Abstract
Context:
Acromegaly is usually treated with surgery as a first-line treatment, although medical therapy has also been used as an alternative primary treatment.
Objective:
We conducted a systematic review and meta-analysis to synthesize the existing evidence comparing these two approaches in treatment-naïve patients with acromegaly.
Data Sources:
This study performed a comprehensive search in multiple databases, including Medline, EMBASE, and Scopus from early inception through April 2014.
Study Selection:
The study used original controlled and uncontrolled studies that enrolled patients with acromegaly to receive either surgical treatment or medical treatment as their first-line treatment.
Data Extraction:
Reviewers extracted data independently and in duplicates. Because of the noncomparative nature of the available studies, we modified the Newcastle-Ottawa Scale to assess the quality of included studies. Outcomes evaluated were biochemical remission and change in IGF-1 or GH levels. We pooled outcomes using the random-effects model.
Data Synthesis:
The final search yielded 35 studies enrolling 2629 patients. Studies were noncomparative series with a follow-up range of 6–360 months. Compared with medical therapy, surgery was associated with a higher remission rate (67% vs 45%; P = .02). Surgery had higher remission rates at longer follow-up periods (≥24 mo) (66% vs 44%; P = .04) but not the shorter follow-up periods (≤6 mo) (53% vs 26%; P = .02). Surgery had higher remission rates in the follow-up levels of GH (65% vs 46%; P = .05). In one study, the IGF-1 level was reduced more with surgery compared with medical treatment (−731 μg/L vs −251 μg/L; P = .04). Studies in which surgery was performed by a single operator reported a higher remission rate than those with multiple operators (71% vs 47%; P = .002).
Conclusions:
Surgery may be associated with higher remission rate; however, the confidence in such evidence is very low due to the noncomparative nature of the studies, high heterogeneity, and imprecision.
Acromegaly is a chronic, rare, and possibly life-threating condition when not treated. More than 95% of cases are due to a GH-secreting pituitary adenoma that leads to hyperproduction of GH. GH circulates and stimulates production of IGF-1 from the liver and systemic tissues. Therefore, serum levels of both GH and IGF-1 are used for biochemical diagnosis and level of control (1).
Surgery, usually through an endonasal approach, is the primary therapy for most patients. Surgical remission rates greater than 85% have been reported for microadenomas (<10 mm) and in 40%–50% of macroadenomas (>10 mm) (2, 3). In a patient who cannot be cured by surgery because of extensive cavernous sinus invasion, does not have chiasmal compression, or is a poor surgical candidate, primary medical therapy, usually with a somatostatin receptor ligand (octreotide or lanreotide), has been used in lieu of surgery (4). There is no conclusive evidence supporting which treatment modality is superior to the other in treatment-naïve patients of acromegaly.
We conducted a systematic review and meta-analysis to synthesize the existing evidence about the efficacy of first-line surgical and medical interventions in treatment-naïve patients with acromegaly.
Materials and Methods
The reporting of this systematic review complies with the statements from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (5). A priori protocol was developed by The Endocrine Society Committee on the Management of Patients With Acromegaly and specified the outcomes of interest, which included the following: 1) biochemical remission (defined by IGF-1 and GH criteria); and 2) the mean difference (change) between pre- and postinterventional IGF-1 or GH levels.
Study eligibility
We included original controlled and uncontrolled studies (prospective and retrospective) that enrolled patients with acromegaly and were recruited to receive either surgical treatment or medical treatment as their first-line treatment (treatment naïve). We restricted medical treatment to somatostatin receptor ligands (SRLs; octreotide or lanreotide) and a transsphenoidal surgery approach (TSS). The follow-up period was defined as 3 months or longer.
Literature search
A comprehensive literature search was conducted by an expert reference librarian with input from the study's principal investigator and Endocrine Society task force members. The search included Medline in-process and other nonindexed citations, Medline, EMBASE, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Scopus. We used a combination of controlled vocabulary and key words to search for patients with acromegaly who received any form of surgical treatment or medical treatments as shown in table 5. Reviewers, working independently and in duplicate, identified original studies eligible for further review by screening abstracts and titles. If a study was deemed relevant, the manuscript was obtained and reviewed in full-text version for further assessment. Any inclusion or exclusion disagreements were discussed and reconciled by a third investigator. Previously described data sources, including citing articles and relevant systematic reviews, were searched manually for possible studies, and duplicates were excluded. We expanded the search to include all languages, with last date of inclusion to be April 2014. Non-English articles were translated and assessed with the help of native or efficient speakers of the corresponding language used in the article.
Data extraction
Two reviewers independently extracted data from each study. We extracted data on patient demographics, baseline characteristics, study design variables, sample size, levels of IGF-1 and GH pre- and posttherapy levels, and remission rate, when reported. In addition, for each study, we extracted variables related to acromegaly in the relevant intervention group.
Risk of bias assessment
Because of the noncomparative nature of the available studies, we modified the Newcastle-Ottawa Scale (6) by removing the comparability criteria that is not applicable for our research question. Quality assessment focused on cohort selection, outcome ascertainment, and attrition. The untreated arms of interventional studies were considered as case series for quality assessment purposes.
Outcomes
Outcomes of interest were biochemical remission (calculated as rates), mean difference (change) between pre- and postinterventional IGF-1 and GH levels, and all-cause mortality, when reported. Primary biochemical end points were defined by a strict cutoff criteria of normal serum IGF-1 levels adjusted to age and gender and serum GH less than 1 μg/L. For the subgroup analysis, we included studies that ascertained remission by IGF-1-only criterion, defined by normal serum levels adjusted to age and gender. We also included studies that ascertained remission by random GH less than 2.5 μg/L or GH nadir after an oral glucose tolerance test (OGTT) less than 1 μg/L. Total remission rates were calculated based on reporting of patients achieving remission based on at least one biochemical remission criterion (GH or IGF-1). Surgeon experience compared if all surgeries reported in the study were performed by the same surgeon (one surgeon experience) vs more than one surgeon. Tumor size was defined as microadenoma (<10 mm) and macroadenoma (>10 mm).
Statistical analysis and data synthesis
For dichotomized outcomes, we calculated a cumulative incidence at a certain time point (event rate) and estimated the 95% confidence intervals (CI) using binomial distribution. We then pooled the log-transformed event rates using the DerSimonian and Laird random-effects models (7) with the heterogeneity estimated from the Mantel-Haenszel model. For continuous outcomes, we calculated the weighted difference in means between the baseline and the longest duration of follow-up for each study. We pooled effect size using the DerSimonian and Laird random-effect models. The Altman and Bland test (8) was used to compare the outcomes between medical and surgical interventions. We conducted subgroup analyses based on different remission criteria, the follow-up period, the surgeon's experience, and the type of adenoma tumor. To measure the overall heterogeneity across the included studies, we used I2 statistic, in which I2 greater than 50% suggests high heterogeneity. All statistical analyses were conducted using STATA version 12.1 (StataCorp LP).
Results
The initial search resulted in 1022 citations and after abstract review; this was limited to 187 potentially relevant articles. The full-text review results in 35 eligible studies enrolling 2629 patients, whereas 152 were excluded as shown in Figure 1. The weighted (overall) κ to evaluate concordance of independent reviewers was 0.78. The characteristics of included studies are summarized in Table 1. The risk of bias was very high, considering the noncomparative nature of the studies. Supplemental Table 1 demonstrates quality indicators of included studies. The included studies were 11 case series, 21 cohort (retrospective and prospective) studies, one nonrandomized study, and two randomized controlled trials. Only 18 studies reported remission rates: 11 surgical and seven medical studies.
Figure 1. Flow chart shows the literature search yield and selected studies.
Table 1.
Characteristics of the Included Studies
| Study Identification (Design) | Patients, n | Age, y (Mean) | Male, % | Location/Settings | Intervention, Patients, n | Follow-Up Period, mo | Inclusion Criteria |
|---|---|---|---|---|---|---|---|
| Karaca et al (12), 2011 (RCT) | 22 | 45 | 63.5 | Outpatient clinic of Endocrinology, at Erciyes University Medical School, Turkey | Oct-LAR | 12 | 1) Newly diagnosed patients with acromegaly. |
| Medical arm | N1 = 11 | ||||||
| Surgical arm | TSS N2 = 11 |
||||||
| Colao et al (13), 2009 (RCT) Medical arm |
81 | 48.2 | 49 | Multicenter study conducted at 46 neuroendocrinology clinics in seven countries (Australia, Belgium, Brazil, France, Germany, Italy, and the United Kingdom) | Oct-LAR | 12 | 1) Patients aged 18–80 y with newly diagnosed or previously untreated acromegaly. 2) Patients were required to have a biochemical diagnosis comprising both a lack of GH suppression to <1 μg/L after a 75-g oral glucose load and serum IGF-1 levels >97th percentile (adjusted for age and gender). |
| Surgical arm | TSS n = 41 |
||||||
| Luque-Ramirez et al (14), 2011 (case series) | 74 | 49 | 49 | Outpatient clinic of multiendocrinology center in Spain | Somatostatin analogs | 28 | 1) The patient was aged 18–80 y with acromegaly. |
| Dopamine agonists | 2) The patient had recently been diagnosed or had undergone surgery in the previous 6 mo with or without drug treatment any longer than 3 mo prior to surgery. | ||||||
| Medical arm | N1 = 18 | 3) There was a minimum follow-up of 20 mo. | |||||
| Surgical arm | TSS | 4) Informed consent. | |||||
| N2 = 56 | 5) The patient had not participated in any clinical trial or other studies with drugs. | ||||||
| Abe and Ludecke (15), 1999 (case series) | 78 | 47 | 42 | Hamburg University, Germany | Primary transnasal surgery n = 78 |
52 | 1) Consecutive acromegalic patients with intraoperative GH measurement who had not previously undergone surgery. |
| Gondim et al (16), 2010 (cohort) | 67 | 54 | 49 | Department of neurosurgery of the general hospital in Brazil | Endoscopic TSS n = 67 |
24 | 1) Clinical diagnosis compatible with acromegaly (GH > 1 mU/L, IGF-1 level > the normal age and sex adjusted level), presence of GH-secreting pituitary adenoma, tumor determined to be positive for GH marker through histological examination, no previous treatment, surgery performed by the senior author (J.A.G.), and at least 1 y of follow-up. |
| Salvatori et al (17), 2010 (cohort) | 59 | 53 | 49 | Multicenter study conducted on 13 endocrinology clinics in the United States | Somatostatin analog and dopamine drugs n = 59 |
6 | 1) Suitably motivated patients with a clinical diagnosis of acromegaly due to a pituitary tumor and age >18 y. Switch patients also had to have taken a constant dose of Oct-LAR for ≥3 mo, with serum IGF-1 no > 10% > normal. Switch patients had to have their last Oct-LAR injection 28–35 d prior to study enrollment. |
| Gondim et al (18), 2009 (cohort) | 33 | 44 | 27 | Outpatient clinic of the neuroendocrine department of the General Hospital of Fortaleza, Brazil | TSS n = 33 |
29 | 1) Patient with acromegaly (GH >1 μg/L , IGF-1 > normal age and sex matcher), compatible imaging with intrasellar tumor, no cavernous sinus invasion, positive immune reactivity of GH, no previous treatment, surgery at the HGF, and ≥1 y of follow-up. |
| Lombardi et al (19), 2009 (cohort) | 51 | 50 | 45 | Outpatient clinic in 24 centers in Italy | Somatostatin analogs Lanreotide-autogel 120 mg n = 39 |
13 | 1) Patients aged >18 y with active acromegaly (serum GH levels >5 μg/L and/or >1 μg/L after OGTI and abnormal IGF-1 values) who signed the written informed consent form. Patients should not have undergone pituitary surgery <3 mo before selection, somatostatin analogs (except for a presurgical treatment of <3 mo) or radiotherapy. |
| Kreutzer et al (20), 2001 (cohort) | 57 | 44 | 35 | Outpatient clinics at the neuroendocrine pituitary service at the University of Virginia Health Sciences Center, Charlottesville, Virginia | TSS n = 57 |
37.7 | 1) Patients with active acromegaly who received surgical intervention as initial treatment. |
| Arita et al (21), 2008 (cohort) | 9 | 74 | 56 | Outpatient clinic of Hiroshima and Kagoshima university hospitals | TSS n = 9 |
66 | 1) Patients with acromegaly who are >70 y old. |
| Sheaves (22), 1996 (case series) | 101 | 46 | 54 | Single center, St Bartholomew's Hospital, London, United Kingdom | TSS n = 101 |
45.6 | 1) Patients assessed preoperatively in the center according to a consistent protocol. |
| Colao (23), 2008 (case series) | 67 | 20–81 | 51 | Single center in Naples, Italy | Oct-LAR | 12 | 1) Patients with active acromegaly to somatostatin analogs as first-line therapy in all macroadenomas with extrasellar extension. |
| n = 67 | 2) Patients with proven cardiomyopathy, hypertension, sleep apnea or other respiratory disorders, and/or other systemic complications potentially making anesthesia less safe than in other patients. | ||||||
| 3) Written consent at the time of hospitalization agreeing with diagnostic procedures and treatment strategy. | |||||||
| Erturk et al (24), 2005 (case series) | 30 | 43 | NR | Multicenter, Department of Endocrinology Uludag University, School of Medicine, Bursa, Turkey | TSS | 42 | 1) Patients with clinical symptoms. |
| n = 30 | 2) Basal GH levels > 25 μg/L. 3) Patients had MRI showed intrasellar adenomas. |
||||||
| Grottoli et al (25), 2005 (case series) | 21 | 46 | 62 | Departments of Endocrinology of Multicenter (15 centers) in Italy (outpatient clinics) | Oct-LAR n = 21 |
12 | 1) Newly diagnosed patients with acromegaly. |
| Mangupli et al (26), 2003 (cohort) | 11 | 41 | 55 | Outpatient clinic of Caracas University Hospital, Caracas, Venezuela | Oct-LAR n = 11 |
12 | 1) Newly diagnosed acromegaly patients. |
| Lucas et al (27), 2003 (case series) | 104 | 48 | 40.4 | Departments of Endocrinology of Multicenter, Spain | Somatostatin analogs, lanreotide n = 104 |
6 | 1) Diagnosis of acromegaly was made using clinical criteria, with high serum GH levels not suppressible < 2 μg/L (4 mU/L) after an oral glucose test (75 g) and serum IGF-1 levels > 450 μg/L. |
| Beauregard et al (28), 2003 (cohort) | 103 | 42 | 58 | Outpatient clinic of the Norte Dame Hospital, Montréal, Canada | TSS | 12–360 | 1) Patients with acromegaly and received TSS during period of 1970–1999 as primary treatment. |
| n = 103 | 2) Patients with at least 1 y of follow-up. | ||||||
| 3) Operation was done by the same surgeon. | |||||||
| Bevan et al (29), 2002 (case series) | 27 | 53 | 62.96 | Multicenter, nine endocrine centers in the United Kingdom | Oct-LAR | 12 | 1) Patients with newly diagnosed acromegaly with biochemically active disease. |
| n = 27 | 2) Mean serum GH concentration > 5 mU/L (2 μg/L) that failed to suppress < 2 mU/L (0.8 μg/L ) after oral 75-g glucose administration. | ||||||
| 3) Visible adenoma on pituitary imaging. | |||||||
| 4) No patient had received previous surgery, radiotherapy, or medical therapy with somatostatin analogs or dopamine agonists. | |||||||
| Biermasz et al (30), 2000 (cohort) | 59 | 44 | 58 | Outpatient clinic for the Department of Endocrinology and Neurosurgery Leiden University, Leiden, The Netherlands | TSS n = 59 |
86 | 1) Acromegalic patients who underwent TSS by the same neurosurgeon during the study period. 2) Patient with no previous treatment. |
| Albarel et al (31), 2013 (case series) | 115 | 45.5 | Reported as ratio of women to men, 1.2/1 | Single center in Timone University Hospital, Marseille, France | TSS n = 115 |
120 | 1) Patients underwent sublabial or nasal TSS, with the exception of two who were operated on by the transfrontal route. |
| Giordano et al (32), 2012 (cohort) | 231 | NR | 35 | Single center University of Naples, Naples, Italy. | Lanreotide | 12 | 1) Untreated patients with acromegaly. |
| Medical arm | N1 = 151 | ||||||
| Surgical arm | TSS N2 = 80 |
||||||
| Ronchi et al (33), 2005 (cohort) | 146 | 57 | 42 | Department of Endocrinology, University of Milan, Milan, Italy | TSS | 228 | 1) Patient with acromegaly who underwent TSS between 1985 and 1996. |
| n = 40 | 2) Patient with record documented imaging studying with their records. 3) All patients who had been considered as in remission. |
||||||
| Bex et al (34), 2007 (case series) | 418 | NR | 51 | 64 endocrinologists from 37 different hospital settings in Belgium and GD-Luxembourg | Short- and long-acting somatostatin analogs, dopamine drugs, bromocriptine, and/or cabergoline | 132 | 1) Newly diagnosed patients with acromegaly who primarily received medical therapy. |
| n = 95 | |||||||
| Baldys-Waligorska et al (35), 2011 (cohort) | 26 | 58 | 15 | Department of Endocrinology, Jagiellonian University Hospital, Krakow, Poland | Oct-LAR | 12 | 1) The diagnosis of acromegaly was based on the Cortina criteria: IGF-1 concentration > normal range for age and gender, human GH nadir during OGTT >1.0 μg/L. Pituitary adenoma was verified by MRI. |
| N1 = 26 | 2) Written consent was obtained from each patient. | ||||||
| Colao et al (36), 2009 (cohort) | 45 | NR | 60 | Outpatient clinic of the Federico II University of Naples, Naples, Italy | Somatostatin analogs | 60 | 1) Patients with active acromegaly primarily treated with octreotide or lanreotide for 5 y. |
| n = 45 | 2) Written consent. | ||||||
| Gilbert et al (37), 2003 (case series)a | 39 | NR | NR | Outpatient clinic in London, United Kingdom | Oct-LAR n = 39 |
6 | 1) Newly diagnosed patients with acromegaly. 2) |
| Mercado et al (38), 2007 (cohort) | 68 | 50 | 41 | Outpatient clinic of 31centers in 15 countries | Somatostatin analogs | 12 | 1) Male or female patients aged 18–80 y with previously untreated acromegaly who provided their written informed consent were eligible to participate in the study. |
| n = 68 | 2) Biochemical diagnosis of acromegaly required both a lack of GH suppression to <1 μg/L after a 75-g oral glucose load and an elevated IGF-1 >97th percentile of the normal range adjusted for age and gender. | ||||||
| Cozzi et al (4), 2006 (cohort) | 67 | 55 | 46 | Outpatients clinic of Divisions of Endocrinology Ospedale Niguarda, Milan, Italy, and Division of Endocrinology, Ospedali Riuniti, Bergamo, Italy | Oct-LAR n = 67 |
48 | 1) Consecutive patients with active acromegaly, according to clinical picture, elevated GH levels not suppressible <1 μg/L after OGTT, and high age-adjusted IGF-1 levels, macroadenoma or invasive microadenoma at MRI scans, and no previous neurosurgery or radiotherapy. |
| Colao et al (39), 2006 (cohort) | 34 | 50 | 59 | Outpatient clinic of the Department of Clinical and Molecular Endocrinology and Oncology, University 'Federico II' of Naples, Naples, Italy | Somatostatin analogs n = 34 |
6 | 1) Patient's age ≥18 y. 2) Newly diagnosed acromegalic patients or those previously untreated. 3) Presence of a pituitary tumor (microadenoma or macroadenoma) on pituitary MRI within 8 wk before enrollment. 4) Lack of suppression of GH nadir to <1 μg/L after oral administration of 75 g of glucose. 5) IGF-1 levels > upper limits of normal. 6) Tolerance to a test dose of 50 μg sandostatin. |
| Luque-Ramirez (40), 2010 (cohort) | 19 | 52 | 45 | Outpatient clinic of multiendocrinology center in Spain | Oct-LAR n = 19 |
12 | 1) Age >18 y. 2) Newly diagnosed previously untreated acromegalic patients. 3) Presence of a pituitary tumor on pituitary MRI within 12 wk before enrollment. 4) Fail to suppress GH levels <1 μg/L after oral administration of 75 mg of glucose. 5) IGF-1 levels > upper limits of normality (ie, > 95th percentile adjusted for age). 6) Demonstrated tolerance and response to an acute test of Oct-LAR sc 7) Written informed consent of all patients to participate in this study and for the scientific use of their data. |
| Jenkins et al (41), 2004 (cohort) | 6 | 53 | NR | Outpatient clinic of the Department of Endocrinology at St Bartholomew's Hospital, London, United Kingdom | Oct-LAR n = 6 |
6 | 1) Patients newly diagnosed with acromegaly. 2) Patient who do not receive any previous treatment. |
| Newman et al (42), 1998 (NRT) | 26 | 50 | 38 | Outpatient clinic of the 14 participating centers, United States | Oct-LAR | NR | 1) Newly diagnosed patients with acromegaly. |
| Carlsen et al (43), 2011 (cohort) | 32 | 50 | 66 | Acromegalic patients in Norway diagnosed as part of POTA (Preoperative Oct-LAR Treatment of Acromegaly) study | Oct-LAR n = 32 |
6 | 1) Newly diagnosed, previously untreated patients with GH nadir >5 μg/L during a standard 75-g, 2-h OGTT. 2) Pituitary adenoma verified by a pituitary MRI scan. 3) Age between 18 and 80 y. |
| Biermasz et al (44), 2004 (cohort) | 164 | 46 | 55 | Outpatient clinic for the Department of Endocrinology and Neurosurgery, Leiden, The Netherlands | TSS n = 164 |
NR | 1) Patients newly diagnosed with acromegaly. 2) Patient who do not receive any previous treatment. |
| Ahmed et al (45) 1999 (cohort) | 139 | 45 | 49 | Single center, United Kingdom | TSS n = 139 |
67 | 1) Patients who agreed to receiving surgery as the first line of treatment. |
Abbreviations: MRI, magnetic resonance imaging; NR, nonrandomized; NRT, nonrandomized trial; Oct-LAR, octreotide long-acting release; RCT, randomized controlled trial; TSS, Transspehnoidal Surgery; GH, growth hormone; IGF-1, Insulin-like growth factor 1; Oct-LAR, Octreotide long-acting repeatable.
Abstract only.
Twenty-three studies included treatment-naïve patients who received medical treatments, whereas 22 studies included treatment-naïve patients who received surgical interventions. SRLs were mainly in the form of octreotide long-acting release (LAR) (15 studies) followed by lanreotide autogel depot (seven studies). The TSS approach was the most common surgical treatment (all surgical intervention studies). Only four studies were comparative studies; they compared treatment-naïve patients receiving medical or surgical interventions directly.
Meta-analysis
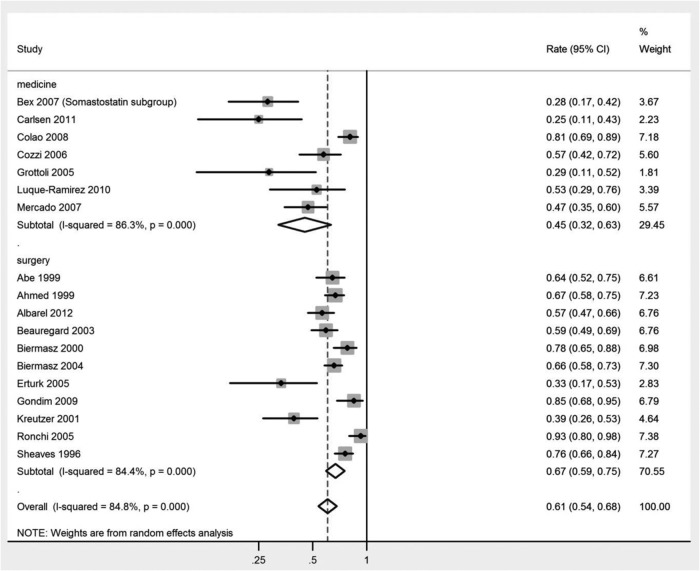
When compared with medical treatment, surgery was associated with higher remission rates at the longest follow-up for each study using the cutoff criterion-based remission (67% vs 45%; P = .02) (Table 2). Results of a meta-analysis of remission rates (reported as cumulative incidence) are shown in Figure 2. This trend continued while using the GH-based remission (65% vs 46%; P = .05) (Table 3), but remission using the IGF-1-only criterion showed no statistical difference (64% vs 45%; P = .06) (Table 4). Surgery had higher remission rates at longer follow-up periods (≥24 mo) (66% vs 44%; P = .04) but not at the shorter follow-up periods [≤6 mo, 53% vs 26% (P = .02) vs ≤24 mo, 57% vs 45% (P = .47)] (Table 2).
Table 2.
Meta-Analysis of Remission Rates as Compared Between Surgical and Medical Interventions
| Outcomes | Effect Sizea | 95% CI | I2, % | Heterogeneity P Value | P Value (Interaction) |
|---|---|---|---|---|---|
| Remission | |||||
| Medical interventions | 0.45 | (0.32, 0.63) | 86.3 | .000 | .02 |
| Surgical interventions | 0.67 | (0.59, 0.75) | 84.4 | .000 | |
| Remission FUT ≤6 mo | |||||
| Medical interventions | 0.26 | (0.16, 0.44) | 0 | .796 | .22 |
| Surgical interventions | 0.37 | (0.29, 0.48) | NA | NA | |
| Remission FUT <24 mo | |||||
| Medical interventions | 0.45 | (0.25, 0.81) | 84.8 | .000 | .47 |
| Surgical interventions | 0.57 | (0.43, 0.67) | 90.8 | .000 | |
| Remission FUT ≥24 mo | |||||
| Medical interventions | 0.44 | (0.31, 0.63) | 72.5 | .026 | .04 |
| Surgical interventions | 0.65 | (0.56, 0.76) | 86.7 | .003 | |
| Remission one-surgeon experienceb | |||||
| Yes | 0.71 | (0.64, 0.78) | 60.6 | .019 | .002 |
| No | 0.47 | (0.28, 0.77) | 68.3 | .076 |
Abbreviations: FUT, follow-up time; NA, not available.
Cumulative incidence (ie, event rate at end of follow-up period).
Surgical intervention studies only.
Figure 2. Meta-analysis of remission rates (reported as cumulative incidence) comparing medical and surgical interventions by the latest follow-up period of the included studies.
Table 3.
Meta-Analysis Results of GH-Defined Outcomes as Compared Between Surgical and Medical Interventions
| Outcomes | Effect Size | 95% CI | I2, % | Heterogeneity P Value | P Value (Interaction) |
|---|---|---|---|---|---|
| Total remissiona | |||||
| Medical Interventions | 0.45 | (0.32, 0.63) | 86.3 | .068 | .02 |
| Surgical Interventions | 0.67 | (0.59, 0.75) | 82.0 | .000 | |
| Remission by cutoff criteriaa | |||||
| Medical interventions | 0.46 | (0.33, 0.64) | 87.8 | .000 | .05 |
| Surgical interventions | 0.65 | (0.57, 0.75) | 85.7 | .000 | |
| GH levelsb | |||||
| Medical interventions | −24.02 | (−31.00, −17.05) | 92.1 | .000 | .78 |
| Surgical interventions | −21.84 | (−35.85, −7.83) | 90.3 | .000 | |
| GH levels FUT ≤24 mob | |||||
| Medical interventions | −24.27 | (−31.16, −17.38) | 90.2 | .000 | .97 |
| Surgical interventions | −24.49 | (−34.10, −14.88) | 77.1 | .004 | |
| GH levels FUT ≥24 monthsb | |||||
| Medical interventions | −19.61 | (−44.24, 5.02) | 95.6 | .000 | .16 |
| Surgical interventions | −38.58 | (−59.69, −38.28) | 70.4 | .034 | |
| Macroadenomac | −24.92 | (−34.09, −15.75) | 54.7 | .085 | |
| GH levels macroadenomab | |||||
| Medical interventions | −21.93 | (−30.55, −13.31) | 42.8 | .174 | .09 |
| Surgical interventions | −37.54 | (−53.83, −21.25) | 0.0 | .000 | |
| GH levels tumor sizeb | |||||
| Microadenomac | −5.85 | (−10.75, −0.95) | 48.5 | .163 | .003 |
| Macroadenomac | −24.92 | (−34.09, −15.75) | 54.7 | .085 |
Abbreviation: FUT, follow-up time.
Cumulative incidence (ie, event rate at end of follow-up period).
Weighted mean difference (micrograms per liter).
Medical interventions only.
Table 4.
Meta-Analysis of IGF-1-Defined Outcomes as Compared Between Surgical and Medical Interventions
| Outcomes | Effect Size | 95% CI | I2, % | Heterogeneity P Value | P Value (Interaction) |
|---|---|---|---|---|---|
| Remission by cutoff criteriaa | |||||
| Medical interventions | 0.45 | (0.32, 0.63) | 86.3 | .000 | .14 |
| Surgical interventions | 0.64 | (0.54, 0.76) | 83.9 | .000 | |
| Remission by IGF-1 FUT ≤24 moa | |||||
| Medical interventions | 0.45 | (0.25, 0.81) | 84.8 | .000 | .49 |
| Surgical interventions | 0.58 | (0.38, 0.88) | 93.3 | .000 | |
| FUT ≥24 moa | |||||
| Medical interventions | 0.44 | (0.31, 0.63) | 72.5 | .026 | .24 |
| Surgical interventions | 0.56 | (0.45, 0.68) | 81.4 | .000 | |
| IGF-1 levelsb | |||||
| Medical interventions | −329.72 | (−496.73, 162.71) | 76.4 | .000 | .14 |
| Surgical interventions | −549.17 | (−791.67, −306.67) | 38.8 | .195 | |
| IGF-I levels FUT ≤24 mob | |||||
| Medical interventions | −355.34 | (−457.54, −253.15) | 0.0 | .866 | .65 |
| Surgical interventions | −412.06 | (−637.14, −186.98) | 0.0 | .661 | |
| IGF-1 levels FUT ≥24 mob | |||||
| Medical interventions | −251 | (−627.30, 125.26) | 85.9 | .001 | .04 |
| Surgical interventions | −731 | (−1007.23, −454.77) | NA | NA | |
| Tumor sizeb | |||||
| Microadenomac | −311.97 | (−571.66, −52.28) | 0.0 | .816 | .80 |
| Macroadenomac | −280.38 | (−456.44, −104.32) | 0.0 | .893 |
Abbreviations: FUT, follow-up time; NA, not applicable (not possible to calculate heterogeneity because the result was based on a single study).
Cumulative incidence.
Weighted mean difference (micrograms per liter).
Medical interventions only.
At the longest follow-up, there was no statistical difference between the surgical vs medical groups when comparing the mean difference (decline from baseline levels) of GH levels (−21.84 μg/L vs −24.02 μg/L; P = .78) or IGF-1 levels (−549.17 μg/L vs −329.72 μg/L; P = .14) as shown in Tables 3 and 4, respectively. This trend changed only when comparing the mean difference of IGF-1 levels in studies with a longer follow-up period (≥24 m) in which surgery was associated with more decline of IGF-1 levels (−731 vs −251; P = .04).
In the surgery interventions studies, one-surgeon experience, in which all surgeries performed by the same surgeon in the study, was associated with higher remission when compared with studies that had more than one surgeon performing the surgeries (71% vs 47%; P = .002) (Table 3). Additionally, surgically treated macroadenoma tumors showed a higher decline of GH levels from baseline levels when compared with microadenoma tumors (−21.93 vs −5.85; P = .003) (Table 3), although the baseline measurements of GH for macroadenoma were generally greater than those of microadenoma. Subgroup analysis results are included in the Supplemental Figures.
Discussion
We conducted a systematic review and meta-analysis to compare surgical and medical interventions in treatment-naïve patients with acromegaly (Table 5). Surgery was associated with a higher remission rate, especially in longer-term studies and when using the GH-based remission. Studies in which surgery was performed by a single surgeon reported a higher remission rate than those with multiple surgeons. Based on a single surgical series, the serum IGF-1 level was reduced more with surgery compared with medical treatment, but there was no statistical difference in the follow-up levels of GH.
Table 5.
Search Strategy
| n | Searches | Results |
|---|---|---|
| 1 | Exp acromegaly/su | 1716 |
| 2 | Exp acromegaly/ | 13 678 |
| 3 | (Acromegaly or somatotropin hypersecretion syndrome* or inappropriate GH secretion syndrome* or inappropriate GH secretion syndrome* or acromegalia or acromegalism or akromegalia or megalakria).mp [mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, kf, ps, rs, ui, tx, ct] | 15 672 |
| 4 | Exp surgery | 2 532 239 |
| 5 | (Surgery or surgical or operation* or operative or resection*).mp [mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, kf, ps, rs, ui, tx, ct] | 3 678 391 |
| 6 | (2 or 3) and (4 or 5) | 4287 |
| 7 | 1 or 6 | 4678 |
| 8 | Exp acromegaly/dm, dt, th (disease management, drug therapy, therapy) | 2217 |
| 9 | Exp acromegaly/dh, dt, th (diet therapy, drug therapy, therapy) | 2055 |
| 10 | Exp acromegaly | 13 678 |
| 11 | (Acromegaly or somatotropin hypersecretion syndrome* or inappropriate GH secretion syndrome* or inappropriate GH secretion syndrome* or acromegalia or acromegalism or akromegalia or megalakria).mp (mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, kf, ps, rs, ui, tx, ct) | 15 672 |
| 12 | Exp somatostatin | 34 699 |
| 13 | Exp therapy | 4 294 400 |
| 14 | Exp octreotide | 21 850 |
| 15 | Exp angiopeptin | 2263 |
| 16 | Exp cabergoline | 3791 |
| 17 | Exp dopamine receptor stimulating agent | 116 840 |
| 18 | Exp dopamine agents | 320 175 |
| 19 | (Medical therapy* or medical treatment* or medical management or diet or dietary or drug or drugs or somatotropin or somatostatin or stilamin or SRIH or somatofalk or aminopan or GH release inhibiting factor* or modustatine or somatotropic or somiaton or SRIF or stylamin or octreotide or sandoz or longastatin* or oncolar or samilstin or sandostatin* or sandstatin or somatuline or lanreotide or angiopeptin or somatulin or cabergoline or cabaser or cabaseril or dostinex or dopamine or dopaminergic).mp (mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, kf, ps, rs, ui, tx, ct) | 7 461 359 |
| 20 | or/12–19 | 10 236 092 |
| 21 | (10 or 11) and 20 | 7567 |
| 22 | 8 or 9 or 21 | 8133 |
| 23 | 7 and 22 | 3158 |
| 24 | (Naive or unselected or not selected or de novo or denovo).mp (mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, kf, ps, rs, ui, tx, ct) | 270 024 |
| 25 | 7 and 24 | 90 |
| 26 | 22 and 24 | 163 |
| 27 | 23 or 25 or 26 | 3251 |
| 28 | Exp controlled study | 4 081 640 |
| 29 | exp randomized controlled trial | 669 451 |
| 30 | [(Control$ or randomized) adjusted2 (study or studies or trial or trials)].mp. [mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, kf, ps, rs, ui, tx, ct) | 5 219 885 |
| 31 | Meta-analysis | 109 105 |
| 32 | Meta-analysis$.mp. | 176 889 |
| 33 | Exp systematic review | 58 731 |
| 34 | (Systematic* adjusted review$).mp. | 136 457 |
| 35 | Exp cohort studies | 1 483 167 |
| 36 | Exp longitudinal study | 956 895 |
| 37 | Exp retrospective study | 749 805 |
| 38 | Exp prospective study | 619 427 |
| 39 | Exp comparative study | 2 366 329 |
| 40 | Exp clinical trial | 1 626 337 |
| 41 | Exp cross-sectional study | 246 723 |
| 42 | Crossover procedure | 36 528 |
| 43 | Exp crossover studies | 91 225 |
| 44 | [(Clinical or comparative or cohort or longitudinal or retrospective or prospective or concurrent or cross-sectional or crossover) adjusted (study or studies or survey or surveys or analysis or analyses or trial or trials)].mp | 6 757 151 |
| 45 | (Crossover procedure).mp (mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, kf, ps, rs, ui, tx, ct) | 40 396 |
| 46 | Or/28–45 | 1 039 9278 |
| 47 | 27 and 46 | 1443 |
| 48 | Limit 27 to (clinical trial, all or clinical trial, phase I or clinical trial, phase II or clinical trial, phase III or clinical trial, phase IV or clinical trial or comparative study or controlled clinical trial or evaluation studies or meta-analysis or multicenter study or randomized controlled trial or systematic reviews or validation studies) (limit not valid in Embase, CCTR, CDSR; records were retained) | 567 |
| 49 | 47 or 48 | 1474 |
| 50 | Limit 49 to y = 1990 to current | 1417 |
| 51 | Limit 50 to [all adult (19 + y) or young adult (19–24 y) or adult (19–44 y) or young adult and adult (19–24 and 19–44 y) or middle aged (45–64 y) or middle aged (45 + y) or all aged (65 y and older) or aged (80 y and older)] (limit not valid in Embase, CCTR, CDSR; records were retained) | 1344 |
| 52 | Limit 51 to (book or book series or editorial or erratum or letter or note or addresses or autobiography or bibliography or biography or comment or dictionary or directory or interactive tutorial or interview or lectures or legal cases or legislation or news or newspaper article or overall or patient education handout or periodical index or portraits or published erratum or video-audio media or webcasts) [limit not valid in Embase, Ovid Medline(R), Ovid Medline(R) In-Process, CCTR, CDSR; records were retained] | 32 |
| 53 | 51, not 52 | 1312 |
| 54 | From 27 keep 3215–3251 | 37 |
| 55 | 53 or 54 | 1318 |
| 56 | Remove duplicates from 55 | 1012 |
Ovid database(s): Embase 1988–2013 week 14; Ovid Medline(R) in-process and other nonindexed citations and ovid Medline(R), 1946 to present; EBM Reviews include Cochrane Central Register of Controlled Trials, April 2014; EBM Reviews include Cochrane Database of Systematic Reviews, 2005 to February 2013. Scopus includes the following: 1) title-ABS-key (acromegaly or somatotropin hypersecretion syndrome* or inappropriate GH secretion syndrome* or inappropriate GH secretion syndrome* or acromegalia or acromegalism or akromegalia or megalakria) and publication year after 1989; 2) title-ABS-key (surgery or surgical or operation* or operative or resection*); 3) title-ABS-key (medical therapy* or medical treatment* or medical management or diet or dietary or drug or drugs or somatotropin or somatostatin or stilamin or SRIH or somatofalk or aminopan or GHRIH or GH release-inhibiting factor* or modustatine or somatotropic or somiaton or SRIF or stylamin or octreotide or sandoz or longastatin* or oncolar or samilstin or sandostatin* or sandstatin or somatuline or lanreotide or angiopeptin or somatulin or cabergoline or cabaser or cabaseril or dostinex or dopamine or dopaminergic); 4) title-ABS-key [(meta-W/1 analysis*) or (systematic* W/2 review*) or (control* W/2 study*) or (control* W/2 trial*) or (randomized W/2 study*) or (randomized W/2 trial*) or comparative study* or comparative survey* or comparative analysis* or cohort study* or cohort survey* or cohort analysis* or longitudinal study* or longitudinal survey* or longitudinal analysis* or retrospective study* or retrospective survey* or retrospective analysis* or prospective study* or prospective survey* or prospective analysis* or concurrent study* or concurrent survey* or concurrent analysis* or clinical study* or clinical trial* or cross-sectional study* or cross-sectional analysis* or crossover study* or crossover analysis* or crossover procedure or crossover study* or crossover analysis* or crossover procedure]; 5) 1 and 2 and 3 and 4; 6) title-ABS-key (naive or unselected or not selected or de novo or denovo); 7) (1 and 2 and 6) or (1 and 3 and 6); 8) 5 or 7; 9) PMID(0*) or PMID(1*) or PMID(2*) or PMID(3*) or PMID(4*) or PMID(5*) or PMID(6*) or PMID(7*) or PMID(8*) or PMID(9*); 10) 8 and not 9; 11) document type(le) or document type(ed) or document type(bk) or document type(er) or document type(no) or document type(sh); and 12) 10 and not 11.
Surgery is generally considered the primary therapy for acromegaly because it can lead to immediate biochemical remission, decompression of local structures, and pathological analysis (1). We found that institutions using one surgeon had improved outcomes compared with sites with multiple surgeons. This confirms an earlier study showing that individual surgeon volume correlates with improved surgical outcomes (9). Therefore, our findings support the finding that surgical experience is key in patients with pituitary tumors. Primary medical therapy in lieu of surgery has been considered in patients with acromegaly, particularly in those subjects in whom surgical cure is unlikely. SRLs are the main form of primary medical therapy, and administration of SRLs to patients with acromegaly has been associated with biochemical control in up to 70% of subjects (4). In addition, SRL administration can result in at least 50% tumor shrinkage in up to 60% of subjects and correlates with biochemical response (10). In a recent study, approximately two-thirds of subjects achieved significant tumor shrinkage over 48 weeks with a SRL (11). These data support a role for primary medical therapy with a SRL in selected patients with acromegaly.
There are no head-to-head studies that assess chronic primary SRL administration vs initial surgery in the approach to a patient with acromegaly. In the current study, we showed that surgery resulted in a higher rate of biochemical remission when using either safe GH or normal IGF-1 levels as end points. Therefore, our study shows that surgery likely does result in a higher proportion of biochemical control rates, especially with long-term treatment. Of note, this finding was not present when IGF-1 alone was used as the criterion for biochemical control. Our meta-analysis revealed IGF-1 control rates after surgery of 66%, which is higher than generally reported in the literature when including both macroadenomas and microadenomas (2, 3). Nevertheless, when a surgical approach is unlikely to be completely effective in normalizing GH/IGF-1 concentrations, our data support a role for primary medical therapy. Indeed, the control rates of medical therapy, particularly when using IGF-1 criteria, are similar to those of surgery.
The main limitation of this systematic review is the noncomparative nature of the included studies, which weakens the evidence based on the results drawn. However, the strengths of this study relate to the rigorous methodological approach: attempts to reduce heterogeneity by stratifying analysis based on remission criteria, length of follow-up, and other variables; the expansive and comprehensive literature search, the inclusion of all publication languages, and selecting studies in duplicate.
In conclusion, surgery was associated with better remission and lower follow-up GH and IGF-1; however, the confidence in such evidence is very low, mainly due to the noncomparative nature of the studies, high heterogeneity, and imprecision.
Acknowledgments
Disclosure Summary: L.K. has financial or business/organizational interests in Novartis, Roche, and Pfizer. The other authors have nothing to declare.
Footnotes
- CI
- confidence interval
- LAR
- long-acting release
- OGTT
- oral glucose tolerance test
- SRL
- somatostatin receptor ligand
- TSS
- transsphenoidal surgery.
References
- 1. Katznelson L, Atkinson JL, Cook DM, et al. . American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of acromegaly—2011 update. Endocr Pract. 2011;4(suppl 17):1–44. [DOI] [PubMed] [Google Scholar]
- 2. Jane JA Jr, Starke RM, Elzoghby MA, et al. . Endoscopic transsphenoidal surgery for acromegaly: remission using modern criteria, complications, and predictors of outcome. J Clin Endocrinol Metab. 2011;96:2732–2740. [DOI] [PubMed] [Google Scholar]
- 3. Starke RM, Raper DM, Payne SC, Vance ML, Oldfield EH, Jane JA Jr. Endoscopic vs microsurgical transsphenoidal surgery for acromegaly: outcomes in a concurrent series of patients using modern criteria for remission. J Clin Endocrinol Metab. 2013;98:3190–3198. [DOI] [PubMed] [Google Scholar]
- 4. Cozzi R, Montini M, Attanasio R, et al. . Primary treatment of acromegaly with octreotide LAR: a long-term (up to nine years) prospective study of its efficacy in the control of disease activity and tumor shrinkage. J Clin Endocrinol Metab. 2006;91:1397–1403. [DOI] [PubMed] [Google Scholar]
- 5. Liberati A, Altman DG, Tetzlaff J, et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wells G, Shay B, O'Connell D., et al. . The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. In: Ottawa, 2012; http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 7. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 8. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 9. Barker FG 2nd, Klibanski A, Swearingen B. Transsphenoidal surgery for pituitary tumors in the United States, 1996–2000: mortality, morbidity, and the effects of hospital and surgeon volume. J Clin Endocrinol Metab. 2003;88:4709–4719. [DOI] [PubMed] [Google Scholar]
- 10. Colao A, Pivonello R, Auriemma RS, et al. . Predictors of tumor shrinkage after primary therapy with somatostatin analogs in acromegaly: a prospective study in 99 patients. J Clin Endocrinol Metab. 2006;91:2112–2118. [DOI] [PubMed] [Google Scholar]
- 11. Caron PJ, Bevan JS, Petersenn S, et al. . Tumor shrinkage with lanreotide Autogel 120 mg as primary therapy in acromegaly: results of a prospective multicenter clinical trial. J Clin Endocrinol Metab. 2014;99:1282–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karaca Z, Tanriverdi F, Elbuken G, et al. . Comparison of primary octreotide-LAR and surgical treatment in newly diagnosed patients with acromegaly. Clin Endocrinol (Oxf). 2011;75:678–684. [DOI] [PubMed] [Google Scholar]
- 13. Colao A, Cappabianca P, Caron P, et al. . Octreotide LAR vs. surgery in newly diagnosed patients with acromegaly: a randomized, open-label, multicentre study. Clin Endocrinol (Oxf). 2009;70:757–768. [DOI] [PubMed] [Google Scholar]
- 14. Luque-Ramirez M, Carreno A, Alvarez Escola C, et al. . The OASIS study: therapeutic management of acromegaly in standard clinical practice. Assessment of the efficacy of various treatment strategies. Endocrinology. 2011;58:478–486. [DOI] [PubMed] [Google Scholar]
- 15. Abe T, Ludecke DK. Recent primary transnasal surgical outcomes associated with intraoperative growth hormone measurement in acromegaly. Clin Endocrinol (Oxf). 1999;50(1):27–35. [DOI] [PubMed] [Google Scholar]
- 16. Gondim JA, Almeida JP, de Albuquerque LAF, Gomes E, Schops M, Ferraz T. Pure endoscopic transsphenoidal surgery for treatment of acromegaly: results of 67 cases treated in a pituitary center. Neurosurgery. 2010;29:E7. [DOI] [PubMed] [Google Scholar]
- 17. Salvatori R, Nachtigall LB, Cook DM, et al. . Effectiveness of self- or partner-administration of an extended-release aqueous-gel formulation of lanreotide in lanreotide-naive patients with acromegaly. Pituitary. 2010;13:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gondim JA, Ferraz T, Mota I, et al. . Outcome of surgical intrasellar growth hormone tumor performed by a pituitary specialist surgeon in a developing country. Surg Neurol. 2009;72:15–19; discussion 19. [DOI] [PubMed] [Google Scholar]
- 19. Lombardi G, Minuto F, Tamburrano G, et al. . Efficacy of the new long-acting formulation of lanreotide (lanreotide Autogel) in somatostatin analogue-naive patients with acromegaly. J Endocrinol Invest. 2009;32:202–209. [DOI] [PubMed] [Google Scholar]
- 20. Kreutzer J, Vance ML, Lopes MB, Laws ER Jr. Surgical management of GH-secreting pituitary adenomas: an outcome study using modern remission criteria. J Clin Endocrinol Metab. 2001;86:4072–4077. [DOI] [PubMed] [Google Scholar]
- 21. Arita K, Hirano H, Yunoue S, et al. . Treatment of elderly acromegalics. Endocr J. 2008;55:895–903. [DOI] [PubMed] [Google Scholar]
- 22. Sheaves R, Jenkins P, Blackburn P, et al. . Outcome of transsphenoidal surgery for acromegaly using strict criteria for surgical cure. Clin Endocrinol (Oxf). 1996;45:407–413. [DOI] [PubMed] [Google Scholar]
- 23. Colao A, Pivonello R, Auriemma RS, et al. . Growth hormone-secreting tumor shrinkage after 3 months of octreotide-long-acting release therapy predicts the response at 12 months. J Clin Endocrinol Metab. [Erratum (2008) 93(10):4162] 2008;93:3436–3442. [DOI] [PubMed] [Google Scholar]
- 24. Erturk E, Tuncel E, Kiyici S, Ersoy C, Duran C, Imamoglu S. Outcome of surgery for acromegaly performed by different surgeons: importance of surgical experience. Pituitary. 2005;8:93–97. [DOI] [PubMed] [Google Scholar]
- 25. Grottoli S, Celleno R, Gasco V, et al. . Efficacy and safety of 48 weeks of treatment with octreotide LAR in newly diagnosed acromegalic patients with macroadenomas: an open-label, multicenter, non-comparative study. J Endocrinol Invest. 2005;28:978–983. [DOI] [PubMed] [Google Scholar]
- 26. Mangupli R, Lisette A, Ivett C, Paul C, de los Rios Victoria C, Luis CJ. Improvement of acromegaly after octreotide LAR treatment. Pituitary. 2003;6:29–34. [DOI] [PubMed] [Google Scholar]
- 27. Lucas T, Astorga R, Catala M, Spanish Multicentre Lanreotide Study Group on Acromegaly. Preoperative lanreotide treatment for GH-secreting pituitary adenomas: effect on tumour volume and predictive factors of significant tumour shrinkage. Clin Endocrinol (Oxf). 2003;58:471–481. [DOI] [PubMed] [Google Scholar]
- 28. Beauregard C, Truong U, Hardy J, Serri O. Long-term outcome and mortality after transsphenoidal adenomectomy for acromegaly. Clin Endocrinol (Oxf). 2003;58:86–91. [DOI] [PubMed] [Google Scholar]
- 29. Bevan JS, Atkin SL, Atkinson AB, et al. . Primary medical therapy for acromegaly: an open, prospective, multicenter study of the effects of subcutaneous and intramuscular slow-release octreotide on growth hormone, insulin-like growth factor-I, and tumor size. J Clin Endocrinol Metab. 2002;87:4554–4563. [DOI] [PubMed] [Google Scholar]
- 30. Biermasz NR, van Dulken H, Roelfsema F. Ten-year follow-up results of transsphenoidal microsurgery in acromegaly. J Clin Endocrinol Metab. 2000;85:4596–4602. [DOI] [PubMed] [Google Scholar]
- 31. Albarel F, Castinetti F, Morange I, et al. . Outcome of multimodal therapy in operated acromegalic patients, a study in 115 patients. Clin Endocrinol (Oxf). 2013;78(2):263–270. [DOI] [PubMed] [Google Scholar]
- 32. Giordano C, Ciresi A, Amato MC, et al. . Clinical and metabolic effects of first-line treatment with somatostatin analogues or surgery in acromegaly: a retrospective and comparative study. Pituitary. 2012;15(4):539–551. [DOI] [PubMed] [Google Scholar]
- 33. Ronchi CL, Varca V, Giavoli C, et al. . Long-term evaluation of postoperative acromegalic patients in remission with previous and newly proposed criteria. J Clin Endocrinol Metab. 2005;90(3):1377–1382. [DOI] [PubMed] [Google Scholar]
- 34. Bex M, Abs R, T'Sjoen G, et al. . AcroBel—the Belgian registry on acromegaly: a survey of the ‘real-life’ outcome in 418 acromegalic subjects. Eur J Endocrinol. 2007;157(4):399–409. [DOI] [PubMed] [Google Scholar]
- 35. Baldys-Waligorska A, Krzentowska-Korek A, Golkowski F, Sokolowski G, Hubalewska-Dydejczyk A. Octreotide lar affects the volume of pituitary adenoma in acromegalic patients. Exp Clin Endocrinol Diabetes. 2011;119:295–299. [DOI] [PubMed] [Google Scholar]
- 36. Colao A, Auriemma RS, Galdiero M, Lombardi G, Pivonello R. Effects of initial therapy for five years with somatostatin analogs for acromegaly on growth hormone and insulin-like growth factor-I levels, tumor shrinkage, and cardiovascular disease: a prospective study. J Clin Endocrinol Metab. 2009;94:3746–3756. [DOI] [PubMed] [Google Scholar]
- 37. Gilbert J, Ketchen M, Kane P, et al. . The treatment of de novo acromegalic patients with octreotide-LAR: efficacy, tolerability and cardiovascular effects. Pituitary. 2003;6:11–18. [DOI] [PubMed] [Google Scholar]
- 38. Mercado M, Borges F, Bouterfa H, et al. . A prospective, multicentre study to investigate the efficacy, safety and tolerability of octreotide LAR (long-acting repeatable octreotide) in the primary therapy of patients with acromegaly. Clin Endocrinol (Oxf). 2007;66:859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Colao A, Pivonello R, Rosato F, et al. . First-line octreotide-LAR therapy induces tumour shrinkage and controls hormone excess in patients with acromegaly: results from an open, prospective, multicentre trial. Clin Endocrinol (Oxf). 2006;64:342–351. [DOI] [PubMed] [Google Scholar]
- 40. Luque-Ramirez M, Portoles GR, Varela C, et al. . The efficacy of octreotide LAR as first-line therapy for patients with newly diagnosed acromegaly is independent of tumor extension: predictive factors of tumor and biochemical response. Horm Metab Res. 2010;42:38–44. [DOI] [PubMed] [Google Scholar]
- 41. Jenkins PJ, Emery M, Howling SJ, Evanson J, Besser GM, Monson JP. Predicting therapeutic response and degree of pituitary tumour shrinkage during treatment of acromegaly with octreotide LAR. Horm Res. 2004;62:227–232. [DOI] [PubMed] [Google Scholar]
- 42. Newman CB, Melmed S, George A, et al. . Octreotide as primary therapy for acromegaly. J Clin Endocrinol Metab. 1998;83:3034–3040. [DOI] [PubMed] [Google Scholar]
- 43. Carlsen SM, Svartberg J, Schreiner T, et al. . Six-month preoperative octreotide treatment in unselected, de novo patients with acromegaly: effect on biochemistry, tumour volume, and postoperative cure. Clin Endocrinol (Oxf). 2011;74:736–743. [DOI] [PubMed] [Google Scholar]
- 44. Biermasz NR, Dekker FW, Pereira AM, et al. . Determinants of survival in treated acromegaly in a single center: predictive value of serial insulin-like growth factor I measurements. J Clin Endocrinol Metab. 2004;89(6):2789–2796. [DOI] [PubMed] [Google Scholar]
- 45. Ahmed S, Elsheikh M, Stratton IM, Page RCL, Adams CBT, Wass JAH. Outcome of transphenoidal surgery for acromegaly and its relationship to surgical experience. Clin Endocrinol (Oxf). 1999;50(5):561–567. [DOI] [PubMed] [Google Scholar]