Abstract
Context:
Endometriosis is one of the most common gynecological diseases in women with a prevalence rate of approximately 10%. Chronic pelvic inflammation has been observed in patients with endometriosis and is associated with disease severity. However, how pelvic inflammation promotes endometriosis progression remains unknown.
Objective:
The objective of the study was to investigate the regulatory network of proinflammatory cytokines in endometriosis progression.
Design, Settings, and Patients:
Immunostaining of human endometrial (n = 21) and endometriotic (n = 36) sections, quantitative RT-PCR, Western blotting, chromatin immunoprecipitation, and luciferase reporter assays in primary culture human endometrial stromal cells were performed. Autologous transplantation of uterine endometrium from control chicken ovalbumin upstream promoter-transcription factor II [(COUP-TFII) flox/flox] and uterus-specific COUP-TFII knockout mice was performed.
Results:
Expression of COUP-TFII was significantly reduced in endometriotic stroma. Reduction of COUP-TFII in endometriotic stromal cells was mediated by proinflammatory cytokines including IL-1β, TNF-α, and TGF-β1 via a common effector, microRNA-302a. Treatment with these proinflammatory cytokines increased the expression of microRNA-302a, which targets the 3′untranslated region of COUP-TFII to cause its down-regulation. Intriguingly, down-regulation of COUP-TFII in endometrial stromal cells resulted in de-repression of cyclooxygenase-2 (COX-2). Further investigation demonstrated that COUP-TFII directly binds to COX-2 promoter to inhibit its transcription. Forced expression of COUP-TFII inhibited IL-1β-induced COX-2 up-regulation, whereas the knockdown of COUP-TFII augmented this effect.
Conclusion:
Because overexpression of COX-2 has been demonstrated to be a master regulator in endometriosis progression, our data demonstrate the critical function of proinflammatory cytokines and the COUP-TFII regulatory gene network in the progression of endometriosis.
Endometriosis is a common and complex gynecological disease associated with chronic pelvic pain and infertility with a prevalence rate of approximately 10% in women of reproductive age. The cost of treating endometriosis is about $22 billion per year in the United States (1); however, there is no effective treatment available to cure this disease. Surgical removal of endometriotic tissues is the gold standard of current treatment regimen. Unfortunately, about 50% of patients relapse within 5 years after surgery (2). The lack of efficacious treatment regimens highlights the urgency for more intensive studies to unravel the molecular and cellular mechanisms underlying the pathological processes of this disease.
Although the etiology of endometriosis remains unclear, elevation of proinflammatory cytokines, such as IL-1β, IL-6, TNF-α, and TGF-β1, has been found in peritoneal fluid of women with endometriosis (3, 4). Although in vitro studies demonstrated that these proinflammatory cytokines may involve in preventing cell apoptosis, promoting cell proliferation, and inducing angiogenesis (5), the underlying mechanisms of how these proinflammatory cytokines contribute to endometriosis development and/or progression remains largely uncharacterized.
Chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII, also known as NR2F2) is an orphan nuclear receptor that plays pivotal roles in cell fate determination and organ development (6). In the female reproductive system, COUP-TFII was found to be indispensable for proper embryo implantation and placentation (7, 8), indicating it is a critical regulator in uterine physiology. Furthermore, COUP-TFII was found to oppose the mitogenic action of estrogen receptor-α (7) and to regulate the expression of steroidogenic acute regulatory protein (StAR) (9, 10) and aromatase (11), proteins that regulate the committed step of estrogen biosynthesis. Because endometriosis is an estrogen-dependent disease and aberrant expression of StAR (12) and aromatase (13) has been found in ectopic endometriotic stromal cells, we hypothesize that the down-regulation of COUP-TFII may play an important role in the pathogenesis of endometriosis. Interestingly, the expression of COUP-TFII transcript was found to be reduced in endometriotic cells (11), providing an important clue to support our hypothesis. However, the function of COUP-TFII in endometriosis progression and the mechanism responsible for COUP-TFII down-regulation in endometriotic cells remain uncharacterized.
COUP-TFII forms a homodimer or heterodimer with COUP-TFI, a close related protein, and binds to a wide range of direct repeat AGGTCA motifs with a variety of spacing and orientation (14). Binding of the homodimer COUP-TFII to the direct repeat sequence results in suppression of gene transcription. In addition, COUP-TFII can also activate gene expression by tethering to specificity protein-1 on a specificity protein-1 binding site (15). Recent studies revealed that the loss of COUP-TFII results in an increase of genes responsible for cell proliferation/differentiation and migration/invasion (16, 17). In lights of the importance of COUP-TFII in female reproduction and its multiple functions in gene regulation, it is necessary to further investigate the regulation and functions of COUP-TFII in normal endometrium and endometriotic lesions. Herein we report the discovery of mechanisms that cause COUP-TFII down-regulation and subsequent pathological processes due to the loss of COUP-TFII in endometriotic cells. Our data provide an important piece of evidence to functionally link chronic inflammation in the peritoneum with pathological processes of endometriosis.
Materials and Methods
Clinical samples
Tissues from individuals without (defined as normal, n = 21) or with (n = 36) endometriosis were collected during laparoscopic or gynecological operations at the National Cheng Kung University Hospital (18). This study was approved by the Clinical Research Ethics Committee at the National Cheng Kung University Medical Center, and informed consent was obtained from each patient. A more detailed description of each study is provided in the Supplemental Methods, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org.
Primary stromal cell isolation and treatments
The procedure for isolation of normal endometrial stromal cells, eutopic endometrial stromal cells of patients with endometriosis, and ectopic endometriotic stromal cells was illustrated previously (12). A more detailed protocol is provided in the Supplemental Methods.
RNA isolation and quantitative RT-PCR
The protocol for the isolation of total RNA was followed by the manufacturer's instructions (TRIsure; Bioline USA Inc). The levels of mRNA were quantified by using SYBR Green PCR master mix (Roche) and specific primers listed in the Supplemental Table 1 using a thermocycler (StepONE plus; Applied Biosciences). To analyze mature microRNA expression, specific RT-PCR primers were purchased from Ambion (Invitrogen), and a TaqMan microRNA reverse transcription kit (Invitrogen) was used to perform the reverse transcription for microRNA.
Western blot analysis and immunohistochemical staining
Standard procedures for Western blot and immunohistochemical staining were performed routinely in the laboratory. The antibodies used are described in the Supplemental Methods.
Chromatin immunoprecipitation (ChIP) assay
The procedure for ChIP was described previously (19). In brief, COUP-TFII protein was fixed with DNA by using 1% formaldehyde for 10 minutes. Cells were harvested and sonicated to fragment DNA (average size of ∼200–500 bp). Then a COUP-TFII antibody was used to pull down the COUP-TFII protein and DNA complexes. DNA-containing COUP-TFII binding motif and control region were amplified by specific primers (Supplemental Table 1) after reverse cross-linking.
Promoter activity assay, small interference RNA (siRNA), microRNA, and cDNA overexpression
A fragment of human cyclooxygenase-2 (COX-2) 5′ flanking region (−916 to +23 bp) was cloned into a pXP1 vector containing the luciferase reporter system. Site-directed mutagenesis of putative COUP-TFII binding sites and promoter activity assay procedures were performed as described before (20). siRNA and microRNA-302a (miR-302a) mimics were purchased from commercial companies. A more detailed description of each procedure is provided in the Supplemental Methods.
Animal experiment
Conditional knockout of COUP-TFII mice was generated by using Cre/Loxp system as described previously (21, 22). All procedures for animal study were approved by the Institutional Committee of Animal Care Guidelines at Baylor College of Medicine. A more detail protocol of the study is provided in the Supplemental Methods.
Gene reporter assay
A 2240-bp fragment of 3′untranslated region (UTR) of COUP-TFII was amplified and cloned into a pIS-2 vector containing the luciferase reporter system. Site-directed mutagenesis was performed to mutate the two miR-302a recognition sites. A more detailed protocol is provided in the Supplemental Methods.
Statistical analysis
The data are expressed as means ± SEM. A Student's t test was used to compare differences between two groups. Differences between groups were analyzed using one-way ANOVA using commercial statistical software (GraphPad Prism 5.02; GraphPad Software) followed by post hoc analysis using a Tukey's multiple analysis test. Statistical significance was set at P < .05.
Results
COUP-TFII is down-regulated in endometriotic stromal cells
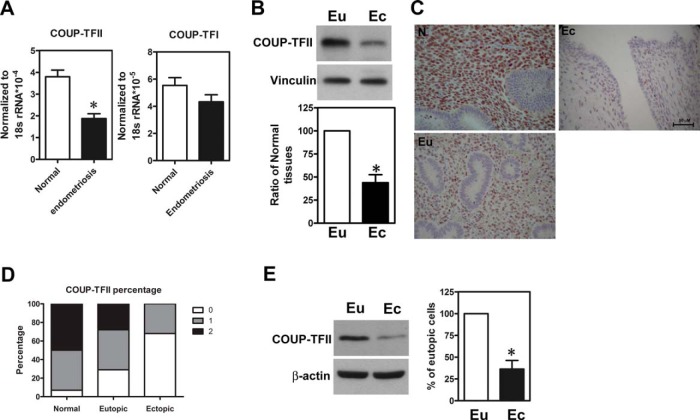
To investigate the function of COUP-TFII in endometriosis, we quantified levels of COUP-TFII in normal endometria and endometriotic tissues. The levels of COUP-TFII mRNA were significantly decreased in the endometriotic tissues (n = 36) compared with the normal endometria (n = 21) (Figure 1A). In contrast, levels of COUP-TFI, a close family member of COUP-TFII in mammals, were not different (Figure 1A). When compared with eutopic tissues collected from the same individual, COUP-TFII expression was markedly reduced in ectopic endometriotic tissues (Figure 1B). Immunohistochemical staining showed that COUP-TFII was mainly expressed in the stromal cells and was significantly reduced in ectopic endometriotic lesions (Figure 1, C and D). Indeed, Western blot results from paired primary cultured stromal cells showed that levels of COUP-TFII were markedly reduced in ectopic stroma compared with the eutopic counterparts (Figure 1E). Most interestingly, COUP-TFII expression was lower in the eutopic endometrial tissues derived from women with endometriosis compared with those derived from women without endometriosis (Figure 1, C and D).
Figure 1.
COUP-TFII is down-regulated in endometriotic stromal cells. A, Levels of COUP-TFII and COUP-TFI mRNA in normal endometria (n = 21) and endometriotic tissues (n = 36) determined by quantitative RT-PCR. 18S ribosomal RNA was quantified as an internal control. *, Significant difference from normal at P < .05 by Student's t test. B, Representative Western blot image (upper panel) and quantitative results (lower panel, n = 6) show levels of COUP-TFII protein in eutopic (Eu) and ectopic (Ec) tissues obtained from the same individuals with endometriosis. *, Significant difference from eutopic tissues at P < .05 by Student's t test. C and D, Representative immunohistochemistry pictures (C) and quantitative results (D) show immunoreactivity of COUP-TFII proteins in normal endometria (N, n = 21), eutopic tissue (Eu, n = 36), and ectopic lesion (Ec, n = 36). Staining results were as follows: 0, negative; 1, weak staining; 2, strong staining. E, Representative Western blot (left panel) and quantitative results (right panel, n = 5) show levels of COUP-TFII proteins in paired eutopic endometrial stromal cells (Eu) and ectopic endometriotic stromal cells (Ec) from patients with endometriosis. *, Significant difference from eutopic stromal cells at P < .05 by Student's t test.
Down-regulation of COUP-TFII is inhibited by proinflammatory cytokines
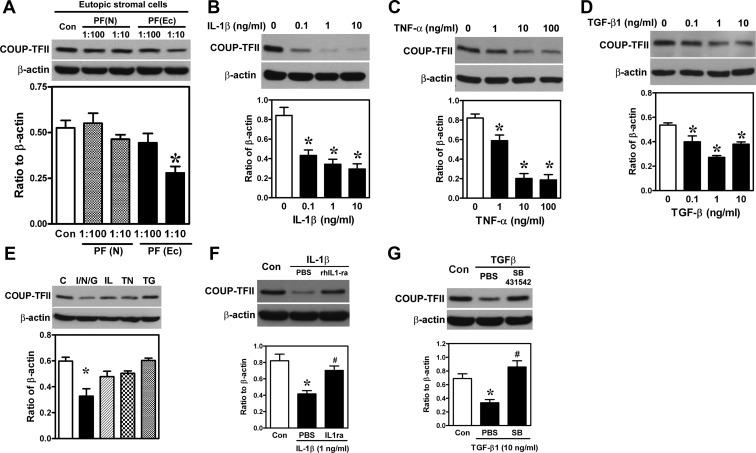
Because endometriosis is an inflammatory disease and many proinflammatory cytokines are elevated in the peritoneal fluid of patients with endometriosis, we sought to determine whether these proinflammatory cytokines affect COUP-TFII expression. As shown in Figure 2A, treatment of normal endometrial stromal cells with endometriotic peritoneal fluid in 1:10 dilution reduced COUP-TFII expression, whereas treatment with normal peritoneal fluid had no effect. To identify factor(s) responsible for such inhibitory effect, several proinflammatory cytokines known to be elevated in the peritoneal fluid of women with endometriosis were used to treat normal endometrial stromal cells and levels of COUP-TFII were quantified. Our results demonstrated that IL-1β, TNF-α, and TGF-β1 dose dependently inhibited COUP-TFII mRNA and protein expression (Figure 2, B–D, and Supplemental Figure 1), whereas prostaglandin E2 (PGE2) and IL-6 had no effect (Supplemental Figure 1). Combined treatment with suboptimal doses of these three proinflammatory cytokines exerted an additive effect on the down-regulation of COUP-TFII (Figure 2E), whereas individual cytokine at this dose has little effect on COUP-TFII expression. Furthermore, treatment with IL-1β receptor antagonist and TGF-β receptor inhibitor (SB431542) blocked the inhibitory effect of IL-1β and TGF-β1, respectively (Figure 2, F and G), suggesting specific receptor-mediated signaling pathways were used. Taken together, these data demonstrate that proinflammatory cytokines in the peritoneal fluid of women with endometriosis are factors causing the loss of COUP-TFII in endometrial stromal cells.
Figure 2.
COUP-TFII is down-regulated by proinflammatory cytokines elevated in peritoneal fluid of individuals with endometriosis. A, Representative Western blot (upper panel) and quantitative results (lower panel, n = 4) show levels of COUP-TFII protein in normal endometrial stromal cells treated with peritoneal fluids (PFs) derived from women with [PF(Ec)] and without [PF(N)] endometriosis. *, Significant difference from control (Con). B–D, Representative Western blots (upper panel) and quantitative results (lower panel, n = 4) show levels of COUP-TFII in normal endometrial stromal cells treated with different doses of IL-1β (B), TNF-α (C), or TGF-β1 (D) for 24 hours. *, Significant difference from vehicle controls. E, Representative Western blot (upper panel) and quantitative result (lower panel, n = 4) show levels of COUP-TFII in normal endometrial stromal cells treated with IL-1β (IL; 0.01 ng/mL), TNF-α (TN; 0.1 ng/mL), TGF-β1 (TG; 0.01 ng/mL) or in combination (I/N/G) for 24 hours. *, Significant difference from vehicle control. F, Representative Western blots (upper panel) and quantitative results (low panel, n = 4) show levels of COUP-TFII in normal endometrial stromal cells treated with IL-1β in the presence or absence of recombinant IL-1β receptor antagonist (rIL1-ra; 200 ng/mL) for 24 hours. *, Significant difference from vehicle control (Con); #, significant difference from PBS-treated group. G, Representative Western blots (upper panel) and quantitative results (low panel, n = 4) show levels of COUP-TFII in normal endometrial stromal cells treated with TGF-β1 in the presence or absence of TGF-β receptor inhibitor (SB431542; 10 μM) for 24 hours. *, Significant difference from vehicle control (Con); #, significant difference from PBS-treated group.
Proinflammatory cytokine-repressed COUP-TFII expression is mediated by miR-302a
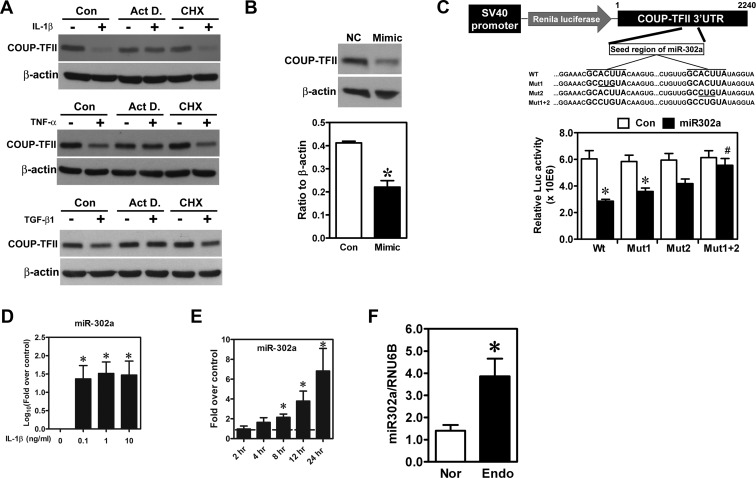
To further investigate the underlying mechanism of how COUP-TFII was repressed, actinomycin D or cycloheximide was administered to the culture media to test whether de novo RNA and/or protein synthesis is required for such inhibitory effect. Our results indicated that cycloheximide treatment did not reverse IL-1β-, TNF-α-, or TGF-β1-inhibited COUP-TFII expression. In contrast, treatment with actinomycin D restored IL-1β-, TNF-α-, and TGF-β1-repressed COUP-TFII expression (Figure 3A). These results imply that the down-regulation of COUP-TFII by IL-1β, TNF-α, and TGF-β1 is not dependent on de novo protein synthesis but is likely mediated by noncoding RNA such as microRNA (miRNA). To this end, we used bioinformatic tools to predict potential miRNA that would target COUP-TFII. After bioinformatic prediction and prescreening, miR-302a was identified as a COUP-TFII-targeting miRNA. Indeed, treatment with miR-302a mimics in normal endometrial stromal cells inhibited COUP-TFII expression (Figure 3B). In addition, two miR-302a seed sequences were identified in the 3′UTR of COUP-TFII, which are highly conserved across different species (Supplemental Figure 2). Transient transfection of miR-302a mimics reduced the luciferase activity of plasmids carrying wild-type COUP-TFII 3′UTR but had no effect on seed sequence-mutated ones (Figure 3C), confirming that miR-302a was able to recognize these two seed sequences and suppressed COUP-TFII expression.
Figure 3.
COUP-TFII is down-regulated by miR-302a. A, Representative Western blot images show levels of COUP-TFII in normal endometrial stromal cells treated with IL-1β (upper panel), TNF-α (middle panel), and TGF-β1 (lower panel) in the presence or absence of actinomycin D (Act D; 1 μM) or cycloheximide (CHX; 1 μM) for 24 hours. These experiments were repeated three times using different batches of cells. B, Representative Western blot (upper panel) and quantitative result (lower panel) show levels of COUP-TFII in normal endometrial stromal cells treated with miR-302a mimics (Mimic). *, Significant difference from control. C, Schematic drawing (upper panel) shows construct of luciferase reporter system containing COUP-TFII 3′UTR. The two seed regions of miR-302a (WT) and mutated sequences (Mut1, Mut2, and Mut1+2) are shown. Lower panel shows relative luciferase activity in endometrial stromal cells transfected with wild-type or miR-302a seed sequence-mutated reporter constructs cotransfected with control miRNA (Con) or miR-302a. *, Significant difference from control miRNAs; #, significant difference compared with wild-type constructs treated with miR-302a. D and E, Quantitative results show miR-302a levels in endometrial stromal cells treated with different doses of IL-1β (D) or 1 ng/ml IL-1β for different lengths of time (E). *, Significant difference from control. F, Quantitative results show levels of miR-302a in normal endometrial stromal cells (Nor; n = 21) and endometriotic stromal cells (Endo; n = 36). *, Significant difference from normal cells by a Student's t test.
Next, we tested whether the levels of miR-302a were induced by IL-1β, TNF-α, and TGF-β1. Results show that miR-302a was up-regulated by IL-1β, TNF-α, and TGF-β1 by as early as 8 hours (Figure 3, D and E, and Supplemental Figure 3), which precedes the down-regulation of COUP-TFII. Quantitative RT-PCR results confirmed that the level of miR-302a was elevated in endometriotic stromal cells compared with the normal counterparts (Figure 3F). Taken together, these data demonstrate that cytokine-repressed COUP-TFII expression is mediated by the induction of miR-302a expression.
COUP-TFII negatively regulates COX-2 expression
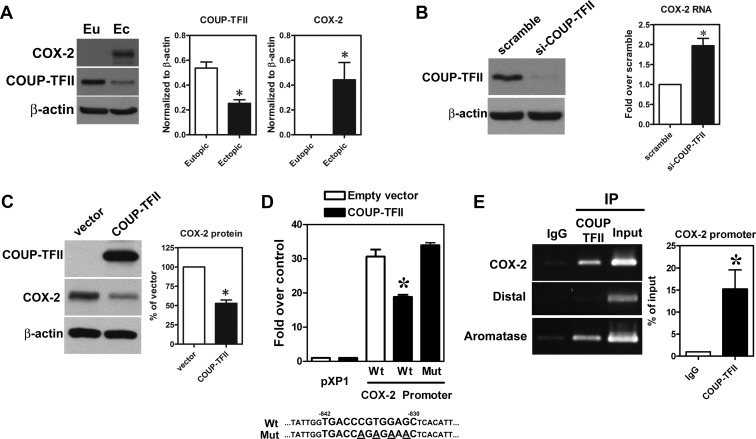
To investigate the pathological function of COUP-TFII down-regulation in endometriosis, we performed a genome-wide prediction of potential COUP-TFII candidate genes using the in-house Bioinformatic platform, the BEST (http://thebest.binfo.ncku.edu.tw/thebest/). Among the long list of the candidate genes, COX-2 caught our eyes immediately because we have previously found that COX-2 and its metabolic product, PGE2, play numerous indispensable roles in endometriosis development and progression (23). Therefore, we investigated the effect of COUP-TFII on COX-2 expression. First, we confirmed the bioinformatic predicted result by Western blotting and showed there was an inverse correlation of COUP-TFII and COX-2 in paired eutopic and ectopic stromal cells (Figure 4A). Next, knockdown of COUP-TFII in eutopic stromal cells (high COUP-TFII, no COX-2) increased COX-2 expression, whereas overexpression of COUP-TFII in ectopic endometriotic stromal cells (low COUP-TFII, high COX-2) reduced COX-2 expression (Figure 4, B and C). Bioinformatic analysis identified a putative COUP-TFII binding site located in the COX-2 promoter region (−842 to −830), and results from the promoter activity assay demonstrated that forced expression of COUP-TFII inhibited wild-type but not mutated COX-2 promoter activity (Figure 4D). Furthermore, ChIP-PCR results showed that COUP-TFII specifically bound to this element in normal endometrial stromal cells (Figure 4E).
Figure 4.
Expression of COX-2 is inhibited by COUP-TFII. A, Representative Western blot (left panel) and quantitative results (middle and right panel) show levels of COX-2 protein in paired eutopic and ectopic endometrial stromal cells from patients with endometriosis (n = 6). *, Significant difference from eutopic endometrial stromal cells. B, Representative Western blot (left panel) shows level of COUP-TFII protein in normal endometrial stromal cells treated with siRNA against COUP-TFII (si-COUP-TFII) or scrambled siRNA. Right panel shows quantitative result of COX-2 mRNA quantified by quantitative RT-PCR (n = 5). *, Significant difference from scrambled control. C, Representative Western blot (left panel) shows level of COUP-TFII and COX-2 proteins in ectopic endometriotic stromal cells transfected with human COUP-TFII plasmid or empty vector. Right panel shows quantitative result of COX-2 protein (n = 4). *, Significant difference from empty vector control. D, Quantitative result shows COX-2 promoter activities in normal endometrial stromal cells transfected with empty vector or COUP-TFII containing plasmids (n = 5). pXP1, basic vector without human COX-2 promoter (−916 to +23); Wt, Cox-2 promoter with wild-type COUP-TFII binding site; Mut, COX-2 promoter with COUP-TFII binding site mutated. Wild-type (Wt) and mutated (Mut) sequences of COUP-TFII binding region in the COX-2 promoter were shown at the bottom of the figure. E, Representative picture (left panel) and quantitative result (right panel) show results of COUP-TFII binding to COX-2 promoter determined by ChIP assay. COX-2, PCR products amplified by specific primer; Distal, PCR products amplified by primer pairs 2000 bp upstream of predicted COUP-TFII binding site; aromatase, PCR products amplified by aromatase primers as a positive control. *, Significant difference from nonspecific control using mouse IgG.
Reduced COUP-TFII enhances COX-2 promoter sensitivity
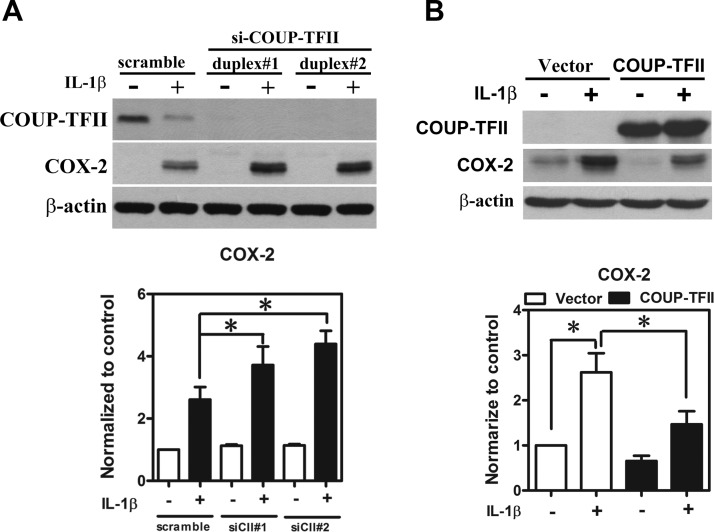
We have previously reported that ectopic endometriotic stromal cells are more sensitive to the IL-1β-induced COX-2 expression compared with the normal endometrial stromal cells (24). We reasoned that the loss of COUP-TFII in endometriotic stromal cells may contribute to the increased sensitivity of COX-2 to the IL-1β challenge. To test this hypothesis, we used COUP-TFII knockdown and overexpression approaches in normal and endometriotic stromal cells, respectively. As shown in Figure 5, A and B, knockdown of COUP-TFII in normal endometrial stromal cells further increased IL-1β-induced COX-2 expression, whereas overexpression of COUP-TFII in endometriotic stromal cells inhibited IL-1β-induced COX-2 expression. Taken together, these results suggest that COUP-TFII plays an important role in determining the degree of responsiveness of COX-2 promoter to the IL-1β challenge.
Figure 5.
COUP-TFII negatively regulates COX-2 promoter activity. A, Representative Western blot (upper panel) and quantitative results (lower panel) show levels of COX-2 protein in COUP-TFII-specific siRNA (siCII#1 and siCII#2) or scrambled siRNA transfected normal endometrial stromal cells treated with or without IL-1β for 24 hours. *, Significant difference between groups as indicated. B, Representative Western blot (upper panel) and quantitative results (lower panel) show levels of COX-2 protein in COUP-TFII plasmids or empty vector-transfected ectopic endometriotic stromal cells treated with or without IL-1β for 24 hours. *, Significant difference between groups as indicated.
Proinflammatory cytokines regulate COX-2 expression via miR-302a and COUP-TFII
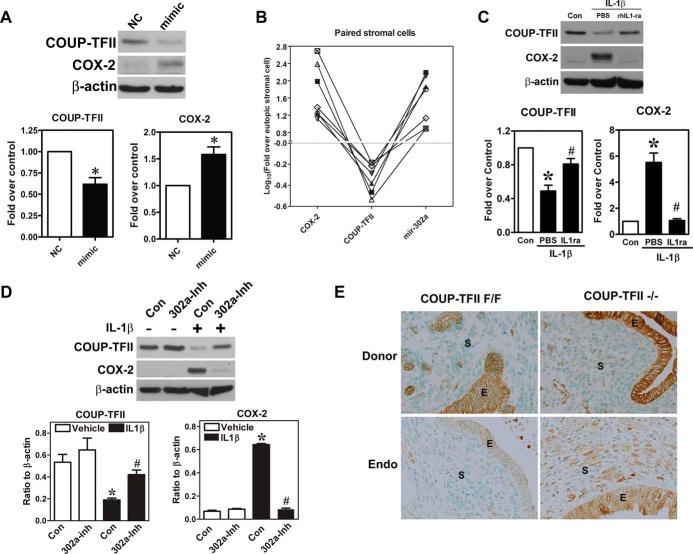
Next, we tested whether induction of miR-302a in normal endometrial stromal cells is sufficient to cause COX-2 overexpression. Indeed, transient transfection of miR-302a mimics inhibited COUP-TFII expression and conversely induced COX-2 expression (Figure 6A). We analyzed the expression of miR-302a, COUP-TFII, and COX-2 in six pairs of eutopic and ectopic stromal cells. Results demonstrated that miR-302a positively correlated with COX-2, whereas both miR-302a and COX-2 negatively correlated with COUP-TFII (Figure 6B). Treatment with IL-1β resulted in the COUP-TFII suppression and COX-2 overexpression, and both were reversed by cotreatment with IL-1β receptor antagonist (Figure 6C). Finally, we demonstrated that the IL-1β-induced COUP-TFII down-regulation and the COX-2 up-regulation can be reversed by pretreatment with miR-302a inhibitor (Figure 6D).
Figure 6.
miR-302a mediates IL-1β-induced COUP-TFII down-regulation and COX-2 up-regulation. A, Representative Western blot (left panel) and quantitative results (middle and right panels, n = 5) show levels of COUP-TFII and COX-2 proteins in normal endometrial stromal cells treated with miR-302a mimics (mimic) or scrambled miRNA (NC). *, Significant difference compared with control. B, Quantitative results show ratios of COX-2, COUP-TFII, and miR-302a in six pairs of endometrial stromal cells and endometriotic stromal cells from patients with endometriosis. Levels of COX-2, COUP-TFII, and miR-302a in eutopic endometrial stromal cells were set as 0.0 after logarithmic transformation. C, Representative Western blot (upper panel) and quantitative results (lower panels, n = 5) show levels of COUP-TFII and COX-2 proteins in normal endometrial stromal cells treated with IL-1β with or without pretreatment with recombinant IL-1β receptor antagonist (rIL-1ra). *, Significant difference from control; #, significant difference from PBS-treated cells. D, Representative Western blot (upper panel) and quantitative results (lower panels, n = 3) show levels of COUP-TFII and COX-2 proteins in normal endometrial stromal cells treated with IL-1β with or without pretreatment with miR-302a inhibitor (302a-Inh). *, Significant difference from control; #, significant difference from IL-1β-treated cells. E, Representative immunohistochemistry pictures show levels of COX-2 in donor endometrial tissues (donor) and endometriotic lesions (Endo) after injection of COUP-TFII-knockout or intact endometrial tissues into the peritoneal cavity of receipt mice for 4 weeks. COUP-TFII F/F, wild-type COUP-TFII; COUP-TFII −/−, uterine stroma-specific COUP-TFII knockout; E, epithelial cells; S, stromal cells.
Loss of COUP-TFII expression increased COX-2 overexpression in endometriotic lesion
Our in vitro experiments demonstrated that knockdown of COUP-TFII leads to aberrant COX-2 expression. To further confirm these findings in vivo, we isolated the endometrial tissues from uterus-specific COUP-TFII knockout mice and control mice (COUP-TFII flox/flox) to set up the mouse model of endometriosis. As shown in Figure 6E, COX-2 expression was mainly in the epithelial cells of donor uterine endometria, irrespective of with or without uterus-specific COUP-TFII knockout. In contrast, the stromal cells in COUP-TFII knockout endometrium expressed slightly more COX-2 protein. After injected into peritoneal cavity and implanted in the abdominal wall of normal recipient mice, COX-2 levels in the endometriotic lesion derived from COUP-TFII knockout-endometrial tissue was markedly increased (Figure 6E). This result indicated clearly that COUP-TFII is essential for the increased COX-2 expression in endometriotic lesion in vivo.
Discussion
It is well acknowledged that endometriosis is a chronic inflammatory disease with elevated proinflammatory cytokines in the peritoneal fluid. However, the pathological roles of many proinflammatory cytokines remain uncharacterized. Here we report that proinflammatory cytokines such as IL-1β, TNF-α, and TGF-β1 individually and cooperatively suppress the multifunctional transcription regulator, COUP-TFII. Inhibition of COUP-TFII leads to de-repression of COX-2 promoter and results in not only increasing basal level of COX-2 but also enhancing the sensitivity of COX-2 promoter to proinflammatory cytokines' stimulation. Our data provide mechanistic insights of the pathological roles of proinflammatory cytokines in the progression of endometriosis and, for the first time, reveal the molecular mechanism of COUP-TFII function in regulating COX-2 expression.
It has been previously demonstrated that COUP-TFII mRNA was reduced in endometriotic tissue, which leads to loss of antagonistic function against steroidogenesis factor-1 in regulating the promoter activity of StAR (9) and aromatase (11). However, the expression of COUP-TFII protein was not examined. More importantly, the mechanism of how COUP-TFII is suppressed in ectopic endometriotic tissue remains unanswered. Herein, by using quantitative RT-PCR, Western blot, and immunohistostaining approaches, we demonstrated that COUP-TFII, but not COUP-TFI, mRNA and protein were reduced in endometriotic tissues. Immunohistostaining data further revealed that, similar to what was reported in mouse uterus (7), COUP-TFII protein was mainly expressed in stromal cells. Of note, our data showed that eutopic stromal cells in individuals with endometriosis expressed lower amount of COUP-TFII as compared with stromal cells of individuals without endometriosis. This result supports the notion that eutopic endometria of individuals with endometriosis are biochemically different from those of normal women. Part of the reason for this difference may be contributed by effects of proinflammatory cytokines (described below) or genetic differences. Further study is necessary to investigate whether a genetic factor is involved in controlling COUP-TFII expression in women with or without endometriosis. Nevertheless, our data provide definitive evidence demonstrating that levels of COUP-TFII mRNA and protein are differentially expressed in normal and endometriotic stroma, which highlights its critical functions in endometriotic pathogenesis. It has been shown that COUP-TFII is indispensable for maintaining normal pregnancy as evidenced by COUP-TFII knockout mice showing severe defects in embryo implantation and placenta formation (7, 8). Thus, our data showing reduced expression of COUP-TFII in eutopic endometrial stroma of individuals with endometriosis may explain, at least in part, why women with endometriosis are less likely to be pregnant.
The reduced expression of COUP-TFII in paired eutopic and ectopic endometrial tissues prompted us to consider that local microenvironmental factors may be responsible for this phenomenon. Because endometriosis is a chronic inflammatory disease, we sought to investigate whether any of these proinflammatory cytokines would affect the expression of COUP-TFII. Our results demonstrated that IL-1β, TNF-α, and TGF-β1 dose dependently inhibited COUP-TFII mRNA and protein expression, whereas IL-6 and PGE2 had no effect. Intriguingly, the inhibition does not require synthesis of new proteins but depends on the biogenesis of RNA molecules because treatment with actinomycin D (blocking transcription) but not cycloheximide (blocking translation) rescued the expression of COUP-TFII. The results of RNA molecules being able to reduce mRNA and protein levels provide a clue to search for possible candidates from the pool of miRNA. Several lines of evidence support that miR-302a is the intermediate effector that executes the inhibitory action of IL-1β, TNF-α, and TGF-β1. First, miR-302a was time and dose dependently induced by all three proinflammatory cytokines; second, transient transfection of miR-302a mimics was enough to inhibit COUP-TFII expression; third, transfection of miR-302a mimics suppressed COUP-TFII 3′UTR reporter activity, whereas pretreatment with the miR-302a inhibitor blocked IL-1β-mediated COUP-TFII down-regulation; and fourth, results from paired stromal cells derived from eutopic and ectopic tissues of individuals with endometriosis provide confirmative evidence showing an inverse correlation between miR-302a and COUP-TFII. Taken all together, our data provide a novel mechanism of gene regulation when multiple proinflammatory cytokines aim to control the same target gene. This is an economical mechanism for controlling gene expression, a mechanism that clearly has the evolutional advantage.
Loss of COUP-TFII was suggested to contribute to aberrant expression of aromatase and StAR protein in endometriotic stromal cells (9–11). Herein we provided evidence to demonstrate a pivotal pathological function caused by loss of COUP-TFII, derepression of COX-2. Overexpression of COX-2 in ectopic endometriotic stromal cells is a key factor leading to acquisition of steroidogenic capacity, increase of cell proliferation, induction of angiogenesis, and suppression of phagocytosis via aberrant production of PGE2 (23). It has been shown that ectopic endometriotic stromal cells aberrantly express COX-2 and are at least 100 times more sensitive to an IL-1β challenge compared with the eutopic counterparts (24). Previously we have demonstrated that loss of the ERK-specific phosphatase, dual-specificity phosphatase-2, contributes to the increase of COX-2 promoter activity (18, 25). Here we showed that knockdown of COUP-TFII in eutopic endometrial stromal cells not only induces COX-2 expression but also enhances sensitivity of IL-1β-induced COX-2 expression, which phenocopies the characteristics of endometriotic stromal cells. This notion is supported by the data that COUP-TFII binds to the COX-2 promoter to suppress its transcription. When COUP-TFII was knocked down in normal endometrial stromal cells or reduced in endometriotic stromal cells, likely due to proinflammatory cytokine-mediated down-regulation as described above, the suppressive effect of COUP-TFII was demolished and the COX-2 promoter becomes more accessible by transcription factors, such as cAMP response element binding protein (24). As a result, the transcription of COX-2 promoter becomes much easier, a phenomenon similar to what was observed in aromatase and StAR promoter (9, 11).
The basal level of COX-2 protein in normal endometrial stromal cells is undetectable but can be induced by proinflammatory cytokines such as IL-1β, TNF-α, and macrophage migration inhibiting factor (24, 26–28). IL-1β-induced COX-2 expression in normal endometrial stromal cells is mediated via increasing its mRNA stability (24, 26). Here we demonstrated another mechanism of how COX-2 was induced by proinflammatory cytokines (Supplemental Figure 4). The elevated proinflammatory cytokines induce miR-302a expression, which targets COUP-TFII mRNA at the 3′UTR to inhibit COUP-TFII expression. Reduced COUP-TFII expression in ectopic endometriotic stromal cells leads to the de-repression of COX-2 promoter activity. This not only induces COX-2 expression in ectopic endometriotic stromal cells but also enhances COX-2 promoter sensitivity in response to other stimuli. Because the peritoneal fluid of individuals with endometriosis contains many factors that can stimulate COX-2, an increase in promoter accessibility enables these factors to activate COX-2 transcription. As a result, levels of COX-2 in the ectopic endometriotic stromal cells are constitutively up-regulated and large amounts of PGE2 are synthesized. Because PGE2 regulates many critical processes for endometriosis development and progression including steroidogenesis, mitogenesis, angiogenesis, and immune suppression (23), the effects of elevated proinflammatory cytokines are amplified like a chain reaction.
Taking all data together, we showed a novel gene-regulatory network that regulates important pathological processes of endometriosis. This gene network involves proinflammatory cytokines (IL-1β, TNF-α, and TGF-β1), an epigenetic regulator (miR-302a), a transcription factor (COUP-TFII), a catabolic enzyme (COX-2), a metabolite of the enzyme (PGE2), and effector genes regulated by that metabolite. This gene-regulatory network scheme provides evidence to support the notion that endometriosis is a multigenic disease affected by gene-gene and microenvironment-gene interactions. It also highlights the difficulty of treating endometriosis with a single drug that aims to block a particular function of gene. With this in mind, future research should concentrate on developing effective prodrugs that can be activated by local microenvironmental factors such as oxygen tension, pH value, or reactive oxygen species. The activated drugs then target on blocking multiple enzyme activities or disrupting a gene regulating pathway to simultaneously attenuate or terminate the pathological signal initiated by inflammation.
Acknowledgments
We are grateful to Ms Mei-Feng Huang and Ms Yi-Shang-Yeh for technical support in isolating primary endometrial stromal cells and performing immunohistochemical staining. We thank the Bioinformatics Center of National Cheng Kung University for providing assistance in analyzing COUP-TFII-regulated candidate genes.
Author contributions included the following: S.-C.L. and Y.-H.L. performed the experiments, analyzed the data, and wrote the manuscript. M.-H.W. and Y.-F.C. collected the clinical samples, performed the immunohistochemistry, and analyzed the data. D.-K.L., S.Y.T., and M.-J.T. designed the mouse experiment and analyzed the data. S.-J.T. coordinated this project and wrote the manuscript. All authors read and approved the final version of this manuscript.
This work was supported by Grant NSC-101–2320-B-006–030-MY3 from the National Science Council of Taiwan.
Disclosure Summary: The authors declare that there is no conflict of interest.
Funding Statement
This work was supported by Grant NSC-101–2320-B-006–030-MY3 from the National Science Council of Taiwan.
Footnotes
- ChIP
- chromatin immunoprecipitation
- COUP-TFII
- chicken ovalbumin upstream promoter-transcription factor II
- COX-2
- cyclooxygenase-2
- miRNA
- microRNA
- miR-302a
- microRNA-302a
- PGE2
- prostaglandin E2
- siRNA
- small interference RNA
- StAR
- steroidogenic acute regulatory protein
- UTR
- untranslated region.
References
- 1. Simoens S, Hummelshoj L, D'Hooghe T. Endometriosis: cost estimates and methodological perspective. Hum Reprod Update. 2007;13:395–404. [DOI] [PubMed] [Google Scholar]
- 2. Cheong Y, Tay P, Luk F, Gan HC, Li TC, Cooke I. Laparoscopic surgery for endometriosis: how often do we need to re-operate? J Obstet Gynaecol. 2008;28:82–85. [DOI] [PubMed] [Google Scholar]
- 3. Koyama N, Matsuura K, Okamura H. Cytokines in the peritoneal fluid of patients with endometriosis. Int J Gynaecol Obstet. 1993;43:45–50. [DOI] [PubMed] [Google Scholar]
- 4. Kupker W, Schultze-Mosgau A, Diedrich K. Paracrine changes in the peritoneal environment of women with endometriosis. Hum Reprod Update. 1998;4:719–723. [DOI] [PubMed] [Google Scholar]
- 5. Harada T, Enatsu A, Mitsunari M, et al. Role of cytokines in progression of endometriosis. Gynecol Obstet Invest. 1999;47(suppl 1):34–39; discussion 39–40. [DOI] [PubMed] [Google Scholar]
- 6. Lin FJ, Qin J, Tang K, Tsai SY, Tsai MJ. Coup d'etat: an orphan takes control. Endocr Rev. 2011;32:404–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kurihara I, Lee DK, Petit FG, et al. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007;3:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petit FG, Jamin SP, Kurihara I, et al. Deletion of the orphan nuclear receptor COUP-TFII in uterus leads to placental deficiency. Proc Natl Acad Sci USA. 2007;104:6293–6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Attar E, Tokunaga H, Imir G, et al. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J Clin Endocrinol Metab. 2009;94:623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takamoto N, Kurihara I, Lee K, Demayo FJ, Tsai MJ, Tsai SY. Haploinsufficiency of chicken ovalbumin upstream promoter transcription factor II in female reproduction. Mol Endocrinol. 2005;19:2299–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zeitoun K, Takayama K, Michael MD, Bulun SE. Stimulation of aromatase P450 promoter (II) activity in endometriosis and its inhibition in endometrium are regulated by competitive binding of steroidogenic factor-1 and chicken ovalbumin upstream promoter transcription factor to the same cis-acting element. Mol Endocrinol. 1999;13:239–253. [DOI] [PubMed] [Google Scholar]
- 12. Tsai SJ, Wu MH, Lin CC, Sun HS, Chen SM. Regulation of steroidogenic acute regulatory protein expression and progesterone production in endometriotic stromal cells. J Clin Endocrinol Metab. 2001;86:5765–5773. [DOI] [PubMed] [Google Scholar]
- 13. Noble LS, Simpson ER, Johns A, Bulun SE. Aromatase expression in endometriosis. J Clin Endocrinol Metab. 1996;81:174–179. [DOI] [PubMed] [Google Scholar]
- 14. Cooney AJ, Tsai SY, O'Malley BW, Tsai MJ. Chicken ovalbumin upstream promoter transcription factor (COUP-TF) dimers bind to different GGTCA response elements, allowing COUP-TF to repress hormonal induction of the vitamin D3, thyroid hormone, and retinoic acid receptors. Mol Cell Biol. 1992;12:4153–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qin J, Chen X, Xie X, Tsai MJ, Tsai SY. COUP-TFII regulates tumor growth and metastasis by modulating tumor angiogenesis. Proc Natl Acad Sci USA. 2010;107:3687–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qin J, Wu SP, Creighton CJ, et al. COUP-TFII inhibits TGF-β-induced growth barrier to promote prostate tumorigenesis. Nature. 2013;493:236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu SP, Cheng CM, Lanz RB, et al. Atrial identity is determined by a COUP-TFII regulatory network. Dev Cell. 2013;25:417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu MH, Lin SC, Hsiao KY, Tsai SJ. Hypoxia-inhibited dual-specificity phosphatase-2 expression in endometriotic cells regulates cyclooxygenase-2 expression. J Pathol. 2011;225:390–400. [DOI] [PubMed] [Google Scholar]
- 19. Lin SC, Chien CW, Lee JC, et al. Suppression of dual-specificity phosphatase-2 by hypoxia increases chemoresistance and malignancy in human cancer cells. J Clin Invest. 2011;121:1905–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu CW, Lin SC, Chen KF, Lai YY, Tsai SJ. Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance. J Biol Chem. 2008;283:28106–28114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takamoto N, You LR, Moses K, et al. COUP-TFII is essential for radial and anteroposterior patterning of the stomach. Development. 2005;132:2179–2189. [DOI] [PubMed] [Google Scholar]
- 22. Chuang PC, Lin YJ, Wu MH, Wing LY, Shoji Y, Tsai SJ. Inhibition of CD36-dependent phagocytosis by prostaglandin E2 contributes to the development of endometriosis. Am J Pathol. 2010;176:850–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu MH, Lu CW, Chuang PC, Tsai SJ. Prostaglandin E2: the master of endometriosis? Exp Biol Med (Maywood). 2010;235:668–677. [DOI] [PubMed] [Google Scholar]
- 24. Wu MH, Wang CA, Lin CC, Chen LC, Chang WC, Tsai SJ. Distinct regulation of cyclooxygenase-2 by interleukin-1β in normal and endometriotic stromal cells. J Clin Endocrinol Metab. 2005;90:286–295. [DOI] [PubMed] [Google Scholar]
- 25. Lin SC, Wang CC, Wu MH, Yang SH, Li YH, Tsai SJ. Hypoxia-induced microRNA-20a expression increases ERK phosphorylation and angiogenic gene expression in endometriotic stromal cells. J Clin Endocrinol Metab. 2012;97:E1515–E1523. [DOI] [PubMed] [Google Scholar]
- 26. Tamura M, Sebastian S, Yang S, Gurates B, Fang Z, Bulun SE. Interleukin-1β elevates cyclooxygenase-2 protein level and enzyme activity via increasing its mRNA stability in human endometrial stromal cells: an effect mediated by extracellularly regulated kinases 1 and 2. J Clin Endocrinol Metab. 2002;87:3263–3273. [DOI] [PubMed] [Google Scholar]
- 27. Kim YA, Kim JY, Kim MR, Hwang KJ, Chang DY, Jeon MK. Tumor necrosis factor-α-induced cyclooxygenase-2 overexpression in eutopic endometrium of women with endometriosis by stromal cell culture through nuclear factor-κB activation. J Reprod Med. 2009;54:625–630. [PubMed] [Google Scholar]
- 28. Carli C, Metz CN, Al-Abed Y, Naccache PH, Akoum A. Up-regulation of cyclooxygenase-2 expression and prostaglandin E2 production in human endometriotic cells by macrophage migration inhibitory factor: involvement of novel kinase signaling pathways. Endocrinology. 2009;150:3128–3137. [DOI] [PMC free article] [PubMed] [Google Scholar]