Abstract
Context:
Cancer stem cells (CSCs) have been recently identified in thyroid neoplasm. Anaplastic thyroid cancer (ATC) contains a higher percentage of CSCs than well-differentiated thyroid cancer. The signaling pathways and the transcription factors that regulate thyroid CSC self-renewal remain poorly understood.
Objective:
The objective of this study is to use two ATC cell lines (KAT-18 and SW1736) as a model to study the role of the sonic hedgehog (Shh) pathway in maintaining thyroid CSC self-renewal and to understand its underlying molecular mechanisms.
Design:
The expression and activity of aldehyde dehydrogenase (ALDH), a marker for thyroid CSCs, was analyzed by Western blot and ALDEFLUOR assay, respectively. The effect of three Shh pathway inhibitors (cyclopamine, HhAntag, GANT61), Shh, Gli1, Snail knockdown, and Gli1 overexpression on thyroid CSC self-renewal was analyzed by ALDEFLUOR assay and thyrosphere formation. The sensitivity of transfected KAT-18 cells to radiation was evaluated by a colony survival assay.
Results:
Western blot analysis revealed that ALDH protein levels in five thyroid cancer cell lines (WRO82, a follicular thyroid cancer cell line; BCPAP and TPC1, two papillary thyroid cancer cell lines; KAT-18 and SW1736, two ATC cell lines) correlated with the percentage of the ALDHHigh cells as well as Gli1 and Snail expression. The Shh pathway inhibitors, Shh and Gli1 knockdown, in KAT-18 cells decreased thyroid CSC self-renewal and increased radiation sensitivity. In contrast, Gli1 overexpression led to increased thyrosphere formation, an increased percentage of ALDHHigh cells, and increased radiation resistance in KAT-18 cells. Inhibition of the Shh pathway by three specific inhibitors led to decreased Snail expression and a decreased number of ALDHHigh cells in KAT-18 and SW1736. Snail gene knockdown decreased the number of ALDHHigh cells in KAT-18 and SW1736 cells.
Conclusions:
The Shh pathway promotes the CSC self-renewal in ATC cell lines by Gli1-induced Snail expression.
The hedgehog pathway is regulated by three ligands: sonic hedgehog (Shh), Indian HH, and Desert HH. In the absence of these ligands, the Shh pathway is inactive because the transmembrane receptor, Patched (Ptch), functions as a tumor suppressor to prevent Smoothened (Smo), a G protein-coupled receptor (1–3), from activating a family of oncogenic Gli transcription factors. Hedgehog binding to Ptch leads to the uncoupling of Ptch from Smo, subsequently leading to the activation of a signal cascade and the translocation of Gli into the nucleus to induce or repress gene expression (1–3). Accumulating evidence suggests that the Shh pathway regulates cancer stem cells (CSCs) (4), which are functionally defined by their capacity to undergo self-renewal and give rise to differentiated progeny that recapitulates the original tumor in an ectopic setting. Loss of Shh signaling by genetically disrupting Smo resulted in the inhibition of BCR-ABL-expressing leukemic stem cells and prolonged their survival (5, 6). In glioblastoma CSCs, inhibition with cyclopamine or small interfering RNA (siRNA) directed against pathway components results in the loss of tumorigenic potential (7, 8). Thus, the Shh pathway may dictate the fate of CSCs, including self-renewal and differentiation by generation of a malignant niche (4).
Thyroid cancer is the most common malignancy of the endocrine system (9). Surgery, thyroid hormone replacement, and radioiodine therapy are effective for treating most well-differentiated thyroid cancers but are less effective for poorly differentiated thyroid cancers. In addition, approximately 15–20% of patients with differentiated thyroid cancer relapse in their lifetime. The undifferentiated anaplastic subtype of thyroid carcinoma is almost always fatal, with a mean survival of 2–6 months. A novel therapeutic strategy is needed for preventing thyroid cancer recurrence and treating poorly differentiated and anaplastic thyroid cancer (ATC). A recent study by Todaro et al (10) has identified thyroid CSCs as a unique population (1–3%) with highly invasive and metastatic behavior (10). Poorly differentiated or undifferentiated thyroid cancers contain a higher percentage of ALDH (aldehyde dehydrogenase)-positive CSCs than benign adenomas and well-differentiated thyroid cancers. AKT and c-Met are highly activated in thyroid CSCs (10). Carina et al (11) reported that CSC-related genes, SOX2, SOX4, NANOG, c-MYC, and ABCG, are highly expressed in ATC. A more recent study by Ma et al (12) showed that the stage-specific embryonic antigen 1 (SSEA-1), a marker of progenitor cells, is present in a small portion of cells with the characteristics of thyroid CSCs in several human thyroid cancer lines. Our recent study demonstrated that the Shh pathway is widely activated in thyroid neoplasms and can promote thyroid tumor cell proliferation (13). Here we report that the Shh pathway promotes thyroid CSC self-renewal in two ATC cell lines by inducing Snail expression and renders KAT-18 ATC cells resistant to radiation killing.
Materials and Methods
Cell lines and plasmid DNA
Five thyroid tumor cell lines used in this study are: WRO82 (BRAF and TP53 mutations), a follicular thyroid carcinoma cell line (provided by Dr Guy J. F. Juillard (University of California at Los Angeles); TPC1 (RET/PTC1 and RAS mutations) and BCPAP (BRAF and TP53 mutations), two papillary thyroid carcinoma (PTC) cell lines; and KAT-18 and SW1736 (BRAF mutation), two ATC cell lines (kindly provided by Dr Kenneth B. Ain, University of Kentucky Medical Center, Lexington, KY) (14). All tumor cell lines were cultured in complete RPMI 1640 medium containing 10% fetal bovine serum. Most experiments were conducted in KAT-18 cells because this anaplastic thyroid tumor cell line lacks BRAF, RAS, RET/PTC1, and TP53 gene mutations (15, 16) and is more sensitive to the inhibitors of the Shh pathway on cell proliferation (13). KAT-18 cells were stably transfected with pcDNA6.2/GW-miR constructs encoding Shh and Gli1 hairpin DNA oligonucleotides according to our prior publication (13). Transfected cells were selected in complete RPMI 1640 medium containing 5 μg/mL blasticidin. pGL3/Gli-Luc is a luciferase reporter gene encoding an eight-copy Gli1 binding site-driven luciferase reporter gene (8 × 3′Gli-BS-Luc) originally constructed by Dr Hiroshi Sasaki (Center for Developmental Biology, RIKEN, Kobe, Japan). pcDNA3.1.HisB expression vector encoding a human Gli1 cDNA (pcDNA/Gli1) was kindly provided by Dr Tsutomu Kume (Northwestern University Feinberg School of Medicine) and was used to stably transfect KAT-18 cells. Stable colonies selected in the complete RPMI 1640 medium containing 5 mg/mL G418 were pooled and used for all experiments.
Thyrosphere culture
Single cell suspensions of KAT-18 cells were cultured in serum-free DMEM/Ham's F-12 (1:1) medium containing B-27 (1:50) (Life Technology) and epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF), 20 ng/mL each, and seeded with 200 cells per well in a 96-well ultralow-attachment plate or 2000 cells per well in a six-well plate. The number of thyrospheres was counted and presented as the mean ± standard deviation of one experiment in triplicate. The experiments were repeated at least twice with similar results.
Western blot
Cells were lysed in Nonidet P-40 lysis buffer (50 mm Tris-HCl [pH 8.0], 150 mm NaCl, 1% Nonidet P-40, 5 mm EDTA, 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 1 mm phenylmethylsulfonyl fluoride). The following proteins were detected with their specific antibodies: Gli1, Snail, and ALDH (Cell Signaling Biotechnology, Inc). For loading control, β-actin was detected by a mouse monoclonal antibody (Santa Cruz Biotechnology Inc)
ALDEFLUOR assay
Single cell suspensions of five thyroid tumor cell lines were prepared and analyzed for ALDH by using an ALDEFLUOER kit (STEMCELL Technologies, Inc) according to the manufacturer's instruction. Diethylaminobenzaldehyde (DEAB), an ALDH-specific inhibitor, was added to the aliquot of each sample before staining as a background control. Cells were analyzed in a Becton Dickson FACScan flow cytometer. Cyclopamine (a Smo inhibitor) (LC Laboratory), HhAntag (an analog of cyclopamine) (kindly provided by Genentech Inc), or GANT61 (a Gli inhibitor) (Sigma-Aldrich) was used to determine the effect of the Shh pathway inhibitors on thyroid CSC self-renewal, KAT-18 and SW1736 cells were incubated in the presence of vehicle (0.5% dimethylsulfoxide), cyclopamine (5 μm), HhAntag (10 μm), or GANT61 (5 μm) for 72 hours. Cells were analyzed for ALDH activity by ALDEFLUOR assay.
Colony survival assay
KAT-18 cells seeded in six-well plates (1 × 104 cells/well) in triplicates were irradiated with a single dose of 10 Gy in a 137Cs resource in a CIS Biointernational generator at a rate of 1.45 Gy/min. Cells were cultured for 2 weeks and replenished with fresh media twice weekly. Surviving colonies were stained with crystal violet. After being rinsed in running water and air-dried, the colonies were photographed and counted. The staining of crystal violet dye was dissolved in 1 mL methanol and read at OD595 for the optical density in a Tecan plate reader.
Luciferase reporter assay
KAT-18 cells seeded in a 24-well plate were transfected with an eight-copy Gli1 binding site-driven luciferase reporter gene (8 × 3′Gli-BS-Luc) by using FuGENE6 (Roche Applied Science) following the manufacturer's instruction. An internal control plasmid, pCMV/SPORT, which encodes a β-galactosidase gene, was included in the transfection mixture. Luciferase reporter gene without a promoter (pGL3/Basic) was included as a negative control. After incubation for 48 hours, cells were harvested and analyzed for luciferase activity by using the luciferin substrate and reading in a Tecan plate reader (Phenix Research Products). The relative light unit in each sample was normalized against the β-galactosidase activity measured by a colorimetric assay as previously reported (17). The means ± standard deviations of the data in triplicate from one experiment are presented. The experiments were repeated at least once with similar results.
Snail knockdown
Snail siRNA ON-TARGETplus SMARTpool were synthesized by Dharmacon and purchased from Fisher Scientific (catalog no. L-010847–01). KAT-18 and SW1736 cells seeded in a six-well plate were transfected with Snail siRNA by using Lipofectamine RNAiMAX (Invitrogen Life Technologies) according to the manufacturer's instruction. After incubation for 72 hours, the cells were analyzed for Snail expression by Western blot with an anti-Snail rabbit antibody (Cell Signaling Biotechnology, Inc) and for the percentage of ALDHHigh cells by ALDEFLUOR assay.
Statistical analysis
The differences in the percentage of ALDHHigh cells between different treatment groups were statistically analyzed by using an unpaired Student t test. A P value of < .05 was considered statistically significant. Analysis of all statistics was performed with SigmaPlot 11 software (Systat Software Inc).
Results
ALDH expression in thyroid tumor cell lines
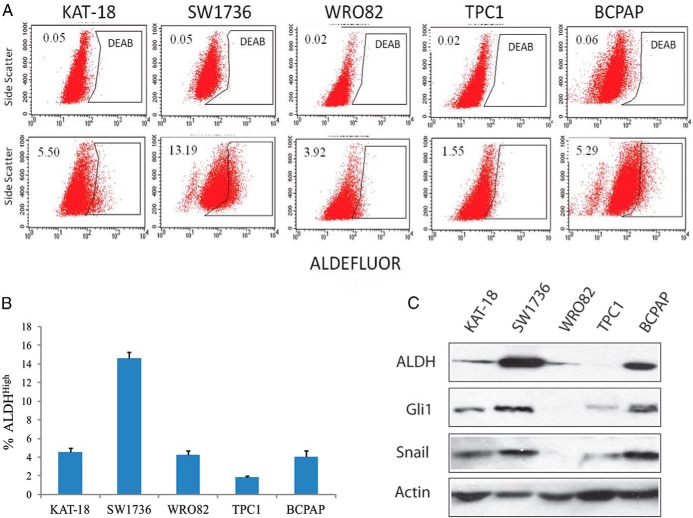
The Shh pathway has been implicated in regulating stem cell self-renewal (4). ALDH is a cytosolic enzyme that detoxifies intracellular aldehydes and confers resistance to chemotherapeutic agents (18). ALDH is highly expressed in breast, colon, and lung CSCs (18) and is considered the most reliable marker for thyroid CSCs (10). We first conducted an ALDEFLUOR assay to analyze the percentage of thyroid CSCs and found that five thyroid tumor cell lines, including SW1736, KAT-18, WRO82, TPC1, and BCPAP, contained variable percentages of ALDHHigh cells (Figure 1, A and B). SW1736, a BRAF-mutated ATC cell line, had the highest percentage of ALDHHigh cells (14.6 ± 0.6%), which is consistent with the observation by Carina et al (11) showing that the SW1736 cell line is enriched with CSCs (10.6%). Western blot analysis revealed that the expression of ALDH levels corresponded to the percentage of ALDHHigh cells in these thyroid tumor cell lines (Figure 1C). Interestingly, the levels of Gli1 expression in these five thyroid tumor cell lines correlated with the levels of Snail and ALDH.
Figure 1. ALDH activity and expression in five thyroid tumor cell lines.
ALDH activity in KAT-18, WRO82, SW1736, TPC1, and BCPAP cells was analyzed by ALDEFLUOR (A). DEAB was added to each sample before staining as a background control. The number in each histogram denotes the percentage of ALDHHigh cells. The percentage of ALDHHigh cells in each cell line was plotted as the mean ± SD value from three independent experiments (B). Cell lysates were prepared from five thyroid tumor cell lines and analyzed by Western blot for the expression of ALDH, Gli1, Snail, and actin (C).
Effect of Shh pathway inhibition on thyroid CSC self-renewal
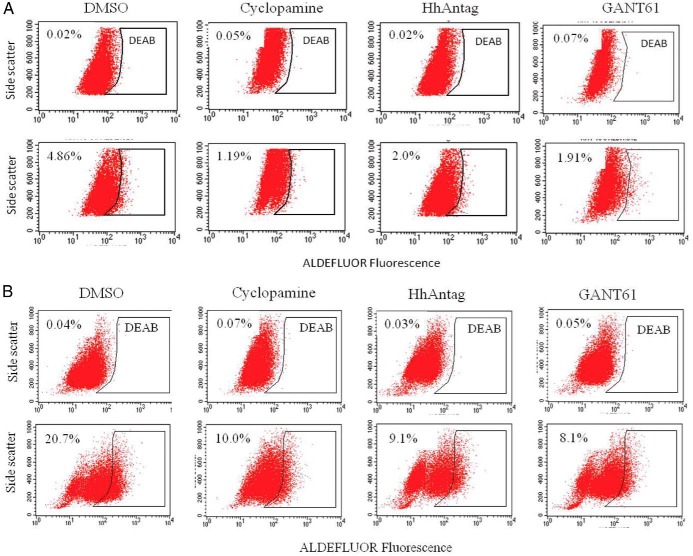
To determine whether the Shh pathway played a role in thyroid CSC self-renewal, we first tested whether three Shh pathway inhibitors affected the proportion of ALDHHigh cells in two anaplastic thyroid tumor cell lines. As shown in Figure 2, the percentage of ALDHHigh CSCs was decreased by >50% in KAT-18 and SW1736 cells treated with cyclopamine, HhAntag, and GANT61. To further confirm the role of the Shh pathway in regulating thyroid CSC self-renewal, we tested whether inhibition of the Shh pathway by gene knockdown led to a decreased number of thyroid CSC cells. Our prior studies showed that two microRNA (miRNA) constructs that target Shh and Gli1 were very effective at suppressing Shh and Gli1 expression in SW1736 and KAT-18 cells (13). The ability of these miRNA constructs to suppress Shh and Gli1 in KAT-18 cells stably transfected with these miRNA constructs was confirmed by Western blot (Figure 3A). Interestingly, Shh knockdown in KAT-18 cells stably transfected with Shh-miRNA led to a modest decrease of Gli1 expression, probably due to an autocrine regulation by the Shh pathway. KAT-18 cells stably transfected with a control miRNA (Ctr-miRNA) construct, a Shh-miRNA construct targeting Shh miRNA at nucleotide 658, and a Gli1-miRNA targeting Gli1 miRNA at nucleotide 1519 were selected for the remaining experiments. As shown in Figure 3B, the percentage of ALDHHigh cells was significantly decreased in Shh-miRNA- and Gli1-miRNA-transfected KAT-18 cells (1.31 and 0.07%, respectively), compared to that in Ctr-miRNA-transfected KAT-18 cells (3.56%). We next measured the clonogenic activity of these transfected cell lines cultured in ultralow affinity six-well plates. The size of the spheres initiated from clonogenic cells appeared to be significantly smaller in KAT-18 cells transfected with Shh-miRNA and Gli1-miRNA than Ctr-miRNA-transfected KAT-18 cells (Figure 3C). The number of the thyrospheres of Shh-miRNA- and Gli1-miRNA-transfected cells were about 50% lower than that of Ctr-miRNA-transfected KAT-18 cells (Figure 3C). These observations suggest that inhibition of the Shh pathway by specific inhibitors of Smo or Gli1 or by Shh and Gli1 knockdown leads to reduced capacity of thyroid CSC self-renewal.
Figure 2. The effect of the Shh pathway inhibitors on CSC self-renewal.
KAT-18 (A) and SW1736 (B) cells were incubated in the presence of vehicle (0.5% dimethylsulfoxide), cyclopamine (5 μm), HhAntag (10 μm), or GANT61 (5 μm) for 72 hours. The cells were harvested and analyzed for ALDHHigh cells by ALDEFLUOR assay. DEAB was added to each sample before staining as a background control. The experiments were repeated at least twice with similar results.
Figure 3. Shh or Gli1 knockdown suppresses thyroid CSC self-renewal.
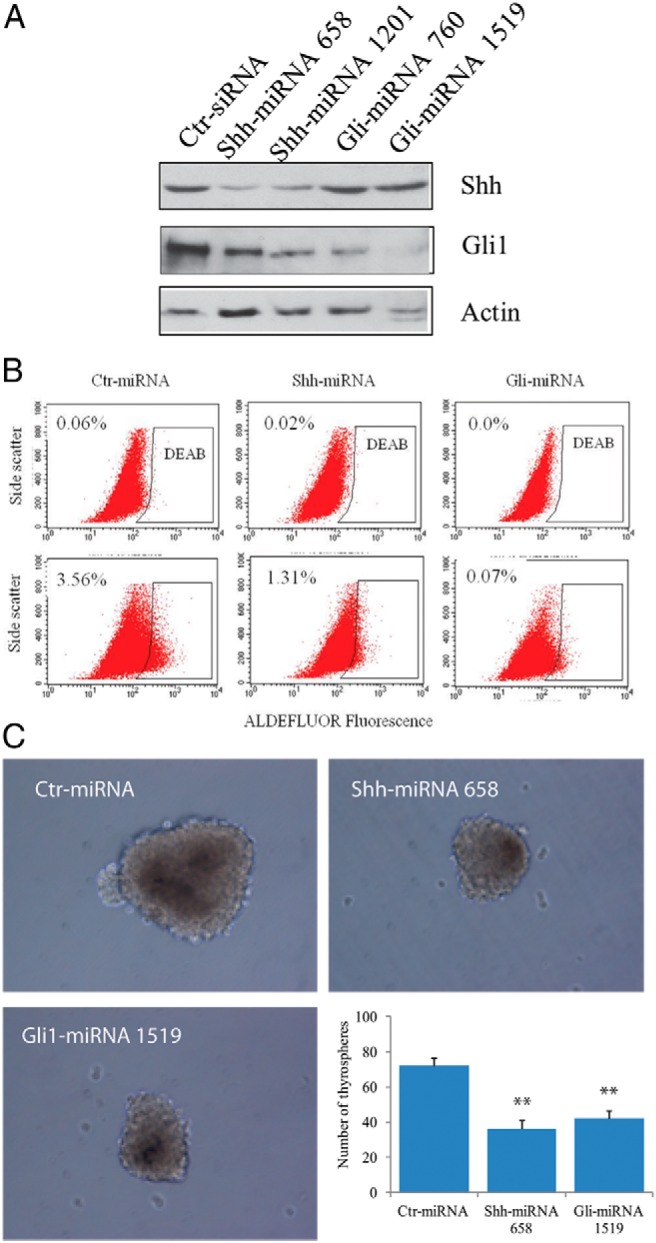
A, Western blot analysis of Gli1 and Shh expression. KAT-18 cells stably transfected with Ctr-miRNA, Shh-miRNA (constructs that target Shh miRNA at the nucleotide 658 or 1201), or Gli1-miRNA (constructs that target Gli1 miRNA at the nucleotide 760 or 1519) were analyzed for Shh, Gli1, and actin expression by Western blot. B, KAT-18 cells stably transfected with Ctr-miRNA, Shh-miRNA 658, or Gli-miRNA 1519 were analyzed for ALDHHigh cells by ALDEFLUOER. The experiments were repeated at least twice with similar results. C, Thyrosphere formation. Single cell suspension of Ctr-miRNA, Shh-miRNA, or Gli1-miRNA-transfected KAT-18 cells was resuspended in serum-free DMEM/Ham's F-12 (1:1) medium containing B-27, EGF, and bFGF 20 ng/mL each and seeded at 2 × 103 cells per well in six-well ultralow-attachment plates. A representative of thyrospheres from these transfected cell lines was photographed 2 weeks after seeding. Thyrospheres were counted and presented as the mean ± SD from one of two experiments in triplicate. **, P < .01.
Effect of Gli1 overexpression on thyroid CSC self-renewal
We next tested whether Gli1 overexpression led to increased thyroid CSC self-renewal. KAT-18 cells were stably transfected with a pcDNA3.1 empty vector or the vector encoding a histidine-tagged human Gli1 cDNA. To test whether Gli1 was functional, we conducted a luciferase reporter assay to determine whether Gli1 overexpression led to increased luciferase reporter gene expression driven by a promoter containing eight copies of the Gli1-binding site. As shown in Figure 4A, pcDNA/Gli1-transfected KAT-18 cells had approximately 20-fold higher promoter activity than that in pcDNA3.1-transfected cells. ALDEFLUOR assay revealed that Gli1 transfection increased the percentage of ALDHHigh cells (Figure 4B). The percentage of ALDHHigh cells in pcDNA3.1-transfected KAT-18 cells was 1.55% vs 8.33% in pcDNA/Gli1-transfected KAT-18 cells. Gli1 overexpression led to almost a 5-fold increase in the number of ALDHHigh cells. Consistently, the mean number of thyrospheres in per 100 pcDNA3.1-transfected KAT-18 cells was 0.79 vs 2.62 in pcDNA/Gli1-transfected KAT-18 cells (Figure 4C). Thus, Gli1 overexpression in KAT-18 cells increased the number of thyrospheres. These results collectively suggest that activation of the Shh pathway plays an important role in thyroid CSC enrichment.
Figure 4. Gli1 overexpression stimulates thyroid CSC self-renewal.
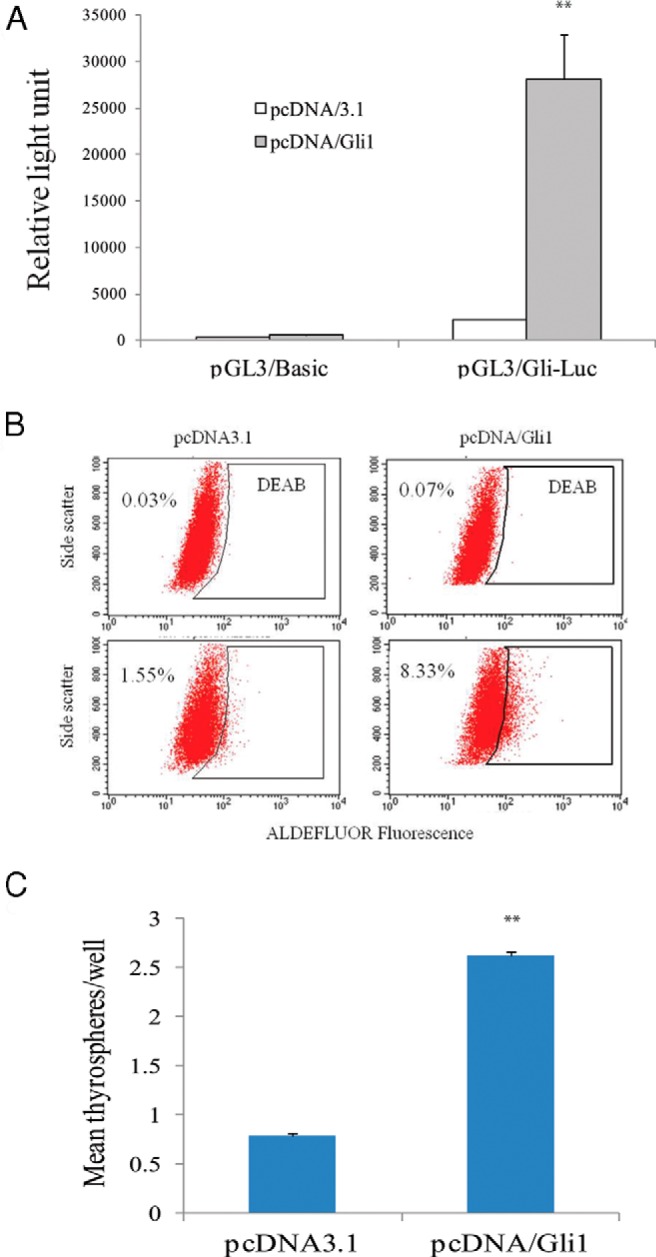
A, Luciferase reporter assay. pcDNA3.1- or pcDNA/Gli1-transfected cells were transfected with the pGL3/Basic empty vector or the same vector encoding an 8XGli1-Luc reporter gene. Luciferase activity was analyzed 48 hours later. **, The P value in comparison with pcDNA3.1 transfected control was < .01. B, ALDEFLUOR assay. Single cell suspensions of KAT-18 cells transfected with pcDNA/3.1 or pcDNA/Gli1 were analyzed for ALDHHigh cells by ALDEFLUOR. The experiments were repeated twice with similar results. C, Thyrosphere formation. Single cell suspension of pcDNA3.1 and pcDNA/Gli1-transfected KAT-18 cells were resuspended in serum-free DMEM/Ham's F-12 (1:1) medium containing B-27, EGF, and bFGF (20 ng/mL each) and seeded with 200 cells per well in a 96-well ultralow-attachment plate. Thyrospheres were counted and presented as the mean ± SD from 48 wells in a representative experiment. **, The P value in comparison with pcDNA3.1 transfected control was < .01.
Role of Snail in regulating thyroid CSC self-renewal
The Shh pathway-activated Gli1 transcription factor induces the expression of Snail (19), a transcriptional factor that has recently been shown to be involved in transforming thyroid tumor cells with CSC-like properties (20). We first tested whether the Shh pathway was involved in regulating Snail expression in thyroid tumor cell lines. Cyclopamine, HhAntag, and GANT61 all drastically inhibited the expression of Snail in KAT-18 cells (Figure 5A) and SW1736 cells (data not shown). Consistent with this observation, Snail expression was down-regulated in KAT-18 cells with Shh or Gli1 knockdown but was up-regulated in Gli1-transfected KAT-18 cells (Figure 5B). We next determined whether Snail knockdown affected thyroid CSC self-renewal. As shown in Figure 5C, Snail expression was effectively suppressed by Snail siRNA in both KAT-18 and SW1736 cells. The concentration of 2.5 nmol was most effective in suppressing Snail expression in KAT-18 cells. The percentage of ALDHHigh cells in Snail siRNA-transfected KAT-18 cells was 6.31, vs 1.5% in a scrambled siRNA-transfected negative control (Figure 5D). The percentage of ALDHHigh cells in Snail siRNA-transfected SW1736 cells was 10.2, vs 7.0% in a scrambled siRNA-transfected negative control (Figure 5E).
Figure 5. The effect of the Shh pathway on Snail expression.
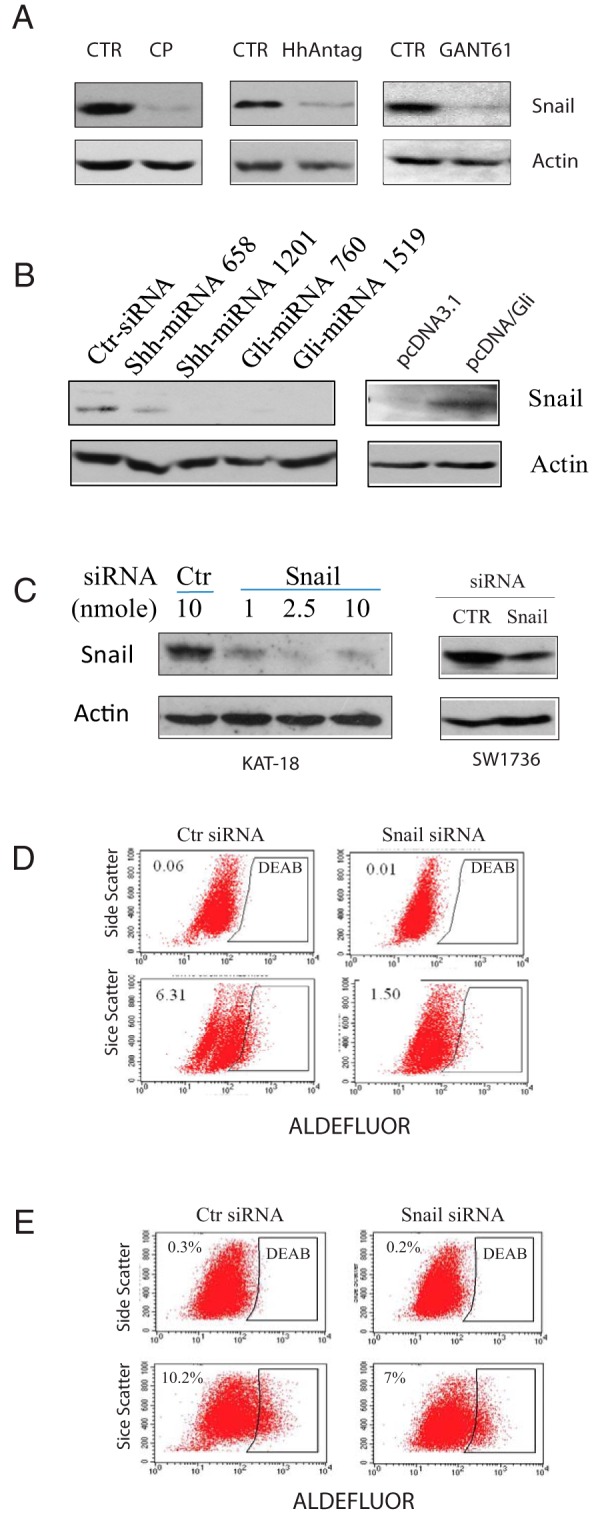
A, The effect of the Shh pathway inhibitors on Snail expression. KAT-18 cells were treated with cyclopamine (CP) (5 μm), HhAntag (10 μm), or GANT61 (10 μm) for 72 hours. The cells were harvested and analyzed for Snail and Actin expression by Western blot with their specific antibodies. B, Regulation of Snail by the Shh pathway. KAT-18 cells transfected with the indicated genes were analyzed for Snail expression by Western blot. C, Snail knockdown. KAT-18 cells were transfected with the indicated amounts of Snail or scrambled siRNA as a control. SW1736 cells were transfected with control (Ctr) or Snail siRNA (2.5 nmol each). After incubation for 48 hours, the cells were harvested and analyzed for Snail and actin expression by Western blot. The experiments were repeated twice with similar results. D and E, The effect of Snail knockdown on KAT-18 (D) and SW1736 (E) CSC self-renewal. Cells seeded in 35-mm dishes were transfected with a scrambled control siRNA or Snail siRNA (2.5 nmol each). After incubation for 72 hours, the cells were analyzed for ALDHHigh thyroid CSCs by ALDEFLUOR assay.
Effect of the Shh pathway on radiation resistance
A hallmark characteristic of CSCs is their ability to resist chemotherapy and radiation therapy (21). We tested whether the manipulation of Shh and Gli1 expression affected the sensitivity of KAT-18 cells to radiation. As shown in Figure 6A, Gli1 expression in KAT-18 cells significantly increased the number of surviving colonies after irradiation (10 Gy). Quantification of the OD reading of dissolved crystal violet dye showed an almost 100% increase of OD595 value in Gli1-transfected cells, compared to pcDNA3.1-transfected KAT-18 cells (Figure 6B). In contrast, Shh and Gli1 knockdown significantly decreased the survival of colonies (Figure 6C), as revealed by a decreased OD595 reading (Figure 6D), compared to that in Ctr-miRNA-transfected KAT-18 cells.
Figure 6. The effect of the Shh pathway on radiation survival.
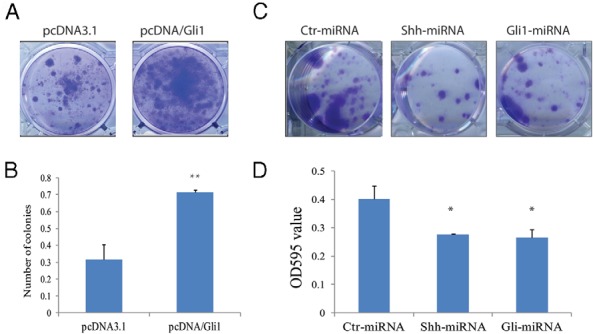
KAT-18 cells transfected with pcDNA3.1 or pcDNA/Gli1 (A and B) or transfected with Ctr-miRNA, Shh-miRNA 658, or Gli1-miRNA 1519 expression vector (C) were seeded in six-well plates (1 × 104 cells/well) and irradiated (10 Gy). Cells were stained 2 weeks later with crystal violet. D, After being rinsed in the running water, the staining of crystal violet dye was dissolved in 1 mL methanol and read at OD595. The data represent the mean ± SD from one experiment in triplicate. The experiments were repeated twice with similar results. **, The P value in comparison with pcDNA3.1-transfected KAT-18 cells was < .01; *, The P value in comparison with Ctr-miRNA-transfected KAT-18 cells was < .01.
Discussion
Thyroid CSCs have been characterized by the expression of several markers such as side population and CD133 (22, 23) or by inducing strong tumorigenicity in NOD/SCID mice (10, 20). A recent study by Todaro et al (10) found that ALDH is a reliable marker of thyroid CSCs. More recently, Ma et al (12) showed that SSEA-1 is also a specific marker for thyroid CSCs, and that SSEA-1-postive thyroid CSCs express high levels of stem cell-related genes such as Nanog, Sox2, and Oct4 and are resistant to 5-fluorouracil cytotoxicity. In our studies, thyroid CSCs were characterized by ALDH positivity and their ability to form thyrospheres. We found that inhibition of the Shh pathway by cyclopamine, HhAntag, and GANT61 significantly reduced the number of ALDHHigh CSCs in two ATC cell lines, KAT-18 and SW1736 cells. Consistently, Shh and Gli1 knockdown in KAT-18 cells decreased the number of ALDHHigh CSCs and the number of thyrospheres, whereas Gli1 overexpression increased the number of ALDHHigh cells and thyrospheres. These observations collectively suggest that the Shh pathway plays an important role in maintaining the CSC self-renewal in ATC cells. In support of this notion, inhibition of the Shh pathway sensitized KAT-18 cells to radiation-induced killing, whereas Gli1 overexpression led to increased radiation resistance. These characteristics are consistent with the high capacity of CSCs to resist killing from chemotherapy and radiation (18).
The mechanisms by which the activation of the Shh pathway promotes CSC self-renewal remain to be fully understood. In breast cancer, Gli1 knockdown leads to the suppression of Bmi-1, a central regulator of self-renewal in normal stem cells (24). A recent study showed that transduction of Snail expression into the immortalized mammary epithelial cells leads to the enrichment of CD44hiCD24lo breast CSCs (25). Snail expression also induces an epithelial to mesenchymal transition and CSC-like properties in a human squamous cell carcinoma cell line (26). Our studies demonstrated that Snail expression was regulated by the Shh pathway, correlated with the expression of Gli1 and ALDH, and corresponded to the number of ALDHHigh CSCs in five thyroid cancer cell lines. Moreover, knockdown of Snail by siRNA led to a decreased number of ALDHHigh thyroid CSCs in two anaplastic thyroid tumor cell lines. These observations collectively suggest that Snail plays a critical role in the Shh pathway-mediated maintenance of the CSC self-renewal in ATC cell lines. Consistently, Ma et al (12) reported that Snail expression is significantly higher in SSEA-1-positive thyroid CSCs than in SSEA-1-negative non-CSCs. Intriguingly, Yasui et al (20) reported that Snail overexpression in an ATC-I thyroid tumor cell line increased the number of thyrospheres but decreased the number of ALDHHigh cells. Because ALDH is considered a reliable marker for thyroid CSCs and thyrospheres are initiated from thyroid CSCs (10), this discrepancy is hard to reconcile.
The Shh pathway plays an important role in tumorigenesis in several types of malignancies and is an important molecular target in cancer therapy (1, 2, 27). Activation of the Shh signaling pathway predisposes individuals to the development of nevoid basal cell carcinoma syndrome, an autosomal dominant disorder characterized by mutations in Ptch (28–30). Sporadic basal cell carcinomas and medulloblastomas display mutations in both Smo and Ptch in the early stage of tumor growth (30–33). Snail is a transcription factor intimately regulated by Gli1. Snail is not expressed in normal thyroid epithelial cells but is highly expressed in thyroid tumor tissues and in thyroid tumor cell lines (34). Snail transgenic mice develop spontaneous PTCs (34). The incidence rate of thyroid cancer in Snail-transgenic mice exposed to radiation is significantly higher than wild-type mice (34). Our present study demonstrated that Gli1 correlated with Snail expression in five thyroid tumor cell lines and that activation of the Shh pathway led to increased Snail expression, whereas the inhibition of the Shh pathway by three inhibitors or by Shh and Gli1 gene knockdown led to decreased Snail expression in two anaplastic thyroid tumor cell lines. Our studies suggest that activation of the Shh pathway in thyroid neoplasm could contribute to high levels of Snail expression, and that the induction of Snail expression by activation of the Shh pathway may confer the susceptibility of thyrocytes with genetic alterations such as RET/PTC rearrangements or BRAF gene mutations to the development of overt thyroid cancer.
It is widely accepted that CSCs are more resistant to chemo- or radiotherapy than non-CSCs. Surviving CSCs play a critical role in tumor dormancy and recurrence. Due to their highly invasive potential, recurrent tumors often take place in a remote organ as a result of metastasis, which is responsible for >90% of cancer-related deaths. Therefore, identification of the signaling pathways and transcription factors involved in CSC self-renewal and the use of these pathway-specific inhibitors are critical for preventing tumor cell spread and cancer recurrence. Kurrey et al (35) reported that Snail and slug mediate radioresistance and chemoresistance in ovarian cancer cells by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype. Preclinical studies revealed that the combination of GDC-0449 and gemcitabine chemotherapy curtails pancreatic CSCs and completely blocks tumor regrowth in mouse models (36, 37). Approximately 15–20% of patients with thyroid cancer relapse in their lifetime. Recurrent thyroid cancers tend to be poorly differentiated and do not respond well to radioiodine and thyroid hormone therapy. Our present studies show that the Shh pathway was required for maintaining thyroid CSC self-renewal in two ATC cell lines and that the activation of the Shh pathway rendered KAT-18 cells resistant to radiation killing, whereas inhibition of the Shh pathway by Shh or Gli1 knockdown sensitized KAT-18 cells to radiation killing. Consistently, SSEA-positive thyroid CSCs are resistant to the cytotoxic activity of 5-fluorouracil (12). These observations collectively suggest that inhibitors of the Shh pathway in combination with radiation or chemotherapy could be useful for treating poorly differentiated or ATC. Although the current study was carried out in two ATC cell lines, we speculate that blocking the Shh pathway could also sensitize thyroid CSC cell lines derived from well-differentiated PTC and follicular thyroid carcinoma and thyroid CSCs isolated from fresh thyroid tumor tissues to the killing effect of radiation therapy.
Acknowledgments
We thank Drs Guy J. F. Juillard (University of California at Los Angeles) and Kenneth B. Ain (University of Kentucky Medical Center, Lexington, KY) for kindly providing three thyroid tumor cell lines; Dr Hiroshi Sasaki (Center for Developmental Biology, RIKEN Kobe, Japan) for 8×3′Gli-BS-Luc for luciferase reporter construct; and Dr Tsutomu Kume (Northwestern University Feinberg School of Medicine) for pcDNA/Gli1 plasmid. HhAntag (GDC-0449) was kindly provided by Genentech Inc (South San Francisco, California).
This work was supported in part by the ThyCa Foundation (to X.X.) and Rush Dean's Fellowship (to A.J.W.).
Present address for Y.W.: Section of General Surgery, First Hospital of Qinhuangdao, Qinhuangdao, Hebei Province 066000, People's Republic of China.
Disclosure Summary: The authors declare that they have no competing interests.
Funding Statement
This work was supported in part by the ThyCa Foundation (to X.X.) and Rush Dean's Fellowship (to A.J.W.).
Footnotes
- ALDH
- aldehyde dehydrogenase
- ATC
- anaplastic thyroid cancer
- bFGF
- basic fibroblast growth factor
- CSC
- cancer stem cell
- Ctr-miRNA
- control miRNA
- DEAB
- diethylaminobenzaldehyde
- EGF
- epidermal growth factor
- miRNA
- microRNA
- PTC
- papillary thyroid carcinoma
- Ptch
- Patched
- Shh
- sonic hedgehog
- siRNA
- small interfering RNA
- Smo
- Smoothened
- SSEA-1
- stage-specific embryonic antigen 1.
References
- 1. Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol. 2005;6:306–317. [DOI] [PubMed] [Google Scholar]
- 2. Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. [DOI] [PubMed] [Google Scholar]
- 3. van den Brink GR. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev. 2007;87:1343–1375. [DOI] [PubMed] [Google Scholar]
- 4. Merchant AA, Matsui W. Targeting Hedgehog–a cancer stem cell pathway. Clin Cancer Res. 2010;16:3130–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Naka K, Hoshii T, Hirao A. Novel therapeutic approach to eradicate tyrosine kinase inhibitor resistant chronic myeloid leukemia stem cells. Cancer Sci. 2010;101:1577–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao C, Chen A, Jamieson CH, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nicolis SK. Cancer stem cells and “stemness” genes in neuro-oncology. Neurobiol Dis. 2007;25:217–229. [DOI] [PubMed] [Google Scholar]
- 8. Xu Q, Yuan X, Liu G, Black KL, Yu JS. Hedgehog signaling regulates brain tumor-initiating cell proliferation and portends shorter survival for patients with PTEN-coexpressing glioblastomas. Stem Cells. 2008;26:3018–3026. [DOI] [PubMed] [Google Scholar]
- 9. Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 2006;6:292–306. [DOI] [PubMed] [Google Scholar]
- 10. Todaro M, Iovino F, Eterno V, et al. Tumorigenic and metastatic activity of human thyroid cancer stem cells. Cancer Res. 2010;70:8874–8885. [DOI] [PubMed] [Google Scholar]
- 11. Carina V, Zito G, Pizzolanti G, et al. Multiple pluripotent stem cell markers in human anaplastic thyroid cancer: the putative upstream role of SOX2. Thyroid. 2013;23:829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ma R, Minsky N, Morshed SA, Davies TF. Stemness in human thyroid cancers and derived cell lines: the role of asymmetrically dividing cancer stem cells resistant to chemotherapy. J Clin Endocrinol Metab. 2014;99:E400–E409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu X, Ding H, Rao G, et al. Activation of the Sonic Hedgehog pathway in thyroid neoplasms and its potential role in tumor cell proliferation. Endocr Relat Cancer. 2012;19:167–179. [DOI] [PubMed] [Google Scholar]
- 14. Ain KB, Taylor KD, Tofiq S, Venkataraman G. Somatostatin receptor subtype expression in human thyroid and thyroid carcinoma cell lines. J Clin Endocrinol Metab. 1997;82:1857–1862. [DOI] [PubMed] [Google Scholar]
- 15. Pilli T, Prasad KV, Jayarama S, Pacini F, Prabhakar BS. Potential utility and limitations of thyroid cancer cell lines as models for studying thyroid cancer. Thyroid. 2009;19:1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schweppe RE, Klopper JP, Korch C, et al. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab. 2008;93:4331–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eustice DC, Feldman PA, Colberg-Poley AM, Buckery RM, Neubauer RH. A sensitive method for the detection of β-galactosidase in transfected mammalian cells. Biotechniques. 1991;11:739–740, 742–743. [PubMed] [Google Scholar]
- 18. Tirino V, Desiderio V, Paino F, et al. Cancer stem cells in solid tumors: an overview and new approaches for their isolation and characterization. FASEB J. 2013;27:13–24. [DOI] [PubMed] [Google Scholar]
- 19. Li X, Deng W, Nail CD, et al. Snail induction is an early response to Gli1 that determines the efficiency of epithelial transformation. Oncogene. 2006;25:609–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yasui K, Shimamura M, Mitsutake N, Nagayama Y. SNAIL induces epithelial-to-mesenchymal transition and cancer stem cell-like properties in aldehyde dehydrogenase-negative thyroid cancer cells. Thyroid. 2013;23:989–996. [DOI] [PubMed] [Google Scholar]
- 21. Baccelli I, Trumpp A. The evolving concept of cancer and metastasis stem cells. J Cell Biol. 198:281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mitsutake N, Iwao A, Nagai K, et al. Characterization of side population in thyroid cancer cell lines: cancer stem-like cells are enriched partly but not exclusively. Endocrinology. 2007;148:1797–1803. [DOI] [PubMed] [Google Scholar]
- 23. Zito G, Richiusa P, Bommarito A, et al. In vitro identification and characterization of CD133(pos) cancer stem-like cells in anaplastic thyroid carcinoma cell lines. PLoS One. 2008;3:e3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu S, Dontu G, Mantle ID, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tam WL, Lu H, Buikhuisen J, et al. Protein kinase C α is a central signaling node and therapeutic target for breast cancer stem cells. Cancer Cell. 2013;24:347–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhu LF, Hu Y, Yang CC, et al. Snail overexpression induces an epithelial to mesenchymal transition and cancer stem cell-like properties in SCC9 cells. Lab Invest. 2012;92:744–752. [DOI] [PubMed] [Google Scholar]
- 27. Kasper M, Schnidar H, Neill GW, et al. Selective modulation of Hedgehog/GLI target gene expression by epidermal growth factor signaling in human keratinocytes. Mol Cell Biol. 2006;26:6283–6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Undén AB, Zaphiropoulos PG, Bruce K, Toftgård R, Ståhle-Bäckdahl M. Human patched (PTCH) mRNA is overexpressed consistently in tumor cells of both familial and sporadic basal cell carcinoma. Cancer Res. 1997;57:2336–2340. [PubMed] [Google Scholar]
- 29. Xie J, Johnson RL, Zhang X, et al. Mutations of the PATCHED gene in several types of sporadic extracutaneous tumors. Cancer Res. 1997;57:2369–2372. [PubMed] [Google Scholar]
- 30. Xie J, Murone M, Luoh SM, et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–92. [DOI] [PubMed] [Google Scholar]
- 31. Raffel C, Jenkins RB, Frederick L, et al. Sporadic medulloblastomas contain PTCH mutations. Cancer Res. 1997;57:842–845. [PubMed] [Google Scholar]
- 32. Zurawel RH, Allen C, Wechsler-Reya R, Scott MP, Raffel C. Evidence that haploinsufficiency of Ptch leads to medulloblastoma in mice. Genes Chromosomes Cancer. 2000;28:77–81. [PubMed] [Google Scholar]
- 33. Zurawel RH, Allen C, Chiappa S, et al. Analysis of PTCH/SMO/SHH pathway genes in medulloblastoma. Genes Chromosomes Cancer. 2000;27:44–51. [DOI] [PubMed] [Google Scholar]
- 34. Hardy RG, Vicente-Dueñas C, González-Herrero I, et al. Snail family transcription factors are implicated in thyroid carcinogenesis. Am J Pathol. 2007;171:1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kurrey NK, Jalgaonkar SP, Joglekar AV, et al. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009;27:2059–2068. [DOI] [PubMed] [Google Scholar]
- 36. Mueller MT, Hermann PC, Witthauer J, et al. Combined targeted treatment to eliminate tumorigenic cancer stem cells in human pancreatic cancer. Gastroenterology. 2009;137:1102–1113. [DOI] [PubMed] [Google Scholar]
- 37. Feldmann G, Dhara S, Fendrich V, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]