Abstract
Background:
Although the incidence of type 2 diabetes (T2D) among persons with prediabetes is well known (∼10%/y), the incidence of prediabetes among normoglycemic persons is unclear. Also, in the Diabetes Prevention Program, no racial/ethnic differences were seen in diabetes incidence, whereas marked racial/ethnic disparities are reported in the prevalence of T2D. We aimed to obtain estimates of incident prediabetes and determine whether racial disparities manifest during transition to prediabetes.
Design and Methods:
We enrolled 376 (217 black, 159 white) nondiabetic offspring of parents with T2D (mean age 44.2 y) and followed them up quarterly for 5.5 years. Assessments included anthropometry, body composition, oral glucose tolerance test, biochemistries, energy expenditure, insulin sensitivity, and insulin secretion. The primary outcome was progression to impaired fasting glucose and/or impaired glucose tolerance (or diabetes).
Results:
Of 343 participants with evaluable data, 101 subjects (49 white, 52 black) developed prediabetes, and 10 (4 white, 6 black) developed diabetes during a mean follow-up of 2.62 years. There was no significant racial difference in the cumulative incidence of prediabetes (32.7% white, 30% black) or combined prediabetes/diabetes (35% white, 30% black). Significant predictors of prediabetes included age, gender, trunk fat, 2-hour postload glucose (2hrPG), insulin sensitivity, and insulin secretion. In a Cox proportional-hazards model, with adjustment for age and sex, the 2hrPG and abdominal obesity were independent predictors of incident prediabetes/diabetes [relative hazards (95% confidence interval [CI]) for the 90th vs 10th percentile: trunk fat mass 2.90 (95% CI 1.74–4.82), P < .0001; 2hrPG 2.54 (95% CI 1.46–4.40), P = .0009]. Having the trunk fat mass and the 2hrPG at the 90th percentile conferred a 7-fold hazard of prediabetes compared with persons at the 10th percentile for both measures.
Conclusion:
Black and white offspring of parents with type 2 diabetes develop prediabetes at a similar high rate of approximately 11% per year. Therefore, close surveillance, with prompt intervention to prevent dysglycemia, is warranted in persons with parental diabetes.
Cross-sectional surveys report higher prevalence rates for type 2 diabetes (T2D) in African Americans (or blacks) and other racial/ethnic groups than European Americans (or whites) (1–3). Prediabetes, defined as impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) (4, 5), represents an intermediate step in the pathogenesis to T2D. In the Diabetes Prevention Program (6), individuals with prediabetes from the different racial/ethnic groups developed T2D at similar rates of approximately 10% per year (6). However, apart from studies in Pima Indians (7), rigorous prospective studies on incidence rates and predictors of prediabetes are lacking for the general population. The lack of racial/ethnic disparities in incident diabetes among persons with prediabetes (despite reports of marked ethnic differences in diabetes prevalence) led us to hypothesize that ethnic disparities manifest during the transition from normal glucose regulation to prediabetes. Theoretically, if more African Americans progress to prediabetes than Caucasians, and both groups then progress at similar rates to diabetes, that could explain the higher prevalence of T2D among African Americans in cross-sectional surveys.
The objective of the Pathobiology of Prediabetes in A Biracial Cohort (POP-ABC) study was to determine the rate and predictors of incident prediabetes among initially normoglycemic black and white subjects. Because such natural history studies are feasible only in populations with high hazard rates (7–11), we assembled a cohort comprising African American and Caucasian offspring of parents with T2D. The rationale, design and methods, recruitment strategies, and baseline data of the POP-ABC study have been described elsewhere (12–14). In this report, we present the main results of the study.
Materials and Methods
Participants
Eligibility criteria included age 18–65 years; self-reported non-Hispanic white or non-Hispanic black race/ethnicity status; one or both biological parents with T2D; no evidence of diabetes; normal fasting plasma glucose (FPG) [<100 mg/dL(5.6 mmol/L)] and/or normal glucose tolerance (NGT) [2 h plasma glucose (2hrPG) < 140 mg/dL (7.8 mmol/L) during a 75-g oral glucose tolerance test (OGTT)]; and good overall health, as previously described (12–14).
Excluded from participation were persons with diabetes or using antidiabetes medications or other medications known to alter glucose metabolism or body weight (12–14). Other exclusion criteria were enrollment in behavioral, pharmacological, or combined weight-loss program; history of liposuction or bariatric surgery; a current pregnancy or being within 12 months postpartum; and a recent hospitalization (within 6 wk of the screening visit) (12–14).
The goal was to enroll a majority of participants who met the dual criteria of having normal FPG and NGT at entry to permit the detection of progression to IFG and/or IGT (or diabetes) during follow-up. We also enrolled offspring (25% of the cohort) who met the single criterion of having either normal FPG (with isolated IGT) or NGT (with isolated IFG) at baseline. Among the latter, subjects enrolled with normal FPG (and isolated IGT) were followed up for progression to IFG (or diabetes), and those enrolled with NGT (and isolated IFG) were followed up for progression to IGT (or diabetes), as described in Definition of outcome measures. We used the revised American Diabetes Association criteria for the diagnosis of IFG [100-125 mg/dL (5.6-6.9 mmol/L)] and diabetes (15) and the World Health Organization criteria [2hrPG 140–199 mg/dL (7.8–11.0 mmol/L)during a 75-g OGTT] for the diagnosis of IGT (16). Self-report of race/ethnicity was based on the 1990 US Census questionnaire (17). Parental history of T2D was documented using a diabetes-focused questionnaire that captured information on the number of affected biological parents, parent's gender and age at diagnosis, use of antidiabetes medications, diabetes complications, and contact information of the parents' physicians (12–14).
The study protocol was approved by the institutional review board; all participants gave written informed consent before initiation of the study, which was conducted at the University of Tennessee General Clinical Research Center (GCRC), in accordance with the principles of the Declaration of Helsinki. Enrollment in the POP-ABC study began in September 2006 and ended in February 2010, and participants were followed up until study closeout on March 31, 2012 (14).
Procedures and measurements
Assessments
Participants made quarterly visits to the GCRC for prespecified assessments: anthropometry, body mass index (BMI), blood pressure (BP), a standard 75-g OGTT, biochemical measurements, energy expenditure, insulin secretion, and insulin sensitivity, following a schedule as previously described (12, 14). In brief, FPG was measured quarterly; glycosylated hemoglobin (HbA1c), lipid profile, OGTT, body composition (dual energy x-ray absorptiometry), and frequently sampled iv glucose tolerance test annually; and resting energy expenditure (indirect calorimetry) and insulin sensitivity (euglycemic clamp) in years 1, 3, and 5. Repository blood, DNA, RNA, and protein specimens were obtained during the baseline visit and stored at −80°C for future analyses.
Participants arrived at the GCRC after an overnight fast. Initial procedures consisted of a structured medical interview and a general physical examination; measurement of weight, height, waist circumference, and BP; and a standard 75-g OGTT (12). Weight was measured in duplicate on a calibrated balance beam scale. Standing height was determined in duplicate with a standard stadiometer. The BMI was calculated as the weight in kilograms divided by the height in meters squared. Waist circumference was determined to the nearest 0.1 cm at the midpoint between the highest point of the iliac crest and the lowest costal margin in the midaxillary line, using a Gulick II tape measure (Country Technology, Inc). Blood pressure was recorded in the seated position, using an automated sphygmomanometer; the average of two readings was used for calculations. The OGTT was preceded by written instructions to consume a usual diet with adequate carbohydrates, refrain from strenuous exercise and alcohol consumption for 24 hours, and avoid smoking on the morning of the test. The test was initiated between 7:00 and 11:00 am in the subjects who had been fasting for approximately 10–14 hours: venous blood specimens for the measurement of glucose and insulin were obtained before (0 min) and at 30 minutes and 120 minutes after ingestion of 75 g flavored glucose (Trutol 75; Custom Laboratories).
Lipid profile, HbA1c, and other analytes in fasting plasma specimens were also measured per protocol (12). Standardized questionnaires were used to obtain data on education, employment, family income, marital status, and menopause. Additional annual assessments included food habits (18) and physical activity (19). The latter was computed by multiplying the duration of each activity in response to the Modifiable Activity Questionnaire (19) by the frequency (in hours per week). The product was then weighted by an estimate of the metabolic equivalent of the particular activity [metabolic equivalent (MET)], added up for all activities reported, and expressed as the average MET hours per week.
Measurement of insulin sensitivity and insulin secretion
The homeostasis models of insulin resistance (HOMA-IR) and β-cell function (HOMA-B) were derived from fasting glucose and insulin values (20). In addition, insulin sensitivity (Si-clamp) was assessed directly using the hyperinsulinemic euglycemic clamp, and insulin secretion was assessed using frequently sampled iv glucose tolerance test, as previously described (12). In brief, the clamp procedure was performed in subjects who had fasted overnight for approximately 12 hours. A primed, continuous iv infusion of regular insulin (2 mU/kg−1·min−1;14.4 pmol/kg−1 · min−1) was administered for 180 minutes, whereas blood glucose concentration was maintained at approximately 100 mg/dL (5.6 mmol/L) with a variable rate dextrose (20%) infusion. Arterialized blood specimens for measurement of glucose and insulin levels were obtained every 10 minutes. The rate of total insulin-stimulated glucose disposal was calculated for the last 60 minutes of the insulin infusion and corrected for steady-state plasma insulin levels to derive the insulin sensitivity index (Si-clamp) (12, 21). For the direct assessment of insulin secretion, overnight fasted subjects received an iv bolus of dextrose (25 g); arterialized blood sampling for the measurement of glucose and insulin were collected 30 minutes before and at 2, 3, 4, 5, 7, and 10 minutes after the iv dextrose bolus (12, 22). The acute insulin response to iv glucose (AIR) was computed as the mean incremental insulin concentration from 3 to 5 minutes after the dextrose bolus (12, 22).
Biochemical measurements
Plasma glucose was measured with a glucose oxidase method (Yellow Spring Instruments Co). Plasma levels of insulin, high-sensitivity C-reactive protein (hsCRP), and adiponectin were measured immunochemically in our Endocrine Research Laboratory, using commercial ELISA kits. HbA1c and fasting plasma lipid profiles were measured in a contract clinical laboratory.
Definition of outcome measures
The primary outcome measure was the occurrence of prediabetes (IFG and/or IGT) or diabetes (15, 16). For all subjects, any occurrence of diabetes, as indicated by an FPG value of 126 mg/dL (7.0 mmol/L) or higher or 2hrPG of 200 mg/dL (11.1 mmol/L) or higher, or a prescription of a diabetes medication, was an end point. By design, 75% of the subjects met the dual criteria of having normal FPG and NGT, and 25% had either normal FPG or NGT at baseline. For participants enrolled with normal FPG and NGT, the occurrence of IFG and/or IGT constituted an end point. For subjects enrolled with normal FPG (and isolated IGT), progression to IFG constituted an end point. For those enrolled with NGT (and isolated IFG), progression to IGT was an end point. If initial tests during a scheduled quarterly visit showed the occurrence of an end point, a confirmatory test was performed, usually within 6 weeks. The 75-g OGTT was the method of confirmation. If the second test was nonconfirmatory, the subject continued in the study as scheduled. For all subjects with confirmed end point occurrence, the date of the initial end point occurrence was recorded as the confirmed end point date. All end points were independently adjudicated by the Institutional Data and Safety Officer (Murray Heimberg, MD, PhD).
Secondary outcomes
The secondary outcome measures included glycemia, HbA1c, body weight, waist circumference, fat distribution, food habits, physical activity, insulin sensitivity, insulin secretion, energy expenditure, lipid profile, adipocytokines, and socioeconomic status.
Statistical analysis
Data are reported as means ± SD unless otherwise specified. Significance level was set as P < .05. The primary analytic outcome is progression to prediabetes/diabetes. For all statistical analyses, Si-clamp and AIR values were log transformed to achieve a normal distribution. The Kaplan-Meier curves for the probability of prediabetes/diabetes in black and white subjects were compared with the use of the log-rank test. General linear regression models were used to analyze baseline demographic, anthropometric, biochemical, behavioral, and socioeconomic variables as predictors of incident diabetes/prediabetes, after adjustments for age and sex. All variables significant at the 15% level were included in a stepwise Cox proportional-hazards regression, which produced a final model that identified variables significant at the 5% level. Thereafter, two-way interactions among the significant variables were tested for significance. All statistical analyses were performed with the use of SAS statistical software, version 9.3 (SAS Institute Inc).
Results
Study cohort, baseline characteristics, and follow-up
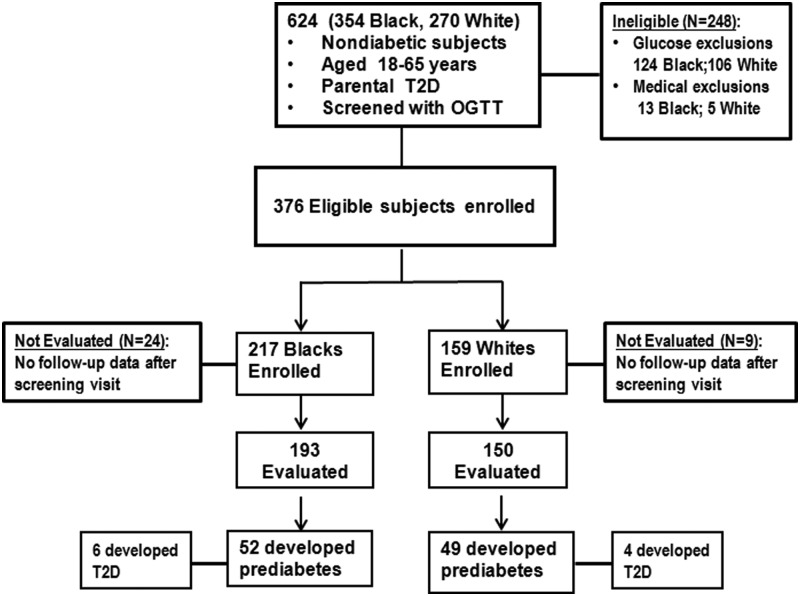
Figure 1 summarizes the enrollment and outcome data. Screening oral glucose tolerance tests were performed in a total of 624 offspring of parents with T2D; 376 subjects (217 black, 159 white) who met all eligibility criteria were enrolled in the study. We excluded 248 subjects because of preexisting diabetes (n = 54; 29 black, 25 white), combined IFG and IGT at baseline (n = 176; 95 black, 81 white), or for other medical reasons (n = 18; 13 black, five white). The latter included anemia, uncontrolled hypertension, poor venous access, and disallowed medications. The mean age of the participants was 44.2 ± 10.6 years, and approximately 70% was female. Most participants (86%) reported having only one parent with diabetes, whereas 14% reported having both affected parents. A similar proportion of black (14.8%) and white (12.6%) offspring reported having both parents with diabetes (Supplemental Table 1) (14). At enrollment, 282 subjects (75%) had normal FPG and NGT and 94 (25%) had either normal FPG [n = 68 (18%)] or normal 2hrPG [n = 26 (7%)]. The distribution of enrollment glycemic status was similar in white and black offspring (Supplemental Table 1).
Figure 1.
Enrollment and outcomes data for the POP-ABC study.
Compared with the white offspring, black offspring had a lower mean age, higher weight and BMI, and lower resting energy expenditure at enrollment (Table 1). The black participants also had a lower mean baseline values for FPG, triglycerides, adiponectin, and Si-clamp and higher values for AIR and hsCRP, as compared with white participants (Table 2). As previously noted (23), the mean HbA1c level was higher in black offspring than white offspring with similar or higher FPG and 2hrPG levels (Table 2). Compared with white participants, black participants had less family income and fewer years of education and were less likely to be current smokers (Supplemental Table 2).
Table 1.
Demographic and Clinical Characteristics at Baseline in 343 Offspring of Parents With Type 2 Diabetes
| Characteristic | White | Black | P Value |
|---|---|---|---|
| n | 150 | 193 | |
| Progression | 53 (35%) | 58 (30%) | .2997 |
| Female | 104 (69%) | 141 (73%) | .4489 |
| Age, y | 47 ± 10 | 43 ± 10 | .0007 |
| Age 18–40/40–65 y | 39/111 | 69/124 | .0538 |
| Premenopause/postmenopause | 54/49 | 95/45 | .0147 |
| Weight, kg | 81 ± 21 | 88 ± 22 | .0040 |
| BMI, kg/m2 | 28.7 ± 6.7 | 31.3 ± 7.5 | .0008 |
| Waist, cm | 93 ± 15 | 96 ± 16 | .0925 |
| Female | 90 ± 16 | 95 ± 15 | .0193 |
| Male | 99 ± 11 | 98 ± 18 | .7238 |
| Total fat mass, kg | 29.3 ± 13.3 | 31.8 ± 13.7 | .1087 |
| Female | 31.6 ± 14.4 | 35.2 ± 13.3 | .0543 |
| Male | 24.2 ± 8.1 | 22.4 ± 10.1 | .3622 |
| Trunk fat mass, kg | 14.7 ± 7.0 | 15.4 ± 7.5 | .4355 |
| Female | 15.4 ± 7.6 | 16.9 ± 7.5 | .1536 |
| Male | 13.2 ± 4.9 | 11.3 ± 5.9 | .1006 |
| SBP, mm Hg | 120 ± 16 | 123 ± 16 | .1469 |
| DBP, mm Hg | 72 ± 9 | 74 ± 9 | .2136 |
| REE, kcal/kg FFM | 30.6 ± 6.1 | 28.9 ± 4.9 | .0159 |
Abbreviations: DBP, diastolic BP; FFM, fat-free mass; REE, resting energy expenditure; SBP, systolic BP. The ± values are means ± SD.
Table 2.
Biochemical and Behavioral Characteristics at Baseline in 343 Offspring of Parents With T2Ds
| Characteristic | White Offspring | Black Offspring | P Value |
|---|---|---|---|
| FPG, mg/dL | 93.1 ± 6.4 | 91.0 ± 6.8 | .0029 |
| 2hrPG, mg/dL | 125 ± 24 | 124 ± 28 | .7203 |
| HbA1c | 5.4 ± 0.4% (36 ± 4.4 mmol/mol) | 5.7 ± 0.5 (39 ± 5.5 mmol/mol) | <.0001 |
| Fasting insulin, μU/mL | 8.4 ± 8.2 | 7.7 ± 6.1 | .4437 |
| HOMA-IR | 1.93 ± 1.62 | 1.86 ± 1.53 | .6984 |
| HOMA-B | 93 ± 76 | 93 ± 62 | .9864 |
| AIR, μU/mL | 61 ± 39 | 107 ± 89 | <.0001 |
| Si-clamp, μmol/kg FFM per min−1/pmol/L | 0.14 ± 0.06 | 0.12 ± 0.07 | .0253 |
| Total cholesterol, mg/dL | 180 ± 31 | 175 ± 34 | .1983 |
| LDL cholesterol, mg/dL | 105 ± 28 | 106 ± 30 | .7671 |
| HDL cholesterol, mg/dL | 52 ± 13 | 53 ± 14 | .3318 |
| Triglycerides, mg/dL | 114 ± 64 | 80 ± 40 | <.0001 |
| hsCRP, mg/L | 2.8 ± 4.6 | 4.7 ± 6.6 | .0024 |
| Adiponectin, μg/mL | 10.7 ± 5.49 | 8.03 ± 4.89 | <.0001 |
| Food habits score | 2.44±.49 | 2.67±.51 | <.0001 |
| Physical activity, MET h/wk | 19.5 ± 27.1 | 17.8 ± 36.9 | .6412 |
The ± values are means ± SD. Si-clamp had 203 subjects (103 black, 100 white). To convert the values for glucose to millimoles per liter, multiply by 0.0555. To convert the values for insulin to picomoles per liter, multiply by 7.175. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129. To convert the values for cholesterol to millimoles per liter, multiply by 0.02586.
Development of prediabetes/diabetes
During a mean follow-up period of 2.62 years, a total of 101 subjects (49 white, 52 black) of 343 participants with evaluable data developed prediabetes and 10 subjects (four white, six black) developed diabetes. The cumulative incidence of prediabetes/diabetes was 35% and 30% among white and black offspring, respectively (P = .2997). The crude incidence of prediabetes was 12.5 cases and 10.3 cases per 100 person-years and that of diabetes was 1.18 and 1.02 cases/100 person-years among white and black offspring, respectively. Of the participants who developed prediabetes, 44% reached that endpoint by the IFG criterion, 35% did so by the IGT criterion, and 20.6% had combined IFG and IGT. The proportion of participants who converted to prediabetes by IFG (42.6% vs 46%), IGT (34% vs 36.2%) or IFG/IGT (20.0% vs 21.3%) criteria was similar in black vs white participants.
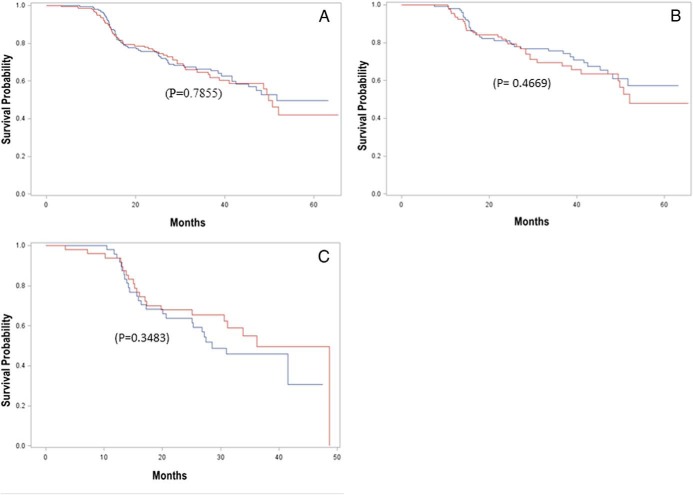
Figure 2 shows the cumulative prediabetes/diabetes survival probability, which did not differ significantly by race in the entire cohort (Figure 2A), among the subjects enrolled with both normal FPG and NGT (Figure 2B), or among those enrolled with either normal FPG or NGT (Figure 2C). The median prediabetes/diabetes survival time was 49.8 months in white offspring and 51.6 months in black offspring for the entire cohort (Figure 2A).
Figure 2.
Kaplan-Meier plot of prediabetes/diabetes survival in black (blue line) and white (red line) offspring of parents with T2D for the entire cohort (A), for participants enrolled with both normal fasting glucose and NGT (B), and for persons enrolled with either normal fasting glucose or NGT (C). There was no racial difference in the survival probability in the entire cohort (P = .7855), among offspring enrolled with both normal fasting glucose and NGT (P = .4669), or those enrolled with either normal fasting glucose or NGT (P = .3483).
Predictors of glycemic progression
The significant baseline demographic and clinical variables predictive of incident prediabetes/diabetes included age (P = .0017), male gender (P = .0003), weight (P = .0036), BMI (P = .0013), waist circumference (P < .0001), total fat mass (P = .0025), and trunk fat mass (P < .0001) (Table 3). Progressors to prediabetes had a mean enrollment weight of 90.0 ± 20.4 kg compared with 82.9 ± 21.7 kg among nonprogressors (P = .006). During a mean follow-up of 2.62 years, the mean weight change among nonprogressors was 0.48 ± 6.18 kg (P = .54), compared with 2.54 ± 6.91 kg (P = .01) among progressors. The mean weight change among nonprogressors did not differ by race or gender but tended to be greater in black offspring than white offspring among progressors (2.10 ± 0.96 kg vs 1.53 ± 1.83 kg in women; 5.77 ± 3.22 kg vs 1.62 ± 1.72 kg in men). The proportion of participants with either one affected parent (86% vs 85%) or both affected parents (13.7% vs 14.7%) was similar among progressors to prediabetes/diabetes vs nonprogressors. Among progressors, 44.1% gave a maternal history of diabetes, compared with 48.5% among nonprogressors. Paternal diabetes was reported by 42.2% of progressors and 36.8% of nonprogressors. The gender of the affected parent did not significantly predict glycemic progression status (P = .3701). Race did not predict incident prediabetes/diabetes before (P = .3745) or after (P = .8630) adjustment for baseline variables.
Table 3.
Demographic and Clinical Characteristics at Baseline in Participants Who Developed Prediabetes or Diabetes (Progressors) Compared With Those Who Remained Free of Incident Dysglycemia (Nonprogressors)
| Characteristic | Progressors | Nonprogressors | P Valuea |
|---|---|---|---|
| N | 111 | 232 | |
| White/black | 53/58 | 97/135 | .2997 |
| Female/male | 65/46 | 180/52 | .0003 |
| Age, y | 47 ± 8.9 | 43.9 ± 10.7 | .0017 |
| Age 18–40/40–65 y | 23/88 | 85/147 | .0030 |
| Premenopause/postmenopause | 35/30 | 114/64 | .1485 |
| Weight, kg | 90 ± 20 | 83 ± 22 | .0036 |
| BMI, kg/m2 | 31.4 ± 6.9 | 29.6 ± 7.4 | .0013 |
| Waist, cm | 99 ± 14 | 92 ± 16 | <.0001 |
| Female | 98 ± 12 | 91 ± 16 | .0006 |
| Male | 101 ± 15 | 96 ± 15 | .1367 |
| Total fat mass, kg | 32.0 ± 12.6 | 29.9 ± 14.0 | .0025 |
| Female | 37.2 ± 12.0 | 32.1 ± 14.4 | .0217 |
| Male | 24.2 ± 9.1 | 22.4 ± 9.1 | .0004 |
| Trunk fat mass, kg | 16.6 ± 6.8 | 14.3 ± 7.3 | <.0001 |
| Female | 18.9 ± 6.7 | 15.1 ± 7.6 | .0064 |
| Male | 13.1 ± 5.4 | 11.5 ± 5.5 | .0795 |
| SBP, mm Hg | 125 ± 16 | 120 ± 16 | .0768 |
| DBP, mm Hg | 73.6 ± 8.8 | 72.7 ± 9.1 | .6729 |
| REE, kcal/kg FFM | 29.6 ± 5.5 | 29.8 ± 5.6 | .6990 |
Abbreviations: DBP, diastolic BP; FFM, fat-free mass; REE, resting energy expenditure; SBP, systolic BP. The ± values are means ± SD.
Adjusted for age and sex, except white/black, female/male, age 18–40/40–65 years, age (years), and premenopause/postmenopause.
The glycemic and glucoregulatory predictors of incident prediabetes included FPG (P = .0027), 2hrPG (P = .0036), HbA1c (P = .019), insulin (P = .0075), HOMA-IR (P = .0012), HOMA-B (0.0392), Si-clamp (P = .0002), AIR corrected for Si-clamp (P = .0222), and the disposition index (P = .0032) (Table 4). Other significant biochemical predictors of glycemic progression were low-density lipoprotein (LDL) cholesterol (P = .026), high-density lipoprotein (HDL) cholesterol (P = .0022), and hsCRP (P = .031) (Table 4). Adiponectin levels tended to be lower in progressors than nonprogressors (P = .065). Further analysis showed that baseline adiponectin levels predicted glycemic progression in black (P = .0488) but not white (P = .5432) offspring, after adjusting for age and gender.
Table 4.
Biochemical and Behavioral Characteristics at Baseline in Persons Who Developed Prediabetes or Diabetes (Progressors) Compared With Those Who Remained Free of Incident Dysglycemia (Nonprogressors)
| Characteristic | Progressors | Nonprogressors | P Valuea |
|---|---|---|---|
| FPG, mg/dL | 94 ± 7 | 91 ± 6 | .0027 |
| 2hrPG, mg/dL | 130 ± 27 | 121 ± 25 | .0036 |
| HbA1c, % | 5.7 ± 0.5 (39 ± 5.5 mmol/mol) | 5.5 ± 0.4 (37 ± 4.4 mmol/mol) | .0190 |
| Fasting insulin, μU/mL | 9.5 ± 7.6 | 7.3 ± 6.7 | .0075 |
| HOMA-IR | 2.26 ± 1.70 | 1.71 ± 1.47 | .0012 |
| HOMA-B | 100 ± 64 | 89 ± 71 | .0392 |
| Si-clamp, μmol/kg FFM per min−1/pmol/L | 0.116 ± 0.066 | 0.147 ± 0.064 | .0002 |
| AIR, μU/mL | 81 ± 74 | 88 ± 74 | .8378 |
| AIR adjusted for Si-clampb | 54 ± 1.0 | 68 ± 1.0 | .0222 |
| Disposition index | 45.1 ± 31.1 | 63.9 ± 50.2 | .0032 |
| Total cholesterol, mg/dL | 181 ± 34 | 176 ± 32 | .2095 |
| LDL cholesterol, mg/dL | 111 ± 30 | 103 ± 28 | .0260 |
| HDL cholesterol, mg/dL | 49 ± 12 | 54 ± 14 | .0022 |
| Triglycerides, mg/dL | 104 ± 55 | 91 ± 54 | .1688 |
| hsCRP, mg/L | 4.3 ± 6.7 | 3.5 ± 5.4 | .0310 |
| Adiponectin, μg/mL | 8.53 ± 4.35 | 9.85 ± 5.68 | .0650 |
| Food habits score | 2.57± .49 | 2.56± .52 | .8900 |
| Physical activity, MET h/wk | 14.5 ± 24.3 | 20.8 ± 35.9 | .0709 |
The ± values are means ± SD except as noted. Si-clamp had 203 subjects (103 black, 100 white). To convert the values for glucose to millimoles per liter, multiply by 0.0555. To convert the values for insulin to picomoles per liter, multiply by 7.175. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129. To convert the values for cholesterol to millimoles per liter, multiply by 0.02586.
Adjusted for age and sex.
Least square means ± SE.
In a Cox proportional-hazards model, with adjustment for age and sex, the 2hrPG and trunk fat mass were independent predictors of incident prediabetes/diabetes. The relative hazards (95% confidence interval [CI]) for the 90th vs 10th percentile were as follows: trunk fat mass 2.90 (95% CI 1.74–4.82), P < .0001; 2hrPG 2.54 (95% CI 1.46–4.40), P = .0009. The combined hazard of both trunk fat mass and 2hrPG at the 90th vs 10th percentile was 7.35. The interaction between these two variables was not significant (P > .05).
Discussion
In this prospective study, we observed that the incidence of prediabetes was 10–12.5 cases per 100 person-years among African-American and Caucasian offspring of parents with T2D. We detected no significant effect of race on incident prediabetes among participants enrolled with normal FPG and NGT. Also, among the offspring who had one marker of prediabetes (IFG or IGT) at baseline, progression to dual markers of prediabetes (IFG+IGT) or diabetes did not differ by race. In a study of 254 Pima Indians, 79 (31%) developed incident prediabetes during a mean follow-up of 4 years (7). In our study, 101 of 343 participants (29.4%) developed prediabetes during a mean follow-up of 2.62 years. Although direct comparison is difficult, the crude incidence rate of prediabetes among the white and black subjects with parental T2D in our study appears to be of the same magnitude as the rate among Pima Indians, the group with the world's highest prevalence of T2D (8–10).
In the Framingham Offspring Study that enrolled predominantly white subjects, offspring with maternal history of diabetes were more likely to have prediabetes than offspring with paternal diabetes, although the subsequent risk of diabetes was similar regardless of parental gender (11). In the POP-ABC study, black offspring were more likely to report maternal diabetes, whereas white offspring were more likely to report paternal diabetes. However, parental gender did not predict incident prediabetes in our biracial cohort. Our POP-ABC study identified several demographic and metabolic predictors of incident prediabetes, including age, male gender, general and abdominal adiposity, glycemia, insulin sensitivity, dyslipidemia, and adipocytokines. Baseline adiponectin levels showed an inverse trend toward prediction of prediabetes; that trend reached significance among black but not white offspring, suggesting possible ethnic disparity in the relationship between adiponectin and dysglycemia. With regard to insulin sensitivity, both Si-clamp (a measure of predominantly muscle insulin sensitivity) and HOMA-IR (a reflection of hepatic insulin sensitivity) were predictive of progression to prediabetes in our study. The male preponderance in incident prediabetes is consistent with national data (2, 3, 24), but the lack of gender difference in diagnosed diabetes indicates that equilibration occurs during transition from prediabetes to T2D (25).
We had hypothesized that ethnic differences might be discernible during the transition to prediabetes, which would then explain the higher prevalence of T2D among African Americans (1–3, 24), despite the lack of racial differences in progression from prediabetes to T2D (6). At baseline, the black and white offspring in our POP-ABC study differed in age, weight, BMI, and several metabolic characteristics associated with the risk of diabetes and prediabetes (Table 1). We found that the occurrence of prediabetes and progression from IFG or IGT to combined IFG+IGT were similar in black and white offspring of parents with T2D, before and after adjusting for baseline differences. The absence of racial disparity in incident prediabetes indicates that, among subjects with parental diabetes, race/ethnicity may not be a major determinant of early dysglycemia.
Notably, the demographic information on prevalent diabetes in the United States was derived mostly from cross-sectional surveys that relied on self-report during telephone interviews. In the 2005–2006 National Health and Nutrition Examination Survey, 516 persons who reported having ever been told by a health care professional that they had diabetes were classified as having diagnosed diabetes, and 3107 persons who did not report preexisting diabetes underwent evaluation with blood glucose measurements to estimate the prevalence of undiagnosed diabetes and prediabetes (24). Using the self-reported information, the prevalence of diagnosed diabetes was 12.8% in non-Hispanic blacks and 6.6% in non-Hispanic whites (24). In contrast, measured blood glucose in National Health and Nutrition Examination Survey 2005–2006 showed no significant racial/ethnic differences in the prevalence of undiagnosed diabetes or prediabetes (24). The 2011 Centers for Disease Control and Prevention estimates for diagnosed and undiagnosed diabetes among US adults were 10.2% in non-Hispanic whites and 18.7% in non-Hispanic blacks (26). The race/ethnicity-specific estimates for diagnosed diabetes were calculated using the 2007–2009 National Health Interview Survey (27) that also relied on self-report. To estimate the prevalence of undiagnosed diabetes and prediabetes, the 2011 National Diabetes Fact Sheet relied on actual blood glucose and HbA1c measurements. Using these hard measures, there was no racial/ethnic difference in the prevalence of undiagnosed diabetes or prediabetes (26). Remarkably, none of the national surveys based on blood glucose measurements has shown a higher prevalence of diabetes, and all have shown a lower prevalence of prediabetes, in black persons compared with white persons (1–3, 24, 26, 28, 29).
Thus, our findings are consistent with national data derived from measured blood glucose but are discordant with the nearly 2-fold black to white ratio of self-reported diagnosed diabetes (1–3, 23). Theoretically, a higher prevalence of self-reported diabetes diagnosis in black persons could occur if the following occurs: 1) black persons were more likely than white persons to admit having been diagnosed with diabetes during interviews; 2) black persons have greater access to care, or greater health resource use, or are more likely to be screened for diabetes than white persons; 3) black persons with diabetes live longer than their white counterparts; or 4) there were nonrandom methodological or communication factors associated with the telephone surveys. African Americans do not enjoy a greater access to health care than Caucasians (30, 31), and it is unclear whether race-based screening for diabetes is a widespread practice. Interestingly, black patients with chronic kidney disease receiving dialysis have been reported to have 13%–45% lower mortality than white patients (32–34). The apparent survival advantage appears to extend to persons with less severe chronic kidney disease (35–38). It is unknown whether the differential survival among black patients with diabetes is of sufficient magnitude to explain the discrepancy between self-reported prevalent diabetes and newly detected diabetes in national surveys (1–3, 24, 26, 27). Thus, no clear mechanisms explain why objective estimates of undiagnosed diabetes would follow a markedly different racial pattern from that of self-reported prevalence of diagnosed diabetes. Owing to the inherent risks of recall bias, health illiteracy, and potential miscommunication during telephone interviews, objective estimates of diabetes prevalence (based on glucose measurement) are likely to be more valid than estimates based on self-report.
One limitation of the present study is that the restriction of the study population to offspring of diabetic parents renders our findings not generalizable. However, data based on measured blood glucose in the general population do show a similar or lower prevalence of prediabetes in black persons than white persons (1–3, 24, 26). The Diabetes Prevention Program, which enrolled people with and without familial diabetes, reported no ethnic/racial differences in incident type 2 diabetes (6), whereas the Atherosclerosis Risk In Communities study (in which 25% of participants gave a family history of diabetes) reported a 2-fold higher incidence of diabetes (including self-reported) in black subjects compared with white subjects (39). A recent study, in which less than 15% of participants had familial history of diabetes, reported that race/ethnicity was not a significant predictors of incident type 2 diabetes (40). In the prospective San Antonio Heart Study, incident prediabetes occurred at a similar rate among obese Mexican-Americans and Caucasians, but Mexican-Americans had a greater risk among nonobese subjects (41). Thus, on balance, our present findings are supported by data from nonoffspring cohorts. Another limitation is the relatively short duration of follow-up. It is, thus, possible that longer follow-up of our cohort could show a different racial pattern among late progressors to prediabetes. Nonetheless, our findings are in accord with objective national data that indicate no racial/ethnic disparities in the rates of biochemically diagnosed diabetes or prediabetes (1–3, 24, 26).
In conclusion, we here report a prediabetes incidence of 10–12.5 cases per 100 person-years among black and white offspring of parents with T2D. Such a high hazard rate argues for the routine, aggressive screening of all persons with parental diabetes, prompt identification of high-risk individuals, and timely intervention to prevent glycemic progression. We found that the predictors of progression from normoglycemia to prediabetes overlap considerably with known risk factors for T2D. Therefore, proven interventions that prevent type 2 diabetes (6) may be effective in slowing, preventing, or even reversing (42) incident prediabetes in high-risk persons.
Acknowledgments
We are indebted to the participants who volunteered for this study.
Authors' contributions included the following: S.D.-J. was the principal investigator, developed the study concept and design, and wrote the manuscript; C.E. collected the data and reviewed and revised the manuscript; S.E. collected the data and reviewed and revised the manuscript; E.N. collected the data and reviewed and revised the manuscript; and J.W. performed the statistical analysis and reviewed and revised the manuscript.
Role of the funding sources included the following: the funding sources (National Institutes of Health, American Diabetes Association, and the State of Tennessee) had no role in the design and execution of the POP-ABC study or the analysis and publication of the data obtained from the study.
POP-ABC Research Group included the following (current): Samuel Dagogo-Jack, MD (principal investigator); Ann Ammons, BS; John Crisler; Chimaroke Edeoga, MBBS, MPH; Sotonte Ebenibo, MBBS, MPH; Ebenezer Nyenwe, MD; and Jim Wan, PhD.
Past members include the following: Emmanuel Chapp-Jumbo, MBBS (2009–2011); Ruben Cuervo, MD (2006–2007); Nonso Egbuonu, MBBS (2007–2010); Nicoleta Ionica, MD (2007–2008); and Dorota Malinowski, MD (2007–2008).
Consultant was Steven Haffner, MD; the data and safety officer was Murray Heimberg, MD, PhD.
The POP-ABC study was supported by Grants R01 DK067269, R01 DK067269-04S1, and MO1 RR00211 from the National Institutes of Health; Grant 7-07-MN-13 from the American Diabetes Association; and the State of Tennessee Clinical Research Center fund (University of Tennessee Health Science Center Clinical Research Center Grant E070166010).
Disclosure Summary: The authors have nothing to declare.
Funding Statement
The POP-ABC study was supported by Grants R01 DK067269, R01 DK067269-04S1, and MO1 RR00211 from the National Institutes of Health; Grant 7-07-MN-13 from the American Diabetes Association; and the State of Tennessee Clinical Research Center fund (University of Tennessee Health Science Center Clinical Research Center Grant E070166010).
Footnotes
- AIR
- acute insulin response to iv glucose
- BMI
- body mass index
- BP
- blood pressure
- CI
- confidence interval
- FPG
- fasting plasma glucose
- GCRC
- General Clinical Research Center
- HbA1c
- glycosylated hemoglobin
- HDL
- high-density lipoprotein
- HOMA-B
- homeostasis model of β-cell function
- HOMA-IR
- homeostasis model of insulin resistance
- 2hrPG
- 2-hour plasma glucose
- hsCRP
- high-sensitivity C-reactive protein
- IFG
- impaired fasting glucose
- IGT
- impaired glucose tolerance
- LDL
- low-density lipoprotein
- MET
- metabolic equivalent
- NGT
- normal glucose tolerance
- OGTT
- oral glucose tolerance test
- POP-ABC
- Pathobiology of Prediabetes in A Biracial Cohort
- Si-clamp
- insulin sensitivity by euglycemic clamp
- T2D
- type 2 diabetes.
References
- 1. Centers for Disease Control and Prevention (CDC). National diabetes fact sheet. Diagnosed and undiagnosed diabetes in the United States, all ages, 2010. 2011. http://www.cdc.gov/diabetes/pubs/estimates11.htm. Accessed January 3, 2014.
- 2. Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the US population: National Health And Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29:1263–1268. [DOI] [PubMed] [Google Scholar]
- 3. Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the US population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bock G, Dalla Man C, Campioni M, Chittilapilly E, et al. Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes. 2006;55:3536–3549. [DOI] [PubMed] [Google Scholar]
- 5. Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes. 1997;46:701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weyer C, Tataranni PA, Bogardus C, Pratley R. Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes Care. 2000;24:89–94. [DOI] [PubMed] [Google Scholar]
- 8. Knowler WC, Pettitt DJ, Savage PJ, Bennett PH. Diabetes incidence in Pima Indians: contributions of obesity and parental diabetes. Am J Epidemiol. 1981;113:144–156. [DOI] [PubMed] [Google Scholar]
- 9. Knowler WC, Pettitt DJ, Saad MF, Bennett PH. Diabetes mellitus in the Pima Indians: incidence, risk factors and pathogenesis. Diabetes Metab Rev. 1990;6:1–27. [DOI] [PubMed] [Google Scholar]
- 10. Baier LJ, Hanson RL. Genetic studies of the etiology of type 2 diabetes in Pima Indians: hunting for pieces to a complicated puzzle. Diabetes. 2004;53:1181–1186. [DOI] [PubMed] [Google Scholar]
- 11. Meigs JB, Cupples LA, Wilson PW. Parental transmission of type 2 diabetes: the Framingham Offspring Study. Diabetes. 2000;49:2201–2207. [DOI] [PubMed] [Google Scholar]
- 12. Dagogo-Jack S, Edeoga C, Nyenwe E, Chapp-Jumbo E, Wan J. Pathobiology of Prediabetes in a Biracial Cohort (POP-ABC): design and methods. Ethn Dis. 2011;21:33–39. [PMC free article] [PubMed] [Google Scholar]
- 13. Ebenibo S, Edeoga C, Ammons A, Egbuonu N, Dagogo-Jack S. Recruitment strategies and yields for the Pathobiology of Prediabetes in a Biracial Cohort: a prospective natural history study of incident dysglycemia. BMC Med Res Methodol. 2013;13:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dagogo-Jack S, Edeoga C, Ebenibo S, Chapp-Jumbo E. Pathobiology of Prediabetes in a Biracial Cohort (POP-ABC) study: baseline characteristics of enrolled subjects. J Clin Endocrinol Metab. 2013;98:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Genuth S, Alberti KG, Bennett P, et al. The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: 2003 follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization. Diabetes mellitus: report of a WHO Study Group. Geneva: World Health Organization (Technical Report Series 1985, no. 727); 1985. [PubMed] [Google Scholar]
- 17. Bureau of the Census. Census of the population. Washington, DC: US Government Printing Office; 1990. [Google Scholar]
- 18. Mayer-Davis EJ, Vitolins MZ, Carmichael SL, et al. Validity and reproducibility of a food frequency interview in a multi-cultural epidemiology study. Ann Epidemiol. 1999;9:314–324. [DOI] [PubMed] [Google Scholar]
- 19. Kriska AM, Caspersen CJ. Introduction to a collection of physical activity questionnaires. Med Sci Sports Exerc. 1997;29(suppl):S5–S9. [PubMed] [Google Scholar]
- 20. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 21. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. [DOI] [PubMed] [Google Scholar]
- 22. Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chapp-Jumbo E, Edeoga C, Wan J, Dagogo-Jack S. Ethnic disparity in hemoglobin A1c levels among normoglycemic offspring of parents with type 2 diabetes mellitus. Endocr Pract. 2012;18:356–362. [DOI] [PubMed] [Google Scholar]
- 24. Centers for Disease Control and Prevention. National Center for Health Statistics: National Health and Nutrition Examination Survey 2005–2006. 2008. http://www.cdc.gov/nchs/about/major/nhanes/nhanes2005–2006/nhanes05_06.htm. Accessed January 3, 2014.
- 25. Perreault L, Ma Y, Dagogo-Jack S, et al. Sex differences in diabetes risk and the effect of intensive lifestyle modification in the Diabetes Prevention Program. Diabetes Care. 2008;31:1416–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Centers for Disease Control and Prevention. National diabetes fact sheet. Diagnosed and undiagnosed diabetes in the United States, all ages, 2010. 2011. http://www.cdc.gov/diabetes/pubs/estimates11.htm. Accessed February 19, 2014.
- 27. Centers for Disease Control and Prevention. National Health Interview Survey (NHIS). http://www.cdc.gov/nchs/nhis.htm. Accessed January 3, 2014.
- 28. Benjamin SM, Valdez R, Geiss LS, Rolka DB, Narayan KM. Estimated number of adults with prediabetes in the US in 2000: opportunities for prevention. Diabetes Care. 2003;26:645–649. [DOI] [PubMed] [Google Scholar]
- 29. Williams DE, Cadwell BL, Cheng YJ, et al. Prevalence of impaired fasting glucose and its relationship with cardiovascular disease risk factors in US adolescents, 1999–2000. Pediatrics. 2005;116:1122–1126. [DOI] [PubMed] [Google Scholar]
- 30. Harris MI. Racial and ethnic differences in health care access and health outcomes for adults with type 2 diabetes. Diabetes Care. 2001;24:454–459. [DOI] [PubMed] [Google Scholar]
- 31. Rhee MK, Cook CB, Dunbar VG, et al. Limited health care access impairs glycemic control in low income urban African Americans with type 2 diabetes. J Health Care Poor Underserved. 2005;16:734–746. [DOI] [PubMed] [Google Scholar]
- 32. Kalantar-Zadeh K, Kovesdy CP, Derose SF, Horwich TB, Fonarow GC. Racial and survival paradoxes in chronic kidney disease. Nat Clin Pract Nephrol. 2007;3:493–506. [DOI] [PubMed] [Google Scholar]
- 33. Kucirka LM, Grams ME, Lessler J, et al. Association of race and age with survival among patients undergoing dialysis. JAMA. 2011;306:620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fernandes NM, Hoekstra T, van den Beukel TO, et al. Association of ethnicity and survival in peritoneal dialysis: a cohort study of incident patients in Brazil. Am J Kidney Dis. 2013;62:89–96. [DOI] [PubMed] [Google Scholar]
- 35. Kovesdy CP, Quarles LD, Lott EH, et al. 2013 Survival advantage in black versus white men with CKD. Effect of estimated GFR and case mix. Am J Kidney Dis. 2013;62:228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jha AK, Shlipak MG, Hosmer W, Frances CD, Browner WS. Racial differences in mortality among men hospitalized in the Veterans Affairs health care system. JAMA. 2001;285:297–303. [DOI] [PubMed] [Google Scholar]
- 37. Conway BN, May ME, Blot WJ. Mortality among low-income African Americans and whites with diabetes. Diabetes Care. 2013;35:2293–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Egede LE, Dagogo-Jack S. Epidemiology of type 2 diabetes: focus on ethnic minorities. Med Clin North Am. 2005;89:949–975. [DOI] [PubMed] [Google Scholar]
- 39. Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA. 2000;283:2253–2259. [DOI] [PubMed] [Google Scholar]
- 40. Rodbard HW, Bays HE, Gavin JR 3rd, et al. Rate and risk predictors for development of self-reported type-2 diabetes mellitus over a 5-year period: the SHIELD study. Int J Clin Pract. 2012;66:684–691. [DOI] [PubMed] [Google Scholar]
- 41. Lorenzo C, Lee R, Haffner SM. Impaired glucose tolerance and obesity as effect modifiers of ethnic disparities of the progression to diabetes: the San Antonio Heart Study. Diabetes Care. 2012;35:2548–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE. Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet. 2012;379:2243–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]