Abstract
Context:
Bile acids (BAs) are newly recognized signaling molecules in glucose and energy homeostasis. Differences in BA profiles with type 2 diabetes mellitus (T2D) remain incompletely understood.
Objective:
The objective of the study was to assess serum BA composition in impaired glucose-tolerant, T2D, and normal glucose-tolerant persons and to monitor the effects of improving glycemia on serum BA composition in T2D patients.
Design and Setting:
This was a cross-sectional cohort study in a general population (cohort 1) and nonrandomized intervention (cohort 2).
Patients and Interventions:
Ninety-nine volunteers underwent oral glucose tolerance testing, and 12 persons with T2D and hyperglycemia underwent 8 weeks of intensification of treatment.
Main Outcome Measures:
Serum free BA and respective taurine and glycine conjugates were measured by HPLC tandem mass spectrometry.
Results:
Oral glucose tolerance testing identified 62 normal-, 25 impaired glucose-tolerant, and 12 T2D persons. Concentrations of total taurine-conjugated BA were higher in T2D and intermediate in impaired- compared with normal glucose-tolerant persons (P = .009). Univariate regression revealed a positive association between total taurine-BA and fasting glucose (R = 0.37, P < .001), postload glucose (R = 0.31, P < .002), hemoglobin A1c (R = 0.26, P < .001), fasting insulin (R = 0.21, P = .03), and homeostatic model assessment-estimated insulin resistance (R = 0.26, P = .01) and an inverse association with oral disposition index (R = −0.36, P < .001). Insulin-mediated glycemic improvement in T2D patients did not change fasting serum total BA or BA composition.
Conclusion:
Fasting taurine-conjugated BA concentrations are higher in T2D and intermediate in impaired compared with normal glucose-tolerant persons and are associated with fasting and postload glucose. Serum BAs are not altered in T2D in response to improved glycemia. Further study may elucidate whether this pattern of taurine-BA conjugation can be targeted to provide novel therapeutic approaches to treat T2D.
Bile acids (BAs) are synthesized from cholesterol in the liver. They are secreted into bile as glycine or taurine conjugates and released into the intestine with meals where they can be further modified by intestinal bacteria. A high proportion of BAs are reabsorbed to undergo enterohepatic recirculation (1). BA function in cholesterol homeostasis is well established. Additionally, BAs participate in glucose and energy regulation, in part through the activation of farnesoid X receptor (FXR) (1). In diabetic mice, FXR activation improves glycemia, and FXR-null mice are glucose intolerant and insulin resistant (2). Additional effects of BAs on glucose homeostasis are mediated via activation of the G protein-coupled bile acid receptor 1 also known G-protein coupled receptor 19, membrane-type receptor for bile acids (TGR5) (3, 4) or by decreasing endoplasmic reticulum stress (5). Circulating BA levels themselves are affected by food intake (6). Furthermore, BA sequestrants, which prevent ileal reabsorption, increase fecal excretion thus promote increased de novo BA synthesis, and effectively lower blood glucose in type 2 diabetes (T2D). Indeed, the BA sequestrant colesevelam is approved by the Food and Drug Administration for T2D treatment (7). Together, these studies highlight the importance of BA in regulation of systemic metabolism.
It is unknown whether alterations in BAs, as seen in rodent models of diabetes (8, 9), also occur in humans and contribute to the pathophysiology of human T2D. BA homeostasis in T2D has been assessed by examining bile, plasma, or serum, with inconsistent results (7, 10–15). In some studies, fasting levels of total BAs in serum or plasma were unchanged (7, 13, 14), whereas in others certain BA subfractions were increased (14, 15) in T2D compared with controls. Recently 12-hydroxylated BAs have been associated with insulin resistance (16).
To determine whether BA profiles differ in normal glucose tolerance (NGT) compared with impaired glucose tolerance (IGT) and T2D, we assessed fasting and postload serum BA composition across a spectrum of glycemia. We also examined the relationship between fasting and postload BAs composition and their relationship to metabolic measures. Additionally, we assessed whether improvement in glycemia via intensification of insulin therapy and lifestyle counseling would alter fasting serum BA levels in patients with T2D and poor metabolic control to determine the effect of insulin and glucose per se on circulating BA composition.
Materials and Methods
Subjects and methods
The Joslin Diabetes Center Institutional Review Board approved the study. All subjects responded to advertisement and provided written informed consent.
Cohort 1
Ninety-nine consecutively recruited subjects were examined. Participants had no risk factor for T2D (n = 10) or one or more risk factors for T2D, including prior borderline abnormal blood glucose, family history of T2D, history of gestational diabetes, body mass index (BMI) greater than 30 kg/m2, hypertension, dyslipidemia, heart disease, irregular menses, or ethnic minority. No participant was receiving oral or injectable diabetes medication. Participants were instructed to refrain from any alcoholic beverages and from intensive physical activity the day prior to the visit. Participants were also instructed to consume an extra serving of carbohydrate (bread, rice, pasta, or potato) with each meal, two snacks daily, and not to miss a meal for 3 days prior to the study visit, which was performed after an overnight fast. Height, weight, and sitting blood pressure (BP) were measured. Glucose and insulin concentrations were measured at baseline and at 30, 60, 90, and 120 minutes after administration of a 75-g oral glucose tolerance test (OGTT). Serum BA composition was measured at baseline and 120 minutes after the load. Glucose tolerance status (NGT, IGT, and T2D) was classified based on Expert Committee on the Diagnosis and Classification of Diabetes Mellitus criteria but assessed on a single day.
Cohort 2
Twelve consecutively recruited subjects with T2D in poor glycemic control (distinct from cohort 1) were enrolled in an intensive glycemic management program. Inclusion criteria included age of 18–65 years; hemoglobin A1c (HbA1c) above 8.5%; current use of or newly prescribed insulin; no donation of blood within 2 months of enrollment; stable hypertension and lipid medications for 6 months; and no history of active infection, inflammatory, or malignant disease. Participants were instructed in home glucose monitoring four to eight times daily (One Touch; Lifescan) and seen weekly over 8 weeks for interim health assessment, review of glucose logs, insulin adjustment, and lifestyle (diet and exercise) counseling. Phone visits occurred between scheduled study visits as needed.
Assays
Plasma glucose was measured by the glucose oxidase method (Synchron CX3 Delta; Beckman Coulter, Inc), total, low-density lipoprotein (LDL)- and high-density lipoprotein (HDL)-cholesterol assessed using a cholesterol esterase assay, triglycerides measured via hydrolysis to glycerol and free fatty acids (Synchron CX9; Beckman Coulter), and HbA1c assessed by HPLC (Tosoh 2.2; Tosoh Bioscience) in the clinical laboratory of the Joslin Diabetes Center. Batch assays for serum insulin and C-peptide were performed in duplicate by a double-antibody RIA (Diagnostic Systems Laboratories Inc) in samples stored at −70°C. The coefficient of variation for the DSL-1600 insulin RIA (Diagnostic Systems Laboratories Inc) for samples with insulin concentration between 4.8 and 55 μU/mL ranges from 4.5% to 8.3% (intraassay), with corresponding interassay values of 4.7%–12.2%. Serum fibroblast growth factor (FGF)-19 was assayed by an ELISA (R&D Systems Europe).
Fasting and postload serum BAs, including cholic acid (CA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), ursodeoxycholic acid (UDCA), muricholic acid (MCA), and their respective taurine (T) and glycine (G) conjugates were batch analyzed in samples stored at −70°C, using HPLC tandem mass spectrometry and quantified using deuterium-labeled internal standards (17). Because CDCA and DCA and their conjugates are not fully separated, they are reported as the sum of CDCA and DCA (CDCA+DCA).
Calculations and statistical analysis
Homeostatic model assessment-estimated insulin resistance (HOMA-IR) and oral disposition index were calculated as previously described (18, 19). Nonalcoholic fatty liver disease was estimated using the alcoholic liver disease from nonalcoholic fatty liver disease index (20). Statistical analysis was performed using SPSS 19.0 (IBM Corp). Demographic characteristics are presented as mean ± SD, with results as mean ± SE. An ANOVA was used for comparison by group, with Fisher's exact test for between-group comparisons. Post hoc Tukey correction was applied to adjust for multiple comparisons. Linear and multivariate regression was used to assess the relationship between BA and metabolic parameters including BMI, triglycerides, total cholesterol, LDL, HDL, HbA1c, fasting plasma glucose, postload glucose, fasting insulin, HOMA-IR, and oral disposition index.
Variables were logarithmically transformed if not normally distributed. Results were considered significant if P < .05.
Results
Study population (cohort 1)
A total of 99 consecutively recruited persons, 62 with NGT, 25 with IGT, and 12 with T2D participated in this cross-sectional cohort study. Patient characteristics are summarized in Table 1. Ethnicity was similar across groups, with 66% Caucasian, 8% black, 11% Asian, and 5% Indian in the NGT group, 88% Caucasian and 12% Asian in the IGT group, and 83% Caucasian and 17% Asian in T2D (P = .271). BMI, total cholesterol, and triglyceride levels were also similar between groups. As expected, individuals with T2D were hyperglycemic, with higher fasting glucose, 120-minute postload OGTT glucose, HbA1c, fasting insulin, HOMA-IR, and lower oral disposition index (all P < .001). T2D subjects were older (P = .023) and had higher systolic (P = .007) and diastolic (P = .023) BP and lower HDL cholesterol (P = .018). These variables were intermediate in IGT.
Table 1.
Subject Demographics and Metabolic Characteristics
| Group | NGT | IGT | T2D | P (ANOVA) |
|---|---|---|---|---|
| Female/male | 34/28 | 19/6 | 8/4 | .156 |
| Age, y | 40 ± 12 | 45 ± 13 | 49 ± 11a | .023 |
| BMI, kg/m2 | 28.6 ± 5.8 | 31.0 ± 8.6 | 32.3 ± 8.7 | .143 |
| Systolic BP, mm Hg | 116 ± 14 | 124 ± 14a | 129 ± 18b | .007 |
| Diastolic BP, mm Hg | 74 ± 9 | 77 ± 7 | 82 ± 10b | .023 |
| Total cholesterol, mg/dL [mmol/L] | 179 ± 35 [4.6 ± 0.9] | 187 ± 28 [4.8 ± 0.7] | 166 ± 29 [4.3 ± 0.8] | .19 |
| LDL cholesterol, mg/dL [mmol/L] | 117 ± 35 [3.0 ± 0.9] | 121 ± 34 [3.1 ± 0.9] | 115 ± 39 [3.0 ± 1.0] | .88 |
| HDL cholesterol, mg/dL [mmol/L] | 46 ± 12 [1.2 ± 0.3] | 49 ± 12 [1.3 ± 0.3] | 38 ± 9 [1.0 ± 0.2]a,d | .018 |
| Triglycerides, mg/dL [mmol/L] | 98 ± 62 [1.1 ± 0.7] | 118 ± 76 [1.3 ± 0.9] | 125 ± 61 [1.4 ± 0.7] | .248 |
| ALT, U/L | 26 ± 16 | 24 ± 9 | 33 ± 30 | .884 |
| AST, U/L | 26 ± 10 | 25 ± 7 | 27 ± 15 | .311 |
| Liver disease index (ANI) | −6.8 ± 0.7 | −9.1 ± 1.4 | −8.3 ± 1.8 | .273 |
| HbA1c, % | 5.3 ± 0.3 | 5.5 ± 0.3 | 6.2 ± 0.8c,e | <.001 |
| Fasting glucose, mg/dL [mmol/L] | 93 ± 7 [5.2 ± 0.4] | 96 ± 8 [5.3 ± 0.4] | 115 ± 17 [6.4 ± 0.9]c,e | <.0001 |
| 120-minute glucose, mg/dL [mmol/L] | 109 ± 21 [6.1 ± 1.2] | 164 ± 20 [9.1 ± 1.1]c | 240 ± 25 [13.3 ± 1.4]c,e | <.0001 |
| Fasting insulin, μU/mL [pmol/L] | 9.3 ± 5.8 [65 ± 40] | 13.3 ± 8.1 [92 ± 56]b | 21.9 ± 20.5 [152 ± 142]c,d | .0001 |
| HOMA-IR | 2.2 ± 1.4 | 3.3 ± 2.1 | 6.7 ± 6.7c,d | <.0001 |
| Disposition index (oral) | 0.15 ± 0.12 | 0.08 ± 0.06b | 0.04 ± 0.05b | <.001 |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; ANI, alcoholic liver disease/nonalcoholic fatty liver disease index. Demographic data of our cross-sectional study cohort (n = 99) of consecutive subjects undergoing an OGTT: mean ± SD.
P < .05 vs NGT.
P < .01 vs NGT.
P < .001 vs NGT.
P < .01 vs IGT.
P < .001 vs IGT.
Taurine-conjugated bile acids are higher in T2D
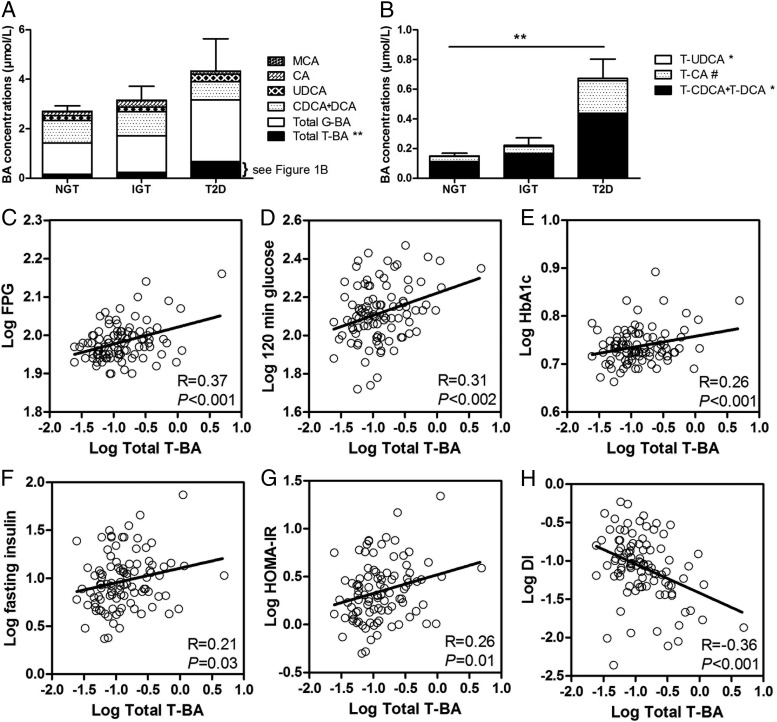
Fasting serum total BA concentrations were similar in all groups (P = .848) (Figure 1A). However, fasting total taurine-conjugated BA (T-BA) absolute (Figure 1A and Table 2) and percentage of total BA concentration (NGT: 5.5% ± 0.4%, IGT: 7.7% ± 0.8%, T2D: 12.3% ± 2.5%, P < .0001) differed between groups, with higher concentrations in T2D than normal glucose tolerant and intermediate concentrations in impaired glucose tolerant persons. T-BA species T-CDCA+DCA and T-UDCA were also higher in T2D compared with normal glucose tolerant and intermediate in impaired glucose-tolerant persons, with similar trends for T-CA (Figure 1B and Table 2). In contrast, fasting total glycine-conjugated BA (G-BA), both absolute and relative concentrations (expressed as percentage of total, NGT: 44% ± 2%, IGT: 45% ± 4%, T2D: 49% ± 5%, P = .705) as well as absolute and relative concentrations of total conjugated BA (NGT: 50% ± 2%, IGT: 53% ± 5%, T2D: 61% ± 6%, P = .185) and total free BA (NGT: 50% ± 2%, IGT: 47% ± 5%, T2D: 39% ± 6%, P=0.190) did not differ across study groups (Table 2). Central tendencies and variance of BA species by subgroup pertinent to power calculations for future investigations are provided in Table 2.
Figure 1.
Fasting serum BA concentrations and glycemic metabolic measures. Total BA concentrations broken down into total T-BA, total G-BA, CDCA+DCA, UDCA, CA, and MCA (A) and total T-BA concentrations broken down into its subsets of T-CDCA+DCA, T-CA, and T-UDCA (B) in NGT, IGT, and T2D subjects. Error bars mark the standard error for total BA (A) and total T-BA (B). C–H, Univariate regression between total T-BA and fasting plasma glucose [95% confidence interval (CI) 0.022–0.066] (C), postload glucose (95% CI 0.045–0.188) (D), HbA1c (95% CI 0.006–0.041) (E), fasting insulin (95% CI 0.012–0.291) (F), HOMA-IR (95% CI 0.046–0.343) (G), and oral disposition index (95% CI −0.575 to −0.181) (H) (all variables were logarithmically transformed). DI, disposition index. *, P (ANOVA) < .05; **, P (ANOVA) < .01; #, P (ANOVA) = .054.
Table 2.
Fasting and 2-Hour Postglucose Load BA and FGF-19 Concentrations
| BA Concentrations, μmol/L |
||||
|---|---|---|---|---|
| NGT (n = 62) | IGT (n = 25) | T2D (n = 12) | P (ANOVA)a | |
| T-CA | ||||
| Fasting | 0.036 ± 0.004 | 0.048 ± 0.007 | 0.213 ± 0.147 | .054 |
| Postload | 0.024 ± 0.006 | 0.031 ± 0.008 | 0.131 ± 0.076 | .307 |
| P value (fasting vs postload) | <0.001 | <0.001 | 0.065 | |
| T-UDCA | ||||
| Fasting | 0.002 ± 0.001 | 0.006 ± 0.003 | 0.014 ± 0.01b | .029 |
| Postload | 0.0003 ± 0.0002 | 0.002 ± 0.001 | 0.003 ± 0.003 | .122 |
| P value (fasting vs postload) | 0.004 | 0.018 | 0.537 | |
| T-MCA | ||||
| Fasting | 0.002 ± 0.001 | 0.012 ± 0.007 | 0.004 ± 0.003 | .078 |
| Postload | 0.0007 ± 0.0005 | 0.005 ± 0.003b | 0.003 ± 0.001 | .012 |
| P value (fasting vs postload) | 0.058 | 0.161 | 0.712 | |
| T-CDCA+DCA | ||||
| Fasting | 0.111 ± 0.016 | 0.166 ± 0.043 | 0.441 ± 0.243b | .03 |
| Postload | 0.163 ± 0.033 | 0.174 ± 0.056 | 0.38 ± 0.176 | .162 |
| P value (fasting vs postload) | 0.22 | 0.593 | 0.713 | |
| Total T-BA | ||||
| Fasting | 0.151 ± 0.02 | 0.233 ± 0.051 | 0.667 ± 0.391c | 0.009 |
| Postload | 0.188 ± 0.039 | 0.212 ± 0.063 | 0.518 ± 0.255 | 0.095 |
| P value (fasting vs postload) | 0.197 | 0.084 | 0.855 | |
| G-CA | ||||
| Fasting | 0.168 ± 0.022 | 0.209 ± 0.039 | 0.664 ± 0.382 | 0.192 |
| Postload | 0.257 ± 0.047 | 0.233 ± 0.037 | 0.592 ± 0.319 | 0.437 |
| P value (fasting vs postload) | 0.217 | 0.324 | 0.716 | |
| G-UDCA | ||||
| Fasting | 0.112 ± 0.018 | 0.127 ± 0.04 | 0.15 ± 0.103 | 0.414 |
| Postload | 0.088 ± 0.013 | 0.085 ± 0.22 | 0.077 ± 0.043 | 0.288 |
| P value (fasting vs postload) | 0.345 | 0.033 | 0.293 | |
| G-CDCA+DCA | ||||
| Fasting | 0.995 ± 0.117 | 1.151 ± 0.254 | 1.673 ± 0.685 | 0.643 |
| Postload | 1.221 ± 0.163 | 0.932 ± 0.16 | 1.293 ± 0.349 | 0.782 |
| P value (fasting vs postload) | 0.267 | 0.55 | 0.935 | |
| Total G-BA | ||||
| Fasting | 1.274 ± 0.147 | 1.488 ± 0.311 | 2.487 ± 1.118 | 0.66 |
| Postload | 1.565 ± 0.211 | 1.25 ± 0.197 | 1.962 ± 0.659 | 0.745 |
| P value (fasting vs postload) | 0.325 | 0.581 | 0.82 | |
| Total conjugated | ||||
| Fasting | 1.425 ± 0.165 | 1.716 ± 0.358 | 3.163 ± 1.482 | 0.481 |
| Postload | 1.754 ± 0.243 | 1.463 ± 0.252 | 2.479 ± 0.899 | 0.551 |
| P value (fasting vs postload) | 0.375 | 0.701 | 0.81 | |
| CA | ||||
| Fasting | 0.161 ± 0.028 | 0.27 ± 0.115 | 0.099 ± 0.035 | 0.429 |
| Postload | 0.075 ± 0.011 | 0.167 ± 0.081 | 0.567 ± 0.022 | 0.293 |
| P value (fasting vs postload) | <0.001 | 0.02 | 0.003 | |
| UDCA | ||||
| Fasting | 0.193 ± 0.034 | 0.193 ± 0.045 | 0.223 ± 0.113 | 0.848 |
| Postload | 0.15 ± 0.027 | 0.143 ± 0.034 | 0.188 ± 0.111 | 0.422 |
| P value (fasting vs postload) | 0.511 | 0.605 | 0.001 | |
| MCA | ||||
| Fasting | 0.008 ± 0.001 | 0.01 ± 0.002 | 0.009 ± 0.003 | 0.657 |
| Postload | 0.004 ± 0.001 | 0.006 ± 0.002 | 0.003 ± 0.001 | 0.676 |
| P value (fasting vs postload) | <0.001 | <0.001 | 0.001 | |
| CDCA+DCA | ||||
| Fasting | 0.913 ± 0.78 | 0.964 ± 0.293 | 0.7 ± 0.163 | 0.203 |
| Postload | 0.492 ± 0.038 | 0.57 ± 0.187 | 0.43 ± 0.099 | 0.447 |
| P value (fasting vs postload) | <0.001 | 0.001 | 0.005 | |
| Total free BA | ||||
| Fasting | 1.274 ± 0.106 | 1.435 ± 0.397 | 1.014 ± 0.23 | 0.366 |
| Postload | 0.722 ± 0.051 | 0.886 ± 0.264 | 0.678 ± 0.167 | 0.567 |
| P value (fasting vs postload) | <0.001 | 0.002 | 0.009 | |
| Total BA | ||||
| Fasting | 2.699 ± 0.22 | 3.152 ± 0.561 | 4.176 ± 1.54 | 0.848 |
| Postload | 2.475 ± 0.252 | 2.349 ± 0.381 | 3.157 ± 0.902 | 0.73 |
| P value (fasting vs postload) | 0.101 | 0.107 | 0.427 | |
| FGF-19 concentrations, pg/mL | ||||
| FGF-19 | ||||
| Fasting | 103 ± 10 | 94 ± 15 | 90 ± 21 | 0.801 |
| Postload | 146 ± 12 | 211 ± 39 | 113 ± 20 | 0.095 |
| P value (fasting vs postload) | <0.001 | <0.001 | 0.095 | |
BA and FGF-19 concentrations of our cross-sectional study cohort (n = 99) are provided for the fasted state and 120 min after a 75-g OGTT (mean ± SE).
Differences between groups at either time point were assessed by ANOVA with post hoc Tukey correction.
P < .05, vs NGT assessed by ANOVA.
P < .01, vs NGT assessed by ANOVA.
T-BAs are associated with measures of glycemia
To assess the relationship of total T-BA, total G-BA, and total BA with metabolic parameters, we performed a linear and multivariate regression analysis. BA concentrations were determined as the independent and metabolic parameters as the dependent variables. Total T-BA were positively correlated with fasting glucose (Figure 1C), postload glucose (Figure 1D), HbA1c (Figure 1E), fasting insulin (Figure 1F), and HOMA-IR (Figure 1G) and negatively with oral disposition index (Figure 1H). Although total G-BA and total BAs were also negatively associated with oral disposition index (R = −0.29, P = .004, and R= −0.32, P = .002, respectively), no other association was significant for total or G conjugates. There was no relationship between total BA, total T-BA, or total G-BA and ethnicity in the entire cohort or any subgroup. There was no relationship between gender, age, or BMI with serum total BA, total conjugated, total T-BA, total G-BA, or total free BA fractions (Supplemental Figures 1–3, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Likewise there was no association with nonalcoholic fatty liver disease index (data not shown). Bivariate regression analysis including gender, age, or BMI did not alter the significant relationship between fasting T-BA and metabolic measures. The sample size was not sufficient to support multivariate analysis of all factors simultaneously. No BA subsets were associated with fasting triglycerides or cholesterol.
Fasting and postload serum BA composition
We next compared fasting with postload serum BA composition. In NGT individuals, postload reductions (Table 2) were seen in T-CA and T-UDCA but not in T-MCA or the combined fractions of T-CDCA+DCA, such that there was no change in total T-BA. Similar patterns were observed in IGT and T2D. G-BAs were not significantly altered after a glucose load. Free BA fractions (CA, MCA, and CDCA+DCA) and the total free BAs were lowered after a glucose load in NGT, IGT, and T2D. Linear regression revealed an association between postload total T-BA and postload glucose (R = 0.23, P = .021) and a trend toward an association of postload total T-BA with the antecedent fasting glucose (R = 0.20, P=0.053). There were no relationships of postload total BA or total G-BA with any measures of glycemia.
Fibroblast growth factor 19
Given that FGF-19 is a regulator of bile acid synthesis, we assessed the relationship of serum FGF-19 with BA composition and glycemic measures. FGF-19 did not differ by glucose tolerance status. Serum FGF-19 increased after a glucose load (102 ± 79 vs 165 ± 145 pg/mL, fasting vs postload in the entire cohort, P < .00001). Neither fasting nor postload FGF-19 correlated with total BAs, total free or conjugated, total T-BA, or total G-BA. Fasting FGF-19 concentrations tended to correlate with BMI (R = 0.20, P = .051) (Supplemental Figure 4), but fasting and/or postload FGF-19 did not associate with age, fasting or postload glucose, HbA1c, or lipids.
Effect of improved glycemia with insulin and lifestyle counseling on serum BAs
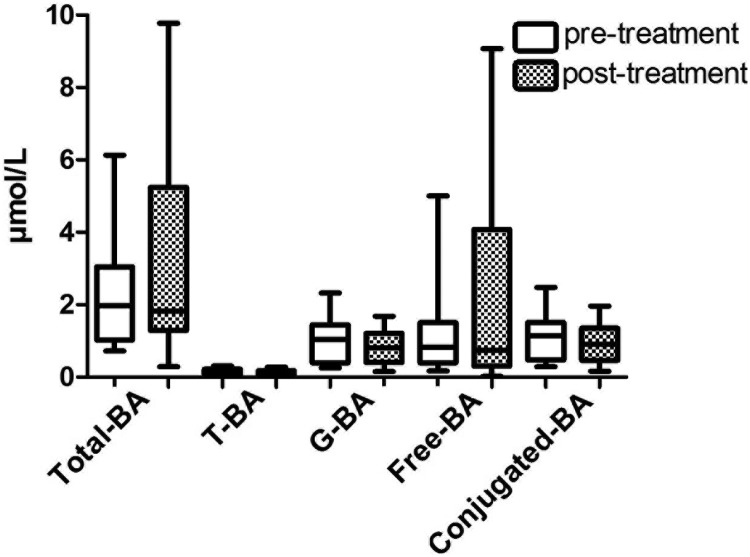
To assess the impact of glycemia per se on BA composition, 12 individuals with T2D and poor glycemic control (four female/eight male, nine Caucasian/three black, age 52 ± 4 y, BMI 38.0 ± 4.7 kg/m2, fasting plasma glucose 176 ± 68 mg/dL, HbA1c 10.6% ± 1.7%) underwent intensified diabetes management with insulin dose titration and lifestyle counseling over 8 weeks. At baseline patients used on average 1.9 ± 0.9 different diabetes medications: 83% were on insulin, 58% on metformin, 33% on thiazolidinedione, and 17% on sulfonylureas, yet insulin was the only medication that was adjusted over the treatment period. There were no episodes of serious hypoglycemia (requiring assistance from others). Postintervention fasting glucose was reduced to 104 ± 32 mg/dL (P < .007) and HbA1c to 7.6% ± 0.8% (P < .0001). Weight was unchanged (118 ± 2 vs 119 ± 2 kg, baseline vs after treatment, P = .168). There were no changes in fasting serum total BAs, total conjugated, total T-BA, total G-BA, or total free BA fractions (Figure 2). There was no relationship between change in HbA1c and change in total T-BA, total G-BA, conjugated BA, or free BA by linear regression.
Figure 2.
Fasting serum BA concentrations before and after intensification of glycemic control in patients with T2D. Fasting serum total BAs (P = .208), T-BA (P = .665), G-BA (P = .286), and total free BAs (free-BA, P = .092) and conjugated BAs (conjugated-BA, P = .284) are shown before and after 8 weeks of glycemic management by intensification of lifestyle and insulin in persons with poorly controlled type 2 diabetes.
Discussion
BAs are important signaling molecules in glucose and energy homeostasis and may positively affect glucose regulation by FXR- and TGR5-dependent mechanisms and by decreasing endoplasmic reticulum stress (1, 3, 5). Moreover, BA sequestrants improve glycemia in T2D (7), highlighting the important role of BAs in the regulation of systemic metabolism in humans in vivo. Whether BA levels or activity is altered in T2D remains incompletely understood. We now report that the circulating concentrations of fasting T-conjugated BAs are higher in T2D and intermediate in IGT compared with normoglycemic controls. T-BAs are the only BA species associated with fasting and postload glucose, HbA1c, fasting insulin, and measures of insulin sensitivity in our study population. Because our cohort was not matched for anthropomorphic characteristics, other factors that differed between groups could be associated with BA differences. However, these metabolic associations do not appear to be directly linked to obesity because total BA, T-BAs and G-BAs are not associated with BMI. It is unlikely that glucose per se is responsible for these differences because improving glycemia via lifestyle and insulin titration does not alter the serum BA concentrations in persons with poorly controlled T2D.
In our study we observed no change in total BA levels as a function of glucose tolerance. These data are consistent with the lack of association between total BA and glycemia in patients treated with BA sequestrants, both at baseline and after treatment (7). By contrast, Steiner et al (13) reported associations of secondary BA with HbA1c and total BA with HbA1c and HOMA-IR in a cohort with a spectrum of insulin sensitivity and dysglycemia. Differences in these relationships might be attributable to the differences in study population; importantly, secondary BA subspecies were not separately assessed. However, our data are consistent with a recent report showing higher levels of most free, T-BA and G-BA subsets in T2D compared with a NGT group with different levels of insulin resistance (16).
Interestingly, we observed a correlation between T-BA and multiple measures of glucose homeostasis. Although T-BAs constitute a small percentage of total BAs, it is possible that such differences in specific BA subfractions could have distinct metabolic effects. For example, more hydrophilic BAs, like T-BA, cannot activate FXR as potently (21), whereas the altered affinity of different subfractions for other receptors (eg, TGR5) could also impact systemic metabolic effects of BAs. Indeed, T-BAs demonstrate slightly increased potency as TGR5 agonists in cultured cells (22). Conversely, differences in T-BAs could also result from differences in glycemia or overall metabolic control. However, we found no change in total or T-BAs after glycemic control by lifestyle and intensification of insulin therapy. Although our cross-sectional analysis cannot conclude whether associations between T-BA and glucose homeostasis are causal in nature, our data are more suggestive of a role for BAs in modulating glycemia under physiological conditions rather than for changes in glycemia to directly modulate BAs.
We observed significant increases only in the T-BA subset in patients with T2D compared with NGT and IGT subjects. This pattern could potentially be caused by higher rates of taurine conjugation or reduced deconjugation in the gut. The gut microbial flora strongly impacts BA metabolism by performing structural modifications through deconjugation, oxidation, or hydroxylation in the intestine (1). BA conjugation with either T or G in the liver is linked to availability of these amino acid substrates. Taurine-rich food such as meat or seafood could increase concentrations of T-BA (23) and thus contribute to differences in fasting serum BAs seen in our study population. Unfortunately, detailed dietary assessments in our group are unavailable. However, serum taurine levels have been previously shown to be lower in T2D and inversely related to HbA1c (23), and glycine levels are reduced in parallel with insulin resistance (24). Thus, increased availability of taurine and glycine are unlikely to be responsible for the observed patterns in T2D; conversely, it is plausible that altered patterns of BA conjugation could contribute to differences in circulating levels of these amino acids. Alternatively, increases in taurine-conjugated BA might be related to lower rates of taurine deconjugation in the gut. Some bile salt hydrolases specifically degrade T-BA (25). One of these hydrolases is active in Bacteroidetes, a species that is reduced in the gut microbial environment of mice with either genetic or diet-induced obesity and diabetes (26). Furthermore, taurine-conjugated BA are greatly increased in the total BA pool of germ-free or antibiotic-treated rats (27, 28). Although we found no relationship between total BAs or T- or G-BA with BMI, these data suggest that alterations in microbial populations in insulin resistance could contribute to reduced taurine deconjugation. Further studies are required to determine potential relationships between serum taurine levels, gut microflora, T-BA, and glycemia.
We also demonstrate strong inverse associations of taurine-conjugated, as well as glycine-conjugated, and total BAs with the oral disposition index, which integrates fasting insulin as well as the change of insulin and glucose in the first 30 minutes after a glucose challenge (19). It is possible that the lack of association between total and G-BA and other measures of glycemia could be due to smaller magnitude of difference between groups, greater variability in concentrations, or sample size. However, the associations between total and G-BA concentrations and glycemic measures appear less robust than with taurine-conjugated fractions. Insulinotropic effects of BAs are thought to be mediated by BA activation of the TGR5 receptor in enteroendocrine cells, promoting glucagon like peptide-1 (GLP-1) secretion (29). This association suggests that total BA and subfractions could play unique roles in the modulation of incretin-dependent insulin secretion; however, it remains unknown how changes in the endogenous BA levels relate to the activation of TGR5 in the enteroendocrine cells. It is possible that high circulating fasting BA render enteroendocrine cells less sensitive to bile acids in the intestine when these levels rise upon feeding. Notably, small intestinal infusion of taurocholic acid did not change fasting GLP-1 concentrations, although postglucose GLP-1 concentrations were increased (30). We previously reported increased total BA concentrations, especially G-CDCA and G-DCA subfractions, were higher in patients with previous Roux-en-Y gastric bypass surgery, positively correlated with GLP-1, and inversely associated with postmeal glucose (31). Anatomic, dietary, and microbiota changes after a gastric bypass may impact greatly on total and relative proportions of BA subfractions, yielding distinct metabolic effects.
Interestingly, we did not find differences in postload serum BA between groups. Comparison of baseline and postload serum BA showed a decrease of total free BA and free BA subfractions in all groups, whereas there were rarely changes in the T- and G-BA. Similarly, it has been shown that free BAs decreased in a cohort of nondiabetic subjects, whereas conjugated BA levels increased (32). Other studies in NGT subjects showed an increase of G-CDCA, G-CA, and T-CDCA after a glucose challenge (6, 33) and a relationship of fasting insulin with G-CDCA (6). Similarly postload concentrations of these BA subsets are increased in our NGT and modestly in the IGT group, although these changes from baseline do not reach significance in our cohort. Despite the lack of change in T-BA during the OGTT, only total T-BAs were associated with measures of glycemia in our study.
Interindividual variability of BA concentrations is high (13) and may be confounded by differences in dietary composition and the interval between nutrient intake and sampling. We demonstrate variability comparing fasting with postglucose load samples. We also show greater variability in free compared with conjugated BAs after glucose load. Moreover, although circulating BA levels correlate with portal venous concentrations, they provide only surrogate information about the complex enterohepatic circulation and total BA pools (34), which are challenging to assess in humans. Prior studies evaluating BA concentration and composition in bile itself also yielded an inconsistent association with glycemia (10–12). Diabetic Pima Indians have reduced BA in bile after initiation of insulin treatment, suggesting insulin and/or glycemia may influence BA metabolism in this pool (11). This contrasts with the lack of change in serum BAs after intensification of glycemic control found in our study. Concomitant medications or previous cholecystectomy might also contribute to altered serum BA concentrations and to the lack of association of BA with lipids in our cohort.
FGF-19 levels increased approximately 1.6-fold after a glucose load, consistent with FGF-19 being secreted from the small intestine after feeding (35). However, FGF-19 levels were assessed only at 120 minutes after glucose load, which might not be sufficient to detect FGF-19 peak levels. FGF-19 concentrations tended to correlate with BMI. Although mouse FGF-15 and the human ortholog FGF-19 are known to participate in regulation of BA biosynthesis, gallbladder motility, enterohepatic signaling, and metabolic homeostasis (36, 37), we did not find strong relationships between FGF-19 and either fasting or postload BA levels or composition or with glycemic measures or lipid levels. These findings are concordant with a study in healthy obese, T2D, and controls in whom no relationship with glucose, insulin, HOMA-IR, or lipids was observed (38). Together these data suggest that circulating concentrations of FGF-19 may not fully reflect its complex and tissue-specific regulation of metabolism (37), and thus, links with circulating BAs are difficult to detect in a correlation analysis. In contrast, Stejskal et al (39) found a relationship between FGF-19 and glucose and lipid levels in NGT and metabolic syndrome, so it is possible this relationship is perturbed with progressive dysglycemia.
We have investigated changes in T- and G-BA concentrations across a spectrum of glycemia in the fasting and postload state. We find fasting serum T-BA and its subsets are higher in T2D, and intermediate in IGT, compared with NGT. Changes in glycemia mediated by lifestyle and intensification of insulin do not alter BAs; thus, these changes are unlikely to be secondary to dysglycemia per se. Although total and glycine conjugates are associated with the oral disposition index, only T-BAs are associated with multiple metabolic measures. Further study is needed to elucidate whether differences in levels of T-BAs play a causal role in dysregulation of metabolic control in T2D to determine potential relationships between serum taurine levels, gut microflora, and glycemia and to determine whether T-BA could be targeted for novel therapeutic approaches to treat T2D.
Acknowledgments
This work was supported by National Institutes of Health Grant DK062948 (to M.-E.P.); Grants DK036836 and DK060837 (to M.-E.P. and A.B.G.); and Grant R56DK095451 (to A.B.G. and M.W.); the Juvenile Diabetes Research Foundation (to M.-E.P. and A.B.G.); Marietta Blau Grant ICM-2010–02797 (to M.W.); an Investigator Initiated Study Grant from Daiichi Sankyo; and support for the Joslin Clinical Research Center from its philanthropic donors.
Present address for S.H.: Associate Professor, Department of Genetics and Genomic Sciences, Icahn Institute for Genomics and Multiscale Biology, Icahn School of Medicine at Mount Sinai, 1425 Madison Avenue, Box 1498, New York, NY 10029. E-mail: sander.houten@mssm.edu.
Present address for M.W.: Medical University of Vienna, Department for Medicine III, Gastroenterology and Hepatology, Waehringer Guertel 18-20, 1090 Vienna, Austria. E-mail: marlene.wewalka@meduniwien.ac.at.
Disclosure Summary: M.-E.P. had commercial support (investigator initiated grants) from Sanofi, Nuclea, and Bristol-Myers Squibb and was a consultant for Janssen and Novartis. A.B.G. had commercial support (investigator initiated grant) from Daiichi Sankyo and Nuclea. M.W., C.B., and S.M.H. have nothing to disclose.
Funding Statement
This work was supported by National Institutes of Health Grant DK062948 (to M.-E.P.); Grants DK036836 and DK060837 (to M.-E.P. and A.B.G.); and Grant R56DK095451 (to A.B.G. and M.W.); the Juvenile Diabetes Research Foundation (to M.-E.P. and A.B.G.); Marietta Blau Grant ICM-2010–02797 (to M.W.); an Investigator Initiated Study Grant from Daiichi Sankyo; and support for the Joslin Clinical Research Center from its philanthropic donors.
Footnotes
- BA
- bile acid
- BMI
- body mass index
- BP
- blood pressure
- CA
- cholic acid
- CDCA
- chenodeoxycholic acid
- DCA
- deoxycholic acid
- FGF
- fibroblast growth factor
- FXR
- farnesoid X receptor
- G
- glycine
- G-BA
- glycine-conjugated BA
- GLP-1
- glucagon like peptide-1
- HbA1c
- hemoglobin A1c
- HDL
- high-density lipoprotein
- HOMA-IR
- homeostatic model assessment of insulin resistance
- IGT
- impaired glucose tolerance
- LDL
- low-density lipoprotein
- MCA
- muricholic acid
- NGT
- normal glucose tolerance
- OGTT
- oral glucose tolerance test
- T
- taurine
- T-BA
- taurine-conjugated BA
- T2D
- type 2 diabetes
- TGR5
- G protein-coupled bile acid receptor 1 also known G-protein coupled receptor 19, membrane-type receptor for bile acids
- UDCA
- ursodeoxycholic acid.
References
- 1. Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–191. [DOI] [PubMed] [Google Scholar]
- 2. Zhang Y, Lee FY, Barrera G, et al. . Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci USA. 2006;103:1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prawitt J, Caron S, Staels B. Bile acid metabolism and the pathogenesis of type 2 diabetes. Curr Diab Rep. 2011;11:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pols TW, Noriega LG, Nomura M, Auwerx J, Schoonjans K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J Hepatol. 2011;54:1263–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Özcan U, Yilmaz E, Özcan L, et al. . Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shaham O, Wei R, Wang TJ, et al. . Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol Sys Biol. 2008;4:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brufau G, Stellaard F, Prado K, et al. . Improved glycemic control with colesevelam treatment in patients with type 2 diabetes is not directly associated with changes in bile acid metabolism. Hepatology. 2010;52:1455–1464. [DOI] [PubMed] [Google Scholar]
- 8. Herrema H, Meissner M, van Dijk TH, et al. . Bile salt sequestration induces hepatic de novo lipogenesis through farnesoid X receptor- and liver X receptor α-controlled metabolic pathways in mice. Hepatology. 2010;51:806–816. [DOI] [PubMed] [Google Scholar]
- 9. Uchida K, Makino S, Akiyoshi T. Altered bile acid metabolism in nonobese, spontaneously diabetic (NOD) mice. Diabetes. 1985;34:79–83. [DOI] [PubMed] [Google Scholar]
- 10. de Leon MP, Ferenderes R, Carulli N. Bile lipid composition and bile acid pool size in diabetes. Am J Dig Dis. 1978;23:710–716. [DOI] [PubMed] [Google Scholar]
- 11. Bennion LJ, Grundy SM. Effects of diabetes mellitus on cholesterol metabolism in man. N Engl J Med. 1977;296:1365–1371. [DOI] [PubMed] [Google Scholar]
- 12. Abrams JJ, Ginsberg H, Grundy SM. Metabolism of cholesterol and plasma triglycerides in nonketotic diabetes mellitus. Diabetes. 1982;31:903–910. [DOI] [PubMed] [Google Scholar]
- 13. Steiner C, Othman A, Saely CH, et al. . Bile acid metabolites in serum: intraindividual variation and associations with coronary heart disease, metabolic syndrome and diabetes mellitus. PLoS One. 2011;6:e25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cariou B, Chetiveaux M, Zaïr Y, et al. . Fasting plasma chenodeoxycholic acid and cholic acid concentrations are inversely correlated with insulin sensitivity in adults. Nutr Metab (Lond). 2011;8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brufau G, Bahr MJ, Staels B, et al. . Plasma bile acids are not associated with energy metabolism in humans. Nutr Metab (Lond). 2010;7:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haeusler RA, Astiarraga B, Camastra S, Accili D, Ferrannini E. Human insulin resistance is associated with increased plasma levels of 12α-hydroxylated bile acids. Diabetes. 2013;62(12):4184–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bootsma AH, Overmars H, van Rooij A, et al. . Rapid analysis of conjugated bile acids in plasma using electrospray tandem mass spectrometry: application for selective screening of peroxisomal disorders. J Inherit Metab Dis. 1999;22:307–310. [DOI] [PubMed] [Google Scholar]
- 18. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 19. Utzschneider KM, Prigeon RL, Faulenbach MV, et al. . Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care. 2009;32:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dunn W, Angulo P, Sanderson S, et al. . Utility of a new model to diagnose an alcohol basis for steatohepatitis. Gastroenterology. 2006;131:1057–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sato H, Macchiarulo A, Thomas C, et al. . Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies. J Med Chem. 2008;51:1831–1841. [DOI] [PubMed] [Google Scholar]
- 23. Hansen SH. The role of taurine in diabetes and the development of diabetic complications. Diabetes Metab Res Rev. 2001;17:330–346. [DOI] [PubMed] [Google Scholar]
- 24. Cheng S, Rhee EP, Larson MG, et al. . Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation. 2012;125:2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jones BV, Begley M, Hill C, Gahan CG, Marchesi JR. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci USA. 2008;105:13580–13585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cani PD, Bibiloni R, Knauf C, et al. . Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet—induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. [DOI] [PubMed] [Google Scholar]
- 27. Swann JR, Want EJ, Geier FM, et al. . Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci USA. 2011;108:4523–4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sayin SI, Wahlstrom A, Felin J, et al. . Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-β-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. [DOI] [PubMed] [Google Scholar]
- 29. Thomas C, Gioiello A, Noriega L, et al. . TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu T, Bound MJ, Standfield SD, Jones KL, Horowitz M, Rayner CK. Effects of taurocholic acid on glycemic, glucagon-like peptide-1, and insulin responses to small intestinal glucose infusion in healthy humans. J Clin Endocrinol Metab. 2013;98:E718–E722. [DOI] [PubMed] [Google Scholar]
- 31. Patti ME, Houten SM, Bianco AC, et al. . Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring). 2009;17:1671–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ho JE, Larson MG, Vasan RS, et al. . Metabolite profiles during oral glucose challenge. Diabetes. 2013;62:2689–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao X, Peter A, Fritsche J, et al. . Changes of the plasma metabolome during an oral glucose tolerance test: is there more than glucose to look at? Am J Physiol Endocrinol Metab. 2009;296:E384–E393. [DOI] [PubMed] [Google Scholar]
- 34. Angelin B, Björkhem I, Einarsson K, Ewerth S. Hepatic uptake of bile acids in man. Fasting and postprandial concentrations of individual bile acids in portal venous and systemic blood serum. J Clin Invest. 1982;70:724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev. 2012;26:312–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jones SA. Physiology of FGF15/19. Adv Exp Med Biol. 2012;728:171–182. [DOI] [PubMed] [Google Scholar]
- 37. Kir S, Beddow SA, Samuel VT, et al. . FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mraz M, Lacinova Z, Kavalkova P, et al. . Serum concentrations of fibroblast growth factor 19 in patients with obesity and type 2 diabetes mellitus: the influence of acute hyperinsulinemia, very-low calorie diet and PPAR-α agonist treatment. Physiol Res. 2011;60:627–636. [DOI] [PubMed] [Google Scholar]
- 39. Stejskal D, Karpisek M, Hanulova Z, Stejskal P. Fibroblast growth factor-19: development, analytical characterization and clinical evaluation of a new ELISA test. Scand J Clin Lab Invest. 2008;68:501–507. [DOI] [PubMed] [Google Scholar]