Abstract
Background:
In endometriosis, the establishment and subsistence of ectopic lesions outside the endometrium suggest an altered cellular state for pathological hyperplasia. Sphingolipids are bioactive compounds, and their biosynthesis and metabolism modulate a range of cellular processes including proliferation, migration and apoptosis. We demonstrate that aberrations in sphingolipid metabolism occur in women with endometriosis.
Methods:
Targeted mass spectrometry on >120 sphingolipids were measured in the sera (n = 62), peritoneal fluid (n = 63), and endometrial tissue (n = 14) of women with and without endometriosis. Quantitative RT-PCR and immunohistochemistry were performed on endometrial tissues determine the expression levels of sphingolipid enzymes.
Results:
Sphingolipidomics identified the in vivo accumulation of numerous sphingolipids, including the functionally antagonistic glucosylceramides and ceramides in the serum and PF of women with endometriosis. We found upregulation of specific sphingolipid enzymes, namely sphingomyelin synthase 1 (SMS1), sphingomyelinase 3 (SMPD3), and glucosylceramide synthase (GCS) in the endometrium of endometriotic women with corresponding increased GlcCer, decreased sphingomyelin levels, and decreased apoptosis in the endometrium.
Conclusions:
Our sphingolipidomics approach provided evidence of altered sphingolipid metabolism flux in serum, peritoneal fluid, and endometrial tissue in women with endometriosis. The results provide new information on how sphingolipids and eutopic endometrium may contribute to the pathophysiology of endometriosis. The results also have implications for the use of sphingolipids as potential biomarkers.
Endometriosis affects an estimated 6% to 10% of women of reproductive age, resulting in debilitating pelvic pain, dysmenorrhea, dyspareunia, painful defecation, and infertility (1). Hereditary components are known risk factors in endometriosis (2–4), whereas sustained inflammation can be observed through elevated macrophages and proinflammatory cytokine levels (5, 6). Treatment modalities for endometriosis are currently inadequate and empirical, with medical therapy resulting in anovulation, a situation unacceptable for patients seeking fertility. Surgical removal or ablation of ectopic endometrial implants are currently most effective not only for alleviation of pain but also for improving fertility. However, both medical and surgical treatments are not without significant side effects or are unsuitable for couples seeking fertility (7) and do not address the issue of disease recurrence (8). Therefore, elucidating the mechanisms of enhanced cell growth will greatly enhance our understanding of the disease pathobiology and generate novel therapeutic targets directed at the pathophysiological mechanisms to ultimately improve the quality of life of affected women.
Although numerous molecular events related to lesion subsistence have been implicated (9), the contribution of sphingolipid metabolism, in particular ceramide (Cer) metabolism, has not been explored. Sphingolipids are increasingly known to be important bioactive signaling molecules and are involved in a diverse range of cellular processes. For example, Cer and sphinghoid bases modulate many apoptosis signaling events, whereas their phosphorylation or glycosylation lead to the production of mitogenic factors such as Cer-1-phosphate (C1P) and glucosylceramide (GlcCer). Elevated levels of GlcCer have been documented in many chronic human diseases such as Gaucher's disease, cancers, type 2 diabetes mellitus, and polycystic kidney disease (10–13). Studies on renal epithelial cells (14), keratinocytes (15, 16) and cancer cell lines (17, 18) further demonstrate the mitogenic properties of GlcCer. In addition, mice with β-glucosidase mutations display systemic inflammation (19). Central among the GlcCer changes is GlcCer synthase (GCS), the enzyme that catalyzes the glycosylation of Cer, and constitutes the first committed step for the biosynthesis of higher glycosphingolipids that are also implicated in cellular proliferation (20). Given the heightened cellular proliferative states associated with endometriosis, we hypothesized that dysregulation in the sphingolipid metabolic pathway may be associated with intrinsic enhanced endometrial cell proliferation, which leads to lesion growth and implantation at ectopic sites.
In this study, we used a targeted quantitative liquid chromatography-tandem mass spectrometry (LC-MS/MS) lipidomics approach to analyze serum, peritoneal fluid (PF), and endometrial tissue sphingolipid concentrations en masse in woman with endometriosis and immunohistochemistry (IHC) to understand dysregulation in sphingolipid metabolism and fluxes.
Materials and Methods
Patient enrollment and ethics
The study population comprising patients presenting with subfertility in addition to a general gynecological case mix was recruited in KK Women's and Children's Hospital, Singapore. Women provided written informed consent for collection of samples under Centralized Institutional Research Board approval (CIRB 2010-167-D). Exclusion criteria included menstruating patients, postmenopausal patients, patients on hormonal therapy for at least 3 months before laparoscopy, and other confounding diseases such as diabetes, other chronic inflammatory diseases (rheumatoid arthritis, inflammatory bowel disease, etc). Serum (n = 62), PF (n = 63), and endometrial samples (n = 14) were collected from women undergoing laparoscopic procedures for suspected endometriosis, infertility, sterilization procedures, and/or pelvic pain. A careful survey of the uterus, fallopian tubes, ovaries, and the pelvic peritoneum was made for any evidence of endometriotic deposits, and the severity of endometriosis was scored according to the revised American Fertility Society (rAFS) classification of endometriosis (21, 22). A total of 38 patients with endometriosis (EM+), and 24 women who did not have endometriosis or have benign gynecological diagnoses (uterine fibroids, benign ovarian cysts, etc) were included as the control group (EM−). For PF analysis, a cohort of 63 subjects, of whom 45 were common to the serum cohort, comprised 39 EM+ subjects and 26 EM− subjects. Further details on patient characteristics can be found in Table 1. For checking specificity of sphingolipids in relation to endometriosis, serum from an additional 5 EM+ and 5 EM− menstruating women were collected and analyzed. The phase of the menstrual cycle (proliferative and secretory) was determined according to cycle history of the patients.
Table 1.
Summary of Clinical Parameters of Study Cohort for Serum and PF Analysis
| Serum |
PF |
|||||
|---|---|---|---|---|---|---|
| EM+ (n = 38) | EM− (n = 24) | P Valuea | EM+ (n = 39) | EM− (n = 26) | P Valuea | |
| Age, y | ||||||
| Median | 33.5 | 34.5 | 0.22 | 34 | 35 | 0.56 |
| Range | 22–44 | 22–47 | 22–44 | 22–51 | ||
| Diagnostic, rAFS grade | ||||||
| I–II | 11 | NA | 13 | NA | ||
| III–IV | 27 | NA | 22 | NA | ||
| Cycle phase | 0.29 | 0.44 | ||||
| Proliferative | 21 | 9 | 20 | 10 | ||
| Secretory | 17 | 14 | 18 | 15 | ||
| Ethnicity | 0.79 | 0.95 | ||||
| Chinese | 27 | 18 | 26 | 18 | ||
| Malay | 7 | 0 | 5 | 3 | ||
| Othersb | 4 | 4 | 7 | 4 | ||
| Fecundity | 0.64 | 0.43 | ||||
| Fertile | 8 | 6 | 8 | 6 | ||
| Infertile | 28 | 18 | 29 | 14 | ||
| Subfertile | 2 | 0 | 1 | 2 | ||
Abbreviation: NA, not available.
Two-tailed Mann-Whitney U test for EM+ and EM−; χ2 and Fisher's exact tests of association for categorical values.
Other races comprised Burmese, Indians, Indonesians, Filipinos, and Vietnamese.
Sample preparation
Blood was collected and serum prepared by centrifugation at 1200g for 10 minutes and the top layer respun at 3600g for 10 minutes. The supernatant was carefully removed and transferred into 1-mL aliquots. PF was collected from the pouch of Douglas, and 1% vol/vol protease inhibitor was added (Roche), spun at 1000g for 10 minutes and the supernatant transferred to 15-mL aliquots. Both sera and PF were stored at −80°C until use. Uterine endometrial curettage tissues were irrigated in saline and split into 2 parts: one section was processed for formalin-fixed-paraffin embedding for histology, and the other section was kept on ice and frozen at −80°C (within 4 hours of collection).
Sphingolipids were extracted from sera, PF, and tissues using a modified Bligh and Dyer method. Briefly, 900 μL chloroform-methanol (1:2, vol/vol) was added to 100 μL serum or PF. Alternatively, endometrial tissues were incubated in chloroform-methanol for 30 minutes and homogenized using TissueLyser LT (QIAGEN) before the addition of 900 μL chloroform-methanol (1:2, vol/vol). After 20 minutes vortexing and incubation at 4°C, 300 μL chloroform and 300 μL ddH2O were added to the mixture. Sphingolipids were removed from the lower organic phase after centrifugation at 9000 rpm at 4°C for 2 minutes. Subsequently, 500 μL chloroform was added and vortexed at 4°C for 20 minutes, and lipids were removed from the lower organic phase after centrifugation and combined with the previous fraction. The lipid extracts were vacuum-dried and stored at −80°C. Before LC-ESI-multiple reaction monitoring (MRM) lipid extracts were reconstituted in 200 or 400 μL chloroform-methanol (1:1, vol/vol). Lipids were analyzed within 2 weeks after extraction.
Mass spectrometry-based sphingolipidomics
Positive ionization-mode LC-MS/MS via MRM on a triple quadrupole 6460 with electrospray ionization source (Agilent Technologies) was used for the quantification of sphingolipids (156 molecular species; Supplemental Table 1). MRM was based on product ion mass to charge ratio (m/z) 264.4 [sphingosine-H2O]+ and 266.4 [sphinganine-H2O]+ for Cer, GlcCer, galatocsylceramide (GalCer), lactosylceramide (LacCer), and C1P and m/z 184.1 [phosphocholine]+ for sphingomyelin (SM) and phosphatidylcholine (PC). The product ion m/z 184.1 for SM is unable to differentiate the N-linked fatty acid and sphingoid base but can be assumed to be the predominant sphingosine base (d18:1) in humans. C-18 reversed-phase LC (Zorbax Eclipse 2.1 × 50 mm inner diameter, 1.8 μm; Agilent Technologies) was used to separate reconstituted lipids at 400 μL/min before entering the mass spectrometer. The mobile phase consisted of 5mM ammonium acetate in water (mobile phase A) and 5mM ammonium acetate in methanol (mobile phase B). Sphingolipids were eluted using linear gradients from 60% to 100% B over 2 minutes, maintained at 100% B for 5 minutes, followed by a linear gradient to 60% B over 2 minutes, and held for another 2 minutes before the next injection. The optimized lipid-class dependent mass spectrometry parameters are shown in Supplemental Table 2. Non-naturally occurring internal standards, namely Cer d18:1/17:0, GlcCer d18:1/8:0, GalCer d18:1/8:0, LacCer d18:1/12:0, PC 14:0/14:0, SM 12:0, and C1P d18:1/8:0 (Avanti Polar Lipids), which corresponded to their sphingolipid classes, were verified for their absence and used for quantification. Further details on optimization of LC-MS/MS parameters can be found in Supplemental Figures 1–3.
Geometric isomeric monohexosylceramides GlcCer and GalCer were separately resolved via normal-phase chromatography (LC-Si, 2.1 × 250 mm; inner diameter, 5 μm; Supelco, Sigma-Aldrich). A 10.5-minute isocratic elution using the mobile phase (CH3CN/CH3OH/CH3COOH, 97/2/1, vol/vol, with 5mM ammonium acetate) at 750 μL/min was used to resolve and identify the isomers using their corresponding internal standards (23). Peaks were integrated, manually inspected, and quantified using MassHunter Quantitative software (versions B.03–05; Agilent).
Quantitative-RT-PCR
Quantitative RT-PCR was performed as previously described (24). Total RNA was extracted from tissue samples obtained from 5 EM+Sev and 5 EM− subjects using TRIzol (Bio-Rad) according to the manufacturer's instructions. Deoxyribonuclease I (Bio-Rad) was used to remove genomic DNA contamination, total RNA concentration was quantified (NanoDrop; Thermo Scientific) and 1 μg RNA was reverse-transcribed by using oligo(dT) and the iScript cDNA synthesis kit (Bio-Rad). Primer sequences for genes Ugcg, Sptl1, Sptl2, Sptl3, Smpd1, Smpd2, Smpd3, Sgms1, and Actb are listed in Supplemental Table 3. PCR was carried out for 95°C for 30 seconds and 40 cycles at 95°C for 1 second and 60°C for 10 seconds. Data were normalized with Actb.
Immunohistochemistry
IHC was performed as previously described (25) with modifications. Proteinase K-induced antigen retrieval (20 μL/mL, pH 8.0) was performed by incubating sections for a half-hour. Alternatively, in preparation for CD10 detection, heat-induced antigen retrieval was carried out by microwaving slides in citrate buffer (10mM citric acid, 0.05% Tween 20, pH 6.0) for 20 minutes, blocked with 5% BSA/0.5% Tween 20 in PBS, and incubated overnight at 4°C with primary antibodies against SM phosphodiesterase 1 (SMPD1) (Abcam), SMPD2 (Abnova), SMPD3 (Abnova), serine palmitoyl transferase (Abcam), GalCer (Abcam), GCS (Abbiotec), SM synthase 1 (SMS1) (Proteintech), and CD10 (Novocastra). Detection of antigens was performed with Alexa Fluor 546 or 3,3′-diaminobenzidine for 1 hour at room temperature. Samples were washed in PBS and stained with Slow Fade Gold Antifade Reagent with 4′,6-diamidino-2-phenylindole (Invitrogen) before imaging through confocal microscopy (FV1000; Olympus). Primary antibodies replaced with blocking buffer acted as negative controls.
Terminal deoxynucleotide transferase mediated dUTP nick-end labeling assay
Terminal deoxynucleotide transferase mediated dUTP nick-end labeling (TUNEL) assay was performed using the TUNEL apoptosis detection kit (GenScript). Section processing was similar to IHC except for endogenous peroxidase activity blocking with 3% H2O2 in methanol. Apoptotic regions were detected Streptavidin-fluorescein isothiocyanate. Positive and negative controls were run in parallel to the samples. Negative controls were processed by omitting terminal deoxynucleotidyl transferase from the TUNEL reaction mixture, whereas positive controls were incubated with deoxyribonuclease before labeling to induce DNA strand degradation.
Image analysis
Image analysis was performed using ImageJ. Fluorescent intensity was measured for the sections in general as well as for the individual cell types (stromal and glandular epithelial cells). For both cases, the intensities were normalized with values obtained from the samples' corresponding negative controls. With respect to analysis of the individual cell type's fluorescent intensities, 5 glands and stromal sites were randomly selected from each sample for measurement, and the mean value obtained after normalization was used for comparison. In addition to comparing the signal intensity across the different samples, comparison was also made between the EM+Sev and EM− group as a whole.
Data preprocessing
Quantification of sphingolipids was performed via MassHunter Quantitative software (versions B.04 and B.05; Agilent). Sphingolipids with a low signal to noise ratio (response <50) were removed because low responses exhibit decreased reproducibility and reliability and therefore may be less quantitatively accurate. Hence, only sphingolipids that were represented in ≥70% of the study cohort were accepted for further analyses. To implement statistical modeling (orthogonal partial least squares [OPLS]), undetectable sphingolipid levels were assumed as reaching the detection limits of their analytical method and were replaced with a randomly generated number that is between the lowest measurable value and 1/10 of the lowest measureable value.
Statistical analysis
Comparisons between groups were achieved using nonparametric analysis, namely two-tailed Wilcoxon rank sum tests and Kruskal-Wallis tests with Dunn's post hoc analysis, unless otherwise stated. Friedman's analysis was used for paired analysis. The P values were tested for multiple hypothesis testing using Bonferroni adjustment or false discovery rate estimation. Lipids with adjusted P ≤ .01 or q value ≤0.15 and fold change ≥1.15 were deemed significant. The R statistical software environment (http://www.r-project.org/) was used to perform false discovery rate estimation. Fisher's exact test was used considering the relatively smaller sample set. Nonparametric tests were chosen to reduce the influence of the imputed values unless otherwise stated. Heatmaps (MeV version 4.6.2) and OPLS (Unscrambler X version 10.1) were analyzed and modeled after the normalization of data by first centering the data to the median and scaling it by division with the SD. Full cross-validation was applied in OPLS to increase model performance and for the calculation of coefficient regression values.
Results
Differential sphingolipidome in endometriosis
Our cohort of 62 subjects used for serum studies and 63 subjects for PF studies (Table 1), of which 45 were common to the serum cohort, were grouped into cases (EM+) and controls (EM−). EM+ subjects were further stratified to mild cases of endometriosis (EM+Mild; rAFS I–II) (21) or severe cases (EM+Sev; rAFS III–IV). Age, ethnicity, fertility, and menstrual phase were not significant parameters (Fisher's exact test, P = .563, .954, .426, and .444, respectively). By capitalizing on the fast chromatographic separation of ultra–high-performance LC and by coupling the selectivity of MRM, we were able to simultaneously measure >120 sphingolipids at sensitivities of 0.01 to 0.1 ng/mL to 10 ng/mL (Supplemental Table 4) at interday coefficient of variation of 6.5% and intraday coefficient of variation of 9% (Supplemental Figure 3, A–C). Monohexosylceramides GalCer and GlcCer are geometric isomers in which specific species share the same MRM transitions. For example, the 588.5 m/z → 264.4 m/z transition is shared by both GalCer and GlcCer d18:1/24:0 (Supplemental Figure 3D). By chiral chromatography, we are able to separate GalCer and GlcCer and showed that GalCer represented a small fraction, ∼0.5% to 5% of total monohexosylceramides (Supplemental Figure 3E), consistent with other reports (26), and therefore only GlcCer was considered for subsequent analyses.
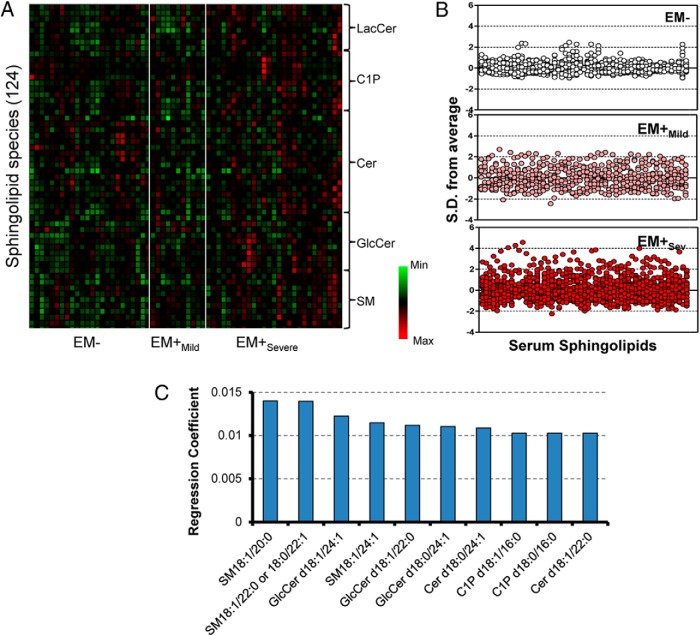
Unbiased serum sphingolipid profiles displayed as a heat map (Figure 1A) showed pattern discrimination in relation to the disease. The z-plots revealed serum sphingolipidome alterations in EM+ patients (z-score range, −3.1 to 5.4) compared with EM− (z-score range, −0.99 to 2.56) and to a larger extent in EM+Sev (Figure 1B). There were widespread elevations of PF and serum sphingolipid levels (Supplemental Figures 4 and 5). Multivariate modeling by OPLS discriminated EM+Sev from EM−, and OPLS-derived regression coefficients ranked medium- to very-long-chain GlcCers and SMs (C16:0–C24:1) as major influencing independent variables (Figure 1C and Supplemental Figure 5B). Supplemental Figure 5C shows a robust exchange of sphingolipids between the 2 biofluids (serum and PF, Spearman r = 0.9, P < .0001).
Figure 1. Serum sphingolipid profile of endometriosis.
A, Heat map representation of median-centered, SD-scaled data from women of differential endometriotic stages as determined by rAFS classification (EM−, n = 24; EM+Mild, n = 11; EM+Sev, n = 27). Each row represents a sphingolipid species (total 124 species), whereas each column represents a subject grouped together according to their endometriotic status. B, Z-score plots of serum sphingolipids in subjects without endometriosis (EM−), mild endometriosis (EM+Mild), severe endometriosis (EM+Sev), and all grades of endometriosis (EM+All). C, Regression coefficients of te top 10 ranked important variables (sphingolipids) from serum that have potential influence on endometriosis.
Serum and PF GlcCers are increased in endometriosis
The strong GlcCer correlation with endometriosis led us to explore this glycosphingolipid because GlcCer, a mitogenic factor, may be a prime candidate for lesion subsistence ectopically. In our broad mass spectrometry profiling of sphingolipids, systematic analysis revealed significantly higher levels of total serological and PF GlcCer (P = .0004 and .005, respectively) in EM+Sev patients compared with controls (Figure 2). Further examination revealed an enrichment of GlcCer and a striking enrichment of very-long-chain monounsaturated sphingolipids (SM, Cer, and GlcCer) of 24 carbons (C24:1) in EM+Sev women (Table 2), suggesting sequential sphingolipid metabolism. Other sphingolipids such as C1P, Cer, and LacCer were not significantly enriched in EM+Sev women (Supplemental Figure 6, A–D). Overall, there were few major sphingolipid changes in EM+Mild relative to EM−.
Figure 2. Accumulation of GlcCer in endometriosis.
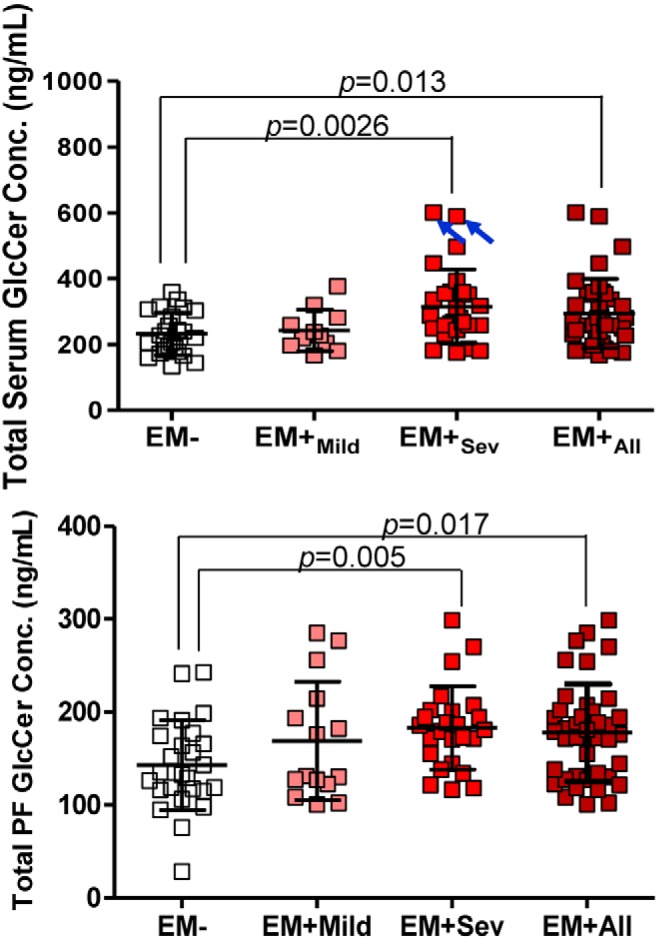
Scatter plots of total serum and PF GlcCer in EM+ and EM− subjects. Open boxes, EM− (n = 24); pink boxes, EM+Mild (n = 11); red boxes, EM+Sev (n = 27); maroon boxes, EM+All (n = 38). Although 2 EM+Sev patients seemingly contribute to the difference (blue arrows), their removal maintained statistical significance (P = .007). P values adjusted by Bonferroni adjustment.
Table 2.
Fold Change of Serum, PF, and Endometrial Tissue Sphingolipidsa
| Sphingolipid | Fold Change (EM+Sev /EM−) |
||
|---|---|---|---|
| Serum | PF | Tissue | |
| SM18.1.24.0 | 1.77 | 1.29 | 0.69 |
| SM18.1.22:1 | 1.12 | 1.07 | 0.66 |
| SM18.1.22.0 | 1.17 | 1.14 | 0.81 |
| SM18.1.20:1 | 1.14 | 1.13 | 0.62 |
| SM18.1.20.0 | 1.18 | 1.08 | 0.78 |
| SM18.1.18:1 | 1.07 | 1.13 | 0.76 |
| SM18.1.18:0 | 1.05 | 1.07 | 0.79 |
| SM18.1.16.1 | 1.12 | 1.06 | 0.68 |
| SM18.1.16.0 | 1.11 | 1.03 | 0.73 |
| Cer d18:1/24:1 | 1.20 | 1.41 | 0.36 |
| Cer d18:1/24:0 | 1.27 | 1.41 | 0.37 |
| Cer d18:1/22:1 | 1.32 | 1.16 | 0.49 |
| Cer d18:1/22:0 | 1.39 | 1.49 | 0.36 |
| Cer d18:1/20:0 | 1.17 | 1.62 | 0.49 |
| Cer d18:1/18:0 | 0.97 | 1.41 | 0.47 |
| Cer d18:1/16:1 | 1.04 | 0.98 | 0.64 |
| Cer d18:1/16:0 | 1.27 | 1.33 | 0.40 |
| Cer d18:0/24:1 | 1.08 | 1.09 | 0.65 |
| Cer d18:0/24:0 | 0.81 | 1.32 | 0.31 |
| Cer d18:0/18:1 | 0.95 | 1.03 | 0.34 |
| Cer d18:0/16:0 | 1.10 | 1.45 | 0.35 |
| GluCer d18:1/24:1 | 1.47 | 1.14 | 1.76 |
| GluCer d18:1/24:0 | 1.47 | 1.22 | 0.40 |
| GluCer d18:1/22:0 | 1.42 | 1.23 | 0.53 |
| GluCer d18:1/20:0 | 1.32 | 1.37 | 0.55 |
| GluCer d18:1/18:0 | 1.47 | 1.32 | 0.58 |
| GluCer d18:1/16:0 | 1.28 | 1.36 | 0.32 |
| GluCer d18:0/24:1 | 1.21 | 1.06 | 2.02 |
| GluCer d18:0/16:0 | 1.25 | 1.16 | 0.57 |
| LacCer d18:1/22:0 | 1.35 | 1.47 | 0.47 |
| LacCer d18:1/16:1 | 1.20 | 1.51 | 0.79 |
| LacCer d18:1/16:0 | 1.24 | 1.41 | 0.49 |
| C1P d18:1/24:1 | 1.35 | 1.35 | 0.27 |
| C1P d18:1/18:1 | 1.23 | 1.14 | 0.60 |
| C1P d18:1/18:0 | 1.11 | 1.46 | 0.60 |
| C1P d18:1/16:0 | 1.32 | 1.20 | 0.49 |
| C1P d18:0/16:0 | 1.32 | 1.17 | 0.46 |
Only sphingolipids that are statistically significant in at least 1 biological compartment are shown.
Role of endometrium in regulating altered sphingolipid flux
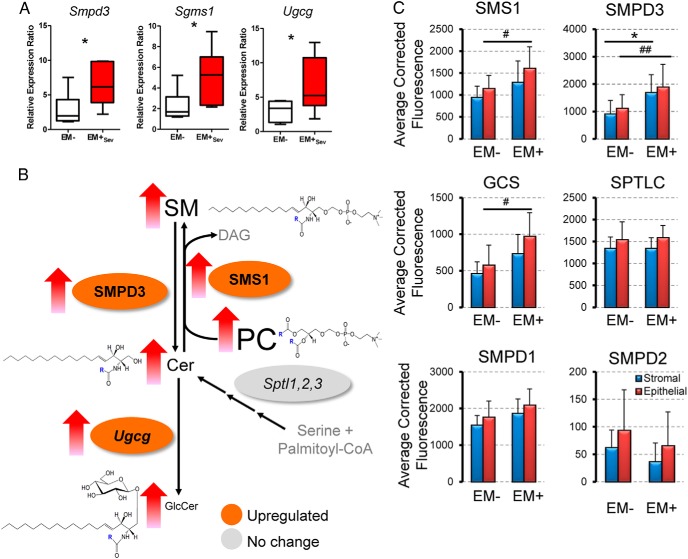
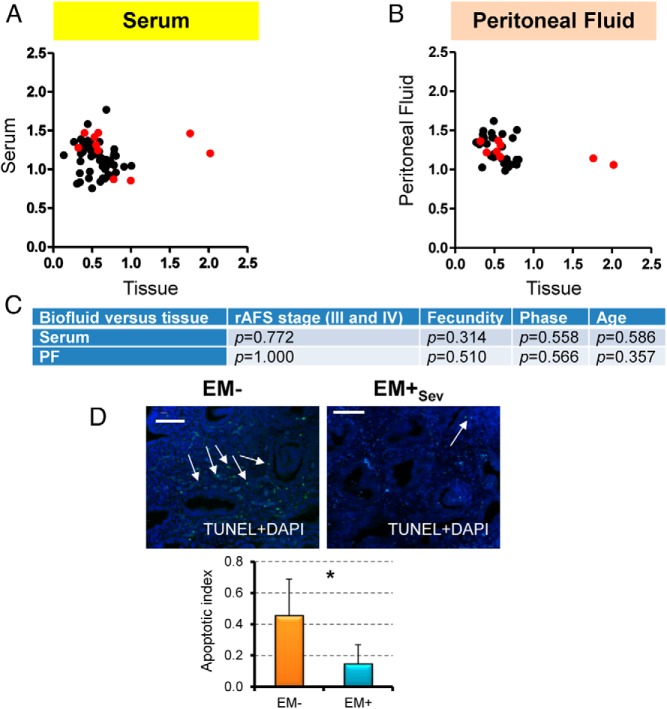
Because there are numerous molecular defects in the endometrium that reflect enhanced survival, evasion of apoptosis, and lesion establishment in endometriosis (27), we determined whether endometrial sphingolipid enzymes were altered. At the transcript level, we found upregulation of SMPD3 (Smpd3) in EM+Sev women (n = 6) relative to EM− (n = 6; P = .026; Figure 3A) but not serine-palmitoyl transferases (Sptl1–3), suggesting SM hydrolysis as the main source of Cer and not through de novo synthesis. SM can be synthesized by the transfer of phosphocholine from PC onto Cers, catalyzed by SMS1e (Sgms1). In ovarian endometriosis, several plasma and PF PCs were found elevated (28, 29). Consistent with this, there was Sgms1 upregulation (P = .041), corresponding decreases in tissue PCs, and increases in serum and PF PCs in EM+Sev relative to EM− (P = .038, P = .0009, P = .039 respectively; Supplemental Figure 6E). Importantly, UDP-glucose Cer glucosyltransferase (Ugcg; GCS) levels were higher in EM+Sev subjects (P = .041). Upregulation of Ugcg transcript in endometriotic lesions of a rat model of endometriosis has been previously reported (30). Our data thus indicated the flux of EM+ endometrial sphingolipid metabolism: PC→SM→Cer→GlcCer (Figure 3B). By IHC, increased SMS1, SMPD3, and GCS expression were detected in EM+Sev glandular epithelial and stromal cells (n = 6), whereas these enzymes were expressed predominantly in the glands in EM− specimens (n = 6; Figure 3C and Supplemental Figure 7). In concordance with our quantitative RT-PCR results, staining intensities for SMPD1, SMPD2, and serine-palmitoyl transferase were similar between EM− and EM+Sev (Supplemental Figure 7). GalCer synthase expression levels were similar between EM− and EM+ subjects, further supporting our LC-MS/MS measurements of GlcCer elevation, and not GalCer in endometriosis (Supplemental Figure 7). Endometriotic endometrial cells are more proliferative (31), and given the upregulation of endometrial GCS, elevated GlcCer should be found in EM+Sev endometrium. EM+Sev/EM− fold change of endometrial very-long-chain GlcCer (C24:1) were high (n = 7 per group; Table 2); by contrast, most sphingolipids were elevated in serum and PF but low in endometrium, suggesting endometrium as the tissue of origin (Table 2 and Figure 4, A–C). There was a corresponding decreased apoptosis in EM+Sev endometrial tissues (Figure 4D) relative to EM− women, consistent with previous reports (32, 33), an observation in line with the crucial role of GlcCer as a proliferative agent. Overall, these findings suggest that the accumulation of GlcCer in endometriosis is due to aberrant sphingolipid metabolism in the endometrium that may render eutopic endometrial cells more proliferative.
Figure 3. Regulation of sphingolipid concentrations in endometriosis in the endometrium.
A, Upregulation of endometrial sphingolipid enzymes in EM+Sev (n = 5 per group). *, P < .05. B, Serum lipid concentrations and lipid enzymes that control their metabolism in the endometrium of EM+Sev and EM−. Colors indicate fold changes in the expression levels of lipid enzymes that regulate the flux of sphingolipids. Font sizes are proportionate to the abundance of the lipids in serum. Unmeasured analytes are shown in gray. C, Endometrial sphingolipid enzyme expression levels as measured by fluorescence intensity in stromal (blue bar) and epithelial (red bar) compartments (n = 6). *, P < .05 for stromal comparison; and #, P < .05; ##, P < .01 for epithelial comparisons.
Figure 4. Accumulation of GlcCer in endometriosis.
A, Scatterplot of mean serum sphingolipid levels against mean endometrial tissue sphingolipid levels. B, Scatter plot of mean PF sphingolipid levels against mean endometrial tissue sphingolipid levels. GlcCers are highlighted in red. C, Because the tissue and PF/serum data were not necessarily from the same patients, Fisher's exact test and Wilcoxon P values of demographics variables in EM+Sev patients were tested and shown to be similar. D, Apoptotic index based on average number of apoptotic bodies/6.4 mm2 detected by TUNEL IHC of EM+ and EM− women (n = 4–5). *, P < .05. Original magnification, ×400; scale bar, 50 μm.
Discussion
Sphingolipids define a distinctive and important lipid class involved in diverse biological functions including signal transduction and cell fate determination, but their association with endometriosis pathophysiology remains poorly studied. Lipidomics is an emerging discipline in systems biology that aims to investigate and model lipids at the global level. This is done in part in an effort to stitch lipids with the rest of the 'omics' sciences such as proteomics and genomics to comprehensively understand biological systems at the systems level. In this study, we used targeted LC-MS/MS-based lipidomics in a case-control study for the profiling of >120 sphingolipids to evaluate sphingolipid changes in endometriosis systematically. This sphingolipidome-wide quantitative analysis identified the in vivo accumulation of GlcCer in endometriotic women as a result of altered sphingolipid metabolic processing by GCS in the endometrium.
The association of GlcCer accumulation and hyperproliferation and organomegaly seen in human and experimental diseases such as Gaucher's disease, polycystic kidney disease, and diabetes demonstrate the effect of dysregulated GlcCer metabolic processing on tissue proliferation (15, 34). In animal models and cellular work, GlcCers possess growth-stimulatory effects, and pharmacological or genetic inhibition of GCS resulted in cell death (13, 16, 35, 36). Consistent with this, we identified increased endometrial GCS expression and corresponding serum and PF GlcCer accumulation in women with endometriosis, raising the possibility of biochemically induced proliferative abnormalities in the endometrium. It is therefore interesting that we found increased serum and PF Cer in EM+ women that would have promoted apoptosis of shed endometrial cells. Endometrial stromal cells originating from EM+ women have blunted responses to the apoptosis-inducing effects of Cer (32, 37). Consequently, the combination of GlcCer to promote cell proliferation and apoptotic-resistant endometriotic cells suggest intrinsic cellular dysregulation conducive for the enhanced cell proliferation and potentially the growth of endometriotic lesions. Recent studies have also shown that sphingosine-1-phosphate receptors are upregulated in EM+ ectopic and eutopic endometrium (38), and it results in increased viability upon exposure to sphingosine analogs (37), further suggesting the role of dysregulated sphingolipid metabolism in this disease. Together, our data shed some light on the importance of dysregulated sphingolipid fluxes and the specific effects of accumulated GlcCer in endometriosis, suggesting a possible pathophysiological role. Whatever the etiological agent of the observed accumulation of GlcCer is, approaches that target this pathway may address both the establishment and sustenance of endometriotic lesions concurrently. Experimental models will be helpful in clarifying the plausible biological roles of these sphingolipids in the development of enhanced cell proliferation and lesion development. It should be recognized that our study is exploratory and requires further studies in an independent population cohort for both biomarker discovery and validation purposes.
In conclusion, our clinical sphingolipidomics approach defines the net outcome of an imbalanced GlcCer that may be crucial for endometriosis pathophysiology. Such heightened proliferative abilities may be key to how menstrual fragments may persist as endometriotic lesions in ectopic sites.
Acknowledgments
We acknowledge the help of Drs. Heng Hao Tan, Mathew Lau and Steven Teo from KK Women's and Children's Hospital in the collection of samples.
This work was supported by the Singapore National Research Foundation (to Singapore-MIT Alliance for Research and Technology). J.K.Y.C. received salary support from the National Medical Research Council (CSA/043/2012).
Current address for S.F.L.: Thomson Fertility Centre, Singapore.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the Singapore National Research Foundation (to Singapore-MIT Alliance for Research and Technology). J.K.Y.C. received salary support from the National Medical Research Council (CSA/043/2012).
Footnotes
- Cer
- ceramide
- C1P
- Cer-1-phosphate
- GalCer
- galatocsylceramide
- GCS
- GlcCer synthase
- GlcCer
- glucosylceramide
- IHC
- immunohistochemistry
- LacCer
- lactosylceramide
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- MRM
- multiple reaction monitoring
- m/z
- mass to charge ratio
- OPLS
- orthogonal partial least squares
- PC
- phosphatidylcholine
- PF
- peritoneal fluid
- rAFS
- revised American Fertility Society
- SM
- sphingomyelin
- SMPD1
- SM phosphodiesterase 1
- SMS1
- SM synthase 1
- TUNEL
- terminal deoxynucleotide transferase mediated dUTP nick-end labeling.
References
- 1. Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility. Fertil Steril. 2004;81:1441–1446. [DOI] [PubMed] [Google Scholar]
- 2. Painter JN, Anderson CA, Nyholt DR, et al. Genome-wide association study identifies a locus at 7p15.2 associated with endometriosis. Nat Genet. 2011;43:51–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stefansson H, Geirsson RT, Steinthorsdottir V, et al. Genetic factors contribute to the risk of developing endometriosis. Hum Reprod. 2002;17:555–559. [DOI] [PubMed] [Google Scholar]
- 4. Zondervan KT, Cardon LR, Kennedy SH. The genetic basis of endometriosis. Curr Opin Obstet Gynecol. 2001;13:309–314. [DOI] [PubMed] [Google Scholar]
- 5. Siedentopf F, Tariverdian N, Rücke M, Kentenich H, Arck PC. Immune status, psychosocial distress and reduced quality of life in infertile patients with endometriosis. Am J Reprod Immunol. 2008;60:449–461. [DOI] [PubMed] [Google Scholar]
- 6. Khan KN, Masuzaki H, Fujishita A, Kitajima M, Sekine I, Ishimaru T. Differential macrophage infiltration in early and advanced endometriosis and adjacent peritoneum. Fertil Steril. 2004;81:652–661. [DOI] [PubMed] [Google Scholar]
- 7. Olive DL, Pritts EA. Treatment of endometriosis. N Engl J Med. 2001;345:266–275. [DOI] [PubMed] [Google Scholar]
- 8. Fedele L, Bianchi S, Zanconato G, Bettoni G, Gotsch F. Long-term follow-up after conservative surgery for rectovaginal endometriosis. Am J Obstet Gynecol. 2004;190:1020–1024. [DOI] [PubMed] [Google Scholar]
- 9. Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. [DOI] [PubMed] [Google Scholar]
- 10. Fuller M. Sphingolipids: the nexus between Gaucher disease and insulin resistance. Lipids Health Dis. 2010;9:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Langeveld M, Aerts JM. Glycosphingolipids and insulin resistance. Prog Lipid Res. 2009;48:196–205. [DOI] [PubMed] [Google Scholar]
- 12. Platt FM, Jeyakumar M, Andersson U, et al. Inhibition of substrate synthesis as a strategy for glycolipid lysosomal storage disease therapy. J Inherit Metab Dis. 2001;24:275–290. [DOI] [PubMed] [Google Scholar]
- 13. Natoli TA, Smith LA, Rogers KA, et al. Inhibition of glucosylceramide accumulation results in effective blockade of polycystic kidney disease in mouse models. Nat Med. 2010;16:788–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shayman JA, Mahdiyoun S, Deshmukh G, Barcelon F, Inokuchi J, Radin NS. Glucosphingolipid dependence of hormone-stimulated inositol trisphosphate formation. J Biol Chem. 1990;265:12135–12138. [PubMed] [Google Scholar]
- 15. Marsh NL, Elias PM, Holleran WM. Glucosylceramides stimulate murine epidermal hyperproliferation. J Clin Invest. 1995;95:2903–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uchida Y, Murata S, Schmuth M, et al. Glucosylceramide synthesis and synthase expression protect against ceramide-induced stress. J Lipid Res. 2002;43:1293–1302. [PubMed] [Google Scholar]
- 17. Liu YY, Han TY, Giuliano AE, Ichikawa S, Hirabayashi Y, Cabot MC. Glycosylation of ceramide potentiates cellular resistance to tumor necrosis factor-alpha-induced apoptosis. Exp Cell Res. 1999;252:464–470. [DOI] [PubMed] [Google Scholar]
- 18. Xie P, Shen YF, Shi YP, et al. Overexpression of glucosylceramide synthase in associated with multidrug resistance of leukemia cells. Leuk Res. 2008;32:475–480. [DOI] [PubMed] [Google Scholar]
- 19. Mizukami H, Mi Y, Wada R, et al. Systemic inflammation in glucocerebrosidase-deficient mice with minimal glucosylceramide storage. J Clin Invest. 2002;109:1215–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Merrill AH. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem Rev. 2011;111:6387–6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–821. [DOI] [PubMed] [Google Scholar]
- 22. Revised American Fertility Society classification of endometriosis: 1985. Fertil Steril. 1985;43:351–352. [DOI] [PubMed] [Google Scholar]
- 23. Shaner RL, Allegood JC, Park H, et al. Quantitative analysis of sphingolipids for lipidomics using triple quadrupole and quadrupole linear ion trap mass spectrometers. J Lipid Res. 2009;50:1692–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dong H, Chang DC, Hua MH, et al. 2′-O methylation of internal adenosine by flavivirus NS5 methyltransferase. PLoS Pathog. 2012;8:e1002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee YH, Goh WW Bin, Ng CK, et al. Integrative toxicoproteomics implicates impaired mitochondrial glutathione import as an off-target effect of troglitazone. J Proteome Res. 2013;12:2933–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quehenberger O, Armando AM, Brown AH, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51:3299–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. [DOI] [PubMed] [Google Scholar]
- 28. Vouk K, Hevir N, Ribić-Pucelj M, et al. Discovery of phosphatidylcholines and sphingomyelins as biomarkers for ovarian endometriosis. Hum Reprod. 2012;27:2955–2965. [DOI] [PubMed] [Google Scholar]
- 29. Murphy AA, Santanam N, Morales AJ, Parthasarathy S. Lysophosphatidyl choline, a chemotactic factor for monocytes/T-lymphocytes is elevated in endometriosis. J Clin Endocrinol Metab. 1998;83:2110–2113. [DOI] [PubMed] [Google Scholar]
- 30. Konno R, Fujiwara H, Netsu S, et al. Gene expression profiling of the rat endometriosis model. Am J Reprod Immunol. 2007;58:330–343. [DOI] [PubMed] [Google Scholar]
- 31. Wingfield M, Macpherson A, Healy DL, Rogers PA. Cell proliferation is increased in the endometrium of women with endometriosis. Fertil Steril. 1995;64:340–346. [DOI] [PubMed] [Google Scholar]
- 32. Dmowski WP, Gebel H, Braun DP. Decreased apoptosis and sensitivity to macrophage mediated cytolysis of endometrial cells in endometriosis. Hum Reprod Update. 1998;4:696–701. [DOI] [PubMed] [Google Scholar]
- 33. Johnson MC, Torres M, Alves A, et al. Augmented cell survival in eutopic endometrium from women with endometriosis: expression of c-myc, TGF-beta1 and bax genes. Reprod Biol Endocrinol. 2005;3:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zador IZ, Deshmukh GD, Kunkel R, Johnson K, Radin NS, Shayman JA. A role for glycosphingolipid accumulation in the renal hypertrophy of streptozotocin-induced diabetes mellitus. J Clin Invest. 1993;91:797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Datta SC, Radin NS. Stimulation of liver growth and DNA synthesis by glucosylceramide. Lipids. 1988;23:508–510. [DOI] [PubMed] [Google Scholar]
- 36. Deng W, Li R, Guerrera M, Liu Y, Ladisch S. Transfection of glucosylceramide synthase antisense inhibits mouse melanoma formation. Glycobiology. 2002;12:145–152. [DOI] [PubMed] [Google Scholar]
- 37. Chrobak A, Sieradzka U, Sozański R, Chełmońska-Soyta A, Gabryś M, Jerzak M. Ectopic and eutopic stromal endometriotic cells have a damaged ceramide signaling pathway to apoptosis. Fertil Steril. 2009;92:1834–1843. [DOI] [PubMed] [Google Scholar]
- 38. Santulli P, Marcellin L, Noël JC, et al. Sphingosine pathway deregulation in endometriotic tissues. Fertil Steril. 2012;97:904–911. [DOI] [PubMed] [Google Scholar]