Abstract
Context:
Several fracture prediction models that combine fractures at different sites into a composite outcome are in current use. However, to the extent individual fracture sites have differing risk factor profiles, model discrimination is impaired.
Objective:
The objective of the study was to improve model discrimination by developing a 5-year composite fracture prediction model for fracture sites that display similar risk profiles.
Design:
This was a prospective, observational cohort study.
Setting:
The study was conducted at primary care practices in 10 countries.
Patients:
Women aged 55 years or older participated in the study.
Intervention:
Self-administered questionnaires collected data on patient characteristics, fracture risk factors, and previous fractures.
Main Outcome Measure:
The main outcome is time to first clinical fracture of hip, pelvis, upper leg, clavicle, or spine, each of which exhibits a strong association with advanced age.
Results:
Of four composite fracture models considered, model discrimination (c index) is highest for an age-related fracture model (c index of 0.75, 47 066 women), and lowest for Fracture Risk Assessment Tool (FRAX) major fracture and a 10-site model (c indices of 0.67 and 0.65). The unadjusted increase in fracture risk for an additional 10 years of age ranges from 80% to 180% for the individual bones in the age-associated model. Five other fracture sites not considered for the age-associated model (upper arm/shoulder, rib, wrist, lower leg, and ankle) have age associations for an additional 10 years of age from a 10% decrease to a 60% increase.
Conclusions:
After examining results for 10 different bone fracture sites, advanced age appeared the single best possibility for uniting several different sites, resulting in an empirically based composite fracture risk model.
Multivariable statistical models combine patient characteristics to create decision tools for clinicians to identify women at increased risk of future fracture. Most widely cited and used are 10-year prediction tools for Fracture Risk Assessment Tool (FRAX) major osteoporotic fracture (clinical spine, forearm, hip, or shoulder) (1) and the Garvan model (hip, symptomatic spine, forearm, metatarsal, humerus, scapula, clavicle, distal femur, proximal tibia, patella, pelvis, or sternum) (2). It is unclear whether these particular fractures were combined based on empirical data or on some other basis, such as how common, severe, or osteoporotic the fractures are. Their model discrimination reflects limited clinical utility (c-statistics <0.7) (3), perhaps because the chosen fractures have distinct risk profiles. Although combining disparate fractures may appear to increase model efficiency by increasing the volume of fractures, model performance may suffer without an empirical rationale for the chosen fracture sites. Models for hip fracture alone, in contrast, provide much better patient discrimination [c-statistics for hip fracture with or without bone mineral density (BMD) ≥0.75 in Study of Osteoporotic Fractures (4) and ≥0.70 for 9 of 10 studies with BMD (5)].
A previous Global Longitudinal Study of Osteoporosis in Postmenopausal Women (GLOW) paper examined associations between characteristics of older women and 10 bone fracture sites [hip, pelvis, upper leg, spine, clavicle, upper arm or shoulder (UAS), rib, lower leg, wrist, ankle] over a 3-year period (6). Several factors were associated with more than one fracture site in adjusted results: advanced age (eight sites), prior fracture of the same or a different bone (all 10 sites), low weight (five sites), falls (nine sites), weight loss of 10 lb or more in a year (three sites), and poor health or reduced activity (six sites). The fracture risk associated with advanced age is, however, unique. No other modeled factor showed comparable variation over fracture sites, from an estimated 20% adjusted decrease in ankle fracture hazard to an estimated 130% adjusted increased hip fracture hazard per 10 additional years of age. Second, no other factor showed comparable discriminative ability in as many different site models, advanced age being the dominant factor in five of 10 sites.
Accordingly, this paper introduces a risk profile-based, 5-year composite fracture model whose five fracture sites (hip, pelvis, upper leg, clavicle, and spine) are all strongly associated with advanced age, whereas the five fracture sites not included (rib, upper arm, lower leg, wrist, and ankle) have moderate to inverse age associations.
Using 5-year data from the GLOW study, the new model's performance and risk factor estimates are compared with a model that combines the four FRAX major osteoporotic fractures, and with new models of seven and 10 fracture sites, to illustrate the principle that increasing the number of fracture outcomes may have unintended model performance consequences.
Materials and Methods
The GLOW study has been described elsewhere (7). In brief, 60 393 women aged 55 years or older were recruited by physician letter from 17 sites in 10 countries. About 90% of the women contributed at least 1 year of follow-up information after baseline. Women were followed up at 1, 2, 3, and 5 years after study baseline, with the 5-year follow-up to include information since the last completed written survey. All study information, including clinical fractures from any cause and their dates, are self-reported. We did not collect information on BMD.
Analytical methods
Women with at least one follow-up survey after baseline were analyzed. To include women with gaps in follow-up surveys, we used the counting process approach (8). Fracture rate estimates use the Kaplan-Meier method, adjusted for estimated deaths. The estimated deaths were obtained using the US Social Security Period Life Table (2007). Fracture rates are approximately 3% lower after adjustment for estimated deaths.
Outcomes are time to first fracture of the specified type. Unadjusted hazard ratios (HRs) are presented for potential fracture risk factors for 10 individual fracture sites and four site combinations. Adjusted risk factor estimates, as well as their model χ2 values (indicative of factors' relative strengths within a given model), are from Cox multiple regression models. All Table 1 risk factors were considered. Factors were grouped (medical history, health/vitality/physical function) to eliminate possible redundant factors before adding other factors. Nonlinear (on log scale) associations with continuous variables were considered, using the methods of fractional polynomials (9) and restricted cubic splines (10). The only interactions considered were with age. Because data extend to 5 years past baseline, we checked the proportional hazards assumption for final model covariates; no violations were found.
Table 1.
Unadjusted Results for 5-Year Fracture Combinations (53 896 Women)
| Fracture Outcome |
||||||
|---|---|---|---|---|---|---|
| F5 | F7 | F10 | Major | |||
| Total observations, n | 55 163 | 55 150 | 55 132 | 55 220 | ||
| Fractures, n | 1783 | 3082 | 5040 | 3169 | ||
| Five-year estimated fracture rate, % | 4.0 | 7.0 | 11.2 | 7.4 | ||
| % Missing | Median | HR | ||||
|---|---|---|---|---|---|---|
| Risk factor at study baseline | ||||||
| Age per 10 y past 55 y | 0 | 67 y | 2.06 | 1.76 | 1.44 | 1.58 |
| Weight per 10 kg | 2.3 | 68 kg | 0.87 | 0.91 | 0.94 | 0.91 |
| BMI per 10 kg/m2 | 4.4 | 26 kg/m2 | 0.74 | 0.84 | 0.89 | 0.83 |
| Height per 10 cm | 3.0 | 161 cm | 0.84 | 0.84 | 0.92 | 0.89 |
| Physical function (0–100) per 10-point decrease | 1.3 | 83 | 1.2 | 1.17 | 1.12 | 1.12 |
| Vitality index (0–100) per 10-point decrease | 1.8 | 63 | 1.21 | 1.18 | 1.13 | 1.12 |
| EQ-5D index (−0.20 to −1.00) per 0.10-point decrease | 8.6 | 0.81 | 1.17 | 1.15 | 1.12 | 1.12 |
| % Missing | % With RF | HR | ||||
|---|---|---|---|---|---|---|
| Risk factor at study baseline | ||||||
| Age 80 y or older | 0 | 12 | 3.29 | 2.66 | 2.03 | 2.28 |
| Prior F10 | 2.3 | 23 | 3.05 | 2.8 | 2.37 | 2.37 |
| Prior hip fracture | 0.8 | 1.7 | 5.52 | 4.34 | 3.03 | 3.59 |
| Prior wrist fracture | 0.7 | 8.4 | 2.37 | 2.28 | 2.19 | 2.37 |
| One fall | 1.1 | 23 | 1.5 | 1.42 | 1.39 | 1.36 |
| Two or more falls | 15 | 2.28 | 2.22 | 2.06 | 1.94 | |
| Maternal hip fracture | 6.0 | 13 | 1.39 | 1.28 | 1.26 | 1.27 |
| Paternal hip fracture | 9.9 | 3.9 | 1.45 | 1.26 | 1.11 | 1.16 |
| Medical history | ||||||
| Asthma | 1.1 | 11 | 1.31 | 1.37 | 1.24 | 1.18 |
| Emphysema | 2.4 | 8.6 | 1.69 | 1.67 | 1.54 | 1.51 |
| Osteoarthritis | 1.9 | 40 | 1.55 | 1.54 | 1.42 | 1.41 |
| Rheumatoid arthritis | 7.9 | 0.8 | 2.11 | 1.84 | 1.55 | 1.44 |
| Colitis | 1.4 | 2.0 | 1.65 | 1.52 | 1.51 | 1.39 |
| Celiac disease | 1.8 | 0.6 | 1.47 | 1.4 | 1.4 | 1.57 |
| Stroke | 1.3 | 3.9 | 2.03 | 1.83 | 1.46 | 1.61 |
| Parkinson's disease | 1.1 | 0.5 | 4.14 | 3.06 | 2.46 | 2.43 |
| Multiple sclerosis | 1.1 | 0.6 | 1.69 | 1.87 | 1.72 | 1.76 |
| Cancer | 1.1 | 14 | 1.4 | 1.3 | 1.24 | 1.15 |
| Type 1 diabetes | 0.4 | 0.8 | 1.56 | 1.46 | 1.44 | 1.64 |
| General health (poor vs excellent) | 1.2 | 2.7 | 5.69 | 4.52 | 3.31 | 3.45 |
| Moderate activities (very limited vs not) | 2.3 | 12 | 2 | 1.76 | 1.5 | 1.52 |
| EQ-5D (unable to perform usual activities) | 0.8 | 1.8 | 2.35 | 2.01 | 1.71 | 1.76 |
| Days walked past month | ||||||
| 0 | 1.8 | 15 | 2.03 | 1.66 | 1.4 | 1.39 |
| 1–9 (vs ≥10) | 37 | 1.43 | 1.28 | 1.13 | 1.05 | |
| Active (not at all vs very) | 1.1 | 4.4 | 2.44 | 2.12 | 1.73 | 1.74 |
| Current smoker | 0.8 | 8.9 | 1.01 | 1.11 | 1.1 | 1.04 |
| Alcohol (>20 vs 0 drinks/wk) | 0.8 | 0.5 | 1.19 | 1.02 | 1.18 | 1.25 |
| Lost 10 lb in last y | 1.5 | 9.2 | 1.98 | 1.77 | 1.51 | 1.57 |
Abbreviations: BMI, body mass index; F5, any 5-year fracture of hip, pelvis, upper leg, clavicle, or spine; F7, any 5-year fracture of hip, pelvis, upper leg, clavicle, spine, UAS, or rib; F10, any 5-year fracture of any of the 10 bones in Table 2; Major, any 5-year fracture of hip, spine, UAS, or wrist (FRAX major osteoporotic fracture).
Model covariates for composite indices [five dimension EuroQol (EQ-5D) (11), physical function, vitality] were compared with individual index components with the largest unadjusted fracture associations; final model choices were determined by the best model log likelihood. The EQ-5D has a maximum value of 1 [excellent quality of life (QOL)] and is negative if the QOL is worse than death. The vitality index scale of 0 (low) to 100 (high) was converted to (100−vitality)/20 to obtain a positive association with fracture. Moderate activity, defined as the ability to move a table, push a vacuum cleaner, bowl, or play golf, is defined as being limited a lot, limited a little, or not at all limited (coded −1, −2, and −3, respectively). In her performance of usual activities (work, study, housework, family, or leisure activities), a woman may have no problems, some problems, or be unable to perform them (coded 1, 2, and 3, respectively).
Prior cancer includes cancer of any kind, including skin cancer.
Weight was modeled as 100/weight in kilograms. No other nonlinear associations were found.
Because prior fracture at the same site is more strongly associated with future fracture than is prior fracture at a different site, each composite model includes baseline fracture of a site in that composite as well as fracture at a different site.
The modeling process was performed once for the composite outcome of the five most strongly age-associated fractures (F5 model: hip, pelvis, upper leg, spine, or clavicle) and once for major osteoporotic fracture to assess for each the best possible model using GLOW survey data. The two other reported models (F7: F5 plus UAS or rib, and F10: F7 plus lower leg, wrist, or ankle) use the same final risk factors as found in the age-associated fracture model because they include the same five fractures and are presented for comparison with that model.
Model performance was assessed by the May-Hosmer goodness-of-fit test (12) and Harrell's c index (13). This index measures whether pairs of model observations with different risk profiles have expected relative outcomes; for example, a pair of women is concordant if a woman with lower modeled risk has not fractured by the time (since study baseline) a higher-risk woman fractures. Its range is typically 0.5 (a model equivalent to random chance) to 1.0. These measures were calculated on the 88% of women with no interruptions in follow-up since study baseline.
Table 2 parameter estimates (β-hats) can be converted into a 5-year cumulative fracture risk for an individual woman as shown in the technical note following the body of this paper.
Table 2.
Unadjusted Results for 5-Year Individual Fractures (53 896 Women)
| Fracture Outcome |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hip | Pelvis | Upper Leg | Clavicle | Spine | UAS | Rib | Wrist | Lower Leg | Ankle | |
| Total observations, n | 55 224 | 55 280 | 55 270 | 55 273 | 55 296 | 55 294 | 55 283 | 55 304 | 55 248 | 55 219 |
| Fractures, n | 513 | 278 | 254 | 226 | 701 | 760 | 878 | 1426 | 362 | 834 |
| Five-year estimated rate, % | 1.2 | 0.6 | 0.6 | 0.5 | 1.6 | 1.7 | 2.0 | 3.2 | 0.8 | 1.8 |
| Hazard ratios | ||||||||||
| Risk factor at study baseline | ||||||||||
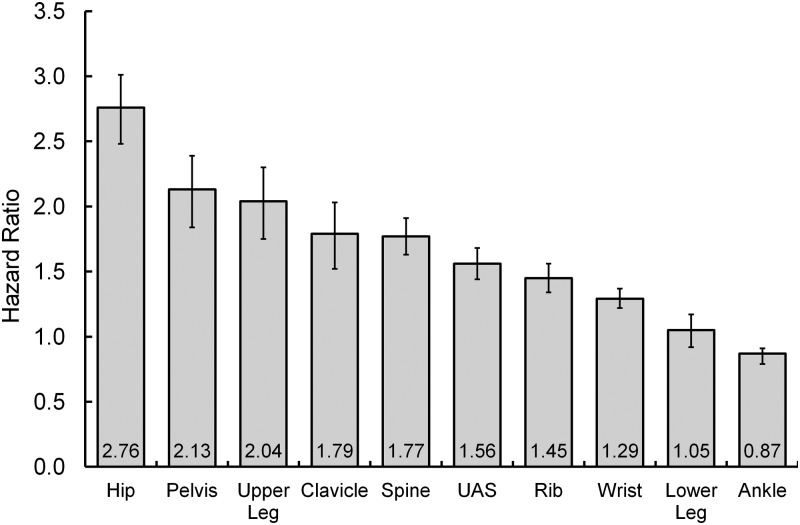
| Age per 10 y past 55 y | 2.76 | 2.13 | 2.04 | 1.79 | 1.77 | 1.56 | 1.45 | 1.29 | 1.05 | 0.87 |
| Weight per 10 kg | 0.78 | 0.81 | 0.96 | 0.87 | 0.92 | 0.98 | 0.92 | 0.91 | 1.09 | 1.12 |
| BMI per 10 kg/m2 | 0.57 | 0.57 | 1.04 | 0.83 | 0.81 | 1.05 | 0.86 | 0.81 | 1.23 | 1.32 |
| Height per 10 cm | 0.85 | 0.86 | 0.77 | 0.70 | 0.89 | 0.78 | 0.85 | 0.94 | 1.07 | 1.07 |
| Physical function per 10-point decrease | 1.24 | 1.18 | 1.24 | 1.21 | 1.19 | 1.12 | 1.16 | 1.06 | 1.15 | 1.07 |
| Vitality index per 10-point decrease | 1.21 | 1.19 | 1.18 | 1.23 | 1.23 | 1.12 | 1.23 | 1.06 | 1.13 | 1.08 |
| EQ-5D index per 0.1-point decrease | 1.20 | 1.16 | 1.19 | 1.17 | 1.16 | 1.10 | 1.19 | 1.09 | 1.15 | 1.08 |
| Age 80 y or older | 5.15 | 3.37 | 3.08 | 2.99 | 2.59 | 1.93 | 2.20 | 1.64 | 1.36 | 0.77 |
| Prior F10 | 2.90 | 3.81 | 3.24 | 2.62 | 3.20 | 2.37 | 3.37 | 2.03 | 2.68 | 1.77 |
| Prior hip fracture | 9.10 | 4.89 | 5.18 | 2.41 | 3.79 | 2.35 | 3.17 | 2.06 | 2.50 | 0.83 |
| Prior wrist fracture | 2.59 | 3.69 | 2.37 | 1.98 | 2.01 | 2.41 | 2.40 | 2.52 | 1.96 | 1.52 |
| One fall | 1.53 | 1.81 | 1.46 | 1.82 | 1.43 | 1.39 | 1.36 | 1.32 | 1.38 | 1.49 |
| Two or more falls | 2.34 | 3.00 | 2.57 | 2.81 | 2.00 | 1.98 | 2.45 | 1.88 | 2.46 | 2.21 |
| Maternal hip fracture | 1.46 | 1.70 | 1.38 | 1.24 | 1.36 | 1.25 | 1.21 | 1.20 | 1.06 | 1.29 |
| Paternal hip fracture | 1.61 | 1.40 | 1.05 | 1.98 | 1.43 | 1.23 | 1.17 | 0.93 | 0.95 | 0.94 |
| Medical history | ||||||||||
| Asthma | 1.02 | 1.02 | 1.55 | 1.11 | 1.59 | 1.25 | 1.74 | 1.05 | 1.32 | 1.34 |
| Emphysema | 1.60 | 1.09 | 1.73 | 1.77 | 1.99 | 1.39 | 2.03 | 1.29 | 1.16 | 1.71 |
| Osteoarthritis | 1.37 | 1.39 | 1.79 | 1.28 | 1.77 | 1.32 | 1.79 | 1.36 | 1.37 | 1.17 |
| Rheumatoid arthritis | 2.43 | 2.88 | 2.17 | 1.21 | 1.77 | 1.79 | 2.01 | 0.66 | 4.64 | 1.30 |
| Colitis | 2.04 | 1.15 | 1.04 | 2.72 | 1.87 | 1.13 | 1.60 | 1.29 | 1.49 | 2.00 |
| Celiac disease | 1.91 | 1.76 | n/e | 1.46 | 1.40 | 1.29 | 1.34 | 1.75 | 0.91 | 0.99 |
| Stroke | 2.30 | 1.87 | 1.39 | 1.32 | 2.16 | 1.64 | 1.82 | 1.10 | 0.97 | 1.13 |
| Parkinson's disease | 4.04 | 3.27 | 4.34 | 4.01 | 3.98 | 1.18 | 3.20 | 1.75 | 1.22 | 1.07 |
| Multiple sclerosis | 2.30 | 0.59 | 1.96 | 2.24 | 1.90 | 2.21 | 1.34 | 1.52 | 3.27 | 1.81 |
| Cancer | 1.31 | 1.58 | 1.78 | 1.30 | 1.33 | 1.11 | 1.33 | 1.05 | 1.29 | 1.32 |
| Type 1 diabetes | 2.04 | 0.94 | 2.06 | 2.34 | 1.31 | 2.07 | 0.89 | 1.66 | 1.45 | 1.57 |
| General health (poor vs excellent) | 3.93 | 3.26 | 3.52 | 3.58 | 12.5 | 2.47 | 4.91 | 2.01 | 5.24 | 2.25 |
| Moderate activity (very limited vs not) | 2.24 | 1.84 | 2.15 | 2.01 | 1.96 | 1.43 | 1.76 | 1.22 | 1.61 | 1.23 |
| EQ-5D (unable to perform usual activities) | 2.75 | 2.03 | 2.50 | 2.34 | 2.32 | 1.58 | 1.92 | 1.40 | 2.01 | 1.43 |
| Days walked past month | ||||||||||
| 0 | 2.58 | 1.66 | 2.48 | 1.97 | 1.83 | 1.29 | 1.59 | 1.04 | 1.70 | 1.25 |
| 1–9 (vs ≥10) | 1.64 | 1.42 | 1.74 | 1.58 | 1.28 | 1.04 | 1.29 | 0.87 | 1.11 | 1.25 |
| Active (not at all vs very) | 3.07 | 1.91 | 3.27 | 2.13 | 2.77 | 1.78 | 2.27 | 1.30 | 3.16 | 1.83 |
| Current smoker | 0.99 | 0.68 | 0.93 | 1.19 | 1.26 | 1.12 | 1.26 | 0.90 | 1.17 | 1.34 |
| Alcohol (>20 vs 0 drinks/wk) | 1.32 | 0.67 | 1.84 | 2.36 | 0.51 | 0.74 | 1.10 | 1.65 | 0.50 | 0.91 |
| Lost 10 lb in last y | 2.24 | 1.66 | 1.71 | 2.26 | 1.85 | 1.54 | 1.61 | 1.23 | 1.04 | 1.24 |
BMI, body mass index; F10, fracture of any of 10 bones listed.
Site of recruitment was not included in these models.
All analyses were conducted using the SAS software package, version 9.2 (SAS Institute).
Results
Estimated 5-year fracture rates among the 53 896 women with at least one follow-up survey range from 0.5% (clavicle) to 3.2% (wrist; Table 2). The median age was 67 years, weight was 68 kg, 23% had a prior baseline fracture of at least one of the 10 individual bones since age 45 years, and 38% reported falling one or more times in the year prior to baseline (Table 1). Only 2.7% reported poor general health, 4.4% were not at all active compared with the same-aged women, 1.8% were unable to perform usual activities, and 12% reported being very limited in moderate activities.
Compared with women with complete 5 years of follow-up, women without complete 5 years of follow-up tended to be older (70 vs 67 y), have lower mean baseline physical function and vitality (56 vs 63 and 65 vs 77, respectively), and worse general health (6.2% vs 10% excellent; data not shown). Fracture risk factors were more likely to be present in women without complete follow-up, in particular a prior fracture of any of the 10 bones (28% vs 21%).
Tables 1 and 2 also present unadjusted HRs for 10 individual bone fractures and four fracture combinations. Individual fractures (Table 2) are listed in descending order of age association, with HRs ranging from 0.9 (ankle) to 2.8 (hip) for 10 additional years after age 55 years (see also Figure 1). Model F5 (Table 1) combines hip, pelvis, upper leg, clavicle, and spine (unadjusted age HRs from 1.8 to 2.8), F7 combines these five plus upper arm/shoulder (UAS) and rib (age HRs 1.5–2.8), F10 combines all 10 bones (age HRs 0.9–2.8). Major osteoporotic fracture combines hip, spine, UAS, and wrist (unadjusted age HRs 1.3–2.8 per 10 y).
Figure 1.
Unadjusted HRs and 95% confidence intervals per 10 years of age (past 55 y) for 10 fracture sites.
Because wrist fractures are relatively common (n = 1426), fracture of at least one major bone is more common than an F5 or F7 fracture (n = 2594 vs 1455 and 2547). Kaplan-Meier 5-year estimated mean fracture rates are 4.0%, 7.0%, 7.4%, and 11.2% for F5, F7, major, and F10 composites, respectively. Median 5-year fracture rates are somewhat lower due to the right-skewed nature of risk (2.4%, 4.9%, 5.4%, and 9.0%, respectively).
As one might expect, the five age-associated fractures show fairly large unadjusted individual associations with reduced physical function, vitality, EQ-5D (QOL), not walking, and prior fractures compared with the remaining five fractures (although rib fracture has an HR of 3.4 with prior F10). Parkinson's disease, although affecting only 0.5% of women, has HRs greater than 3 for each F5 fracture (3.3–4.3), less than 2 for all other fractures except rib (HR 3.2). Hip, pelvis, and clavicle are most highly associated with lower weight (HRs from 0.78 to 0.87 per 10 additional kg), whereas lower leg and ankle fractures are associated with higher weight (HR 1.1).
Table 3 presents results for the four composite fracture Cox regression models. Approximately 85% of available data were used to fit the models, the remainder having one or more missing covariate values. Because F5 fractures were selected based on their relatively strong unadjusted associations with age, this composite outcome has the largest adjusted association with age (linear age HR of 1.6 per additional 10 y, ignoring age interactions; data not shown), and age is the strongest model factor. F7 and major fracture composites have somewhat smaller adjusted linear age HRs of 1.4, whereas F10's linear age HR is 1.2 per additional 10 years (ignoring age interactions; data not shown). In F7, F10, and major fracture models, the single strongest risk factor is not age but prior fracture of at least one bone in the composite (based on χ2 values ignoring age interactions; data not shown). F5 risk factors remain statistically significant in the F7 and F10 models, with somewhat reduced HRs for associations with age, weight, maternal hip fracture, Parkinson's disease, unintended weight loss, and reduced usual activity. Baseline falls appear similarly strong in all four composite fracture models.
Table 3.
Cox Regression Models for 5-Yr Fracture Combinations
| Model Summary | F5 | F7 | F10 | Major |
|---|---|---|---|---|
| Total observations, n | 47 066 | 47 069 | 47 245 | 47 429 |
| Fractures, n | 1455 | 2547 | 4199 | 2638 |
| May-Hosmer goodness of model fit (P) | .44 | .28 | .09 | .99 |
| Harrell's c index | 0.753 | 0.709 | 0.653 | 0.667 |
| β-Hat | χ2 | HR (CI) | β-Hat | χ2 | HR (CI) | β-Hat | χ2 | HR (CI) | β-Hat | χ2 | HR (CI) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model covariates at study baseline | ||||||||||||
| 1. 10 y of age past 55 y | 0.9939 | 154 | a | 0.7068 | 133 | a | 0.3750 | 58 | a | 0.4660 | 60 | a |
| 2. 20 points less vitality | 0.1156 | 14 | 1.1 (1.1–1.2) | 1.1297 | 30 | 1.1 (1.1–1.2) | 0.0788 | 18 | 1.1 (1.0–1.1) | NA | NA | NA |
| 3. Inverse weight (100/weight, kg) | 0.5233 | 43 | a | 0.4111 | 43 | a | 0.3443 | 47 | a | 0.3701 | 34 | a |
| 4. Past fracture, same type | 2.8367 | 27 | a | 2.1910 | 37 | a | 1.3492 | 27 | a | 0.6852 | 220 | 2.0 (1.8–2.2) |
| 5. Past fracture, different type | 0.4173 | 53 | 1.5 (1.4–1.7) | 0.2932 | 38 | 1.3 (1.2–1.5) | NA | NA | NA | 0.3432 | 49 | 1.4 (1.3–1.6) |
| 6. Single fall | 0.185 5 | 28 | 1.2 (1.1–1.4) | 0.1841 | 64 | 1.2 (1.1–1.3) | 0.2364 | 142 | 1.3 (1.2–1.4) | 0.1760 | 56 | 1.2 (1.1–1.3) |
| Two or more falls | 0.3580 | 1.4 (1.3–1.6) | 0.4114 | 1.5 (1.4–1.7) | 0.4748 | 1.6 (1.5–1.7) | 0.3824 | 1.5 (1.3–1.6) | ||||
| 7. Maternal hip fracture | 0.2729 | 16 | 1.3 (1.2–1.5) | 0.1847 | 12 | 1.2 (1.1–1.3) | 0.1955 | 22 | 1.2 (1.1–1.3) | 0.1805 | 12 | 1.2 (1.1–1.3) |
| 8. Parkinson's disease | 0.6788 | 12 | 2.0 (1.4–2.9) | 0.5204 | 9 | 1.7 (1.2–2.4) | 0.3380 | 5 | 1.4 (1.0–1.9) | NA | NA | NA |
| 9. Decreased moderate activity | 0.1999 | 20 | 1.2 (1.1–1.3) | 0.1687 | 23 | 1.2 (1.1–1.3) | 0.1185 | 18 | 1.1 (1.1–1.2) | NA | NA | NA |
| 10. Lost 10 lb in a year | 0.2370 | 10 | 1.3 (1.1–1.5) | 0.2076 | 12 | 1.2 (1.1–1.4) | 0.1598 | 10 | 1.2 (1.1–1.3) | 0.1637 | 7 | 1.2 (1.0–1.3) |
| 11. Decreased usual activity | 2.4357 | 44 | a | 1.5764 | 30 | a | 0.8217 | 13 | a | 1.040 | 13 | a |
| 12. Interaction of 1 and 4 | −0.2607 | 13 | a | −0.1772 | 13 | a | −0.1014 | 8 | a | NA | NA | NA |
| 13. Interaction of 1 and 11 | −0.3030 | 38 | a | −0.2048 | 28 | a | −0.0960 | 9 | a | −0.1195 | 10 | a |
| 14. Osteoarthritis | NA | NA | NA | NA | NA | NA | NA | NA | NA | 0.0921 | 5 | 1.1 (1.0–1.2) |
| 15. Reduced general health | NA | NA | NA | NA | NA | NA | NA | NA | NA | 0.1351 | 33 | 1.1 (1.1–1.2) |
Abbreviations: β-hat, model estimate; CI, confidence interval for HR; F5, any 5-year fracture of hip, pelvis, upper leg, clavicle, or spine; F7, any 5-year fracture of hip, pelvis, upper leg, clavicle, spine, UAS, or rib; F10, any 5-year fracture of any of the 10 bones in Table 2; Major, any 5-year fracture of hip, spine, UAS, or wrist (FRAX major osteoporotic fracture); HR, hazard ratio, which is exp(β-hat), except for interactions; NA, not applicable; χ2, model χ-square, an indication of relative strength of a risk factor in a given model (each χ2 has 1 degree of freedom, except for falls (2 degrees of freedom).
See Figure 2 for clarification of F5 model's nonlinear terms; other models have nonlinear effects that are similar but less pronounced than in F5.
The major osteoporotic fracture model was fitted independently of the F5 model, allowing for the possibility of identifying a different set of risk factors. Vitality, Parkinson's disease, and decreased moderate activity were not statistically significant, whereas osteoarthritis and reduced general health were.
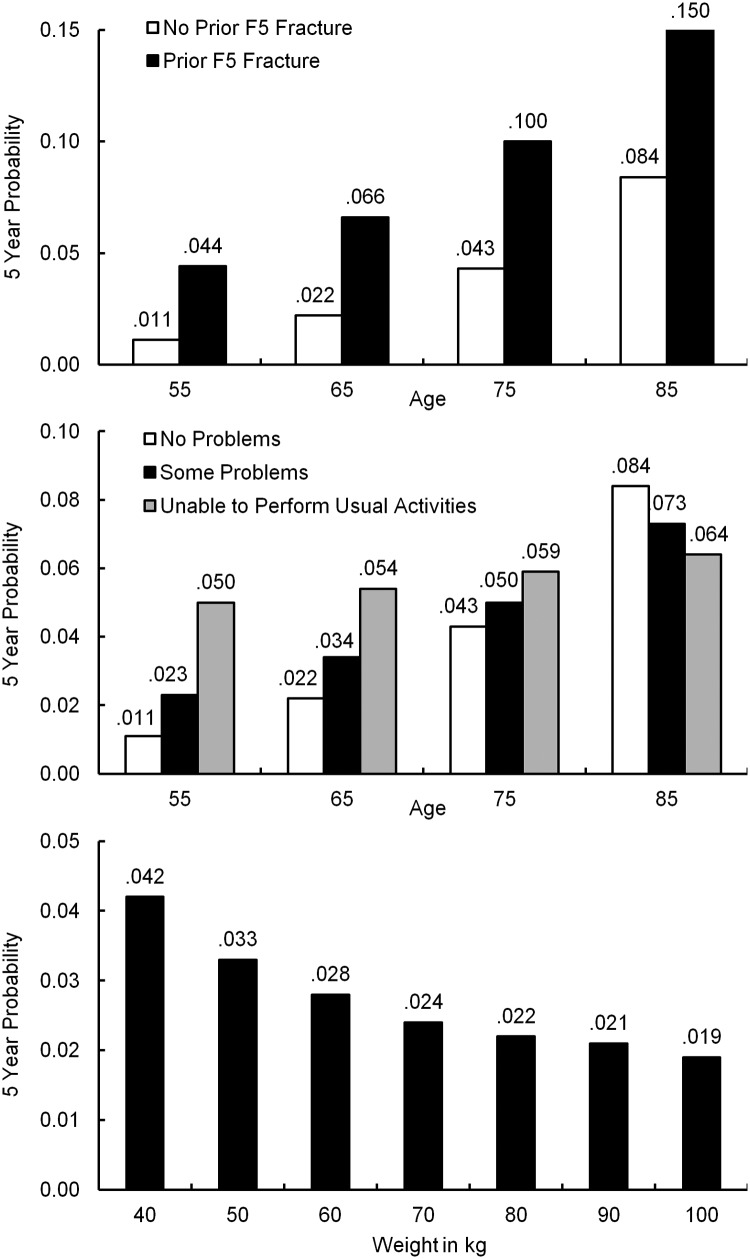
F5, F7, and F10 models have two statistically significant age interactions (Table 3 and Figure 2). Advanced age has a stronger F5 fracture association for women with no prior F5 fracture (estimated cumulative F5 fracture probability increases nearly 8-fold from 1.1% at age 55 y to 8.4% at age 85 y) than for those with prior F5 fracture (a less than 4-fold increase from 4.4% at age 55 y to 15% at age 85 y). A 55-year-old woman with a prior F5 fracture has a similar expected F5 fracture risk to a 75-year-old with no prior F5 fracture (4.4% and 4.3%). The association of reduced ability to perform usual activities such as work, study, and housework with F5 fracture is strongest for younger women (estimated risk increases from age 55 y to 85 y from 1.1% if no problems to 5% if unable to perform usual activities), becoming irrelevant somewhere between age 75 and 85 years (no increased risk with reduced performance). Similar age interaction effects appear in the other composite models but are less dramatic (model estimates, β-hats, are smaller).
Figure 2.
Estimated 5-year cumulative probability of F5 fracture (hip, pelvis, upper leg, spine, or clavicle fracture) for nonlinear model terms.
All four composite models were improved by using inverse, instead of linear, weight (Table 3 and Figure 2). The most pronounced asymmetry appears in the F5 model, whose estimated 5-year cumulative fracture rates drop from 4.2% to 2.4% for 40- and 70-kg women, respectively, to 1.9% for a 100-kg woman. A similar but smaller asymmetry exists in the other composite models.
All four composite fracture models passed the goodness-of-fit test (P > .05), indicating observed and model-expected fractures are similar across the range of fracture risk. Harrell's c index is highest for F5 and F7 (c = 0.75 and c = 0.71), lowest for F10 and major fracture models (c = 0.65 and c = 0.67). Refitting the models using logistic instead of Cox regression yields c statistics (areas under receiver operator curve) nearly identical to Harrell's c indices. If wrist fracture is removed from the major fracture model, the number of fractures (hip, spine, or forearm) drops to 1557, whereas the c index increases to 0.72 (data not shown).
Results adjusting for bone medication use were similar to those reported above (data not shown), as were results using multiple imputation for missing data (data not shown).
Discussion
Existing composite fracture models, although clinically appealing, have not exhibited strong model discrimination. The FRAX-New Zealand risk tool reported c statistics of 0.62–0.64 for FRAX osteoporotic fracture and 0.64 for Garvan osteoporotic fracture (14); among nine population-based FRAX validation studies, all but three c statistics for other osteoporotic fractures without BMD were less than 0.60 and all but one less than 0.63 for other osteoporotic fractures with BMD (5); Study of Osteoporotic Fractures has reported c statistics from 0.63 to 0.70 for FRAX osteoporotic fracture with and without BMD for all women and among obese and nonobese subgroups (4, 15). Such composite models may accumulate large numbers of fractures, but the risk profiles for the individual fracture sites differ enough that, when sites are combined, the models fail to discriminate well. In the GLOW data, the c index of 0.75 for the F5 model drops to 0.71 when UAS and rib are added and to 0.65 when lower leg, wrist, and ankle are added. The FRAX major osteoporotic fracture model's c index of 0.67 increases to 0.72 when wrist fractures are removed because women who fracture a wrist tend to be younger.
A tool with a c statistic or c index less than 0.70, in which the possible range is from 0.50 to 1.0, has limited model predictive ability. On the other hand, a model with impressive discrimination may not appear clinically useful or may have accumulated too few fractures to be useful.
The F5 composite fracture model features a new approach to combining fractures. It has an empirical, objective basis, and natural interpretation. No determination as to which fracture sites are osteoporotic or major is needed. Because the F5 model's component fractures are all strongly associated with advanced age, the model has better discrimination, as measured by its c index, than a composite model whose individual patient risk profiles are less compatible.
In clinical practice, however, the utility of a composite fracture model depends on the effectiveness of intervention in reducing the risk of the fractures included in the model. All of the approved pharmacological interventions have been shown to reduce vertebral fracture, and most also reduce hip fracture. However, pelvic, clavicle, and upper leg fractures are usually combined into a general nonvertebral fracture category, and data for the efficacy of treatment against these fractures have not been separately reported. Reduction in the risk of falling is likely to be important in decreasing the risk of hip, pelvis, upper leg, and clavicle fracture, although less so in the risk of vertebral fracture. The similarity in individual patient risk profile between fractures in the F5 model suggests that treatment should be similarly effective across the range of included fractures, although this remains to be formally tested.
Other possible fracture combinations may be posited based on similarity of patient risk profiles, but it is difficult to see a unifying thread with the strength of age. Most individual fractures have some unadjusted association with age, weight, falling, adverse health, reduced activity, and prior fracture, but ankle, wrist, and lower leg fractures are especially difficult to characterize, with the exception that lower leg and ankle fractures are similarly associated with higher weight (16, 17).
Limitations
Data are self-reported. Outcomes and risk factors are therefore, to some extent, misclassified. To that extent, model associations are underestimated. We did not have information on BMD. Other risk factors that GLOW did not collect may exist, but they may be idiosyncratic or difficult to collect in a survey.
We could not directly assess the impact of study deaths on fracture rate estimates because deaths were not uniformly reported.
We did not choose to consider the possible effect of bone medication because women at high fracture risk are those most likely to take medication. An F5 model controlling for the use of baseline bone medication showed little change from the reported F5 risk factor estimates (data not shown).
We also elected not to include glucocorticoids associated with bone loss in the modeling. Unlike risk factors inherent in a woman, medications can be started and stopped, thus changing risk. If the current use of glucocorticoids at baseline were added to the F5 model, discrimination would not change appreciably (c index increases from 0.753 to 0.754).
Data were missing for about 15% of model F5 observations. Results using imputation were similar to those shown (data not shown), but we cannot be certain that the missing data had no influence on model estimates. It seems unlikely the risk factor associations would differ for women who failed to answer a question from those who answered, but it is possible.
Similarly, about 10% of women contributed no information after study baseline. Because women without complete follow-up tended to be older and in poorer health, our absolute fracture rates are likely to be underestimated.
Model discrimination (c index) is not impressive for any of the composite fracture models, although greater than 0.7 for F5 and F7 models. It may not be possible to form a composite outcome from different fractures and expect excellent discrimination because risk factor profiles vary to some extent by individual fracture type. Any model with more than one fracture site will be, to some extent, a compromise between individual site-specific models; F5 is a compromise between individual hip, pelvis, upper leg, clavicle, and spine models.
If some F5 model risk factors are unavailable, model discrimination will be reduced to some extent. A model containing age, prior fracture, weight, falls, and some measures of health, physical function, and vitality would be expected to perform nearly as well as the full F5 model (factors with relatively large χ2 values contribute most to discrimination).
Finally, our results are from a single, although large and multinational, study. External validation from independent sources is desirable. Average fracture rates in different regions and even sites within a region may be lower or higher than those observed in GLOW, but estimates of risk factor effects (HRs) and relative model performance among the four described models are expected to be reasonably universal.
Strengths
The F5 model is simple and does not require BMD. Five-year fracture risk is clinically relevant compared with the longer duration of the 10-year FRAX assessment. The findings are multinational and practice based, thus more generalizable than prediction tools not so based. The model incorporates risk associated with prior falls, general health and activity, weight loss, and disease, factors that are not commonly considered in other risk tools.
Conclusions
Several possible individual fracture sites may be combined to form a fracture outcome. Rather than a priori define a composite fracture outcome and then create a best-fit model for this outcome, we present an alternate approach. After examining results for 10 individual sites, we found advanced age to be the single best possibility for uniting several different fracture sites, and formed the F5 composite of hip, pelvis, upper leg, spine, or clavicle fracture. Results from the F5 model may be used to predict an individual woman's 5-year F5 fracture risk, if aged 55 years or older, with an expected mean 5-year probability of about 4%. Such a model may be particularly pertinent for fractures associated with involutional osteoporosis. It is hoped that, in addition to its possible clinical utility, the F5 model may stimulate curiosity and investigation into why different fractures have distinctive risk profiles.
Technical note on how to convert Table 2 results into estimates of an individual's 5-year cumulative fracture probability
To estimate a particular woman's 5-year cumulative composite fracture risk using Table 2 results, one may use the following formula: 1 − [Ŝo(5)exp(Xβ̂)](8).
Ŝo(5) is baseline survival at 5 years. Xβ̂ is the combination of a woman's observed covariate value (such as age), and the estimate associated with the observed covariate value (labeled β-hat in Table 2).
Baseline survivals (where all covariates have a value of zero) at 5 years are 0.999 990 37, 0.999 813 85, 0.996 531 35, and 0.999 467 29 for F5, F7, F10, and major fracture models, respectively.
For a woman aged 78 years, vitality index 25, weight 57 kg, prior F5 fracture, prior fracture of a different bone, two or more falls at baseline, maternal hip fracture, no Parkinson's disease, limited a lot in moderate activity, did not lose 10 lb unintentionally in the past year, and no problems performing usual activities, the F5 model's Xβ̂ = {((78/10) × 0.9939) + ((100−25)/20 × 0.1159) + ((100/57) × 0.5233) + (1 × 2.8367) + (1 × 0.4173) + (1 × 0.3580) + (1 × 0.2729) + (0 × 0.6788) + (−1 × 0.1999) + (0 × 0.2370) + (1 × 2.4357) + ((78/10) × 1 × −0.2607) + ((78/10) × 1 × −0.3030)} = 10.829.
Her expected risk of suffering a fracture of one or more of the five bones within 5 years of baseline is 1 − [0.99999037**exp(10.829)] = 0.385, much higher than the 4% average risk.
Dedication
We dedicate this work to Dr Steven Boonen, who was helpful and encouraging in our various fracture risk model efforts and approved an early version of this paper.
Acknowledgments
We thank the various study sites and coordinators as well as those who helped process the data including the following: Diane McBride, Christine Vigeant, Joan Lovell, and Themia Pappas-Fillmore (Worcester, Massachusetts). Sophie Rushton-Smith, PhD, coordinated the revisions and provided editorial assistance.
Financial support for the GLOW study is provided by the Warner Chilcott Company, LLC, and Sanofi (to the Center for Outcomes Research, University of Massachusetts Medical School, Worcester, Massachusetts).
Disclosure Summary: G.F. received research and salary support from the Alliance for Better Bone Health (Procter & Gamble Pharmaceuticals, Sanofi). J.C. consulted for Servier, Shire, Nycomed, Novartis, Amgen, Procter & Gamble, Wyeth, Pfizer, The Alliance for Better Bone Health, Roche, and GlaxoSmithKline; has received lecture fees, travel, and accommodation from Servier, Procter & Gamble, and Lilly; and has received grant support from Nycomed (2009–2012) and Acuitas (2009–2011). R.C. received funding from the French Ministry of Health, Merck, Servier, Lilly, and Procter & Gamble; has received honoraria from Amgen, Servier, Novartis, Lilly, Roche, and Sanofi; and has previously consulted/acted as an advisory board member for Amgen, Merck, Servier, Nycomed, and Novartis. J.P. received grant support from Amgen, Kyphon, Novartis, and Roche; has received grant support for equipment from GE Lunar; has served on speakers' bureaus for Amgen, Sanofi, GlaxoSmithKline, Roche, Lilly Deutschland, Orion Pharma, Merck, Merckle, Nycomed, and Procter & Gamble; and has acted as an advisory board member for Novartis, Roche, Procter & Gamble, and Teva. C.C. consulted for/received lecture fees from Amgen, The Alliance for Better Bone Health (Sanofi and Warner Chilcott), Lilly, Merck, Servier, Novartis, and Roche-GSK. J.A. has received consulting fees or other remuneration from Amgen, Eli Lilly, Merck, Novartis, and Warner Chilcott; has received research grants from Amgen, Eli Lilly, Merck, and Novartis; has held a nonremunerative position of influence on the International Osteoporosis Foundation Board of Directors, Osteoporosis Canada; and has been on speakers' bureaus for Amgen, Eli Lilly, Merck, Novartis, and Warner Chilcott. F.A. has received funding from Pfizer. A.D.-P. has received consulting fees and lectured for Eli Lilly, Amgen, Procter & Gamble, Servier, and Daiichi-Sankyo; has been an expert witness for Merck; consults for/is an advisory board member for Novartis, Eli Lilly, Amgen, and Procter & Gamble; has received honoraria from Novartis, Lilly, Amgen, Procter & Gamble, and Roche; has previously been an expert witness for Merck; and has previously consulted/acted as an advisory board member for Novartis, Lilly, Amgen, and Procter & Gamble. S.G. has previously consulted/been an advisory board member for Amgen, Lilly, and Merck; and has received grant support from The Alliance for Better Bone Health (Sanofi and Proctor & Gamble) and Lilly. J.C.N. has undertaken paid consultancy work for Roche Diagnostics, Daiichi-Sankyo, Proctor & Gamble, and Nycomed; has been a paid speaker for and received reimbursement, travel, and accommodation from Roche Diagnostics, Novartis, Daiichi-Sankyo, and Procter & Gamble; and has received research grants from The Alliance for Better Bone Health and Amgen. M.R. has been on the speakers' bureau for Roche. N.W. has received honoraria for lectures in the past year from Amgen, Novartis, and Warner Chilcott; has acted as a consultant in the past year for Amgen, Arena, Baxter, InteKrin, Johnson & Johnson, Lilly, Medpace, Merck, NPS, Orexigen, Pfizer/Wyeth, Takeda, Vivus, and Warner Chilcott; has received research support (through University of Cincinnati) from Amgen, Merck, and NPS; and cofounded, has stock options in, and is a director of OsteoDynamics. F.H. has received funding from Warner Chilcott, Sanofi, and Pfizer. A.L. has received funding from The Alliance for Better Bone Health (Sanofi and Warner Chilcott) and is an advisory board member for Amgen. L.M. has been an advisory board member for Servier and received speakers' bureau fees and support to travel to scientific meetings from Servier, Merk, and Pfizer. C.R. has received honoraria from and consults/acts as an advisory board member for Alliance, Amgen, Lilly, Merck, Novartis, Nycomed, Roche, GlaxoSmithKline, Servier, and Wyeth. K.S. has received consulting fees or other remuneration from Eli Lilly & Co, Merck, Novartis, and Amgen; and has conducted paid research for Eli Lilly & Co, Merck, Novartis, and Amgen. E.S. has consulted for Amgen, Lilly, Novartis, Merck, and Pfizer; and has served on speakers' bureaus for Amgen and Lilly. S.S. has received grant support from Wyeth, Lilly, Novartis, and Alliance; has served on speakers' bureaus for Lilly, Novartis, Pfizer, and Procter & Gamble; has received honoraria from Procter & Gamble; and has previously consulted/acted as an advisory board member for Lilly, Argen, Wyeth, Merck, Roche, and Novartis. K.G.S. has consulted for or received other remuneration from Merck, Amgen, and Eli Lilly; has received research grants from Merck; and has held nonremunerative positions of influence on the National Osteoporosis Foundation Board of Trustees and as American College of Rheumatology Chair on the Quality of Care Committee. S.S. has received research grants from Wyeth, Lilly, Novartis, and Alliance; has served on speakers' bureaus for Lilly, Novartis, Pfizer, and Procter & Gamble; has received honoraria from Procter & Gamble; and has acted as a consultant/advisory board member for Lilly, Argen, Wyeth, Merck, Roche, and Novartis. S.G. has received funding from Warner Chilcott, Sanofi, and Pfizer. D.H. and J.N. have no conflicts of interest to declare.
Funding Statement
Financial support for the GLOW study is provided by the Warner Chilcott Company, LLC, and Sanofi (to the Center for Outcomes Research, University of Massachusetts Medical School, Worcester, Massachusetts).
Footnotes
- BMD
- bone mineral density
- EQ-5D
- five dimension EuroQol
- FRAX
- Fracture Risk Assessment Tool
- GLOW
- Global Longitudinal Study of Osteoporosis in Postmenopausal Women
- HR
- hazard ratio
- QOL
- quality of life
- UAS
- upper arm or shoulder.
References
- 1. Kanis JA, Johnell O, Oden A, Dawson A, De Laet C, Jonsson B. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001;12:989–995. [DOI] [PubMed] [Google Scholar]
- 2. Nguyen ND, Frost SA, Center JR, Eisman JA, Nguyen TV. Development of prognostic nomograms for individualizing 5-year and 10-year fracture risks. Osteoporos Int. 2008;19:1431–1444. [DOI] [PubMed] [Google Scholar]
- 3. Ohman EM, Granger CB, Harrington RA, Lee KL. Risk stratification and therapeutic decision making in acute coronary syndromes. JAMA. 2000;284:876–878. [DOI] [PubMed] [Google Scholar]
- 4. Ensrud KE, Lui LY, Taylor BC, Schousboe JT, Donaldson MG, Fink HA, Cauley JA, Hillier TA, Browner WS, Cummings SR. A comparison of prediction models for fractures in older women: is more better? Arch Intern Med. 2009;169:2087–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kanis JA, Oden A, Johnell O, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18:1033–1046. [DOI] [PubMed] [Google Scholar]
- 6. FitzGerald G, Boonen S, Compston JE, et al. Differing risk profiles for individual fracture sites: evidence from the Global Longitudinal Study of Osteoporosis in Women (GLOW). J Bone Miner Res. 2012;27:1907–1915. [DOI] [PubMed] [Google Scholar]
- 7. Hooven FH, Adachi JD, Adami S, et al. The Global Longitudinal Study of Osteoporosis in Women (GLOW): rationale and study design. Osteoporos Int. 2009;20:1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hosmer DW Jr, Lemeshow S, May S. Applied Survival Analysis: Regression Modeling of Time to Event Data. 2nd ed Hoboken, NJ: Wiley-Blackwell; 2008. [Google Scholar]
- 9. Royston P, Altman DG. Regression using fractional polynomials of continuous covariates: parsimonious parametric modelling. Appl Statistics. 1994;43:429–467. [Google Scholar]
- 10. Harrell FE., Jr Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. 1st ed New York: Springer; 2001. [Google Scholar]
- 11. The EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 12. May S, Hosmer DW. A cautionary note on the use of the Gronnesby and Borgan goodness-of-fit test for the Cox proportional hazards model. Lifetime Data Anal. 2004;10:283–291. [DOI] [PubMed] [Google Scholar]
- 13. Harrell FE Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. [PubMed] [Google Scholar]
- 14. Bolland MJ, Siu AT, Mason BH, et al. Evaluation of the FRAX and Garvan fracture risk calculators in older women. J Bone Miner Res. 2011;26:420–427. [DOI] [PubMed] [Google Scholar]
- 15. Premaor M, Parker RA, Cummings S, et al. Predictive value of FRAX for fracture in obese older women. J Bone Miner Res. 2013;28:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prieto-Alhambra D, Premaor MO, Fina Aviles F, et al. The association between fracture and obesity is site-dependent: a population-based study in postmenopausal women. J Bone Miner Res. 2012;27:294–300. [DOI] [PubMed] [Google Scholar]
- 17. Premaor MO, Pilbrow L, Tonkin C, Parker RA, Compston J. Obesity and fractures in postmenopausal women. J Bone Miner Res. 2010;25:292–297. [DOI] [PubMed] [Google Scholar]