Abstract
Concept:
Redifferentiation of thyroid carcinoma cells has the potential to increase the efficacy of radioactive iodine therapy in treatment-refractory, nonmedullary thyroid carcinoma (TC), leading to an improved disease outcome. Mammalian target of rapamycin (mTOR) is a key regulator of cell fate affecting survival and differentiation, with autophagy and inflammation as prominent downstream pathways.
Methods:
The effects of mTOR inhibition were studied for its redifferentiation potential of the human TC cell lines BC-PAP, FTC133, and TPC1 by assessment of mRNA and protein expression of thyroid-specific genes and by performance of iodine uptake assays.
Results:
In thyroid transcription factor 1 (TTF1)-expressing cell lines, mTOR inhibition promoted redifferentiation of TC cells by the up-regulation of human sodium-iodine symporter mRNA and protein expression. Furthermore, these cells exhibited markedly elevated iodine uptake capacity. Surprisingly, this redifferentiation process was not mediated by autophagy induced during mTOR inhibition or by inflammatory mediators but through transcriptional effects at the level of TTF1 expression. Accordingly, small interfering RNA inhibition of TTF1 completely abrogated the induction of human sodium-iodine symporter by mTOR inhibition.
Conclusion:
The present study has identified the TTF1-dependent molecular mechanisms through which the inhibition of mTOR leads to the redifferentiation of TC cells and subsequently to increased radioactive iodine uptake.
Conventional treatment modalities for patients with nonmedullary thyroid carcinoma (TC) include surgical removal of the thyroid and subsequent ablation of thyroid (cancer) remnants by radioactive iodine (131I). Effective eradication of TC critically depends on the ability of the tumor cells to actively internalize and trap radioactive iodine by organification. In 20%–30% of patients with metastatic disease, this capacity is lost due to tumor cell dedifferentiation (1, 2). Mechanisms that underlie the process of dedifferentiation comprise the loss of thyroid-specific gene expression, including the human sodium-iodine symporter (hNIS), and/or defective trafficking of hNIS to the basal membrane and are frequently caused by genetic aberrations activating the BRAF, RET, and phosphatidylinositol 3-kinase-AKT pathways (3–6).
Redifferentiation of TC cells that restores the sensitivity of the tumor to radioactive iodine therapy is considered an important potential therapeutic approach. In recent years, multiple strategies have been investigated for their potential to induce redifferentiation of TC cells, with limited success for nonspecific modalities such as retinoic acid (7–9) and histone modification agents (10–12). A much higher therapeutic efficacy was reached by treatment with (combinations of) specific oncogene-guided kinase inhibitors, including MAPK, MAPK kinase, mammalian target of rapamycin (mTOR), and Akt kinases (13–17), of which the MAPK kinase inhibitor selumetinib is particularly promising. Ho et al reported recently that a short course treatment with selumetinib resulted in an increase of 131I uptake sufficient to enable 131I therapy in 12 of 20 patients (14). Although the advent of kinase inhibitors offers new perspectives, no complete responses have been observed, and most responses were temporary. Therefore, the development of alternative treatments for these patients is warranted.
The mTOR pathway has emerged as a key regulator of multiple downstream pathways that act on basic biological processes of protein synthesis, cell division, and cell death (18). Not surprisingly, mTOR signaling is strongly implicated in malignant transformation and tumor cell behavior including TC (19, 20), and the efficacy of mTOR inhibition as anticancer treatment has been shown in renal cell carcinoma, advanced pancreatic neuroendocrine tumor, and lymphoma clinical trials (21–23). Of particular interest to TC, inhibition of mTOR was demonstrated to increase the capacity of physiological thyroid follicular cells to accumulate iodine (19), which, however, remains to be addressed in TC tumor cells. We therefore hypothesize that this effect may also be present in TC.
Two of the pathways that are strongly modulated by mTOR are autophagy and inflammation (24). Autophagy is the process of recycling cellular components, such as cytosolic organelles and protein aggregates, through the degradation mediated by lysosomes and may be relevant for the susceptibility and clinical course of TC (25). Furthermore, TC patients bearing the risk variant of the ATG16L1 T300A (rs2241880) polymorphism, which influences the inflammatory response (26), had tumors requiring higher activity doses of 131I to achieve remission, possibly due to less sensitivity to radioactive iodine (25). We therefore hypothesized that modulation of inflammatory and/or autophagy pathways through mTOR inhibition influences the differentiation status of TC cells and may restore their capacity for iodine uptake.
To examine the role of the mTOR pathway in TC redifferentiation, we studied three human TC cell lines with different genetic backgrounds [BRAF mutated BC-PAP, the phosphatase and tensin homolog deleted from chromosome 10 (PTEN) deficient cell line FTC133, and TPC1 with RET/PTC rearrangement)] that are all associated with dedifferentiation and loss of the capacity to accumulate iodine (3, 27, 28).
Materials and Methods
Cell culture
The TC cell lines BC-PAP (papillary, BRAF V600E mutation), FTC133 (follicular, PTEN deficient), and TPC1 (papillary, RET/PTC rearrangement) were obtained from the sources previously described and were authenticated by short tandem repeat profiling (29). Cell lines were cultured in DMEM medium (Invitrogen) supplemented with gentamicin 10 μg/mL, L-glutamine 10 mM, pyruvate 10 mM, and 10% fetal calf serum (Invitrogen). Earle's balanced salt solution (EBSS) starvation medium was purchased from Invitrogen. Cells were incubated with the mTOR inhibitor rapamycin, several inflammatory stimuli, autophagy modulators, and combinations of these stimuli for the indicated time points: granulocyte macrophage colony-stimulating factor (50 ng/mL; R&D Systems), macrophage colony-stimulating factor (50 ng/mL; R&D Systems), interferon-γ (2.5 μg/mL; Boehringer), IL-1β (100 ng/mL; R&D Systems), TNFα (100 ng/mL; Boehringer), rapamycin (for concentration, see figure legends; Biovision), 3-methyl adenine (10 mM; Sigma), and wortmannin (100 nM; Biolegend).
Real-time quantitative PCR
TC cell lines were treated with TRIzol reagent (Invitrogen), and total RNA purification was performed according to the manufacturer's instructions. Isolated RNA was subsequently transcribed into cDNA using an iScript cDNA synthesis kit (Bio-Rad Laboratories) followed by quantitative PCR using the SYBR Green method (Applied Biosystems). The following primers were used: hNIS forward, 5′-TCC-TGT-CCA-CCG-GAA-TTA-TCT-3′, and reverse, 5′-ACG-ACC-TGG-AAC-ACA-TCA-GTC-3′; thyroid transcription factor 1 (TTF1) forward, 5′-AGC-ACA-CGA-CTC-CGT-TCT-C-3′, and reverse, 5′-GCC-CAC-TTT-CTT-GTA-GCT-TTC-C-3′; thyroid transcription factor 2 (TTF2) forward, 5′-CAC-GGT-GGA-CTT-CTA-CGG-G-3′, and reverse, 5′-GGA-CAC-GAA-CCG-ATC-TAT-CCC-3′; paired box 8 (PAX8) forward, 5′-AGT-CAC-CCC-AGT-CGG-ATT-C-3′, and reverse 5′-CTG-CTC-TGT-GAG-TCA-ATG-CTT-A-3′; and thyroid-stimulating hormone receptor (TSH-R) forward, 5′-TTC-CCT-GAC-CTG-ACC-AAA-GTT-3′, and reverse, 5′-ACG-TCA-TGT-AAG-GGT-TGT- CTG-T-3′.
Data were corrected for expression of the housekeeping gene β2 microglobulin, for which the primers forward, 5′-ATG-AGT-ATG-CCT-GCC-GTG-TG-3′, and reverse, 5′-CCA-AAT-GCG-GCA-TCT-TCA-AAC-3′, were used. For gene silencing, cells were transfected with scrambled or TTF1 (NKX2–1) directed SMARTpool small interfering RNA (siRNA) oligonucleotides (Dharmacon, Thermo Fisher Scientific) by RNAiMAX transfection reagent (Invitrogen) according to the manufacturers' instructions. Target sequences of the TTF1-directed siRNA oligonucleotides were GGA-CGU-GAG-CAA-GAA-CAU-G, GCU-ACA-AGA-AAG-UGG-GCA-U, GCU-ACA-AAA-UGA-AGC-GCC-A, and AGG-CCA-AAC-UGC-UGG-ACG-U.
Western blots
For Western blotting, 5 × 106 cells were lysed in 50 μL of lysis buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 10% glycerol, 1% Triton X-100, 40 mM α-glycerophosphate, 50 mM sodium fluoride, 200 mM sodium vanadate, 10 mg/mL leupeptin, 10 mg/mL aprotinin, 1 mM pepstatin A, and 1 mM phenylmethylsulfonyl fluoride]. The homogenate was frozen and then thawed and centrifuged at 4°C for 10 minutes at 15 000 × g, and the supernatant was mixed with a loading buffer containing dithiothreitol, incubated at 37°C for 1 hour (no boiling), and taken for Western blot analysis.
Equal amounts of protein (quantified by BCA assay; Thermo Scientific) were subjected to SDS-PAGE using 10% polyacrylamide gels at a constant voltage of 100 V. After SDS-PAGE, proteins were transferred to nitrocellulose membrane (0.2 mm). The membrane was blocked with 5% (wt/vol) milk powder in TBS/Tween 20 for 1 hour at room temperature, followed by incubation overnight at 4°C with an hNIS antibody (1:500, 250552; Abbiotec) in 5% milk powder in TBS/Tween 20 or with an actin antibody (loading control, 1:1000, A2066; Sigma) in 5% milk powder in TBS/Tween 20. After overnight incubation, the blots were washed three times with TBS/Tween 20 and then incubated with horseradish peroxidase-conjugated swine antirabbit antibody at a dilution of 1:5000 in 5% (wt/vol) milk powder in TBS/Tween 20 for 1 hour at room temperature. After being washed three times with TBS/Tween 20, the blots were developed with enhanced chemiluminescence (GE Healthcare) according to the manufacturer's instructions.
Iodine uptake
Iodine uptake was determined as described previously (17). During 48 hours before the iodine uptake assay, cells were cultured in serum-free DMEM medium. For these experiments, the medium was not supplemented with serum because serum hormones such as TSH influence the cellular uptake of iodine. After culturing with rapamycin for 48 hours, cells were incubated for 30 minutes with 1 kBq Na125I and 20 μM nonradioactive NaI, with or without 80 μM of sodium perchlorate to control for specific uptake. The radioactive medium was aspirated, and the cells were washed with ice-cold PBS and lysed in 0.1 M NaOH buffer at room temperature. Radioactivity was measured in the cell lysates in a γ-counter. In parallel experiments, DNA was isolated from the cells by standard procedures (Puregene kit; Gentra Systems) and quantified by Nanodrop measurements (Thermo Fisher Scientific). Accumulated radioactivity was expressed as picomoles of NaI per nanogram of DNA.
Statistical analysis
Statistical significance of gene expression and the iodine uptake results were tested by the use of the Mann-Whitney U tests, and a value of P < .05 was considered statistically significant.
Results
mTOR inhibition leads to increased expression of thyroid-specific genes and proteins in BC-PAP and FTC133 cell lines but not in the TPC1 cell line
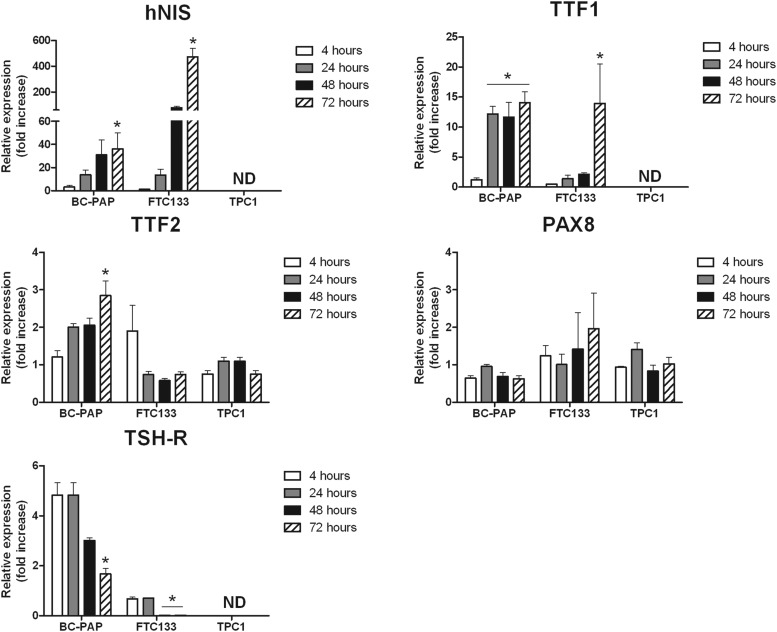
In an initial experiment, we incubated the TC cell lines BC-PAP (papillary TC, BRAF V600E mutated), FTC133 (PTEN deficient follicular TC), and TPC1 (papillary TC with RET/PTC rearrangement) for 4, 24, 48, and 72 hours with the mTOR inhibitor rapamycin (Figure 1). Rapamycin potently up-regulated hNIS (up to 400-fold increase in FTC133 and up to 40-fold increase in BC-PAP after 72 h), TTF1 (thyroid transcription factor 1) (up to 15-fold), and to a lesser extent TTF2 gene expression in both BC-PAP and FTC133 cell lines, whereas PAX8 gene expression was unaffected. Interestingly, the mRNA expression of TSH-R was also induced in BC-PAP cells during the first 24 hours of rapamycin treatment but decreased after 48 and 72 hours in both BC-PAP and FTC133 cell lines. In contrast, rapamycin did not induce mRNA expression of thyroid-specific genes in TPC1. In a subsequent dose-response experiment, the three cell lines were exposed to different concentrations of rapamycin. These experiments showed that the aforementioned effects of rapamycin on thyroid-specific genes were not found with rapamycin concentrations below 50 μg/mL (Supplemental Figure 1).
Figure 1.
Transcriptional profiling BC-PAP, FTC133, and TPC1. The TC cell lines BC-PAP, FTC133, and TPC1 were incubated with the mTOR inhibitor rapamycin for 4, 24, 48, and 72 hours and were assessed for mRNA expression of the thyroid-specific genes hNIS, TTF1, TTF2, PAX8, and TSH-R (rapamycin dose 50 μg/mL). Results are based on three separate experiments (n = 9). The data are given as means ± SD. *, P < .05 with negative control condition as the reference (Mann-Whitney U test). ND, not detectable.
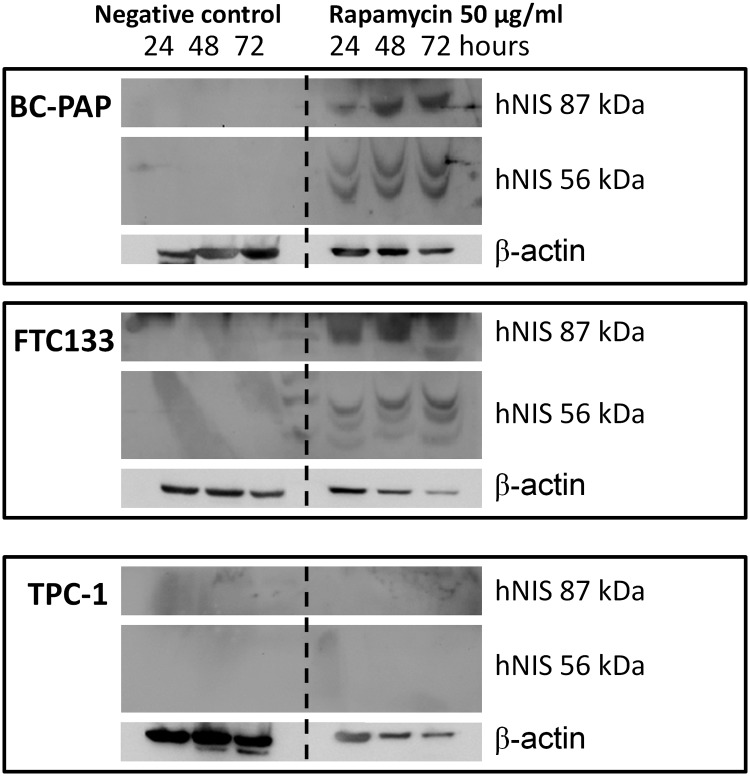
In parallel with the increased hNIS mRNA expression after rapamycin treatment, we also found increased hNIS protein expression in BC-PAP and FTC133 cell lines (Figure 2). Two different bands of hNIS were detected on the Western blots, being the inactive precursor protein (56 kDa) and the glycosylated active symporter (87 kDa), as reported previously (30, 31). No hNIS protein was found in the TPC1 cell line.
Figure 2.
Rapamycin-induced hNIS protein expression in TC cell lines. The TC cell lines BC-PAP, FTC133, and TPC1 were stimulated with 50 μg/mL rapamycin for 24, 48, and 72 hours. After the rapamycin treatment, cell lysates (5 μg loaded) were assessed for hNIS protein expression by Western blot. The picture is representative of three independent experiments.
mTOR inhibition activates iodine uptake in BC-PAP and FTC133 cell lines
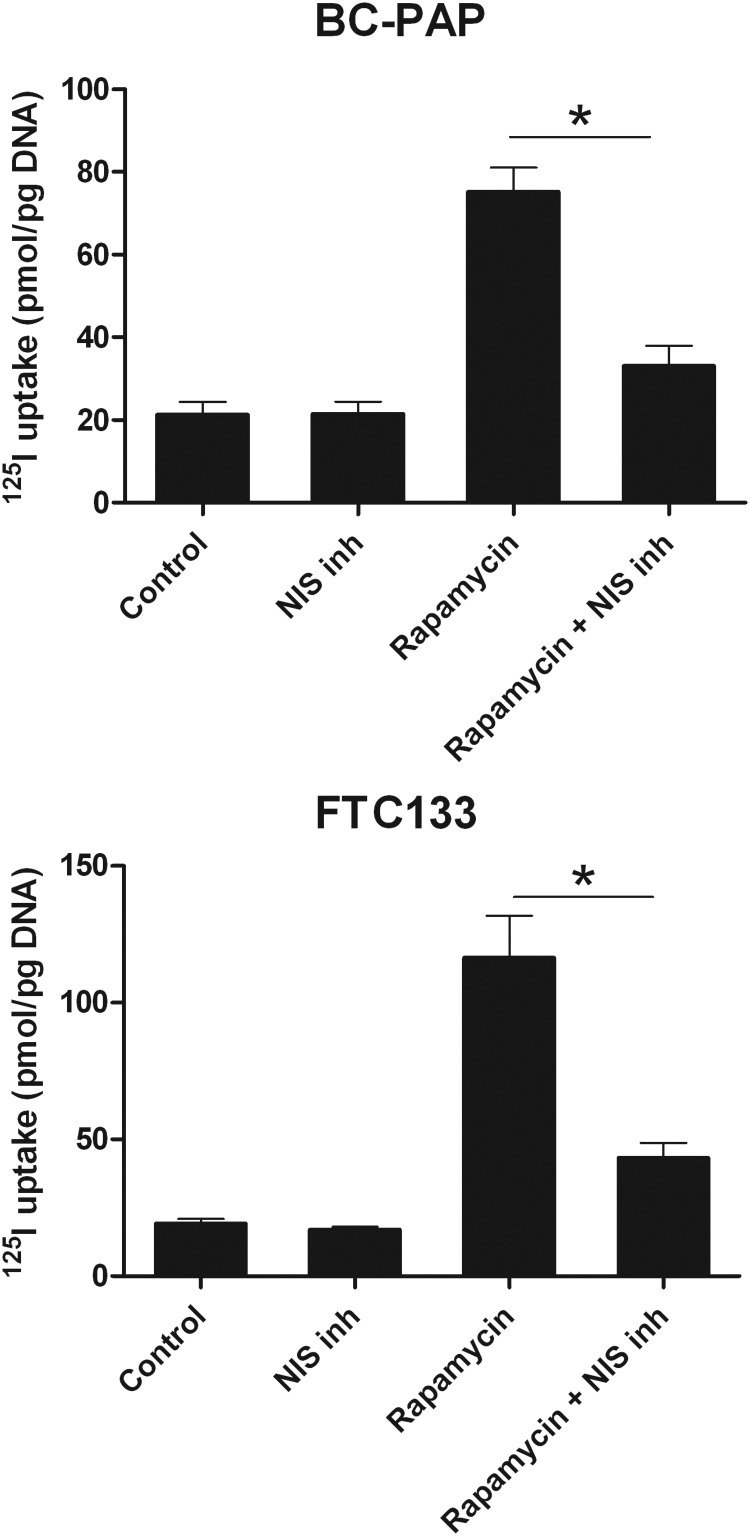
Because mTOR inhibition activates thyroid-specific gene expression in BC-PAP and FTC133 cell lines, we next studied whether this results in iodine accumulation. BC-PAP and FTC133 cells were treated with rapamycin for 48 hours after which the 125I uptake in these cells was measured. Both BC-PAP and FTC133 cells accumulated 3- to 5-fold higher amounts of 125I after pretreatment with rapamycin. This effect could be prevented by treatment with an hNIS specific inhibitor, sodium perchlorate (NaClO4), which demonstrates that the observed increase in iodine uptake was modulated through the effects of rapamycin on hNIS (Figure 3).
Figure 3.
Rapamycin-induced iodine uptake by TC cell lines. The TC cell lines BC-PAP and FTC133 were stimulated with 50 μg/mL rapamycin for 48 hours. After the rapamycin treatment, the cells were incubated for 30 minutes with 125I, with or without the addition of the hNIS blocker NaClO4, and the amount of intracellularly accumulated 125I was measured with a γ-counter. Results are based on four independent experiments (n = 12). The data are given as means ± SD, *, P < .05 (Mann-Whitney U test).
Induction of redifferentiation of TC cell lines by mTOR inhibition is autophagy independent
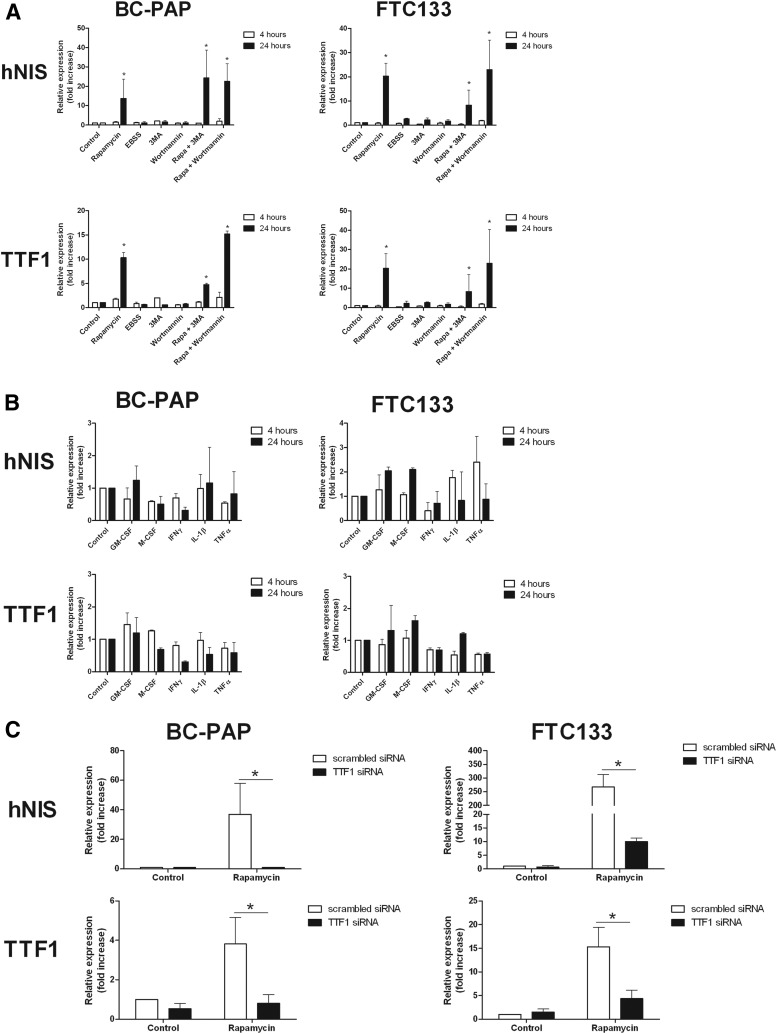
To study whether the effects of the rapamycin-induced redifferentiation are related to autophagy, BC-PAP and FTC133 cell lines were incubated with rapamycin for 4 and 24 hours in the presence or absence of the autophagy inhibitors 3-methyl adenine and wortmannin. Despite the presence of autophagy inhibitors, both cell lines were still capable of inducing TTF1 and hNIS mRNA expression after treatment with rapamycin. In addition, the autophagy-activating starvation medium EBSS was unable to up-regulate hNIS and TTF1 expression (Figure 4A), although autophagy was efficiently induced as reflected by increased WIPI-1 mRNA expression (Supplemental Figure 2), a validated marker of autophagy activity (32).
Figure 4.
Mechanism of rapamycin-induced redifferentiation. A, mRNA expression of hNIS and TTF1 in BC-PAP and FTC133 stimulated with 50 μg/mL rapamycin or cultured in starvation medium (EBSS) for 4 and 24 hours in the presence or absence of autophagy inhibitors. Results are derived from three independent experiments (n = 9). Data are given as means ± SD. *, P < .05 with negative control condition as the reference (Mann-Whitney U test). B, mRNA expression of hNIS and TTF1 in BC-PAP and FTC133 stimulated with inflammatory agents for 4 and 24 hours. Results are derived from three independent experiments (n = 9). Data are given as means ± SD. C, Gene expression knockdown of TTF1 by specific siRNA for 24 hours in BC-PAP and FTC133 and measurement of TTF1 and hNIS mRNA expression after another 48 hours of rapamycin treatment (50 μg/mL). Results are based on three independent experiments (n = 9). Data are given as means ± SD. *, P < .05 (Mann-Whitney U test).
Induction of redifferentiation of TC cell lines by mTOR inhibition is inflammation independent
Because inflammatory pathways are strongly influenced by mTOR signaling, several compounds involved in inflammatory pathways were assessed for their capacity to induce redifferentiation of the cell lines BC-PAP and FTC133 after stimulation for 4 and 24 hours. Although some effects could be observed, none of the inflammatory stimuli were capable of inducing hNIS expression to the same extent as mTOR inhibition (Figure 4B). As a positive control, the inflammatory stimuli were able to increase IL-6 production in at least one of the cell lines (Supplemental Figure 3).
Rapamycin-induced redifferentiation of TC cell lines is TTF1 dependent
The observation that rapamycin induced redifferentiation in the BC-PAP and FTC133 cell lines, but not the TTF1-negative TPC1 cell line, suggested that this process might be TTF1 mediated. To address this hypothesis, siRNA experiments were performed to down-regulate TTF1 expression. Subsequently, cells were stimulated with rapamycin for 48 hours and mRNA expression of TTF1 and hNIS was measured. The down-regulation of TTF1 entirely suppressed the rapamycin-induced hNIS expression in both the BC-PAP and FTC133 cell lines (Figure 4C).
Discussion
A poor prognosis in TC is associated with the lost capacity of TC to accumulate radioactive iodine, resulting in subtherapeutic radiation doses to TC cells. Despite the promising results of novel kinase inhibitors in TC, most responses are temporary and additional therapies are still needed. Given the close association between genetic alterations in TC and dedifferentiation, targeted therapy in TC has the potential not only to halt tumor growth but also to restore the expression of thyroid specific proteins. Recently selectively targeting the MAPK using selumetinib has been shown to induce clinically relevant increases in iodine uptake in a subgroup of patients with TC refractory to radioactive iodine (14). Because most genetic alterations in TC either directly or indirectly activate the mTOR pathway, we performed the present study to investigate the effect of inhibition of mTOR-activated pathways on redifferentiation of thyroid carcinoma cell lines. We found that mTOR inhibition restores functional hNIS expression in selected TC cell lines and demonstrated that this effect is independent of autophagy or inflammation but rather is related to TTF1 expression.
Previous studies have shown that treatment with rapamycin strongly inhibits the mTOR complex 1 in TC cell lines bearing mutations in MAPK (33). In addition, rapamycin can induce growth inhibition and cell cycle arrest in TC cell lines and in xenograft animal models (33). Moreover, de Souza et al (19) have shown that mTOR down-regulates iodine uptake in thyrocytes. Therefore, we hypothesized that mTOR inhibition might restore the iodine uptake in TC cells. Indeed, treatment of the cell lines with rapamycin resulted in markedly increased expression of hNIS, TTF1, and TSH-R in both the BC-PAP and FTC133 cell lines. The up-regulation of the thyroid-specific expression profile in these cell lines also resulted in a restored capacity to accumulate iodine specifically through hNIS. Of note, because PTEN-deficient TC carcinoma is generally known for its reduced ability to accumulate iodine (34, 35) and because mTOR inhibition leads to a 5-fold increase in iodine uptake by the PTEN-deficient cell line FTC133, this further reinforces the clinical potential of mTOR inhibition in restoring 131I sensitivity, even in the most difficult cases of differentiated TC.
Follow-up experiments were conducted to identify the intracellular mechanisms driving the redifferentiation effect induced by inhibiting mTOR. Because mTOR inhibition by rapamycin is a potent activator of the autophagy machinery and mTOR also strongly modulates inflammatory pathways, the roles of autophagy and inflammation were addressed in mediating the redifferentiation process. However, the results indicate that autophagy is not involved in this process because autophagy inhibitors were not able to prevent the induction of the thyroid-specific expression profile by rapamycin. Similarly, redifferentiation of the TC cells was not found by culturing the cells in EBSS starvation medium, which activates autophagy. Furthermore, in addition, inflammation was not the driving force as reflected by the fact that the inflammatory agents we used did not induce redifferentiation in the TC cell lines.
An important issue that has risen from the present study is the fact that the concentration of the mTOR inhibitors required for inducing the redifferentiation of thyroid carcinoma cell lines is higher than currently obtained when administered to patients. However, this dose is within the range being used in experimental murine models (36). Furthermore, and most importantly, tissue concentrations of rapamycin are significantly higher than those found in the circulation, indicating that a lower rapamycin dose is sufficient to reach the desired tissue concentration in vivo (37). Future studies are needed to determine the dosing regimen and efficacy in vivo of mTOR inhibitors for inducing TC redifferentiation.
Another interesting observation is that at early time points (12–24 h) of rapamycin administration, the expression of TSH-R was elevated, whereas at later time points (48–72 h), TSH-R expression was strongly decreased. Because the proliferation signal of TSH is mediated by mTOR signaling (38), the down-regulation of TSH-R by mTOR inhibition might have additional beneficial effects, beyond redifferentiation, by dampening TSH-induced proliferation. On the other hand, the down-regulation of TSH-R could decrease the iodine uptake capacity by impairing TSH signaling.
All together, in the present study, it is demonstrated for the first time that mTOR inhibition results in the restoration of functional sodium-iodine symporter expression in the TTF1-expressing TC cell lines BC-PAP and FTC133, indicating that mTOR inhibition alone is sufficient to induce TTF1-dependent TC redifferentiation, including the restoration of iodine uptake. In an additional set of experiments, the underlying mechanisms are unraveled, indicating that autophagy and inflammation, both strongly modulated by the mTOR kinase, are not involved in the redifferentiation process. On the other hand, we demonstrate the role of transcriptional events at the level of TTF1 as the mechanism of action. These findings highlight an important novel concept of TC redifferentiation evoked by mTOR inhibition and provide in-depth mechanistic insights into the underlying molecular pathways including autophagy, inflammation, and TTF1 reactivation.
Our data show that the process of rapamycin-induced redifferentiation might be driven by the TTF1 transcription factor, which is known to potently induce the expression of hNIS (39, 40). The observation that in the TPC1 cell line, which lacks TTF1 expression (29), treatment with rapamycin had no influence on the expression of the thyroid-specific genes, including hNIS, is in line with this hypothesis. Several mechanisms might explain the link between mTOR inhibition and the induction of TTF1. Previous studies into the architecture of the TTF1 promoter have revealed hepatocyte nuclear factor-3, specificity protein-1, specificity protein-3, GATA-6, and homeobox protein B3 as transcription factors activating TTF1 expression in lung tissue, which could all be potential targets of mTOR signaling (41). Other than transcription factors, epigenetic modifications also could underlie the effect of mTOR on TTF1 expression because it has been reported that histone acetylation is positively correlated with TTF1 expression and that DNA demethylating agents can restore the expression of TTF1 in TC cell lines (42). Future studies are warranted to investigate the exact pathways through which mTOR affects TTF1 expression and whether other TTF1-positive TC cell lines respond to rapamycin in a similar fashion.
In conclusion, treatment of TTF1-positive TC cell lines with rapamycin leads to the activation of a thyroid follicular cell differentiation program, which results in increased intracellular accumulation of iodine. Therefore, inhibition of mTOR is emerging as a promising target for adjunctive therapy for improving the efficacy of 131I treatments and thereby the clinical outcome of TC patients. These novel findings set the stage for clinical application of mTOR inhibitors in 131I-refractory TC patients.
Acknowledgments
Author contributions included the following: T.S.P., B.H., and D.G. performed the experiments and the data analysis; R.E.S. and B.R.H. provided the cell lines, protocols, and experimental guidance; T.S.P., M.G.N., L.A.B.J., O.C.B., J.W.A.S., and R.T.N.-M. designed the study and wrote the manuscript. All authors read and approved the final manuscript, and all authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
T.S.P. was supported by a Veni grant of The Netherlands Organization for Scientific Research. M.G.N. was also supported by a Vici grant of The Netherlands Organization for Scientific Research.
Disclosure Summary: The authors have nothing to declare.
Funding Statement
T.S.P. was supported by a Veni grant of The Netherlands Organization for Scientific Research. M.G.N. was also supported by a Vici grant of The Netherlands Organization for Scientific Research.
Footnotes
- EBSS
- Earle's balanced salt solution
- hNIS
- human sodium-iodine symporter
- mTOR
- mammalian target of rapamycin
- PAX8
- paired box 8
- PTEN
- phosphatase and tensin homolog deleted from chromosome 10
- siRNA
- small interfering RNA
- TC
- thyroid carcinoma
- TSH-R
- thyroid-stimulating hormone receptor
- TTF1
- thyroid transcription factor 1
- TTF2
- thyroid transcription factor 2.
References
- 1. Schlumberger M. Target therapies for radioiodine refractory advanced thyroid tumors. J Endocrinol Invest. 2012;35:40–44. [PubMed] [Google Scholar]
- 2. Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Durante C, Puxeddu E, Ferretti E, et al. BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J Clin Endocrinol Metab. 2007;92:2840–2843. [DOI] [PubMed] [Google Scholar]
- 4. Riesco-Eizaguirre G, Gutierrez-Martinez P, Garcia-Cabezas MA, Nistal M, Santisteban P. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I− targeting to the membrane. Endocr Relat Cancer. 2006;13:257–269. [DOI] [PubMed] [Google Scholar]
- 5. Henderson YC, Shellenberger TD, Williams MD, et al. High rate of BRAF and RET/PTC dual mutations associated with recurrent papillary thyroid carcinoma. Clin Cancer Res. 2009;15:485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kogai T, Sajid-Crockett S, Newmarch LS, Liu YY, Brent GA. Phosphoinositide-3-kinase inhibition induces sodium/iodide symporter expression in rat thyroid cells and human papillary thyroid cancer cells. J Endocrinol. 2008;199:243–252. [DOI] [PubMed] [Google Scholar]
- 7. Malehmir M, Haghpanah V, Larijani B, et al. Multifaceted suppression of aggressive behavior of thyroid carcinoma by all-trans retinoic acid induced re-differentiation. Mol Cell Endocrinol. 2012;348:260–269. [DOI] [PubMed] [Google Scholar]
- 8. Coelho SM, Vaisman F, Buescu A, Mello RC, Carvalho DP, Vaisman M. Follow-up of patients treated with retinoic acid for the control of radioiodine non-responsive advanced thyroid carcinoma. Braz J Med Biol Res. 2011;44:73–77. [DOI] [PubMed] [Google Scholar]
- 9. Fernandez CA, Puig-Domingo M, Lomena F, et al. Effectiveness of retinoic acid treatment for redifferentiation of thyroid cancer in relation to recovery of radioiodine uptake. J Endocrinol Invest. 2009;32:228–233. [DOI] [PubMed] [Google Scholar]
- 10. Zhang M, Guo R, Xu H, Zhang M, Li B. Retinoic acid and tributyrin induce in vitro radioiodine uptake and inhibition of cell proliferation in a poorly differentiated follicular thyroid carcinoma. Nucl Med Commun. 2011;32:605–610. [DOI] [PubMed] [Google Scholar]
- 11. Yuan GB, Kuang AR, Fan Q, Yu LB, Mi YX. Combined effects of all-trans-retinoic acid and trichostatin A on the induction of differentiation of thyroid carcinoma cells. Chin J Cancer. 2010;29:379–384. [DOI] [PubMed] [Google Scholar]
- 12. Vivaldi A, Miasaki FY, Ciampi R, et al. Re-differentiation of thyroid carcinoma cell lines treated with 5-aza-2′-deoxycytidine and retinoic acid. Mol Cell Endocrinol. 2009;307:142–148. [DOI] [PubMed] [Google Scholar]
- 13. Beyer S, Lakshmanan A, Liu YY, et al. KT5823 differentially modulates sodium iodide symporter expression, activity, and glycosylation between thyroid and breast cancer cells. Endocrinology. 2011;152:782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim K, Cabanillas M, Lazar AJ, et al. Clinical responses to vemurafenib in patients with metastatic papillary thyroid cancer harboring V600EBRAF mutation. Thyroid. 2013;23(10):1277–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu D, Hu S, Hou P, Jiang D, Condouris S, Xing M. Suppression of BRAF/MEK/MAP kinase pathway restores expression of iodide-metabolizing genes in thyroid cells expressing the V600E BRAF mutant. Clin Cancer Res. 2007;13:1341–1349. [DOI] [PubMed] [Google Scholar]
- 17. Hou P, Bojdani E, Xing M. Induction of thyroid gene expression and radioiodine uptake in thyroid cancer cells by targeting major signaling pathways. J Clin Endocrinol Metab. 2010;95:820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Souza EC, Padron AS, Braga WM, et al. mTOR downregulates iodide uptake in thyrocytes. J Endocrinol. 2010;206:113–120. [DOI] [PubMed] [Google Scholar]
- 20. Faustino A, Couto JP, Populo H, et al. mTOR pathway overactivation in BRAF mutated papillary thyroid carcinoma. J Clin Endocrinol Metab. 2012;97:E1139–E1149. [DOI] [PubMed] [Google Scholar]
- 21. Molina AM, Feldman DR, Voss MH, et al. Phase 1 trial of everolimus plus sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2012;118:1868–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ansell SM, Tang H, Kurtin PJ, et al. Temsirolimus and rituximab in patients with relapsed or refractory mantle cell lymphoma: a phase 2 study. Lancet Oncol. 2011;12:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dazert E, Hall MN. mTOR signaling in disease. Curr Opin Cell Biol. 2011;23:744–755. [DOI] [PubMed] [Google Scholar]
- 25. Huijbers A, Plantinga TS, Joosten LA, et al. The effect of the ATG16L1 Thr300Ala polymorphism on susceptibility and outcome of patients with epithelial cell-derived thyroid carcinoma. Endocr Relat Cancer. 2012;19:L15–L18. [DOI] [PubMed] [Google Scholar]
- 26. Plantinga TS, Crisan TO, Oosting M, et al. Crohn's disease-associated ATG16L1 polymorphism modulates pro-inflammatory cytokine responses selectively upon activation of NOD2. Gut. 2011;60:1229–1235. [DOI] [PubMed] [Google Scholar]
- 27. Knauf JA, Kuroda H, Basu S, Fagin JA. RET/PTC-induced dedifferentiation of thyroid cells is mediated through Y1062 signaling through SHC-RAS-MAP kinase. Oncogene. 2003;22:4406–4412. [DOI] [PubMed] [Google Scholar]
- 28. Maximo V, Lima J, Prazeres H, Soares P, Sobrinho-Simoes M. The biology and the genetics of Hurthle cell tumors of the thyroid. Endocr Relat Cancer. 2012;19:R131–R147. [DOI] [PubMed] [Google Scholar]
- 29. Schweppe RE, Klopper JP, Pugazhenthi U, et al. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab. 2008;93:4331–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Levy O, Dai G, Riedel C, et al. Characterization of the thyroid Na+/I− symporter with an anti-COOH terminus antibody. Proc Natl Acad Sci USA. 1997;94:5568–5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dohan O, Paroder V, Riedel C, et al. The sodium/iodide Symporter (NIS): characterization, regulation, and medical significance. Endocr Rev. 2003;24:48–77. [DOI] [PubMed] [Google Scholar]
- 32. Tsuyuki S, Takabayashi M, Kawazu M, et al. Detection of mRNA as an indicator of autophagosome formation. Autophagy. 2014;10(3):497–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jin N, Jiang T, Rosen DM, Nelkin BD, Ball DW. Dual inhibition of mitogen-activated protein kinase kinase and mammalian target of rapamycin in differentiated and anaplastic thyroid cancer. J Clin Endocrinol Metab. 2009;94:4107–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sugino K, Ito K, Mimura T, Kameyama K, Iwasaki H, Ito K. Hurthle cell tumor of the thyroid: analysis of 188 cases. World J Surg. 2001;25:1160–1163. [DOI] [PubMed] [Google Scholar]
- 35. McDonald MP, Sanders LE, Silverman ML, Chan HS, Buyske J. Hurthle cell carcinoma of the thyroid gland: prognostic factors and results of surgical treatment. Surgery. 1996;120:1000–1004. [DOI] [PubMed] [Google Scholar]
- 36. O'Reilly T, McSheehy PM, Kawai R, et al. Comparative pharmacokinetics of RAD001 (everolimus) in normal and tumor-bearing rodents. Cancer Chemother Pharmacol. 2010;65:625–639. [DOI] [PubMed] [Google Scholar]
- 37. Napoli KL, Wang ME, Stepkowski SM, Kahan BD. Distribution of sirolimus in rat tissue. Clin Biochem. 1997;30:135–142. [DOI] [PubMed] [Google Scholar]
- 38. Brewer C, Yeager N, Di Cristifano A. Thyroid-stimulating hormone initiated proliferative signals converge in vivo on the mTOR kinase without activating AKT. Cancer Res. 2007;67:8002–8006. [DOI] [PubMed] [Google Scholar]
- 39. Ohmori M, Endo T, Harii N, Onaya T. A novel thyroid transcription factor is essential for thyrotropin-induced up-regulation of Na+/I− symporter gene expression. Mol Endocrinol. 1998;12:727–736. [DOI] [PubMed] [Google Scholar]
- 40. Damante G, Di Lauro R. Thyroid-specific gene expression. Biochim Biophys Acta. 1994;1218:255–266. [DOI] [PubMed] [Google Scholar]
- 41. Boggaram V. Thyroid transcription factor-1 (TTF-1/Nkx2.1/TITF1) gene regulation in the lung. Clin Sci (Lond). 2009;116:27–35. [DOI] [PubMed] [Google Scholar]
- 42. Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 2006;6:292–306. [DOI] [PubMed] [Google Scholar]