex steroids in bone physiology and suggest that sex steroids may influence bone mass independently of GHD (25). In addition, the present study findings suggest that both endogenous and exogenous sex steroids appear to be protective for BMD.
The mechanisms underlying the association between either corticotropin or TSH deficiency and lower sBMD are not clear and might involve a consequence of either lack of respective target gland hormones (as a result of under replacement) or excess hormone replacement. Glucocorticoid therapy or replacement has been associated with decreased BMD, particularly in the lumbar spine, in a dose-dependent manner (26). In addition, excess thyroid replacement has been associated with decreased BMD (27). Although it is possible that excessive replacement with glucocorticoids or thyroid hormone may explain the findings of the present study, additional data are needed to clarify this issue. However, the present findings suggest that careful titration of glucocorticoid and thyroid hormone replacement is prudent to minimize the potential deleterious effects of glucocorticoid or thyroid hormone excess in this population.
The present study findings include a negative association between IGF-I SDS and sBMD in FN, in agreement with physiological data indicating a significant role of IGF-I in bone (9, 11–13). Further studies are needed to examine whether baseline IGF-I SDS predicts BMD responses to GH replacement.
Subjects with Cushing's disease had significantly lower sBMD and z scores in the FN as well as increased fracture rate, in agreement with previous data on the deleterious effects of endogenous glucocorticoid excess on bone (28). Subjects with a history of fracture had longer estimated GHD duration and showed a trend toward lower sBMD in the LS and FN but were not more likely to have additional pituitary hormone deficiencies. These findings affirm the important role of GH on bone strength and suggest that lower BMD might be associated with increased fracture risk in this population.
Strengths of the present study include the large population size, stringent definition of GHD, and the inclusion of GH-naïve subjects. However, the study has some limitations arising from its retrospective design, which necessitates the inclusion of subjects with available data, reflecting local clinical practice. Because the study population was predominantly Caucasian, the findings may not apply to patients in other racial groups. Data on calcium intake and vitamin D levels, which can affect BMD, were not available. Similarly, data on thyroid and adrenocortical hormone replacement doses were not available.
Estimating the duration of GHD as the difference between age at entry into KIMS minus the age at diagnosis of GHD likely underestimated the true duration of GHD because the underlying disease or interventions leading to GHD had predated the time of GH stimulation testing. However, it is unlikely that systematic differences in GHD duration would result between subject subgroups, based on these calculations.
In the present study, BMD values were converted to sBMD to facilitate comparisons between data obtained on different densitometers. It may be noted, however, that z scores were pooled across different densitometers. There have been secular changes in normative databases used by different densitometer manufacturers, which could affect z scores. Nevertheless, the present study findings on sBMD and z scores mirrored each other fairly closely, suggesting that pooling z scores likely led to valid results in this population. All regression analyses were conducted using sBMD (rather than z scores) as the dependent variable, suggesting that the major findings of the present study would not be affected by the inclusion of z scores.
The findings of the present study have explained a relatively small proportion of the total variability in BMD, highlighting the importance of other factors affecting BMD, which may include genetic, nutritional, and lifestyle variables. However, our aim was to investigate endocrine influences on BMD in this population.
In conclusion, the present study investigated endocrine variables independently associated with sBMD in a large population of adults with untreated GHD. The present study data indicate that lower IGF-I SDS as well as deficiencies of sex steroids, corticotropin, and TSH may predict lower sBMD in this population. These findings may be of substantial clinical significance. Further data are needed to examine whether these factors predict bone response to GH replacement in adult GHD.
Acknowledgments
We all express our gratitude to the clinicians who provided the primary data on their patients.
KIMS is sponsored by Pfizer Inc. D.K. and M.P.W. are full-time employees of Pfizer Inc. P.J.J. and M.K.-H. are employed by Pfizer Health AB. N.A.T., S.L.G., A.H., D.M.C., and B.M.K.B. were not compensated for their contributions to this manuscript.
Disclosure Summary: N.A.T., A.H., D.M.C., and B.M.K.B. are KIMS Investigators. N.A.T. has been a recipient of lecture fees from Pfizer Inc., and his spouse is an employee of Pfizer Inc. S.L.G. has been a recipient of research funding from Eli Lilly, Warner Chilcott, and Tarsa and has consulted and served on an advisory board for Amgen and Merck. A.H. has been a recipient of lecture fees from Pfizer Inc. and has consulted for Novo Nordisk. D.M.C. has received lecture fees from Pfizer, has received research funding from Serono, Eli Lilly, and Indevus, and served on an advisory board for Novo Nordisk. D.K. and M.P.W. are full-time employees of Pfizer Inc. P.J.J. and M.K.-H. are employed by Pfizer Health AB. B.M.K.B. has been a recipient of research funding from Eli Lilly and Novo Nordisk and has served on an advisory board and received consulting fees from Pfizer and Novo Nordisk.
Footnotes
- AOGHD
- Adult-onset GHD
- BMD
- bone mineral density
- BMI
- body mass index
- FN
- femoral neck
- GHD
- GH deficiency
- KIMS
- Pfizer International Metabolic Study
- LS
- posterior anterior lumbar spine
- sBMD
- standardized bone mineral density
- SDS
- sd score.
References
- 1. Baroncelli GI , Bertelloni S , Sodini F , Saggese G. 2003. Acquisition of bone mass in normal individuals and in patients with growth hormone deficiency. J Pediatr Endocrinol Metab 16(Suppl 2):327–335 [PubMed] [Google Scholar]
- 2. Koranyi J , Svensson J , Götherström G , Sunnerhagen KS , Bengtsson B , Johannsson G. 2001. Baseline characteristics and the effects of five years of GH replacement therapy in adults with GH deficiency of childhood or adulthood onset: a comparative, prospective study. J Clin Endocrinol Metab 86:4693–4699 [DOI] [PubMed] [Google Scholar]
- 3. Tritos NA , Biller BM. 2009. Growth hormone and bone. Curr Opin Endocrinol Diabetes Obes 16:415–422 [DOI] [PubMed] [Google Scholar]
- 4. Abs R , Mattsson AF , Bengtsson BA , Feldt-Rasmussen U , Góth MI , Koltowska-Häggström M , Monson JP , Verhelst J , Wilton P. 2005. Isolated growth hormone (GH) deficiency in adult patients: baseline clinical characteristics and responses to GH replacement in comparison with hypopituitary patients. A sub-analysis of the KIMS database. Growth Horm IGF Res 15:349–359 [DOI] [PubMed] [Google Scholar]
- 5. Mazziotti G , Bianchi A , Bonadonna S , Nuzzo M , Cimino V , Fusco A , De Marinis L , Giustina A. 2006. Increased prevalence of radiological spinal deformities in adult patients with GH deficiency: influence of GH replacement therapy. J Bone Miner Res 21:520–528 [DOI] [PubMed] [Google Scholar]
- 6. Rosén T , Wilhelmsen L , Landin-Wilhelmsen K , Lappas G , Bengtsson BA. 1997. Increased fracture frequency in adult patients with hypopituitarism and GH deficiency. Eur J Endocrinol 137:240–245 [DOI] [PubMed] [Google Scholar]
- 7. Wüster C , Abs R , Bengtsson BA , Bennmarker H , Feldt-Rasmussen U , Hernberg-Ståhl E , Monson JP , Westberg B , Wilton P. 2001. The influence of growth hormone deficiency, growth hormone replacement therapy, and other aspects of hypopituitarism on fracture rate and bone mineral density. J Bone Miner Res 16:398–405 [DOI] [PubMed] [Google Scholar]
- 8. Hutchison MR , Bassett MH , White PC. 2007. Insulin-like growth factor-I and fibroblast growth factor, but not growth hormone, affect growth plate chondrocyte proliferation. Endocrinology 148:3122–3130 [DOI] [PubMed] [Google Scholar]
- 9. Lupu F , Terwilliger JD , Lee K , Segre GV , Efstratiadis A. 2001. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol 229:141–162 [DOI] [PubMed] [Google Scholar]
- 10. Ohlsson C , Nilsson A , Isaksson OG , Lindahl A. 1992. Effect of growth hormone and insulin-like growth factor-I on DNA synthesis and matrix production in rat epiphyseal chondrocytes in monolayer culture. J Endocrinol 133:291–300 [DOI] [PubMed] [Google Scholar]
- 11. Sims NA , Clément-Lacroix P , Da Ponte F , Bouali Y , Binart N , Moriggl R , Goffin V , Coschigano K , Gaillard-Kelly M , Kopchick J , Baron R , Kelly PA. 2000. Bone homeostasis in growth hormone receptor-null mice is restored by IGF-I but independent of Stat5. J Clin Invest 106:1095–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang M , Xuan S , Bouxsein ML , von Stechow D , Akeno N , Faugere MC , Malluche H , Zhao G , Rosen CJ , Efstratiadis A , Clemens TL. 2002. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem 277:44005–44012 [DOI] [PubMed] [Google Scholar]
- 13. Zhao G , Monier-Faugere MC , Langub MC , Geng Z , Nakayama T , Pike JW , Chernausek SD , Rosen CJ , Donahue LR , Malluche HH , Fagin JA , Clemens TL. 2000. Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology 141:2674–2682 [DOI] [PubMed] [Google Scholar]
- 14. Drinkwater BL , Nilson K , Chesnut CH , Bremner WJ , Shainholtz S , Southworth MB. 1984. Bone mineral content of amenorrheic and eumenorrheic athletes. N Engl J Med 311:277–281 [DOI] [PubMed] [Google Scholar]
- 15. Colao A , Di Somma C , Pivonello R , Loche S , Aimaretti G , Cerbone G , Faggiano A , Corneli G , Ghigo E , Lombardi G. 1999. Bone loss is correlated to the severity of growth hormone deficiency in adult patients with hypopituitarism. J Clin Endocrinol Metab 84:1919–1924 [DOI] [PubMed] [Google Scholar]
- 16. Hitz MF , Jensen JE , Eskildsen PC. 2006. Bone mineral density in patients with growth hormone deficiency: does a gender difference exist? Clin Endocrinol (Oxf) 65:783–791 [DOI] [PubMed] [Google Scholar]
- 17. Mazziotti G , Bianchi A , Cimino V , Bonadonna S , Martini P , Fusco A , De Marinis L , Giustina A. 2008. Effect of gonadal status on bone mineral density and radiological spinal deformities in adult patients with growth hormone deficiency. Pituitary 11:55–61 [DOI] [PubMed] [Google Scholar]
- 18. Murray RD , Columb B , Adams JE , Shalet SM. 2004. Low bone mass is an infrequent feature of the adult growth hormone deficiency syndrome in middle-age adults and the elderly. J Clin Endocrinol Metab 89:1124–1130 [DOI] [PubMed] [Google Scholar]
- 19. Ho KK. 2007. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur J Endocrinol 157:695–700 [DOI] [PubMed] [Google Scholar]
- 20. Riis P. 2003. Thirty years of bioethics: the Helsinki Declaration 1964–2003. New Rev Bioeth 1:15–25 [DOI] [PubMed] [Google Scholar]
- 21. Underwood LE , Attie KM , Baptista J. 2003. Growth hormone (GH) dose-response in young adults with childhood-onset GH deficiency: a two-year, multicenter, multiple-dose, placebo-controlled study. J Clin Endocrinol Metab 88:5273–5280 [DOI] [PubMed] [Google Scholar]
- 22. Brabant G , von zur Mühlen A , Wüster C , Ranke MB , Kratzsch J , Kiess W , Ketelslegers JM , Wilhelmsen L , Hulthén L , Saller B , Mattsson A , Wilde J , Schemer R , Kann P. 2003. Serum insulin-like growth factor I reference values for an automated chemiluminescence immunoassay system: results from a multicenter study. Horm Res 60:53–60 [DOI] [PubMed] [Google Scholar]
- 23. Genant HK , Grampp S , Glüer CC , Faulkner KG , Jergas M , Engelke K , Hagiwara S , Van Kuijk C. 1994. Universal standardization for dual x-ray absorptiometry: patient and phantom cross-calibration results. J Bone Miner Res 9:1503–1514 [DOI] [PubMed] [Google Scholar]
- 24. Lu Y , Fuerst T , Hui S , Genant HK. 2001. Standardization of bone mineral density at femoral neck, trochanter and Ward's triangle. Osteoporos Int 12:438–444 [DOI] [PubMed] [Google Scholar]
- 25. Venken K , Callewaert F , Boonen S , Vanderschueren D. 2008. Sex hormones, their receptors and bone health. Osteoporos Int 19:1517–1525 [DOI] [PubMed] [Google Scholar]
- 26. Debono M , Ross RJ , Newell-Price J. 2009. Inadequacies of glucocorticoid replacement and improvements by physiological circadian therapy. Eur J Endocrinol 160:719–729 [DOI] [PubMed] [Google Scholar]
- 27. Greenspan SL , Greenspan FS. 1999. The effect of thyroid hormone on skeletal integrity. Ann Intern Med 130:750–758 [DOI] [PubMed] [Google Scholar]
- 28. Fernandez-Rodriguez E , Stewart PM , Cooper MS. 2009. The pituitary-adrenal axis and body composition. Pituitary 12:105–115 [DOI] [PubMed] [Google Scholar]