Abstract
Context:
Bone metastases (BM) can cause severe pain, spinal cord compression, pathological fractures, and/or hypercalcemia. These skeletal-related events (SREs) may cause immobilization, loss of independence, poor quality of life, and reduced survival. There is limited information on the clinical effects of BM and SREs in patients with malignant pheochromocytoma or sympathetic paraganglioma (PHEO/sPGL).
Objectives:
We studied the prevalence and clinical characteristics of BM and SREs in patients with PHEO/sPGL and investigated the risk factors for SRE development.
Design:
Using a large institutional database, we conducted a retrospective study of 128 patients with malignant PHEO/sPGL at The University of Texas MD Anderson Cancer Center from 1967 through 2011.
Results:
Of the patients, 91 (71%) had BM, and 57 of these (63%) developed metachronous BM at a median time of 3.4 years (range, 5 months to 23 years) after the primary tumor diagnosis. Metastatic disease was confined exclusively to the skeleton in 26 of 128 (20%) patients. Sufficient information to assess SRE occurrence was available for 67 patients, and 48 of 67 (72%) patients had at least 1 SRE. The median overall survival for the 128 patients was 12 years for patients with only BM, 7.5 years for patients with nonosseous metastases, and 5 years for patients with both BM and nonosseous metastases (log rank test P value = .005). We were unable to identify factors predictive of SRE development, but the occurrence of a first SRE was associated with the development of subsequent SREs in 48% of subjects. In responsive patients, the use of systemic therapy was associated with fewer SREs (P < .0001).
Conclusions:
BM and SREs are frequent in patients with malignant PHEO/sPGL. SREs often develop shortly after the diagnosis of BM; severe pain is the most frequent SRE. These patients should be followed long-term by a multidisciplinary team to promptly identify the need for medical or surgical intervention.
Skeletal metastases are frequently observed in patients with solid tumors. Approximately two thirds of patients with breast and prostate cancer (1, 2) and one third of patients with kidney and lung cancer will develop bone metastases (BM) (1–3). BM weaken and destroy skeletal tissue and predispose cancer patients to acute and chronic skeletal-related events (SREs) such as severe bone pain, spinal cord compression, pathological fractures, and/or hypercalcemia. Patients with BM are at risk of complications such as immobilization, loss of dependence, and poor quality of life as well as reduction in survival. Prompt diagnosis and intervention are needed to decrease the risk of morbidity and complications related to BM.
The incidence and characteristics of BM in patients with neuroendocrine tumors are less well known. In patients with medullary thyroid cancer, the bones are the third most common site of distant metastases after the liver and the lungs (4). Midgut carcinoids and pancreatic neuroendocrine tumors are associated with BM in up to 10% of patients (5); in these patients, BM usually occur after the development of liver metastases and are usually a late event. The incidence of BM in patients with pheochromocytoma or sympathetic paraganglioma (PHEO/sPGL) seems to be high, but information on the clinical effects of BM in this setting is limited (6–8). The intent of the current study was to review the prevalence and clinical characteristics of BM and SREs in a large cohort of patients with PHEO/sPGL and to investigate risk factors associated with the development of SREs.
Patients and Methods
After approval from The University of Texas MD Anderson Cancer Center's institutional review board, we conducted a retrospective study of a large institutional database that included all patients with malignant PHEO/sPGL seen at our institution from 1967 through 2011.
A diagnosis of BM required that at least 1 of the 2 following criteria be satisfied: (1) BM identified on a plain standard X-ray, computed tomography (CT) scan, magnetic resonance imaging (MRI) scan, bone scan, or metaiodobenzylguanidine (MIBG) or fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT scan or (2) BM identified in a pathologic specimen from a biopsy or open surgery.
BM were considered synchronous if they were identified within 3 months of the diagnosis of the primary tumor. If identified beyond 3 months after diagnosis of the primary tumor, BM were considered metachronous. We consider BM widespread when more than 3 skeletal sites were involved.
For the purpose of this study, we considered the following as SREs: severe pain requiring radiotherapy or orthopedic surgery, spinal cord compression, pathological fracture, hypercalcemia, and asymptomatic disease that required intervention (either radiotherapy or surgery) because of an impending fracture or impending cord compression.
We summarized our findings using descriptive statistics. The Kaplan-Meier method was used to estimate overall survival. The log rank test was used to evaluate differences between patient groups. Patients were censored on the dates of last follow-up if death had not occurred. To identify risk factors for the development of SREs, a multivariate Cox proportional hazards model that included important patient and clinical variables was fitted. All tests were 2-sided, and P values <.05 were considered statistically significant. We used SAS version 9.3 (SAS Institute, Inc, Cary, North Carolina) for all the analyses.
Results
Patient demographics
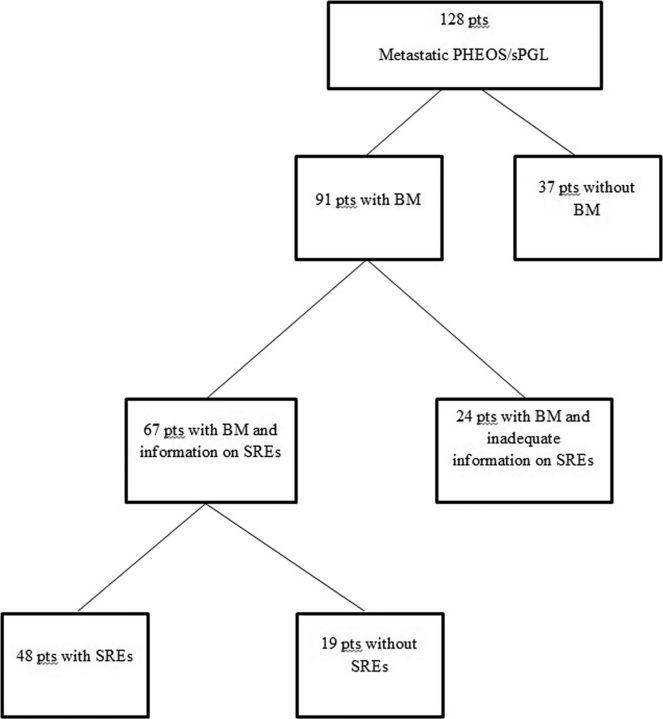
We identified 137 subjects with malignant PHEO/sPGL; of these 9 had inadequate documentation regarding the presence or absence of BM and were therefore excluded from further analysis. BM were identified in 91 (71%) patients. Of these, 24 patients did not have sufficient information in their records for the analysis of SREs (Figure 1).
Figure 1.
Distribution of patients (pts) with metastatic PHEO/sPGL, BM, and SREs.
The median patient age at diagnosis of BM was 47 years (range, 9–83 years). Of the 91 patients, 53 (58%) were men. The median follow-up time from BM diagnosis was 3 years (range, less than 1 month–17 years).
Overall survival
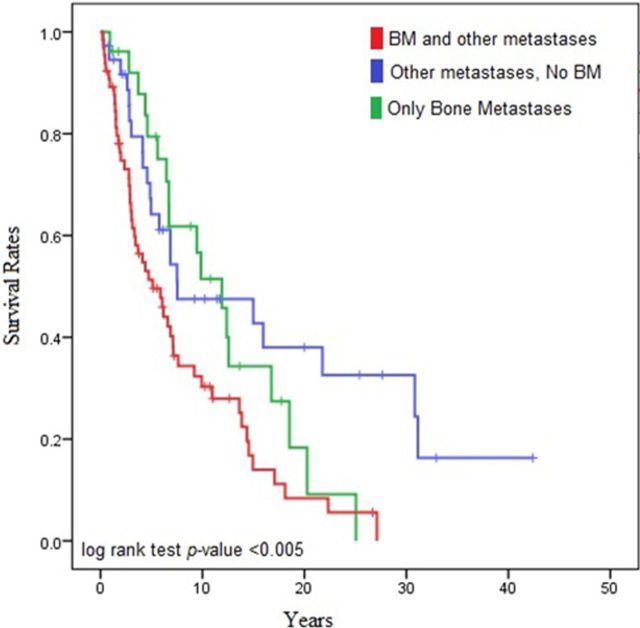
Of the 128 patients with malignant PHEO/sPGL, 26 (20%) had metastatic disease located exclusively in the skeleton. The median overall survival was 12 years for patients with only BM, 7.5 years for patients with nonosseous metastases, and 5 years for patients with both BM and nonosseous metastases (log rank test P = .005) (Figure 2).
Figure 2.
Median overall survival in patients with BM and other metastases, without BM, and with only BM.
BM identification and location
BM were predominantly lytic (>90%). Of the 60 patients who had information from a bone scan available, 49 (82%) had BM. Of the 75 patients who had information from CT and/or MRI scans, 61 (81%) had BM. Functional imaging studies such as FDG-PET/CT were performed in 21 patients and showed evidence of BM in 20 (95%). MIBG testing was positive for BM in 29 of the 41 (70%) patients in whom the study was performed.
Of the 91 patients with BM, 70 (77%) had widespread BM. The most frequent sites of BM in the 91 patients were the spine (74 of 91 [81%]), sacrum and pelvis (61 of 91 [67%]), proximal and distal long bones (45 of 91 [49%]), and skull (19 of 91 [21%]).
Of the 91 patients with BM, 34 (38%) patients had BM synchronous with the primary tumor, and 57 (63%) patients developed metachronous BM in a median time of 3.4 years (range, 5 months to 23 years) after diagnosis of the primary tumor.
SREs and prognostic factors
In the 67 patients included in the SRE analysis, 48 (72%) had at least 1 SRE and 23 (48%) of those 48 developed 2 or more SREs during follow-up. Of the patients with SREs, 15 (31%) had their SRE as the presenting sign of their BM; in the remaining 33 patients with SREs, the median time from BM diagnosis to the development of their first SRE was 4.3 months (range, 1.1 months–6.5 years). In the 23 patients who had more than 1 SRE, the median time from development of the first SRE to development of the second SRE was 9.8 months (range, 1 day–6.9 years).
Of the evaluable patients, 19 did not experience SREs. The median follow-up time from BM diagnosis in the SRE group was 3 years (range, 1 month–17 years), and the median follow-up time from BM diagnosis in the non-SRE group was 1.25 years (range, less than 1 month–6.3 years) (P < .001).
The most common SRE was severe pain (33%), followed by pathological fractures (27%) and spinal cord compression (25%). Of the patients with pathological fractures and spinal cord compression, 2 presented with paraplegia and 8 with paraparesis. Pathological fractures occurred in the spine (cervical spine, 2 patients; thoracic spine, 4 patients; lumbar spine, 1 patient, and sacrum, 1 patients), clavicle (2 patients), femur (1 patient), rib (1 patient), and scapula (1 patients). Hypercalcemia (defined as a serum calcium level greater than the upper normal limit) due to osteolysis was found in only 1 patient (serum calcium >12 mg/dL) and was a late event in the patient's life.
The characteristics of the group of patients with and without SREs are described in Table 1. In the group with SREs, half of the patients who were tested had mutations in the succinate dehydrogenase B (SDHB) gene, which was a rate similar to that of the patients without SREs. Most of the patients who did not develop SREs received some type of systemic or antiresorptive treatment (15 of 19 [79%]), whereas most of the patients who did develop SREs did not receive similar therapies (38 of 48 [79%]) (P < .0001).
Table 1.
Clinical Characteristics of Patients With Bone Metastases With and Without SREs
| Patients With SREs (n = 48) | Patients Without SREs (n = 19) | P Value | |
|---|---|---|---|
| Median (range) age, y | 50 (15–79) | 50 (9–70) | NS |
| Sex, n (%) | |||
| Female | 19 (40) | 8 (42) | NS |
| Male | 29 (60) | 11 (58) | |
| Median (range) primary tumor size, cm | 7.7 (1.1–17.3) | 10 (2.5–24) | NS |
| Type of metastases, n (%) | |||
| Only BM | 15 (31) | 3 (16) | NS |
| BM and nonosseous metastases | 33 (69) | 16 (84) | |
| Had SDHB genetic testing, n (%) | 10 (21) | 11 (58) | NS |
| SDHB-positive, n (%) | 5 (50) | 4 (36) | |
| Systemic treatment, n (%)a | <.0001 | ||
| Chemotherapy | 4 (8) | 7 (37) | |
| MIBG | 0 | 1 (5) | |
| Chemotherapy + MIBG | 0 | 1 (5) | |
| MIBG + bisphosphonates | 0 | 2 (11) | |
| Chemotherapy + MIBG + bisphosphonates | 4 (8) | 1 (5) | |
| Bisphosphonates | 2 (4) | 1 (5) | |
| Chemotherapy + bisphosphonates | 0 | 1 (5) | |
| TKI + denosumab | 0 | 1 (5) | |
| Died before treatment | 0 | 4 (21) | |
| No systemic treatment | 38 (79) | ||
| Functioning tumors, n (%)c | NS | ||
| ≥4 times higher | 23/34 (68)b | 12/17 (71)b | |
| 1–3 times higher | 6/34 (18)b | 2/17 (12)b | |
| Nonfunctioning tumors | 5/34 (15)b | 3/17 (18)b | |
| Status at last follow-up, n (%) | NS | ||
| Alive | 10 (22) | 5 (26) | |
| Deceased | 38 (79) | 14 (74) |
Abbreviations: NS, nonsignificant; TKI, Tyrosine kinase inhibitors.
For patients with SREs: systemic treatment at any time before SRE diagnosis.
Available samples.
Urine/plasma catecholamine or metanephrines 4-fold upper normal limit.
In the multivariate analysis, age, sex, primary tumor size and location, SDHB status, and catecholamine secretion were not associated with an increased risk for SREs.
Treatment
Various surgical procedures and/or treatments were performed for patients who developed SREs (Table 2). Of the 16 patients with severe pain who required intervention, 14 (88%) received radiotherapy and 2 (13%) had surgery. In the 14 patients who received radiotherapy for pain, a clinical response was documented in 8 patients: complete pain relief was observed in 2 (25%), a partial decrease in pain in 4 (50%), and persistent pain after radiotherapy in 2 (25%). Interestingly, in 2 patients with severe pain, monthly subcutaneous treatment with denosumab (120 mg) was associated with complete pain relief.
Table 2.
Treatment for the First SRE
| SRE (n = 48) | Procedure |
|---|---|
| Severe pain, n = 16 (33%) | Radiotherapy = 14 (88%) |
| Surgery = 2 (12.5%) | |
| Spinal cord compression, n = 12 (25%)a | Laminectomy = 5 (42%) |
| Radiotherapy = 2 (17%) | |
| Laminectomy and radiotherapy = 2 (17%) | |
| Resection of metastases = 2 (17%) | |
| Fracture, n = 13 (27%) | Radiotherapy = 3 (25%) |
| Radiotherapy + chemotherapy = 2 (17%) | |
| Surgery + radiotherapy = 3 (25%) | |
| Observation = 1 (8%) | |
| Surgery = 2 (17%) | |
| Laminectomy = 1 (8%) | |
| Stereotactic radiosurgery = 1 (8%) | |
| No symptoms at the time of intervention, n = 7 (15%) | Surgery + radiotherapy = 3 (42%) |
| Surgery = 2 (29%) | |
| Radiotherapy = 2 (29%) |
One patient with spinal cord compression did not receive further treatment because the SRE occurred while the patient was on chemotherapy.
Of the 7 patients who were asymptomatic but received treatment for their BM, 2 had surgery to remove rib and skull metastases, 1 had a craniotomy followed by radiotherapy to remove a skull metastasis that was pressing on the cerebral cortex, 2 had surgery followed by radiotherapy to remove sacral and pelvic bone metastases, and 2 received RT alone.
Discussion
In our large institutional database, approximately 70% of evaluable patients with malignant PHEO/sPGL were identified as having synchronous or metachronous BM. BM were predominantly lytic and predisposed patients to serious SREs such as pain, fractures, spinal cord compression, and, rarely, hypercalcemia. These SREs usually happened after the BM were diagnosed, and SREs frequently were also the first manifestation of malignant disease (31%). After the first SRE, there was an increased predisposition to develop more SREs. In fact, 48% of patients with SREs had 2 or more SREs during follow-up, with a median time to development of the second SRE of 10 months. Patients with SREs exhibited impaired mobility and decreased performance status, required emergency intervention, and had a high rate of hospitalizations. Furthermore, the median overall survival rate of patients with BM was decreased, with patients with bone and visceral metastases exhibiting shorter overall survival than patients with either visceral metastases or BM alone.
Our findings indicate that malignant PHEO/sPGL are among the solid tumors that most frequently spread to the skeleton. The present study demonstrates a BM rate of 71%, which is similar to the rates observed in patients with breast cancer (9). It has been recently proposed that breast and prostate cancer cells are predominantly recruited to the skeleton because they express the chemokine receptors CXCR4 and CCR9 (10–12), whereas bone marrow cells and osteoblasts produce the cognate ligands CXCL12 and CCL25, respectively (13–15). The reasons that PHEO/sPGL cells spread to the skeleton are still unclear, but a similar homing mechanism could take place because they also strongly express CXCR4 and CCR9 (16).
The most frequent SRE observed in our cohort was severe pain, followed by fractures and spinal cord compression. Pain from BM affects up to 45% of all patients with advanced cancer (9, 17, 18) and is a well-known cause of performance status impairment and psychological distress (19, 20). Although an evaluation of the different therapies used for BM pain is beyond the scope of the present study, our findings raise awareness of the importance of assessing bone pain in patients with a history of PHEO/sPGL.
The high frequency of fractures and spinal cord compression in our study can be explained by the high frequency of lytic spinal BM in our population. The prompt evaluation by neurosurgeons or orthopedic surgeons can help prevent further neurological compromise and/or restore function. Because there are great variations in the clinical presentations of SREs, each treatment choice should be individualized (21).
SREs can occur very early after the discovery of BM. The median time to development of the first SRE was 4.4 months, which is similar to that for other cancers that frequently spread to the skeleton, such as breast cancer (9). In our series, the second SRE occurred in a median time of 10 months. These findings should be useful to clinicians for designing interventions to prevent or delay a first SRE or subsequent SRE in patients with PHEO/sPGL. When patients with SREs are compared with patients without them, the only statistically significant difference was that most patients who did not develop SREs had previously received systemic therapy. Our results suggest that in some patients with BM, the use of chemotherapy, radiopharmaceutical agents, and/or antiresorptive therapies may delay or prevent the appearance of SREs. The use of parenteral bisphosphonates to treat cancer patients with lytic BM has been a common practice since the late 1990s when 2 studies demonstrated that pamidronate might prevent SREs in patients with breast cancer and multiple myeloma (22, 23). However, in our cohort, only 17% of patients received preventive antiresorptive therapy. Now that we have recognized the strong association of PHEO/sPGL BM with SREs, we recommend close follow-up and initiation of antiresorptive therapy in any patient with BM and systemic therapy in any patient with progressive bone disease despite the lack of symptoms.
In the multivariate analysis, risk factors for the development of an SRE could not be identified. Age, sex, primary tumor size, primary tumor location, and the presence of an SDHB mutation did not differ between the 2 groups. The excessive production of catecholamines such as adrenaline and noradrenaline was not associated with an increased risk of SREs. This result contrasts with a recent report (24) suggesting that catecholamines increase bone turnover, favoring bone destruction over bone formation in patients with PHEO/sPGL. Of interest, in our study, all the reported SREs were due to BM, and we did not find patients with fragility fractures due to osteoporosis.
An overwhelming majority of patients with metastatic PHEO/sPGL developed BM (71%), frequently many years after diagnosis of the primary tumor. Therefore, we recommend initial and long-term surveillance with radiographic studies that could identify BM for patients with clinical predictors of malignancy (pheochromocytoma ≥ 5 cm in maximum diameter, extraadrenal tumor, and the presence of an SDHB mutation) (25, 26). Bone scans may be the most cost-effective follow-up test in these patients (6). FDG-PET/CT scans may be considered for carriers of SDHB mutations (27). In addition, FDG-PET may be helpful to evaluate progression and the response to treatment in patients with already established BM (28).
Our study has limitations inherent to its retrospective nature, differing approaches to medical care over the decades of the study, and possible referral and ascertainment bias. The prevalence of BM could have been underestimated because some patients with clinical predictors of metastases (primary tumor size and location) or metastatic disease in other organs were not evaluated and followed with radiographic studies such as bone or FDG-PET scans. Furthermore, in a few patients, metastatic PHEO/sPGL was diagnosed when some of the currently available radiographic modalities to study BM did not exist. In addition, our institution has adopted a protocol that combines multiple imaging modalities (CT, MRI, bone, and MIBG and FDG-PET scans) to identify BM only recently. The incidence of bone pain as an SRE could also have been underestimated because assessment of pain was not uniform over the 45-year period studied. Furthermore, follow-up of patients without SREs was shorter than that of patients with SREs. Nevertheless, our findings raise awareness of the importance of BM and SREs in patients with metastatic PHEO/sPGL.
In conclusion, patients with malignant PHEO/sPGL have a high rate of BM and SREs and require a thorough bone evaluation and long-term follow-up. The identification of a first SRE is often associated with a subsequent SRE, underscoring the need to proactively monitor and treat patients with an SRE. A multidisciplinary approach with specialists in endocrinology, oncology, palliative care, radiotherapy, orthopaedic surgery, and neurosurgery is important.
Acknowledgments
We thank Mr and Mrs Clarence Cazalot, Mr and Mrs William Granek, Natalie Papadam, and the Team NAT for their generous support and Zach Bohannan and Melissa Burkett, scientific editors from the Department of Scientific Publications, for their editorial assistance.
This work was supported by the University of Texas MD Anderson Cancer Center Core (Grant CA16672).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BM
- bone metastases
- CT
- computed tomography
- FDG
- fluorodeoxyglucose
- MIBG
- metaiodobenzylguanidine
- MRI
- magnetic resonance imaging
- PET
- positron emission tomography
- PHEO/sPGL
- pheochromocytoma or sympathetic paraganglioma
- SDHB
- succinate dehydrogenase B
- SRE
- skeletal-related event.
References
- 1. Coleman RE , Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bubendorf L , Schopfer A , Wagner U , et al. . Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–583. [DOI] [PubMed] [Google Scholar]
- 3. Maldazys JD , deKernion JB. Prognostic factors in metastatic renal carcinoma. J Urol. 1986;136:376–379. [DOI] [PubMed] [Google Scholar]
- 4. Schlumberger M , Tubiana M , De Vathaire F , et al. . Long-term results of treatment of 283 patients with lung and bone metastases from differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1986;63:960–967. [DOI] [PubMed] [Google Scholar]
- 5. Zuetenhorst JM , Hoefnageli CA , Boot H , Valdes Olmos RA , Taal BG. Evaluation of 111In-pentetreotide, 131I-MIBG and bone scintigraphy in the detection and clinical management of bone metastases in carcinoid disease. Nucl Med Commun. 2002;23:735–741. [DOI] [PubMed] [Google Scholar]
- 6. Zelinka T , Timmers HJ , Kozupa A , et al. . Role of positron emission tomography and bone scintigraphy in the evaluation of bone involvement in metastatic pheochromocytoma and paraganglioma: specific implications for succinate dehydrogenase enzyme subunit B gene mutations. Endocr Relat Cancer. 2008;15:311–323. [DOI] [PubMed] [Google Scholar]
- 7. James RE , Baker HL , Scanlon PW. The roentgenologic aspects of metastatic pheochromocytoma. Am J Roentgenol Radium Ther Nucl Med. 1972;115:783–793. [DOI] [PubMed] [Google Scholar]
- 8. Lynn MD , Braunstein EM , Wahl RL , Shapiro B , Gross MD , Rabbani R. Bone metastases in pheochromocytoma: comparative studies of efficacy of imaging. Radiology. 1986;160:701–706. [DOI] [PubMed] [Google Scholar]
- 9. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. [DOI] [PubMed] [Google Scholar]
- 10. Ibrahim T , Sacanna E , Gaudio M , et al. . Role of RANK, RANKL, OPG, and CXCR4 tissue markers in predicting bone metastases in breast cancer patients. Clin Breast Cancer. 2011;11:369–375. [DOI] [PubMed] [Google Scholar]
- 11. Johnson-Holiday C , Singh R , Johnson E , et al. . CCL25 mediates migration, invasion and matrix metalloproteinase expression by breast cancer cells in a CCR9-dependent fashion. Int J Oncol. 2011;38:1279–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shiozawa Y , Pedersen EA , Havens AM , et al. . Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121:1298–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kucia M , Reca R , Miekus K , et al. . Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–894. [DOI] [PubMed] [Google Scholar]
- 14. Schuettpelz LG , Link DC. Niche competition and cancer metastasis to bone. J Clin Invest. 2011;121:1253–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dezso Z , Nikolsky Y , Sviridov E , et al. . A comprehensive functional analysis of tissue specificity of human gene expression. BMC Biol. 2008;6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dahia PL , Ross KN , Wright ME , et al. . A HIF1α regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 2005;1:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Banning A , Sjogren P , Henriksen H. Pain causes in 200 patients referred to a multidisciplinary cancer pain clinic. Pain. 1991;45:45–48. [DOI] [PubMed] [Google Scholar]
- 18. Mercadante S , Armata M , Salvaggio L. Pain characteristics of advanced lung cancer patients referred to a palliative care service. Pain. 1994;59:141–145. [DOI] [PubMed] [Google Scholar]
- 19. Portenoy RK , Payne D , Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81:129–134. [DOI] [PubMed] [Google Scholar]
- 20. Clohisy DR , Mantyh PW. Bone cancer pain. Cancer. 2003;97:866–873. [DOI] [PubMed] [Google Scholar]
- 21. Eastley N , Newey M , Ashford RU. Skeletal metastases—the role of the orthopaedic and spinal surgeon. Surg Oncol. 2012;21:216–222. [DOI] [PubMed] [Google Scholar]
- 22. Berenson JR , Lichtenstein A , Porter L , et al. . Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med. 1996;334:488–493. [DOI] [PubMed] [Google Scholar]
- 23. Hortobagyi GN , Theriault RL , Porter L , et al. . Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med. 1996;335:1785–1791. [DOI] [PubMed] [Google Scholar]
- 24. Veldhuis-Vlug AG , El Mahdiui M , Endert E , Heijboer AC , Fliers E , Bisschop PH. Bone resorption is increased in pheochromocytoma patients and normalizes following adrenalectomy. J Clin Endocrinol Metab. 2012;97:E2093–E2097. [DOI] [PubMed] [Google Scholar]
- 25. Ayala-Ramirez M , Feng L , Johnson MM , et al. . Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab. 2011;96:717–725. [DOI] [PubMed] [Google Scholar]
- 26. Amar L , Baudin E , Burnichon N , et al. . Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab. 2007;92:3822–3828. [DOI] [PubMed] [Google Scholar]
- 27. Timmers HJ , Kozupa A , Chen CC , et al. . Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. J Clin Oncol. 2007;25:2262–2269. [DOI] [PubMed] [Google Scholar]
- 28. Ayala-Ramirez M , Chougnet CN , Habra MA , et al. . Treatment with sunitinib for patients with progressive metastatic pheochromocytomas and sympathetic paragangliomas. J Clin Endocrinol Metab. 2012;97:4040–4050. [DOI] [PMC free article] [PubMed] [Google Scholar]