Abstract
Context:
Severe hypoglycemia is a rare and challenging complication of Roux-en-Y gastric bypass (RYGB), which is characterized by hypersecretion of insulin and incretin hormones in the postprandial state.
Objective:
The objective of the study was to determine the clinical and hormonal responses to a mixed-meal challenge after the reversal of RYGB in 2 patients with post-RYGB hypoglycemia. We hypothesized that the reversal of RYGB would lead to clinical improvement in hypoglycemia through the attenuation of incretin hormone secretion.
Design/Setting/Subjects/Outcome Measures:
Two patients with post-RYGB hypoglycemia underwent a standardized meal tolerance test prior to and 8 and 18 months after RYGB reversal, respectively, with the measurement of glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), insulin, and glucose levels. Gastric bypass was reversed by reattaching the small gastric pouch to the bypassed distal stomach and resecting the Roux limb to restore the normal flow of food bolus.
Results:
Both subjects showed persistent evidence of hypoglycemia with marked hyperinsulinemia after the RYGB reversal. GLP-1 levels after the RYGB reversal decreased by 76% and 70%, respectively, from their prereversal levels and to the level of nonhypoglycemic post-RYGB controls. In contrast, GIP levels after the RYGB reversal increased by 3–10 times the level before the reversal and 8–26 times that of the nonhypoglycemic post-RYGB controls.
Conclusions:
Reversal of RYGB did not alleviate hyperinsulinemic hypoglycemia upon a mixed-meal challenge in our patients, thus suggesting its limited clinical benefit as treatment of post-RYGB hypoglycemia. The marked increase in GIP levels and concurrent decrease in GLP-1 levels in our patients suggest a possible role of GIP in persistent hyperinsulinemic hypoglycemia after the reversal of RYGB.
Severe hypoglycemia is increasingly recognized as a rare and serious complication occurring after Roux-en-Y gastric bypass (RYGB) with reports of associated seizure, syncope, and motor vehicle accidents (1, 2). The prevalence of post-RYGB hypoglycemia appears less than 1% (3, 4), typically occurring 1–5 years after surgery and almost exclusively after a meal (3, 5, 6). The mechanisms behind post-RYGB hypoglycemia are unclear, but the following etiologies have been proposed: 1) β-cell hyperfunction due to presurgical hypertrophy; 2) hypersecretion of incretin hormones such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP); 3) improved insulin sensitivity after weight loss; 4) abnormal counterregulatory hormonal (glucagon) responses; and 5) dumping syndrome caused by early entry of ingested nutrients into the small intestine (1, 7–9).
The optimal treatment for post-RYBG hypoglycemia is unknown. Recently a case of post-RYGB hypoglycemia that resolved after placement of a feeding tube was reported, suggesting the role of uncontrolled distal delivery of nutrients into the bowel as the cause of post-RYGB hypoglycemia (10). Therefore, we hypothesized that the reversal of RYGB would ameliorate the uncontrolled delivery of nutrients to the distal gut and subsequently attenuate exaggerated incretin hormone secretions, leading to less hypoglycemia.
We report 2 patients with severe hypoglycemia after RYGB who underwent a reversal of their bypass procedures. The 2 patients underwent a meal tolerance test (MTT) before and after the reversal of RYGB to measure hormonal responses.
Subjects and Methods
The 2 subjects provided written informed consent, and studies were approved by the Johns Hopkins Institutional Review Boards.
Subject 1
The first subject was a 35-year-old African American woman with a history of morbid obesity [body mass index (BMI) of 40 kg/m2] and no history of diabetes who underwent RYGB with a subsequent loss of 45 kg. Approximately 2 years after undergoing RYGB, she developed postprandial hypoglycemia with 1 severe episode causing a seizure and a subsequent hospitalization. She had since experienced recurrent hypoglycemia with a blood glucose level as low as 1.4 mmol/L (25 mg/dL), with episodes as frequent as 1–2 per week. Subsequent endocrine evaluation included a normal 72-hour fast and evidence of hyperinsulinemic postprandial hypoglycemia. Computed tomography of the abdomen did not show evidence of a pancreatic mass. She failed to improve with dietary modifications. Approximately 7 years after her RYGB, she underwent RYGB reversal, given the persistent hypoglycemia. Gastric bypass was reversed by reattaching the small gastric pouch to the bypassed distal stomach and resecting the Roux limb (distal jejunum attached to the small gastric pouch), thereby restoring the normal anatomy and flow of food bolus.
Subject 2
The second subject was a 62 year-old Caucasian woman with a history of obesity (BMI 38 kg/m2) and impaired glucose tolerance who underwent RYGB with a subsequent loss of 36 kg. Approximately a year after RYGB, she developed postprandial hypoglycemia including 2 severe episodes leading to a loss of consciousness and hospitalization. Subsequent endocrine evaluation included a normal 72-hour fast and the measurement of hormones during an episode of postprandial hypoglycemia showing a glucose level of 2.7 mmol/L (49 mg/dL), with concurrent serum insulin of 259 pmol/L (normal < 175) and a C-peptide level of 13.6 ng/mL (normal 1.1–5), consistent with inappropriate endogenous insulin production. A computed tomography of the abdomen did not show evidence of a pancreatic mass. An intraarterial calcium gluconate stimulation test was negative, with no evidence of localized insulin production within the pancreas and an 18F-DOPA positron emission tomography scan revealed uniform uptake throughout the pancreas. The subject tried dietary modification as well as medications including octreotide and acarbose with persistent hypoglycemia. Therefore, 3 years after her RYGB, she underwent a distal pancreatectomy based on intraoperative palpation of a small mass suspicious for insulinoma at the tail of the pancreas, instead of initially planned near-total pancreatectomy for presumed nesidioblastosis. However, the histology of the resected tissue did not show evidence of insulinoma. Symptoms improved immediately after the surgery but soon recurred. Given the persistent hypoglycemia and unrevealing endoscopic ultrasound evaluation for a pancreatic mass, she underwent RYGB reversal 5 years after her RYGB.
Meal tolerance test
The MTT was administered before and after the RYGB reversal in each subject. The results of the MTT prior to the reversal of RYGB as well as the control group of 5 nonhypoglycemic patients who had a MTT 12 months after their RYGB have been previously reported (11). The post-RYGB MTT occurred 18 months after the reversal for subject 1 and 8 months after the reversal for subject 2. After an overnight fast, the subjects underwent blood sampling via a peripheral iv catheter. Two cans of Ensure Plus (Abbott Nutrition, Abbott Nutrition, Columbus, Ohio) containing 40 g sugar, 26 g protein, and 22 g fat in a total volume of 475 cc were ingested over 15 minutes. Blood samples were obtained at 10 minutes prior to baseline, at baseline, and at 5, 10, 15, 20, 30, 40, 60, 80, 100, 120, 150, and 180 minutes. Subjects began drinking Ensure at time 0 minutes and finished by time 15 minutes. Samples were stored at −80°C to measure the hormones secreted by the alimentary tract and pancreas. Plasma insulin levels, active GLP-1, and total human GIP were measured using an ELISA (insulin: Mercodia, Uppsala, Sweden; GLP-1: ALPCO Diagnostics, Salem, New Hampshire; GIP: Millipore Corporation, Billerica, Massachusetts). The area under the curve (AUC) for GLP-1 and GIP was calculated using the trapezoidal method. At the time of the postreversal MTT, the subjects were asked on a standard questionnaire to rate the severity of overall hypoglycemic symptoms since undergoing the RYGB reversal on a scale providing 6 choices with a range of much improved, improved, the same, worse, much worse, and very much worse.
Results
Subject 1
The weight at the time of the post-RYGB reversal MTT for subject 1 was 91 kg (BMI 33 kg/m2) compared with her peak weight of 105 kg prior to the RYGB. At time 60 minutes, she had an episode of emesis of approximately 100 cc in volume with subsequent omission of serum measurement at time 60 and 80 minutes. Fingerstick glucose was obtained at time 60 minutes instead.
Hyperinsulinemic hypoglycemia persisted in subject 1 in both pre- and post-RYGB reversal MTT, with a nadir glucose of 3 mmol/L (54 mg/dL) in pre-RYGB reversal and 2.4 mmol/L (43 mg/dL) in post-RYGB reversal (Figure 1). The AUC for GIP increased by 288% after the RYGB reversal compared with the pre-RYGB reversal. In contrast, GLP-1 decreased to the level of nonhypoglycemic RYGB controls at 27 pmol/L at 40 minutes. The AUC comparison of GLP-1 between pre- and post-RYGB reversal showed a 76% reduction upon the reversal of RYGB (Figure 2). At the time of the postreversal MTT, subject 1 indicated on the questionnaire that her hypoglycemic symptoms were much improved.
Figure 1.
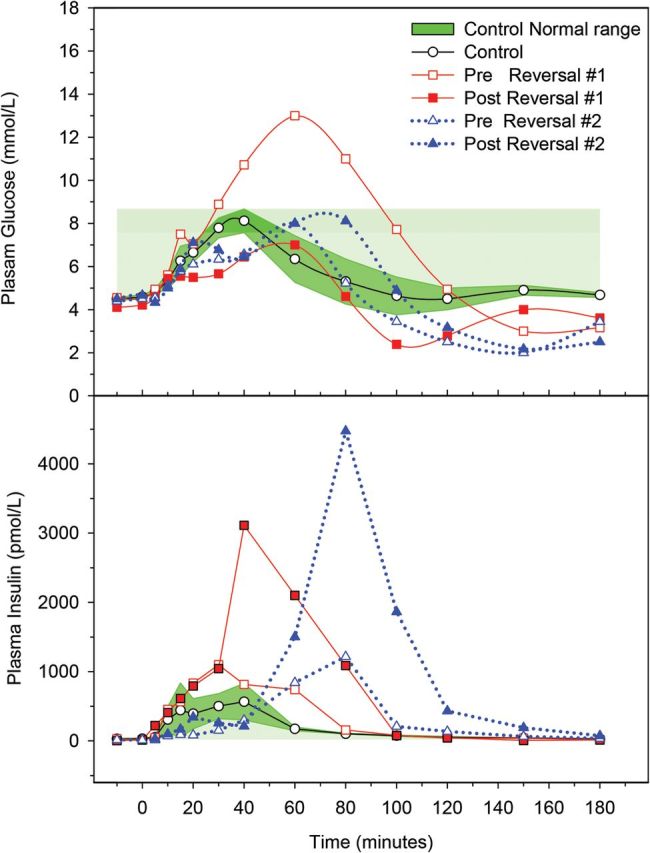
Plasma glucose (top panel) and insulin concentrations (bottom panel) after MTT in subjects with post-RYGB hypoglycemia before and after RYGB reversal compared with nonhypoglycemic post-RYGB controls.
Figure 2.
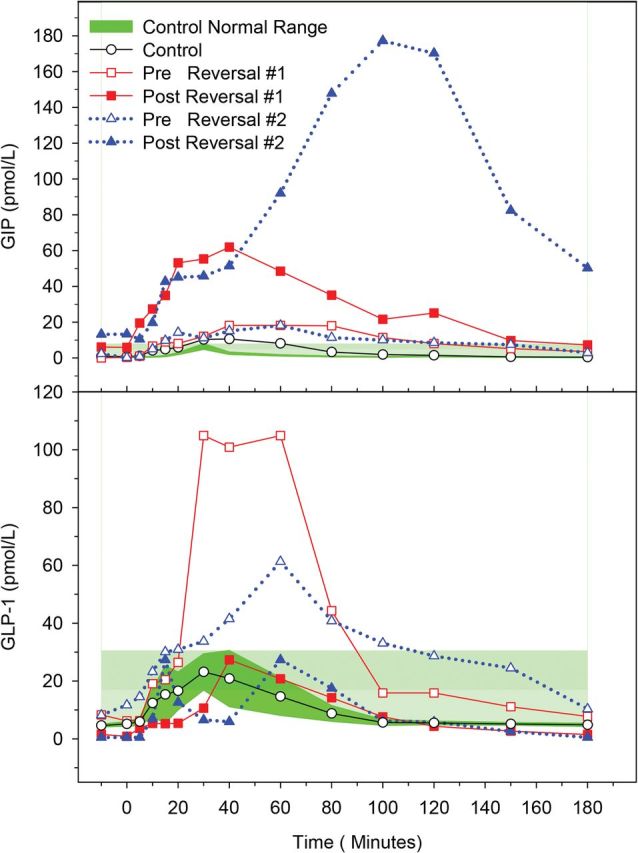
Plasma GIP concentrations (top panel) and GLP-1 concentrations (bottom panel) after MTT in subjects with post-RYGB hypoglycemia before and after RYGB reversal compared with nonhypoglycemic post-RYGB controls.
Subject 2
The weight at the time of post-RYGB reversal MTT for subject 2 was 73 kg (BMI 27 kg/m2) compared with her peak weight of 105 kg. Hyperinsulinemic hypoglycemia also persisted in subject 2, with a nadir glucose of 2 mmol/L (36 mg/dL) prior to the RYGB reversal and 2.1 mmol/L (38 mg/dL) after the RYGB reversal (Figure 1). The AUC for GIP increased by 988% after the RYGB reversal compared with the prereversal. In contrast, the AUC for GLP-1 showed a 70% reduction upon the reversal of RYGB compared with the prereversal (Figure 2). At the time of the postreversal MTT, subject 2 indicated on the standard questionnaire that her hypoglycemic symptoms were minimally improved. The histological evaluation of subject 2's pancreatic tissue did not show evidence of nesidioblastosis.
Discussion
We present 2 subjects with severe post-RYGB hypoglycemia who underwent reversal of RYGB with variable clinical improvement. Our results showed that the reversal of RYGB did not lead to an improvement in hyperinsulinemic postprandial hypoglycemia. In fact, MTT showed a similar degree of postprandial hypoglycemia before and after the reversal of RYGB. Current therapies proposed for the treatment of post-RYGB hypoglycemia include a low-carbohydrate diet; a trial of acarbose, diazoxide, octreotide, calcium-channel blocker, or glucagon; or surgical options including subtotal or total pancreatectomy and reduction of gastric pouch size and gastrostomy tube placement (1, 4, 10, 12–14). Our findings suggest that the reversal of RYGB is not effective as a therapeutic alternative to improve the postprandial hyperinsulinemic hypoglycemia related to RYGB.
Despite the lack of clinical benefit, RYGB reversal did lead to marked changes in incretin hormones. In both patients, RYGB reversal led to an approximately 70% reduction in GLP-1 concentrations such that the concentrations were similar to nonhypoglycemic post-RYGB controls. Prior to the RYGB reversal, the GLP-1 concentrations were elevated in both patients, consistent with reports of increased GLP-1 concentrations up to 2–5-fold after RYGB (6, 15). In post-RYGB hypoglycemia, insulin sensitivity, the β-cell GLP-1 receptor expression level and the sensitivity to GLP-1 were shown to be normal (11, 16, 17). The decrease in GLP-1 observed after the RYGB reversal confirms our hypothesis that the restoration of normal passage of nutrients through RYGB reversal would alleviate the exaggerated GLP-1 response seen after RYGB. The role of GLP-1 in glucose metabolism related to RYGB, however, appears to be minor, given persistent hyperinsulinemic hypoglycemia despite the decrease in GLP-1 after RYGB reversal as well as a recent study showing limited deterioration in glucose metabolism upon blocking GLP-1 action (18).
In contrast to the changes in GLP-1, we found that GIP concentrations prior to the RYGB reversal were similar to the nonhypoglycemic post-RYGB controls and increased after the reversal of RYGB. These findings differ from the reduction in GIP level recently reported in a patient with post-RYGB hypoglycemia who underwent a gastrostomy tube insertion into the remnant stomach, thus restoring food flow into the bypassed limb (10). Although one would expect reduced GIP concentrations after RYGB by bypassing the proximal small bowel in which GIP is released and increased GIP concentrations upon restoration of normal nutrient flow after the reversal of RYGB, the pattern of GIP alteration after RYGB is not clearly established, given conflicting literature (8, 15). Additionally, the role of supraphysiological concentrations of GIP in human glucose metabolism is unknown, and therefore, further studies focused on GIP are needed to understand its role in post-RYGB hypoglycemia.
Our study had several limitations including a small sample number, missing hormone measurements in subject 1 during emesis, and a lack of pre-RYGB glucose tolerance data and measurement of counterregulatory hormones such as glucagon and catecholamines. A recent study of GIP in healthy subjects demonstrated the bifunctional role of GIP in its insulinotropic effect during hyperglycemia and glucagonotropic effect during hypoglycemia (19). This raises the possibility of glucagon deficiency in subject 2 with markedly elevated GIP in the setting of hyperinsulinemic hypoglycemia. Also, we did not assess the gut microbiota in our patients, which may play a role in post-RYGB glucose metabolism (20) .
In conclusion, our study showed a lack of clinical benefit of RYGB reversal in the treatment of post-RYGB hypoglycemia. The persistent hyperinsulinemic hypoglycemia after RYGB reversal, despite marked decreases in GLP-1, suggests that GLP-1 is not a major factor in altered glucose metabolism related to RYGB. In contrast to the decreases in GLP-1 concentrations, GIP responses were markedly elevated after the RYGB reversal, possibly leading to persistent hyperinsulinemic hypoglycemia and suggesting a differential regulation of GIP and GLP-1 secretion. The mechanisms underlying this effect on GIP concentrations deserve further investigation.
Acknowledgments
We thank the research participants for their extraordinary dedication. We also thank Melissa Scudder, Margene Kennedy, and Ann Munson for their excellent assistance in implementing the protocol.
This study was partially supported by the Johns Hopkins Institute of Clinical and Translational Research UL1 TR000424 (National Center for Advancing Translational Sciences).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the curve
- BMI
- body mass index
- GIP
- glucose-dependent insulinotropic polypeptide
- GLP-1
- glucagon-like peptide-1
- MTT
- meal tolerance test
- RYGB
- Roux-en-Y gastric bypass.
References
- 1. Patti ME , McMahon G , Mun EC, et al. Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia. 2005;48(11):2236–2240. [DOI] [PubMed] [Google Scholar]
- 2. Service GJ , Thompson GB , Service FJ , Andrews JC , Collazo-Clavell ML , Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353(3):249–254. [DOI] [PubMed] [Google Scholar]
- 3. Marsk R , Jonas E , Rasmussen F , Naslund E. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5,040 patients undergoing surgery for obesity in 1986–2006 in Sweden. Diabetologia. 2010;53(11):2307–2311. [DOI] [PubMed] [Google Scholar]
- 4. Kellogg TA , Bantle JP , Leslie DB, et al. Postgastric bypass hyperinsulinemic hypoglycemia syndrome: characterization and response to a modified diet. Surg Obes Relat Dis. 2008;4(4):492–499. [DOI] [PubMed] [Google Scholar]
- 5. Service FJ , Natt N , Thompson GB, et al. Noninsulinoma pancreatogenous hypoglycemia: a novel syndrome of hyperinsulinemic hypoglycemia in adults independent of mutations in Kir6.2 and SUR1 genes. J Clin Endocrinol Metab. 1999;84(5):1582–1589. [DOI] [PubMed] [Google Scholar]
- 6. Goldfine AB , Mun EC , Devine E, et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92(12):4678–4685. [DOI] [PubMed] [Google Scholar]
- 7. Meier JJ , Butler AE , Galasso R , Butler PC. Hyperinsulinemic hypoglycemia after gastric bypass surgery is not accompanied by islet hyperplasia or increased β-cell turnover. Diabetes Care. 2006;29(7):1554–1559. [DOI] [PubMed] [Google Scholar]
- 8. Korner J , Bessler M , Inabnet W , Taveras C , Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3(6):597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cummings DE. Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. Int J Obes (Lond). 2009;33(suppl 1):S33–S40. [DOI] [PubMed] [Google Scholar]
- 10. McLaughlin T , Peck M , Holst J , Deacon C. Reversible hyperinsulinemic hypoglycemia after gastric bypass: a consequence of altered nutrient delivery. J Clin Endocrinol Metab. 2010;95(4):1851–1855. [DOI] [PubMed] [Google Scholar]
- 11. Rabiee A , Magruder JT , Salas-Carrillo R, et al. Hyperinsulinemic hypoglycemia after roux-en-Y gastric bypass: unraveling the role of gut hormonal and pancreatic endocrine dysfunction. J Surg Res. 2011;167(2):199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Halperin F , Patti ME , Goldfine AB. Glucagon treatment for post-gastric bypass hypoglycemia. Obesity (Silver Spring). 2010;18(9):1858–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clancy TE , Moore FD , Zinner MJ. Post-gastric bypass hyperinsulinism with nesidioblastosis: subtotal or total pancreatectomy may be needed to prevent recurrent hypoglycemia. J Gastrointest Surg. 2006;10(8):1116–1119. [DOI] [PubMed] [Google Scholar]
- 14. Won JG , Tseng HS , Yang AH, et al. Clinical features and morphological characterization of 10 patients with noninsulinoma pancreatogenous hypoglycaemia syndrome (NIPHS). Clin Endocrinol (Oxf). 2006;65(5):566–578. [DOI] [PubMed] [Google Scholar]
- 15. Laferrere B , Teixeira J , McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93(7):2479–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim SJ , Nian C , McIntosh CH. Glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 modulate beta-cell chromatin structure. J Biol Chem. 2009;284(19):12896–12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reubi JC , Perren A , Rehmann R, et al. Glucagon-like peptide-1 (GLP-1) receptors are not overexpressed in pancreatic islets from patients with severe hyperinsulinaemic hypoglycaemia following gastric bypass. Diabetologia. 2010;53(12):2641–2645. [DOI] [PubMed] [Google Scholar]
- 18. Jimenez A , Casamitjana R , Viaplana-Masclans J , Lacy A , Vidal J. GLP-1 action and glucose tolerance in subjects with remission of type 2 diabetes mellitus after gastric bypass surgery [published online January 28, 2013]. Diabetes Care. doi:10.2337/dc12-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Christensen M , Vedtofte L , Holst JJ , Vilsboll T , Knop FK. Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes. 2011;60(12):3103–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aron-Wisnewsky J , Dore J , Clement K. The importance of the gut microbiota after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2012;9(10):590–598. [DOI] [PubMed] [Google Scholar]


