Abstract
Background:
Basal ganglia calcification (BGC) is an interesting example of ectopic calcification in patients with hypoparathyroidism. Its pathogenesis and reasons for predilection of calcification at basal ganglia are not clear.
Objective:
To assess the expression of osteogenesis-related molecules in the caudate nucleus and surface gray matter (an area spared from calcification) and discuss potential relevance of the results in context of BGC in idiopathic hypoparathyroidism.
Methods:
Caudate nucleus and gray matter were obtained from 14 autopsies performed in accidental deaths. The mRNA expression of bone transcription factors (RUNX2/osterix), bone morphogenetic proteins (BMPs) 2 and 4, osteonectin, osteopontin, osteocalcin, vitamin D receptor, calcium sensing-receptor, Na phosphate transporters (PiTs) 1 and 2, N-methyl-D-aspartate receptor 2B (NMDAR2B), carbonic anhydrase II (CA-II), PTH1 receptor (PTH1R), PTH2R, and PTHrP were assessed by RT-PCR. Western blot, spot densitometry, and immunohistochemistry were performed to assess protein expression of molecules showing differences in mRNA expression between caudate and gray tissues.
Results:
The mean mRNA expression of PiT1 (11.0 ± 10.39 vs 32.9 ± 20.98, P = .003) and PTH2R (1.6 ± 1.47 vs 13.7 ± 6.11, P = .001) were significantly lower in the caudate nucleus than the gray matter. The expression of osteonectin, osteopontin, and CA-II were significantly higher in the caudate nucleus than the gray matter (P = .01, .001, and .04, respectively). The mRNA expression of other molecules was comparable in the 2 tissues. The protein expression of both CA-II and osteonectin was 24% higher and PiT1 17% lower in caudate than the gray matter. The differences in the PTH2R and osteopontin protein expression were not appreciable.
Conclusions:
The presence of several osteogenic molecules in caudate nucleus indicates that BGC would probably be the outcome of an active process. The differences in expression of these molecules in caudate over gray matter could favor BGC at this site in the unique biochemical milieu of hypoparathyroid state.
Basal ganglia calcification (BGC) is an interesting example of ectopic calcification in patients with hypoparathyroidism (1, 2). Its pathogenesis and reasons for predilection of calcification at the basal ganglia are not clear. Recently, we reported an association of hyperphosphatemia with the presence and progression of BGC in patients with idiopathic hypoparathyroidism (3). Despite a high frequency of calcification in these patients at the basal ganglia (72%), gray-white junction (39.8%), cerebellum (31.2%), and thalamus (29.0%), none of them had calcification within the gray matter at the surface of the brain (3). A similar distribution is reported in several other disorders associated with intracranial calcifications such as osteopetrosis, type 1 diabetes, and Fahr's syndrome (4–6). Autopsies in patients with hypoparathyroidism and Fahr's disease have revealed lamellar deposition of calcium-hydroxyapatite crystals in the region of the basal ganglia (7–9). This observation coupled with association of BGC with hyperphosphatemia suggests an osteogenic mechanism in its pathogenesis. Wang et al (10) reported an inactivating mutation of the PiT2 gene in patients with familial BGC. The present study assessed the presence of osteogenic molecules in the caudate nucleus and its comparison with other areas of the brain with a lesser predilection for calcification.
In a previous study, we had suggested the possibility of differential expression of several molecular factors facilitating BGC in hypoparathyroidism (3). These factors included overexpression of calcification-promoting and underexpression of mineral-resorbing molecules. Here, we report expression profile of bone transcription factors (Runt-related transcription factor 2 [RUNX2] and osterix), bone morphogenetic proteins (BMPs) 2 and 4, osteonectin, osteopontin, osteocalcin, vitamin D receptor (VDR), calcium-sensing receptor (CaSR), phosphate transporters (PiTs) 1 and 2, N-methyl-D-aspartate receptor 2B (NMDAR2B), carbonic anhydrase-II (CA-II), PTH1 receptor (PTH1R), PTH2R, and PTHrP in human caudate nucleus. Their expression was compared with that of surface gray matter, an area usually spared from calcification.
Materials and Methods
Autopsy tissues
Caudate nucleus and gray matter of the brain were collected from 14 human autopsies conducted for sudden and accidental deaths at All India Institute of Medical Science, New Delhi. Autopsies from patients dying in the hospital and cases with history of poisoning and or those conducted more than 12 hours after death were excluded. The dissection of the caudate nuclei was carried out by an anatomist and the forensic medicine experts (T.M. and T.S.R.). Gray matter was scraped from the occipital cortex because of the maximum thickness of gray matter in this area. Caudate tissue was obtained from the head and body of the nucleus, which was identified by its location lateral to the third ventricle and caudal to the floor of the anterior horn (Figure 1A). Tissues were immediately transported from the mortuary after dissection on ice to the endocrine laboratory and stored in liquid nitrogen in multiple aliquots. The dissected tissues were examined histologically to confirm the presence of the neuronal tissues. The study protocol was approved by the institutional ethics committee, and all the tissues were collected after written informed consent of the legal heir of the deceased.
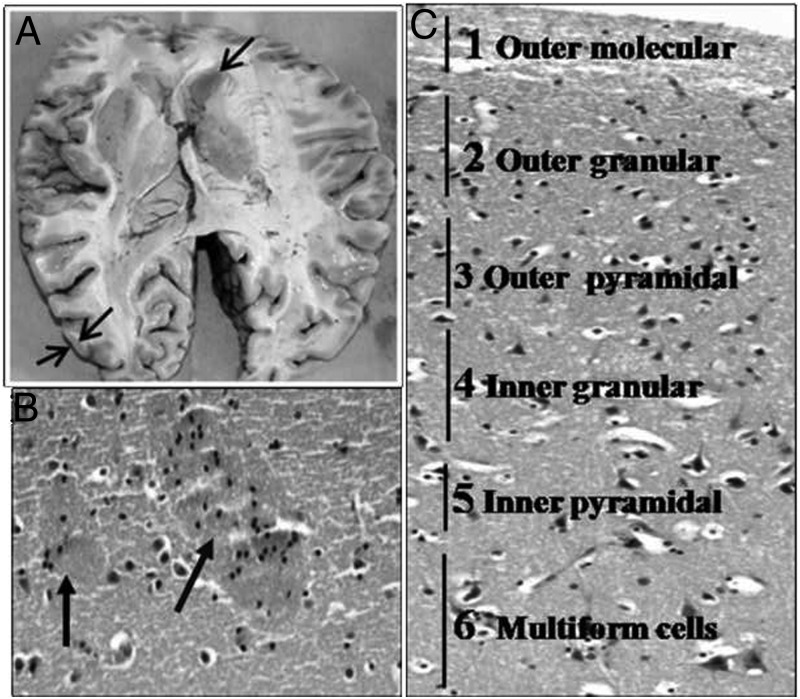
Figure 1.
A, Area showing sites of collection of gray and caudate tissue from the brain. B and C, Hematoxylin and eosin-stained photomicrographs from 2 tissues showing mature neurons embedded in a background of glial cells and neurofibrillary material with 2 patches of striatopallidal/pencil fibers (arrows) in the caudate nucleus (B) and organization of neuron into 6 layers (outer molecular, outer granular, outer pyramidal, inner granular, and multiform layer) in the gray matter (C) (magnification, ×100).
RNA isolation, cDNA preparation, and RT-PCR for gene expression analysis
Total RNA was extracted from the tissues using the RNA binding column (Eppendorf AG-22331) as described previously (11, 12). The quality of RNA was checked by presence of 28S and 18S ribosomal bands on agarose gel electrophoresis and quantified using a UV spectrophotometer (GeneQuant; Amersham). The first strand of the cDNA was prepared using random hexamers and Moloney Murine Leukemia Virus Reverse Transcriptase (RevertAidH Minus; Fermentas) and 2.0 μg of total RNA at 42°C.
The mRNA expression of molecules associated with normal bone mineral calcification, ie, RUNX2, osterix, BMP4, BMP2, VDR, PiT1, PiT2 osteonectin, osteopontin, osteocalcin, CaSR, and those suggested to be linked with BGC in hypoparathyroidism such as CA-II, NMDAR2B, PTH1R, PTH2R, and PTHrP were assessed using RT-PCR (11). The expression of dopamine- and cAMP-regulated phosphoprotein (DARPP-32) mRNA was also assessed to show its generally higher expression in human caudate nucleus than the gray tissues (13).
Table 1 shows the primers used for specific gene expression, annealing conditions used, and the size of the amplified products and their references (11, 14–25). The conditions for RT-PCR were 20-μL reaction volume with 2 μL cDNA, 3.0mM MgCl2, 0.2mM dNTP, 1.0 U Taq DNA polymerase, and 0.1μM each of forward and reverse primers. Initial denaturation was carried out at 94°C for 3 minutes, followed by 35 cycles of denaturation at 94°C for 30 seconds, annealing for 30 seconds, and extension at 72°C for 30 seconds and fluorescence capture in each cycle (Table 1). GAPDH was amplified in separate tubes along with respective genes using SYBR-I dye (Sigma) fluorescence signals on RT-PCR (CFX98 real-time system; Bio-Rad) as described earlier (11). All the reactions were done in duplicate, and the specificity of molecules amplified was checked by post-PCR melting curve analysis and 1.5% agarose gel electrophoresis. DNA sequencing of all the amplified products was also performed by eluting DNA from the agarose gel as described earlier (26). PCR for assessing mRNA expression of various molecules in the gray and caudate tissues from each of the cases were put in the same PCR plates to minimize differences related to interassay variations. The mRNA expression of each of the molecules studied was measured in relation to that of GAPDH transcripts.
Table 1.
Forward and Reverse Primers, Annealing Temperatures, and the Product Size of Various Molecules During RT-PCR
| Gene | Product, bp | Annealing Temperature (°C) | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Ref. |
|---|---|---|---|---|---|
| GAPDH | 381 | 55 | ccaaggtcatccatgacaactttggt | tgttgaagtcagaggagaccacctg | 11 |
| NMDAR2B | 465 | 60 | attggtggcagagtggattc | ggcaaaagaatcatggctgt | 14 |
| VDR | 404 | 60 | gacatcggcatgatgaagg | ctagggtcacagaagggtcatc | 11 |
| PiT1 | 410 | 55 | taccatcctcatctcggtgg | tgacggcttgactgaactgg | 15 |
| BMP2 | 349 | 55 | atggattcgtggtggaagtg | gtggagttcagatgatcagc | 16 |
| BMP4 | 399 | 55 | agcatgtcaggattagccga | tggagatggcactcagttca | 16 |
| PTHR2 | 386 | 59 | aatggagaggttcaggcaga | tctccttggcatccttcagt | 17 |
| PTHrP | 249 | 59 | ccctctcccaacacaaagaa | ggaggtgtcagacaggtggt | 18 |
| RUNX2 | 288 | 55 | ccccacgacaaccgcaccat | cactccggcccacaaatc | 19 |
| Osterix | 307 | 60 | cgggactcaacaactct | ccataggggtgtgtcat | 20 |
| Osteonectin | 291 | 55 | aacgtcctggtcaccctgta | ccaggtcacaggtctcgaa | 21 |
| Osteopontin | 148 | 55 | tggccgaggtgatagtgtg | cggggatggccttgtatg | 22 |
| CA-II | 124 | 55 | caatggtcatgctttcaacg | tccatcaagtgaaccccagt | 23 |
| CaSR | 194 | 55 | attgagggggagcccacctgct | aaagagggtgagtgcgatcccaaagg | 24 |
| Osteocalcin | 297 | 59 | atgagagccctcacactcctc | cgtagaagcgccgataggc | 25 |
| PTH1R | 361 | 55 | ggaagcccaggaaagataagg | gagtagcccacggtgtaaatc | |
| PiT2 | 388 | 55 | gtgttactaggcgccaaagtag | cagcatagaatactgggagtgc | |
| DARPP-32 | 385 | 55 | gcaccatctcaagtcgaagag | ctcacttagtgctgggtcttcc |
Western blot
Western blots were carried out to assess expression of proteins for molecules that showed significant differences in the mRNA expression between caudate and gray matter. The protein for loading in the SDS-PAGE was prepared from a 5 × 5-mm fraction each from a pair of caudate nuclei and gray tissues. Briefly, the tissues were finely cut with a sterile scalpel blade and homogenized manually in a glass douncer in 1.0 mL lysis buffer (50mM Tris and 1% SDS containing protease inhibitor cocktail). The homogenates were sonicated 3 times under ice (1 minute burst and 1 minute cooling) at 20% amplitude and pulse frequency of 5 seconds (Sonics; Vibra Cell). The protein content was measured by a UV spectrophotometer at 280 nm, and Western blot was performed using 10% SDS-PAGE with 25 μg protein loaded per lane as described earlier (12, 27). After transfer of the protein to the polyvinylidene difluoride, blots were put on incubation manifold for incubating with different primary antibodies on the same blot (Deca-probe; GE, Amersham). Primary antibodies used to detect specific proteins were various rabbit polyclonal antibodies raised against 1) 24 to 84 amino acids in the N-terminal extracellular domain of human PTH2R (SC 30005, 1:500 dilution, H60; Santa Cruz Biotechnology, Inc), 2) 191 to 260 amino acids of human CA-II (SC 25596, 1:1500 dilution; Santa Cruz), 3) middle region of PiT1 protein (catalog item 27570002, 1:500 dilution; Novus Biologicals), and 4) 21 to 145 amino acids of osteonectin protein (NBP1–80971, 1:300 dilution; Novus Biologicals). Monoclonal antibodies were used to detect osteopontin protein expression (NB110–89062, 1:500 dilution; Novus Biologicals). All the blots were developed using alkaline phosphatase-conjugated antirabbit or antimouse IgG (DAKO) and nitroblue tetrazolium-5-bromo-4-chloro-3-indolyl-phosphate substrates. The integrated density value (IDV) of the band for proteins was calculated by spot density analysis software using the α-imager (Alpha Innotech Corp) analysis system (27). The ratio of the IDV of the band was used to assess the differences in relative expression of the proteins between caudate and gray matter.
Immunohistochemistry
Caudate and gray tissues obtained from the autopsy were frozen in liquid nitrogen. Immunohistochemistry was performed on 10-μm thin cryostat sections of the tissues taken on gelatin-coated slides. The primary antibodies used for assessing PTH2R, CA-II, PiT1, osteonectin, and osteopontin protein expression were the same as described above. Sections were incubated with primary antibodies in 1:50 dilutions for 60 minutes in humidified conditions, washed with Tris-buffered saline, and incubated again for another 30 minutes with UltraVision one-step horseradish peroxidase polymer system common for detection of both antirabbit and antimouse IgG (Thermo Scientific, Lab Vision Corporation). Color was developed with, 3,3′-diaminobenzidine, and sections were counterstained with hematoxylin. Slides for both gray matter and caudate nucleus for all 5 antigens were stained in one batch along with negative controls (no primary antibody) for both the tissues. Images were taken with a bright-field microscope (Nikon Instruments Inc).
Statistical analysis
Data are shown as mean ± SD, median and interquartile range (25th–75th percentile). The pairwise comparison between the expression of various molecules in the caudate and gray matter was carried out using a 2-sample paired t test on log-transformed data. Two-tailed P < .05 was considered significant. Statistical analysis was performed using SPSS version 11.0.
Results
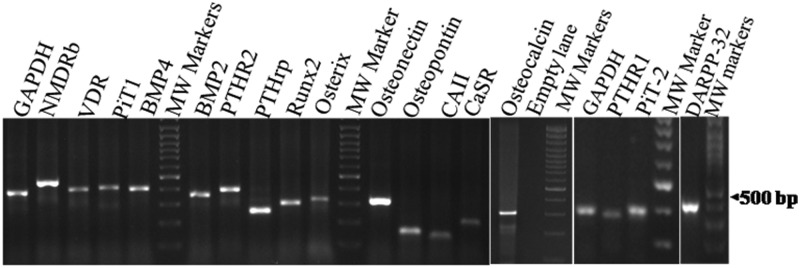
Figure 1 shows the hematoxylin and eosin staining of the caudate and gray matter obtained from a representative case showing the presence of mature neurons and glial cells in both the tissues along with organization into 6 layers in gray matter and the presence of pencil fibers (arrows) typical of the caudate nucleus (28). Expression of DARPP32 assessed in 2 subjects was higher in the caudate than in the gray matter by 2- and 2–3 fold. Sufficient RNA with intact 28S and 18S bands was obtained in 11 cases from paired caudate nucleus and gray tissues (9 males and 2 females; mean age 36.1 ± 14.5 years), and the results are reported on these cases only. The mRNA expression of PiT2 and PTH1R is reported for 5 subjects in view of the limitation of the cDNA available in paired samples from all the subjects. Figure 2 shows the specific amplification product for various molecules at endpoint RT-PCR. DNA sequencing confirmed the specificity of the molecules amplified (data not shown).
Figure 2.
Agarose gel electrophoresis showing specific amplification of various molecules during real-time PCR. MW, molecular weight.
The mean mRNA copy number of various molecules and their difference between caudate and gray tissues are given in Table 2. The mean mRNA expression of PiT1 (11.0 ± 10.39 vs 32.9 ± 20.98, P = .003) and PTH2R (1.6 ± 1.47 vs 14.3 ± 6.11, P = .005) was significantly lower in the caudate nucleus as compared with the gray matter. The differences in the expression remained significant even after Bonferroni correction for multiple testing (ie, multiplying with n = 14 molecules tested). In contrast, the expression of osteonectin, osteopontin, and CA-II were significantly higher in the caudate nucleus as compared with gray matter (corrected P values = .01, .001, and .04, respectively; Table 2). The mRNA expression of all other molecules was comparable between caudate nucleus and gray matter.
Table 2.
Comparison of mRNA Expression of Candidate Molecules Between Caudate Nuclei and Gray Matter
| Molecules | mRNA Copies/103 GAPDH |
Pa | Pb | |
|---|---|---|---|---|
| Caudate Nuclei | Gray Matter | |||
| NMDAR2B | ||||
| Mean ± SD | 194.9 ± 190.92 | 113.9 ± 147.29 | .04 | .56 |
| Median (IQR) | 132 (37–274) | 57.8 (18–177) | ||
| VDR | ||||
| Mean ± SD | 5.9 ± 8.62 | 2.2 ± 3.06 | .35 | |
| Median (IQR) | 0.48 (0.02–14.9) | 0.44 (0.1–3.0) | ||
| PiT1 | ||||
| Mean ± SD | 11.0 ± 10.39 | 32.9 ± 20.98 | .0002 | .003 |
| Median (IQR) | 6.71 (1.7–20.4) | 31.3 (13.7–59.1) | ||
| BMP4 | ||||
| Mean ± SD | 1.4 ± 1.32 | 1.4 ± 0.77 | .33 | |
| Median (IQR) | 0.84 (0.6–2.8) | 1.79 (0.7–2.1) | ||
| BMP2 | ||||
| Mean ± SD | 10.1 ± 4.94 | 9.2 ± 6.61 | .34 | |
| Median (IQR) | 9.61 (7.0–13.5) | 11.6 (2.8–13.8) | ||
| PTH2R | ||||
| Mean ± SD | 1.6 ± 1.47 | 13.7 ± 6.12 | .0001 | .001 |
| Median (IQR) | 1.23 (0.6–1.83) | 14.8 (7.7–16.3) | ||
| PTHrP | ||||
| Mean ± SD | 67.8 ± 39.37 | 71.6 ± 38.24 | .53 | |
| Median (IQR) | 78.6 (31.03–91.2) | 80.2 (33.1–115.4) | ||
| RUNX2 | ||||
| Mean ± SD | 1.4 ± 1.81 | 1.2 ± 0.79 | .72 | |
| Median (IQR) | 0.71 (0.5–1.4) | 0.76 (0.6–1.8) | ||
| Osteorix | ||||
| Mean ± SD | 0.5 ± 0.41 | 0.8 ± 0.58 | .08 | |
| Median (IQR) | 0.49 (0.3–0.7) | 0.62 (0.4–1.0) | ||
| Osteonectin | ||||
| Mean ± SD | 3920 ± 2734 | 1097 ± 1597 | .0007 | .01 |
| Median (IQR) | 3162 (2203–5063) | 576 (204–939) | ||
| Osteopontin | ||||
| Mean ± SD | 4083 ± 2197 | 859 ± 686 | .0001 | .001 |
| Median (IQR) | 3959 (2612–4362) | 555 (409–1532) | ||
| CA-II | ||||
| Mean ± SD | 270.3 ± 185.40 | 94.6 ± 68.71 | .003 | .04 |
| Median (IQR) | 205 (162–349) | 90.9 (38.5–140.6) | ||
| CaSR | ||||
| Mean ± SD | 5.0 ± 16.06 | 0.28 ± 0.55 | .48 | |
| Median (IQR) | 0.15 (0.1–0.3) | 0.11 (0.1–0.2) | ||
| Osteocalcin | ||||
| Mean ± SD | 2.7 ± 4.66 | 1.9 ± 2.80 | .37 | |
| Median (IQR) | 0.73 (0.2–2.7) | 0.50 (0.1–2.7) | ||
| PTH1R | ||||
| Mean ± SD | 0.07 ± 0.02 | 0.2 ± 0.02 | .13 | |
| Median (IQR) | 0.08 (0.05–0.09) | 0.1 (0.07–0.44) | ||
| PiT2 | ||||
| Mean ± SD | 10.9 ± 5.82 | 15.2 ± 12.86 | .40 | |
| Median (IQR) | 9.9 (5.6–16.6) | 16.1 (2.5–27.3) | ||
Abbreviation: IQR, interquartile range.
P values based on paired t test for the log-transformed values.
P values adjusted for multiple comparisons.
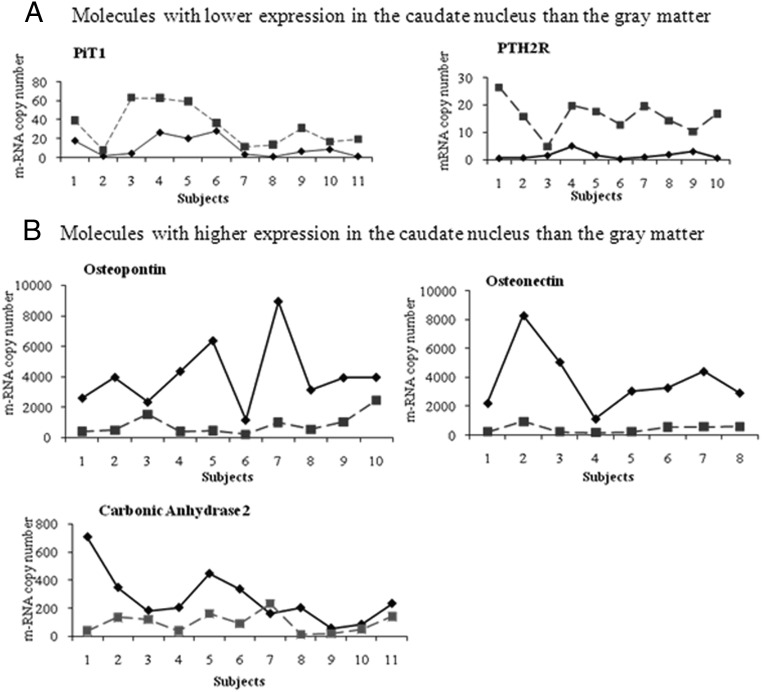
Figure 3 shows the pairwise comparison of the mRNA transcripts in the caudate nucleus and gray matter. There was consistency in the pattern of lower expression of PiT1 and PTH2R and higher expression of osteonectin, osteopontin, and CA-II in the caudate nucleus in all samples as compared with gray tissue except in 1 pair of CA-II.
Figure 3.
Pairwise comparison of mRNA expression of molecules in the caudate nucleus and gray matter (solid lines represent caudate nuclei; interrupted lines represent gray matter). Molecules with higher expression in the gray matter (A) and caudate (B).
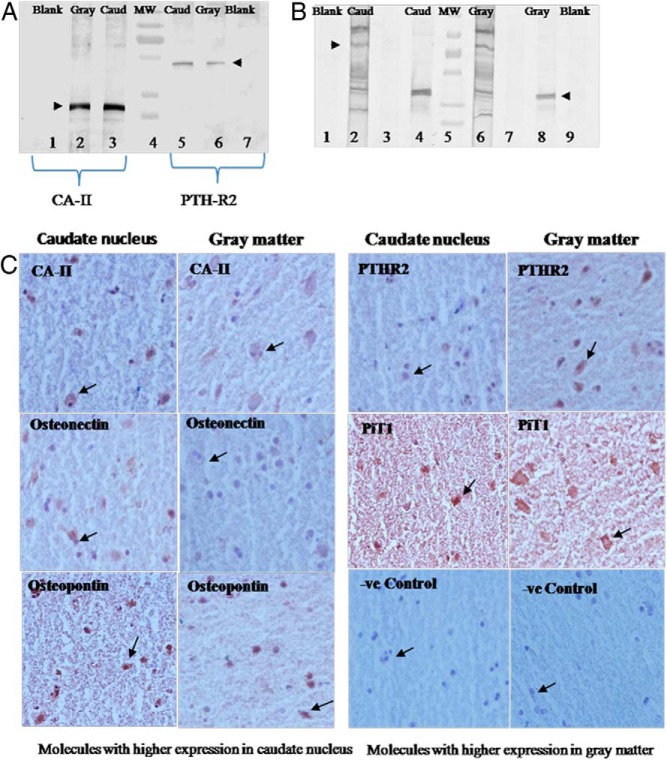
Western blot for protein expression was performed for PTH2R, CA-II, PiT1, osteopontin, and osteonectin because these showed significant differences in the mRNA expression between caudate and gray matter (Figure 4, A and B). All 5 proteins were expressed in both caudate and gray matter. However, the expression of both CA-II and osteonectin proteins as reflected in IDVs was 24% higher in the caudate tissue than the gray matter. The differences in PiT1 expression was 17% lesser in caudate than the gray matter. These differences conformed to the pattern of mRNA expression for these molecules. However, the differences in the PTH2R and osteopontin protein expression could not be appreciated on the IDVs on spot densitometry.
Figure 4.
Western blots. A, Expression of CA-II protein (lanes 2 and 3) and PTH2R (lanes 5 and 6) in the caudate and gray matter of the brain; lanes 1 and 7 are blanks for caudate and gray matter, respectively (ie, without primary antibody), and lane 4 is a molecular weight (MW) marker. B, Lanes 2 and 6 showing PiT1 protein in the caudate and gray matter, respectively; lanes 4 and 8 showing osteonectin expression; lanes 1 and 9 are blank for caudate and gray matter, respectively, and lane 5 is a molecular weight (MW) marker. C, Immunohistochemistry showing comparison of specific staining for expression of proteins in the neurons of the caudate nucleus and gray matter (magnification, ×400).
Immunohistochemistry (Figure 4C) showed specific staining for PTH2R, CA-II, PiT1, osteopontin, and osteonectin in the cytoplasm of the neuronal cells. The specific staining also confirmed the mRNA pattern obtained, with higher staining for CA-II, osteonectin, and osteopontin proteins in the caudate nucleus and higher expression of PTH2R and PiT1 protein in the gray matter.
Discussion
BGC is an interesting example of ectopic calcification observed in more than 24 disorders and can also be observed in normal subjects with aging (6). Although there is substantial information on molecular mechanisms of ectopic calcification in vessels and the aortic cusp in chronic renal disease, similar information is not available for BGC (29–32). In idiopathic hypoparathyroidism, BGC is observed in up to 70% of cases, and its progression correlates with hyperphosphatemia (3). Although hyperphosphatemia has also been implicated in the ectopic calcification in chronic kidney disease, the molecular biology of BGC is likely to be different because BGC is not common in patients with chronic renal failure (33). The results of the present study provide information on molecules that could be involved in the process of intracranial calcification and predisposition of basal ganglia to such calcifications.
Expression of osteogenesis related molecules in intracranial tissues
The mRNA expression profile of the caudate nucleus and gray matter revealed the presence of several osteogenesis-promoting molecules including BMP2 and RUNX2 molecules. BMP2 and BMP4 in vascular, bone, and aortic cusp tissues promote expression of transcription factor RUNX2 and endochondral ossification (29, 30). Similarly, molecules supporting osteogenesis in bones such as osterix, VDR, CaSR, and type III phosphate transporters (PiT1) were also expressed in caudate and gray matter. In addition, there was a high expression of matrix-associated calcification-promoting osteonectin in both tissues. The profile of mRNA expression suggests the presence of molecules favoring active osteogenesis rather than passive metastatic or dystrophic deposition of minerals. In fact, the active process of calcification is also supported by previous studies showing lamellar deposition of hydroxyapatite crystals in the media and adventitia of vessels in regions of BGC (7–9).
Molecules likely to predispose basal ganglia for calcification
To understand the predisposition of the basal ganglia for calcification over other intracranial regions, surface gray matter was selected for comparison. Calcification at the surface of the gray matter is rare and observed only in a few diseases with underlying susceptibility (34). For example, gyral calcification in Sturge-Weber syndrome and after intrathecal methotrexate/cranial radiation therapy could be explained by leptomeningeal capillary-venous malformations and chemical/physical insult, respectively. Rarity of calcification of surface gray matter is also supported by autopsy studies in patients with extensive intracranial calcification. These studies revealed calcifications at the deeper gray-white junction but not at surface of the gyri (7, 8).
The pairwise comparison of caudate nucleus and surface gray matter in all study subjects revealed interesting differences in the expression of molecules of osteogenesis and resorption. There was a 3-fold reduced mRNA expression of the PiT1 and similar increased expression of osteonectin in the caudate nucleus as compared with the gray matter. These findings assume significance in view of a recent report of inactivating mutation of the PiT2 gene in patients with familial BGC (10, 35). PiT1 and PiT2 are type III Na-phosphate transporters with ubiquitous distribution and are involved in transport of phosphorus in the body tissues, including brain cells (36). It is possible that reduced expression of PiT1 and/or PiT2 would not allow phosphate to enter the neuronal cells of the basal ganglia, pericytes, and medial vascular cells and remain trapped in the interstitium or adventitia of the vessels. Thus, in diseases associated with hyperphosphatemia such as hypoparathyroidism or PiT2 mutation, a nidus with phosphate in the center is available for calcium hydroxyapatite deposition in the matrix of the vessels around basal ganglia. The mechanism for predisposition of basal ganglia for calcification can be analogous to a 2-hit mechanism. The first hit could be constitutively reduced expression of PiT1 and increased expression of osteonectin in caudate region as seen in the present study. The second hit could either be genetic as with PiT2 mutation in familial BGC or acquired such as hyperphosphatemia in hypoparathyroidism. A combination of these 2 would synergistically facilitate calcification, especially in the basal ganglia region.
High prevalence of BGC in hypoparathyroidism
Although the above mechanism might explain BGC in idiopathic hypoparathyroidism, it would not explain the lack of predisposition to BGC in other hyperphosphatemic diseases like chronic renal failure and tumoral calcinosis (33). In this regard, an 8-fold reduced expression of PTH2R in the caudate nucleus compared with the gray matter provides an important clue. The subnormal circulating PTH in hypoparathyroidism coupled with reduced PTH2R would have a compounding effect on predisposition to BGC. Although tuberoinfundibular peptide of 39 residues (TIP-39) is a natural ligand for PTH2R, PTH can also bind to the same receptor in humans (37). On the contrary, high PTH in chronic kidney disease would have an inhibitory influence on osteogenesis in the vascular tissues (29). The importance of subnormal PTH action in facilitation of BGC is also supported by a report of reduced cAMP response after PTH injection in the cerebrospinal fluid of patients with Fahr's disease (38).
Significantly higher expression of CA-II and osteopontin mRNA and protein in the caudate than in the gray matter in the present study provides another reason for the predisposition of BGC in hypoparathyroidism. The increased prevalence of BGC in osteopetrosis with CA-II mutation indicates an important role of this protein in ossification process (4). A PTH-dependent increase in CA-II action and the resulting hydrogen ion production normally promotes mineral resorption in the bone (4). The high expression of CA-II normally observed in caudate nucleus as seen in the present study could be an example of natural adaptive response to prevent BGC. The subnormal PTH activity and resultant suppression of CA-II expression in idiopathic hypoparathyroidism would theoretically facilitate BGC. The high expression of the osteogenesis-inhibitory molecule osteopontin in the caudate region over gray matter observed in the present study could also be a natural adaptive response to protect against excessive calcification in the basal ganglia region.
In the present study, levels of NMDAR2b, CaSR, VDR, and PTHrP mRNA expression were comparable in the basal ganglia and gray matter. Thus, these molecules do not seem to be the major determining factor for the predilection of basal ganglia region to calcification. In this study, differences in their protein expression were not studied and are a limitation of the study. The inability to assess the expression of osteogenic molecules in the caudate and gray matter from subjects with BGC and lack of information on the biochemical parameters in the study subjects are other limitations. Although in the present work we have studied several factors possibly related to the process of osteogenesis in BGC, molecules such as receptor activator of nuclear factor κ-B ligand/receptors related to osteoclastogenesis, Wnt-catenin pathways, Msx transcription factor linked with membranous calcification, various calcium binding proteins, pyrophosphate, tissue nonspecific alkaline phosphatase, ectonucleotide pyrophosphatase/phosphodiesterase-1, ecto-5′-nucleotidase/CD73 enzyme, matrix Gla protein, fetuin-A, ligand for PTHR2 (ie, tuberoinfundibular peptide-39), klotho, and fibroblast growth factor-23 and its receptor were not studied. Further studies at cellular and molecular levels are needed to understand the role of these molecules in BGC
Thus, using principles of iterative hypothesis, the present study adds novel information on the presence of various osteogenic molecules in the caudate nucleus. The interplay of these factors in combination with unique biochemical abnormalities of the hypoparathyroid state can theoretically explain the increased prevalence of BGC in patients with hypoparathyroidism.
Acknowledgments
This work has been funded by an intramural grant of the All India Institute of Medical Sciences, New Delhi.
Disclosure Summary: The authors have nothing to disclose, and there is no conflict of interest among the authors.
Funding Statement
This work has been funded by an intramural grant of the All India Institute of Medical Sciences, New Delhi.
Footnotes
- BGC
- basal ganglia calcification
- BMP
- bone morphogenetic protein
- CA-II
- carbonic anhydrase II
- CaSR
- calcium-sensing receptor
- IDV
- integrated density value
- NMDAR2B
- N-methyl-D-aspartate receptor 2B
- PiT
- phosphate transporter
- PTH1R
- PTH1 receptor
- RUNX2
- Runt-related transcription factor 2
- VDR
- vitamin D receptor.
References
- 1. Sachs C, Sjöberg HE, Ericson K. Basal ganglia calcifications on CT: relation to hypoparathyroidism. Neurology. 1982;32:779–782. [DOI] [PubMed] [Google Scholar]
- 2. Rubin MR, Bilezikian JP. Hypoparathyroidism: clinical features, skeletal microstructure and parathyroid hormone replacement. Arq Bras Endocrinol Metabol. 2010;54:220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goswami R, Sharma R, Sreenivas V, Gupta N, Ganapathy A, Das S. Prevalence and progression of basal ganglia calcification and its pathogenic mechanism in patients with idiopathic hypoparathyroidism. Clin Endocrinol (Oxf). 2012;77:200–206. [DOI] [PubMed] [Google Scholar]
- 4. Ohlsson A, Cumming WA, Paul A, Sly WS. Carbonic anhydrase II deficiency syndrome: recessive osteopetrosis with renal tubular acidosis and cerebral calcification. Pediatrics. 1986;77:371–381. [PubMed] [Google Scholar]
- 5. Reske-Nielsen E, Lundbæk K, Rafaelsen OJ. Pathological changes in the central and peripheral nervous system of young long-term diabetics. I. Diabetic encephalopathy. Diabetologia. 1965;1:233–344. [DOI] [PubMed] [Google Scholar]
- 6. Bonazza S, La Morgia C, Martinelli P, Capellari S. Strio-pallido-dentate calcinosis: a diagnostic approach in adult patients. Neurol Sci. 2011;32:537–545. [DOI] [PubMed] [Google Scholar]
- 7. Norman RM, Urich H. The influence of a vascular factor on the distribution of symmetrical cerebral calcifications. J Neurol Neurosurg Psychiatry. 1960;23:142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miklossy J, Mackenzie IR, Dorovini-Zis K, et al. Severe vascular disturbance in a case of familial brain calcinosis. Acta Neuropathol. 2005;109:643–653. [DOI] [PubMed] [Google Scholar]
- 9. Eaton ML, Camp JD, Love JG. Symmetric cerebral calcification, particularly of the basal ganglia, demonstrable roentgenographically calcification of the finer cerebral blood vessels. Arch Neurol Psychiatry. 1939;41:921–942. [Google Scholar]
- 10. Wang C, Li Y, Shi L, et al. Mutations in SLC20A2 link familial idiopathic basal ganglia calcification with phosphate homeostasis. Nat Genet. 2012;44:254–256. [DOI] [PubMed] [Google Scholar]
- 11. Goswami R, Mondal AM, Tomar N, et al. Presence of 25(OH)D deficiency and its effect on vitamin D receptor mRNA expression. Eur J Clin Nutr. 2009;63:446–449. [DOI] [PubMed] [Google Scholar]
- 12. Tomar N, Gupta N, Goswami R. Calcium-sensing receptor autoantibodies and idiopathic hypoparathyroidism. J Clin Endocrinol Metab. 2013;98:3884–3891. [DOI] [PubMed] [Google Scholar]
- 13. Brené S, Lindefors N, Ehrlich M, et al. Expression of mRNAs encoding ARPP-16/19, ARPP-21, and DARPP-32 in human brain tissue. J Neurosci. 1994;14:985–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee MC, Ting KK, Adams S, Brew BJ, Chung R, Guillemin GJ. Characterisation of the expression of NMDA receptors in human astrocytes. PLoS One. 2010;5:E14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li X, Yang HY, Giachelli CM. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res. 2006;98:905–912. [DOI] [PubMed] [Google Scholar]
- 16. Kochanowska I, Chaberek S, Wojtowicz A, et al. Expression of genes for bone morphogenetic proteins BMP-2, BMP-4 and BMP-6 in various parts of the human skeleton. BMC Musculoskelet Disord. 2007;8:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bagó AG, Palkovits M, Usdin TB, Seress L, Dobolyi A. Evidence for the expression of parathyroid hormone 2 receptor in the human brainstem. Ideggyogy Sz. 2008;61:123–126. [PMC free article] [PubMed] [Google Scholar]
- 18. Feeley BT, Liu NQ, Conduah AH, et al. Mixed metastatic lung cancer lesions in bone are inhibited by noggin overexpression and Rank:Fc administration. J Bone Miner Res. 2006;21:1571–1580. [DOI] [PubMed] [Google Scholar]
- 19. Kanno T, Takahashi T, Ariyoshi W, Tsujisawa T, Haga M, Nishihara T. Tensile mechanical strain up-regulates Runx2 and osteogenic factor expression in human periosteal cells: implications for distraction osteogenesis. J Oral Maxillofac Surg. 2005;63:499–504. [DOI] [PubMed] [Google Scholar]
- 20. Huang L, Teng XY, Cheng YY, Lee KM, Kumta SM. Expression of preosteoblast markers and Cbfa-1 and Osterix gene transcripts in stromal tumour cells of giant cell tumour of bone. Bone. 2004;34:393–401. [DOI] [PubMed] [Google Scholar]
- 21. Bonanno G, Perillo A, Rutella S, et al. Clinical isolation and functional characterization of cord blood CD133+ hematopoietic progenitor cells. Transfusion. 2004;44:1087–1097. [DOI] [PubMed] [Google Scholar]
- 22. Kadkol SS, Lin AY, Barak V, et al. Osteopontin expression and serum levels in metastatic uveal melanoma: a pilot study. Invest Ophthalmol Vis Sci. 2006;47:802–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yoshiura K, Nakaoka T, Nishishita T, et al. Carbonic anhydrase II is a tumor vessel endothelium-associated antigen targeted by dendritic cell therapy. Clin Cancer Res. 2005;22:8201–8207. [DOI] [PubMed] [Google Scholar]
- 24. Cañadillas S, Canalejo A, Santamaría R. Calcium-sensing receptor expression and parathyroid hormone secretion in hyperplastic parathyroid glands from humans. J Am Soc Nephrol. 2005;16:2190–2197. [DOI] [PubMed] [Google Scholar]
- 25. Gronthos S, Zannettino AC, Hay SJ, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116(Pt 9):1827–1835. [DOI] [PubMed] [Google Scholar]
- 26. Tomar N, Bora H, Singh R, et al. Presence and significance of a R110W mutation in the DNA-binding domain of GCM2 gene in patients with isolated hypoparathyroidism and their family members. Eur J Endocrinol. 2010;162:407–421. [DOI] [PubMed] [Google Scholar]
- 27. Goswami R, Brown EM, Kochupillai N, et al. Prevalence of calcium sensing receptor autoantibodies in patients with sporadic idiopathic hypoparathyroidism. Eur J Endocrinol. 2004;150:9–18. [DOI] [PubMed] [Google Scholar]
- 28. Hagan CE, Bolon B, Keene CD. Nervous system. In: Treuting PM, Dintzis SM, Frevert CW, Montine KS, eds. Comparative Anatomy and Histology: A Mouse and Human Atlas. 1st ed San Diego, CA: Academic Press; 2012:339–394. [Google Scholar]
- 29. Vattikuti R, Towler DA. Osteogenic regulation of vascular calcification: an early perspective. Am J Physiol Endocrinol Metab. 2004;286:E686–E696. [DOI] [PubMed] [Google Scholar]
- 30. Chester AH. Molecular and cellular mechanisms of valve calcification. Aswan Heart Centre Sci Pract Ser. 2011:4. [Google Scholar]
- 31. Doherty TM, Asotra K, Fitzpatrick LA, et al. Calcification in atherosclerosis: bone biology and chronic inflammation at the arterial crossroads. Proc Natl Acad Sci U S A. 2003;100:11201–11206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ketteler M, Floege J. Calcification and the usual suspect phosphate: still guilty but there are other guys behind the scenes. Nephrol Dial Transplant. 2006;21:33–35. [DOI] [PubMed] [Google Scholar]
- 33. Savazzi GM, Cusmano F, Musini S. Cerebral imaging changes in patients with chronic renal failure treated conservatively or in hemodialysis. Nephron. 2001;89:31–36. [DOI] [PubMed] [Google Scholar]
- 34. Kırolu Y, Callı C, Karabulut N, Oncel C. Intracranial calcifications on CT. Diagn Interv Radiol. 2010;16:263–269. [DOI] [PubMed] [Google Scholar]
- 35. Chen WJ, Yao XP, Zhang QJ, et al. Novel SLC20A2 mutations identified in southern Chinese patients with idiopathic basal ganglia calcification. Gene. 2013;529:159–162. [DOI] [PubMed] [Google Scholar]
- 36. Miyamoto K, Haito-Sugino S, Kuwahara S, et al. Sodium-dependent phosphate cotransporters: lessons from gene knockout and mutation studies. J Pharm Sci. 2011;100:3719–3730. [DOI] [PubMed] [Google Scholar]
- 37. Usdin TB, Gruber C, Bonner TI. Identification and functional expression of a receptor selectively recognizing parathyroid hormone, the PTH2 receptor. J Biol Chem. 1995;270:15455–15458. [DOI] [PubMed] [Google Scholar]
- 38. Smits MG, Gabreels FJ, Froeling PG, de Abreu RA, Thijssen HO, Renier WO. Calcium-phosphate metabolism in autosomal recessive idiopathic strio-pallido-dentate calcinosis and Cockayne's syndrome. Clin Neurol Neurosurg. 1983;85:145–153. [DOI] [PubMed] [Google Scholar]