Abstract
Context:
18-Oxocortisol (18-oxoF) is a derivative of cortisol (F) that is produced by aldosterone synthase (CYP11B2). The potential for this steroid as a biomarker for differentiating patients with aldosterone-producing adenoma (APA) from those with idiopathic hyperaldosteronism (IHA) has not been examined.
Objectives:
We measured 18-oxoF, aldosterone, and F in plasma from adrenal vein sampling (AVS) of patients with primary aldosteronism. We compared 18-oxoF levels and 18-oxoF/F ratios for their potential to differentiate APA from IHA.
Design, Setting, and Subjects:
This study measured 18-oxoF, F, and aldosterone in AVS obtained from patients with unilateral APA (14 cases) or bilateral IHA (seven cases, 14 samples total) using liquid chromatography-tandem mass spectrometry and RIA analyses.
Results:
The levels of 18-oxoF and the ratios of 18-oxoF/F, before and after ACTH stimulation, were significantly higher in blood-draining APA than in those from the contralateral adrenal glands and from adrenal glands with IHA.
Conclusions:
The 18-oxoF levels and ratios of 18-oxoF/F in AVS samples can be a clinically useful biomarker for differentiating APA from IHA and for determining the localization or lateralization of APA in patients with primary aldosteronism.
Primary aldosteronism (PA) is one of the most common causes of secondary hypertension, with an estimated prevalence that has increased among hypertensive patients as a result of improved detection (1). Due to differences in the clinical management of PA, various attempts are made to distinguish unilateral aldosterone-producing adenoma (APA) from idiopathic hyperaldosteronism (IHA) (1–3). Recently, adrenal vein sampling (AVS) has come to be recognized as a definitive procedure for biochemical differentiation between APA and IHA (2, 3). Although AVS is a cumbersome and laborious diagnostic technique, the potential development of improved biomarkers obtained for this method could contribute to the more accurate lateralization of disease and differential diagnosis of patients with APA and IHA.
18-Oxocortisol (18-oxoF) is a derivative of cortisol (F) that was initially described as a biomarker for glucocorticoid remediable aldosteronism (4). This steroid has been detected in the serum of patients with PA, and the synthesis of 18-oxoF requires aldosterone synthase (CYP11B2) (5–7). Therefore, it is important to evaluate whether the measurement of 18-oxoF in AVS samples may enhance the ability to differentiate APA from IHA. In our study, we measured 18-oxoF in AVS samples and correlated them with histopathology of resected adrenal specimens.
Patients and Methods
Cases and AVS
Unilateral APA (14 cases) and bilateral IHA (seven cases) patients underwent AVS at Tohoku University Hospital from 2008 to 2010, following the previously reported protocol (8). The diagnosis for PA was established by measuring the plasma aldosterone concentration/plasma renin activity ratio, which was greater than 20 at both 1 and 2 h after oral administration of 50 mg captopril, and dexamethasone suppression tests were performed in all patients to exclude PA patients with F-producing adenomas before AVS (8). The study was approved by the Institutional Review Board of Tohoku University School of Medicine. Bilateral adrenal veins were simultaneously catheterized in all patients (8). After baseline samples were simultaneously obtained from both adrenal veins, a second set of blood samples was collected from the same sites 15 min after iv bolus injection of 0.25 mg (10 IU) of ACTH (2, 8). Successful adrenal venous cannulation was based on an AVS F level that was greater than 5-fold compared with that in the iliac vein sample after ACTH stimulation (2, 8).
Reagents
Cortisol and fusaric acid were obtained from Sigma-Aldrich (St. Louis, MO). 18-oxoF was kindly provided from Dr. Gomez-Sanchez. Cortisol-2H4 (F-d4) and aldosterone-2H7 (Aldo-d7) were purchased from Isosciences (King of Prussia, PA) and C/D/N Isotopes (Pointe-Claire, Canada), respectively. InertSep Pharma cartridge (60 mg) and InertSep SI cartridge (500 mg) were obtained from GL Sciences (Tokyo, Japan). 4-Dimethylaminopyridine and 2-methyl-6-nitrobenzoic anhydride were purchased from Tokyo Chemical Industry (Tokyo, Japan). Liquid chromatography-tandem mass spectrometry (LC/MS/MS) grade acetonitrile was obtained from Wako Pure Chemicals (Osaka, Japan). All other reagents and solvents were of analytical grade.
LC/MS/MS
An API 4000 triple stage quadrupole mass spectrometer equipped with electrospray ionization ion source (MDS Sciex, Toronto, Canada), an Agilent 1100 HPLC system (Agilent Technologies, Waldbronn, Germany), and a HTC Pal auto-sampler (CTC Analytics, Zwingen, Switzerland) were employed for steroid analysis. The column was a Cadenza CD-C18 (250 mm × 3 mm, internal diameter 3 μm; Imtact, Kyoto, Japan) and was used at 40 C. The mobile phase consisting of acetonitrile (solvent A) and 0.1% formic acid (solvent B) was used with a gradient elution of A:B = 50:50 to 100:0 at a flow rate of 0.5 ml/min. Positive electrospray ionization ion polarity was used. For quantification of steroids, transition of m/z 526/508, 528/510, 566/520, and 557/511 were selected for F, F-d4, 18-oxoF, and Aldo-d7, respectively.
Derivatizations of F, F-d4, 18-oxoF, and Aldo-d7 were performed according to the mixed anhydride using fusaric acid (9). To plasma (0.1 ml) was added to F-d4 (250 pg) and Aldo-d7 (50 pg) as internal standard and acetonitrile (0.2 ml), and the mixture was centrifuged to remove protein. The diluted supernatant was applied to an InertSep Pharma cartridge, and the steroidal fraction was eluted with 80% acetonitrile (1 ml). After the eluate was evaporated to dryness, the residue was treated with 0.25 ml of 35% HCl in ethanol (1:5, vol/vol) at room temperature for 30 min. The obtained residue was reacted with mixed anhydride method using fusaric acid reagent with the triethylamine in acetonitrile for 20 min at 50 C. The resulting mixture was transferred to an InertSep SI cartridge and then was washed successively with 50% hexane-ethyl acetate (3 ml) and 50% ethyl acetate-acetonitrile (1 ml). The desired fraction was eluted with 50% ethyl acetate-acetonitrile (1.5 ml) and evaporated. The residue was dissolved in 40% acetonitrile (0.1 ml) and subjected to LC/MS/MS. The accuracy and precision in intraassay were 95–106 and 2–15% for F and 18-oxoF, respectively. Those in interassay were 90–107 and 1–11% for F and 18-oxoF, respectively. The lower limits of quantification for F and 18-oxoF were 250 and 5 pg/ml, respectively.
Plasma aldosterone was measured by the SPAC-S Aldosterone Kit (TFB Inc., Tokyo, Japan). For aldosterone measurement, the minimal detectable quantity was 25 pg/ml, and the intra- and interassay coefficients of variation were 4.7 and 4.5%, respectively.
Data analysis and statistical methods
Statistical analyses were done by one-way ANOVA, followed by post hoc test for comparisons. Results are given as mean ± sem where appropriate. Significance was accepted at the 0–0.05 level of probability (P ≤ 0.05).
Results
After unilateral adrenalectomy, plasma aldosterone concentrations decreased from 38.55 ± 3.67 to 8.41 ± 0.86 ng/dl in APA cases (n = 14) and from 14.53 ± 0.91 to 5.87 ± 0.81 ng/dl in IHA cases (n = 7). The plasma aldosterone concentration/plasma renin activity ratio fell from 242.3 ± 55.90 to 12.54 ± 2.05 in APA cases (n = 14) and from 56.21 ± 16.25 to 2.59 ± 0.82 in IHA cases (n = 7). The entire specimen of each resected adrenal was examined histopathologically, and all patients were diagnosed as either APA or IHA (Fig. 1, A–D, and Tables 1 and 2) (8, 10, 11). In all APA cases, the lesions were diagnosed as benign adenoma predominantly formed by clear cells (Fig. 1A), as well as having an adjacent zona glomerulosa (ZG) that had negative immunoreactivity for 3β-hydroxysteroid dehydrogenase (3β-HSD) (Fig. 1B) (10, 11). In all IHA cases, immunoreactivity for 3β-HSD was positive in the hyperplastic ZG (Fig. 1, C and D) (10). Before ACTH stimulation, the highest levels of 18-oxoF among all IHA and APA contralateral adrenals were 157 and 74 ng/dl, respectively (Tables 1 and 2). The 18-oxoF levels were greater than 157 ng/dl in all samples of blood-draining APA except in cases 3 and 12 (Tables 1 and 2 and Fig. 1E). Before ACTH stimulation, the maxima 18-oxoF/F ratios in IHA and APA contralateral adrenals were 0.364 and 0.255%, respectively, whereas the ratios were greater than 0.364% in all samples from APA except case 3 (Tables 1 and 2 and Fig. 1F). After ACTH stimulation, the maxima of 18-oxoF levels in all IHA and APA contralateral adrenals were 1249 and 229 ng/dl, respectively, and the levels were greater than 1249 ng/dl in all 14 samples from APA (Tables 1 and 2 and Fig. 1E). After ACTH stimulation, the maxima 18-oxoF/F ratios in IHA and APA contralateral adrenals were 0.145 and 0.044%, respectively, and the ratios were greater than 0.145% in all 14 samples from APA (Tables 1 and 2 and Fig. 1F). Both before and after ACTH stimulation, the levels of 18-oxoF and mean ratios of 18-oxoF/F were significantly higher in samples from APA than in those from IHA and APA contralateral adrenals (P ≤ 0.05) (Fig. 1, E and F).
Fig. 1.
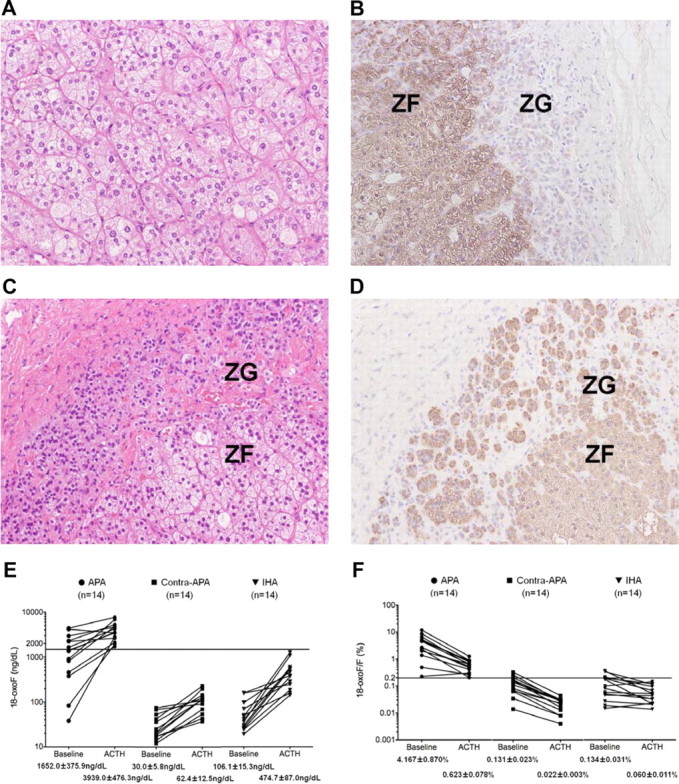
A and B, Hematoxylin-eosin staining of the APA demonstrated the presence of predominantly clear cells (A), and immunohistochemical localization of 3β-HSD (B) demonstrated low expression in adjacent hyperplastic ZG (case 6). C and D, Hematoxylin-eosin staining (C) and immunohistochemical localization of 3β-HSD (D) showing the hyperplasia and distinct immunoreactivity of 3β-HSD in the ZG of the IHA adrenal gland (case 16). E and F, The levels of 18-oxoF (E) and the ratio of 18-oxoF/F (panel F) in adrenal vein samples of APA (14 samples), the APA contralateral adrenal (14 samples), and IHA (14 samples) both before and after ACTH stimulation. Before ACTH stimulation, the mean levels of 18-oxoF and the mean ratios of 18-oxoF/F were significantly higher in blood-draining APA (1652.0 ± 375.9 ng/dl and 4.167 ± 0.870%, respectively) than in those of their contralateral adrenal glands (30.0 ± 5.8 ng/dl and 0.131 ± 0.023%, respectively) and the adrenal glands associated with IHA (62.4 ± 12.5 ng/dl and 0.134 ± 0.031%, respectively) (P ≤ 0.05). After ACTH stimulation, the mean levels of 18-oxoF and the mean ratios of 18-oxoF/F were significantly higher in blood-draining APA (3939.0 ± 476.3 ng/dl and 0.623 ± 0.078%, respectively) than in those of the contralateral-APA adrenal glands (106.1 ± 15.3 ng/dl and 0.022 ± 0.003%, respectively) and adrenal glands with IHA (474.7 ± 87.0 ng/dl and 0.060 ± 0.011%, respectively) (P ≤ 0.05). The horizontal lines show 1500 ng/dl (E) and 0.200% (F), respectively.
Table 1.
Clinical and pathological findings and levels of select adrenal steroids from APA and IHA patients before ACTH
| Case no. | Age (yr) | Sex | CT tumor size (mm) |
Localization of APA | Pre-ACTH |
Pre-ACTH |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A (ng/dl) |
F (μg/dl) |
F (μg/dl) Ilv | F ratio |
A/F (%) |
A/F L-R | 18-oxoF (ng/dl) |
18-oxoF/F (%) |
18-oxoF/F L-R | |||||||||||||
| R | L | R | L | R | L | R/Ilv | L/Ilv | R | L | R | L | R | L | ||||||||
| APA (n = 14) | 1 | 35 | M | 8 | 18 | Lt | 50 | 1250 | 24 | 41 | 12 | 1.945 | 3.285 | 0.205 | 3.047 | 14.860 | 17 | 816 | 0.071 | 1.988 | 28.072 |
| 2 | 38 | M | 23 | 10 | Rt | 2738 | 39 | 26 | 21 | 11 | 2.356 | 1.851 | 10.347 | 0.186 | 55.727 | 2292 | 42 | 8.661 | 0.203 | 42.770 | |
| 3 | 30 | M | n.d. | 11 | Lt | 14 | 134 | 21 | 17 | 7 | 3.109 | 2.538 | 0.067 | 0.788 | 11.791 | 3 | 38 | 0.014 | 0.224 | 15.516 | |
| 4 | 53 | M | n.d. | 18 | Lt | 43 | 4842 | 50 | 85 | 12 | 4.211 | 7.240 | 0.087 | 5.672 | 65.194 | 17 | 2232 | 0.034 | 2.615 | 76.820 | |
| 5 | 46 | M | n.d. | 19 | Lt | 21 | 1036 | 20 | 19 | 9 | 2.299 | 2.164 | 0.103 | 5.385 | 52.410 | 14 | 902 | 0.066 | 4.688 | 70.982 | |
| 6 | 71 | M | n.d. | 14 | Lt | 41 | 2235 | 15 | 25 | 5 | 2.982 | 4.899 | 0.271 | 9.069 | 33.424 | 21 | 1648 | 0.139 | 6.686 | 48.218 | |
| 7 | 53 | F | 10 | n.d. | Rt | 487 | 22 | 16 | 13 | 5 | 3.083 | 2.439 | 2.988 | 0.169 | 17.683 | 376 | 18 | 2.303 | 0.140 | 16.508 | |
| 8 | 31 | F | n.d. | 12 | Lt | 372 | 5695 | 33 | 56 | 15 | 2.229 | 3.823 | 1.140 | 10.189 | 8.936 | 53 | 2960 | 0.164 | 5.295 | 32.315 | |
| 9 | 68 | F | 15 | n.d. | Rt | 7711 | 41 | 37 | 14 | 5 | 7.692 | 2.948 | 20.839 | 0.286 | 72.783 | 4391 | 36 | 11.866 | 0.255 | 46.483 | |
| 10 | 58 | M | n.d. | 20 | Lt | 75 | 1420 | 31 | 102 | 4 | 7.222 | 25.568 | 0.241 | 1.391 | 5.769 | 25 | 1408 | 0.080 | 1.380 | 17.332 | |
| 11 | 65 | M | 22 | n.d. | Rt | 349 | 13 | 17 | 12 | 6 | 2.656 | 1.875 | 2.050 | 0.109 | 18.779 | 457 | 20 | 2.688 | 0.167 | 16.129 | |
| 12 | 70 | M | n.d. | 16 | Lt | 44 | 2141 | 19 | 17 | 6 | 3.393 | 3.036 | 0.230 | 12.595 | 54.760 | 12 | 83 | 0.063 | 0.488 | 7.730 | |
| 13 | 34 | F | n.d. | 30 | Lt | 37 | 749 | 23 | 28 | 13 | 1.797 | 2.154 | 0.159 | 2.675 | 16.824 | 74 | 1369 | 0.322 | 4.890 | 15.186 | |
| 14 | 37 | F | 18 | n.d. | Rt | 3596 | 76 | 91 | 62 | 12 | 7.583 | 5.167 | 3.952 | 0.123 | 32.130 | 4150 | 68 | 4.560 | 0.110 | 41.455 | |
| IHA (n = 7) | 15 | 29 | M | n.d. | n.d. | 1630 | 245 | 26 | 30 | 9 | 2.954 | 3.407 | 6.186 | 0.807 | 7.670 | 96 | 25 | 0.364 | 0.081 | 4.496 | |
| 16 | 24 | M | n.d. | n.d. | 1782 | 766 | 338 | 305 | 20 | 13.397 | 12.109 | 0.528 | 0.251 | 2.102 | 157 | 156 | 0.046 | 0.051 | 1.099 | ||
| 17 | 40 | M | n.d. | n.d. | 270 | 402 | 263 | 257 | 21 | 11.502 | 11.214 | 0.103 | 0.157 | 1.527 | 50 | 69 | 0.019 | 0.027 | 1.429 | ||
| 18 | 43 | M | n.d. | 14 | 1620 | 216 | 165 | 158 | 23 | 7.165 | 6.850 | 0.979 | 0.136 | 7.180 | 96 | 24 | 0.058 | 0.015 | 3.856 | ||
| 19 | 43 | F | n.d. | n.d. | 278 | 449 | 17 | 13 | 4 | 4.120 | 3.289 | 1.650 | 3.339 | 2.023 | 36 | 47 | 0.214 | 0.346 | 1.617 | ||
| 20 | 43 | F | n.d. | n.d. | 60 | 182 | 27 | 21 | 12 | 2.250 | 1.750 | 0.222 | 0.867 | 3.905 | 27 | 41 | 0.100 | 0.195 | 1.950 | ||
| 21 | 35 | F | n.d. | n.d. | 379 | 92 | 16 | 11 | 4 | 4.000 | 2.750 | 2.369 | 0.836 | 2.834 | 30 | 19 | 0.188 | 0.173 | 1.087 | ||
A, Aldosterone; R, right adrenal vein; L, left adrenal vein; Ilv, iliac vein; n.d., not detectable; L-R, the ratio of L/R or R/L divided by the lower values; localization of APA, APA histopathologically detected in the right (Rt) or left (Lt) adrenal gland. The adrenal tumor in Case 18 was histopathologically and immunohistochemically diagnosed as nonfunctional adrenal adenoma.
Table 2.
Clinical and pathological findings and levels of select adrenal steroids from APA and IHA patients after ACTH
| Case no. | Age (yr) | Sex | CT tumor size (mm) |
Localization of APA | Pre-ACTH |
Post-ACTH |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A (ng/dl) |
F (μg/dl) |
F (μg/dl) Ilv | F ratio |
A/F (%) |
A/F L-R | 18-oxoF (ng/dl) |
18-oxoF/F (%) |
18-oxoF/F L-R | |||||||||||||
| R | L | R | L | R | L | R/Ilv | L/Ilv | R | L | R | L | R | L | ||||||||
| APA (n = 14) | 1 | 35 | M | 8 | 18 | Lt | 266 | 8671 | 285 | 756 | 16 | 18.025 | 47.854 | 0.093 | 1.147 | 12.279 | 37 | 4813 | 0.013 | ||
| 2 | 38 | M | 23 | 10 | Rt | 8326 | 399 | 590 | 731 | 18 | 32.224 | 39.945 | 1.412 | 0.055 | 25.893 | 5291 | 229 | 0.897 | 0.031 | 28.617 | |
| 3 | 30 | M | n.d. | 11 | Lt | 496 | 13824 | 975 | 946 | 15 | 67.207 | 65.255 | 0.051 | 1.461 | 28.687 | 41 | 2847 | 0.004 | 0.301 | 71.511 | |
| 4 | 53 | M | n.d. | 18 | Lt | 227 | 8909 | 563 | 495 | 16 | 34.515 | 30.337 | 0.040 | 1.802 | 44.613 | 43 | 2623 | 0.008 | 0.531 | 70.063 | |
| 5 | 46 | M | n.d. | 19 | Lt | 194 | 9888 | 300 | 802 | 16 | 18.609 | 49.839 | 0.065 | 1.232 | 19.012 | 69 | 6837 | 0.023 | 0.852 | 36.998 | |
| 6 | 71 | M | n.d. | 14 | Lt | 559 | 7968 | 740 | 712 | 13 | 59.176 | 56.984 | 0.076 | 1.119 | 14.811 | 198 | 4229 | 0.027 | 0.594 | 22.134 | |
| 7 | 53 | F | 10 | n.d. | Rt | 3968 | 328 | 838 | 366 | 12 | 70.403 | 30.739 | 0.474 | 0.090 | 5.276 | 1699 | 117 | 0.203 | 0.032 | 6.340 | |
| 8 | 31 | F | n.d. | 12 | Lt | 550 | 9976 | 757 | 603 | 16 | 46.710 | 37.222 | 0.073 | 1.654 | 22.781 | 123 | 4652 | 0.016 | 0.771 | 47.306 | |
| 9 | 68 | F | 15 | n.d. | Rt | 23546 | 287 | 608 | 539 | 14 | 43.429 | 38.500 | 3.874 | 0.053 | 72.829 | 7652 | 143 | 1.259 | 0.027 | 47.371 | |
| 10 | 58 | M | n.d. | 20 | Lt | 423 | 5823 | 516 | 843 | 12 | 44.128 | 72.043 | 0.082 | 0.691 | 8.426 | 115 | 3454 | 0.022 | 0.410 | 18.459 | |
| 11 | 65 | M | 22 | n.d. | Rt | 2431 | 317 | 448 | 413 | 14 | 31.766 | 29.255 | 0.543 | 0.077 | 7.062 | 2128 | 115 | 0.475 | 0.028 | 17.100 | |
| 12 | 70 | M | n.d. | 16 | Lt | 359 | 15000 | 656 | 728 | 14 | 45.542 | 50.576 | 0.055 | 2.060 | 37.634 | 54 | 1924 | 0.008 | 0.264 | 31.850 | |
| 13 | 34 | F | n.d. | 30 | Lt | 260 | 7645 | 249 | 406 | 18 | 14.142 | 23.080 | 0.105 | 1.882 | 17.997 | 110 | 3552 | 0.044 | 0.875 | 19.717 | |
| 14 | 37 | F | 18 | n.d. | Rt | 9650 | 541 | 534 | 315 | 16 | 33.572 | 19.830 | 1.808 | 0.172 | 10.536 | 3440 | 91 | 0.645 | 0.029 | 22.430 | |
| IHA (n = 7) | 15 | 29 | M | n.d. | n.d. | 5053 | 5213 | 883 | 1126 | 13 | 67.954 | 86.631 | 0.572 | 0.463 | 1.236 | 462 | 364 | 0.052 | 0.032 | 1.620 | |
| 16 | 24 | M | n.d. | n.d. | 2955 | 1933 | 872 | 731 | 21 | 41.524 | 34.810 | 0.339 | 0.264 | 1.281 | 382 | 242 | 0.044 | 0.033 | 1.322 | ||
| 17 | 40 | M | n.d. | n.d. | 1807 | 2791 | 1090 | 1329 | 28 | 38.893 | 47.464 | 0.166 | 0.210 | 1.266 | 157 | 280 | 0.014 | 0.021 | 1.459 | ||
| 18 | 43 | M | n.d. | 14 | 4200 | 3111 | 866 | 788 | 27 | 32.074 | 29.185 | 0.485 | 0.395 | 1.229 | 462 | 184 | 0.053 | 0.023 | 2.284 | ||
| 19 | 43 | F | n.d. | n.d. | 7719 | 10470 | 1125 | 1083 | 13 | 87.891 | 84.578 | 0.686 | 0.967 | 1.410 | 1065 | 1249 | 0.095 | 0.115 | 1.219 | ||
| 20 | 43 | F | n.d. | n.d. | 4152 | 4663 | 371 | 437 | 18 | 20.396 | 23.989 | 1.118 | 1.068 | 1.047 | 537 | 535 | 0.145 | 0.122 | 1.181 | ||
| 21 | 35 | F | n.d. | n.d. | 3108 | 1984 | 867 | 639 | 15 | 59.807 | 44.041 | 0.358 | 0.311 | 1.153 | 586 | 141 | 0.068 | 0.022 | 3.063 | ||
A, Aldosterone; R, right adrenal vein; L, left adrenal vein; Ilv, iliac vein; n.d., not detectable; L-R, the ratio of L/R or R/L divided by the lower values; localization of APA, APA histopathologically detected in the right (Rt) or left (Lt) adrenal gland. The adrenal tumor in case 18 was histopathologically and immunohistochemically diagnosed as nonfunctional adrenal adenoma.
Discussion
The major finding of our study is that the levels of 18-oxoF and the 18-oxoF/F ratios were significantly higher in adrenal vein blood samples derived from APA than those from contralateral adrenals in patients with APA and from IHA adrenals.
Surgical treatment is generally recommended for APA patients, whereas for IHA patients more conservative medical treatment is suggested to avoid the possible progression of cardiac disease caused by aldosterone excess (12). However, in our IHA cases, the patients were relatively young, and to prevent progression of organ damage in the future, unilateral adrenalectomy was performed to lower circulating aldosterone levels. A previous study postulated that unilateral adrenalectomy can be beneficial in some patients with apparent bilateral PA (13). Besides, we could not rule out the possibilities of APA in a couple of IHA cases based on the aldosterone/F ratio and aldosterone level before ACTH stimulation, although lateralization ratio became suppressed after ACTH stimulation (14). Therefore, all IHA patients in this study agreed to surgical treatment and were finally diagnosed histopathologically and immunohistochemically.
18-oxoF production is restricted by the localization of CYP11B2 (7). Freel et al. (7) suggested that 18-oxoF is synthesized in the ZG from either circulating F or F locally produced. One possibility is the intraadrenal location of APA. Unlike the normal ZG, APA are not lining the outside of adrenals, which causes them to receive blood from surrounding zona fasciculata (ZF) that would have high levels of F that CYP11B2 could use as substrate for 18-oxoF. Alternatively, Auchus et al. (15) previously described a model that explains the different expression pattern of CYP11B2 in APA and IHA. This model suggested that APA tumors express abundant CYP11B2, which yields relatively large amounts of 18-hydroxycorticosterone (18OHB) (15). In contralateral-APA adrenal gland, aldosterone synthesis by CYP11B2 is suppressed, but under ACTH stimulation, sufficient amounts of 18OHB are possibly derived from the ZF via CYP11B1 activity (15). However, IHA adrenal glands produce 18OHB with features generally similar to APA, although the proportions appear to vary among individual subjects (15). It remains unclear whether the expression levels of CYP11B2 are different between APA and IHA cases, resulting in a difference in 18-oxoF production. Mulatero et al. (16), however, suggested that the unique polymorphism of the CYP11B2 gene may contribute to dysregulation of aldosterone synthesis in IHA but not in APA, possibly explaining the difference of aldosterone and 18-oxoF levels between APA and IHA. It requires further study to clarify the explanation for higher production of 18oxoF in APA vs. IHA.
There is still considerable controversy with regard to the interpretation of AVS, including the specific criteria for lateralization of unilateral vs. bilateral adrenal disease (17). For example, should samples be evaluated before and after stimulation by ACTH? It has been postulated that ACTH administration does not improve the diagnostic accuracy of AVS when it is given as a bolus injection (3, 18, 19). Young et al. (2) suggested that ACTH infusion during AVS is preferred because of the small F gradient seen between the adrenal vein and the inferior vena cava and the coefficient of variation of some F assays. Based on our results, ACTH stimulation can make the interpretation of AVS 18-oxoF results easier to differentiate among APA and IHA patients. Although our study shows great promise for 18-oxoF as a tool for lateralization of APA, the costs of LC/MS/MS analysis would limit widespread use of this assay. Larger retrospective and planned prospective studies are needed to determine sensitivity and selectivity of 18-oxoF in PA lateralization, which will determine whether less expensive 18-oxoF measurement systems should be developed or whether the cost of LC/MS/MS is warranted.
Our results indicate that 18-oxoF measurement may provide a useful marker for distinguishing APA from IHA and the lateralization of APA.
Acknowledgments
Disclosure Summary: The authors have nothing to declare.
Footnotes
- Aldo-d7
- Aldosterone-2H7
- APA
- aldosterone-producing adenoma
- AVS
- adrenal vein sampling
- F
- cortisol
- F-d4
- cortisol-2H4
- 3β-HSD
- 3β-hydroxysteroid dehydrogenase
- IHA
- idiopathic hyperaldosteronism
- LC/MS/MS
- liquid chromatography-tandem mass spectrometry
- 18-OHB
- 18-hydroxycorticosterone
- 18-oxoF
- 18-oxocortisol
- PA
- primary aldosteronism
- ZF
- zona fasciculata
- ZG
- zona glomerulosa.
References
- 1. Mulatero P , Stowasser M , Loh KC , Fardella CE , Gordon RD , Mosso L , Gomez-Sanchez CE , Veglio F , Young WF. 2004. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab 89:1045–1050 [DOI] [PubMed] [Google Scholar]
- 2. Young WF , Stanson AW. 2009. What are the keys to successful adrenal venous sampling (AVS) in patients with primary aldosteronism? Clin Endocrinol (Oxf) 70:14–17 [DOI] [PubMed] [Google Scholar]
- 3. Omura M , Sasano H , Saito J , Yamaguchi K , Kakuta Y , Nishikawa T. 2006. Clinical characteristics of aldosterone-producing microadenoma, macroadenoma, and idiopathic hyperaldosteronism in 93 patients with primary aldosteronism. Hypertens Res 29:883–839 [DOI] [PubMed] [Google Scholar]
- 4. Williams GH. 1994. Genetic approaches to understanding the pathophysiology of complex human traits. Kidney Int 46:1550–1553 [DOI] [PubMed] [Google Scholar]
- 5. Ulick S , Chu MD. 1982. Hypersecretion of a new corticosteroid, 18-hydroxycortisol in two types of adrenocortical hypertension. Clin Exp Hypertens A 4:1771–1777 [DOI] [PubMed] [Google Scholar]
- 6. Fisher A , Friel EC , Bernhardt R , Gomez-Sanchez C , Connell JM , Fraser R , Davies E. 2001. Effects of 18-hydroxylated steroids on corticosteroid production by human aldosterone synthase and 11β-hydroxylase. J Clin Endocrinol Metab 86:4326–4329 [DOI] [PubMed] [Google Scholar]
- 7. Freel EM , Shakerdi LA , Friel EC , Wallace AM , Davies E , Fraser R , Connell JM. 2004. Studies on the origin of circulating 18-hydroxycortisol and 18-oxocortisol in normal human subjects. J Clin Endocrinol Metab 89:4628–4633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Satoh F , Abe T , Tanemoto M , Nakamura M , Abe M , Uruno A , Morimoto R , Sato A , Takase K , Ishidoya S , Arai Y , Suzuki T , Sasano H , Ishibashi T , Ito S. 2007. Localization of aldosterone-producing adrenocortical adenomas: significance of adrenal venous sampling. Hypertens Res 30:1083–1095 [DOI] [PubMed] [Google Scholar]
- 9. Yamashita K , Takahashi M , Tsukamoto S , Numazawa M , Okuyama M , Honma S. 2007. Use of novel picolinoyl derivatization for simultaneous quantification of six corticosteroids by liquid chromatography-electrospray ionization tandem mass spectrometry. J Chromatogr A 1173:120–128 [DOI] [PubMed] [Google Scholar]
- 10. Sasano H. 2000. The adrenal cortex. Molecular and cellular endocrine pathology. London: Arnold; 221–252 [Google Scholar]
- 11. Weiss LM. 1984. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am J Surg Pathol 8:163–169 [DOI] [PubMed] [Google Scholar]
- 12. Giacchetti G , Ronconi V , Turchi F , Agostinelli L , Mantero F , Rilli S , Boscaro M. 2007. Aldosterone as a key mediator of the cardiometabolic syndrome in primary aldosteronism: an observational study. J Hypertens 25:177–186 [DOI] [PubMed] [Google Scholar]
- 13. Sukor N , Gordon RD , Ku YK , Jones M , Stowasser M. 2009. Role of unilateral adrenalectomy in bilateral primary aldosteronism: a 22-year single center experience. J Clin Endocrinol Metab 94:2437–2445 [DOI] [PubMed] [Google Scholar]
- 14. Seccia TM , Miotto D , De Toni R , Pitter G , Mantero F , Pessina AC , Rossi GP. 2009. Adrenocorticotropic hormone stimulation during adrenal vein sampling for identifying surgically curable subtypes of primary aldosteronism: comparison of three different protocols. Hypertension 53:761–766 [DOI] [PubMed] [Google Scholar]
- 15. Auchus RJ , Chandler DW , Singeetham S , Chokshi N , Nwariaku FE , Dolmatch BL , Holt SA , Wians FH , Josephs SC , Trimmer CK , Lopera J , Vongpatanasin W , Nesbitt SD , Leonard D , Victor RG. 2007. Measurement of 18-hydroxycorticosterone during adrenal vein sampling for primary aldosteronism. J Clin Endocrinol Metab 92:2648–2651 [DOI] [PubMed] [Google Scholar]
- 16. Mulatero P , Schiavone D , Fallo F , Rabbia F , Pilon C , Chiandussi L , Pascoe L , Veglio F. 2000. CYP11B2 gene polymorphisms in idiopathic hyperaldosteronism. Hypertension 35:694–698 [DOI] [PubMed] [Google Scholar]
- 17. Auchus RJ , Wians FH , Anderson ME , Dolmatch BL , Trimmer CK , Josephs SC , Chan D , Toomay S , Nwariaku FE. 2010. What we still do not know about adrenal vein sampling for primary aldosteronism. Horm Metab Res 42:411–415 [DOI] [PubMed] [Google Scholar]
- 18. Rossi GP , Ganzaroli C , Miotto D , De Toni R , Palumbo G , Feltrin GP , Mantero F , Pessina AC. 2006. Dynamic testing with high-dose adrenocorticotrophic hormone does not improve lateralization of aldosterone oversecretion in primary aldosteronism patients. J Hypertens 24:371–379 [DOI] [PubMed] [Google Scholar]
- 19. Rossi GP , Pitter G , Bernante P , Motta R , Feltrin G , Miotto D. 2008. Adrenal vein sampling for primary aldosteronism: the assessment of selectivity and lateralization of aldosterone excess baseline and after adrenocorticotropic hormone (ACTH) stimulation. J Hypertens 26:989–997 [DOI] [PubMed] [Google Scholar]


