Abstract
Context:
Treatment of congenital adrenal hyperplasia (CAH) is suboptimal. Inadequate suppression of androgens and glucocorticoid excess are common and current glucocorticoid formulations cannot replace the cortisol circadian rhythm.
Objectives:
The primary objective was to characterize the pharmacokinetic profile of Chronocort, a modified-release hydrocortisone formulation, in adults with CAH. Secondary objectives included examining disease control following 6 months of Chronocort with dose titration.
Design, Setting, and Patients:
Sixteen adults (eight females) with classic CAH participated in an open-label, nonrandomized, Phase 2 study at the National Institutes of Health Clinical Center. Twenty-four-hour blood sampling was performed on conventional glucocorticoids and following 6 months of Chronocort. Chronocort was initiated at 10 mg (0700 h) and 20 mg (2300 h). Dose titration was performed based on androstenedione and 17-hydroxyprogresterone (17-OHP) levels and clinical symptomatology.
Main Outcome Measures:
The primary outcome was cortisol pharmacokinetics of Chronocort and secondary outcomes included biomarkers of CAH control (androstenedione and 17-OHP).
Results:
In patients with CAH, Chronocort cortisol profiles were similar to physiologic cortisol secretion. Compared with conventional therapy, 6 months of Chronocort resulted in a decrease in hydrocortisone dose equivalent (28 ± 11.8 vs 25.9 ± 7.1 mg/d), with lower 24-hour (P = .004), morning (0700–1500 h; P = .002), and afternoon (1500–2300 h; P = .011) androstenedione area under the curve (AUC) and lower 24-hour (P = .023) and morning (0700–1500 h; P = .02) 17-OHP AUC.
Conclusions:
Twice-daily Chronocort approximates physiologic cortisol secretion, and was well tolerated and effective in controlling androgen excess in adults with CAH. This novel hydrocortisone formulation represents a new treatment approach for patients with CAH.
Congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency is characterized by cortisol and aldosterone deficiency and androgen excess (1). Goals of treatment are to replace deficient hormones and control excess androgen, while avoiding the adverse effects of exogenous glucocorticoid excess. Physicians caring for patients with CAH often face management challenges. Thrice-daily immediate-release short-acting hydrocortisone is the recommended therapy in growing children (2). However, there are no standard clinical guidelines for glucocorticoid therapy in adults. Several regimens using short- or long-acting glucocorticoid formulations are commonly used in practice, given once to thrice daily as a fixed, or weight-adjusted dose (3–5).
In addition to the lack of management consensus, it is difficult to achieve satisfactory outcome with conventional glucocorticoid formulations (6). These regimens are suboptimal because they cannot replace the normal cortisol circadian rhythm, and inadequate suppression of adrenal androgens and glucocorticoid excess are common (3, 7). New hydrocortisone formulations are being produced. Plenadren, marketed in Europe, is an immediate-release tablet with sustained-release hydrocortisone core taken first thing in the morning and provides once daily treatment but does not address the overnight increase in adrenocorticotrophic hormone (ACTH) (8). Chronocort, a modified-release formulation of hydrocortisone in clinical development, aims to mimic the cortisol circadian rhythm and address the overnight ACTH increase driving the increase in androgens in CAH (9).
We previously compared conventional immediate-release hydrocortisone administered thrice daily (10, 5, and 15 mg) to a pilot modified-release hydrocortisone tablet formulation (30 mg at night) (Phoqus pharmaceuticals) (10). This pilot modified-release hydrocortisone tablet was able to achieve physiologic cortisol levels and good control of ACTH and androgens overnight and in the early morning, but failed to provide adequate cortisol in the afternoon and evening, leading to a increase in adrenal androgens. Pharmacokinetic modeling suggested twice-daily dosing would achieve better hormonal control, but additional studies using this formulation were not possible due to manufacturing issues (11).
To address this shortcoming, Chronocort, a novel modified-release hydrocortisone formulation using scalable technology was developed (Diurnal Ltd). This new multiparticulate formulation has an enteric coat that has a pH trigger of 6.8, allowing small bowel dissolution. Phase 1 pharmacokinetic analysis showed that a twice-daily regimen (10 mg at 0700 h and 20 mg at 2300 h) approximated physiologic cortisol rhythm (12).
The aim of this study was to evaluate the pharmacokinetics of Chronocort in adults with CAH and test the hypothesis that providing near-physiologic cortisol replacement will improve control of androgenic precursors—androstenedione and 17-hydroxyprogresterone (17-OHP).
Patients and Methods
Patients
Sixteen adult patients (eight females) with classic CAH participated in this open label, single center, Phase 2 study (www.clinicaltrials.gov trial NCT01735617). Diagnosis of classic 21-hydroxylase deficiency was established based on medical records and genotype (13). All patients were on stable glucocorticoid and mineralocorticoid dosage for at least 3 months, in good general health and had laboratory evaluation within 12 weeks of enrollment with plasma renin activity (PRA) <1.5 times the upper normal range. Exclusion criteria included pregnancy, lactation, taking spironolactone or glucocorticoids (oral, inhaled or nasal) apart from treatment of CAH, significant medical or psychiatric illness, receiving medications that induce hepatic enzymes or interfere with glucocorticoid metabolism, history of bilateral adrenalectomy, or participation in a clinical trial within 3 months.
The study was approved by Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board. All patients provided written informed consent.
Study design
The primary objective was to characterize the pharmacokinetic profile of short-term Chronocort treatment in adults with CAH. Secondary objectives included examining the effect of Chronocort on hormone control following 6 months of dose titration.
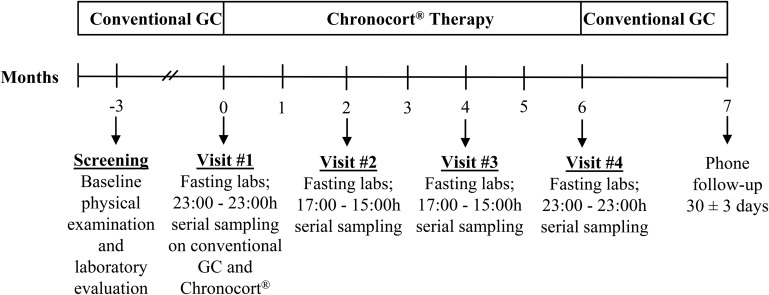
Patients were screened for eligibility up to 12 weeks prior to the first visit. All patients were admitted to the National Institutes of Health (NIH) Clinical Center every 2-months for a total of 6 months of treatment (Figure 1). Prior to Chronocort administration at visit 1, a 24-hour hormonal profile (2300–2300h, 2-hourly) was obtained while on conventional therapy.
Figure 1. Phase 2 study of Chronocort, a modified-release formulation of hydrocortisone.
GC, glucocorticoid. Fasting labs obtained on visit 1 (day 2, day 5 and day 6), visit 2 - visit 4 (day 2) included insulin, glucose, testosterone, supine plasma renin activity, bone turn-over markers (cross-linked telopeptide, osteocalcin) and biochemistry panels. Serial blood sampling included: adrenocorticotropic hormone (ACTH), androstenedione, 17-hydroxyprogesterone (17-OHP) and cortisol. Chronocort was initiated on visit 1, day 2 at 2300 h.
Patients received their last dose of conventional therapy until 2300 of day 2 and were started on twice daily Chronocort (2300 h, 20 mg; 0700 h, 10 mg) following completion of sampling on conventional therapy. This initial starting dose was chosen based on data from a prior Phase 1 study in healthy subjects (12). On day 4 (48 h post dose initiation), 24-hour (2300–2300 h) serial sampling (2-hourly from 2300–0500 h; 1-hourly from 0600–1600 h and 2-hourly from 1700–2300 h) was performed to evaluate Chronocort pharmacokinetics. 2-hourly serum samples (2300–2300 h) were also drawn to evaluate ACTH, androstenedione, and 17-OHP. Serial sampling was repeated after 2, 4, and 6 months. Telephone contact was made within 2-weeks of each visit for dose adjustments and adverse event monitoring. After 6-months of Chronocort treatment, patients were discharged to home on their prior conventional regimen with telephone followup.
Body composition was measured by Dual-energy x-ray absorptiometry prior to study medication administration at visit 1 and at the end of the study. Following breakfast at every visit, four questionnaires were administered to assess fatigue (Multidimensional Assessment of Fatigue), health-related quality-of-life [36-item short-form health survey (SF36), AddiQoL] and signs and symptoms of adrenal insufficiency.
Study medication and dose modification
Chronocort, a modified-release capsule formulation of hydrocortisone, consists of uniform multiparticulate beads which have an inert core, a hydrocortisone drug layer, and a delayed-release enteric outer coat (12). Chronocort was designed to mimic physiologic cortisol circadian rhythm through a delayed-release and sustained absorption profile of hydrocortisone after administration. Chronocort was available in 20, 10, and 5 mg capsules for this study.
Dose adjustments were made in 5-mg increments based on clinical symptoms of adrenal insufficiency or cortisol excess, and androstenedione and 17-OHP levels. Hormone levels obtained between 0100 and 0900 h and between 1100 and 1900 h were considered to reflect the 2300 h and the 0700 h Chronocort doses, respectively. Dose adjustments were made if three or more of the five sample times showed out of range values for androstenedione and 17-OHP. Optimal androstenedione levels were based on the normal range (men: 40–150 ng/dL; women: 30–200 ng/dL); 17-OHP levels were categorized (optimal: 300–1200 ng/dL; suppressed: ≤ 300 ng/dL; elevated, ≥ 1200 ng/dL).
When androstenedione and 17-OHP showed inconsistent trends, androstenedione took precedence in directing dose adjustment because androstenedione levels are less variable (6). Daily Chronocort dosage was not to exceed 50 mg or be less than 10 mg. Dose adjustments were made within 2 weeks following the visits by telephone. Patients were recontacted within 1 week after each dose change.
Compliance was assessed at each visit by accounting for number of capsules dispensed and number returned.
Hormonal assays
Serum concentrations of cortisol (Simbec Research Limited), 17-OHP and androstenedione (Mayo Medical Laboratories) were determined by HPLC–tandem mass spectrometry. The cortisol assay had an analytical sensitivity of 0.05 μg/dL, interassay coefficient of variation (CV) of 2.6, 4.5, 2.4%; intra-assay CV of 3.7, 1.1, 1.9% at mean concentration of 0.8, 7.9 and 19.7 μg/dL respectively. The androstenedione assay had a sensitivity of 15 ng/dL; interassay CV of 7.9, 7.2, 8.7%; intra-assay CV of 13.9, 5.9, 2.6 at mean concentration of 112, 916, and 2281 ng/dL, respectively, and normal range of 40–150 ng/dL for males and 30–200 ng/dL for females. The 17-OHP assay had an analytical sensitivity of 40 ng/dL, interassay CV of 9.7, 8.7, 6.8%; intra-assay CV of 6.8, 2.9, 4.4% with a mean concentration of 111, 751, and 2006 ng/dL, respectively, and normal range of less than or equal to 220 ng/dL for males and less than or equal to 285 ng/dL for females.
Plasma ACTH was measured using a chemiluminescence immunoassay on Siemens Immulite 2000 XPi analyzer (NIH Clinical Center), with a sensitivity of 5 pg/mL, intra- and interassay CVs of 2.5% and 3.6% respectively, and normal range of 0–46 pg/mL.
Statistical analyses
Data are presented as mean ± SD, unless otherwise indicated, and were analyzed using SAS version 9.2 (SAS Institute). All P-values were two sided and P < 0.05 was considered significant. Pharmacokinetic parameters for cortisol were calculated via noncompartmental analysis using Phoenix WinNonlin software (version 6.3, Pharsight Corp). The area under the curve (AUC) was computed using the linear-up, log-down trapezoidal rule for 24-hour (2300–2300 h) and three 8-hour time intervals (night, 2300–0700 h; morning, 0700–1500 h; afternoon, 1500–2300 h) chosen to approximate physiologic periods of low, high, and intermediate cortisol, respectively. Peak concentration (Cmax) and time to peak concentration (Tmax) were determined using actual collection time points. Twenty-four-hour hormonal exposure was also evaluated based on the number of time points that androgens were within elevated, optimal, and suppressed ranges according to the categorization used for dose adjustments.
Pharmacokinetic parameters from this Phase 2 study were compared with prior Phase I study results in healthy volunteers and to cortisol profiles (using tandem mass spectrometry) obtained in healthy controls not receiving any medication (n = 28) who underwent every-20-minute cortisol sampling over 24-hours as an approximation of normal circadian rhythm (12, 14).
SF-36 score was computed using SF-36 version 2 OptumInsight software as norm-based score, which employs a linear T-score transformation with mean of 50 and SD of 10 (15). AddiQoL, a disease-specific questionnaire developed for patients with adrenal insufficiency, has scores ranging from 30 (worst possible) to 120 (best possible) (16). Global Fatigue Index (GFI) score was calculated using the Multidimensional Assessment of Fatigue data and GFI scores range from 1 (no fatigue) to 50 (severe fatigue) (17).
Depending on the data distribution, parametric (paired t test) and nonparametric (Wilcoxon signed rank) tests were used to compare changes in AUCs, biomarkers and metabolic indices from baseline (conventional treatment) to 6 months of Chronocort therapy. Categorical data between these intervals were compared using McNemar's test.
Results
Twenty-one patients were screened. Four patients failed to meet the inclusion criteria for PRA level and one patient was eligible but chose not to participate due to time constraints. Sixteen adults (eight females; median age: 24 y; range 18–60 y) with classic CAH (12 salt wasting, four simple virilizing) participated. Patients were on a variety of glucocorticoid regimens (Table 1).
Table 1.
Baseline Clinical Characteristics of 16 Adults With Classic CAH in a Phase 2 study of Chronocort, a Modified-Release Hydrocortisone Formulation
| Baseline Characteristics | n (%) or Mean ± sd |
|---|---|
| Sex | |
| Female | 8 (50.0) |
| CAH phenotype | |
| Salt wasting | 12 (75) |
| Simple virilizing | 4 (25) |
| Age, y | 28.7 ± 13.0a |
| Body mass index, kg/m2 | 25.6 ± 5.7 |
| Systolic blood pressure, mm Hg | 114 ± 10 |
| Diastolic blood pressure, mm Hg | 66 ± 9 |
| Glucocorticoid medicationb | |
| Dexamethasone, mg/d (n = 5) | 0.34 ± 0.12 |
| Hydrocortisone, mg/d (n = 3) | 20.8 ± 8.0 |
| Prednisone, mg/d (n = 7) | 6.6 ± 2.8 |
| Glucocorticoid-equivalent dosec, mg/d (n = 16) | 28.0 ± 11.8 |
| Fludrocortisone dose, mcg/d | 98.4 ± 32.2 |
Age range, 18–60 y.
One patient on hydrocortisone/prednisolone combination was omitted.
Glucocorticoid-equivalent dose: hydrocortisone × 1, prednisone and prednisolone × 5, and dexamethasone × 80 (1).
Medication accountability revealed that one patient was noncompliant with taking the study medication. Results were similar when analyses were repeated excluding this patient. At study completion, 75% of patients expressed an interest in continuing Chronocort if it were available.
Pharmacokinetic profile of Chronocort
The cortisol pharmacokinetic profile 48 hours post Chronocort initiation and following 6 months of therapy was similar to the normal circadian rhythm of cortisol observed in healthy subjects and the pharmacokinetic profile of Chronocort observed in a Phase 1 study (Table 2) (12, 14).
Table 2.
Pharmacokinetic Parameters for Cortisol in Healthy Volunteers and in Patients With CAH
| Pharmacokinetic Parameter | AUC_24, (hr*μg/dL) (sd) | Cmax μg/dL (sd) | Tmax Clock Time (sd) |
|---|---|---|---|
| Healthy volunteers | |||
| Normal circadian rhythm (11) | 171.4 (41.4) | 21.5 (7.4) | 0800 h (1.6) |
| Phase 1 (12) | 203.3 (42.8) | 24.0 (4.6) | 0706 h (3.5) |
| CAH patients | |||
| Chronocort at 48 ha | 175.2 (1.3) | 21.4 (4.2) | 0654 h (2.9) |
| Chronocort at 6 mob | 141 (1.6) | 17.3 (6.5) | 0706 h (3.1) |
Abbreviations: Cmax, peak concentration; Tmax, time to peak concentration.
Patients receiving Chronocort, 30 mg/d (20 mg at 2300 h, 10 mg at 0700 h).
Following dose titration, average daily dose was 25.9 ± 7.1 mg/d.
Ten patients required Chronocort dose adjustments (decrease in eight, increase in two) resulting in an overall decrease in glucocorticoid dose (glucocorticoid equivalent dose: conventional vs 6 mo: 28 ± 11.8 vs 25.9 ± 7.1 mg/d). After 6 months of Chronocort, there was no evidence of dose accumulation or nonlinear pharmacokinetics. Overall exposure to cortisol (AUC) over 24 hours was similar following the first Chronocort dose and at 6 months (dose-normalized to 10 mg). However, peak cortisol levels were lower by approximately 10% at 6-month therapy (90% confidence interval, 0.742–1.057), which was expected due to reduced doses following dose titration.
Biomarkers of disease control
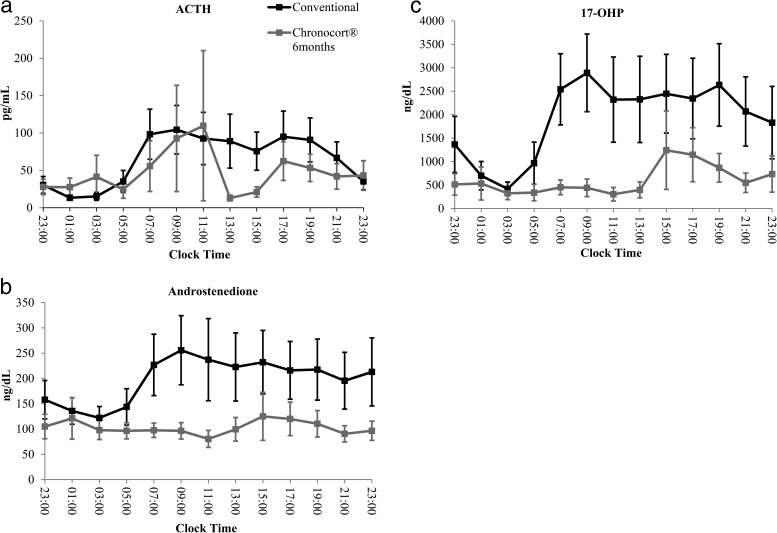
On conventional therapy, ACTH levels began to rise at 0500 h, plateaued between 0700 and 1500 h, and declined after 1700 h. Six months of Chronocort therapy resulted in lower ACTH levels throughout the day; however, changes in ACTH were not significant (Figure 2).
Figure 2. Comparison of 24-hour hormone profiles on Conventional (baseline) versus Chronocort therapy (6 months) (mean ± SEM) for a, ACTH, b, androstenedione and c, 17-OHP.
Most patients had elevated androstenedione and 17-OHP during the day while receiving conventional therapy. In comparison with baseline, androstenedione at 6 months showed a decrease in the percent of time points with elevated levels (33.7 vs 12.0%, P < .0001) and a higher percent of time points in the normal range (55.8 vs 73.1%, P < .0001). Likewise compared with baseline, 17-OHP at 6 months showed a decrease in the percent of time points with elevated levels (33.2 vs 12.0%, P < .0001) and an increase in the number of time points in the suppressed range (46.2 vs 69.2%, P < .0001). In fact, the majority of patients (59%) had 17-OHP values in the normal range (males: 40–220 ng/dL; females: 40–285 ng/dL) following 6 months of Chronocort therapy.
Similarly, at 6 months, Chronocort resulted in lower 24-hour (P = .003), morning (0700–1500 h; P = .0008) and afternoon (1500–2300 h; P = .009) AUC androstenedione and lower 24-hour (P = .021) and morning (0700-–1500 h; P = .018) AUC 17-OHP compared with conventional therapy.
Disease-related metabolic indices and quality-of-life estimates
Following 6 months of Chronocort there were no significant changes in body mass index but there was an increase in lean mass (P = .003); homeostasis model assessment–estimated insulin resistance (HOMA-IR) measured in the morning (2300–0700 h) increased (1.91 ± 0.7 vs 2.98 ± 1.7; P = .02); and the bone turnover marker osteocalcin, increased (P = .01) (Table 3).
Table 3.
Metabolic Parameters and Other Hormones in 16 Adult Patients With Classic CAH in a Phase 2 Study of Chronocort, a Modified-Release Formulation of Hydrocortisone
| Clinical Characteristic | Baseline | 6 Months | P Value |
|---|---|---|---|
| Weight, kg | 69.7 ± 19.7 | 70.6 ± 19.9 | .208 |
| BMI, kg/m2 | 25.6 ± 5.7 | 25.8 ± 5.9 | .623 |
| HOMA-IRa | 1.91 ± 0.7b | 2.98 ± 1.7 | .02 |
| Total T, ng/dL | 246 ± 252 | 206 ± 217 | .121 |
| Free T, ng/dL | 6 ± 6 | 4 ± 5 | .055 |
| PRA, ng/mL/h | 3.2 ± 1.6 | 2.7 ± 1.4 | .386 |
| Osteocalcinc, ng/mL | 21 ± 10 | 30 ± 12 | .010 |
| C-telopeptide, pg/mL | 685 ± 322 | 772 ± 297 | .231 |
| Subtotal BMC, g | 1695 ± 262 | 1684 ± 264 | .138 |
| Subtotal fat massd, g | 20 723 ± 11 972 | 20 495 ± 13 100 | .739 |
| Subtotal lean masse, g | 43 840 ± 10 051 | 45 376 ± 9998 | .003 |
| Whole-body BMDf, grams/cm2 | 1.13 ± 0.09 | 1.11 ± 0.09 | .007 |
| T-scoreg | −0.25 ± 0.97 | −0.55 ± 0.99 | .003 |
| Z-score | −0.20 ± 0.84 | −0.45 ± 0.88 | .004 |
Abbreviation: BMC, body mineral content. Data are mean ± sd. Bold indicates P < .05. To convert T to SI units (nmol/L) multiply by 0.0347.
HOMA-IR, fasting insulin (μIU/mL) × fasting glucose (mmol/L)/22.5.
HOMA-IR following 48 h of Chronocort: 2.45 ± 1.4
n = 15, one patient was excluded due to a laboratory issue.
Decrease in males only, P = .036.
Increase in females only, P = .006.
Subgroup analysis showed a decrease in BMD in females only, P = .015.
n = 13, three patients were <20 y.
Sex differences were observed in some measurements of body composition. Although body composition showed an overall increase in lean mass (P = .003) and no changes in fat mass, subgroup analysis revealed that an increase in lean mass occurred in females only (P = .006) and males experienced a decrease in fat mass (P = .036). Whole-body bone mineral density (BMD) showed a slight decrease (P = .007), and subgroup analysis by sex showed a decrease in BMD in females only (P = .015) (Table 3).
No significant changes were noted in quality of life or fatigue (baseline vs 6 mo: SF-36: 54.2 ± 4.6 vs 53.7 ± 5.5; AddiQoL: 96.1 ± 10.9 vs 97.4 ± 12.5; GFI: 14.3 ± 8.8 vs 12.6 ± 9.3). Of note, at baseline, mean SF-36 score across all domains was greater than 50, the mean of a healthy population (15). Similarly, AddiQoL and GFI scores were similar to a healthy population (16, 17) .
Adverse events
Chronocort was well tolerated. No serious adverse events occurred. Common short-term adverse events resolved and may have been associated with changes in glucocorticoid medication and/or the frequent blood sampling (Table 4). Six patients received stress dosing for acute viral illnesses of short duration. Two patients received stress dosing related to incidental surgical diagnosis and treatment (inguinal hernia, benign breast nodule). One patient experienced symptoms of adrenal insufficiency 1 week after starting the study and received stress dosing for a few days followed by an increase in Chronocort dose. One patient was diagnosed with tenosynovitis and one patient had worsening of trigger finger.
Table 4.
Adverse Events During Chronocort Treatment
| Adverse Event | No. Patients |
|---|---|
| Fatigue/generalized weakness | 14 |
| Headache | 14 |
| Acne | 9 |
| Dizziness | 8 |
| Weight gain | 8 |
| Infusion site pain/bruise | 7 |
| Increase in appetite | 7 |
| Acute insomnia | 6 |
| Decrease in appetite | 4 |
| Vivid dreams | 4 |
| Arthralgia (pre-existing) | 4 |
| Anemiaa | 8 |
| High blood pressure readingb | 3 |
| Acute adrenal insufficiency symptoms | 3 |
| Musculoskeletal pain | 3 |
| Nausea | 2 |
| Early awakening | 2 |
| Increased hair | 2 |
| Ear pain | 2 |
| Epistaxis | 2 |
| Palpitations | 1 |
| Acute phlebitis | 1 |
| Thinning of hair | 1 |
Incidental finding in three patients and possibly secondary to blood sampling in the remaining patients.
Two patients had high blood pressure readings at admission that resolved spontaneously. One patient had history of hypertension and was off treatment. Anti-hypertensive medication was restarted.
Three patients had unexpected carpal tunnel syndrome, but two had a prior history. In one patient symptoms self resolved while still receiving Chronocort. The other two patients had symptomatic improvement with wrist splints. Changes in PRA were not observed.
Discussion
Our study is the first to demonstrate that it is possible to safely replace cortisol in a near-physiologic manner using Chronocort, an oral, modified-release hydrocortisone formulation in patients with CAH. This novel hydrocortisone formulation was well tolerated and effective in controlling androgen excess in adults with CAH when administered twice daily.
Chronocort is a multiparticulate, modified-release capsule formulation of hydrocortisone, developed in an attempt to overcome the challenges observed with conventional glucocorticoid therapy, such as inability to adequately control androgen secretion without the complications of supraphysiologic glucocorticoids (12). At baseline, while receiving conventional glucocorticoid therapy, 24-hour sampling revealed inadequate androgen control throughout the day in most our patients, despite receiving long-acting glucocorticoids. Although all of our Patients with CAH were receiving stable glucocorticoid doses for at least 3 months prior to study entry, inclusion criteria was not based on level of hormonal control. Overall, our patients tended to have mildly elevated androgens on their conventional treatment, none were grossly overtreated and none had hypothalamic-pituitary-adrenal axis suppression. Large cohort studies report that only approximately one third of adult patients with classic CAH have hormones within target ranges (3, 18).
Based on the data obtained in our study of classic patients with CAH, Chronocort provides a stable peak-to-trough ratio of plasma cortisol concentrations that more closely mimics the normal circadian rhythm over a 24-hour period than conventional glucocorticoid replacement therapy. In particular, overnight cortisol increase (2300-–0700 h) and cortisol peak after awakening into the early afternoon (0700-–1500 h) had the most confluence with estimated physiologic cortisol secretion. However, even with the twice-daily regimen of Chronocort, cortisol levels were low in the evening hours but the ACTH and androgen levels remained in an acceptable range and all patients exhibited persistence of an endogenous diurnal variation in their hormone levels. This supports the concept that less cortisol replacement is needed at this time of the day because naturally cortisol and ACTH levels are low (10, 19, 20).
As the cortisol profile approximated physiologic cortisol secretion, Chronocort therapy effectively controlled the androgen excess characteristic of CAH. Compared with conventional therapy at baseline, Chronocort at 6 months showed improved serial androstenedione levels with lower 24-hour, morning, and afternoon androstenedione and improved 17-OHP levels over 24 hours and in the morning. Interestingly, most 17-OHP levels were within the normal range, rather than in the mildly elevated range typically used for management (3, 21). Single time-point morning androgen measurement is frequently used for monitoring treatment in CAH (22). An advantage of our study was our ability to perform 24-hour serial sampling and this lowering of androgens observed throughout the day was achieved with lower average daily glucocorticoid doses.
Our findings must be considered within the context of the population of patients being studied and may be confounded by the multiple hormonal imbalances characteristic of CAH. On Chronocort, there was a decrease in overall glucocorticoid dose based on hydrocortisone dose equivalency, increase in lean body mass, decrease in fat mass (males only), and insulin resistance assessed by morning HOMA-IR increased. The increase in HOMA-IR was observed after the first dose of Chronocort and is therefore unlikely to reflect a change in body composition and might be due to an increase in early-morning cortisol, which does not occur with conventional glucocorticoid therapy. In healthy individuals, insulin sensitivity decreases before awakening, associated with the physiological cortisol increase. Patients with adrenal insufficiency on hydrocortisone have low concentrations of metabolic fuels throughout the night, related to decreased overnight cortisol levels, and this might play a role in nonspecific symptoms such as fatigue, early-morning headache, and risk of hypoglycemia (23). Exogenous glucocorticoids in pharmacologic doses have negative effects on the bone and decrease osteocalcin, a marker of bone formation (24). We observed an increase in osteocalcin reflecting lower glucocorticoid exposure during 6 months of Chronocort therapy. Conversely, we observed a slight decrease in whole-body BMD, significant in females only. This finding is possibly due to a decrease in androgen exposure. Region-specific BMD changes were not assessed. Future studies of Chronocort therapy in patients with CAH should involve more-detailed assessments of metabolic parameters and BMD.
Patients with CAH have increased morbidity which is due in part to the limitations of currently available glucocorticoid therapy (3, 7, 25–27). As a result, novel therapies are being developed and studied by our group and others in an attempt to improve patient outcomes (10, 28, 29). Short-term proof-of-concept studies and case reports have demonstrated promising results using continuous sc hydrocortisone infusion to achieve circadian cortisol replacement in patients with CAH (28, 30–32). In patients with primary autoimmune adrenal insufficiency, once-daily Plenadren, the dual-release hydrocortisone, achieved normal morning cortisol levels but resulted in lower cortisol exposure over 24 hours and lack of normalization of early-morning cortisol levels prior to awakening (33). Thus, it is doubtful that this type of hydrocortisone formulation would improve androgen excess in patients with CAH because it does not provide overnight cortisol replacement. In contrast, the pharmacokinetic profile of cortisol on twice-daily Chronocort showed good 24-hour bioavailability resulting in lowering androgens in patients with CAH.
Although Chronocort overall achieved a near-physiological 24-hour cortisol profile, cortisol secretion exhibits a distinct circadian and ultradian rhythm that is influenced by the sleep-wake cycle and cannot be replicated with oral glucocorticoid replacement (11, 34, 35). The clinical significance of this is unknown. The hypothalamic-pituitary axis has a significant role in the sleep-wake cycle (36, 37), and a subset of our patients experienced sleep disturbances while receiving Chronocort. Dose adjustments helped with early awakening in both patients but complaints of odd dreams persisted in one patient. Qualitative or quantitative assessments of sleep were not part of our study. We did not find any changes in quality of life, which was not surprising given the small sample size, short study duration, and relatively normal baseline quality-of-life scores.
There were no serious adverse events. Three patients had the unexpected adverse event of carpal tunnel syndrome. Two of these patients had a history of similar complaints in the past. The etiology of these three cases of median nerve entrapment syndrome is not clear. Although increased mineralocorticoid activity of Chronocort compared with prestudy treatments (prednisone, dexamethasone) is a possibility, all three patients were receiving fludrocortisone and no changes were seen in PRA.
In addition to the small sample size, our study had a few important limitations. First, the study was an open-label, nonrandomized design. Although majority of patients were being followed at the NIH prior to study enrollment and were known to be compliant on their conventional medication, it is possible that improved compliance occurred secondary to the close scrutiny characteristic of being enrolled in a clinical trial. Moreover, an additional potential advantage during Chronocort therapy was the frequent dose adjustments to optimize treatment. These potential biases are due to the nonrandomized study design. Second, although the study design allowed dosage adjustments, the options were limited as the smallest available dose was 5 mg. Having smaller dose formulations would allow for more flexible and precise dose adjustments.
It is anticipated that the ability of Chronocort therapy to mitigate the drastic fluctuations in cortisol levels observed with conventional glucocorticoid therapy while more closely mirroring physiologic diurnal variation will result in improved patient outcomes. This newly developed modified-release oral hydrocortisone formulation regimen given as a twice-daily dosing represents a new treatment approach for patients with CAH. Further studies, and studies including children, are necessary to determine the long-term outcomes of Chronocort therapy.
Acknowledgments
We thank our patients for participating in the study and our nursing staff of the 5SWN Metabolic Unit for their assistance in conducting this study. We extend our thanks to Judith Starling (Investigational Drug Specialist, NIH Clinical Center Pharmacy) for her expert assistance in conducting this study and CARES Foundation for patient referrals.
This study was registered in ClinicalTrials.gov as trial number NCT01735617.
This work was supported in part by the Intramural Research Program of the National Institutes of Health and in part by Diurnal Ltd UK.
Disclosure Summary: D.P.M. received research funds from Diurnal Ltd through National Institutes of Health Cooperative Research and Development Agreement; D.D., D.J.A.E., and W.A. are consultants to Diurnal Ltd; M.J.W. and R.J.R. are Directors of Diurnal Ltd.
Funding Statement
This work was supported in part by the Intramural Research Program of the National Institutes of Health and in part by Diurnal Ltd UK.
Footnotes
- 17-OHP
- 17-hydroxyprogresterone
- SF36
- 36-item short-form health survey
- AUC
- area under the curve
- BMD
- bone mineral density
- CAH
- congenital adrenal hyperplasia
- CV
- coefficient of variation
- GFI
- global fatigue index
- HOMA-IR
- homeostasis model assessment–estimated insulin resistance
- NIH
- National Institutes of Health
- PRA
- plasma renin activity.
References
- 1. Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet. 2005;365:2125–2136. [DOI] [PubMed] [Google Scholar]
- 2. Speiser PW, Azziz R, Baskin LS, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4133–4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Finkielstain GP, Kim MS, Sinaii N, et al. Clinical characteristics of a cohort of 244 patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2012;97:4429–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Han TS, Stimson RH, Rees DA, et al. Glucocorticoid treatment regimen and health outcomes in adults with congenital adrenal hyperplasia. Clinical endocrinology. 2013;78:197–203. [DOI] [PubMed] [Google Scholar]
- 5. Mah PM, Jenkins RC, Rostami-Hodjegan A, et al. Weight-related dosing, timing and monitoring hydrocortisone replacement therapy in patients with adrenal insufficiency. Clin Endocrinol. 2004;61:367–375. [DOI] [PubMed] [Google Scholar]
- 6. Auchus RJ, Arlt W. Approach to the patient: The adult with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2013;98:2645–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Han TS, Walker BR, Arlt W, Ross RJ. Treatment and health outcomes in adults with congenital adrenal hyperplasia. Nature reviews Endocrinology. 2014;10:115–124. [DOI] [PubMed] [Google Scholar]
- 8. Johannsson G, Bergthorsdottir R, Nilsson AG, Lennernas H, Hedner T, Skrtic S. Improving glucocorticoid replacement therapy using a novel modified-release hydrocortisone tablet: A pharmacokinetic study. Eur J Endocrinol. 2009;161:119–130. [DOI] [PubMed] [Google Scholar]
- 9. Newell-Price J, Whiteman M, Rostami-Hodjegan A, et al. Modified-release hydrocortisone for circadian therapy: A proof-of-principle study in dexamethasone-suppressed normal volunteers. Clin Endocrinol. 2008;68:130–135. [DOI] [PubMed] [Google Scholar]
- 10. Verma S, Vanryzin C, Sinaii N, et al. A pharmacokinetic and pharmacodynamic study of delayed- and extended-release hydrocortisone (Chronocort) vs. conventional hydrocortisone (Cortef) in the treatment of congenital adrenal hyperplasia. Clin Endocrinol. 2010;72:441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Debono M, Ghobadi C, Rostami-Hodjegan A, Huatan H, et al. Modified-release hydrocortisone to provide circadian cortisol profiles. J Clin Endocrinol Metab. 2009;94:1548–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Whitaker MJ, Debono M, Huatan H, Merke DP, Arlt W, Ross RJ. An oral multiparticulate, modified-release, hydrocortisone replacement therapy that provides physiological cortisol exposure. Clin Endocrinol (Oxf). 2014;80:554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Finkielstain GP, Chen W, Mehta SP, et al. Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2011;96:E161–E172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arlt W, Ross RJ, Taylor A, Debono M. Comparison of Parameters Defining Cortisol Diurnal Rhythm as Measured by Liquid Chromatography Tandem Mass Spectrometry and Immunoassay. Program of the 94th Annual Meeting of The Endocrine Society, Houston, TX, 2012 (Abstract MON-480). [Google Scholar]
- 15. sf98norms.pdf.
- 16. Øksnes M, Bensing S, Hulting AL, et al. Quality of life in European patients with Addison's disease: Validity of the disease-specific questionnaire AddiQoL. J Clin Endocrinol Metab. 2012;97:568–576. [DOI] [PubMed] [Google Scholar]
- 17. Belza BL. Comparison of self-reported fatigue in rheumatoid arthritis and controls. Journal Rheumatol. 1995;22:639–643. [PubMed] [Google Scholar]
- 18. Arlt W, Willis DS, Wild SH, et al. Health status of adults with congenital adrenal hyperplasia: A cohort study of 203 patients. J Clin Endocrinol Metab. 2010;95:5110–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frisch H, Parth K, Schober E, Swoboda W. Circadian patterns of plasma cortisol, 17-hydroxyprogesterone, and testosterone in congenital adrenal hyperplasia. Arch Dis Child. 1981;56:208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sólyom J. Diurnal variation in blood 17-hydroxyprogesterone concentrations in untreated congenital adrenal hyperplasia. Arch Dis Child. 1984;59:743–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Merke DP. Approach to the adult with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2008;93:653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Speiser PW, Azziz R, Baskin LS, et al. A summary of the Endocrine Society clinical practice guidelines on congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency. Int J Pediatr Endocrinol. 2010;2010:494173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Al-Shoumer KA, Ali K, Anyaoku V, Niththyananthan R, Johnston DG. Overnight metabolic fuel deficiency in patients treated conventionally for hypopituitarism. Clin Endocrinol (Oxf). 1996;45:171–178. [DOI] [PubMed] [Google Scholar]
- 24. Seibel MJ, Cooper MS, Zhou H. Glucocorticoid-induced osteoporosis: Mechanisms, management, and future perspectives. Lancet Diabetes Endocrinol. 2013;1:59–70. [DOI] [PubMed] [Google Scholar]
- 25. Falhammar H, Filipsson H, Holmdahl G, et al. Metabolic profile and body composition in adult women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2007;92:110–116. [DOI] [PubMed] [Google Scholar]
- 26. Falhammar H, Filipsson Nystrom H, Wedell A, Thoren M. Cardiovascular risk, metabolic profile, and body composition in adult males with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur J Endocrinol. 2011;164:285–293. [DOI] [PubMed] [Google Scholar]
- 27. Debono M, Ross RJ, Newell-Price J. Inadequacies of glucocorticoid replacement and improvements by physiological circadian therapy. Eur J Endocrinol. 2009;160:719–729. [DOI] [PubMed] [Google Scholar]
- 28. Hindmarsh PC. The child with difficult to control Congenital Adrenal Hyperplasia: Is there a place for continuous subcutaneous hydrocortisone therapy. Clin Endocrinol. 2014;22:12453. [DOI] [PubMed] [Google Scholar]
- 29. Auchus RJ, Buschur EO, Chang AY, et al. Abiraterone acetate to lower androgens in women with classic 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2014:jc20141258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Merza Z, Rostami-Hodjegan A, Memmott A, et al. Circadian hydrocortisone infusions in patients with adrenal insufficiency and congenital adrenal hyperplasia. Clin Endocrinol. 2006;65:45–50. [DOI] [PubMed] [Google Scholar]
- 31. Bryan SM, Honour JW, Hindmarsh PC. Management of altered hydrocortisone pharmacokinetics in a boy with congenital adrenal hyperplasia using a continuous subcutaneous hydrocortisone infusion. J Clin Endocrinol Metab. 2009;94:3477–3480. [DOI] [PubMed] [Google Scholar]
- 32. Tuli G, Rabbone I, Einaudi S, et al. Continuous subcutaneous hydrocortisone infusion (CSHI) in a young adolescent with congenital adrenal hyperplasia (CAH). J Pediatr Endocrinol Metab. 2011;24:561–563. [DOI] [PubMed] [Google Scholar]
- 33. Johannsson G, Nilsson AG, Bergthorsdottir R, et al. Improved cortisol exposure-time profile and outcome in patients with adrenal insufficiency: A prospective randomized trial of a novel hydrocortisone dual-release formulation. J Clin Endocrinol Metab. 2012;97:473–481. [DOI] [PubMed] [Google Scholar]
- 34. Russell GM, Durant C, Ataya A, et al. Subcutaneous pulsatile glucocorticoid replacement therapy. Clin Endocrinol (Oxf). 2014;81:289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lightman S, Terry JR. The importance of dynamic signalling for endocrine regulation and drug development: Relevance for glucocorticoid hormones. Lancet Diabetes Endocrinol. 2014;2:593–599. [DOI] [PubMed] [Google Scholar]
- 36. Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: Normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab. 2005;90:3106–3114. [DOI] [PubMed] [Google Scholar]
- 37. Postnova S, Fulcher R, Braun HA, Robinson PA. A minimal physiologically based model of the HPA axis under influence of the sleep-wake cycles. Pharmacopsychiatry. 2013;46(Suppl 1):S36–S43. [DOI] [PubMed] [Google Scholar]