Abstract
Context:
Non-islet cell tumor hypoglycemia (NICTH) is a rare but serious paraneoplastic syndrome in which a tumor secretes high molecular weight IGF-II, causing hypoglycemia. Complete tumor resection is curative but is often delayed or unfeasible. There is no clear “standard of care” for managing these patients.
Evidence Acquisition:
PubMed searches were conducted for: “non-islet-cell tumor hypoglycemia,” “NICTH,” “Doege-Potter,” “Doege-Potter syndrome,” “high molecular weight IGF-II,” and “big IGF-II.” Relevant articles were reviewed in detail. We limited our review to English-language articles, focusing on 1988–2013 (corresponding with the elucidation of the pathophysiology of NICTH).
Evidence Synthesis:
The available literature exists as case reports or small case series, with a void of higher-order treatment studies. Thus, an evidence-based approach to data synthesis was difficult. Nevertheless, the available literature is presented objectively with an attempt to describe clinically useful trends and findings in the management of NICTH.
Conclusions:
Appropriate identification of NICTH and prompt and complete tumor resection represents ideal management. However, when prompt resection is not feasible, iv glucose or dextrose often does not suffice to prevent hypoglycemia. In such cases, we suggest consideration of local antitumor therapies for disease control and trial of glucocorticoids alone or in combination with GH. Continuous glucagon infusion can be successful if the patient has a positive response to a glucagon stimulation test, and parenteral nutrition may allow higher glucose delivery, but both are limited by the need for continuous iv infusion. Diazoxide and octreotide have no role in NICTH.
Non-islet-cell tumor hypoglycemia (NICTH) is a rare paraneoplastic syndrome encountered in the setting of a wide variety of benign and malignant tumors (1). NICTH is eponymously known as Doege-Potter syndrome when the tumor is a fibrous tumor located in the thorax (2). This syndrome has been recognized for decades (3, 4) and was initially attributed to increased glucose utilization by large tumors (5). The true mechanism by which non-islet cell tumors cause hypoglycemia remained elusive into the 1970s and early 1980s when circulating insulin-like peptides were described (6, 7). In the 1980s and early 1990s, abnormal (incompletely processed) IGF-II was described (8, 9) and was ultimately characterized as a high molecular weight or “big” IGF-II (10, 11) with potent insulin-like activity causing hypoglycemia. This led to numerous reports describing a wide variety of management options. However, most of the available literature exists as case reports or small case series. The three largest series we identified reported on clinical features of NICTH but focused largely on epidemiology and diagnosis, with minimal comment on management (12–14). This review seeks to summarize the available literature on management of hypoglycemia in NICTH, with special focus on preoperative management and management in the setting of nonresectable disease.
Methods
PubMed searches were conducted for the following terms: “non-islet-cell tumor hypoglycemia,” “NICTH,” “Doege-Potter,” “Doege-Potter syndrome,” “high molecular weight IGF-II,” and “big IGF-II.” All articles identified by these searches were reviewed if the article text was available in English. Case reports and case series were included in our detailed review and summary if they were published between January 1, 1988, and August 15, 2013. This time period was chosen to correspond with the elucidation of the mechanism of NICTH. (In reading earlier published cases, it was more difficult to discern whether those cases were true cases of NICTH or whether the hypoglycemia was due to other etiologies). Additional articles were identified from the reference sections of articles found via the above searches, particularly from review articles.
Epidemiology
NICTH is rare, with our own search revealing 98 case reports or small series (fewer than 10 patients) totaling 130 cases in the English language medical literature between January 1, 1988, and August 15, 2013. Additionally, there are several larger series providing more aggregate dates but fewer individual patient details. Tsuro et al (14), in addition to detailing a case report included in our summary, also reviewed details of 20 additional Japanese cases. Fukuda et al (12) summarized 78 patients with NICTH, Hizuka et al (13) reported on 44 cases, and Miraki-Moud et al (15) published 16 cases. Therefore, there are nearly 290 cases of NICTH reported in the English language medical literature in the past quarter century. We did not encounter familial cases or links with genetic tumor syndromes.
Pathophysiology
The gene product of the IGF-II locus is a 180-amino acid residue molecule termed prepro-IGF-II. This molecule consists of an N-terminus 24-amino acid peptide, a 67-amino acid mature IGF-II, and an 89-amino acid C-terminus extension, defined as the E-domain. All pro-IGF-II-related molecules are designated as “big” IGF-II. The big IGF-II including amino acid residues 1–87 is associated with NICTH. Additional big IGF-II molecules have been described, such as the big IGF-II (amino acid residues 1–104), which is linked to the hepatitis C-associated osteosclerosis syndrome (16).
IGF-II is normally a 7.5-kDa peptide, but in cases of NICTH, most circulating IGF-II is a high molecular weight form in the 10- to 20-kDa range (8, 17). This big IGF-II is formed due to abnormal processing of an IGF-II precursor in tumors with aberrant IGF-II gene transcription and gene expression (18, 19). Although many reported cases of NICTH detail high IGF-II levels (9), low and normal IGF-II levels are also reported (20). It is thought that this discrepancy in reporting lies in assay variation in the ability of different laboratories to detect abnormal IGF-II forms (17). IGF-I and IGF-II are capable of lowering glucose levels but typically fail to do so because they are normally trapped within the vascular space in a high molecular weight protein complex. Under normal circumstances, 7.5-kDa IGF-II binds with 40-kDa IGF-binding protein-3 (IGFBP-3) to create a roughly 50-kDa binary complex. This complex then binds with 85-kDa acid-labile subunit (ALS) to create a roughly 140- to 150-kDa ternary complex. A normal subject would have approximately 20% of IGF-II in the binary complex and 80% in the ternary complex (16).
In NICTH, elevated total IGF-II leads to a greater concentration of free IGF-II. Additionally, the high molecular weight precursor big IGF-II tends to reside in the binary complex with IGFBP-3 (ALS is not able to bind due to steric hindrance with the altered IGF-II forms; ALS concentrations may also be lower). The ratio of binary:ternary complexes is typically reversed in NICTH, with 80% binary and 20% ternary (16). The big IGF-II:IGFBP-3 binary complex is approximately 60 kDa and is thought to be able to cross the endothelial barrier and exert hypoglycemic effects (17, 21, 22). Additionally, the activity of IGF-II suppresses both insulin and GH (with resultant low IGF-I). Low GH also leads to low ALS and IGFBP-3, allowing less binding of big IGF-II (16, 17).
In a similar fashion to insulin, IGF-II determines hypoglycemia by inhibiting glucose output from the liver and by enhancing glucose uptake by skeletal muscle. In particular, the activation of insulin receptors by IGF-II promotes continued glucose utilization mainly by the skeletal muscle and suppression of free fatty acid release by adipocytes. This also leads to inhibition of glucose release, glycogenolysis, gluconeogenesis, and ketogenesis in the liver (16). Furthermore, the release of the counter-regulatory hormones glucagon and GH is suppressed by IGF-II, which in turn magnifies the vulnerability to hypoglycemia in NICTH (23).
Tumor Characteristics
Tumors of mesenchymal or hepatic origin are most commonly described with NICTH (24), although it is now recognized that a wide variety of tumor types can result in production of big IGF-II (25). We found similar variability. Of the 288 total cases reviewed, solitary fibrous tumor (SFT) and/or mesothelioma was the histopathology reported in 64 cases (22%). In 38 of these cases, the pleura was the clear origin of the tumor, with retroperitoneum, abdomen, and pelvis also commonly reported, including a uterine SFT (26) and a bladder SFT (27). The next most common mesenchymal tumor type was hemangiopericytoma, with 19 of 288, or 7%. Hepatocellular carcinoma (HCC) was the most common nonmesenchymal tumor by far, with 50 cases out of 288, or 17%. Note that HCC is well-documented to produce IGF-II (ie, hypoglycemia in HCC cases is not only via hepatic failure of gluconeogenesis) (28). Other commonly reported tumor types include: adenocarcinomas (at least 20 cases—precise determination difficult due to sparse documentation in some reports), gastrointestinal stromal tumors (GISTs) (11 cases) (19, 29–34), various types of sarcomas, and renal cell carcinoma (35–37). Of interest to endocrinologists, NICTH has been reported with both adrenal cortical carcinoma (12, 38, 39) and thyroid cancer (40). Other unusual tumors reported include Burkitt's lymphoma (41), plasmacytoma (42), yolk cell tumor (43), Leydig cell tumor (44), and phyllodes tumor of the breast (45). Due to the predominance of pleural tumors and tumors of hepatic and gastric origin, the vast majority of reported cases of NICTH involve primary tumor origin from the chest, abdomen, or pelvis.
Note that even the most common tumor type and location, pleural SFT, does not typically result in NICTH. Of approximately 800 cases of pleural SFT reported as of 2009, only 5% are estimated to cause NICTH (46, 47). With other tumor types, given reports of only a handful of cases in the past quarter century, the rate of NICTH is likely to be much lower.
In the 288 cases we reviewed, we identified 192 (67%) that were reported as malignant. However, note that malignancy vs benignity was not described well in a large number of cases, and in a few cases it was difficult to even determine the tumor type from the published report.
Details on tumor size vary, but it is generally acknowledged that tumors must become quite large before hypoglycemia manifests (16, 24). Fukuda et al (12) found that tumor diameter was greater than 10 cm in 70% of their 78 cases, and Kalebi et al (48) reviewed 65 cases of pleural SFT and NICTH and reported a mean diameter of 20 cm.
Patient Demographics
Of the 285 cases for which gender was reported, 131 were female (46%), implying either a slight male predominance, or more likely, no gender predilection. Reported ages ranged from 2–87 years, with at least four cases in the pediatric age range (ages 2, 5, 9, and 11—based on three pediatric case reports and the lower end of the age range in the largest series) (12, 41, 49, 50). Mean reported age could not be determined for the entire group of cases reviewed due to the lack of exact ages reported for all cases, but of the 128 cases for which exact ages were available, the mean age was 56.4 years.
Presenting Symptoms
Fukuda et al (12) found that only 48% of the 65 cases presented with hypoglycemia as the initial manifestation of the tumor, whereas 52% had known tumors before the onset of hypoglycemia. Indeed, presentation with symptoms due to the tumor mass itself before the onset of hypoglycemia is well-described (51). Three cases documented dramatic improvement or complete resolution of pre-existing diabetes mellitus (42, 52, 53). One pregnant patient was described (54). Hypokalemia is also frequently described with NICTH and is attributed to the insulin-like activity of big IGF-II (12, 38, 55). In one case, subclinical Cushing syndrome was even described concomitantly with NICTH (56). Acromegaloid features have also been described in NICTH (16), with documented resolution after tumor resection (57). Dynkevich et al (16) postulate that neuroglycopenic symptoms are more commonly seen than autonomic symptoms due to repeated hypoglycemic events and insidious progression seen with NICTH.
Diagnosis
NICTH should be suspected in any patient with hypoglycemia without clear etiology. Initial evaluation of hypoglycemia should proceed according to usual practice. The Endocrine Society Guidelines recommend investigation in patients in whom Whipple's triad is fulfilled (58). This includes evaluating and pursuing the possibilities of medication-induced hypoglycemia, critical illness, organ failure, and/or hormone deficiencies (eg, liver failure, kidney failure, adrenal insufficiency, GH deficiency), as well as endogenous hyperinsulinism (with differential diagnosis of insulinoma, post-gastric bypass hypoglycemia, insulin autoimmune hypoglycemia, and accidental or surreptitious insulin secretagogue ingestion). If there are clues to NICTH (eg, known malignancy, identification of large new mass), this can be pursued early. Otherwise, we would consider this rare diagnosis if the workup of the preceding causes was unrevealing. When performing laboratory investigation of hypoglycemia, it is imperative to draw a serum glucose level (not just a finger-stick capillary blood glucose level) to confirm hypoglycemia with simultaneous measurement of levels for insulin, proinsulin, C-peptide, β-hydroxybutyrate, and an oral hypoglycemic agent screen (58). Evaluation of liver and kidney function and a cosyntropin stimulation test round out the usual evaluation of hypoglycemia. Additional investigation when NICTH is suspected includes measurement of IGF-I, IGF-II, and GH levels. In a hypoglycemic patient with low insulin and C-peptide, a low β-hydroxybutyrate level suggests an agent mimicking insulin and is therefore an indication to measure IGF-I and IGF-II (59).
The typical pattern for NICTH on the above laboratory studies includes low glucose (serum glucose < 55 mg/dL) with simultaneous low insulin/proinsulin/C-peptide/β-hydroxybutyrate levels, and the absence of positive results on an oral hypoglycemic agent screen. Note that GH levels are typically low (unlike brief episodes of hypoglycemia that trigger a surge in GH). Depending on the specific IGF-II assay used, the IGF-II levels may or may not be elevated in NICTH (17, 25). Even if IGF-II levels are normal (approximate normal range, 275–750 ng/mL, depending on the laboratory used), the IGF-I levels are suppressed under 100 ng/mL (12), and therefore the IGF-II:IGF-I ratio is elevated (5) above the normal molar ratio of 3:1 (16) and often approaching or exceeding 10:1 (12). This ratio may be an important screening tool for NICTH in cases of hypoglycemia (13). For the initial laboratory studies above, we advocate using the cutoffs advocated by The Endocrine Society: insulin level < 3 μU/mL, proinsulin level < 5 pmol/L, C-peptide level < 0.2 nmol/L, and β-hydroxybutyrate level < 2.7 mmol/L (58).
To our knowledge, there is no commercially available assay for big IGF-II, and measurement of high molecular weight precursor forms of IGF-II must be done in a research laboratory setting at this time. Several such assays have been summarized previously (17). As a recent example, Miraki-Moud et al (15) detailed their rapid method for separating pro-IGF-II from mature IGF-II using tricine-SDS-PAGE, followed by IGF-II immunoblot. This method could be reproduced using their descriptions. Detection of a high percentage of IGF-II as pro-IGF-II (big IGF-II) would then allow for diagnosis of NICTH.
The lack of widely available measurement of big IGF-II is likely because NICTH, a very rare disease, is the only established clinical indication for testing big IGF-II (59). Nevertheless, a widely available assay would assist all practitioners, especially those without access to a research laboratory. As research on the clinical utility of IGF-II measurement in cancer screening, diagnosis, and monitoring evolves, we may see an expanded list of indications prompting further assay development (59).
In cases in which it is unclear whether hypoglycemia is due to IGF-II or another etiology (eg, liver failure due to metastatic disease with depleted glycogen stores), a glucagon-stimulation test can be employed. A rise in glucose suggests a hormonal cause of hypoglycemia, whereas the lack of appropriate rise suggests the absence of sufficient liver stores (41, 60).
As with evaluation of insulinoma, once you have the biochemical evidence, the next step is localization/visualization. If laboratory results suggest NICTH (eg, high IGF-II:IGF-I ratio and absence of evidence for hyperinsulinemia), a reasonable next step is cross-sectional imaging of the chest, abdomen, and pelvis to identify a tumor, given that the vast majority of reported cases of NICTH involve a tumor in one of these sites. If a tumor is identified, evaluation should proceed as one normally would proceed based on tumor location, imaging characteristics, and other patient characteristics. See Table 1 for comparison of laboratory test results in various etiologies of hypoglycemia.
Table 1.
Comparison of Laboratory Test Results for Various Etiologies of Hypoglycemia
| Diagnosis | Insulin | Proinsulin | C-Peptide | IGF-I | IGF-II | IGF-II:IGF-I Ratio | Pro-IGF-II | OHA Screen | Insulin Antibody |
|---|---|---|---|---|---|---|---|---|---|
| Exogenous insulin | High | Low | Low | Normal | Normal | Normal | Normal | − | − |
| Insulinoma, post-gastric bypass | High | High | High | Normal | Normal | Normal | Normal | − | − |
| OHA | High | High | High | Normal | Normal | Normal | Normal | + | − |
| Insulin autoimmune syndrome | High | High | High | Normal | Normal | Normal | Normal | − | + |
| IGF-mediateda | Low or low-normal | Low or low-normal | Low or low-normal | Low | High or normal | High | High | − | − |
Abbreviation: OHA, Oral hypoglycemic agent.
In cases of NICTH, we expect the following laboratory values: insulin level < 3 μU/mL, proinsulin level < 5 pmol/L, C-peptide level < 0.2 nmol/L, IGF-I level < 100 ng/mL, IGF-II level > 275 ng/mL, and an IGF-II:IGF-I ratio > 3:1, with true cases of NICTH frequently having a ratio > 10:1.
Management
Initial treatment of hypoglycemia is accomplished by oral glucose and/or iv glucose- or dextrose-containing fluids as necessary. In many cases, this suffices to avoid further hypoglycemia (47, 55, 61, 62). Once NICTH is identified and a primary tumor is found, the mainstay of treatment is surgical resection, which is curative for hypoglycemia if resection is complete (63–69). Resolution of hypoglycemia has also been described in cases of subtotal resection (70). In rare instances, hypoglycemia recurs with tumor recurrence after what was thought to be complete resection (71, 72). However, in many cases, total resection is either delayed or not feasible. Reasons for nonresectability include large tumor burden (73), widely metastatic disease (14, 74), compromised local structures necessitating subtotal resection (34, 75), physical characteristics of the tumor and/or its relationship to surrounding structures necessitating abortion of resection (76, 77), and patient preference (78, 79).
Often the hypoglycemia in NICTH is severe enough to require further treatment beyond iv glucose or dextrose, either in lieu of surgical resection or while awaiting surgical resection. Management in these cases has varied widely. An increase in oral food intake (whether in amount of food, caloric density, and/or frequency) is often tried in addition to iv glucose or dextrose, with mixed results (37, 57, 80, 81). Increases in calories and total carbohydrate delivery have sometimes been accomplished with iv nutrition (commonly known as partial parenteral nutrition or total parenteral nutrition) (82, 83). However, the use of partial parenteral nutrition or total parenteral nutrition is not a desirable long-term strategy given the necessity of long-term venous access with its inherent risks of complications and concomitant risks of bloodstream infections, liver toxicity, and electrolyte imbalances, not to mention cost (84).
Local antitumor therapy has been successful in selected cases, including resolution of NICTH due to HCC with two courses of intrahepatic adriamycin (85); resolution of NICTH due to GIST after selective use of embolization of a GIST before subtotal resection (34); preoperative glucose stabilization using a combination of selective embolization, chemotherapy, and radiation therapy (76); and resolution of NICTH with radiation therapy of a large leiomyosarcoma (despite untreated pulmonary metastases, although the patient later died due to tumor bleeding) (72). Meanwhile, systemic antitumor therapy has been reported with very limited success. Zachariah et al (22) described “multiple doses of chemotherapy” used for a 55-year-old man with NICTH due to a large retroperitoneal undifferentiated mesenchymal tumor, but noted that the patient “deteriorated and died” over the next few months; and Ishikura et al (39) reported failure of multiple chemotherapy regimens for NICTH due to adrenocortical carcinoma. However, Tsuro et al (14) detailed temporary improvement in hypoglycemia with chemotherapy, and Rosario et al (40) reported resolution of NICTH due to undifferentiated thyroid cancer after use of doxorubicin and cisplatin. Imatinib, a more targeted systemic therapy, has been used for GIST, with successful resolution of hypoglycemia (30). However, Hamberg et al (32) describe a case of NICTH due to GIST in which they suggest that imatinib worsened the hypoglycemia.
Glucagon via injection may ameliorate hypoglycemia in cases of NICTH, but the effect with this route is short-lived (81, 86) and is probably best reserved as an adjunctive therapy in the setting of acute hypoglycemia. However, continuous glucagon infusion (iv) has been tried in several cases, with success as monotherapy predicted by a positive glucagon stimulation test (41, 60). The effect may be limited (14, 87), however, especially as tumor burden increases in terminal patients (33, 88).
Diazoxide is a nondiuretic benzothiadiazine derivative initially introduced as an antihypertensive but found to have hyperglycemia as a side effect. Diazoxide has subsequently been used with moderate success in hypoglycemia due to insulinoma, thought due to reduction in insulin secretion by β-cells (89). Use in NICTH was not found to be successful in any case we reviewed (33, 36, 41, 53, 90). Additionally, use is often limited by fluid retention and edema (41).
Octreotide, a nonspecific somatostatin analog, has been used unsuccessfully in multiple cases (39, 91). Even tumor positivity on octreotide scintigraphy did not predict success, with reports of octreotide leading to blood glucose stabilizing “somewhat” allowing reduction in concomitant dexamethasone (77) and absolutely no resolution of hypoglycemia or suppression of big IGF-II with “maximal doses of octreotide” (92). The only case with any success reported “a slight reduction in the frequency of hypoglycemic episodes” (78).
Recombinant human GH (rhGH) at supraphysiological doses of 3–12 mg daily has been successful in many cases (43, 44, 92–96), including one pediatric case (49). Use of rhGH in NICTH is likely successful via multiple mechanisms. GH suppresses peripheral glucose uptake (96) and leads to increased levels of IGF-I, IGFBP-3, and ALS, with resultant promotion of the normal ternary-complexed IGF-II (17, 79, 94). This makes it a much more targeted therapy for NICTH despite continued high IGF-II levels (17) and perhaps increased IGF-II levels (25, 94). However, rhGH is not universally successful in resolving NICTH (36, 85), and even where successful, use may be limited by the need for high doses (25, 93) and resultant side effects of fluid retention (79), orthostatic hypotension, and others, especially in older patients (97). Additionally, we propose that cost could be a limiting factor, especially for long-term therapy. Finally, without the benefit of randomized, controlled trial data, we cannot exclude the possibility that rhGH may stimulate the growth of tumor cells.
Glucocorticoids (including dexamethasone, hydrocortisone, prednisolone, and prednisone, typically in doses equivalent to prednisone 30–60 mg/d) are the most extensively described medical therapy for NICTH (98). High-dose glucocorticoid therapy has immediate beneficial effect on symptomatic hypoglycemia and, unlike other therapeutic regimens, can be effective in correcting the underlying biochemical dysfunction if long-term side effects do not occur. We found that 32 of 129 or 25% of individual cases reviewed (excluding the larger series and our own cases) included glucocorticoid therapy, and Tsuro et al (14) note 30% in their series of 20 Japanese patients with NICTH. Glucocorticoids have been successfully used as “bridge” therapy to resection (19, 98, 99). Complete freedom from iv glucose or dextrose using glucocorticoid monotherapy in nonresectable cases was also reported (14, 35, 73, 79, 90, 98), although in some cases hypoglycemia later recurred as tumor burden progressed (36, 51, 81). However, there are several cases where glucocorticoids failed as monotherapy (44, 87), even with extremely high doses (equivalent to > 200 mg prednisone daily) (43), and in combination therapy, although typically in cases of widespread disease/high tumor burden and imminent demise of the patient (29, 33, 100–102).
Unlike rhGH, glucocorticoids treat NICTH via reduction in IGF-II levels (79, 92, 98). Teale and Marks (25) demonstrated the biochemical differences between the therapies by measuring levels of glucose, insulin, C-peptide, GH, IGF-I, IGF-II, IGFBPs, and ALS before and after therapy in eight patients with NICTH, four of whom were treated with rhGH and four of whom were treated with glucocorticoids. Therapy with rhGH, while treating hypoglycemia largely via transition to ternary-complexed IGF-II, failed to produce normal insulin and C-peptide levels. This was thought to be due to a large remaining amount of unsequestered IGF-II with resultant ongoing activity at insulin receptors. Glucocorticoid therapy also led to hypoglycemia resolution, but did so via increased IGF-I and decreased big and total IGF-II (via suppressed production and/or increased clearance of big IGF-II), with resultant restoration of normal insulin and C-peptide levels. Glucocorticoids were more effective than rhGH in raising ALS levels but less effective in raising IGFBP-3 levels (25).
Combination therapy with glucocorticoids and rhGH may help minimize the doses and side effects of each (and mitigate some of the rhGH cost). Combination therapy was successful (including freedom from iv glucose or dextrose) in multiple cases where monotherapy with either failed (44, 92, 96), although one report suggested an antagonistic effect when the two were combined (98).
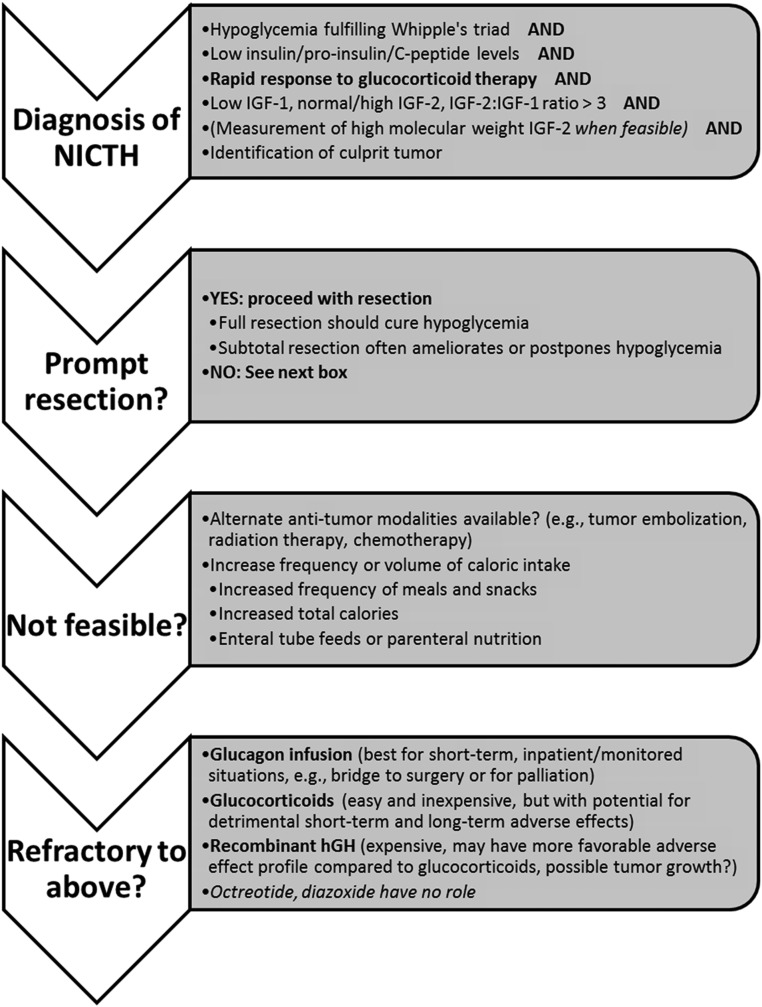
Although there are no randomized controlled trials to guide therapy, ample case reports and series are available in the literature over the past quarter century. For further review, Table 2 details the various treatment modalities utilized in the case reports and small case series reviewed. Additionally, Supplemental Table 1 (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org) expands on the detail provided in Table 2. Total resection is curative for NICTH, but prompt and/or total resection is not always feasible. In this setting, there is no clear “standard of care.” Nutritional approaches may provide relief from hypoglycemia but often do not suffice. Local or systemic targeted antitumor therapy may be an option for some patients and may be successful. In cases where this is not available or does not free the patient from hypoglycemia, the literature suggests the careful use of glucocorticoids. In certain cases, glucagon infusion and/or rhGH may be additive or glucocorticoid-sparing when side effects are unfavorable. Our management strategy is outlined in Figure 1.
Table 2.
Treatment Modalities Utilized in the Case Reports/Series Reviewed
| Treatment Modalities | Refs. |
|---|---|
| Resection only | 1, 11, 18, 20, 21, 26, 28, 38, 45, 47, 48, 50, 52, 54, 55, 61–71, 98, 103–121 |
| Nutritional approaches (eg, hyperalimentation, enteral tube feeds, parenteral nutrition) | 8, 36, 37, 51, 57, 72, 82, 83, 91, 92, 102 |
| Local therapies (eg, embolization, radiation) | 34, 36, 60, 72, 76, 87, 102, 120 |
| Systemic therapies (eg, chemotherapy, targeted antitumor therapy such as imatinib) | 1, 22, 30–32, 36, 40, 76, 77, 85, 87 |
| Glucocorticoids | 1, 14, 19, 29, 33, 35, 36, 43, 44, 46, 51, 56, 73, 75, 77, 79, 81, 87, 90–92, 96, 98–102 |
| rhGH | 36, 43, 44, 49, 79, 85, 92–96, 98 |
| Glucagon | 14, 33, 41, 60, 81, 86–88 |
| Octreotide | 39, 77, 78, 91, 92 |
| Diazoxide or bendrofluazide | 33, 36, 41, 53, 90, 92 |
A more detailed representation of the information in Table 2 can be found in Supplemental Table 1.
Figure 1.
Management strategy for NICTH.
Summary
NICTH is a rare but serious paraneoplastic syndrome involving progressive hypoglycemia in the setting of a wide variety of benign and malignant tumors, due to tumor production of high molecular weight IGF-II. A biochemical diagnosis is made when more common etiologies have been ruled out; hypoinsulinemic hypoglycemia is accompanied by an elevated IGF-II:IGF-I ratio (with low IGF-I and normal-to-high IGF-II). When possible, measurement of high molecular weight IGF-II further confirms the diagnosis. When a tumor is not previously known or readily apparent, imaging of the chest, abdomen, and pelvis is likely to be high yield. Prompt and complete surgical resection is curative, and subtotal resection and other local modalities such as radiation therapy may be successful. When resection is not feasible or is delayed, initial management of hypoglycemia involves increased caloric intake and frequency and/or iv glucose or dextrose. When conservative measures fail, medical therapy can be effective in alleviating hypoglycemia, although the degree of success likely correlates with overall tumor burden and progression. Based on the available literature, a trial of glucocorticoids at the lowest possible dose is a reasonable first step. Providers should anticipate the need to titrate to the equivalent of prednisone 30–60 mg per day, and even at these supraphysiological doses, success is not assured. When glucocorticoids fail to adequately control hypoglycemia or to reduce glucocorticoid exposure, addition of rhGH (again, at the lowest possible doses) to glucocorticoid therapy may provide greater therapeutic success with a more acceptable side effect profile than either agent alone. A reasonable alternative, acknowledging the limitations of continuous iv therapy, is continuous glucagon infusion. Octreotide and diazoxide have no role in NICTH.
Acknowledgments
T.W.B. was supported by the University of Michigan Health System's Metabolism, Endocrinology, and Diabetes Fellowship Program. M.P. was supported by the Michigan Institute for Clinical & Health Research and the Clinical and Translational Science Award program (UL1RR024986).
Disclosure Summary: The authors do not have any conflicts to disclose.
Funding Statement
T.W.B. was supported by the University of Michigan Health System's Metabolism, Endocrinology, and Diabetes Fellowship Program. M.P. was supported by the Michigan Institute for Clinical & Health Research and the Clinical and Translational Science Award program (UL1RR024986).
Footnotes
- ALS
- acid-labile subunit
- GIST
- gastrointestinal stromal tumor
- HCC
- hepatocellular carcinoma
- IGFBP-3
- IGF-binding protein-3
- NICTH
- non-islet cell tumor hypoglycemia
- rhGH
- recombinant human GH
- SFT
- solitary fibrous tumor.
References
- 1. de Groot JW, Rikhof B, van Doorn J, et al. Non-islet cell tumour-induced hypoglycaemia: a review of the literature including two new cases. Endocr Relat Cancer. 2007;14:979–993. [DOI] [PubMed] [Google Scholar]
- 2. Baldwin RS. Hypoglycemia with neoplasia (Doege-Potter Syndrome). Wis Med J. 1965;64:185–189. [PubMed] [Google Scholar]
- 3. Doege KW. Fibro-sarcoma of the mediastinum. Ann Surg. 1930;92:955–960. [PMC free article] [PubMed] [Google Scholar]
- 4. Marks V, Samols E. Hypoglycaemia of non-endocrine origin (non-islet cell tumours). Proc R Soc Med. 1966;59:338–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Teale JD. Non-islet cell tumour hypoglycaemia. Clin Endocrinol (Oxf). 1999;51:147. [DOI] [PubMed] [Google Scholar]
- 6. Megyesi K, Kahn CR, Roth J, Gorden P. Hypoglycemia in association with extrapancreatic tumors: demonstration of elevated plasma NSILA-s by a new radioreceptor assay. J Clin Endocrinol Metab. 1974;38:931–934. [DOI] [PubMed] [Google Scholar]
- 7. Zapf J, Walter H, Froesch ER. Radioimmunological determination of insulinlike growth factors I and II in normal subjects and in patients with growth disorders and extrapancreatic tumor hypoglycemia. J Clin Invest. 1981;68:1321–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Daughaday WH, Emanuele MA, Brooks MH, Barbato AL, Kapadia M, Rotwein P. Synthesis and secretion of insulin-like growth factor II by a leiomyosarcoma with associated hypoglycemia. N Engl J Med. 1988;319:1434–1440. [DOI] [PubMed] [Google Scholar]
- 9. Teale JD, Marks V. Inappropriately elevated plasma insulin-like growth factor II in relation to suppressed insulin-like growth factor I in the diagnosis of non-islet cell tumour hypoglycaemia. Clin Endocrinol (Oxf). 1990;33:87–98. [DOI] [PubMed] [Google Scholar]
- 10. Zapf J, Futo E, Peter M, Froesch ER. Can “big” insulin-like growth factor II in serum of tumor patients account for the development of extrapancreatic tumor hypoglycemia? J Clin Invest. 1992;90:2574–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fukuda I, Hizuka N, Takano K, Asakawa-Yasumoto K, Shizume K, Demura H. Characterization of insulin-like growth factor II (IGF-II) and IGF binding proteins in patients with non-islet-cell tumor hypoglycemia. Endocr J. 1993;40:111–119. [DOI] [PubMed] [Google Scholar]
- 12. Fukuda I, Hizuka N, Ishikawa Y, et al. Clinical features of insulin-like growth factor-II producing non-islet-cell tumor hypoglycemia. Growth Horm IGF Res. 2006;16:211–216. [DOI] [PubMed] [Google Scholar]
- 13. Hizuka N, Fukuda I, Takano K, Okubo Y, Asakawa-Yasumoto K, Demura H. Serum insulin-like growth factor II in 44 patients with non-islet cell tumor hypoglycemia. Endocr J. 1998;45(suppl):S61–S65. [DOI] [PubMed] [Google Scholar]
- 14. Tsuro K, Kojima H, Okamoto S, et al. Glucocorticoid therapy ameliorated hypoglycemia in insulin-like growth factor-II-producing solitary fibrous tumor. Intern Med. 2006;45:525–529. [DOI] [PubMed] [Google Scholar]
- 15. Miraki-Moud F, Grossman AB, Besser M, Monson JP, Camacho-Hübner C. A rapid method for analyzing serum pro-insulin-like growth factor-II in patients with non-islet cell tumor hypoglycemia. J Clin Endocrinol Metab. 2005;90:3819–3823. [DOI] [PubMed] [Google Scholar]
- 16. Dynkevich Y, Rother KI, Whitford I, et al. Tumors, IGF-2 and hypoglycemia: insights from the clinic, the laboratory and the historical archive. Endocr Rev. 2013;34:798–826. [DOI] [PubMed] [Google Scholar]
- 17. Baxter RC. The role of insulin-like growth factors and their binding proteins in tumor hypoglycemia. Horm Res. 1996;46:195–201. [DOI] [PubMed] [Google Scholar]
- 18. Bertherat J, Logié A, Gicquel C, Mourriéras F, Luton JP, Le Bouc Y. Alterations of the 11p15 imprinted region and the IGFs system in a case of recurrent non-islet-cell tumour hypoglycaemia (NICTH). Clin Endocrinol (Oxf). 2000;53:213–220. [DOI] [PubMed] [Google Scholar]
- 19. Tani Y, Tateno T, Izumiyama H, Doi M, Yoshimoto T, Hirata Y. Defective expression of prohormone convertase 4 and enhanced expression of insulin-like growth factor II by pleural solitary fibrous tumor causing hypoglycemia. Endocr J. 2008;55:905–911. [DOI] [PubMed] [Google Scholar]
- 20. Cotterill AM, Holly JM, Davies SC, Coulson VJ, Price PA, Wass JA. The insulin-like growth factors and their binding proteins in a case of non-islet-cell tumour-associated hypoglycaemia. J Endocrinol. 1991;131:303–411. [DOI] [PubMed] [Google Scholar]
- 21. Hoekman K, van Doorn J, Gloudemans T, Maassen JA, Schuller AG, Pinedo HM. Hypoglycaemia associated with the production of insulin-like growth factor II and insulin-like growth factor binding protein 6 by a haemangiopericytoma. Clin Endocrinol (Oxf). 1999;51:247–253. [DOI] [PubMed] [Google Scholar]
- 22. Zachariah S, Brackenridge A, Shojaee-Moradie F, Camuncho-Hubner C, Umpleby AM, Russell-Jones D. The mechanism of non-islet cell hypoglycaemia caused by tumour-produced IGF-II. Clin Endocrinol (Oxf). 2007;67:637–638. [DOI] [PubMed] [Google Scholar]
- 23. LeRoith D, Roberts CT Jr. The insulin-like growth factor system and cancer. Cancer Lett. 2003;195:127–137. [DOI] [PubMed] [Google Scholar]
- 24. Phillips LS, Robertson DG. Insulin-like growth factors and non-islet cell tumor hypoglycemia. Metabolism. 1993;42:1093–1101. [DOI] [PubMed] [Google Scholar]
- 25. Teale JD, Marks V. Glucocorticoid therapy suppresses abnormal secretion of big IGF-II by non-islet cell tumours inducing hypoglycaemia (NICTH). Clin Endocrinol (Oxf). 1998;49:491–498. [DOI] [PubMed] [Google Scholar]
- 26. Wakami K, Tateyama H, Kawashima H, et al. Solitary fibrous tumor of the uterus producing high-molecular-weight insulin-like growth factor II and associated with hypoglycemia. Int J Gynecol Pathol. 2005;24:79–84. [PubMed] [Google Scholar]
- 27. Corti B, Carella R, Gabusi E, D'Errico A, Martorana G, Grigioni WF. Solitary fibrous tumour of the urinary bladder with expression of bcl-2, CD34, and insulin-like growth factor type II. Eur Urol. 2001;39:484–488. [DOI] [PubMed] [Google Scholar]
- 28. Shapiro ET, Bell GI, Polonsky KS, Rubenstein AH, Kew MC, Tager HS. Tumor hypoglycemia: relationship to high molecular weight insulin-like growth factor-II. J Clin Invest. 1990;85:1672–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rikhof B, Van Den Berg G, Van Der Graaf WT. Non-islet cell tumour hypoglycaemia in a patient with a gastrointestinal stromal tumour. Acta Oncol. 2005;44:764–766. [DOI] [PubMed] [Google Scholar]
- 30. Pink D, Schoeler D, Lindner T, et al. Severe hypoglycemia caused by paraneoplastic production of IGF-II in patients with advanced gastrointestinal stromal tumors: a report of two cases. J Clin Oncol. 2005;23:6809–6811. [DOI] [PubMed] [Google Scholar]
- 31. Rikhof B, van Doorn J, Suurmeijer AJ, et al. Insulin-like growth factors and insulin-like growth factor-binding proteins in relation to disease status and incidence of hypoglycaemia in patients with a gastrointestinal stromal tumour. Ann Oncol. 2009;20:1582–1588. [DOI] [PubMed] [Google Scholar]
- 32. Hamberg P, de Jong FA, Boonstra JG, van Doorn J, Verweij J, Sleijfer S. Non-islet-cell tumor induced hypoglycemia in patients with advanced gastrointestinal stromal tumor possibly worsened by imatinib. J Clin Oncol. 2006;24:e30–e31. [DOI] [PubMed] [Google Scholar]
- 33. Davda R, Seddon BM. Mechanisms and management of non-islet cell tumour hypoglycaemia in gastrointestinal stromal tumour: case report and a review of published studies. Clin Oncol (R Coll Radiol). 2007;19:265–268. [DOI] [PubMed] [Google Scholar]
- 34. Escobar GA, Robinson WA, Nydam TL, et al. Severe paraneoplastic hypoglycemia in a patient with a gastrointestinal stromal tumor with an exon 9 mutation: a case report. BMC Cancer. 2007;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fernando HS, Hawkyard SJ, Poon P, Musa M. Renal cell carcinoma with non-islet cell tumor hypoglycemia. Int J Urol. 2006;13:985–986. [DOI] [PubMed] [Google Scholar]
- 36. Berman J, Harland S. Hypoglycaemia caused by secretion of insulin-like growth factor 2 in a primary renal cell carcinoma. Clin Oncol R Coll Radiol. 2001;13:367–369. [DOI] [PubMed] [Google Scholar]
- 37. Holt RI, Teale JD, Jones JS, Quin JD, McGregor AM, Miell JP. Gene expression and serum levels of insulin-like growth factors (IGFs) and IGF-binding proteins in a case of non-islet cell tumour hypoglycaemia. Growth Horm IGF Res. 1998;8:447–454. [DOI] [PubMed] [Google Scholar]
- 38. Eguchi T, Tokuyama A, Tanaka Y, et al. Hypoglycemia associated with the production of insulin-like growth factor II in adrenocortical carcinoma. Intern Med. 2001;40:759–763. [DOI] [PubMed] [Google Scholar]
- 39. Ishikura K, Takamura T, Takeshita Y, et al. Cushing's syndrome and big IGF-II associated hypoglycaemia in a patient with adrenocortical carcinoma. BMJ Case Rep. 2010;2010:pii:bcr07.2009.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rosário PW, Furtado MS, Castro AF, Purisch S. Non-islet cell tumor hypoglycemia in a patient with poorly differentiated thyroid cancer. Thyroid. 2007;17:84–85. [DOI] [PubMed] [Google Scholar]
- 41. Rastogi MV, Desai N, Quintos JB. Non-islet-cell tumor hypoglycemia and lactic acidosis in a child with congenital HIV and Burkitt's lymphoma. J Pediatr Endocrinol Metab. 2008;21:805–810. [DOI] [PubMed] [Google Scholar]
- 42. Snowden JA, Greaves M, Page K. Reversal of diabetes associated with escape of myeloma: evidence for inappropriate IGF-II secretion. Br J Haematol. 1994;87:202–204. [DOI] [PubMed] [Google Scholar]
- 43. Powter L, Phillips S, Husbands E. A case report of non-islet cell tumour hypoglycaemia associated with ovarian germ-cell tumour. Palliat Med. 2013;27:281–283. [DOI] [PubMed] [Google Scholar]
- 44. Mukherjee S, Diver M, Weston PJ. Non islet cell tumor hypoglycaemia in a metastatic Leydig cell tumor. Acta Oncol. 2005;44:761–763. [DOI] [PubMed] [Google Scholar]
- 45. Hino N, Nakagawa Y, Ikushima Y, Yoshida M, Tsuyuguchi M. A case of a giant phyllodes tumor of the breast with hypoglycemia caused by high-molecular-weight insulin-like growth factor II. Breast Cancer. 2010;17:142–145. [DOI] [PubMed] [Google Scholar]
- 46. Rosseel L, De Leu N, Van Hecke W, Unuane D. A rare case of hypoglycemia in a patient with elevated right hemidiaphragm. BMJ Case Rep. 2012;2012:pii:bcr0320125972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zafar H, Takimoto CH, Weiss G. Doege-Potter syndrome: hypoglycemia associated with malignant solitary fibrous tumor. Med Oncol. 2003;20:403–408. [DOI] [PubMed] [Google Scholar]
- 48. Kalebi AY, Hale MJ, Wong ML, Hoffman T, Murray J. Surgically cured hypoglycemia secondary to pleural solitary fibrous tumour: case report and update review on the Doege-Potter syndrome. J Cardiothorac Surg. 2009;4:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Agus MS, Katz LE, Satin-Smith M, Meadows AT, Hintz RL, Cohen P. Non-islet-cell tumor associated with hypoglycemia in a child: successful long-term therapy with growth hormone. J Pediatr. 1995;127:403–407. [DOI] [PubMed] [Google Scholar]
- 50. Korn E, Van Hoff J, Buckley P, Daughaday WH, Carpenter TO. Secretion of a large molecular-weight form of insulin-like growth factor by a primary renal tumor. Med Pediatr Oncol. 1995;24:392–396. [DOI] [PubMed] [Google Scholar]
- 51. Schutt RC, Gordon TA, Bhabhra R, et al. Doege-Potter syndrome presenting with hypoinsulinemic hypoglycemia in a patient with a malignant extrapleural solitary fibrous tumor: a case report. J Med Case Rep. 2013;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. O'Loughlin A, Waldron-Lynch F, Cronin KC, et al. When a nephrectomy cures hypoglycaemia. BMJ Case Rep. 2009;2009:bcr0220091617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sturrock ND, Selby C, Hosking DJ. Spontaneous hypoglycaemia in a noninsulin-dependent diabetes mellitus patient with disseminated pancreatic carcinoma. Diabet Med. 1997;14:324–326. [DOI] [PubMed] [Google Scholar]
- 54. Schweichler M, Hennessey JV, Cole P, Perdue JF, Le Roith D. Hypoglycemia in pregnancy secondary to a non-islet cell tumor of the pleura and ectopic insulin-like growth factor II hormone production. Obstet Gynecol. 1995;85:810–813. [DOI] [PubMed] [Google Scholar]
- 55. Li Z, Wang J, Zhu Q, Li H, Chen Y, Chen L. Huge solitary fibrous tumor of the pleura with hypoglycemia and hypokalemia: a case report [published online January 31, 2013]. Ann Thorac Cardiovasc Surg. doi:10.5761/atcs.cr.12.01913. [DOI] [PubMed] [Google Scholar]
- 56. Ikeda K, Mizuguchi M, Yoshida H, et al. Preclinical Cushing's syndrome associated with non-islet cell tumor hypoglycemia; an additional report. Intern Med. 2003;42:1151–1152. [DOI] [PubMed] [Google Scholar]
- 57. Trivedi N, Mithal A, Sharma AK, et al. Non-islet cell tumour induced hypoglycaemia with acromegaloid facial and acral swelling. Clin Endocrinol (Oxf). 1995;42:433–435. [DOI] [PubMed] [Google Scholar]
- 58. Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94:709–728. [DOI] [PubMed] [Google Scholar]
- 59. Livingstone C. Insulin-like growth factor-II and cancer. Endocr Relat Cancer. 2013;20:R321–R339. [DOI] [PubMed] [Google Scholar]
- 60. Hoff AO, Vassilopoulou-Sellin R. The role of glucagon administration in the diagnosis and treatment of patients with tumor hypoglycemia. Cancer. 1998;82:1585–1592. [PubMed] [Google Scholar]
- 61. Kishi K, Homma S, Tanimura S, Matsushita H, Nakata K. Hypoglycemia induced by secretion of high molecular weight insulin-like growth factor-II from a malignant solitary fibrous tumor of the pleura. Intern Med. 2001;40:341–344. [DOI] [PubMed] [Google Scholar]
- 62. Lucas CE, Ledgerwood AM. Malignant solitary fibrous tumor of the intestine with refractory hypoglycemia (Doege Potter syndrome). J Am Coll Surg. 2006;203:398. [DOI] [PubMed] [Google Scholar]
- 63. Kameyama K, Okumura N, Kokado Y, Miyoshi K, Matsuoka T, Nakagawa T. Solitary fibrous tumor associated with non-islet cell tumor hypoglycemia. Ann Thorac Surg. 2007;84:292–294. [DOI] [PubMed] [Google Scholar]
- 64. Chaugle H, Parchment C, Grotte GJ, Keenan DJ. Hypoglycaemia associated with a solitary fibrous tumour of the pleura. Eur J Cardiothorac Surg. 1999;15:84–86. [DOI] [PubMed] [Google Scholar]
- 65. Famà F, Le Bouc Y, Barrande G, et al. Solitary fibrous tumour of the liver with IGF-II-related hypoglycaemia. A case report. Langenbecks Arch Surg. 2008;393:611–616. [DOI] [PubMed] [Google Scholar]
- 66. Yamakawa-Yokota F, Ozaki N, Okajima A, Nishio H, Nagasaka T, Oiso Y. Retroperitoneal solitary fibrous tumor-induced hypoglycemia associated with high molecular weight insulin-like growth factor II. Clin Med Res. 2010;8:159–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chang JC, Su KY, Chao SF, Hsu YH, Yang GG, Chang BS. Hypoglycemia in a patient with a huge malignant solitary fibrous tumor of the pleura. Pathol Int. 2007;57:791–793. [DOI] [PubMed] [Google Scholar]
- 68. Okabe R, Sonobe M, Bando T, Date H. Large solitary fibrous tumor with overexpression of insulin-like growth factor-2. Interact Cardiovasc Thorac Surg. 2010;11:688–690. [DOI] [PubMed] [Google Scholar]
- 69. Yang CY, Chou CW, Hao LJ. Malignant solitary fibrous tumor with hypoglycemia (Doege-Potter syndrome). J Postgrad Med. 2013;59:64–66. [DOI] [PubMed] [Google Scholar]
- 70. Tan G, Teo M, Choo SP. Hypoglycaemia in a 63-year-old female with a large, recurrent, metastatic gastrointestinal stromal tumour (GIST). J Gastrointest Cancer. 2011;42:263–265. [DOI] [PubMed] [Google Scholar]
- 71. Matsuda S, Usui M, Sakurai H, Suzuki H, Ogura Y, Shiraishi T. Insulin-like growth factor II-producing intra-abdominal hemangiopericytoma associated with hypoglycemia. J Gastroenterol. 2001;36:851–855. [DOI] [PubMed] [Google Scholar]
- 72. Kishi K, Sonomura T, Sato M. Radiotherapy for hypoglycaemia associated with large leiomyosarcomas. Br J Radiol. 1997;70:306–308. [DOI] [PubMed] [Google Scholar]
- 73. Kageyama K, Moriyama T, Hizuka N, et al. Hypoglycemia associated with big insulin-like growth factor II produced during development of malignant fibrous histiocytoma. Endocr J. 2003;50:753–758. [DOI] [PubMed] [Google Scholar]
- 74. Bessell EM, Selby C, Ellis IO. Severe hypoglycaemia caused by raised insulin-like growth factor II in disseminated breast cancer. J Clin Pathol. 1999;52:780–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Barra WF, Castro G, Hoff AO, Siqueira SA, Hoff PM. Symptomatic hypoglycemia related to inappropriately high igf-ii serum levels in a patient with desmoplastic small round cell tumor. Case Rep Med. 2010;2010:684045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. de Boer J, Jager PL, Wiggers T, et al. The therapeutic challenge of a nonresectable solitary fibrous tumor in a hypoglycemic patient. Int J Clin Oncol. 2006;11:478–481. [DOI] [PubMed] [Google Scholar]
- 77. Höög A, Sandberg Nordqvist AC, Hulting AL, Falkmer UG. High-molecular weight IGF-2 expression in a haemangiopericytoma associated with hypoglycaemia. APMIS. 1997;105:469–482. [DOI] [PubMed] [Google Scholar]
- 78. Ma RC, Tong PC, Chan JC, Cockram CS, Chan MH. A 67-year-old woman with recurrent hypoglycemia: non-islet cell tumour hypoglycemia. CMAJ. 2005;173:359–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Baxter RC, Holman SR, Corbould A, et al. Regulation of the insulin-like growth factors and their binding proteins by glucocorticoid and growth hormone in nonislet cell tumor hypoglycemia. J Clin Endocrinol Metab. 1995;80:2700–2708. [DOI] [PubMed] [Google Scholar]
- 80. Kanzaki M, Kashihara H, Kiura K, et al. Severe hypoglycemia induced by IGF-II producing non-islet cell tumor. Intern Med. 2007;46:1061. [DOI] [PubMed] [Google Scholar]
- 81. Scott K. Non-islet cell tumor hypoglycemia. J Pain Symptom Manage. 2009;37:e1–e3. [DOI] [PubMed] [Google Scholar]
- 82. Ishida S, Noda M, Kuzuya N, et al. Big insulin-like growth factor II-producing hepatocellular carcinoma associated with hypoglycemia. Intern Med. 1995;34:1201–1206. [DOI] [PubMed] [Google Scholar]
- 83. Tominaga N, Kawarasaki C, Kanemoto K, et al. Recurrent solitary fibrous tumor of the pleura with malignant transformation and non-islet cell tumor-induced hypoglycemia due to paraneoplastic overexpression and secretion of high-molecular-weight insulin-like growth Factor II. Intern Med. 2012;51:3267–3272. [DOI] [PubMed] [Google Scholar]
- 84. Kirkland LL, Kashiwagi DT, Brantley S, Scheurer D, Varkey P. Nutrition in the hospitalized patient. J Hosp Med. 2013;8:52–58. [DOI] [PubMed] [Google Scholar]
- 85. Hunter SJ, Daughaday WH, Callender ME, et al. A case of hepatoma associated with hypoglycaemia and overproduction of IGF-II (E-21): beneficial effects of treatment with growth hormone and intrahepatic adriamycin. Clin Endocrinol (Oxf). 1994;41:397–401; discussion 402. [DOI] [PubMed] [Google Scholar]
- 86. Föger B, Zapf J, Lechleitner M, Konwalinka G, Patsch JR. Prevention with glucocorticoids of extrapancreatic tumour-hypoglycaemia as a result of increased 'big' insulin-like growth factor II. J Intern Med. 1994;236:692–693. [DOI] [PubMed] [Google Scholar]
- 87. Tietge UJ, Schöfl C, Ocran KW, et al. Hepatoma with severe non-islet cell tumor hypoglycemia. Am J Gastroenterol. 1998;93:997–1000. [DOI] [PubMed] [Google Scholar]
- 88. Kuenen BC, van Doorn J, Slee PH. Non-islet-cell tumour induced hypoglycaemia: a case report and review of literature. Neth J Med. 1996;48:175–179. [DOI] [PubMed] [Google Scholar]
- 89. Goode PN, Farndon JR, Anderson J, Johnston ID, Morte JA. Diazoxide in the management of patients with insulinoma. World J Surg. 1986;10:586–592. [DOI] [PubMed] [Google Scholar]
- 90. Krishnan L, Clark J. Non-islet cell tumour hypoglycaemia. BMJ Case Rep. 2011;2011:pii:bcr0220113914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Morbois-Trabut L, Maillot F, De Widerspach-Thor A, Lamisse F, Couet C. “Big IGF-II”-induced hypoglycemia secondary to gastric adenocarcinoma. Diabetes Metab. 2004;30:276–279. [DOI] [PubMed] [Google Scholar]
- 92. Perros P, Simpson J, Innes JA, Teale JD, McKnight JA. Non-islet cell tumour-associated hypoglycaemia: 111In-octreotide imaging and efficacy of octreotide, growth hormone and glucocorticosteroids. Clin Endocrinol (Oxf). 1996;44:727–731. [DOI] [PubMed] [Google Scholar]
- 93. Silveira LF, Bouloux PM, MacColl GS, Camacho-Hubner C, Miraki-Moud F. Growth hormone therapy for non-islet cell tumor hypoglycemia. Am J Med. 2002;113:255–257. [DOI] [PubMed] [Google Scholar]
- 94. Teale JD, Blum WF, Marks V. Alleviation of non-islet cell tumour hypoglycaemia by growth hormone therapy is associated with changes in IGF binding protein-3. Ann Clin Biochem. 1992;29:314–323. [DOI] [PubMed] [Google Scholar]
- 95. Drake WM, Miraki F, Siddiqi A, et al. Dose-related effects of growth hormone on IGF-I and IGF-binding protein-3 levels in non-islet cell tumour hypoglycaemia. Eur J Endocrinol. 1998;139:532–536. [DOI] [PubMed] [Google Scholar]
- 96. Bourcigaux N, Arnault-Ouary G, Christol R, Périn L, Charbonnel B, Le Bouc Y. Treatment of hypoglycemia using combined glucocorticoid and recombinant human growth hormone in a patient with a metastatic non-islet cell tumor hypoglycemia. Clin Ther. 2005;27:246–251. [DOI] [PubMed] [Google Scholar]
- 97. Sullivan DH, Carter WJ, Warr WR, Williams LH. Side effects resulting from the use of growth hormone and insulin-like growth factor-I as combined therapy to frail elderly patients. J Gerontol A Biol Sci Med Sci. 1998;53:M183–M187. [DOI] [PubMed] [Google Scholar]
- 98. Teale JD, Wark G. The effectiveness of different treatment options for non-islet cell tumour hypoglycaemia. Clin Endocrinol (Oxf). 2004;60:457–460. [DOI] [PubMed] [Google Scholar]
- 99. Ndzengue A, Deribe Z, Rafal RB, et al. Non-islet cell tumor hypoglycemia associated with uterine leiomyomata. Endocr Pract. 2011;17:e109–e112. [DOI] [PubMed] [Google Scholar]
- 100. Maruyama H, Tatsumi M, Kitayama H, et al. A case of gastric cancer with non-islet cell tumor hypoglycemia detected by insulin-like growth factor II. Pathol Int. 2010;60:595–597. [DOI] [PubMed] [Google Scholar]
- 101. Kato A, Bando E, Shinozaki S, et al. Severe hypoglycemia and hypokalemia in association with liver metastases of gastric cancer. Intern Med. 2004;43:824–828. [DOI] [PubMed] [Google Scholar]
- 102. Rose MG, Tallini G, Pollak J, Murren J. Malignant hypoglycemia associated with a large mesenchymal tumor: case report and review of the literature. Cancer J Sci Am. 1999;5:48–51. [PubMed] [Google Scholar]
- 103. Alkemade GM, Bakker M, Rikhof B, et al. Hypoglycemia in a patient with a big “big”-IGF-II-producing tumor. J Clin Endocrinol Metab. 2013;98:3113–3114. [DOI] [PubMed] [Google Scholar]
- 104. Balduyck B, Lauwers P, Govaert K, Hendriks J, De Maeseneer M, Van Schil P. Solitary fibrous tumor of the pleura with associated hypoglycemia: Doege-Potter syndrome: a case report. J Thorac Oncol. 2006;1:588–590. [PubMed] [Google Scholar]
- 105. Chamberlain MH, Taggart DP. Solitary fibrous tumor associated with hypoglycemia: an example of the Doege-Potter syndrome. J Thorac Cardiovasc Surg. 2000;119:185–187. [DOI] [PubMed] [Google Scholar]
- 106. Christofilis MA, Remacle-Bonnet M, Atlan-Gepner C, et al. Study of serum big-insulin-like growth factor (IGF)-II and IGF binding proteins in two patients with extrapancreatic tumor hypoglycemia, using a combination of Western blotting methods. Eur J Endocrinol. 1998;139:317–322. [DOI] [PubMed] [Google Scholar]
- 107. Cole FH Jr, Ellis RA, Goodman RC, Weber BC, Courington DP. Benign fibrous pleural tumor with elevation of insulin-like growth factor and hypoglycemia. South Med J. 1990;83:690–694. [DOI] [PubMed] [Google Scholar]
- 108. Daughaday WH, Kapadia M. Significance of abnormal serum binding of insulin-like growth factor II in the development of hypoglycemia in patients with non-islet-cell tumors. Proc Natl Acad Sci USA. 1989;86:6778–6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Fukasawa Y, Takada A, Tateno M, et al. Solitary fibrous tumor of the pleura causing recurrent hypoglycemia by secretion of insulin-like growth factor II. Pathol Int. 1998;48:47–52. [DOI] [PubMed] [Google Scholar]
- 110. Herrmann BL, Saller B, Kiess W, et al. Primary malignant fibrous histiocytoma of the lung: IGF-II producing tumor induces fasting hypoglycemia. Exp Clin Endocrinol Diabetes. 2000;108:515–518. [DOI] [PubMed] [Google Scholar]
- 111. Hirai A, Nakanishi R. Solitary fibrous tumor of the pleura with hypoglycemia associated with serum insulin-like growth factor II. J Thorac Cardiovasc Surg. 2006;132:713–714. [DOI] [PubMed] [Google Scholar]
- 112. Kim JH, Kim JO, Kim SY, Na MH, Lim SP, Kim JM. Two cases of large solitary fibrous tumors of the pleura associated with fasting hypoglycemia. Eur Radiol. 2001;11:819–824. [DOI] [PubMed] [Google Scholar]
- 113. Lawson EA, Zhang X, Crocker JT, Wang WL, Klibanski A. Hypoglycemia from IGF2 overexpression associated with activation of fetal promoters and loss of imprinting in a metastatic hemangiopericytoma. J Clin Endocrinol Metab. 2009;94:2226–2231. [DOI] [PubMed] [Google Scholar]
- 114. Lee CE, Zanariah H, Masni M, Pau KK. Solitary fibrous tumour of the pleura presenting with refractory non-insulin mediated hypoglycaemia (the Doege-Potter syndrome). Med J Malaysia. 2010;65:72–74. [PubMed] [Google Scholar]
- 115. Masson EA, MacFarlane IA, Graham D, Foy P. Spontaneous hypoglycaemia due to a pleural fibroma: role of insulin like growth factors. Thorax. 1991;46:930–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Moat NE, Teale JD, Lea RE, Matthews AW. Spontaneous hypoglycaemia and pleural fibroma: role of insulin like growth factors. Thorax. 1991;46:932–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Roy TM, Burns MV, Overly DJ, Curd BT. Solitary fibrous tumor of the pleura with hypoglycemia: the Doege-Potter syndrome. J Ky Med Assoc. 1992;90:557–560. [PubMed] [Google Scholar]
- 118. Thabit H, Healy ML, Royston D, et al. A case of spontaneous hypoglycaemia and impaired glucose tolerance in the same patient. Ann Clin Biochem. 2011;48:183–185. [DOI] [PubMed] [Google Scholar]
- 119. Wagner S, Greco F, Hamza A, Hoda RM, Holzhausen HJ, Fornara P. Retroperitoneal malignant solitary fibrous tumor of the small pelvis causing recurrent hypoglycemia by secretion of insulin-like growth factor 2. Eur Urol. 2009;55:739–742. [DOI] [PubMed] [Google Scholar]
- 120. Hu Y, Mahar TJ, Hicks DG, et al. Malignant solitary fibrous tumor: report of 3 cases with unusual features. Appl Immunohistochem Mol Morphol. 2009;17:451–457. [DOI] [PubMed] [Google Scholar]
- 121. Hata T, Tsuruta Y, Takamori S, Shishikura Y. Non-islet cell tumor hypoglycemia at the second recurrence of malignant solitary fibrous tumor in the retroperitoneum and pelvis: a case report. Case Rep Oncol. 2012;5:420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]