Abstract
In early 2011, a committee convened by the Institute of Medicine issued a report on the Dietary Reference Intakes for calcium and vitamin D. The Endocrine Society Task Force in July 2011 published a guideline for the evaluation, treatment, and prevention of vitamin D deficiency. Although these reports are intended for different purposes, the disagreements concerning the nature of the available data and the resulting conclusions have caused confusion for clinicians, researchers, and the public. In this commentary, members of the Institute of Medicine committee respond to aspects of The Endocrine Society guideline that are not well supported and in need of reconsideration. These concerns focus on target serum 25-hydroxyvitamin D levels, the definition of vitamin D deficiency, and the question of who constitutes a population at risk vs. the general population.
The Journal of Clinical Endocrinology and Metabolism recently published a Clinical Practice Guideline for the Evaluation, Treatment, and Prevention of Vitamin D Deficiency (1). The guideline, written by an Endocrine Society Task Force, disagrees on several points with the Institute of Medicine (IOM) Committee's 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D (2). Although there are some agreements, the differences generate confusion for clinicians, researchers, and the public and raise concerns among members of the IOM committee. In this commentary, we respond to a number of the guideline's conclusions that we find to be unsubstantiated and in need of reconsideration. We recognize that the intent of the guideline is to target different populations than those covered by the IOM report, notably diseased and high risk populations, but we find that normal populations have been inappropriately declared at risk and subsumed into the guideline.
Regarding agreement, the guideline and our report agree that vitamin D is essential for skeletal health, and there is no convincing evidence to link vitamin D with benefits for nonskeletal outcomes such as cardiovascular disease, death, and quality of life. There is agreement that there is no need to screen the general population routinely. The guideline's discussions of the vitamin D needs of the general population are consistent with dietary reference intakes established by the IOM committee, and both acknowledge the interrelationship between calcium and vitamin D relative to skeletal health.
However, there are three major points of disagreement. First, we disagree that serum 25-hydroxyvitamin D (25OHD) levels of 30 ng/ml or higher compared with 20 ng/ml provide increased health benefits. Second, we disagree that all persons are deficient if serum 25OHD levels are below 20 ng/ml. Third, the guideline incorrectly characterizes several large subgroups as at risk that, as discussed below, do not fit this category. In the end, for reasons that cannot be supported by the available evidence, the guideline recommends screening a multitude of persons. With no evidence for benefit, it is difficult to justify the billions in health care dollars that would be required as well as the administration of high doses of vitamin D in the absence of data to demonstrate safety.
The IOM committee was charged with identifying an end point that could be used as a basis for establishing the requirements for vitamin D for the U.S. and Canadian populations. This determination was based on the ability to convincingly link vitamin D to relevant changes in the endpoint. Once that end point was established, the committee was further charged with specifying the amount of vitamin D that produces the optimal effect on that end point. We also determined an upper level of vitamin D, that is, a level beyond which the risk of harm could be expected to increase for the general population. Per the procedures of the National Academy of Sciences, the approach used to develop our report ensured that the totality of the evidence was taken into account, the conclusions were justified, and the documentation was presented in a clear and transparent manner. The IOM committee considered more than 1000 publications and did not selectively focus on the work of any one researcher or research group. This comprehensive, evidence-based approach, which informed the committee about the totality of the evidence as well as its strengths and weaknesses within the context of the needed scientific judgment, is different from what was used by the authors of the guideline which by its nature is driven by expert opinion regarding a selective set of data. Although these approaches can lead to different outcomes, the comments below focus on the consistency of the available data that can be used to inform expert opinion. Our concerns about The Endocrine Society guideline are outlined below.
Disagreement: should target serum 25OHD levels be different for the general and at-risk populations?
We find that the discussions presented in the guideline fail to support the conclusion that at-risk populations benefit from higher serum 25OHD levels compared with the general population.
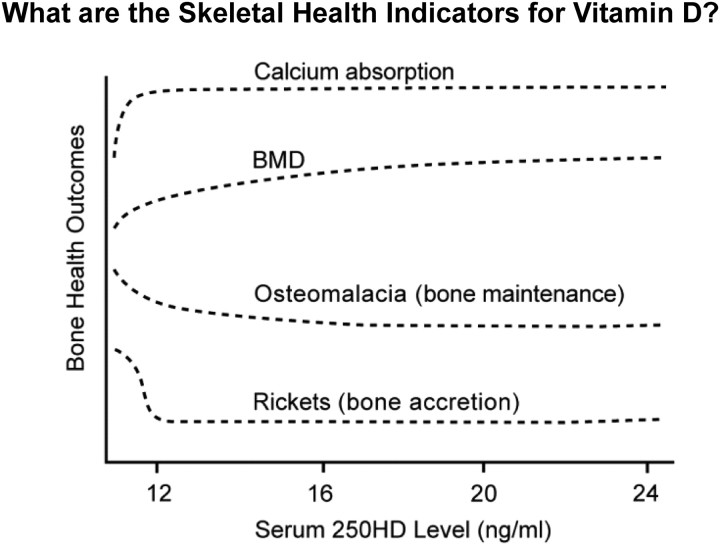
The IOM committee considered in depth the level of serum 25OHD that is associated with bone health. We integrated measures derived from the literature on calcium absorption, bone mineral density, osteomalacia, and rickets. When the totality of the evidence was considered, there was a notable congruence of data to indicate little or no additional benefit with serum levels above 20 ng/ml and a clear plateau of the effect between 12 and 16 ng/ml (see Fig. 1). We did not find that a higher level of serum 25OHD, i.e. 30 ng/ml, would be beneficial for bone health. Our review of the guideline fails to identify the evidence base for the conclusion that appropriate treatment for underlying conditions such as osteoporosis, chronic kidney disease, hepatic failure, malabsorption syndromes, obesity, and pregnancy or lactation requires higher serum 25OHD levels compared with the general population. We did not specifically consider these disease-related conditions in detail because our focus was the general population, but the available literature on these disorders was summarized in our report. Although such topics may be valid research questions, we are concerned about a guideline that asserts benefit from a higher level in the apparent absence of data to establish the basis for the recommendation.
Fig. 1.
IOM committee integration of optimal bone health outcomes (y-axis) and achieved serum 250HD levels (x-axis) revealing congruence of benefit between 16 and 20 ng/ml. BMD, Bone mineral density. [Reproduced from IOM (Institute of Medicine): Dietary reference intakes for calcium and vitamin D, p 293. Washington DC: The National Academies Press 2011 (2), with permission.]
We are aware that 30 ng/ml has been noted as a desirable serum 25OHD level. Our review of the available publications suggested that this conclusion is based on three lines of purported evidence: 1) elevated PTH is consistently lowered to a plateau when serum 25OHD is at 30 ng/ml or higher; 2) there is reduction in the risk of falls among older persons at serum 25OHD levels of 30 ng/ml or higher; and 3) calcium absorption is maximal at serum 25OHD levels of 30 ng/ml. As discussed below, the IOM committee examined the data for these three tenets and could find little basis for the conclusions of the guideline.
PTH levels
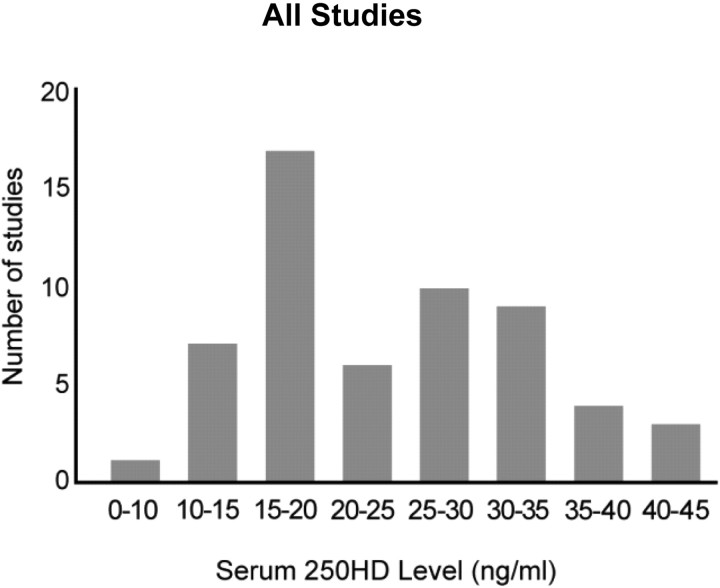
The guideline inappropriately concludes that reductions in serum PTH levels are optimized only when serum 25OHD levels are 30 ng/ml or higher. However, available evidence shows that PTH values decline to a plateau at different levels of serum 25OHD, ranging between 15 and 50 ng/ml. This varies widely among individuals and appears to be dependent on age, race, ethnicity, body composition, renal function, and geographic locations. The guideline states that “several, but not all, studies …” show that PTH begins to plateau in adults who have blood levels of 25OHD between 30 and 40 ng/ml and as evidence references four studies. Our report made use of more than 14 studies (3), and it is noteworthy that meta-analysis published in this journal in 2011 (4) highlighted more than 70 citations that do not support 30 ng/ml as consistent with a plateau effect, as shown in Fig. 2.
Fig. 2.
Levels of serum 250HD at which serum PTH plateaus and/or is maximally suppressed, as demonstrated by 59 studies reviewed by Sai et al. (4). Not included are eight studies in which serum PTH continually decreased with increasing serum 25OHD and three studies that found no relationship between serum 250HD and serum PTH. [Reproduced from A. Sai et al.: Relationship between vitamin D, parathyroid hormone, and bone health. J Clin Endocrinol Metab 96:E436–E446, 2011 (4), with permission. ©The Endocrine Society.]
The four studies referred to by the guideline relative to PTH levels are the IOM report (2), the work of Chapuy et al. (5), and reports from Holick et al. (6) and Thomas et al. (7). We presume that the phrase “but not all” is referring to the IOM report citation in which we argue against a plateau at 30–40 ng/ml. The remaining three studies do not provide strong or compelling support for a plateau at 30 ng/ml of 25OHD. Chapuy et al. (5) used a simple linear regression to show correlation between PTH and 25OHD, but this approach is not statistically appropriate considering the curvilinear nature of the data. In addition, the r2 for the correlation was only 0.04, suggesting that there must be other mechanisms responsible for the increase in PTH in this otherwise healthy elderly cohort. The report from Holick et al. (6) was based solely on women treated for osteoporosis, the vast majority of whom were receiving bisphosphonates, which are known to raise PTH levels due to suppressed bone resorption. In the case of Thomas et al. (7), it would appear from the figure in the publication that there may be a plateau at 20–30 ng/ml. However, the accompanying text indicates that patients were categorized according to their serum 25OHD levels in increments of 5 ng/ml. As reported by Thomas et al. (7), the slope of the relation between serum 25OHD and PTH concentrations was not significantly different from zero for patients with serum 25OHD concentrations greater than 15 ng/ml. It is unclear whether the guideline has based its conclusion merely on the appearance of the figure or whether this study constitutes one of the “but not all” studies.
Incidence of falls among older persons
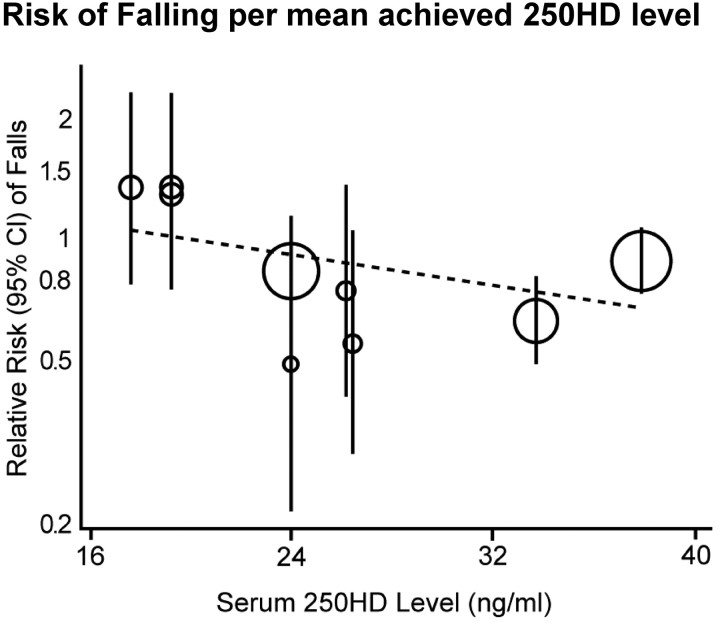
The guideline recommends vitamin D supplementation for fall prevention, a so-called noncalcemic benefit, relying heavily on a 2009 meta-analysis that demonstrated a dose-response between falls and serum 25OHD (8). We do not think this conclusion can be supported. The 2009 meta-analysis contains significant inconsistencies and misrepresentations of results. Critical problems included the inaccurate reporting of the methods, internal inconsistencies in the paper, inappropriate and misleading visual display of the quantitative information, and the selectivity of the dose-response analyses. Therefore, conclusions from the meta-analysis appear questionable. The IOM committee reanalyzed these data, the results of which are illustrated in Fig. 3 and described in the IOM report (9). The analysis used the STATA program (STATA Corp., College Station, TX), and analyses were repeated by fitting a random effects metaregression with the log(RR) of sustaining at least one fall as the response variable and the mean achieved serum 25OHD level in the vitamin D supplementation arm as the predictor variable (continuous). The results indicate that there is no significant dose-response relationship between the risk of sustaining at least one fall and the achieved 25OHD level (β-coefficient = −0.0087 ± 0.0056 se; relative risk reduction = 0.92 for risk of falls for every 4-ng/ml increase in 25OHD level, P = 0.17). This reanalysis argues against a dose-response relationship between falls and serum levels of 25OHD.
Fig. 3.
Relative risk of falls and mean achieved serum 25OHD concentrations. The IOM committee reanalysis of 2009 metaregression data on falls (8) demonstrates correct metaregressions with continuous predictors showing nonsignificance. [Reproduced from IOM (Institute of Medicine) Dietary reference intakes for calcium and vitamin D, p 161. Washington DC: The National Academies Press (2), with permission.]
Calcium absorption
The IOM committee carefully examined the data related to serum 25OHD levels and calcium absorption. The guideline relies on a 2003 study (10) based on 34 subjects, of which 45% were taking estrogen and 50% did not undergo formal calcium absorption tests. The guideline suggests that the 2003 study was compelling because calcium absorption was higher in 24 women with mean serum 25OHD of 32 ng/ml compared with women with a mean serum 25OHD level of 20ng/ml; however, only 14 of 34 subjects were common to both absorption studies. The guideline chose this single study and ignored trials in more than 1300 subjects who underwent formal calcium absorption tests, and a key study of 319 subjects with low serum 25OHD clearly shows that calcium absorption reaches near maximum at serum 25OHD levels of 8 ng/ml (11). Overall, the IOM committee's examination of the totality of the evidence (12) showed that the data contradict the 2003 study, revealing that calcium absorption reaches near maximum between serum 25OHD levels of 8–20 ng/ml.
Disagreement: how should deficiency be defined?
The guideline specifies that vitamin D deficiency is present when serum levels of 25OHD are below 20 ng/ml. This conclusion is stated as a fact for the general population, not as a special consideration for diseased populations as is the purported purpose of the guideline. The guideline attributes the conclusion to several groups, including incorrectly to the IOM committee, and does not relate such levels to those with underlying conditions and disease states. Moreover, the guideline authors appear to have selected a subgroup of available data without providing an explanation as to why these studies are more compelling than others that also address the question of insufficient levels of vitamin D.
The work of the IOM to define human requirements for vitamin D for the general population was based on assuring bone health, the same basis used by the guideline. A comprehensive review of the literature was undertaken as described in the IOM report. To be useful for judging bone health outcomes relative to requirements and recommended intakes of vitamin D, the available evidence was considered in the context of its relevance to bone accretion, bone maintenance, and bone loss (see Fig. 1). Studies were weighted for quality, and outcomes integrated on that basis. Evidence of a dose-response relationship was pivotal and the reliance on certain studies as well as the concerns about others are documented in the literature review. The outcomes revealed the following: 1) practically all persons, 97.5% of the general population, are assured bone health when serum levels of serum 25OHD are 20 ng/ml (50 nmol/liter); and 2) serum 25OHD concentrations of 16 ng/ml (40 nmol/liter) reflect a level that is sufficient to ensure bone health for approximately half the general population.
For the IOM, the 20-ng/ml level is the upper range of human requirements, and therefore, on a population basis, 20 ng/ml reflects a level that more than meets the needs of almost all of the general population. The guideline's use of 20 ng/ml as the point for defining deficiency in the general population is inconsistent with the data and inflates the number of persons deemed to be vitamin D deficient. Less than 3% of the general population is likely to have vitamin D needs that require levels to be higher than 20 ng/ml; more than 97% are adequate in vitamin D with serum levels of 20 ng/ml and 50% are adequate at levels of 16 ng/ml.1 These delineations were defined for the general population for the United States and Canada. Although more study is necessary to clarify the nature of the serum response to dietary vitamin D, it would appear that intakes between 400 and 800 IU/d are likely to increase serum levels to at least 20 ng/ml and probably higher, depending on a variety of factors.
In clinical situations in which the true requirement of an individual member of the general population cannot be known, practitioners' interest may focus on maintaining a serum 25OHD level of 20 ng/ml for that individual. This is not the same as declaring the general population to be deficient if average serum values are less than 20 ng/ml.2 Rather, it is reasonable to expect that for practitioners there are likely to be alternative definitions of vitamin D deficiency for a patient with a disease condition or a health-related consideration. The goal of the guideline therefore should be to provide a rationale for such a definition specific to the evidence associated with the condition of interest. The guideline has not provided the rationale.
Disagreement: who constitutes a population at risk vs. the general population?
The IOM report is focused on the general population, and the guideline recommendations are intended for at-risk individuals. However, the guideline incorporates some conditions that are appropriately part of the general population. The IOM committee regarded the groups discussed below as members of the general population. Our review took into account the relevant physiological states of these groups, and the Dietary Reference Intakes are intended to be adequate to meet their needs.
African-Americans, Hispanics, and dark-skinned populations
Persons likely to experience reduced vitamin D synthesis from sun exposure are those with dark skin pigmentation and include certain immigrant groups (for example, those from South Asia and the Middle East) who now reside in North America as well as dark-skinned, exclusively breast-fed infants. African-Americans present a conundrum because, although their serum values are lower than those of their white counterparts, their rates of osteoporosis and fractures are lower than Caucasians. In establishing the intake requirements for populations that would include these subgroups, the IOM committee determined intake requirements on the basis of minimal to no sun exposure (13). That is, the data used were from studies that were conducted under conditions of very limited or no sun exposure. Thus, the reference values for vitamin D for the general population overcome the variability that may be introduced by melanin levels in the skin, and in turn are adequate for dark-skinned subgroups that may be at risk of limited synthesis due to sun exposure. In short, this approach resulted in estimates of required intake that are not dependent on skin pigmentation and vitamin D synthesis.
Pregnant or lactating women
The guideline misinterprets and overlooks data related to pregnancy and lactation. In the case of pregnancy, available evidence does not suggest that pregnant women are at increased risk relative to vitamin D compared with the nonpregnant population, and a recent commentary by a member of the IOM committee may be helpful to those working in the area (14). In terms of bone health, the existing data set is insufficient to link serum 25OHD levels with maternal bone mineral density during pregnancy, as noted in the IOM report. Furthermore, no effect of maternal 25OHD levels has been demonstrated relative to fetal calcium homeostasis or skeletal outcomes. The guideline focuses on the levels of 1,25 dihydroxyvitamin D and then on a study (15), which associates vitamin D deficiency with preeclampsia. Although preventing vitamin D and other nutrient deficiencies during pregnancy is wise, there is not a sound basis to conclude that the risk for preeclampsia is reduced with intakes or serum levels of vitamin D higher than those appropriate for nonpregnant females. Therefore, a blanket conclusion that pregnant women are at high risk for vitamin D deficiency and preeclampsia is unwarranted. Two observational studies (16, 17), neither of which is cited by the Guideline, identified associations between supplementary vitamin D and incidence of preeclampsia, but the associations between serum 25OHD level and preeclampsia were not conclusive. On the other hand, two case-control studies, one in the United States (18) and one in Denmark (19), found no significant difference in serum 25OHD levels between women with preeclampsia and those without. To date, no mechanism of action has been articulated to posit a relationship.
In the case of lactation, the IOM report found no basis for increased vitamin D requirements during lactation compared with nonlactating states. The guideline discussion concerning lactation acknowledges that the related metabolic changes do not increase the requirements for vitamin D. Then, as is the case for virtually all population groups discussed by the guideline, the authors stipulate the need to achieve serum levels of 30 ng/ml. Vitamin D and its metabolites are present at very low levels in milk, and to transfer vitamin D from the mother to the infant, the mother's dietary intake must be raised to extraordinarily high levels. As discussed in the IOM report (20), there are eight randomized clinical trials that are consistent with the six existing observational studies, all indicating that increased maternal vitamin D intakes increase maternal serum 25OHD levels but have no effect on the neonatal serum 25OHD levels of breast-fed infants unless, as mentioned, the maternal intake of vitamin D is extremely high (i.e. 4000–6400 IU/d) (21). The 1400–1500 IU/d recommended by the guideline is not likely to result in transfer to the infant in any case. The 4000–6000 IU/d indicated as necessary to transfer enough to the infant to avoid supplementation is worrisome and surpasses the tolerable upper intake level identified by the IOM committee. Although not highlighted by the guideline, Prentice et al. (22) found no relationship between vitamin D and breast milk calcium content (9i), nor has a relationship been reported by others. Wagner et al. (21) (9h) failed to demonstrate any alteration in the calcium content of breast milk, even when 6400 IU/d of vitamin D were fed. The guideline authors' assertion that vitamin D is needed to increase the dietary absorption of calcium during lactation suggests that this is the main route of the calcium that comprises breast milk. But, in fact, intestinal calcium absorption is not the main supply of calcium to breast milk. Instead most of the calcium in breast milk is derived from skeletal resorption (23, 24), a process that has been remarkably conserved, even at high and low extremes of maternal calcium intakes.
Older adults with history of nontraumatic fracture
For the IOM committee, the reduction in fracture risk was an important indicator of interest in establishing the Dietary Reference Intakes for older adults, not only because of the actual event but also because of the high mortality and morbidity associated with fractures. Overall, an intake of 600–800 IU/d of vitamin D has been supported in the literature relative to fracture risk reduction when calcium supplementation was also part of the treatment. However, when studies involving only the use of vitamin D were included, a meta-analysis found no effect of vitamin D supplementation on fracture risk (25). There are few studies of dose-response relationships for vitamin D and even fewer that relate intake to serum vitamin D. Noting a report that 800 IU/d vitamin D provided some benefit in fracture risk reduction and in the face of no dose-response data to demonstrate that the effect also occurred at a lower level of intake, the IOM committee set dietary reference intakes for this group based on fracture risk reduction (26). Although the set of dietary reference intakes for older adults stipulates the same average requirement as for the rest of the population because there is wide variability in age-related physiological changes, sun exposure, and skin morphology among older individuals, leading to greater variability around the average requirement, the recommended intake (i.e. a value generally 2 sd above the average requirement) was set higher than for other age groups.
Obese persons
The basis for the guideline's recommendation to supplement obese persons is not derived from evidence that increased vitamin D intake among these persons improves bone health, only on the observation that their 25OHD levels are lower than nonobese persons. The lower levels of serum 25OHD observed in obese persons may be due to storage of vitamin D in adipose tissue, although the dynamics of this effect has not been firmly established (27). Studies of modest weight loss have found circulating serum 25OHD levels to increase despite constant intakes of vitamin D (27), and this increase may be proportional to weight loss. In short, there is no evidence that increases in vitamin D intake beyond the requirements for nonobese persons can affect bone health or other health conditions among obese persons.
Summary
Although the IOM committee welcomes efforts to identify the role of vitamin D in treating special and diseased populations outside the category of the general population, the guideline defines a number of population groups to constitute those at risk when such groups clearly were considered as part of the general population for establishing Dietary Reference Intakes. The guideline goes on to inappropriately conclude that the benefits of vitamin D for a large segment of our population occur only when serum levels are at 30 ng/ml 25OHD and above, and mistakenly concludes that all persons with serum 25OHD levels below 20 ng/ml are deficient in vitamin D. In turn, the guideline conclusion that at least half of our population requires routine testing represents a large, unnecessary cost. Finally, the guideline's recommendation for high levels of intake for a wide range of conditions in which benefits have not been demonstrated is disconcerting and does not reflect an evidence-based approach.
We support the Endocrine Society efforts to produce clinical practice guidelines targeted to persons with underlying health conditions, but this needs to be done using a systematic, evidence-based approach that assesses the strength of evidence for both benefits and risks of supplementation. The current guideline fails in both respects and therefore is in need of reexamination.
Acknowledgments
The IOM Committee on Dietary Reference Intakes for Vitamin D and Calcium was, as is pro forma and customary, decommissioned at the time its completed report was released in 2011. The authors of this manuscript worked independently and voluntarily to develop the manuscript; no funding sources were used.
Dr. Taylor served as Study Director (2008–2011) for the committee's report.
Disclosure Summary: All authors received reimbursement for travel to the Institute of Medicine committee meetings. C.J.R., S.A.A., J.F.A., S.K.C., R.A.D.-A., J.C.G., R.L.G., C.S.K., J.E.M., S.T.M., A.C.R., S.A.S., and C.L.T. have nothing to disclose. P.M.B. has received speaker honoraria from the American Dietetic Association. G.J. is a member of the Scientific Advisory Board of Cytochroma, Inc. and Receptor Therapeutics and has served as a speaker for Genzyme Speaker's Bureau.
As presented in Appendix I of the IOM committee report (2), data from national surveys reveal that approximately 81% of the population in the United States (National Health and Nutrition Examination Survey) have serum values greater than 16 ng/ml, and values for 87% of the population in Canada (Canadian Health Measures Survey) surpass 16 ng/ml.
It is important to distinguish between applications for a population as compared with applications for individual patients. Many clinicians are well versed in what might be referred to as the clinical model in which the goal is to address the needs of a specific individual presenting to the clinician and requiring specific advice about treatment. This is quite distinct from a public health model in which the goal is to understand the needs of a population, make conclusions about the status of the population, and apply those understandings to a range of activities.
- IOM
- Institute of Medicine
- 25OHD
- 25-hydroxyvitamin D.
References
- 1. Holick MF , Binkley NC , Bischoff-Ferrari HA , Gordon CM , Hanley DA , Heaney RP , Murad MH , Weaver CM. 2011. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:1911–1930 [DOI] [PubMed] [Google Scholar]
- 2. IOM (Institute of Medicine) 2011. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; [PubMed] [Google Scholar]
- 3. IOM (Institute of Medicine) 2011. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 260–262 [PubMed] [Google Scholar]
- 4. Sai AJ , Walters RW , Fang X , Gallagher JC. 2011. Relationship between vitamin D, parathyroid hormone, and bone health. J Clin Encocrinol Metab 96:E436–E446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chapuy MC , Schott AM , Garnero P , Hans D , Delmas PD , Meunier PJ. 1996. Healthy elderly French women living at home have secondary hyperparathyroidism and high bone turnover in winter: EPIDOS Study Group. J Clin Endocrinol Metab 81:1129–1133 [DOI] [PubMed] [Google Scholar]
- 6. Holick MF , Siris ES , Binkley N , Beard MK , Khan A , Katzer JT , Petruschke RA , Chen E , de Papp AE. 2005. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab 90:3215–3224 [DOI] [PubMed] [Google Scholar]
- 7. Thomas MK , Lloyd-Jones DM , Thadhani RI , Shaw AC , Deraska DJ , Kitch BT , Vamvakas EC , Dick IM , Prince RL , Finkelstein JS. 1998. Hypovitaminosis D in medical inpatients. N Engl J Med 338:777–783 [DOI] [PubMed] [Google Scholar]
- 8. Bischoff-Ferrari HA , Dawson-Hughes B , Staehelin HB , Orav JE , Stuck AE , Theiler R , Wong JB , Egli A , Kiel DP , Henschkowski J. 2009. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis or randomized controlled trials. BMJ 339:b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. IOM (Institute of Medicine) 2011. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 158–161 [PubMed] [Google Scholar]
- 10. Heaney RP , Dowell MS , Hale CA , Bendich A. 2003. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr 22:142–146 [DOI] [PubMed] [Google Scholar]
- 11. Need AG , O'Loughlin PD , Morris HA , Coates PS , Horowitz M , Nordin BE. 2008. Vitamin D metabolites and calcium absorption in severe vitamin D deficiency. J Bone Miner Res 23:1859–1863 [DOI] [PubMed] [Google Scholar]
- 12. IOM (Institute of Medicine) 2011. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 264–267 [PubMed] [Google Scholar]
- 13. IOM (Institute of Medicine) 2011. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 370–371 [PubMed] [Google Scholar]
- 14. Abrams SA. 2011. Vitamin D supplementation during pregnancy. J Bone Miner Res 26:2338–2340 [DOI] [PubMed] [Google Scholar]
- 15. Bodnar LM , Simhan HN , Powers RW , Frank MP , Cooperstein E , Roberts JM. 2007. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr 137:447–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haugen M , Brantsaeter AL , Trogstad L , Alexander J , Roth C , Magnus P , Meltzer HM. 2009. Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology 20:720–726 [DOI] [PubMed] [Google Scholar]
- 17. Hyppönen E , Hartikainen AL , Sovio U , Järvelin MR , Pouta A. 2007. Does vitamin D supplementation in infancy reduce the risk of pre-eclampsia? Eur J Clin Nutr 61:1136–1139 [DOI] [PubMed] [Google Scholar]
- 18. Seely EW , Wood RJ , Brown EM , Graves SW. 1992. Lower serum ionized calcium and abnormal calciotrophic hormone levels in preeclampsia. J Clin Endocrinol Metab 74:1436–1440 [DOI] [PubMed] [Google Scholar]
- 19. Frølich A , Rudnicki M , Storm T , Rasmussen N , Hegedüs L. 1992. Impaired 1,25-dihydroxyvitmain D production in pregnancy-induced hypertension. Eur J Obstet Gynecol Reprod Biol 47:25–29 [DOI] [PubMed] [Google Scholar]
- 20. IOM (Institute of Medicine) 2011. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 258. [PubMed] [Google Scholar]
- 21. Wagner CL , Hulsey TC , Fanning D , Ebeling M , Hollis BW. 2006. High-dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: a 6-month follow-up pilot study. Breastfeed Med 1:59–70 [DOI] [PubMed] [Google Scholar]
- 22. Prentice A , Yan L , Jarjou LM , Dibba B , Laskey MA , Stirling DM , Fairweather-Tait S. 1997. Vitamin D status does not influence the breast-milk calcium concentration of lactating mothers accustomed to a low calcium intake. Acta Paediatr 86:1006–1008 [DOI] [PubMed] [Google Scholar]
- 23. Sowers M , Eyre D , Hollis BW , Randolph JF , Shapiro B , Jannausch ML , Crutchfield M. 1995. Biochemical marker of bone turnover in lactating and nonlactating postpartum women. J Clin Endocrinol Metab 80:2210–2216 [DOI] [PubMed] [Google Scholar]
- 24. Kalkwarf HJ , Specker BL , Bianchi DC , Ranz J , Ho M. 1997. The effect of calcium supplementation on bone density during lactation and after weaning. N Engl J Med 337:523–528 [DOI] [PubMed] [Google Scholar]
- 25. Avenell A , Gillespie WJ , Gillespie LD , O'Connell D. 15 April 2009. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database Syst Rev 10.1002/14651858.CD000227.pub3 [DOI] [PubMed] [Google Scholar]
- 26. IOM (Institute of Medicine) 2011. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 391–392 [PubMed] [Google Scholar]
- 27. IOM (Institute of Medicine) 2011. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 490–491 [PubMed] [Google Scholar]