Abstract
Introduction:
RAS mutations are common in thyroid tumors and confer a high risk of cancer when detected in fine-needle aspiration (FNA) specimens. Specific characteristics of RAS-positive thyroid cancers are not well described.
Methods:
From April 2007 to April 2009, 921 consecutive patients undergoing FNA were evaluated prospectively with a panel of molecular markers. Ultrasonographic, cytological, histological, and surgical outcomes were retrospectively assessed.
Results:
Sixty-eight aspirates from 66 patients were positive for RAS mutations including 63 cytologically indeterminate (93%), 3 malignant (4%), and 2 benign (3%) specimens. Cancer was histologically confirmed in 52 of 63 aspirates (83%) including the following: 46 papillary thyroid cancers, 4 follicular thyroid cancers, 1 medullary cancer, and 1 anaplastic cancer. All 46 RAS-positive papillary thyroid cancers, including 1 metastatic cancer, had follicular variant histology papillary thyroid cancer; only 11 tumors demonstrated vascular/capsular invasion and 4 had infiltrative growth. Of 48 patients with differentiated thyroid cancer, lymph node metastasis was uncommon and bilateral cancer was present in 48%. Only 33% of malignant nodules were suspicious by preoperative ultrasonography. At a mean follow-up of 22 months, 31 of 35 differentiated thyroid cancer patients (89%) have no evidence of recurrence, 4 patients (9%) have detectable thyroglobulin, 1 patient has bone metastases, and both patients with medullary and anaplastic cancer have died.
Conclusion:
Most RAS-positive thyroid cancers have indeterminate cytology, lack suspicious ultrasound features, and are histologically low-grade follicular variant histology papillary thyroid cancer. Lymph node and distant metastases are uncommon but bilateral disease is frequent. Total thyroidectomy should be considered for initial surgical management of most patients with RAS-positive FNA results. The role of prophylactic lymphadenectomy remains unclear.
Thyroid nodules can be detected in up to 50% of otherwise healthy patients by the fifth or sixth decade (1). Despite this high prevalence, only 5%–15% of thyroid nodules are malignant (2, 3). Fine-needle aspiration (FNA) cytology remains the gold standard for definitive nonsurgical evaluation of nodules. However, a rapidly growing literature has demonstrated that molecular genetic testing not only improves diagnostic accuracy of FNA but also shows that mutations identified during FNA are independent predictors of malignancy in specimens with indeterminate cytology (4–7). Many of these mutations occur in genes coding for intermediates of the pathway that plays a central role in thyroid tumorigenesis (8). The BRAFV600E mutation is the most common and perhaps best-studied mutation of the MAPK pathway. RAS mutations are also common, accounting for 10%–20% of papillary and 40%–50% of follicular thyroid cancers (9). Compared with BRAF mutations, far less is known about the role of RAS mutation in thyroid tumorigenesis and the clinical behavior of thyroid cancers that harbor RAS mutations.
RAS functions downstream from mitogenic growth factor receptors as a key membrane bound GTPase signal transduction protein. RAS thereby acts as a molecular switch, signaling along the MAPK, phosphatidylinositol 3-kinase/AKT, and other intracellular signaling pathways, controlling diverse cellular processes such as differentiation, proliferation, and cell survival (10, 11). Three highly homologous human RAS genes, NRAS, KRAS, and HRAS, have been described (12). Oncogenic alleles of RAS carry mutations in specific hotspots of the 3 genes in codons 12, 13, and 61 (11). These mutations cause loss of intrinsic GTPase activity, leading to constitutive activation of RAS and increased activation of MAPK and alternate pathway signaling (11, 13).
Activating mutations of RAS in thyroid epithelial cells were first reported in the 1980s, but only recently have been directly implicated as early and frequent events in transformation and proliferation of thyroid carcinoma (14, 15). However, the diagnostic and prognostic importance of RAS mutations has been obscured because these mutations are often reported not only in thyroid cancers but also in follicular adenomas (FAs) and histologically hyperplastic nodules (HNs) as well (16). Better understanding of the role of RAS mutation in thyroid carcinogenesis is important in light of the expanding use of mutational analysis in preoperative thyroid FNA evaluation. In this report, we provide the first comprehensive characterization of the clinical, histopathological, and radiographic features of the largest series to date of thyroid tumors harboring RAS mutations detected prospectively in thyroid FNA samples.
Materials and Methods
Study cohort
Under a University of Pittsburgh Institutional Review Board protocol, we retrieved and examined the records associated with 68 FNAs from 66 consecutive patients who had molecular genetics results positive for any type of RAS mutation from April 2007 through April 2009. Additionally, 187 consecutive cytologically malignant specimens were also tested for RAS mutations. RAS mutations were assessed as part of a panel of molecular genetic tests including RAS, BRAF, RET/PTC, and PAX8/PPARγ that were collected prospectively on all thyroid FNAs performed at our institution during this period. Molecular testing was performed reflexively on all specimens with indeterminate cytology or cytology positive for malignant cells or by the request of the treating physician. Molecular testing was not routinely performed in cases with unsatisfactory or benign cytology. The reported results represent a total of 1320 consecutive FNAs from 921 patients, collected during the study period. A portion of the subjects in this series was included in a previous study that established the predictive value of RAS mutations for malignancy in cytologically indeterminate FNA specimens (7).
All patients and records were queried preoperatively for a positive family history of primary thyroid cancer in a first-degree relative and a history of head or neck radiation (XRT). Thyroid surgery was recommended when FNA cytology was malignant, suspicious for malignant cells, follicular neoplasm/suspicious for follicular neoplasm (FN/SFN), atypical, repeatedly atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS), or repeatedly nondiagnostic or for indications such as significant nodule enlargement, nodule size greater than 4 cm, or 20% or greater tracheal compression by substernal goiter (17–19). When thyroid surgery was indicated, total thyroidectomy (TT) rather than diagnostic lobectomy and isthmusthectomy was recommended for features including malignant or suspicious cytology, indeterminate cytology in nodules 4 cm or greater, XRT history, positive family history, and chronic hypothyroidism. Additionally, when lobectomy alone would have been indicated by standard criteria, TT was offered to all patients with RAS-positive nodules after detailed counseling about the increased risk of cancer conveyed by the presence of a RAS mutation. Compartment-oriented central compartment node dissection (CND) was performed together with TT according to preoperative findings or surgeon preference.
Preoperative ultrasonography images available for 49 patients with 51 nodules with known tumor histology underwent blinded review by a single radiologist (A.K.D.). The presence of 1 or more of the following established radiographic features was considered suspicious for papillary thyroid cancer: markedly hypoechoic appearance, increased intranodular vascularity on Doppler flow interrogation, irregular borders, microcalcifications, taller than wide nodule shape, and presence of edge-refractive shadow (2, 18).
Mutational analysis
Sample collection and testing for RAS, BRAF, RET/PTC, and PAX8/PPARγ was performed as described previously (7). Briefly, after slide preparation after routine FNA, residual material in a standard 25–27G needle was ejected into 20 μL of nucleic acid preservative solution. Total nucleic acids were extracted and sample adequacy for molecular analysis was assessed based on the general quantity and quality of isolated nucleic acids and the proportion of epithelial cells within the sample. NRAS codon 61, HRAS codon 61, KRAS codons 12 and 13 point mutations were detected using real-time LightCycler PCR (Roche Applied Science, Indianapolis, Indiana) and fluorescence melting curve analysis as part of a larger mutational panel that also included BRAFV600E point mutation and RET/PTC1, RET/PTC3, and PAX8/PPARγ rearrangements. The sensitivity of mutation detection for RAS was 10% of mutant alleles.
After surgery, all histological specimens were retested for RAS mutations using DNA isolated from formalin-fixed and paraffin-embedded tissue following the same detection approach. All detected RAS mutations were further confirmed by Sanger sequencing.
Cytological and histological review
All aspirated nodules were 1 cm or greater, and FNA was performed for indications based on American Thyroid Association guidelines that were current at the time of care (18, 20). FNA cytology samples processed prior to September 2008 when Bethesda reporting terminology was implemented at our institution were reviewed and reclassified based on Bethesda consensus nomenclature by a single cytopathologist (N.P.O.) (21). All tumors were staged based on American Joint Committee on Cancer criteria (22). Histological slides were subsequently reviewed by a single pathologist (Y.E.N.) to confirm the diagnosis, evaluate the distribution of the nuclear features of papillary carcinoma within each nodule, and establish the presence of tumor encapsulation and capsular or vascular invasion.
Results
Patient characteristics, FNA results, and FNA mutational status
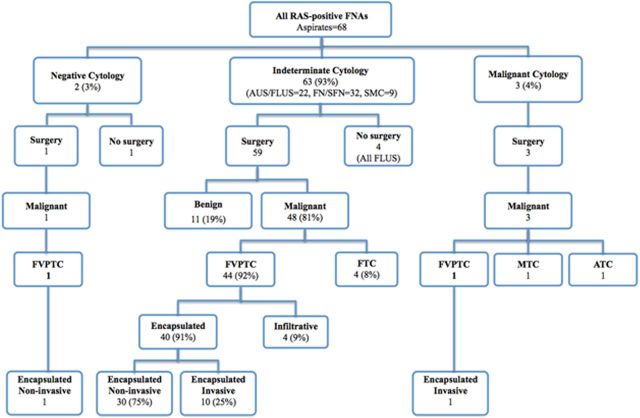
During the study interval, 68 aspirates from 66 patients were positive for RAS mutations (Figure 1). All RAS mutations detected in the 68 cytological specimens were reconfirmed in the corresponding histological specimens with 100% concordance in this study. Point mutations were identified in NRAS codon 61 in 49 (72%), HRAS codon 61 in 15 (22%), and KRAS codons 12 of 13 in 4 (6%) aspirates. These included 67 aspirates of thyroid nodules and a single aspirate from an NRAS61-positive metastatic papillary thyroid cancer (PTC) in a patient who presented with bulky cervical adenopathy and metastasis to the C3 vertebral body; given the presence of distant metastasis, FNA of the thyroid itself was not done. A family history of thyroid cancer was found in 7 patients (12%), and there was a prior history of XRT to the head and neck for 2 patients (3%).
Figure 1.
Cytological and histopathological diagnosis of 68 RAS-positive aspirates from 66 patients.
Based on the Bethesda classification nomenclature for thyroid FNA cytology, 63 of 68 RAS-positive aspirates (93%) had a diagnosis within indeterminate categories, 3 (4%) were malignant, and 2 (3%) were negative for malignant cells (Figure 1) (21). Of the 63 cytologically indeterminate aspirates, 32 (51%) were classified as follicular neoplasm/suspicious for follicular neoplasm, 22 (35%) as AUS/FLUS, and 9 (14%) as suspicious for malignant cells. None of the 187 additional cytologically malignant specimens or their corresponding histological specimens contained RAS mutations.
Operative management
Demographic information and histological outcomes for 61 surgically managed patients who contributed 63 RAS-positive aspirates appear in Table 1. A conventional indication for surgery was present in 60 of 61 patients including 3 with FNA cytology-positive for malignant cells, 8 aspirates suspicious for malignant cells, 31 aspirates FN/SFN, or repeated AUS/FLUS cytology in 18 cases, and 1 patient with benign cytology noted in a 2.5-cm thyroid nodule positive for an NRAS61 mutation underwent TT for intractable thyroid pain. Of 15 patients with a RAS-positive FNA and a standard indication for lobectomy, 10 chose initial TT instead. All 10 (100%) had differentiated thyroid carcinoma (DTC) on histology of which 6 of 10 (60%) were bilateral. Bilateral cancers consisted of microcarcinoma ranging in size from 0.1 to 0.9 cm in 5 cases and a single patient with a 3.1-cm follicular variant PTC (FVPTC). Of 14 patients who had RAS-positive cancer at lobectomy, 11 received completion thyroidectomy. Of these, 3 patients (27%) had a contralateral cancer, including 2 incidental papillary microcarcinomas and a 3-cm FVPTC. Completion thyroidectomy was deferred for the remaining 3 patients who at initial lobectomy had single foci of encapsulated FVPTC, measuring 1.2 cm, 0.9 cm, and 0.6 cm, respectively.
Table 1.
Demographic and Histological Features of 61 RAS-Positive Patients With Surgical Follow-Up
| FVPTC |
FTC | MTC | ATC | Benign | Total | |||
|---|---|---|---|---|---|---|---|---|
| Encapsulated |
Infiltrative | |||||||
| Noninvasive | Invasive | |||||||
| Patients, n | 29 | 11 | 4 | 4 | 1 | 1 | 11 | 61 |
| Aspirates, n | 31 | 11 | 4 | 4 | 1 | 1 | 11 | 63 |
| Age, y | ||||||||
| Mean (range) | 39 (19–77) | 51 (19–74) | 46 (28–65) | 55 (23–73) | 76 (n/a) | 65 (n/a) | 53 (23–76) | |
| ≤45 | 17 | 3 | 2 | 2 | n/a | n/a | 4 | 28 |
| >45 | 12 | 8 | 2 | 2 | 1 | 1 | 7 | 33 |
| Gender | ||||||||
| Female | 25 | 10 | 2 | 4 | n/a | n/a | 10 | 51 |
| Male | 4 | 1 | 2 | 0 | 1 | 1 | 1 | 10 |
| Tumor | ||||||||
| Mean size, cm (range) | 1.8 (0.4–5.0) | 2.5 (0.9–4.3) | 1.25 (1.0–6.3) | 3.1 (2.1–4.0) | 3.2 | 6.5 | ||
| T1 | 18 | 4 | 3 | 0 | n/a | n/a | n/a | 25 |
| T2 | 10 | 4 | 0 | 3 | n/a | n/a | n/a | 17 |
| T3 | 1 | 2 | 1 | 1 | n/a | 1 | n/a | 6 |
| T4 | 0 | 1 | 0 | 0 | 1 | n/a | n/a | 2 |
| Lymph node status | ||||||||
| N0 | 13 | 3 | 3 | 1 | 0 | 0 | n/a | 20 |
| N1 | 0 | 2 | 0 | 0 | 1 | 0 | n/a | 3 |
| NX | 16 | 6 | 1 | 3 | 0 | 1 | n/a | 27 |
| Metastases at diagnosis | ||||||||
| MX | 29 | 10 | 4 | 4 | 1 | 1 | n/a | 49 |
| M1 | 0 | 1 | 0 | 0 | n/a | n/a | n/a | 1 |
| Laterality | ||||||||
| Unilateral | 15 | 5 | 2 | 4 | n/a | n/a | n/a | 26 |
| Bilateral | 14 | 6 | 2 | 0 | 1 | 1 | n/a | 24 |
| Extent of surgery | ||||||||
| Lobectomy | 11 | 2 | 1 | 1 | n/a | n/a | 5 | 20 |
| Total thyroidectomy | 18 | 9 | 3 | 3 | 1 | 1 | 6 | 41 |
Abbreviation: n/a, not available.
Histological analysis
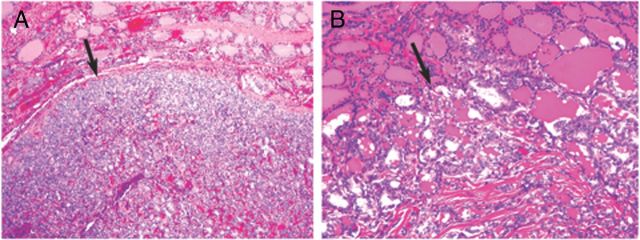
Of 63 aspirates from 61 RAS-positive patients managed surgically, cancer was histologically confirmed in 52 specimens from 50 patients (Table 1 and Figure 1). The presence of a RAS mutation was associated with cancer in 52 of 63 aspirates, yielding a positive predictive value (PPV) of 83% in this series. RAS-positive cancers included 46 FVPTCs, 4 follicular thyroid carcinomas (FTCs), 1 medullary thyroid carcinoma (MTC), and 1 anaplastic thyroid cancer (ATC). Of the 46 FVPTCs, 42 had a smooth border and a well-defined capsule. Of these, 31 FVPTCs had no invasion and 11 had tumor capsule or vascular invasion (Figure 2A). The remaining 4 of 46 FVPTC had infiltrative growth and no tumor capsule (Figure 2B). The patient who presented with distant metastasis to C3 had an encapsulated invasive FVPTC positive for NRAS61 mutation. Two patients with RAS-positive cancers also had discrete ipsilateral cancers positive for BRAFV600E.
Figure 2.
A, Encapsulated FVPTC. Note the smooth thin capsule surrounding the tumor (arrow). B, Infiltrative FVPTC. Note an irregular, invasive tumor border (arrow).
One patient with RAS-positive MTC had CND and a left modified radical neck dissection demonstrating 13 of 17 metastatic lymph nodes. Among the 48 patients with histological DTC, 22 had 1 or more lymph nodes resected at thyroidectomy (Table 1). These included the patient with encapsulated invasive FVPTC with distant metastasis to C3 treated by central and lateral compartment dissection, 2 DTC patients with CND by surgeon choice, and 19 DTC patients with 1 or more lymph nodes removed incidentally. Lymph node (LN) metastasis was noted in 2 of 22 patients with RAS-positive DTC including the patient with distant metastasis to C3, and 1 patient with both an encapsulated invasive 2.8-cm NRAS61 FVPTC with capsular and vascular invasion and a 1.2-cm tall cell variant PTC positive for BRAFV600E. Because of the small size of the LN metastases in this latter case, we were not able to determine whether the metastatic LNs contained BRAF or RAS mutations.
For 3 cases of RAS-positive cancer noted at diagnostic lobectomy, completion thyroidectomy was deferred, and these cases are reported as unilateral cancers. Bilateral cancer was present in 24 of 50 cases (48%). Excluding the MTC and ATC patients, of the remaining 22 bilateral DTC cases, 15 contralateral tumors (68%) were 1 cm or less, 7 (32%) were greater than 1 cm; RAS mutations were present in 10 (45%), 1 (5%) was positive for BRAFV600E, and 11 (50%) were not tested because of small tumor size.
Genetic microdissection analysis
Microscopic examination of the encapsulated and noninvasive FVPTC tumors revealed that some tumors had nuclear features of papillary carcinoma diffusely throughout the nodule, whereas other specimens showed significant variability in the presence of these nuclear features. We hypothesized that if RAS mutations were an early event in progression of these cancers, they would be present in all areas within each tumor. We therefore selected 4 representative cases (2 positive for NRAS61 and 2 for HRAS61) and microdissected 3 separate areas in each tumor, which demonstrated a range of nuclear features of PTC, varying from minimal to clear-cut (Figure 3). Molecular analysis demonstrated that RAS mutations were uniformly present in all dissected areas. Despite the heterogeneity in the histological appearance of the cancer in these lesions, the common finding of homogeneously distributed RAS mutation confirms that these selected lesions were clonal neoplasms. This finding supports the role of RAS mutation as an early and key event in thyroid tumorigenesis.
Figure 3.
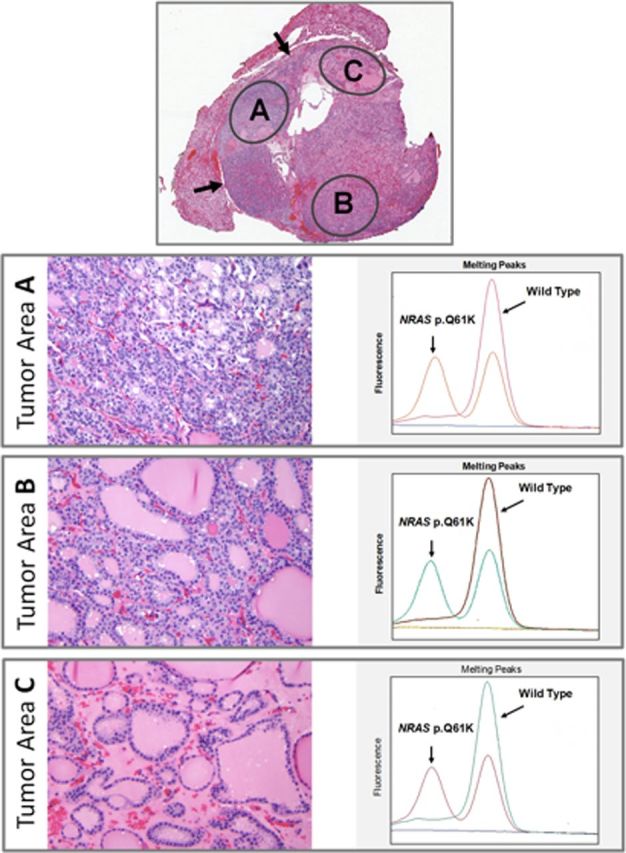
The tumor has a thin capsule (upper panel arrows) and shows microfollicular areas with well-developed nuclear features of PTC (tumor area A), areas composed of larger follicles with borderline nuclear features of PTC (tumor area B), and areas with large follicles lacking nuclear features of PTC (tumor area C). When tested separately, all 3 areas were positive for the same NRAS61 mutation.
Of 63 RAS-positive aspirates, 11 (17%) with histological correlation were benign. Of these, 7 nodules were initially classified as FA based on predominantly microfollicular architecture and 4 others were classified as HN based on the presence of normal to large-sized follicles with abundant colloid. To determine whether the lesions diagnosed as HN were indeed nonclonal hyperplastic lesions, we microdissected 3 different areas in a representative HN positive for an NRAS61 mutation and a second HN positive for an HRAS61 mutation. High levels of RAS mutations were found in all 3 microdissected regions within each nodule. Therefore, despite the histological appearance suggestive of a nonclonal HN, the homogeneous distribution of the RAS mutations within these nodules indicates that they are clonal neoplasms driven by a RAS mutation and would be more correctly classified as FA.
Preoperative ultrasound features and correlation with diagnosis
Among 51 RAS-positive thyroid nodules for which preoperative neck ultrasound images were available, blinded review showed that 19 nodules (37%) had at least 1 suspicious feature. Of these, 17 were histologically malignant, yielding a PPV of 89% and a specificity of 67%. However, 28 of 32 nodules without suspicious features (88%) also proved to be malignant on final histology. Negative predictive value (NPV) and sensitivity were therefore only 13% and 38%, respectively, demonstrating the significant limitations of ultrasound evaluation of nodules with RAS mutations. Even inclusion of Doppler flow assessment for increased intranodular vascularity, which has been reported to improve the diagnostic yield in histologically follicular lesions such as FVPTC and FTC, was of only limited benefit (23–25). In a subset of 10 of 51 nodules with this finding, malignancy was present in 9 of 10 hypervascular nodules. For 8 malignant nodules, hypervascularity was the only suspicious finding. However, hypervascularity was absent in the other 41 RAS lesions, of which 36 were malignant. Despite a specificity and a PPV of 83% and 90%, respectively, the poor sensitivity of 20% and NPV of 12% also limited the usefulness of Doppler flow assessment of intranodular vascularity.
Postoperative management and follow-up
Follow-up data were available for 37 of the 50 patients with histologically proven cancer. Excluding the cases of MTC and ATC, 31 of 35 patients received radioiodine (RAI) ablation with a mean activity of 87.5 (±32.9) mCi for the 30 M0 patients and an activity of 317 mCi for the single patient who presented with C3 metastases. This patient also received external beam radiation. To date, with a mean follow-up interval of 21.8 months (SD ± 8.7, range 12–38 months) 31 of 35 patients (89%) have no evidence of recurrence based on undetectable serial thyroglobulin (Tg) measurements and negative neck ultrasonography. Detectable Tg has been noted in 4 of 35 patients (11%). Two of these also had discrete BRAFV600E cancers, one of whom has an ungenotyped distant metastasis noted on follow-up RAI imaging. A patient with an NRAS61-encapsulated invasive FVPTC continues to have detectable Tg on follow-up without evidence of structural disease. The patient with C3 metastases continues to have a Tg of 1600 ng/mL on suppressive doses of T4 with an 18F-fluorodeoxyglucose-avid C3 lytic lesion. One patient with ATC presented with a T4 tumor with local invasion into skeletal muscle and died while receiving palliative treatment. The patient with MTC had positive surgical margins, continued postoperative calcitoninemia, and died after a prolonged postoperative course.
Discussion
In this study we not only reconfirm a previously described strong association between RAS mutations detected in thyroid FNA specimens and thyroid cancer risk (4, 5, 7) but also report for the first time a comprehensive characterization of biological, clinical, and histopathological features of tumors associated with RAS mutations. Our data also show that although RAS mutations can be found all types of thyroid tumors including MTC and ATC, the vast majority of RAS-positive tumors are FVPTC with characteristic phenotypic and clinical features. A smaller but significant group of these tumors are FTC.
Histologically, follicular lesions including FVPTC, FTC, and FA are notoriously difficult to resolve as benign or malignant by cytology (26, 27). In the case of FVPTC, this is due to an absence of papillary growth as well as the typically limited expression of convincing nuclear features of PTC (28, 29). For FTC, diagnosis is usually not possible because identification of the capsular and vascular invasion that are the sine qua non of these cancers requires a histological specimen (28, 29). It should therefore not be surprising that the preponderance of histologically follicular-patterned lesions reported here resulted in 93% of nodules with RAS mutations being classified as indeterminate on presurgical FNA. Importantly, during the same period of study, 187 additional cytologically malignant specimens were also tested, none of which was positive for RAS mutations. This study provides the reason for this finding, ie, RAS-positive cancers are almost exclusively follicular variant of papillary carcinoma or follicular carcinomas, both of which are rarely diagnosed as cancer on cytology. The strong predictive value of RAS mutations as well as their predominance in histologically follicular pattern lesions emphasizes the importance of RAS mutations as a valuable diagnostic marker for tumors that may be difficult or impossible to diagnose conclusively by cytology.
Of the 11 benign nodules in this series, 7 were initially designated as FA and 4 others as HN. Histological features of FA and HN have significant overlap and the diagnostic distinction is primarily based on subjective impression of colloid abundance and follicle size (30). However, all of these nodules contained RAS mutations and 2 representative microdissected HN had RAS mutations present uniformly throughout the nodule. This finding suggests that these tumors would be better classified as clonal neoplasms, ie, as FA, despite the histological appearance suggestive of nonclonal HN. It is likely that many such RAS-positive clonal FA have similarly been misclassified as nonclonal histologically benign HN, obscuring the importance of RAS as a marker of not only malignancy but of neoplasia as well (16). Furthermore, the RAS-positive tumors in this study are distributed along a histological continuum from benign FA, through ATC, and include histologically intermediate carcinomas such as encapsulated and histologically bland-appearing FVPTC with and without capsular invasion, as well as infiltrative nonencapsulated FVPTC, and FTC. These observations along with data from multiple studies showing that RAS mutations occur in 20%–40% of ATC align with experimental evidence supporting the role of RAS mutation as an early transformative event associated with step-wise tumorigenesis leading from adenoma to carcinoma (31, 32). Additionally, this histological continuum suggests that RAS mutations may be critical for the development of invasive histology and, rarely, further progression to ATC (33).
A single case of MTC was positive for an HRAS61 mutation. This mutation was noted both on presurgical cytology and confirmed with repeat genetic analysis on the histological specimen. Although not typically thought of as a common mutation in MTC, a growing literature suggests that RAS also plays a role in MTC tumorigenesis. Expression of v-Ha-ras has been shown to cause MTC in rascal transgenic mice, and a recent report notes that up to 68% of sporadic RET-negative human MTCs contain mutations of RAS (34, 35).
By incorporating neck ultrasonography to assess nodules for the presence of high-risk features, many have proposed that FNA can be used more selectively with improved diagnostic yield (2, 18). Although numerous ultrasonographic features identifying nodules with an increased risk of malignancy have been reported, these are frequently absent, thus limiting sensitivity and NPV (2). More importantly, these features apply most specifically to the diagnosis of conventional PTC. Radiographic features suggestive of malignancy in histologically follicular lesions, the type predominating in this study, are far less well characterized. The use of Doppler flow ultrasonography for the assessment of intranodular vascularity was reported to improve the detection of otherwise bland-appearing lesions with follicular histology (23, 25, 36). Although we found a relatively high specificity and PPV for the preoperative ultrasonographic identification of nodules with suspicious features, even when including Doppler flow assessment, most of the histologically follicular cancers evaluated in this series were missed by ultrasound. Our findings show that although suspicious ultrasonographic features are useful when present, most RAS-positive cancers lack suspicious features of any type, including the increased intranodular vascularity on Doppler flow assessment.
Because molecular testing has been routinely used at our institution only since 2007, follow-up is limited to a mean only 21.8 months and only for 35 of 48 patients with DTC. Despite these limitations, the vast majority of patients have no evidence of persistent or recurrent disease, suggesting that RAS-positive DTC is typically low risk. Among patients with RAS-positive DTC, lymph node metastasis was rare and only 1 patient with an invasive FVPTC presented with a distant metastasis. The encapsulated noninvasive RAS-positive FVPTCs that predominate in this series appear to be especially low risk. Although LN dissection was not done systematically, all patients with encapsulated noninvasive FVPTCs who underwent CND were N0 and none had distant metastases. These findings are consistent with other large studies that show the very low metastatic potential of FVPTC (28, 37, 38). One of these reports from Liu et al (37) suggests that based on the absence of long-term recurrence in the subset of patients with encapsulated noninvasive FVPTC, lobectomy alone was sufficient treatment for these patients. However, we believe that our observation of bilateral cancer in almost half of all patients with RAS-positive cancers suggests that total thyroidectomy is a more appropriate surgical management option.
Prior reports concerning FVPTC have also demonstrated that encapsulated tumors with invasion, and infiltrative types of FVPTC are more clinically aggressive, with an increased prevalence of locoregional LN and distant metastasis (28, 37, 38). Both of these variants were uncommon in our series. Nonetheless, the invasive encapsulated FVPTC we report were aggressive, accounting for 1 patient who presented with locoregional LN metastases and a second patient who presented with both cervical metastases and distant metastases to the cervical spine. Because RAS mutations are also found in a significant proportion of poorly differentiated and anaplastic cancers, as was also documented in this study, it is justifiable to consider surgical management of all RAS-positive nodules to establish the invasiveness of the tumors and prevent cancer progression. We also believe that unless gross disease is evident either by presurgical neck ultrasonography or based on intraoperative findings, LN dissection is not necessary for most RAS-positive cancers. However, additional studies with a more systematic approach to CND will be needed to definitively answer this question. Whether RAI therapy is necessary for the treatment of RAS-positive thyroid cancers is unclear. Most cancers in this series containing RAS mutations were fully encapsulated and showed no invasion. However, 4 patients had FTC, 2 patients continue to have persistently detectable Tg without evidence of structural disease, and 2 other patients with RAS-positive cancers have distant metastases. These findings suggest that therapy with adjuvant RAI and TSH suppression may be unnecessary in most cases, particularly those cases with completely encapsulated noninvasive cancer. However, more aggressive management including RAI ablation and TSH suppression may be appropriate in a subset of RAS-positive tumors with histologically aggressive features.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ATC
- anaplastic thyroid cancer
- AUS
- atypia of undetermined significance
- CND
- compartment node dissection
- DTC
- differentiated thyroid carcinoma
- FA
- follicular adenoma
- FLUS
- follicular lesion of undetermined significance
- FNA
- fine-needle aspiration
- FN/SFN
- follicular neoplasm/suspicious for follicular neoplasm
- FTC
- follicular thyroid carcinoma
- FVPTC
- follicular variant PTC
- HN
- hyperplastic nodule
- LN
- lymph node
- MTC
- medullary thyroid carcinoma
- NPV
- negative predictive value
- PPV
- positive predictive value
- PTC
- papillary thyroid cancer
- RAI
- radioiodine
- Tg
- thyroglobulin
- TT
- total thyroidectomy
- XRT
- head or neck radiation.
References
- 1. Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328:553–559. [DOI] [PubMed] [Google Scholar]
- 2. Gharib H , Papini E , Paschke R , et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association Medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: executive summary of recommendations. Endocr Pract. 2010;16:468–475. [DOI] [PubMed] [Google Scholar]
- 3. Yassa L , Cibas ES , Benson CB , et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer. 2007;111:508–516. [DOI] [PubMed] [Google Scholar]
- 4. Nikiforov YE , Steward DL , Robinson-Smith TM , et al. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab. 2009;94:2092–2098. [DOI] [PubMed] [Google Scholar]
- 5. Cantara S , Capezzone M , Marchisotta S , et al. Impact of proto-oncogene mutation detection in cytological specimens from thyroid nodules improves the diagnostic accuracy of cytology. J Clin Endocrinol Metab. 2010;95:1365–1369. [DOI] [PubMed] [Google Scholar]
- 6. Ohori NP , Nikiforova MN , Schoedel KE , et al. Contribution of molecular testing to thyroid fine-needle aspiration cytology of “follicular lesion of undetermined significance/atypia of undetermined significance.” Cancer Cytopathol. 2010;118:17–23. [DOI] [PubMed] [Google Scholar]
- 7. Nikiforov YE , Ohori NP , Hodak SP , et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab. 2011;96:3390–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fagin JA. Minireview: branded from the start-distinct oncogenic initiating events may determine tumor fate in the thyroid. Mol Endocrinol. 2002;16:903–911. [DOI] [PubMed] [Google Scholar]
- 9. Nikiforov YE. Thyroid carcinoma: molecular pathways and therapeutic targets. Modern Pathology. 2008;2(suppl 21):S37–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fehrenbacher N , Bar-Sagi D , Philips M. Ras/MAPK signaling from endomembranes. Mol Oncol. 2009;3:297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karnoub AE , Weinberg RA. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol. 2008;9:517–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moon A. Differential functions of Ras for malignant phenotypic conversion. Arch Pharmacal Res. 2006;29:113–122. [DOI] [PubMed] [Google Scholar]
- 13. Kyriakis JM. Thinking outside the box about Ras. J Biol Chem. 2009;284:10993–10994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poghosyan Z , Wynford-Thomas D. Analysis of Ras transformation of human thyroid epithelial cells. Methods Enzymol. 2006;407:648–660. [DOI] [PubMed] [Google Scholar]
- 15. Namba H , Rubin SA , Fagin JA. Point mutations of ras oncogenes are an early event in thyroid tumorigenesis. Mol Endocrinol. 1990;4:1474–1479. [DOI] [PubMed] [Google Scholar]
- 16. Vasko V , Ferrand M , Di Cristofaro J , Carayon P , Henry JF , de Micco C. Specific pattern of RAS oncogene mutations in follicular thyroid tumors. J Clin Endocrinol Metab. 2003;88:2745–2752. [DOI] [PubMed] [Google Scholar]
- 17. Stang MT , Armstrong MJ , Ogilvie JB , et al. Positional dyspnea and tracheal compression as indications for goiter resection. Arch Surg. 2012;147(7):621–626. [DOI] [PubMed] [Google Scholar]
- 18. Cooper DS , Doherty GM , Haugen BR , et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. [DOI] [PubMed] [Google Scholar]
- 19. McCoy KL , Jabbour N , Ogilvie JB , Ohori NP , Carty SE , Yim JH. The incidence of cancer and rate of false-negative cytology in thyroid nodules greater than or equal to 4 cm in size. Surgery. 2007;142:837–844; discussion 844.e831–e833. [DOI] [PubMed] [Google Scholar]
- 20. Cooper DS , Doherty GM , Haugen BR , et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109–142. [DOI] [PubMed] [Google Scholar]
- 21. Ali SZ , Cibas ES. The Bethesda System for Reporting Thyroid Cytopathology. New York: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 22. Edge SB , Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. [DOI] [PubMed] [Google Scholar]
- 23. Ozdemir D , Ersoy R , Cuhaci N , et al. Classical and follicular variant papillary thyroid carcinoma: comparison of clinical, ultrasonographical, cytological, and histopathological features in 444 patients. Endocr Pathol. 2011;22:58–65. [DOI] [PubMed] [Google Scholar]
- 24. Iared W , Shigueoka DC , Cristofoli JC , et al. Use of color Doppler ultrasonography for the prediction of malignancy in follicular thyroid neoplasms: systematic review and meta-analysis. J Ultrasound Med. 2010;29:419–425. [DOI] [PubMed] [Google Scholar]
- 25. Yoon JH , Kim EK , Hong SW , Kwak JY , Kim MJ. Sonographic features of the follicular variant of papillary thyroid carcinoma. J Ultrasound Med. 2008;27:1431–1437. [DOI] [PubMed] [Google Scholar]
- 26. Elsheikh TM , Asa SL , Chan JK , et al. Interobserver and intraobserver variation among experts in the diagnosis of thyroid follicular lesions with borderline nuclear features of papillary carcinoma. Am J Clin Pathol. 2008;130:736–744. [DOI] [PubMed] [Google Scholar]
- 27. Lloyd RV , Erickson LA , Casey MB , et al. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am J Surg Pathol. 2004;28:1336–1340. [DOI] [PubMed] [Google Scholar]
- 28. Zhu Z , Gandhi M , Nikiforova MN , Fischer AH , Nikiforov YE. Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. Am J Clin Pathol. 2003;120:71–77. [DOI] [PubMed] [Google Scholar]
- 29. Adeniran AJ , Zhu Z , Gandhi M , et al. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol. 2006;30:216–222. [DOI] [PubMed] [Google Scholar]
- 30. Nikiforov YE , Ohori NP. Follicular adenoma. In: , Nikiforov YE , Biddinger PW , Thompson LDR eds. Diagnostic Pathology and Molecular Genetics of the Thyroid: A Comprehensive Guide for Practicing Thyroid Pathology. 2nd ed Philadelphia: Wolters Kluwer/Lippincott Williams and Wilkins; 2012;119–142. [Google Scholar]
- 31. Burns JS , Blaydes JP , Wright PA , et al. Stepwise transformation of primary thyroid epithelial cells by a mutant Ha-ras oncogene: an in vitro model of tumor progression. Mol Carcinog. 1992;6:129–139. [DOI] [PubMed] [Google Scholar]
- 32. Nikiforov YE , Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;7:569–580. [DOI] [PubMed] [Google Scholar]
- 33. Smallridge RC , Marlow LA , Copland JA. Anaplastic thyroid cancer: molecular pathogenesis and emerging therapies. Endocr Relat Cancer. 2009;16:17–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnston D , Hatzis D , Sunday ME. Expression of v-Ha-ras driven by the calcitonin/calcitonin gene-related peptide promoter: a novel transgenic murine model for medullary thyroid carcinoma. Oncogene. 1998;16:167–177. [DOI] [PubMed] [Google Scholar]
- 35. Moura MM , Cavaco BM , Pinto AE , Leite V. High prevalence of RAS mutations in RET-negative sporadic medullary thyroid carcinomas. J Clin Endocrinol Metab. 2011;96:E863–E868. [DOI] [PubMed] [Google Scholar]
- 36. De Nicola H , Szejnfeld J , et al. Flow pattern and vascular resistive index as predictors of malignancy risk in thyroid follicular neoplasms. J Ultrasound Med. 2005;24:897–904. [DOI] [PubMed] [Google Scholar]
- 37. Liu J , Singh B , Tallini G , et al. Follicular variant of papillary thyroid carcinoma: a clinicopathologic study of a problematic entity. Cancer. 2006;107:1255–1264. [DOI] [PubMed] [Google Scholar]
- 38. Rivera M , Ricarte-Filho J , Knauf J , et al. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol. 2010;23:1191–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]