Abstract
Context:
Familial combined hypolipidemia causes a global reduction of plasma lipoproteins. Its clinical correlates and metabolic implications have not been well defined.
Objective:
The objective of the study was to investigate the genetic, clinical, and metabolic characteristics of a cohort of subjects with familial combined hypolipidemia.
Design:
The design of the study included candidate gene screening and the comparison of the clinical and metabolic characteristics between carrier and noncarrier individuals.
Setting:
The study was conducted in a general community.
Subjects:
Participants in the study included individuals belonging to nine families with familial combined hypolipidemia identified in a small town (Campodimele) as well as from other 352 subjects living in the same community.
Main Outcomes Measures:
Serum concentrations of lipoproteins, Angiopoietin-like 3 (Angptl3) proteins, and noncholesterol sterols were measured.
Results:
The ANGPTL3 S17X mutation was found in all probands, 20 affected family members, and 32 individuals of the community. Two additional frame shift mutations, FsE96del and FsS122, were also identified in two hypocholesterolemic individuals. Homozygotes for the ANGPTL3 S17X mutation had no circulating Angptl3 and a marked reduction of all plasma lipids (P < 0.001). Heterozygotes had 42% reduction in Angptl3 level compared with noncarriers (P < 0.0001) but a significant reduction of only total cholesterol and high-density lipoprotein cholesterol. No differences were observed in the plasma noncholesterol sterols between carriers and noncarriers. No association between familial combined hypolipidemia and the risk of hepatic or cardiovascular diseases were detected.
Conclusions:
Familial combined hypolipidemia segregates as a recessive trait so that apolipoprotein B- and apolipoprotein A-I-containing lipoproteins are comprehensively affected only by the total deficiency of Angptl3. Familial combined hypolipidemia does not perturb whole-body cholesterol homeostasis and is not associated with adverse clinical sequelae.
Familial combined hypolipidemia is a recently discovered dyslipidemic phenotype characterized by reduced levels of both apolipoprotein (Apo) B-[very low-density lipoprotein (VLDL) and low-density lipoprotein (LDL)] and apoA-1-containing [high-density lipoprotein (HDL)] lipoproteins (1). It belongs to the group of familial hypobetalipoproteinemia syndrome (FHBL; online inheritance in man no. 107730), which includes several genetically heterogeneous disorders of lipid metabolism (2, 3). A form of FHBL is due to the presence of mutations in the gene encoding for ApoB (APOB), leading to the reduced secretion of VLDL by the liver (4). FHBL is inherited as a dominant trait and is usually considered a benign condition, even though patients with ApoB deficiency are likely to develop hepatic steatosis and fat malabsorption (5). Loss-of-function mutations in the proprotein convertase subtilisin kexin type 9 (PCSK9), a protein involved in the posttranslational regulation of the LDL receptor, also cause FHBL (6, 7). The genetic cause of familial combined hypolipidemia has been attributed to mutations in the ANGPTL3 gene, which encodes a protein involved in the regulation of extracellular lipases (8). Due to paucity of cases investigated so far (9, 10), the mode of inheritance and the clinical implications of familial combined hypolipidemia are not well defined. In particular, no data have been reported about the potential association of this condition with clinical manifestation of atherosclerosis or liver disease. Therefore, further clinical and biochemical characterization of this new form of hypolipidemia is warranted.
The aims of this work was to define the role of the ANGPTL3 gene as a determinant of the combined hypolipidemia phenotype in a large cohort of subjects with primary hypocholesterolemia identified in a small town in Italy and to investigate the clinical and metabolic characteristics of subjects carrying ANGPTL3 variants.
Materials and Methods
Family study
In 1991, as a part of a screening for cardiovascular risk factors, we identified in Campodimele, a small town located in the province of Latina (Italy), some subjects with reduced plasma levels of LDL-cholesterol (C) and ApoB. A family linkage analysis showing the absence of cosegregation between APOB alleles and the hypocholesterolemic trait allowed us to rule out mutations in APOB as the cause of this phenotype (11). An additional eight unrelated families were characterized in this town, bringing to nine the total number of families considered for the present study. Diagnostic criteria to select families were the presence of an index case and at least one relative with LDL-C less than the fifth age- and gender-specific percentile for the general Italian population and without evidence of secondary causes of hypolipidemia (12). Fifty-two family members (nine index cases, 28 first degree relatives, 11 second degree relatives, and four spouses) were investigated.
The Ethical Committee of Sapienza University of Rome approved the study protocol and all subjects provided their informed consent.
Population study
To evaluate the prevalence of hypocholesterolemia in the population, all residents in the town of Campodimele were asked to voluntarily participate into a general program of preventive medicine not specifically dedicated to dyslipidemias. This screening was carried out in 2008–2009.
Examination was carried out in the morning after an overnight fast according to a standardized protocol. Medical history (with particular reference to liver and cardiovascular diseases), coronary artery disease risk factors, and current medications (including vitamins and supplements) were recorded using a structured questionnaire. Alcohol consumption was assessed using a semiquantitative scale (0, abstainers; 1, less than two glasses per day; and 2, more than two glasses per day). Measurements included height and weight and systolic and diastolic blood pressures. A standard 12-lead electrocardiogram was performed in all subjects. Fasting blood samples for laboratory determinations and the isolation of DNA were also obtained.
Genetic analysis
DNA from peripheral blood was extracted with the Wizard genomic DNA purification kit (Promega, Madison, WI) according to the manufacturer's instructions. First, the known genes causing hypocholesterolemia, MTTP, PCSK9, NPC1L1, and ANXA2 were screened by direct sequencing in probands III2, II2, III1, and II2 belonging to families no. 1, no. 2, no. 3, and no. 5, respectively (13–16). For the ANGPTL3 analysis, exons and intron-exon boundaries were PCR amplified with oligonucleotides and the procedure reported in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org. All the sequence variants identified were verified by manual inspection of the chromatograms and changes were confirmed by an independent resequencing reaction.
To assess the possibility of shared ancestry for the S17X mutation, we did a haplotype analysis with a series of polymorphic markers obtained from HapMap. Five single-nucleotide polymorphisms (rs656297, rs3850634, rs12563308, rs6678483, and rs12130333) encompassing a 293-kb block containing the ANGPTL3 gene (http://www.ncbi.nlm.nih.gov/SNP/) were selected. We assayed these single-nucleotide polymorphisms in 33 unrelated S17X carriers (four homozygotes and 29 heterozygotes).
Measurements of serum levels of angiopoietin-like 3 (Angptl3)
Angptl3 serum levels were determined using an ELISA assay in ANGPTL3 mutation carriers and in 49 age-, gender-, and body mass index (BMI)-matched noncarriers (17). Serum samples were diluted 50-fold and measured in duplicate. Repeated measurements were made in homozygotes after 10-fold dilution of serum samples. The detection limit of the assay is 1–0.5 ng/ml.
Measurement of noncholesterol sterols
Noncholesterol sterols that correlate with relative levels of cholesterol absorption (cholestanol, campesterol, and sitosterol) and synthesis (lanosterol and lathosterol) as well as bile acid precursors (7α-hydroxycholesterol and 24S- and 27-hydroxycholesterol) were measured by a highly sensitive and specific gas chromatography-mass spectrometry procedure, as previously described (18). The variability of within-day and between-day accuracy and precision for all analytes was less than 4% of the nominal and mean values, respectively. Absolute plasma sterol concentrations were corrected for total cholesterol (TC) (sterols/TC) to account for the difference in the plasma cholesterol levels; the TC-independent lathosterol to sitosterol ratio, which measures the balance of cholesterol synthesis and absorption, was also calculated.
Lipoprotein profile by fast-phase liquid chromatography (FPLC)
Serum aliquots of 100 μl were applied on a Superose 6HR gel filtration column (Pharmacia Biotech, Uppsala, Sweden) at a flow rate of 0.3 ml/min in PBS (pH 7.4) containing 1 mm EDTA. During chromatography fractions (0.6 ml) were collected and analyzed for cholesterol and triglyceride concentrations.
Plasma lipids and other laboratory determinations
Blood samples were collected in EDTA-containing tubes and plasma was immediately obtained by centrifugation at 4 C. Aliquots were added with EDTA (0.04%), NaN3 (0.05%), and phenylmethylsulfonyl fluoride (0.015%) to prevent lipid and lipoprotein modifications. Some were used within 2–4 h for lipids and blood glucose determination, whereas others were stored at −80 C for additional measurements. Plasma lipids, ApoB, fasting blood glucose, and liver enzymes were assayed as reported (19). ApoE genotyping was performed as previously described (20).
Statistical analysis
All statistical analyses were performed with SPSS/WIN program (version 12.0; SPSS Inc., Chicago, IL). Descriptive statistics such as means, sd, and ranges were undertaken for all the variables. Continuous variables were compared by the Mann-Whitney U test, whereas the categorical variables were compared by χ2 or Fisher's exact tests, when appropriate. Bonferroni's correction was performed for multiple comparisons. Differences in lipid levels between carriers and noncarriers were tested for significance by using ANOVA including age, sex, and BMI as covariates (analysis of covariance). The size effect of mutant genotypes on plasma lipids was also evaluated by comparing lipid residuals between carriers and noncarriers. To calculate residuals, age-, sex-, and BMI-adjusted coefficients for all lipid variables were calculated in the screened population by linear regression. The obtained equation model was used to calculate the expected lipid values for each individual, and residuals were obtained by subtracting expected from observed values.
As an exploratory analysis, we calculated the standardized mortality rates (SMR) for ischemic heart disease (IHD) (International Classification of Diseases (ICD), ninth revision, codes 410-414 and ICD, 10th revision, X 120-125) and for tumors (ICD, ninth revision, codes140-239 and ICD, 10th revision, codes C00-D48) in the Campodimele population during the period 1998-2007. SMR represent the ratio of deaths observed, and those expected, on the basis of the mortality rates of the general Italian population (http://demo.istat.it) 95% confidence intervals were calculated using the exact Poisson probability. A two-sided P < 0.05 was considered statistically significant.
Results
Family study
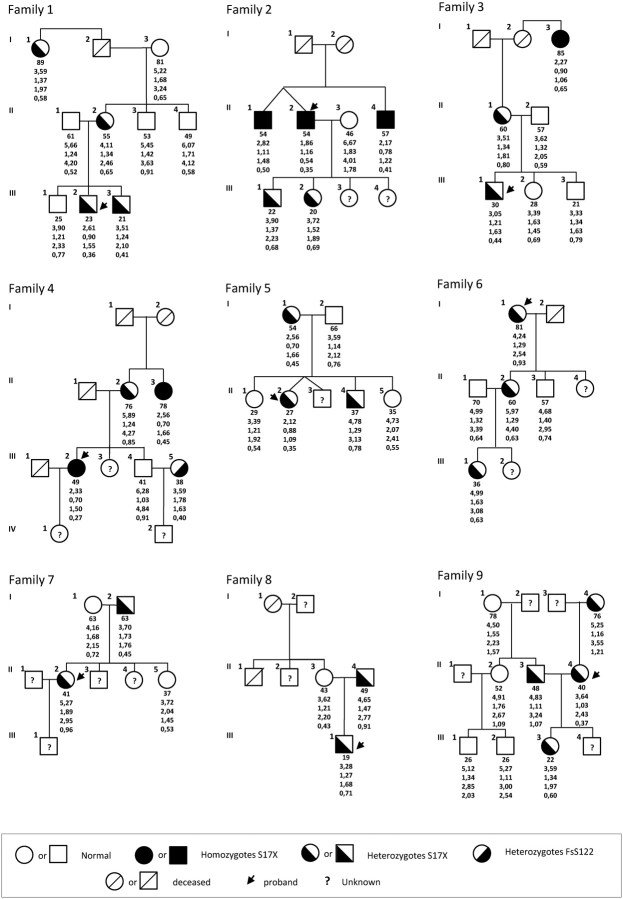
The pedigrees and the lipid values of family members are reported in Fig. 1. Twenty-three family members were classified as hypocholesterolemic (mean LDL-C levels 1.85 ± 0.61 mmol/liter) and 29 normocholesterolemic (mean LDL-C levels 2.94 ± 0.94 mmol/liter). Compared with normocholesterolemic, hypocholesterolemic family members tended to be older (45.7 ± 19.7 vs. 50.9 ± 20.7 yr, respectively; P = 0.311) and had significantly reduced plasma levels of HDL-C (1.44 ± 0.28 vs. 1.23 ± 0.34 mmol/liter, respectively; P = 0.027) and triglycerides (TG) (0.91 ± 0.50 vs. 0.55 ± 0.19 mmol/liter, respectively; P < 0.001).
Fig. 1.
Pedigrees of the family study. Squares indicate male family members and circles female family members. Slashes indicate deceased persons and arrows indicate probands. Roman numerals to the left of the pedigree indicate the generation; numerals to the upper left of each symbol indicate the individual family member. For the ANGPTL3 genotype, the X denotes a stop codon. The columns under each symbol indicate, from top to bottom, age, the total cholesterol, triglycerides, LDL, and HDL-C concentration (millimoles per liter). The values are the means of measurements from multiple fasting lipid profiles.
The screening of MTTP, PCSK9, NPC1L1, and ANXA2 revealed several known nonsynonimous variations (see Supplemental Table 2), but none of these segregated with the hypocholesterolemic phenotype, thus allowing their exclusion as causative mutations. Conversely, the sequencing of the ANGPTL3 led to the identification in all hypocholesterolemic probands of a 2-bp change at nucleotide 50 of exon 1 (c.50 CC>GA) determining a nonsense mutation at codon 17 (S17X) (seven heterozygotes and two homozygotes). The screening of family members identified 20 additional subjects carrying the S17X mutation (four homozygotes and 16 heterozygotes) (Fig. 1). All but three heterozygous carriers (family no. 5, II-4; family 6, II-2; and family no. 9, II-3) showed a hypocholesterolemic phenotype (LDL-C less than the fifth age and gender specific percentile). In a hypocholesterolemic spouse (LDL-C 1.63 mmol/liter) (family no. 4, III-5), a 2-bp deletion (c.363delCT) in exon 1 was also detected. This mutation was predicted to cause a frame shift variation at codon 122 (FsS122). Unfortunately, her 10-yr-old son was not available for screening so that we were unable to determine the segregation of this variant with the hypocholesterolemic trait. With the exception of one E2/E2 individual (family no. 4, III-5), all the family members were carriers of the common E3/E3 genotype.
Population study
Three hundred fifty-two of Campodimele's residents (58.5%) were examined [165 men (46.8%) and 187 women (53.1%)], ranging from 14 to 96 yr of age (mean 53.3 ± 20.0 yr). Among them, 46 individuals (13.1%) had hypocholesterolemia defined as LDL-C less than the fifth age- and gender-specific percentile (mean LDL-C 1.73 ± 0.43 mmol/liter). These individuals also presented lower HDL-C (P = 0.01) and TG levels (P < 0.001) compared with normocholesterolemic population individuals.
The sequencing of ANGPTL3 in these 46 hypocholesterolemic individuals identified six carriers of the S17X mutation (four heterozygous and two homozygous); in one individual we identified a novel mutation in exon 1 caused by the deletion of three nucleotides at position 286 (c.286delGAA). This variation predicts a deletion of codon 96 (FsE96del). Unfortunately, we were unable to enroll the subject's relatives to assess the cosegregation of this ANGPTL3 variation with the low LDL-C phenotype within his family. PolyPhen analysis (http://genetics.bwh.harvard.edu/pph/) predicted this variant to be possibly damaging. No ANGPTL3 mutations were identified in the remaining 39 hypocholesterolemic individuals.
We also screened the remaining population for mutations in the ANGPTL3 and identified additional 26 individuals carrying the S17X variant as heterozygotes. Overall, 32 individuals presenting the S17X (30 heterozygotes and two homozygotes) and one presenting the FsE96 mutation were detected, and the prevalence of carriers of ANGPTL3 variants in the whole population sample was calculated to be 9.4%. When the estimate was restricted to those individuals with a hypocholesterolemic phenotype, the prevalence of ANGPTL3 variants was 15.2%.
To determine whether the high frequency of the S17X mutation in this community was due to shared ancestry, we did haplotype analysis with polymorphic markers around the ANGPTL3 gene. We found that all the S17X alleles shared the same haplotype over an approximately 162-kb interval between rs656297 and rs12563308. These results strongly suggest that the S17X mutation arises from a common ancestry.
Plasma lipids and lipoproteins in ANGPTL3 mutation carriers
The comparison of demographic characteristics and lipid levels in all carriers and noncarriers of ANGPTL3 mutations is reported in Table 1. No difference in adiposity or age was observed between groups. Only homozygous carriers showed a comprehensive reduction of plasma lipoproteins (all P < 0·001), whereas heterozygous carriers presented a significant reduction limited to TC and HDL-C levels. This was observed in individuals identified throughout both the family and population cohort (Table 1), and it was further confirmed by analysis of lipid residues, which indicated that the presence of two ANGPTL3 mutated alleles reduced LDL-C levels by 48%, TG by 62%, HDL-C by 46%, ApoB by 44%, and apoA1 by 48% (data not shown).
Table 1.
Comparison of clinical characteristics and plasma lipids in carriers and noncarriers of mutations in the ANGPTL3 gene
| Age (yr) | Sex (M/F) | BMI | TC (mmol/liter) | LDL-C (mmol/liter) | HDL-C (mmol/liter) | TG (mmol/liter) | ApoB (g/liter) | ApoAI (mmol/liter) | |
|---|---|---|---|---|---|---|---|---|---|
| Family study (n = 52) | |||||||||
| Noncarriers (n = 21)a | 47.1 ± 18.1 (21–81) | 11/10 | 28.2 ± 4.1 (20.9–36.6) | 4.71 ± 1.03 (2.69–6.68) | 2.80 ± 0.96 (1.45–4.84) | 1.48 ± 0.30 (0.62–2.07) | 0.94 ± 0.56 (0.43–2.54) | 0.84 ± 0.21 (0.55–1.23) | 1.63 ± 0.22 (1.16–1.98) |
| Heterozygotes (n = 24) | 45.1 ± 21.1 (19–89) | 9/15 | 27.8 ± 5.8 (19.3–45.1) | 4.11 ± 0.97 (2.12–5.98) | 2.45 ± 0.84 (1.09–4.40) | 1.35 ± 0.27 (0.88–1.89) | 0.65 ± 0.24 (0.35–1.21) | 0.83 ± 0.24 (0.39–1.31) | 1.52 ± 0.26 (0.83–1.98) |
| Homozygotes (n = 6)b | 63.2 ± 15.9 (49–88) | 3/3 | 28.7 ± 2.7 (25.5–30.6) | 2.34 ± 0.33c,d (1.86–2.82) | 1.24 ± 0.40c,d (0.54–1.65) | 0.89 ± 0.20c,d (0.70–1.17) | 0.43 ± 0.13e (0.27–0.66) | 0.48 ± 0.10c,f (0.28–0.59) | 0.85 ± 0.13c,d (0.68–1.00) |
| Population study (n = 352) | |||||||||
| Noncarriers (n = 318) | 53.3 ± 20.1 (14–96) | 149/169 | 27.4 ± 5.0 (16.6–45.8) | 4.73 ± 0.84 (2.74–7.80) | 2.62 ± 0.83 (1.04–5.23) | 1.64 ± 0.42 (0.83–2.82) | 1.04 ± 0.64 (0.29–4.25) | 0.88 ± 0.24 (0.30–1.75) | 1.73 ± 0.28 (1.08–2.39) |
| Heterozygotes (n = 31) | 53.5 ± 19.3 (18–88) | 15/16 | 28.8 ± 4.4 (20.6–36.7) | 4.54 ± 0.62 (3.23–5.59) | 2.60 ± 0.61 (1.27–3.73) | 1.47 ± 0.41 (0.67–2.17) | 1.04 ± 0.52 (0.37–2.20) | 0.86 ± 0.17 (0.53–1.22) | 1.67 ± 0.31 (1.12–2.23) |
| Homozygotes (n = 2)b | 69.5 ± 9.1 (63–76) | 1/1 | 30.9 ± 12.8 (21.0–39.9) | 2.44 ± 0.35g,h (2.20–2.69) | 1.66 ± 0.35 (1.42–1.89) | 0.61 ± 0.02g,h (0.59–0.62) | 0.40 ± 0.02 (0.37–0.40)h | 0.62 ± 0.17 (0.50–0.74) | 0.63 ± 0.30 (0.42–0.84)e,h |
| All (n = 404) | |||||||||
| Noncarriers (n = 339)a | 52.9 ± 20.0 (14–96) | 161/180 | 27.5 ± 4.9 (16.6–45.8) | 4.73 ± 0.85 (2.74–2.80) | 2.63 ± 0.83 (1.03–5.23) | 1.63 ± 0.41 (0.83–2.82) | 1.03 ± 0.64 (0.29–4.25) | 0.88 ± 0.24 (0.30–1.75) | 1.72 ± 0.28 (1.08–2.39) |
| Heterozygotes (n = 55) | 49.8 ± 20.4 (18–89) | 24/31 | 28.7 ± 5.1 (19.3–45.1) | 4.35 ± 0.81 (2.12–5.98)i | 2.53 ± 0.72 (1.09–4.40) | 1.42 ± 0.35 (0.67–2.17)j | 0.87 ± 0.45 (0.35–2.20) | 0.85 ± 0.21 (0.39–1.31) | 1.61 ± 0.30 (0.83–2.23)i |
| Homozygotes (n = 8)b | 64.8 ± 14.2 (49–88) | 4/4 | 29.4 ± 6.2 (21.0–39.9) | 2.37 ± 0.31 (1.86–2.82)c,d | 1.35 ± 0.41 (0.54–1.89)c,d | 0.82 ± 0.22 (0.62–1.17)c,d | 0.42 ± 0.11 (0.27–0.88)c,f | 0.51 ± 0.13 (0.28–0.74)c,d | 0.78 ± 0.19 (0.42–1.00)c,d |
Values are expressed as mean ± sd. The minimum-maximum values are reported in parentheses. Differences between variables were evaluated using the Mann-Whitney test.
One noncarrier subject was excluded because he was taking lipid-lowering medication.
Homozygotes refer only to carriers of S17X mutation.
P < 0.001 for comparison between homozygous carriers vs. noncarriers.
P < 0.001 for comparison between heterozygous vs. homozygous carriers.
P < 0.01 for comparison between homozygous carriers vs. noncarriers.
P < 0.01 for comparison between heterozygous vs. homozygous carriers.
P < 0.05 for comparison between homozygous carriers vs. noncarriers.
P < 0.05 for comparison between heterozygous vs. homozygous carriers.
P < 0.05 for comparison between heterozygous carriers vs. noncarriers.
P < 0.01, for comparison between heterozygous carriers vs. noncarriers.
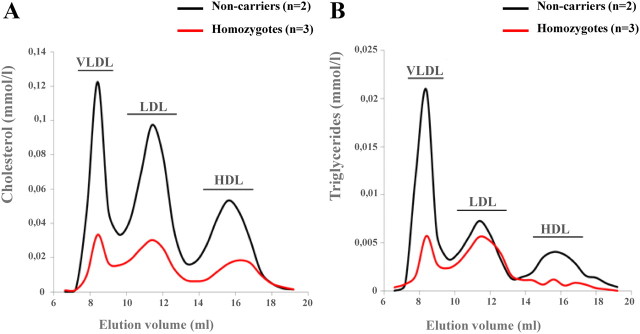
FPLC analysis confirmed the severely disturbed, hypolipidemic lipoprotein profile in S17X homozygotes, compared with noncarriers (Fig. 2A). Furthermore, in contrast to noncarriers, homozygotes showed reduced TG levels mainly in VLDL and HDL and to a lesser extent in LDL particles (Fig. 2B). A reduction in HDL size was also observed in homozygotes compared with noncarriers.
Fig. 2.
Serum lipoprotein profiles for noncarriers vs. homozygous carriers of S17X mutation. Average FPLC elution profiles for noncarriers (n = 2) and for homozygotes (n = 3) as assessed by cholesterol (A) and TG (B) in elution fractions are shown. Elution positions for VLDL, LDL, and HDL are also depicted.
Serum concentration of Angptl3 protein according to genotypes
Serum concentration of Angptl3 protein in mutation carriers and noncarriers are shown in Fig. 3. Compared with noncarriers, heterozygotes showed a 42% reduced levels of Angptl3 (228 ± 159 vs. 133.9 ± 116.9 ng/ml, respectively; P < 0.0001). In addition, Angptl3 levels in carriers of the FsS122 and FsE96del mutations (25.6 and 53.3 ng/ml, respectively) were lower than in controls, confirming that these variants may be functional. Homozygous carriers had little to no detectable Angptl3 in their plasma. Because all homozygotes were carrying the S17X variant, these data clearly indicate that this variant can be characterized as a loss-of-function (LOF) mutation.
Fig. 3.
Serum Angptl3 levels according to the number of mutant ANGPTL3 alleles. Shown are the levels of Angptl3 according to the ANGPTL3 genotype. The box plots give the median levels (middle horizontal line in each box), the interquartile ranges (delineated by the top and bottom of each box), and outliers falling below the fifth percentile or above the 95th percentile (points below or above the vertical lines, respectively).
Association between mutations in the ANGPTL3 gene and noncholesterol sterols
Plasma levels of noncholesterol sterols and their ratios in carriers and noncarriers of mutant ANGPTL3 alleles are shown in Table 2. No differences were observed in all indices of cholesterol absorption and bile acid metabolism except for a higher lanosterol to TC ratio in homozygous carriers compared with the other groups. However, the significance of this finding is limited by the observation that the lathosterol to TC ratio, the other major marker of cholesterol synthesis, was unchanged.
Table 2.
Serum levels of sterol markers of cholesterol metabolism in carriers and noncarriers of ANGPTL3 mutations
| Noncholesterol sterols | Carriers (n = 57) |
Noncarriers (n = 318) | |
|---|---|---|---|
| Homozygous (n = 6) | Heterozygous (n = 51) | ||
| Cholesterol absorption | |||
| Cholestanol/TC (μg/mg) | 1.30 ± 0.54 | 1.16 ± 0.57 | 1.23 ± 0.61 |
| Campesterol/TC (μg/mg) | 1.30 ± 0.54 | 1.16 ± 0.57 | 1.23 ± 0.61 |
| Sitosterol/TC (μg/mg) | 1.93 ± 0.89 | 1.47 ± 0.68 | 1.54 ± 0.69 |
| Cholesterol synthesis | |||
| Lanosterol/TC (ng/mg) | 201.1 ± 42.9a,b | 144.2 ± 56.2 | 142.1 ± 87.1 |
| Lathosterol/TC (μg/mg) | 1.09 ± 0.19 | 1.24 ± 0.49 | 1.22 ± 0.62 |
| Bile acids synthesis | |||
| 24-Hydroxycholesterol/TC (ng/mg) | 415.5 ± 103.2 | 347.1 ± 81.7 | 357.7 ± 90.9 |
| 7α-Cholesterol/TC (ng/mg) | 1172.2 ± 355.1 | 1225.1 ± 583.6 | 1143.8 ± 666.2 |
| 27-Hydroxycholesterol/TC (ng/mg) | 1065.8 ± 225.8 | 996.1 ± 234.8 | 965.1 ± 248.9 |
| Lathosterol/sitosterol | 0.70 ± 0.40 | 1.06 ± 0.69 | 1.01 ± 0.77 |
Noncholesterol sterol concentrations are standardized for TC. All ratios are expressed as × 103. Values are expressed as mean ± sd. Differences between variables were evaluated using the Mann-Whitney test.
P < 0.01 for comparison between homozygous carriers vs. noncarriers.
P < 0.05 for comparison between homozygous vs. heterozygous carriers.
Overall, these data suggest that the markedly reduced plasma lipids in homozygous carriers of ANGPTL3 mutations did not associate with significant alterations of whole-body cholesterol metabolism.
Clinical conditions associated to ANGPTL3 mutations
The clinical characteristics of all carriers and non-carriers of ANGPTL3 mutations are reported in Table 3. No significant difference in adiposity or age was observed between groups. The distribution of coronary risk factors did not differ between carriers and noncarriers with the exception of blood glucose levels, which were significantly lower (P < 0.05) in homozygotes compared with noncarriers. In addition, the prevalence of elevated liver enzyme was not significantly different between carriers and noncarriers. The presence of mutations in the ANGPTL3 was not associated with diabetes mellitus or history of liver diseases and coronary artery disease. As an exploratory analysis, we estimated the 1998–2007 SMR for IHD and cancer in the population of Campodimele (Supplemental Fig. 1). Based on these estimates, no signals of increased mortality in this community could be detected.
Table 3.
Prevalence of risk factors and arterial and liver diseases among carriers and noncarriers of ANGPTL3 S17X mutation
| Variables | Carriers (n = 63) |
Noncarriers (n = 341) | |
|---|---|---|---|
| Homozygous (n = 8) | Heterozygous (n = 55) | ||
| Age (yr) | 64.8 ± 14.2 | 49.8 ± 20.4 | 52.9 ± 20.0 |
| Sex (M/F) | 4/4 | 24/31 | 161/180 |
| BMI (kg/m2) | 29.4 ± 6.2 | 28.7 ± 5.1 | 27.5 ± 4.9 |
| Menopause, n (%) | 3 (37.5) | 16 (29.1) | 95 (27.9) |
| Fasting blood glucose (mmol/liter) | 4.6 ± 0.5a,b | 5.5 ± 1.5 | 5.5 ± 1.3 |
| Systolic blood pressure (mm Hg) | 135.5 ± 30.5 | 129.9 ± 20.9 | 130.6 ± 19.7 |
| Diastolic blood pressure (mm Hg) | 81.2 ± 9.8 | 81.3 ± 10.3 | 81.1 ± 10.0 |
| Smokers, n (%) | 2 (25.0) | 12 (21.8) | 70 (20.5) |
| Alcohol consumption, n (%) | |||
| Moderate drinkers | 6 (75.0) | 37 (67.3) | 213 (62.5) |
| Abstainers | 2 (25.0) | 18 (32.7) | 128 (37.5) |
| Hypertension, n (%) | 2 (25.0) | 15 (27.3) | 85 (24.9) |
| Diabetes, n (%) | 0 | 4 (7.3) | 34 (10.0) |
| Coronary heart disease, n (%) | 0 | 5 (9.1) | 16 (4.7) |
| Cerebro vascular disease, n (%) | 0 | 4 (7.3) | 24 (7.0) |
| Liver disease, n (%) | |||
| Hypertransaminasemia | 2 (25.0) | 7 (12.7) | 59 (17.4) |
| Cholelithiasis | 0 | 0 | 12 (3.5)c |
| Chronic hepathitis | 0 | 0 | 3 (0.9) |
| Cirrhosis | 0 | 1 (1.8) | 0 |
Hypertransaminasemia was defined as plasma concentration of aspartate aminotransferase and/or alanine aminotransferase greater than 40 U/liter. The diagnosis of type 2 diabetes mellitus was based on history of treatment with hypoglycemic agents and/or fasting blood glucose greater than 126 mg/dl and that of hypertension on the presence of elevated systolic (>140 mm Hg) and/or diastolic (>90 mm Hg) blood pressure and/or the current use of antihypertensive drugs. M, Male; F, female.
P < 0.01 for comparison between homozygous carriers vs. noncarriers.
P < 0.05 for comparison between homozygous vs. heterozygous carriers.
P < 0.05 for comparison between homozygous carriers vs. noncarriers.
Discussion
Within a population showing an increased prevalence of hypocholesterolemia, we found three ANGPTL3 variant alleles, S17X, FsS122, and FsE96del, to be associated with reduced LDL-C levels. The first two mutations have been already reported, whereas the last is novel and estimated to be functional (1, 21). It has been very recently reported that the prevalence of ANGPTL3 gene mutations responsible for a combined hypolipidemia phenotype is about 10% in a cohort of subjects with severe primary hypobetalipoproteinemia (22). When we calculated this prevalence among individuals with hypobetalipoproteinemia (LDL-C less than the fifth percentile) identified in our population (n = 46), we found that it was higher (15.2%). It is likely that this high prevalence is caused by consanguinity and/or genetic isolation. The demonstration that carriers of the S17X allele share a common haplotype strongly supports this option.
A key finding of our study is that only individuals presenting two nonsense S17X alleles and no detectable Angptl3 in the serum had a significant reduction of LDL-C, TG, and HDL-C levels, a lipid phenotype compatible with familial combined hypolipidemia. Conversely, heterozygous carriers showed a significant reduction of only TC and HDL-C. This is in contrast with Musunuru et al. (1), who reported that in their family also, heterozygous carriers expressed lower serum LDL-C and TG levels. The reason for this discrepancy is not immediately evident and may depend on other environmental or genetic differences between individuals in the different studies. In fact, Musunuru et al. (1) reported two different variant ANGPTL3 alleles, S17X and E129X, in their family, but the distinct effect of each allele on plasma lipids was not described. Because our series of hypolipidemic individuals mainly included carriers of the S17X mutations, it is possible that this variant when present in the heterozygous state has only a partial influence on plasma lipids. On the other hand, we noted that, compared with noncarriers, heterozygous individuals showed only a partial, although significant, reduction of plasma levels of Angptl3 protein, thus suggesting that a moderate reduction of this protein may affect only TC and HDL-C concentrations.
Several variants in the ANGPTL3 have been previously associated with either lower TG or LDL-C levels (21, 23), and some of them were described in subjects with familial combined hypolipidemia (1, 9, 10, 22). The catalog of all these latter variations is reported in the Supplemental Fig. 2. It is possible to note that those causing combined hypolipidemia were in the vast majority nonsense mutations, always present at the homozygous (or compound heterozygous) state and determining the total absence of Angplt3 in the plasma (LOF mutations). In addition, with the only exception of the G400V, all of them were located in the N terminal of the protein. All together, these observations support the recessive nature of familial combined hypolipidemia in which only homozygous carriers of LOF mutations in the ANGPTL3 gene present the full expression of the lipid phenotype.
The knowledge of the physiological role of Angptl3 protein may help in interpreting these findings. Angptl3 protein is expressed mostly in the liver and released into the circulation in which it acts as a partial suppressor of lipoprotein lipase (LPL) and by this increases plasma levels of VLDL (24–28). Subsequent experimental studies have demonstrated that Angptl3 also inhibits endothelial lipase (EL), an enzyme that hydrolyzes HDL phospholipids and promotes the catabolism of HDL particles (29). These effects have been partially confirmed also in humans in which polymorphisms or rare loss-of-function variants in the ANGPTL3 have been associated with reduced TG levels, and reduced concentrations of Angptl3 have been correlated to lower HDL-C concentration (17–21). Our observation that the absence of Angptl3 due to inactivating mutations causes a marked reduction of VLDL and HDL lipoproteins is in accordance with the role of Angptl3 in inhibiting the activity of both LPL and EL.
The impact of Angptl3 deficiency on LDL-C and ApoB levels is more difficult to explain. Increased LDL catabolism has been demonstrated by in vivo studies of lipoprotein metabolism in members of family studied by Musunuru et al. (30), but the reason for this is not defined. The possibility that this is due to increased LDL receptor activity secondary to changes in intracellular cholesterol balance is ruled out by our observation that the Angptl3 deficiency, differently from that reported in other forms of FHBL, does not affect whole-body cholesterol synthesis, absorption, or bile acid synthesis (31). An alternative hypothesis is that the LDL-C-lowering effect of Angptl3 deficiency may be related to its inhibition of hepatic lipase (HL). In fact, HL displays both triglyceride and phospholipase activity and enhances LDL and HDL catabolism. Interestingly, the lipoprotein profile in subjects lacking Angptl3 demonstrated a dramatic reduction of HDL-associated TG, pointing toward an increased HL activity.
Taken together, these data indicate that Angptl3 may be a key regulator of plasma lipid metabolism by controlling the activity of the extracellular lipase family, composed of LPL, HL, and EL. It can be hypothesized that in humans with total deficiency in Angptl3, there is an overactivation of these lipases leading to an accelerated catabolism of lipoproteins reflected by low levels of VLDL, LDL, and HDL in plasma. On the other hand, the partial loss of Angptl3 may affect only LPL or EL activities, thus determining a partial and variable reduction of TG and HDL-C levels. Additional studies are required to confirm this hypothesis.
The present study including a large collection of subjects with combined hypolipidemia allowed us to explore the potential clinical correlates of this syndrome. We found that homozygous carriers of the S17X mutation showed lower levels of plasma glucose and absence of type 2 diabetes mellitus. A similar observation was reported by Romeo et al. (21), who showed a significantly higher prevalence of nonsynonymous variations in ANGPTL3 among subjects in the lowest quartile of blood glucose levels. Overall, these observations indicate that individuals with Angptl3 deficiency might have more efficient glucose use. Even though mechanistic explanations for this effect are lacking, it must be noted that Angptl3 stimulates adipose tissue lipolysis, thereby raising plasma free fatty acids (FFA) and glycerol levels. Because increased FFA have been linked to insulin resistance, one might speculate that the lack of Angptl3 may improve tissue insulin sensitivity by reducing FFA availability (32). Further investigations on this point are needed.
We did not find evidence that familial combined hypolipidemia is associated with liver abnormalities. This is in agreement with others, who showed that ANGPTL3 sequence variants were not associated with increased body or liver fat (21). The lack of association with fatty liver may be a distinctive characteristic of familial combined hypolipidemia because hepatic fat accumulation has been reported with a high frequency in other forms of FHBL (5).
We noted that carriers of ANGPTL3 variants, despite markedly reduced HDL-C levels, did not show increased risk of ischemic cardiovascular disease. The low number of subjects prevents any definitive conclusion on this aspect, but the observation that also the mortality rate for IHD was low in the Campodimele population might support the notion that familial combined hypolipidemia may protect against atherosclerosis. This may be in agreement with the observation in mice that the reduction of Angptl3 expression protects against atherosclerosis, even in the absence of ApoE, owing to the enhanced catabolism and clearance of TG-rich lipoproteins (25).
Previous reports have suggested that a marked reduction of plasma lipids, namely total cholesterol, might increase cancer risk (33). The Campodimele population showed a lower rate of tumors compared with the general Italian population, which reached statistical significance in males. This observation strongly argues against the possibility that this form of hypolipidemia may be associated with increased risk of neoplasms.
Finally, it must be underlined that we were unable to identify mutations in the ANGPTL3 in three hypocholesterolemic family members and in 39 hypocholesterolemic individuals from the population. These individuals may have a mutation in a region of ANGPTL3 required for the transcription or mRNA splicing not sequenced in this study. Alternatively, their hypocholesterolemia may be caused by mutations in a novel gene that remains to be discovered. Additional work is needed to elucidate this aspect.
In conclusion, our data demonstrate that LOF mutations in the ANGPTL3, common in the Campodimele population, are associated with reduced plasma lipid levels. However, only individuals homozygous for LOF alleles causing the complete Angptl3 deficiency showed the full combined hypolipidemia phenotype. Conversely, partial Angptl3 deficiency was associated with significantly lower HDL-C but only moderate reduction of LDL-C. The present analysis did not provide any indication for an association between familial combined hypolipidemia and changes in the risk of liver disease, tumors, or IHD, thus allowing to conclude that this lipid disorder is a benign condition.
Acknowledgments
Ilenia Minicocci, Fabiana Quagliarini, Vincenzo Censi performed genetic studies. Anna Montali, Giancarlo Labbadia, Claudia Gabiati, Giovanni Pigna, Maria Laura Sepe, and Fabio Pannozzo carried out the family and population screening; Marius R. Robciuc, Matti Jauhiainen, and Christian Ehnholm determined the serum Angptl3 levels, performed the lipoprotein compositions in carriers and noncarriers, and reviewed the manuscript; Sergio Fazio collected the families and reviewed the manuscript; Dieter Lütjohann performed plasma sterols measurements; Marcello Arca designed the study, coordinated the family and population screening, and prepared the manuscript. We thank Professor Alessandro Menotti for providing us with data of plasma lipid distribution in the Italian population, Ms. Anja Kerksiek for laboratory assistance in noncholesterol, sterols and Professor Franco Culasso (Sapienza University of Rome, Italy) for his help in revising statistical analyses. We also thank Professor Helen Hobbs (University of Texas Southwestern Medical Center, Dallas, TX) for the helpful discussion. Finally, we thank all the subjects for their participation in this study.
This work was supported by Sapienza University of Rome (Progetto Ateneo 2006) (to M.A.); the European Union Project Lipididiet FP7/2007-2013 (to D.L.), Finska Läkaresällskapet (to C.E.), the Magnus Ehrnrooth Foundation (to M.R.R.), and the Finnish Foundation for Cardiovascular Research (to M.J.). I.M. was receiving the PhD fellowship in Medical Genetics at Sapienza University of Rome.
Disclosure Summary: No potential conflicts of interest relevant to this article must be disclosed.
Footnotes
- Angptl3
- Angiopoietin-like 3
- Apo
- apolipoprotein
- BMI
- body mass index
- C
- cholesterol
- EL
- endothelial lipase
- FFA
- free fatty acid
- FHBL
- familial hypobetalipoproteinemia syndrome
- FPLC
- fast-phase liquid chromatography
- HDL
- high-density lipoprotein
- HL
- hepatic lipase
- ICD
- International Classification of Diseases
- IHD
- ischemic heart disease
- LDL
- low-density lipoprotein
- LOF
- loss of function
- LPL
- lipoprotein lipase
- PCSK9
- proprotein convertase subtilisin kexin type 9
- SMR
- standardized mortality rate
- TC
- total cholesterol
- TG
- triglycerides
- VLDL
- very low-density lipoprotein.
References
- 1. Musunuru K , Pirruccello JP , Do R , Peloso GM , Guiducci C , Sougnez C , Garimella KV , Fisher S , Abreu J , Barry AJ , Fennell T , Banks E , Ambrogio L , Cibulskis K , Kernytsky A , Gonzalez E , Rudzicz N , Engert JC , DePristo MA , Daly MJ , Cohen JC , Hobbs HH , Altshuler D , Schonfeld G , Gabriel SB , Yue P , Kathiresan S. 2010. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N Engl J Med 363:2220–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schonfeld G , Lin X , Yue P. 2005. Familial hypobetalipoproteinemia: genetics and metabolism. Cell Mol Life Sci 62:1372–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hooper AJ , van Bockxmeer FM , Burnett JR. 2005. Monogenic hypocholesterolaemic lipid disorders and apolipoprotein B metabolism. Crit Rev Clin Lab Sci 42:515–545 [DOI] [PubMed] [Google Scholar]
- 4. Wu J , Kim J , Li Q , Kwok PY , Cole TG , Cefalu B , Averna M , Schonfeld G. 1999. Known mutations of apoB account for only a small minority of hypobetalipoproteinemia. J Lipid Res 40:955–959 [PubMed] [Google Scholar]
- 5. Schonfeld G , Patterson BW , Yablonskiy DA , Tanoli TS , Averna M , Elias N , Yue P , Ackerman J. 2003. Fatty liver in familial hypobetalipoproteinemia: triglyceride assembly into VLDL particles is affected by the extent of hepatic steatosis. J Lipid Res 44:470–478 [DOI] [PubMed] [Google Scholar]
- 6. Cohen J , Pertsemlidis A , Kotowski IK , Graham R , Garcia CK , Hobbs HH. 2005. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet 37:161–165 [DOI] [PubMed] [Google Scholar]
- 7. Maxwell KN , Breslow JL. 2005. Proprotein convertase subtilisin kexin 9: the third locus implicated in autosomal dominant hypercholesterolemia. Curr Opin Lipidol 16:167–172 [DOI] [PubMed] [Google Scholar]
- 8. Mattijssen F , Kersten S. 2012. Regulation of triglyceride metabolism by Angiopoietin-like proteins. Biochim Biophys Acta 1821:782–789 [DOI] [PubMed] [Google Scholar]
- 9. Pisciotta L , Favari E , Magnolo L , Simonelli S , Adorni MP , Sallo R , Fancello T , Zavaroni I , Ardigò D , Bernini F , Calabresi L , Franceschini G , Tarugi P , Calandra S , Bertolini S. 2012. Characterization of three kindred with familial combined hypolipidemia due to loss of function mutations of ANGPTL3. Circ Cardiovasc Genet 5:42–50 [DOI] [PubMed] [Google Scholar]
- 10. Martín-Campos JM , Roig R , Mayoral C , Martinez S , Martí G , Arroyo JA , Julve J , Blanco-Vaca F. 2012. Identification of a novel mutation in the ANGPTL3 gene in two families diagnosed of familial hypobetalipoproteinemia without APOB mutation. Clin Chim Acta 413:552–555 [DOI] [PubMed] [Google Scholar]
- 11. Fazio S , Sidoli A , Vivenzio A , Maietta A , Giampaoli S , Menotti A , Antonini R , Urbinati G , Baralle FE , Ricci G. 1991. A form of familial hypobetalipoproteinaemia not due to a mutation in the apolipoprotein B gene. J Intern Med 229:41–47 [DOI] [PubMed] [Google Scholar]
- 12. Menotti A , Seccareccia F , Lanti M , Farchi G , Conti S , Dima F , Scanga M , Marenco G , Falchero M , Ideo G , et al. 1995. Mean levels and distribution of some cardiovascular risk factors in Italy in the 1970's and 1980's: the Italian RIFLE Pooling Project: risk factors and life expectancy. G Ital Cardiol 25:1539–1572 [PubMed] [Google Scholar]
- 13. Di Leo E , Lancellotti S , Penacchioni JY , Cefalù AB , Averna M , Pisciotta L , Bertolini S , Calandra S , Gabelli C , Tarugi P. 2005. Mutations in MTP gene in abeta- and hypobetalipoproteinemia. Atherosclerosis 180:311–318 [DOI] [PubMed] [Google Scholar]
- 14. Abifadel M , Rabès JP , Devillers M , Munnich A , Erlich D , Junien C , Varret M , Boileau C. 2009. Mutations and polymorphisms in the proprotein convertase subtilisin kexin 9 (PCSK9) gene in cholesterol metabolism and disease. Hum Mutat 30:520–529 [DOI] [PubMed] [Google Scholar]
- 15. Wang LJ , Wang J , Li N , Ge L , Li BL , Song BL. 2011. Molecular characterization of the NPC1L1 variants identified from cholesterol low absorbers. J Biol Chem 286:7397–7408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mayer G , Poirier S , Seidah NG. 2008. Annexin A2 is a C-terminal PCSK9-binding protein that regulates endogenous low density lipoprotein receptor levels. J Biol Chem 283:31791–33801 [DOI] [PubMed] [Google Scholar]
- 17. Robciuc MR , Tahvanainen E , Jauhiainen M , Ehnholm C. 2010. Quantitation of serum angiopoietin-like proteins 3 and 4 in a Finnish population sample. J Lipid Res 51:824–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weingärtner O , Lütjohann D , Böhm M , Laufs U. 2010. Relationship between cholesterol synthesis and intestinal absorption is associated with cardiovascular risk. Atherosclerosis. 210:362–365 [DOI] [PubMed] [Google Scholar]
- 19. Campagna F , Montali A , Baroni MG , Maria AT , Ricci G , Antonini R , Verna R , Arca M. 2002. Common variants in the lipoprotein lipase gene, but not those in the insulin receptor substrate-1, the β3-adrenergic receptor, and the intestinal fatty acid binding protein-2 genes, influence the lipid phenotypic expression in familial combined hyperlipidemia. Metabolism 51:1298–1305 [DOI] [PubMed] [Google Scholar]
- 20. Seet WT , Mary Anne TJ , Yen TS. 2004. Apolipoprotein E genotyping in the Malay, Chinese and Indianethnic groups in Malaysia—a study on the distribution of the different apoE alleles and genotypes. Clin Chim Acta 340:201–205 [DOI] [PubMed] [Google Scholar]
- 21. Romeo S , Yin W , Kozlitina J , Pennacchio LA , Boerwinkle E , Hobbs HH , Cohen JC. 2009. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J Clin Invest 119:70–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Noto D , Cefalù AB , Valenti V , Fayer F , Pinotti E , Ditta M , Spina R , Vigna G , Yue P , Kathiresan S , Tarugi P , Averna MR. 2012. prevalence of ANGPTL3 and APOB gene mutations in subjects with combined hypolipidemia. Arterioscler Thromb Vasc Biol 32:805–809 [DOI] [PubMed] [Google Scholar]
- 23. Teslovich TM , Musunuru K , Smith AV , Edmondson AC , Stylianou IM , Koseki M , Pirruccello JP , Ripatti S , Chasman DI , Willer CJ , Johansen CT , Fouchier SW , Isaacs A , Peloso GM , Barbalic M , Ricketts SL , Bis JC , Aulchenko YS , Thorleifsson G , Feitosa MF , Chambers J , Orho-Melander M , Melander O , Johnson T , Li X , et al. 2010. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466:707–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li C. 2006. Genetics and regulation of angiopoietin-like proteins 3 and 4. Curr Opin Lipidol 17:152–156 [DOI] [PubMed] [Google Scholar]
- 25. Köster A , Chao YB , Mosior M , Ford A , Gonzalez-DeWhitt PA , Hale JE , Li D , Qiu Y , Fraser CC , Yang DD , Heuer JG , Jaskunas SR , Eacho P. 2005. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology 146:4943–4950 [DOI] [PubMed] [Google Scholar]
- 26. Ando Y , Shimizugawa T , Takeshita S , Ono M , Shimamura M , Koishi R , Furukawa H. 2003. A decreased expression of angiopoietin-like 3 is protective against atherosclerosis in apoE-deficient mice. J Lipid Res 44:1216–1223 [DOI] [PubMed] [Google Scholar]
- 27. Lee EC , Desai U , Gololobov G , Hong S , Feng X , Yu XC , Gay J , Wilganowski N , Gao C , Du LL , Chen J , Hu Y , Zhao S , Kirkpatrick L , Schneider M , Zambrowicz BP , Landes G , Powell DR , Sonnenburg WK. 2009. Identification of a new functional domain in angiopoietin-like 3 (ANGPTL3) and angiopoietin-like 4 (ANGPTL4) involved in binding and inhibition of lipoprotein lipase (LPL). J Biol Chem 284:13735–13745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shimizugawa T , Ono M , Shimamura M , Yoshida K , Ando Y , Koishi R , Ueda K , Inaba T , Minekura H , Kohama T , Furukawa H. 2002. ANGPTL3 decreases very low density lipoprotein triglyceride clearance by inhibition of lipoprotein lipase. J Biol Chem 277:33742–33748 [DOI] [PubMed] [Google Scholar]
- 29. Shimamura M , Matsuda M , Yasumo H , Okazaki M , Fujimoto K , Kono K , Shimizugawa T , Ando Y , Koishi R , Kohama T , Sakai N , Kotani K , Komuro R , Ishida T , Hirata K , Yamashita S , Furukawa H , Shimomura I. 2007. Angiopoietin-like protein3 regulates plasma HDL cholesterol through suppression of endothelial lipase. Arterioscler Thromb Vasc Biol 27:366–372 [DOI] [PubMed] [Google Scholar]
- 30. Elias N , Patterson BW , Schonfeld G. 2000. In vivo metabolism of ApoB, ApoA-I, and VLDL triglycerides in a form of hypobetalipoproteinemia not linked to the ApoB gene. Arterioscler Thromb Vasc Biol 20:1309–1315 [DOI] [PubMed] [Google Scholar]
- 31. Noto D , Cefalù AB , Barraco G , Fayer F , Minà M , Yue P , Tarugi P , Schonfeld G , Averna MR. 2011. Plasma non-cholesterol sterols in primary hypobetalipoproteinemia. Atherosclerosis 216:409–413 [DOI] [PubMed] [Google Scholar]
- 32. Boden G. 2003. Effects of free fatty acids (FFA) on glucose metabolism: significance for insulin resistance and type 2 diabetes. Exp Clin Endocrinol Diabetes 111:121–124 [DOI] [PubMed] [Google Scholar]
- 33. Jacobs D , Blackburn H , Higgins M , Reed D , Iso H , McMillan G , Neaton J , Nelson J , Potter J , Rifkind B. 1992. Report of the conference on low blood cholesterol: mortality associations. Circulation 86:1046–1060 [DOI] [PubMed] [Google Scholar]