Abstract
Context:
Exogenous dehydroepiandrosterone (DHEA) therapy has been proposed to replenish the depletion of endogenous DHEA and its sulfate form, which occurs with advancing age and is thought to be associated with loss of libido and menopausal symptoms.
Objective:
We conducted a systematic review and meta-analysis to summarize the evidence supporting the use of systemic DHEA in postmenopausal women with normal adrenal function.
Methods:
We searched MEDLINE, EMBASE, PsycInfo, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Scopus through January 2014. Pairs of reviewers, working independently, selected studies and extracted data from eligible randomized controlled trials (RCTs). We used the random-effects model to pool across studies and evaluated heterogeneity using the I2 statistic.
Results:
We included 23 RCTs with moderate to high risk of bias enrolling 1188 women. DHEA use was not associated with significant improvement in libido or sexual function (standardized mean difference, 0.35; 95% confidence interval, −0.02 to 0.73; P value = .06; I2 = 62%). There was also no significant effect of DHEA on serious adverse effects, serum lipids, serum glucose, weight, body mass index, or bone mineral density. This evidence warranted low confidence in the results, mostly due to imprecision, risk of bias, and inconsistency across RCTs.
Conclusions:
Evidence warranting low confidence suggests that DHEA administration does not significantly impact sexual symptoms or selected metabolic markers in postmenopausal women with normal adrenal function.
As women advance in age, they experience a decline in T and dehydroepiandrosterone sulfate (DHEAS) (1). The reduction in DHEAS is more prominent than that of T (2). The drop in the levels of these hormones has been linked to postmenopausal symptoms, decline in the sexual function, and reduction in bone mineral density (BMD). A cross-sectional study conducted in 2005 reported decreased sexual responsiveness in women 45 years old or more who were having DHEAS levels below the 10th centile for their age (odds ratio, 3.9; 95% confidence interval [CI], 1.54–9.81; P = .004) (3). A case-control study conducted in 2010 further supported these findings and highlighted this association (4).
Dehydroepiandrosterone (DHEA) is one of the precursors in the biosynthesis process of steroid hormones. Because DHEA exerts its effect through conversion to androgen and/or estrogen, it has been proposed as a replacement therapy that yields clinically beneficial effects mediated by both hormones (5). The proposed androgenic effects are increased libido and improved well-being via conversion to T, whereas its transformation to estrogen is thought to result in improvements in menopausal vasomotor symptoms.
The safety profile of DHEA and its impact on menopausal symptoms, sexual performance, and bone health have been studied in several randomized controlled trials (RCTs). Some of these trials showed a beneficial effect of DHEA on sexual function (6–11), whereas others found no positive effect (12, 13). The same conflicting results have been reported for the effect of DHEA on bone health as assessed by BMD because some studies revealed beneficial effects (6, 12, 14) whereas others did not (15, 16). Menopausal symptoms assessed by the Menopause-Specific Quality of Life Questionnaire (MENQOL) showed no improvement when oral DHEA was compared to placebo (10), whereas the sexual domain score of the same questionnaire showed improvement when vaginal DHEA was studied in women with vaginal atrophy in a different study (17).
In light of these conflicting results, we aimed to conduct this systematic review and meta-analysis to evaluate the effect of DHEA on postmenopausal women. This review was conducted to support The Endocrine Society guideline on androgen use in postmenopausal women.
Materials and Methods
This review was based on a predefined protocol and conformed to the standards set in the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement (18).
Eligibility criteria
Eligible studies were RCTs that enrolled women with surgical or natural menopause and normal adrenal function who were assigned to receive systemic DHEA or placebo and evaluated the outcomes of interest. Trials were included regardless of their size or duration of patient follow-up.
Outcomes of interest were: quality of life and general well-being, sexual function, psychological symptoms related to menopause, lipid profile, glucose tolerance, body measurements, and bone health.
Literature search
An expert reference librarian (L.P.) designed and conducted the electronic search strategy with input from study investigators with expertise in conducting systematic reviews (M.H.M. and T.E.). The search involved multiple databases including Medline In-Process & Other Non-Indexed Citations, MEDLINE, EMBASE, PsycInfo, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Scopus, from their inception through January 2014. Controlled vocabulary supplemented with keywords was used to search for DHEA therapy for postmenopausal women, limited to RCTs. We also searched the bibliographies of the included studies to identify any candidate studies that might be missed by the electronic search. Content experts from The Endocrine Society were also queried for potential references. The full search strategy is provided in the Supplemental Data.
Study selection
All relevant abstracts were downloaded into an Endnote library and uploaded to an online reference management system (DistillerSR; Evidence Partners Inc). Reviewers working independently and in duplicate screened the abstracts for eligibility. Disagreements from this level were automatically upgraded to the next level of screening. Full text of eligible abstracts were retrieved and screened in duplicate. Disagreements at this level were resolved by consensus. We calculated the inter-reviewer agreement beyond chance (kappa) during the full-text screening level (19).
Data extraction
Data were extracted in duplicates using a standardized, piloted, web-based form. For each study, we abstracted the following descriptive data: detailed description of baseline characteristics of the participants (ie, age, ethnicity, and patient's description at baseline) and study characteristics (ie, location and setting, follow-up duration, and interventions). A third reviewer compared the reviewers' entered data and resolved inconsistencies by referring to the full text of the article.
Author contact
We contacted the authors of the original studies when data required for analysis were missing or when more clarification was needed. Author contact was done by e-mail. If we did not receive any response, we sent another e-mail 2 weeks later.
Methodological quality and risk of bias assessment
Two reviewers independently assessed the quality of each RCT using the Cochrane Risk of Bias assessment tool (20). We determined the following: how the randomization sequence was generated; how allocation was concealed; whether there were important imbalances at baseline; which groups were blinded (patients, caregivers, data collectors, outcome assessors, data analysts); whether there were any baseline imbalances; whether the analysis was by intention to treat; and how the patients adhered to the assigned medication. The quality of evidence was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methods (21).
Statistical analysis
Meta-analysis was done using random effect meta-analysis described by DerSimonian and Laird (22) to account for the heterogeneity between studies as well as within-study variability. Effect sizes were pooled using weighted difference in means (WMD) when outcomes were measured using the same scale and using the standardized mean difference (SMD) when outcomes were measured using multiple scales. Between-study inconsistency was measured by I2 statistics, which estimate the proportion of variation in results across studies that is not due to chance (19). We planned to assess publication bias using the Egger regression asymmetry test and visual inspection using funnel plots whenever we had an adequate number of studies and low heterogeneity (23). Due to multiple testing, we implemented the false discovery rate-controlled procedures proposed by Benjamini and Hochberg (24) and set the P value for significance at .015 instead of the usual .05. All analyses were conducted using STATA, version 12.1 (StataCorp LP).
Subgroup analysis
We conducted subgroup analyses, which were determined a priori, to explain between-study heterogeneity. These subgroups were based on the length of follow-up (<12 mo vs ≥12 mo) and study design (parallel vs crossover). For each subgroup analysis, we conducted a test for interaction (P < .015 was considered to be statistically significant).
Results
Search results and study description
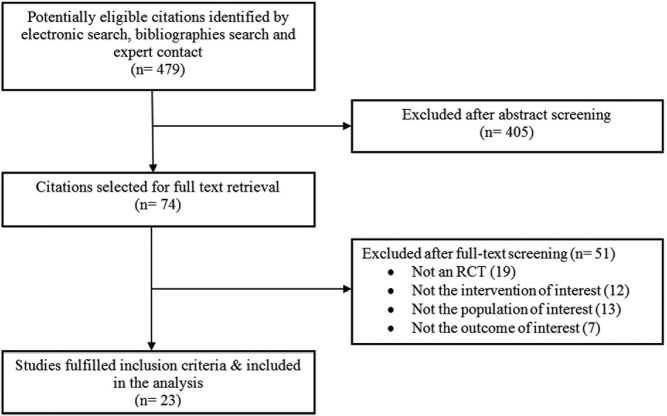
The search identified 479 potentially relevant citations, from which 23 trials were found relevant and were eventually included in our analysis. Figure 1 describes the selection process in more detail. The trials enrolled a total of 1188 postmenopausal women who were assigned to either DHEA or placebo. The average age of participants was 53 years, and they were followed up for various periods of time, ranging from 1.5 to 24 months. Table 1 describes the characteristic of the trials and the interventions.
Figure 1. Selection process.
Table 1.
Description of Included Trials
| First Author, Year (Ref.) | Location, Setting | Sample, n | Population | Age, y | Ethnicity | Duration, mo | Interventions |
|---|---|---|---|---|---|---|---|
| Barnhart, 1999 (27) | United States, academic hospital | 66 | Perimenopausal women with altered mood and well-being | 45–55 | 82% CC, 15% AA, 2% AS | 6 | DHEA (oral, 50 mg) vs placebo |
| Baulieua, 2000 (6) | France, NR | 140 | Healthy postmenopausal women | 60–79 | NR | 12 | DHEA (oral, 50 mg) vs placebo |
| Bloch, 2013 (28) | Israel, academic hospital | 25 | Health postmenopausal women with HSDD | 54 | NR | 1.5 | DHEA (oral, 100 mg) vs placebo |
| Carranza-Lira, 2002 (29) | Mexico, academic hospital | 20 | Healthy postmenopausal women | 54 | NR | 1 | DHEA (oral, 35 mg) vs placebo |
| Casson, 1995 (30) | United States, academic hospital | 11 | Healthy postmenopausal women | 56 | NR | 1 | DHEA (oral, 50 mg) vs placebo |
| Dayal, 2005 (31) | United States, academic hospital | 50 | Healthy postmenopausal women | 57 | NR | 3 | DHEA (oral, 50 mg) vs placebo |
| Finckh, 2005 (32) | Switzerland, academic hospital | 52 | Postmenopausal women with fibromyalgia | 59 | NR | 3 | DHEA (oral, 50 mg) vs placebo |
| Gómez-Santos, 2012 (33) | Spain, academic hospital | 61 | Healthy postmenopausal women | 51.5 | NR | 3 | DHEA (oral, 100 mg) vs placebo |
| Igwebuike, 2008 (34) | United States, academic hospital | 31 | Sedentary, postmenopausal women | 64 | 100% CC | 3 | DHEA (oral, 50 mg) vs placebo |
| Jankowski, 2006 (35) | United States, academic hospital | 70 | Healthy postmenopausal women | 68 | NR | 12 | DHEA (oral, 50 mg) vs placebo |
| Kenny, 2010 (36) | United States, major medical institution | 99 | Postmenopausal women with low DHEAS levels, low bone mass, and frailty | 76.6 | 91% CC, 6% AA, 1% HI, 2% other | 6 | DHEA (oral, 50 mg) vs placebo |
| Kritz-Silverstein, 2008 (7) | United States, clinical research facility | 115 | Healthy postmenopausal women | 68 | NR | 12 | DHEA (oral, 50 mg) vs placebo |
| Lasco, 2001 (37) | Italy, academic hospital | 20 | Healthy adrenal-androgen-deficient postmenopausal women | 57 | NR | 12 | DHEA (oral, 25 mg) vs placebo |
| Morales, 1994 (8) | United States, academic hospital | 17 | Healthy perimenopausal women | 54.5 | NR | 6 | DHEA (oral, 50 mg) vs placebo |
| Morales, 1998 (16) | United States, academic hospital | 10 | Healthy nonobese, advanced age, postmenopausal women | 54.5 | NR | 6 | DHEA (oral, 100 mg) vs placebo |
| Mortola, 1990 (9) | United States, academic hospital | 6 | Healthy postmenopausal women | 46–61 | NR | 1 | DHEA (oral, 1600 mg) vs placebo |
| Nair, 2006 (14) | United States, academic hospital | 57 | Elderly postmenopausal women with low DHEA levels | 69 | NR | 24 | DHEA (oral, 50 mg) vs placebo |
| Panjari, 2009 (10, 38) | Australia, academic hospital | 93 | Healthy postmenopausal women with low libido | 54 | NR | 12 | DHEA (oral, 50 mg) vs placebo |
| Srinivasan, 2010 (39) | United States, academic hospital | 57 | Healthy postmenopausal women with low levels of DHEAS | 69 | NR | 24 | DHEA (oral, 50 mg) vs placebo |
| von Mühlen, 2008 (40) | United States, academic hospital | 115 | Healthy postmenopausal women | 68.5 | NR | 12 | DHEA (oral, 50 mg) vs placebo |
| Weiss, 2009 (41) | United States, academic hospital | 58 | Healthy postmenopausal women | 70 | 96% CC, 4% AA | 12 | DHEA (oral, 50 mg) vs placebo |
| Wolf, 1997 (11) | Germany, academic hospital | 15 | Healthy postmenopausal women | 69.1 | NR | 1.5 | DHEA (oral, 50 mg) vs placebo |
Abbreviations: NR, not reported; HSDD, hypoactive sexual desire disorder; CC, Caucasian; AA, African American; AS, Asian; HI, Hispanic. Age is expressed as mean or range.
Methodological quality
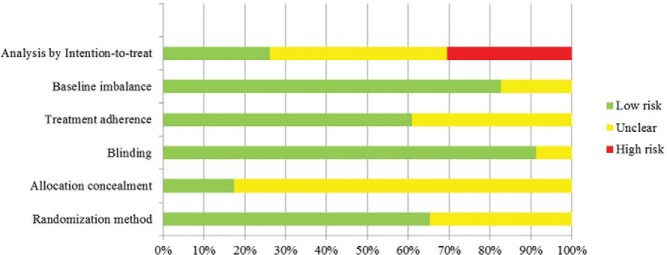
The overall risk of bias of the included trials was moderate to high (Figure 2). Although 65% of the trials implemented adequate randomization methods, 90% blinded study investigators and participants, and 60% measured the patients' adherence to treatment, less than 20% clearly described an allocation concealment technique. Analysis followed the intention-to-treat principle in less than one-third of the trials. More details about the methodological quality are described in Supplemental Table 1.
Figure 2. Risk of bias.
Meta-analysis
Table 2 provides the pooled estimates and 95% CI values for the relevant outcomes, as well as heterogeneity and statistical significance.
Table 2.
Meta-analysis Results
| Outcome | No. of Studies | Difference in Meansb | Lower Limit of 95% CI | Upper Limit of 95% CI | P Valuec | I2, % |
|---|---|---|---|---|---|---|
| Sexual and psychological outcomes | ||||||
| QoL/general well-beinga | 7 | −0.19 | −0.38 | 0.00 | .054 | 0 |
| Depressed mooda | 3 | −0.30 | −0.67 | 0.06 | .107 | 55 |
| Anxiety/distressa | 3 | −0.11 | −0.40 | 0.18 | .470 | 0 |
| Libido/sexual functiona | 5 | 0.35 | −0.02 | 0.73 | .064 | 62 |
| Lipid profile and blood glucose | ||||||
| Total cholesterol, mg/dL | 8 | −6.76 | −15.27 | 1.76 | .120 | 26 |
| HDL, mg/dL | 11 | −2.36 | −4.81 | 0.08 | .058 | 11 |
| LDL, mg/dL | 11 | −2.11 | −7.35 | 3.13 | .430 | 0 |
| Triglyceride, mg/dL | 10 | −7.96 | −18.35 | 2.42 | .133 | 29 |
| Blood glucose, mg/dL | 5 | 0.01 | −0.93 | 0.95 | .978 | 0 |
| Bone health | ||||||
| Total body BMD, g/cm2 | 1 | 0.00 | −0.11 | 0.12 | .973 | NA |
| Total spine BMD, g/cm2 | 4 | 0.01 | 0.002 | 0.027 | .020 | 0 |
| Total hip BMD, g/cm2 | 7 | 0.00 | −0.00 | 0.01 | .159 | 13 |
| Body composition | ||||||
| Weight, kg | 6 | 0.41 | −0.41 | 1.23 | .328 | 0 |
| BMI, kg/m2 | 5 | −0.07 | −0.71 | 0.57 | .826 | 0 |
Abbreviations: QoL, quality of life; LDL, low-density lipoprotein; HDL, high-density lipoprotein; NA, Not applicable (I2 is not meaningful if number of studies is less than three).
Results are presented as a SMD.
Difference in means is calculated as DHEA group minus placebo group.
Significance level is set at P = .015 due to multiple testing.
Sexual and psychological outcomes
DHEA was associated with no statistically significant improvement in libido and sexual function (SMD, 0.35; 95% CI, −0.02 to 0.73; I2 = 62%; n = 5 RCTs). No significant improvement was found in any of the other sexual-related outcomes that were tested, including quality of life and general well-being (SMD, −0.19; 95% CI, −0.38 to 0.00; I2 = 0%; n = 7); depressed mood (SMD, −0.30; 95% CI, −0.67 to 0.06; I2 = 55%; n = 3); and anxiety and distress (SMD, −0.11; 95% CI, −0.40 to 0.18; I2 = 0%; n = 3). Quality of evidence overall (confidence in estimates) was low, rated down due to the risk of bias, imprecision of the estimates, and significant inconsistency for the outcomes of depressed mood and libido.
Lipid profile and blood glucose
There was no significant difference between DHEA and placebo in any of the lipid profile and glucose outcomes. The quality of evidence for all these outcomes is low due to the risk of bias and imprecision.
Bone health
We could not find RCTs assessing the effect of DHEA on fracture risk. Four studies evaluated its effect on spine BMD, seven on hip, and only one on total body BMD. The pooled estimates from these studies did not show any beneficial effect of DHEA on BMD. No significant differences between the intervention and the placebo group were detected for spine BMD (WMD, 0.01 g/cm2; 95% CI, 0.00 to 0.03; I2 = 0%; n = 4), total hip BMD (WMD, 0.00 g/cm2; 95% CI, −0.00 to 0.01; I2 = 13%; n = 7), or whole-body BMD (WMD, 0.00 g/cm2; 95% CI, −0.11 to 0.12; n = 1). The quality of evidence for all these outcomes is low due to the risk of bias and imprecision.
Body composition
The two parameters we tested to assess the effect of DHEA on body composition were body weight and body mass index (BMI). The administration of DHEA was not associated with any significant change in either parameter compared to placebo. The WMD for body weight was 0.41 kg (95% CI, −0.41 to 1.23; I2 = 0%;, n = 6), whereas the WMD for BMI was −0.07 kg/m2 (95% CI, −0.71 to 0.57; I2 = 0%; n = 5). The quality of evidence for all these outcomes is low due to the risk of bias and imprecision.
Other adverse events
Most of the trials included in our review concluded that DHEA use was not associated with any significant adverse effects. The most frequently reported adverse effects were dermatological or androgenic symptoms. One study reported chest pain and palpitations in five women (four receiving DHEA and one receiving placebo). Data on adverse effects were in general sparse and heterogeneous; thus, inappropriate for meta-analysis. Data on adverse effects are summarized in Supplemental Table 3. In brief, it didn't seem that the use of DHEA was associated with any significant adverse effects.
Subgroup analysis
We tested the effect of DHEA based on the duration of follow-up and study design; no significant subgroup interactions were found for any of the subgroups. Subgroup analyses are presented in Supplemental Table 2.
Publication bias
Due to the small number of studies per each outcome, methods to detect publication bias could not be applied because these methods usually require at least 10 to 20 studies to reliably test for bias (23, 25).
Discussion
Main findings
We conducted this systematic review to assess the benefits and harm of DHEA use in postmenopausal women. We found that DHEA was associated with no significant improvement in libido and sexual function. There was no significant effect on serum lipids, glucose, or BMD (except for a small change in lumber spine BMD). Also, no difference was detected when different subgroups were evaluated.
Limitations and strengths
The strength of this systematic review relates to its systematic nature with predefined protocol-driven methods and outcomes. We carried out multiple techniques to reduce the chance of error and bias, including duplicate independent study selection, data extraction, and assessment of the risk of bias. A comprehensive search of multiple databases and collaboration with content experts from The Endocrine Society further strengthened this evidence synthesis.
The overall confidence in the results warranted by this body of evidence is low due to increased risk of bias, imprecision, and inconsistency for some key outcomes (depressed mood and libido).
Comparison with previous reviews
Our conclusions are similar to those of previous reviews. In 2010, Panjari and Davis (5) systematically reviewed the literature and provided a qualitative summary stating that the recent trials do not support a benefit of oral DHEA therapy for postmenopausal women. A more recent literature review conducted by Davis et al (26) in 2011 concluded that oral DHEA did not show any benefit with regard to impaired sexual function, well-being, and cognitive performance in postmenopausal women. They also reported that it has no favorable effects on lipids and carbohydrate metabolism. Our review updates the evidence to the present time and offers meta-analytic estimates.
Implications
Because the evidence is too imprecise, inconsistent, and open to bias, larger and longer randomized trials able to precisely measure not only intermediate outcomes but also patient-important outcomes that directly inform postmenopausal women's decisions to use or not to use DHEA will be necessary. The associated clinical practice guidelines from The Endocrine Society will provide practical recommendations regarding androgen use in postmenopausal women.
Conclusion
Evidence warranting low confidence in estimates suggests that DHEA cannot significantly improve sexual symptoms or other metabolic parameters in postmenopausal women with normal adrenal function.
Acknowledgments
This review was partially funded by a contract from The Endocrine Society.
Disclosure Summary: T.E., M.B.S., Z.W., T.K., N.A., C.U., M.N., O.A., L.P., V.M.M., and M.H.M. have nothing to declare.
Funding Statement
This review was partially funded by a contract from The Endocrine Society.
Footnotes
- BMD
- bone mineral density
- BMI
- body mass index
- CI
- confidence interval
- DHEA
- dehydroepiandrosterone
- DHEAS
- DHEA sulfate
- RCT
- randomized controlled trial
- SMD
- standardized mean difference
- WMD
- weighted difference in means.
References
- 1. Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847–3853. [DOI] [PubMed] [Google Scholar]
- 2. Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59:551–555. [DOI] [PubMed] [Google Scholar]
- 3. Davis SR, Davison SL, Donath S, Bell RJ. Circulating androgen levels and self-reported sexual function in women. JAMA. 2005;294:91–96. [DOI] [PubMed] [Google Scholar]
- 4. Basson R, Brotto LA, Petkau AJ, Labrie F. Role of androgens in women's sexual dysfunction. Menopause. 2010;17:962–971. [DOI] [PubMed] [Google Scholar]
- 5. Panjari M, Davis SR. DHEA for postmenopausal women: a review of the evidence. Maturitas. 2010;66:172–179. [DOI] [PubMed] [Google Scholar]
- 6. Baulieu EE, Thomas G, Legrain S, et al. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: contribution of the DHEAge Study to a sociobiomedical issue. Proc Natl Acad Sci USA. 2000;97:4279–4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kritz-Silverstein D, von Mühlen D, Laughlin GA, Bettencourt R. Effects of dehydroepiandrosterone supplementation on cognitive function and quality of life: the DHEA and Well-Ness (DAWN) Trial. J Am Geriatr Soc. 2008;56:1292–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morales AJ, Nolan JJ, Nelson JC, Yen SS. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab. 1994;78:1360–1367. [DOI] [PubMed] [Google Scholar]
- 9. Mortola JF, Yen SS. The effects of oral dehydroepiandrosterone on endocrine-metabolic parameters in postmenopausal women. J Clin Endocrinol Metab. 1990;71:696–704. [DOI] [PubMed] [Google Scholar]
- 10. Panjari M, Bell RJ, Jane F, et al. A randomized trial of oral DHEA treatment for sexual function, well-being, and menopausal symptoms in postmenopausal women with low libido. J Sex Med. 2009;6:2579–2590. [DOI] [PubMed] [Google Scholar]
- 11. Wolf OT, Neumann O, Hellhammer DH, et al. Effects of a two-week physiological dehydroepiandrosterone substitution on cognitive performance and well-being in healthy elderly women and men. J Clin Endocrinol Metab. 1997;82:2363–2367. [DOI] [PubMed] [Google Scholar]
- 12. Hackbert L, Heiman JR. Acute dehydroepiandrosterone (DHEA) effects on sexual arousal in postmenopausal women. J Womens Health Gend Based Med. 2002;11:155–162. [DOI] [PubMed] [Google Scholar]
- 13. Schmidt PJ, Daly RC, Bloch M, et al. Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Arch Gen Psychiatry. 2005;62:154–162. [DOI] [PubMed] [Google Scholar]
- 14. Nair KS, Rizza RA, O'Brien P, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355:1647–1659. [DOI] [PubMed] [Google Scholar]
- 15. Casson PR, Santoro N, Elkind-Hirsch K, et al. Postmenopausal dehydroepiandrosterone administration increases free insulin-like growth factor-I and decreases high-density lipoprotein: a six-month trial. Fertil Steril. 1998;70:107–110. [DOI] [PubMed] [Google Scholar]
- 16. Morales AJ, Haubrich RH, Hwang JY, Asakura H, Yen SS. The effect of six months treatment with a 100 mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clin Endocrinol (Oxf). 1998;49:421–432. [DOI] [PubMed] [Google Scholar]
- 17. Labrie F, Archer D, Bouchard C, et al. Effect of intravaginal dehydroepiandrosterone (Prasterone) on libido and sexual dysfunction in postmenopausal women. Menopause. 2009;16:923–931. [DOI] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higgins JP, Altman DG. Assessing risk of bias in included studies. In: Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: John Wiley & Sons, Ltd; 2008;187–241. [Google Scholar]
- 21. Swiglo BA, Murad MH, Schünemann HJ, et al. A case for clarity, consistency, and helpfulness: state-of-the-art clinical practice guidelines in endocrinology using the grading of recommendations, assessment, development, and evaluation system. J Clin Endocrinol Metab. 2008;93:666–673. [DOI] [PubMed] [Google Scholar]
- 22. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 23. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 24. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B. 1995;57:289–300. [Google Scholar]
- 25. Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333:597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davis SR, Panjari M, Stanczyk FZ. Clinical review: DHEA replacement for postmenopausal women. J Clin Endocrinol Metab. 2011;96:1642–1653. [DOI] [PubMed] [Google Scholar]
- 27. Barnhart KT, Freeman E, Grisso JA, et al. The effect of dehydroepiandrosterone supplementation to symptomatic perimenopausal women on serum endocrine profiles, lipid parameters, and health-related quality of life. J Clin Endocrinol Metab. 1999;84:3896–3902. [DOI] [PubMed] [Google Scholar]
- 28. Bloch M, Meiboom H, Zaig I, Schreiber S, Abramov L. The use of dehydroepiandrosterone in the treatment of hypoactive sexual desire disorder: a report of gender differences. Eur Neuropsychopharmacol. 2013;23:910–918. [DOI] [PubMed] [Google Scholar]
- 29. Carranza-Lira S, Nájera Mojica JL, Herrera J, et al. Changes in hormones, lipids and symptoms after the administration of a commercial preparation with dehydroepiandrosterone in postmenopausal women. Proc West Pharmacol Soc. 2002;45:181–183. [PubMed] [Google Scholar]
- 30. Casson PR, Faquin LC, Stentz FB, et al. Replacement of dehydroepiandrosterone enhances T-lymphocyte insulin binding in postmenopausal women. Fertil Steril. 1995;63:1027–1031. [PubMed] [Google Scholar]
- 31. Dayal M, Sammel MD, Zhao J, Hummel AC, Vandenbourne K, Barnhart KT. Supplementation with DHEA: effect on muscle size, strength, quality of life, and lipids. J Womens Health (Larchmt). 2005;14:391–400. [DOI] [PubMed] [Google Scholar]
- 32. Finckh A, Berner IC, Aubry-Rozier B, So AK. A randomized controlled trial of dehydroepiandrosterone in postmenopausal women with fibromyalgia. J Rheumatol. 2005;32:1336–1340. [PubMed] [Google Scholar]
- 33. Gómez-Santos C, Hernández-Morante JJ, Tébar FJ, Granero E, Garaulet M. Differential effect of oral dehydroepiandrosterone-sulphate on metabolic syndrome features in pre- and postmenopausal obese women. Clin Endocrinol (Oxf). 2012;77:548–554. [DOI] [PubMed] [Google Scholar]
- 34. Igwebuike A, Irving BA, Bigelow ML, Short KR, McConnell JP, Nair KS. Lack of dehydroepiandrosterone effect on a combined endurance and resistance exercise program in postmenopausal women. J Clin Endocrinol Metab. 2008;93:534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jankowski CM, Gozansky WS, Schwartz RS, et al. Effects of dehydroepiandrosterone replacement therapy on bone mineral density in older adults: a randomized, controlled trial. J Clin Endocrinol Metab. 2006;91:2986–2993. [DOI] [PubMed] [Google Scholar]
- 36. Kenny AM, Boxer RS, Kleppinger A, Brindisi J, Feinn R, Burleson JA. Dehydroepiandrosterone combined with exercise improves muscle strength and physical function in frail older women. J Am Geriatr Soc. 2010;58:1707–1714. [DOI] [PubMed] [Google Scholar]
- 37. Lasco A, Frisina N, Morabito N, et al. Metabolic effects of dehydroepiandrosterone replacement therapy in postmenopausal women. Eur J Endocrinol. 2001;145:457–461. [DOI] [PubMed] [Google Scholar]
- 38. Panjari M, Bell RJ, Jane F, Adams J, Morrow C, Davis SR. The safety of 52 weeks of oral DHEA therapy for postmenopausal women. Maturitas. 2009;63:240–245. [DOI] [PubMed] [Google Scholar]
- 39. Srinivasan M, Irving BA, Frye RL, et al. Effects on lipoprotein particles of long-term dehydroepiandrosterone in elderly men and women and testosterone in elderly men. J Clin Endocrinol Metab. 2010;95:1617–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. von Mühlen D, Laughlin GA, Kritz-Silverstein D, Bergstrom J, Bettencourt R. Effect of dehydroepiandrosterone supplementation on bone mineral density, bone markers, and body composition in older adults: the DAWN trial. Osteoporos Int. 2008;19:699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weiss EP, Shah K, Fontana L, Lambert CP, Holloszy JO, Villareal DT. Dehydroepiandrosterone replacement therapy in older adults: 1- and 2-y effects on bone. Am J Clin Nutr. 2009;89:1459–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]