Abstract
Context:
Clinical evidence supports a role for progestins in the growth of leiomyomata (fibroids). The mechanism(s) for this is thought to involve gene regulation via the nuclear progesterone receptors. Recently a mitochondrial progesterone receptor (PR-M) has been identified with evidence of a progesterone/progestin-dependent increase in cellular respiration. This observation raises a possible new mechanism whereby progesterone/progestin may affect the growth of fibroids.
Objective:
The goals of this research were to determine differential expression of PR-M in normal myometrium compared with the edge of a fibroid within the same uterus, to demonstrate a progestin-dependent increase in mitochondria membrane potential using an immortalized human myometrial cell line and to examine mitochondrial membrane potential in transfected cells expressing the complete coding sequence of PR-M.
Design:
Protein levels of PR-M, PR-B, PR-A, mitochondrial porin, and glyceraldehyde-3-phosphate dehydrogenase were determined in the myometrium and adjacent edge of a fibroid in 10 subjects undergoing hysterectomy for benign indications. Mitochondrial membrane potential was determined by fluorescent emission of 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolecarbocyanide iodine in hTERT-HM cells treated with R5020 and in transfected hTERT-HM cells determined by the fluorescent emission of tetramethylrhodamine methyl ester.
Results:
Higher levels of PR-M and mitochondrial porin were found in the fibroid edge compared with adjacent myometrium. Progestin increased mitochondrial membrane potential in hTERT-HM cells, which was not affected by a translation inhibitor. This effect was exaggerated in hTERT-HM cells expressing PR-M after transient transfection.
Conclusion:
These studies suggest a mechanism whereby progesterone/progestin may affect the growth of fibroids by altering mitochondrial activity.
Fibroids are mechanically stiff, fibrotic tumors with increased water content. They are not only composed of cells but contain a varied, increased amount of structurally altered extracellular matrix proteins and glycoproteins compared with the myometrium (1, 2). The result of these alterations in the fibroid cell microenvironment is increased mechanical stress (3) and altered cell signaling. Interestingly, there is an attenuated response of fibroid cells to mechanical signaling clues (4). Furthermore, apoptosis of fibroid cells is down-regulated (5).
The influence of estrogen and progesterone on the growth and development of fibroid tumors continues to be a field with conflicting data. Epidemiological data suggest sex steroids do not cause fibroids, although they appear to contribute to fibroid growth in a paracrine manner (6). Studies actually show a protective effect with an increasing number of term pregnancies, increasing duration of oral contraceptive use and smoking (7, 8). These observations are contradictory with regard to estrogen levels being high in the first two cases but low in the latter (9). There is more convincing evidence that a decrease in estrogen and/or progesterone decreases the growth of fibroids. Hypoestrogenemia induced by a GnRH agonist (10) or a GnRH antagonist (11) significantly decreases myometrial and fibroid volume. A GnRH agonist also reduces fibroid volume by inducing changes in osmotic gene expression and fluid volume (12). The use of an aromatase inhibitor also results in diminution of fibroid size (13). Hypoestrogenemia results in a lack of progesterone because ovulation is inhibited. Yet progesterone or at least synthetic progestins play a role in fibroid growth independent of estrogen. The addition of medroxyprogesterone acetate to the GnRH agonist treatment obviates the decrease in the uterine volume (14). The blocking of progesterone receptors with mifepristone (RU 486) results in a significant decrease in the size of fibroids (15) as does treatment with selective progesterone receptor modulators (16).
These clinical observations have led to basic science investigations of the role of progesterone/progestin in the growth of myometrium and fibroids. Estrogen receptor (ER)-α, ERβ, progesterone receptor (PR)-A and PR-B have been identified in myometrium and fibroids (17, 18). In general, expression of ER and PR receptors are greater in fibroids compared with myometrium but with substantial heterogeneity. In a histological study of tumor samples and adjacent myometrium from women of different ethnicities (black, Asian, Hispanic, white), PR-A was up-regulated in 52.5%, down-regulated in 24.2%, and with no change in 15.8% of tumors compared with myometrium. PR-A levels were twice that of ERα, and greater PR-A levels were found in the fibroids of blacks compared with other ethnicities. Proliferation rates, as determined by proliferating cell nuclear antigen staining, were higher in fibroids compared with myometrium (19, 20). Primary culture of fibroid cells shows a different response to progesterone compared with normal myometrial cells. Progesterone treatment, in the absence of estradiol, increased proliferating cell nuclear antigen, epidermal growth factor, and B cell lymphoma 2 protein expression by Western blot analyses, which was not seen in normal myometrial cells (19). These observations support a proliferation/survival role for progesterone in fibroids.
The main investigation of progesterone action focuses on direct gene regulation via the nuclear progesterone receptors (nPR) A and B. In contrast, this work focuses on the role of a recently identified mitochondrial progesterone receptor (PR-M) in the growth of fibroids. Progestin action via PR-M increases cellular respiration (21). We believe that progestin induced increase in cellular energy production may provide a mechanism for the growth of fibroids. In this paper we show a greater expression of PR-M protein in the edge of fibroids compared with adjacent myometrium. Additional studies are performed with an immortalized human myometrial cell line (hTERT-HM) demonstrating a progestin-dependent increase in mitochondrial membrane potential (ψm).
Materials and Methods
Cells
Human telomerase reverse transcriptase human myometrial cells (hTERT-HM; gift of William Rainey, PhD) were grown in DMEM:nutrient mixture F12 (DMEM/F12; Sigma-Aldrich) with 10% fetal bovine serum (FBS; Thermoscientific) and antimicrobials of penicillin, streptomycin, and amphotericin B (Invitrogen) at 37°C in 5% CO2. Myometrial cells were obtained from a deidentified woman undergoing hysterectomy for benign indications. For primary myometrial cells, normal myometrium tissue was incubated in HEPES (Sigma-Aldrich) balanced salt solution without magnesium or calcium and digested with 0.1% type I collagenase (Sigma-Aldrich) in serum-free culture medium. The cells were cultured in DMEM/F12 medium with 10% FBS and antimicrobials at 37°C in 5% CO2. Primary myometrial cells prior to passage were used for real-time RT-PCR and Western blot analysis. T47D breast cancer cells, characterized by the expression of ERα and PR, were grown in RPMI 1640 supplemented with 2 mM L-glutamine, 0.2 IU/mL bovine insulin (Invitrogen), and 10% FBS at 37°C in 5% CO2.
Tissue
Myometrial and fibroid tissue were obtained from 10 women aged 39–49 years undergoing hysterectomy for benign disease. Five subjects, recruited from Duke University Hospital, signed an institutional review board-approved consent for tissue procurement. Deidentified tissues from the other five subjects were obtained from the Cooperative Human Tissue Network, which is funded by the National Cancer Institute. Other investigators may have received specimens from the same subjects. Typically, 2–6 g of tissue from each site was obtained. Based on the presence of cyclic menses, nine women were premenopausal and one was menopausal. Ten paired samples of the outer edge of a fibroid (FE) and adjacent myometrium (MA) were obtained. Tissues were flash frozen in liquid nitrogen, transported to the laboratory on dry ice, and stored at −80°C. Characteristics of the subjects are found in Supplemental Methods, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org.
Western blot analysis
Western blot analyses were used to compare protein levels for nuclear PR-A and PR-B, PR-M, and mitochondrial porin (voltage dependent anion channel 1) in the edge of fibroids and adjacent myometrium. Frozen fibroid and myometrial tissue were pulverized with a stainless steel biopluverizer chilled in liquid nitrogen. Total protein was isolated by homogenization and sonication of pulverized tissue or cells in radioimmunoprecipitation assay buffer with protease inhibitors. Total protein concentration was determined by a detergent compatible protein assay kit (Bio-Rad Laboratories). For Western blot analysis, 80–100 μg protein was separated on a 4%–15% or 10% polyacrylamide gel in Tris/glycine/sodium dodecyl sulfate buffer and transferred to polyvinylidene difluoride membrane in Tris/glycine buffer. The membrane was blocked with 5% milk for 1 hour at room temperature and incubated overnight at 4°C with primary antibody (Supplemental Materials).
After the primary antibody, membranes were hybridized with the appropriate antirabbit or antimouse peroxidase-conjugated secondary antibody for 1 hour at room temperature. The membrane was developed with enhanced chemiluminescent reagent (Amersham) per the manufacture's recommendation and exposed to film. Western blot analyses of protein from the 10 subjects were performed. Four Western blots were performed with protein from subjects 1–5, and five Western blots were performed with protein from subjects 6–10. Each Western blot was sequentially analyzed with a PR antibody, porin antibody, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody. Hybridization for PR in samples from subjects 1–5 was performed with the MAB 462 antibody, whereas in samples from subjects 6–10, the C-19 antibody was used. Western blots were analyzed semiquantitatively by densitometry performed on an AlphaImager 2200 scanner (Alpha Innotech). The images were scanned to obtain integrated density value (IDV). The IDV of PR-M, PR-A, PR-B, and porin was corrected by the IDV value of GAPDH (22). Within each subject, bands were considered suitable for analysis if both the FE and MA bands were present, at the correct size, had clear margins, and were not missing density due to incomplete transfer.
RNA isolation, real-time RT-PCR, and sequencing
RT-PCR was used to identify the PR-M transcript and compare levels in hTERT-HM cells and primary myocytes. Total RNA was isolated from hTERT-HM, primary myometrial, and T47D cells with TRIzole reagent (Invitrogen). Total RNA (1 μg) was used for first-strand cDNA synthesis by SuperScript III reverse transcripase with an oligo(deoxythymidine) primer. SYBR Green real-time PCR was performed for amplifying PR-M, nuclear PR-A and PR-B, and GAPDH cDNA. Melt curve analysis was performed on each assay in addition to three negative controls including the absence of RNA in first-strand synthesis, the absence of cDNA, and the absence of polymerase enzyme. Data were analyzed by the 2-ΔΔ cycle threshold method in which the T47D reaction is the reference and the GAPDH reaction is the control. A single primer extension reaction was performed followed by two real-time PCR experiments on different days. Within each real-time PCR experiment, determinations were made in duplicate. All products were verified by sequence analysis (Supplemental Materials).
Non-real-time RT-PCR was also used to analyze PR-M and GAPDH transcript levels in transfected and nontransfected hTERT-HM cells. PCR was performed for 40 cycles using the same parameters as described for real-time PCR. PCR products were separated by 1.2% agarose gel electrophoresis and visualized under UV light after ethidium bromide staining.
Vector creation and transfection
Transient expression of PR-M in hTERT-HM cells was performed to further define the action of this protein. Two plasmids were engineered to yield the complete coding sequence of PR-M (23) with a preceding Kozak sequence and a control plasmid without protein production. Plasmid enhanced green fluorescent protein-amino terminus (pEGFP-N1; CLONTECH), characterized by a cytomegalovirus promoter, was used as the core vector. The enhanced green fluorescent protein coding sequence was removed with BamH1/Not1 digestion, whereas the coding sequence for PR-M was removed with the same enzymes from pTRE-TIGHT-PR-M (CLONTECH) (21). After gel purification, the insert was ligated into the vector with T4 DNA Ligase (New England BioLabs) and propagated with transformation in One Shot Top 10 Escherichia coli (Invitrogen). After a miniplasmid preparation, the complete coding sequence of the PR-M insert was verified by sequence analysis. For the control vector, the enhanced green fluorescent protein coding sequence was removed with BamH1/Not1 digestion. A 953-bp insert was created from the kanamycin/neomycin resistance sequence by PCR, incorporating a BamH1 sequence on the 5′ end and a Not1 sequence on the 3′ end (see Supplemental Methods for primer sequences). After enzyme digestion of the PCR product and gel purification, the insert was ligated as above.
hTERT-HM cells were transfected with the PR-M expressing or control plasmids with Lipofectamine 2000 (Invitrogen) in basal DMEM/F12 medium without serum or antibiotics at 80% confluence in six-well plates. Serum-containing media were added at 4–6 hours after transfection.
Mitochondrial membrane potential assay (ψm)
Progestin-induced changes in ψm were determined in nontransfected and transfected hTERT-HM cells to define an action of PR-M. ψm was determined with two fluorometric dyes, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolecarbocyanide iodine (JC-1) dye and tetramethylrhodamine methyl ester (TMRM) (Invitrogen). Nontransfected hTERT-HM cells (50 000/well) were seeded into Costar 48-well plates (Corning). At approximately 80% confluence, cells were placed in modified Krebs-Ringer-HEPES buffer containing 25 mM Na-HEPES, 115 mM NaCl, 5 mM KCl, 1 mM KH2PO4, 1.2 mM MgSO4, 0.5 mM CaCl2, and 5 mM glucose at pH 7.4 for 2 hours at 37°C before experimentation. Cells were then treated with varying doses of R5020 (promegestone; PerkinElmer) for 60 minutes followed by a 10-minute incubation with 3.75 μM JC-1. Using a Tecan Safire multichannel plate reader, cells were excited at 488 nm (band width 12 nm), and emission was determined at 529 nm (band width 5 nm) and 585 nm (band width 5 nm). Fluorescence was read from the bottom. To determine the possible interaction of a glucocorticoid receptor, cells were similarly treated with varying doses of dexamethasone. Experiments were also performed in the presence of cycloheximide (CHX). Cells were pretreated with CHX at 5 μg/mL for 2 hours while in buffer in addition to the 60 minutes with ligand. Control reactions included treatments with ADP, A23187 (Calcimycin), and carbonyl cyanide m-chlorophenyl hydrazine (CCCP; Sigma-Aldrich).
ψm was determined with TMRM in hTERT-HM cells 72 hours after transfection. Cells were treated in basal DMEM/F12 medium with varying doses of R5020 for 60 minutes. Twenty nanomoles of TMRM was then added for a 10-minute incubation at 37°C in a CO2-free incubator. Cells were then rinsed in PBS, trypsinized with 0.25% trypsin-EDTA, and placed in a 96-well Nunclon Surface black plate (Nalge Nunc) at 43 000 cells/well. Cells were immediately evaluated using a Tecan Safire multichannel plate reader with excitation at 544 nm (band width 12 nm) and emission at 630 nm (band width 12 nm) wavelength. Fluorescence was read from the top using optimum gain with Z-position calculated from the first well. Control included treatment with 10 μM oligomycin A.
Statistical analysis
Statistical tests were all performed at the two-sided α = .05 level. All statistical analyses were performed using IBM SPSS version 20. Densitometry values for Western analyses of fibroid edge and adjacent myometrium were normally distributed and evaluated with a paired t test. The relationship between protein expressions was determined with Spearman rank-order correlation coefficient. A repeated-measures ANOVA was performed to compare the effect of treatment and time (day of treatment) on ψm values (within subjects variable) of cells treated with various ligands and day by treatment interaction. Pairwise comparisons for treatment were performed with a Tukey honestly significant difference. Nonnormal ψm values were transformed to a log base 10 value to obtain a normal distribution prior to statistical analysis. The ψm values for transfected cells were transformed to a natural log value to obtain a normal distribution. The same treatment was compared between recombinant PR-M (rPR-M) transfected and control transfected cells by paired t testing. Real-time PCR fold-change values for nPR and PR-M in hTERT-HM and primary myometrial cells were compared with an independent t test. Results are reported of the statistical parameter with the degrees of freedom in parentheses.
Results
Expression of PR-M in the edge of fibroids and the adjacent myometrium
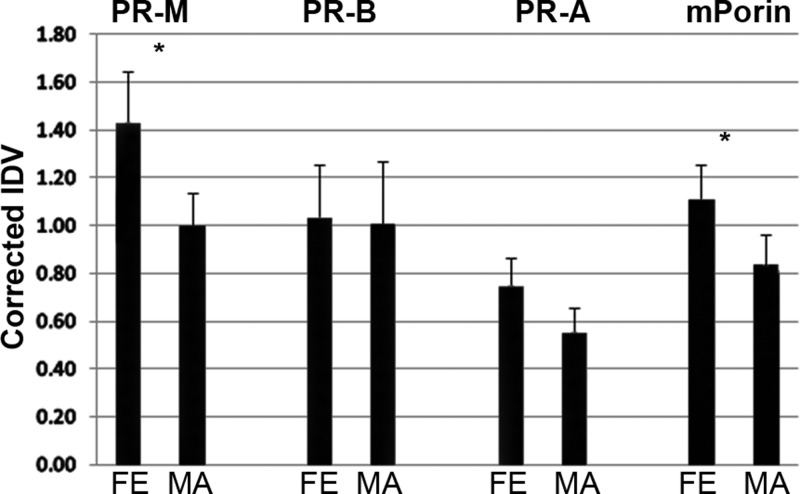
Figure 1 shows PR-M, nPR, and mitochondrial porin protein levels in 10 paired samples of protein from the FE and MA of 10 women undergoing hysterectomy. The mean levels of PR-M [t(9) = 3.67, P = .005] and porin [t(9) = 2.24, P = .052] were higher in the FE compared with the MA, whereas the level of PR-A approached statistical significance [t(9) = 2.19, P = .056]. There was no difference in the PR-B levels [t(9) = 0.24, P = .815]. Individual differences showed higher levels of PR-M in the FE in 9 of 10 samples, higher levels of porin in the FE in 8 of 10 samples, and higher levels of PR-A in the FE in 7 of 10 samples. Interestingly, the only patient showing substantially higher levels of PR-M in the MA compared with the FE was menopausal (Supplemental Figures 1 and 2). There was a significant correlation between PR-M in the FE and MA [r(18) = 0.86, P = .001], between PR-B in the FE and MA [r(18) = 0.90, P < .001], and between PR-A in the FE compared with the MA [r(18) = 0.67, P = .04]. The correlation between porin in the FE and MA did not reach statistical significance [r(18) = 0.56, P = .09].
Figure 1.
Western blot analyses of PR-M, nPR, and mitochondrial porin in the FE and MA. Ten paired samples of FE and MA showed higher levels of PR-M protein in the FE vs MA (P = .005) and porin protein in the FE vs MA (P = .052). A higher level of PR-A in the FE vs MA approached statistical significance (P = .056), whereas there was no difference in PR-B (P = .815). For PR, samples from subjects 1–5 were hybridized with a monoclonal antibody to the HBD (MAB 462), whereas samples from subjects 6–10 were hybridized with a polyclonal antibody to the HBD (C-19). PR bands include PR-B (118 kDa), PR-A (92 kDa), and PR-M (38 kDa). Individual differences are shown in Supplemental Figure 2. All results are expressed as mean ± SEM.
Progestin-dependent increase in ψm in untransfected hTERT-HM cells
The presence of a transcript for PR-M was verified in human myometrial cells and an immortalized human myometrial cell line (hTERT-HM) (24) using real-time RT-PCR with primers directed to the 5′ untranslated region (UTR) and second exon of PR-M. Because the 5′ UTR sequence is unique to PR-M, the RT-PCR would not identify PR-A and PR-B with these primers (Supplemental Methods and Supplemental Figure 3). Similar levels of transcripts for PR-M [t(2) = −0.84, P = .49] and PR-A and PR-B [t(2) = −3.02, P = .097] were seen in the hTERT-HM cells and myometrial cells. Western blot analysis demonstrated a 38-kDa protein consistent with PR-M (23), a 118-kDa band consistent with PR-B, and a 92-kDa band consistent with PR-A in hTERT-HM and myometrial cells. A 60-kDa band, commonly seen with antibodies to the hormone-binding domain (HBD) of PR and referred to as PR-C, was also identified. The fidelity of this band continues to be debated (25, 26).
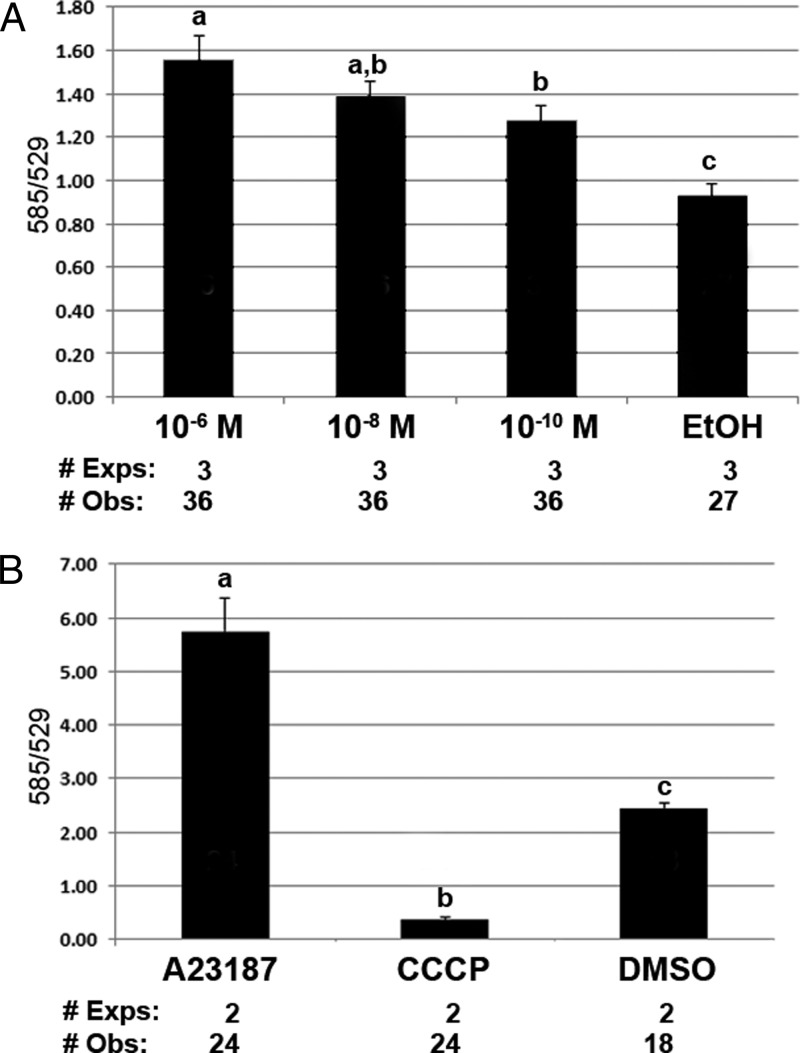
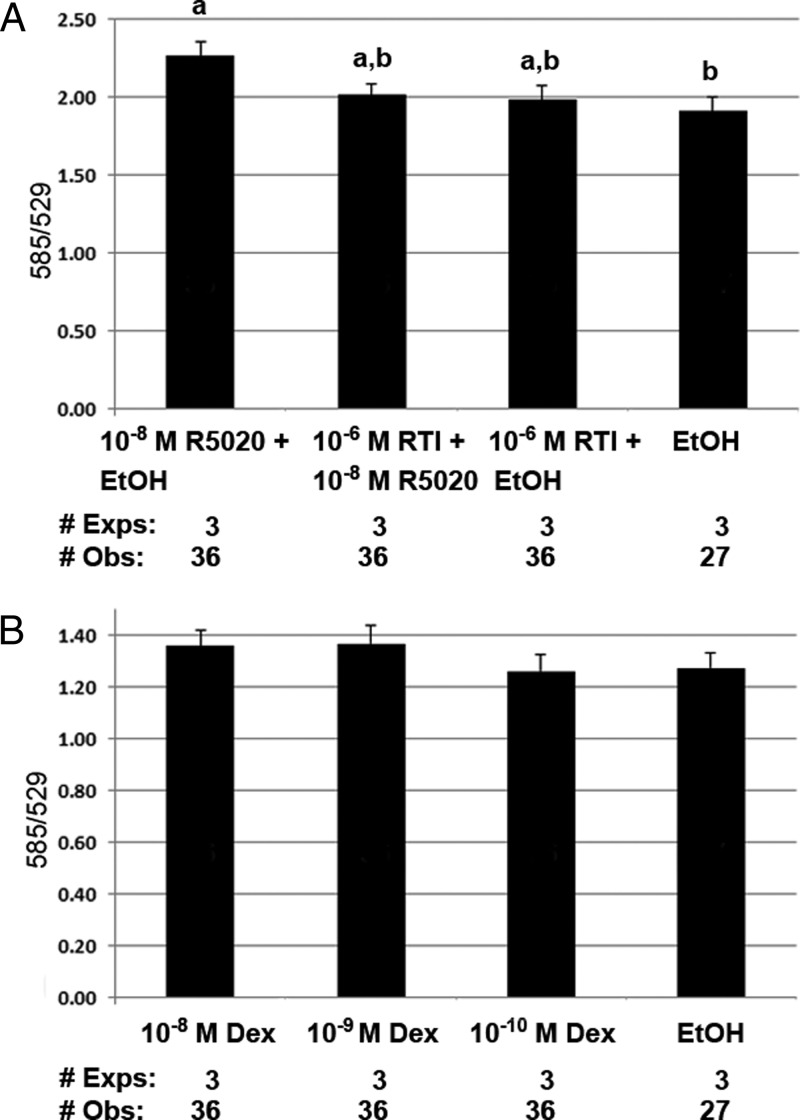
hTERT-HM cells showed high levels of α-smooth muscle actin, characteristic of a muscle cell line (Supplemental Figure 4). Figure 2A shows an increase in ψm with a 60-minute treatment of varying doses of the progestin R5020 [F(1.7, 38) = 24.6, P < .001]. ψm was determined by the fluorescent emission at 585 and 529 nm of JC-1. An increase in 585/529 is consistent with mitochondrial membrane hyperpolarization, whereas a decrease in 585/529 suggests depolarization. Figure 2B shows control reactions with an increase in ψm after treatment with the calcium ionophore, A23187, and a decrease in ψm after treatment with the proton pump uncoupler CCCP [F(2, 32) = 419, P < .001]. Figure 3A evaluates inhibition of the R5020 mediated increase in ψm with the PR antagonist RTI-6413–049b (27). Compared with RU-486, RTI-6413–049b lacks a PR agonist effect; does not bind androgen receptor, ERα, and ERβ; and has a much lower antiglucocorticoid effect. Although an overall treatment effect was seen [F(3, 72) = 4.0, P = .01], the inhibition of R5020-induced ψm did not reach statistical significance (P = .125). Additional studies excluded a possible role of the glucocorticoid receptor (GR) in this reaction. Figure 3B shows no change in ψm in cells treated for 60 minutes with the potent glucocorticoid dexamethasone [F(3, 72) = 2.6, P = .058].
Figure 2.
Changes in ψm with progestin (R5020) treatment in hTERT-HM cells. A, A significant effect of R5020 treatment for 60 minutes was seen (P < .001). Pairwise comparisons of an increase in ψm include the following: P < .001, 10−6 M vs EtOH; P < .001, 10−8 M vs EtOH; P = .006, 10−10 M vs EtOH; and P = .006, 10−6 M vs 10−10 M. A significant interaction was seen between day of treatment and ψm (P = .004). B, Control reactions show an increase in ψm with the calcium ionophore A23187 and a decrease in ψm with the protonophore CCCP compared with dimethylsulfoxide (DMSO) vehicle. A significant effect of treatment was seen (P < .001). All pairwise comparisons were significant (P < .001). A significant interaction was seen between day of treatment and ψm (P = .002). All results were expressed as mean ± SEM. Treatments with different superscripts are significantly different. Exps, number of experiments done on different days; Obs, total number of observations in all experiments.
Figure 3.
Inhibition of progestin (R5020) induced an increase in ψm with the PR antagonist, RTI-6413–049b. A, An overall significant treatment effect was seen (P = .01). Pairwise comparisons of a difference in ψm include the following: P = .023, 10−8 M R5020 vs EtOH; and P = .068, 10−8 M R5020 vs RTI. The decrease in R5020 induced ψm by the antagonist did not reach statistical significance (P = .125). There was no interaction between day of treatment and ψm (P = .857). B, hTERT-HM cells treated for 60 minutes with varying doses of dexamethasone showed no change in ψm (P = .058). There was no interaction between day of treatment and ψm (P = .139). All results were expressed as mean ± SEM. Treatments with different superscripts are significantly different. Exps, number of experiments done on different days; Obs, total number of observations in all experiments.
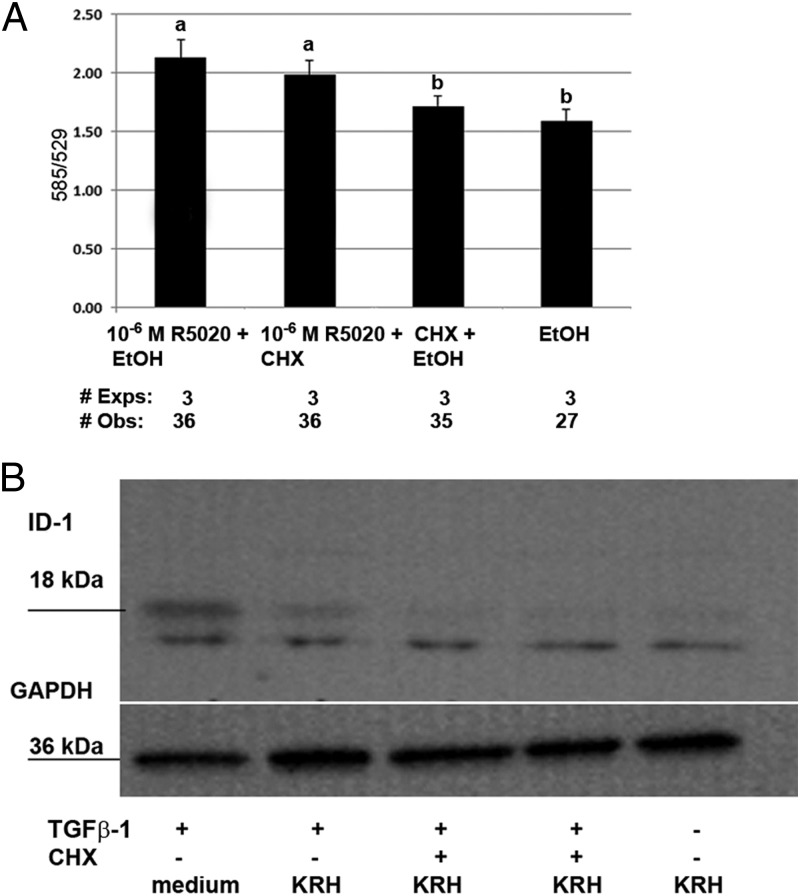
Next, the possible need of protein synthesis for the progestin-mediated increase in ψm was investigated. Figure 4A shows cotreatment with the translational inhibitor CHX. An overall treatment effect was seen [F(3, 69) = 19.9, P < .001], but there was no difference in the response to R5020 with CHX compared with R5020 alone [P = .385]. We verified that the 5-μg/mL concentration of CHX was adequate to block translation in these cells. Figure 4B shows a Western blot analysis demonstrating inhibition of TGFβ induction of the DNA-binding protein inhibitor, inhibitor of DNA binding-1. Because experiments were repeated on different days, repeated-measures ANOVA, with post hoc Tukey honestly significant difference testing, was performed to evaluate the interaction of time (day of treatment) and ψm values of cells treated with various ligands. A significant effect of time was seen in experiments shown in Figures 2A [F(6, 80) = 3.55, P = .004] and 4A [F(4, 49) = 2.9, P = .034]. In both cases, a significant difference in mean values was seen for each of 3 days. Because the experimental protocol did not vary between days, the cause of the difference is not known but may relate to subtle changes in the cells with passage.
Figure 4.
Progestin (R5020) mediated increase in ψm is not dependent on protein synthesis. A, An overall significant treatment effect was seen (P < .001). Pairwise comparisons of a difference in ψm include the following: P < .001, R5020 vs EtOH; P < .001, R5020 vs CHX; and P = .385 CHX vs CHX + R5020. A significant difference was seen between day of treatment and ψm (P = .034). B, The efficacy of CHX was determined by the inhibition of the known induction of the DNA-binding protein inhibitor, inhibitor of DNA binding 1 (ID-1), by TGFβ. Reactions were performed in the medium or Krebs-Ringers-HEPES (KRH) plus glucose buffer. Western blot analyses of hTERT-HM cell protein after 60 minutes of treatment with 5 ng/mL TGFβ-1, with or without 2 hours of pretreatment with 5 μg/mL CHX, were conducted. All results are expressed as mean ± SEM. Treatments with different superscripts are significantly different. Exps, number of experiments done on different days; Obs, total number of observations in all experiments.
Progestin-dependent increase in ψm in transfected hTERT-HM cells expressing PR-M
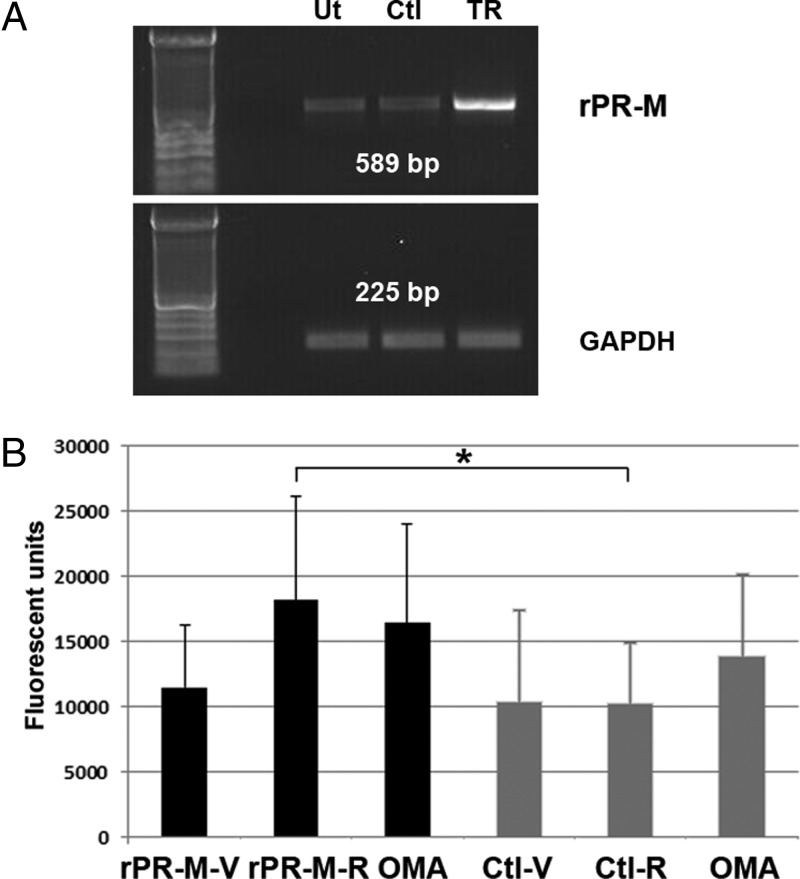
To provide additional evidence that PR-M is responsible for the progestin-induced changes in ψm, we induced the expression of recombinant PR-M in hTERT-HM cells. We also corroborated the ψm changes seen with JC-1 with the use of another indicator, TMRM. At a low loading concentration of 20 nM, the fluorescent emission of TMRM directly correlates with ψm (28). Figure 5A shows the results of a RT-PCR experiment demonstrating the expression of rPR-M transcript in transfected hTERT-HM cells. Figure 5B shows a significant increase in the ψm of cells expressing PR-M treated with progestin (10−7 M R5020) compared with control vector-transfected cells treated with the same ligand [t(2) = 7.15, P = .019]. Oligomycin, an inhibitor of proton extrusion, was used as a positive control to increase ψm.
Figure 5.
Progestin (R5020) increases ψm in hTERT-HM cells expressing PR-M. A, RT-PCR showing high expression of rPR-M transcript in transfected hTERT-HM cells (TR) compared with untransfected (Ut) and control (Ctl) transfected hTERT-HM cells (top panel). There was no difference in GAPDH transcript levels (bottom panel). B, R5020 (10−7 M) increases ψm in hTERT-HM cells (black bars) expressing PR-M (rPR-M-R) compared with R5020-treated control (gray bars) transfected cells (Ctl-R) (P = .019). Results are from three assays with six to eight replicates per assay. Fluorescent units (x-axis) for TMRM emission were corrected for background. All results are expressed as mean ± SEM. rPR-M-V, recombinant PR-M-expressing cells treated with vehicle; rPR-M-R, recombinant PR-M-expressing cells treated with R5020; OMA, oligomycin; Ctl-V, control transfected cells treated with vehicle; Ctl-R, control-transfected cells treated with R5020.
Discussion
The cDNA for PR-M was originally cloned from human aortic and adipose cDNA libraries with expression in Spodoptera frugiperda (Sf 9) insect cells showing a 38-kDa protein (AY212933) (23). The predicted protein structure contains 16 novel amino terminus amino acids, consistent with a mitochondrial localization signal, encoded by sequence within the third intron of the PR gene. This is followed by a sequence identical to nPR encoded by exons 4–8, consisting of the hinge and HBD. Thus, PR-M is a truncated isoform of nPR lacking the amino terminus and DNA-binding domain. Mitochondrial localization of PR-M was shown by confocal imaging of a green fluorescent protein-tagged recombinant protein and Western blot analysis of purified human heart mitochondrial protein (21). Specifically, binding to the outer mitochondrial membrane was shown with immunoelectron microscopy and Western blot analysis of fractionated mitochondrial protein enriched with outer membrane proteins and mitoplast (inner membrane and matrix) proteins. Tissue distribution of PR-M protein showed a strong correlation with mitochondrial content, thus being highest in the heart ventricle and myometrium. Progesterone/progestin via PR-M controls mitochondrial function. In cell models of transient overexpression or gene silencing of PR-M, a progestin-dependent increase in ψm was seen, corresponding to increased extracellular oxygen consumption (21). Because progesterone is a reproductive hormone, produced in substantial amounts during the luteal phase of the menstrual cycle and pregnancy, we theorize that PR-M provides a mechanism to meet the increased energy demands of pregnancy. In contrast, PR-M may also function in the pathology of sex steroid-dependent neoplasms such as fibroid tumors.
Because energy requirements increase with cell growth and proliferation, we investigated the expression of PR-M in the edge of fibroids and adjacent normal myometrium. In 9 of 10 specimens analyzed, the PR-M protein level was greater in fibroid tissue, as was mitochondrial porin. These studies do not differentiate as to whether there is increased PR-M and porin per mitochondrion or the increased PR-M and porin represents an increased number of mitochondria. The latter is supported by two observations. Because porin is the most abundant protein in the mitochondrial outer membrane, protein levels are believed to reflect mitochondrial mass (29). Additionally, previous studies with electron microscopy (EM) showed an increased number of mitochondria per cell in fibroids vs normal myometrium (30). Ideally, additional patient characteristics such as chronic illnesses, medications, and ovulatory history would have been obtained. Using deidentified tissues, we were reliably able to obtain only pathology information.
hTERT-HM cells were used as a model to investigate PR-M control of mitochondrial function. This immortalized cell line was developed by retroviral infection of human myometrial cells with the catalytic subunit of human telomerase (24). Characterization of these cells includes expression of ERα, nPR, and smooth muscle proteins including α-smooth muscle actin, calponin, caldesmon, and oxytocin receptor. In this study, hTERT-HM cells were shown to express PR-M, with a dose-dependent increase in ψm occurring with R5020 treatment. The R5020-induced increase in ψm was diminished by cotreatment with a specific PR antagonist, RTI-6413–049b (31) but did not reach statistical significance in this study. Prior study has shown a significant inhibition by this antagonist in HeLa cells, with transfected expression of PR-M, treated with 10−7 M R5020 (21). The increase in ψm persisted in the presence of the translation inhibitor CHX excluding a process dependent on protein synthesis. Additionally, treatment with the potent glucocorticoid, dexamethasone, failed to illicit a response, thus obviating the possible cross-reactivity of R5020 with a GR (32). Additional evidence that PR-M mediated the increase in ψm was gained by the transient overexpression in these cells. The transfected hTERT-HM cells expressing PR-M also demonstrated a progestin-dependent increase in ψm.
Two different fluorescent dyes were used in experiments to determine ψm. JC-1 is a cationic membrane permeant compound that distributes within the cell according to electrical charge. In the cytoplasm JC-1 is a monomer with primary emission in the green fluorescent range. In the mitochondrial matrix, low potential causes dye stacking (JC-1 aggregates), resulting in a shift to red fluorescence. The ratio of red/green emission shows a direct correlation with ψm. The use of this dye has been both supported (33, 34) and criticized (35, 36). A major concern includes the fact that changes in plasma membrane potential without a change in the ψm may lead to changes in the emission ratio of JC-1 (36). Due to criticism of JC-1, we corroborated our findings with another dye, TMRM. When used at a low concentration of 10–30 nM, TMRM distributes within the cell based on the electrical potential and shows a linear fluorescent emission with concentration (37). Thus, the progestin-induced increase in ψm shown by JC-1 in nontransfected hTERT-HM cells was corroborated with the use of TMRM in transfected cells expressing PR-M. The cause of an increased ψm was not determined in these experiments and can be associated with several processes including the following: 1) an increase in proton pumping by the increased activity of respiratory enzyme complexes and consequential ATP production; 2) a transient block of electron transfer; 3) a transient block of proton use by enzymes within the mitochondria; and 4) a change in the mitochondrial pH, leading to a change in the membrane potential (38).
In previous publications we have demonstrated a progestin-dependent increase in ψm in nPR-negative MCF-10A breast epithelial cells corresponding to an increased level of total cellular ATP (39). Additionally, progestin treatment of HeLa cells overexpressing PR-M resulted in increased ψm corresponding to increased oxygen consumption consistent with increased cellular respiration (21). It is important to note that this is not a kinetic assay but a comparison of treatments at a given time point. In a kinetic assay, a transient decrease in ψm is seen with increased ATP production as the proton gradient is decreased. In the presence of adequate substrate, this quickly returns to a new steady state (40). Our results show a more energized cell after progestin treatment, most likely consistent with increased cellular respiration.
Both the existence of PR-M protein and the fidelity of commercial PR antibodies directed to the HBD has been questioned by Samalecos and Gellersen (26). These authors corroborated the finding of the PR-M transcript by RT-PCR with Southern hydridization in T47D breast cancer cells and myometrial cells. They were unable to identify the endogenous protein with Western blot analysis or express the protein in a rabbit lysate reticulocyte system. These studies were conducted before the knowledge of PR-M as a mitochondrial protein. The lack of identification of endogenous protein may be due to differences in the amount of loaded protein. The investigators used whole-cell protein preparations, typically with 30 μg protein, whereas our experiments commonly used 80–120 μg total protein for Western blot analysis. Additionally, the TNT T7 quick coupled transcription/translation system (Promega Corp) used by these investigators is primarily designed for soluble proteins and requires the addition of pancreatic microsomes for membrane protein expression (41). In regard to antibodies, there is no question that Western analyses with PR antibodies directed to the HBD results in a greater number of bands compared with antibodies directed to the amino terminus. Previously we have shown that the commercial antibodies C-19 and MAB 462 recognize a histadine-tagged rPR-M. Additionally, the antibodies identified a 38-kDa protein in a myometrial, mitochondrial protein preparation after immunoprecipitation, shown by mass spectrometry to contain a peptide fragment of PR-M (21).
Little research exists regarding cellular respiration or mitochondrial function in fibroids. The discovery of a mutation resulting in decreased function of fumarate hydratase has provided insight into how alterations in energy production may be tumorigenic. Fumarate hydratase converts fumarate to malate in the citric acid cycle. A heterozygous germline mutation predisposes to a syndrome consisting of cutaneous and uterine leiomyomata and renal cell carcinoma (42). Compared with spontaneous fibroids, the fumarate hydratase deficiency-related tumors showed increased cellularity with overexpression of glycolytic enzymes and increased glycolysis.
A shift in metabolism from oxidative phosphorylation to glycolysis is well recognized in malignancies (Warburg effect) (43). Originally thought to represent a defect in oxidative phosphorylation, it is now apparent that proliferating cells in general shift to glycolysis, even with normal functioning oxidative phosphorylation capacity. This strategy may allow the use of tricarboxylic acid cycle intermediates for the synthesis of nucleic acids, proteins, and lipids for proliferation (44).
Because fibroid cells appear to be adapted to their stiff microenvironment and thus respond less to moderate mechanical stress than other cells and are apparently resistant to apoptosis, it is possible that the energy necessary to allow for these adaptations is provided by PR-M. Antiprogestins have been reported to increase the apoptotic response in many but not all fibroid cells and tissues, raising the intriguing possibility for a role of PR-M in altered mitochondrial function in the development of fibroids (45, 46). It could be postulated that to meet the energy demands of growth, fibroids develop cells with increased numbers of mitochondria, which are resistant to apoptosis. The effect of progesterone on mechanical signaling is not well studied, but it is reasonable to assume that its effect on cellular respiration would impact this signaling mechanism as well.
The function of the PR-M receptor makes teological sense as a mechanism to control cellular energy production during pregnancy. In this respect, myometrial cells undergo marked proliferation during pregnancy, requiring significant cellular energy followed by apoptosis postpartum corresponding to the fall in progesterone.
Acknowledgments
hTERT-HM cells were generously donated by William E. Rainey, PhD. We thank Donald McDonnell, PhD, for the kind gift of RTI-6413–049b and technical assistance. We also thank Richard Auten, MD, for technical assistance with the mitochondrial membrane potential assays. Dr Maragatha Kuchibhatla kindly provided assistance with the statistical analysis.
This work was supported by the Charles Hammond Foundation of Duke University and the Susan Fiery-Hughes Foundation of Duke University.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the Charles Hammond Foundation of Duke University and the Susan Fiery-Hughes Foundation of Duke University.
Footnotes
- CHX
- cycloheximide
- ER
- estrogen receptor
- FBS
- fetal bovine serum
- FE
- outer edge of a fibroid
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- hTERT-HM
- human myometrial cell line
- IDV
- integrated density value
- JC-1
- 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolecarbocyanide iodine
- ψm
- mitochondrial membrane potential
- MA
- adjacent myometrium
- nPR
- nuclear PR
- PR
- progesterone receptor
- PR-M
- mitochondrial PR
- TMRM
- tetramethylrhodamine methyl ester.
References
- 1. Catherino W, Leppert P, Stenmark M, et al. Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes Chromsomes Cancer. 2004;40:204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leppert P, Baginski T, Prupas C, Catherino W, Pletcher S, Segars J. Comparative ultrastructure of collagen fibrils in uterine leiomyomas and normal myometrium. Fertil Steril. 2004;82:1182–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rogers R, Norian J, Malik M, et al. Mechanical homeostasis is altered in uterine leiomyoma. Am J Obstet Gynecol. 2008;198:474.e1–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Norian J, Owen C, Taboas J, et al. Characterization of tissue biomechanics and mechanical signaling in uterine leiomyoma. Matrix Biol. 2012;31:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leppert P, Catherino W, Segars J. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am J Obstet Gynecol. 2006;195:415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peddada S, Laughlin S, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA. 2008;105:19887–19892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marshall L, Spiegleman D, Goldman M, et al. A prospective study of reproductive factors and oral contraceptive use in relation to the risk of uterine leiomyomata. Fertil Steril. 1998;70:432–439. [DOI] [PubMed] [Google Scholar]
- 8. Chiaffarino F, Parazzini F, La Vecchia C, Marsico S, Surace M, Ricci E. Use of oral contraceptives and uterine fibroids: results from a case-control study. Br J Obstet Gynaecol. 1999;106:857–860. [DOI] [PubMed] [Google Scholar]
- 9. Soldin OP, Makambi KH, Soldin SJ, O'Mara DM. Steroid hormone levels associated with passive and active smoking. Steroids. 2011;76:653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Filicori M, Hall D, Loughlin J, Rivier J, Vale W, Crowley W Jr. A conservative approach to the management of uterine leiomyoma: pituitary desensitization by a luteinizing hormone-releasing hormone analogue. Am J Obstet Gynecol. 1983;147:726–727. [DOI] [PubMed] [Google Scholar]
- 11. Kettel L, Murphy A, Morales A, Rivier J, Vale W, Yen S. Rapid regression of uterine leiomyomas in response to daily administration of gonadotropin-releasing hormone antagonist. Fertil Steril. 1993;60:342–346. [DOI] [PubMed] [Google Scholar]
- 12. McCarthy-Keith DM, Malik M, Britten J, Segars J, Catherino WH. Gonadotropin-releasing hormone agonist increases expression of osmotic response genes in leiomyoma cells. Fertil Steril. 2011;95:2383–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parsanezhad ME, Azmoon M, Alborzi S, et al. A randomized, controlled clinical trial comparing the effects of aromatase inhibitor (letrozole) and gonadotropin-releasing hormone agonist (triptorelin) on uterine leiomyoma volume and hormonal status. Fertil Steril. 2010;93:192–198. [DOI] [PubMed] [Google Scholar]
- 14. Carr B, Marshburn P, Weatherall P, et al. An evaluation of the effect of gonadotropin-releasing hormone analogs and medroxyprogesterone acetate on uterine leiomyomata volume by magnetic resonance imaging: a prospective, randomized, double blind, placebo-controlled, crossover trial. J Clin Endocrinol Metab. 1993;76:1217–1223. [DOI] [PubMed] [Google Scholar]
- 15. Murphy A, Kettel L, Morales A, Roberts V, Yen S. Regression of uterine leiomyomata in response to the antiprogesterone RU 486. J Clin Endocrinol Metab. 1993;76:513–517. [DOI] [PubMed] [Google Scholar]
- 16. Chwalisz K, Perez M, DeManno D, Winkel C, Schubert G, Elger W. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocr Rev. 2005;26:423–438. [DOI] [PubMed] [Google Scholar]
- 17. Pedeutour F, Quade BJ, Weremowicz S, Dal Cin P, Ali S, Morton CC. Localization and expression of the human estrogen receptor β gene in uterine leiomyomata. Genes Chromosomes Cancer. 1998;23:361–366. [DOI] [PubMed] [Google Scholar]
- 18. Viville B, Charnock-Jones D, Sharkey A, Wetzka B, Smith S. Distribution of the A and B forms of the progesterone receptor messenger ribonucleic acid and protein in uterine leiomyomata and adjacent myometrium. Hum Reprod. 1997;12:815–822. [DOI] [PubMed] [Google Scholar]
- 19. Maruo T, Matsuo H, Samoto T, et al. Effects of progesterone on uterine leiomyoma growth and apoptosis. Steroids. 2000;65:585–592. [DOI] [PubMed] [Google Scholar]
- 20. Kayisli UA, Berkkanoglu M, Kizilay G, Senturk L, Arici A. Expression of proliferative and preapoptotic molecules in human myometrium and leiomyoma throughout the menstrual cycle. Reprod Sci. 2007;14:678–686. [DOI] [PubMed] [Google Scholar]
- 21. Dai Q, Shah AA, Garde RV, et al. A truncated progesterone receptor (PR-M) localizes to the mitochondrion and controls cellular respiration. Mol Endocrinol. 2013;27:741–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu X, Englund K, Lindblom B, Blanck A. mRNA-expression of often used housekeeping genes and the relation between RNA and DNA are sex steroid-dependent parameters in human myometrium and fibroids. Gynecol Obstet Invest. 2003;55:225–230. [DOI] [PubMed] [Google Scholar]
- 23. Saner K, Welter B, Zhang F, et al. Cloning and expression of a novel, truncated progesterone receptor. Mol Cell Endocrinol. 2003;200:155–163. [DOI] [PubMed] [Google Scholar]
- 24. Condon J, Yin S, Mayhew B, et al. Telomerase immortalization of human myometrial cells. Biol Reprod. 2002;67:506–514. [DOI] [PubMed] [Google Scholar]
- 25. Wei LL, Hawkins P, Baker C, Norris B, Sheridan PL, Quinn PG. An amino-terminal truncated progesterone receptor isoform, PRc, enhances progestin-induced transcriptional activity. Mol Endocrinol. 1996;10:1379–1387. [DOI] [PubMed] [Google Scholar]
- 26. Samalecos A, Gellersen B. Systematic expression analysis and antibody screening do not support the existence of naturally occurring progesterone receptor (PR)-C, PR-M, or other truncated PR isoforms. Endocrinology. 2008;149:5872–5887. [DOI] [PubMed] [Google Scholar]
- 27. Sathya G, Jansen M, Nagel S, Cook C, McDonnell D. Identification and characterization of novel estrogen receptor-β-sparing antiprogestins. Endocrinology. 2002;143:3071–3082. [DOI] [PubMed] [Google Scholar]
- 28. Esposti M. Assessing functional integrity of mitochondria in vitro and in vivo. Methods Cell Biol. 2001;65:75–96. [DOI] [PubMed] [Google Scholar]
- 29. Hanson BJ, Carrozzo R, Piemonte F, Tessa A, Robinson BH, Capaldi RA. Cytochrome c oxidase-deficient patients have distinct subunit assembly profiles. J Biol Chem. 2001;276:16296–16301. [DOI] [PubMed] [Google Scholar]
- 30. Ferenczy A, Richart RM, Okagaki T. A comparative ultrastructural study of leiomyosarcoma, cellular leiomyoma, and leiomyoma of the uterus. Cancer. 1971;28:1004–1018. [DOI] [PubMed] [Google Scholar]
- 31. Giannoukos G, Szapary D, Smith CL, Meeker JEW, Simons SS Jr. New antiprogestins with partial agonist activity: potential selective progesterone receptor modulators (SPRMs) and probes for receptor- and coregulator-induced changes in progesterone receptor induction properties. Mol Endocrinol. 2001;15:255–270. [DOI] [PubMed] [Google Scholar]
- 32. Sethna F, Tyson-Capper A, Shiells E, Robson S. Expression of glucocorticoid receptor in human myometrium during pregnancy and labour. J Steroids Hormon Sci. 2011;2:104. [Google Scholar]
- 33. Cossarizza A, Ceccarelli D, Masini A. Functional heterogeneity of an isolated mitochondrial population revealed by cytofluorometric analysis at the single organelle level. Exp Cell Res. 1996;222:84–94. [DOI] [PubMed] [Google Scholar]
- 34. Mathur A, Hong Y, Kemp B, Barrientos A, Erusalimsky J. Evaluation of fluorescent dyes for the detection of mitochondrial membrane potential changes in cultured cardiomyocytes. Cardiovasc Res. 2000;46:126–138. [DOI] [PubMed] [Google Scholar]
- 35. Bernardi P, Scorrano L, Colonna R, Petronilli V, Di Lisa F. Mitochondria and cell death. Eur J Biochem. 1999;264:687–701. [DOI] [PubMed] [Google Scholar]
- 36. Duchen MR, Surin A, Jacobson J. Imaging mitochondrial function in intact cells. Methods Enzymol. 2003;361:353–389. [DOI] [PubMed] [Google Scholar]
- 37. Duchen MR, Surin A, Jacobson J. Imaging mitochondrial function in intact cells. Methods Enzymol. 2003;361:353–389. [DOI] [PubMed] [Google Scholar]
- 38. Zorov D, Juhaszova M, Sollott S. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta. 2006;1757:509–517. [DOI] [PubMed] [Google Scholar]
- 39. Behera MA, Dai Q, Garde R, Saner C, Jungheim E, Price TM. Progesterone stimulates mitochondrial activity with subsequent inhibition of apoptosis in MCF-10A benign breast epithelial cells. Am J Physiol Endocrinol Metab. 2009;297:E1089–E1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nicholls D, Ward M. Mitochondrial membrane potential and neuronal glutamate exitotoxicity: mortality and millivolts. Trends Neurosci. 2000;23:166–174. [DOI] [PubMed] [Google Scholar]
- 41. Falk MM, Buehler LK, Kumar NM, Gilula NB. Cell-free synthesis and assembly of connexins into functional gap junction membrane channels. EMBO J. 1997;16:2703–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vanharanta S, Pollard PJ, Lehtonen HJ, et al. Distinct expression profile in fumarate-hydratase-deficient uterine fibroids. Hum Mol Genet. 2006;15:97–103. [DOI] [PubMed] [Google Scholar]
- 43. Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 44. DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. [DOI] [PubMed] [Google Scholar]
- 45. Luo X, Yin P, Coon V JS, Cheng Y-H, Wiehle RD, Bulun SE. The selective progesterone receptor modulator CDB4124 inhibits proliferation and induces apoptosis in uterine leiomyoma cells. Fertil Steril. 2010;93:2668–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Catherino WH, Malik M, Driggers P, Chappel S, Segars J, Davis J. Novel, orally active selective progesterone receptor modulator CP8947 inhibits leiomyoma cell proliferation without adversely affecting endometrium or myometrium. J Steroid Biochem Mol Biol. 2010;122:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]